Volume 11, Number 4—April 2005

Recurring Methicillin-resistant Staphylococcus aureus Infections in a Football Team
Cite This Article
An outbreak of community-associated methicillin-resistant Staphylococcus aureus (MRSA) skin and soft tissue infection (SSTI) occurred in a college football team from August to September 2003. Eleven case-players were identified, and boils were the most common sign. Linemen had the highest attack rate (18%). Among 99 (93% of team) players with cultured specimens, 8 (8%) had positive MRSA nasal cultures. All available case-players’ MRSA isolates characterized had the community-associated pulsed-field type USA300. A case-control study found that sharing bars of soap and having preexisting cuts or abrasions were associated with infection. A carrier-control study found that having a locker near a teammate with an SSTI, sharing towels, and living on campus were associated with nasal carriage. Successful outbreak control measures included daily hexachlorophene showers and hygiene education.
Football-related skin infections have gained national notoriety and public interest ( 1 , 2 ). Media coverage of high-profile athletes and teams with skin and soft tissue infection (SSTI) has provided more impetus for research of these infections. Annually, 60,000 college football players compete among 600 teams ( 3 ). The community-associated methicillin resistant Staphylococcus aureus (CA-MRSA) strains have been a cause of SSTI outbreaks among athletes participating in football, wrestling, rugby, soccer, fencing and canoeing ( 4–7 ; Jon Rosenberg, pers. comm.; Los Angeles County Department of Health Services, unpub. data). SSTIs (pustules, “insect bites,” boils, and abscesses) are the hallmarks of CA-MRSA infections ( 8 , 9 ). CA-MRSA causes disease in young, otherwise healthy persons without the usual risk factors for MRSA infections ( 9 ). In addition, CA-MRSA has unique molecular markers (SCC mec IV and Panton-Valentine leukocidin) and fewer resistance genes to non β-lactam antimicrobial drugs than healthcare-associated MRSA strains ( 10 , 11 ).
In August 2002, the Los Angeles County Department of Health Services (LACDHS) received reports of 2 college football players (players X and Y) on team A hospitalized for SSTIs due to MRSA, which was later identified as a community-associated strain (USA300) ( 12 ). No other MRSA SSTI was reported on team A until 1 year later. On August 25, 2003, an infectious disease physician notified LACDHS of the hospitalizations of 4 different players on team A with MRSA SSTIs. Despite the lack of background SSTI data on this team, the recurrence of infections prompted an investigation with objectives of identifying players with MRSA SSTIs and nasal carriage, conducting epidemiologic studies, implementing outbreak-control measures, and determining the genotype of the outbreak strain.
Team A was a college football program with 107 players on the roster at the time of the outbreak. The team practiced and played 11 of their 13 games on grass fields. Players began their football season with training camp from August 5 to 18, 2003. In camp, players were sequestered and lived together, in suites of 4 per dormitory, to foster camaraderie among teammates. Rigorous practices were held twice daily in the hot, summer weather.
Case Finding
Case-players were defined as team A members with MRSA culture-confirmed SSTIs or SSTIs presumably caused by the USA300 strain in the outbreak period August 5 to September 5, 2003. Because we suspected that disease exposure occurred during camp, we chose the study period from the start of training camp to ≈2 weeks after the end. Our experience with other SSTI outbreaks found that in most persons lesions develop within 2 weeks postexposure to CA-MRSA. To find case-players, we reviewed the trainer’s treatment log to identify players with skin lesions who required medical or surgical interventions. We asked the staff to conduct skin inspections of all players. Players were encouraged to report any skin lesion. In addition, we queried the student health center to determine if these infections were prevalent on campus.
Nasal Carriage Study
As soon as the current outbreak was recognized, a returning player (player X) was suspected to be the source of infection. Player X had 1 of the 2 cases of CA-MRSA SSTIs discovered in 2002. His locker was directly across from the index case-player, and he was a roommate, during camp, of another case-player. Trainers obtained a nasal culture from player X on August 25. On September 3, trainers obtained cultures from the anterior nares of 99 available team members for a nasal carriage study.
Laboratory Study
MRSA isolates from case-players and nasal carriers were characterized by using pulsed-field gel electrophoresis (PFGE) with the Sma I and Eag I restriction enzymes ( 12 , 13 ). PFGE patterns of the isolates were compared with the USA300 strain responsible for other SSTI outbreaks in Los Angeles County ( 14 ). This strain was previously determined to contain SCC mec IV by the Centers for Disease Control and Prevention (L. Yasuda, pers. comm.). We also characterized a sample of methicillin-susceptible Staphylococcus aureus (MSSA) isolates from players’ nasal cultures.
Case-Control and Carrier-Control Studies
On the basis of anecdotal reports of players sleeping in the locker room on used towels and delaying treatment of cuts and abrasions, we hypothesized that poor hygiene habits and compromised skin integrity might predispose players to infection. We designed a standard questionnaire to collect data on player demographics, living situation, football activities, exposure to persons with skin infections, hygiene practices, histories of skin lesions, and clinical symptoms. Trained health department employees administered the questionnaires in person.
We conducted unmatched case-control and carrier-control studies. Controls were selected by jersey numbers, by using a random-number generator, from asymptomatic teammates without nasal carriage of MRSA. Teammates with positive nasal cultures for MRSA were considered carriers. Carrier-players were defined as carriers with matching PFGE pattern to the USA300 strain. We excluded non-USA300 strains carriers to remove players who might represent the background prevalence of MRSA in the community. Players who were not available for interviews were not included. Bivariate analysis was completed by using Fisher exact test in Epi Info version 3.3 (CDC, Atlanta, GA, USA). Statistical significance was defined as p values <0.05. Because of the small sample size and zero-valued cells, similar risk factors from the bivariate analysis were grouped into categories. Multivariate analysis was completed by using the conditional exact test in SAS version 8 (SAS Institute Inc., Cary, NC, USA).
Outbreak Control Interventions
Upon recognition of the outbreak on August 25, team A instituted daily hexachlorophene showers for all players, increased the frequency of cleaning the facilities and athletic gear, disinfected the whirlpool tubs, provided more towels, and posted hand-hygiene signs in the locker room. Once nasal culture results were available, team physicians attempted to decolonize carriers with intranasal mupirocin ( 15 ). We recommended improving the timeliness of wound care, barring case-players from playing unless wounds were covered, discouraging the sharing of personal items and tubs, prohibiting sleeping in the locker room, and checking laundry procedures. We also disseminated CA-MRSA educational materials to staff and team members ( 16 ).
Characteristics of Case-players

Figure 1 . Epidemic curve of clinical and methicillin-resistant Staphylococcus aureus skin and soft tissue infections among players on a college football team by date of diagnosis, Los Angeles County, August–September 2003.

Figure 2 . Football field positions; see Table 2 for position-specific attack rates. S, safety; LB, linebacker; CB, cornerback; L, lineman; WR, wide receiver; TE, tight end; QB, quarterback; TB, tailback,...
We identified 11 case-players out of 107 team members for an attack rate of 10%. Cases were diagnosed during or within 2 weeks of the end of training camp ( Figure 1 ). The first case was diagnosed on August 15, the last on September 1. With 1 exception, infections occurred before the first scheduled game on August 30. The most common sign was a boil ( Table 1 ). The elbow was the most common body site infected. No infection was at a current site of skin trauma or occurred at >1 body location simultaneously. Before hospitalization, the index and second case-players were given cephalexin and levofloxacin, respectively, for their infections without any clinical improvement. In total, 4 case-players were hospitalized and treated with parenteral vancomycin. Subsequent nonhospitalized case-players were treated with doxycycline and rifampin. Lesions of 9 players required surgical incision and drainage. All case-players ultimately responded to treatment with resolution of their infections. The median age of case-players was 20 years, with a median tenure of 2 years on team A. Linemen had the highest attack rate (18%) among all field positions ( Figure 2 , Table 2 ). No quarterbacks, wide receivers, or special team players (kickers, punters) were affected. All were healthy men without underlying illnesses. Eight (80%) case-players interviewed reported having never worn elbow pads, and 6 (60%) usually did not have cuts or abrasions covered until >1 hour postinjury.
Characteristics of Carriers
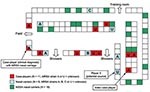
Figure 3 . Distribution of locker locations for case-players, methicillin-resistant Staphylococcus aureus (MRSA) nasal carriers, and methicillin-susceptible S. aureus (MSSA) nasal carriers.
Nasal cultures were obtained from 99 (93%) of 107 team members. Twenty-six (26%) cultures were positive for Staphylococcus aureus , among which 8 (8%) were positive for MRSA, including player X. Player Y’s nasal culture was negative. The median age of carriers was 20 years (range 18–21 years), and median tenure on the team was 2.5 years (range 1–5 years). MRSA carriage was highest in linemen (38%). We identified 1 case-player with nasal carriage of MRSA. However, trainers obtained nasal cultures after all case-players had begun antimicrobial treatment. Locker room assignments showed clustering of case-players and carrier-players, notably the proximity of the potential source player (player X) to the index case-player ( Figure 3 ). Among MSSA carriers (n = 18), no clustering of locker locations was seen. MSSA carriage was highest among linemen (28%) and cornerbacks/safeties (28%).
Laboratory Results
Four (57%) of seven MRSA isolates from culture-confirmed case-players were available for PFGE analysis. All were indistinguishable from each other, the USA300 strain found in Los Angeles County, and the isolates from 2 cases (players X and Y) in 2002. We denoted this genotype as strain A. Of 6 (75%) available MRSA isolates from 8 carriers, 4 (67%) were indistinguishable from strain A. Two carriers had unique MRSA genotypes (strains B and C) with > 7 bands difference between them and between strain A. Strains A, B, and C, player X and Y’s isolates, demonstrate community-associated antimicrobial susceptibility phenotypes ( Table 3 ). Among 5 MSSA isolates characterized, all had > 7 bands difference among themselves as well as from the USA300 strain.
Case-control and Carrier-control Study Results
Ten of 11 case-players were enrolled in the study; 1 was unavailable for interview. During camp, case-players were 15 times more likely than controls to have shared bars of soap with teammates and more likely to have had preexisting cuts or abrasions ( Table 4 ).
Five of 6 carrier-players were available for interviews. Carrier-players were 60 times more likely than controls to have had a locker adjacent to or across from a teammate with an SSTI and 47 times more likely to have shared towels with teammates ( Table 4 ). Carrier-players were more likely than controls to lived on campus in a dormitory or fraternity house. Among carrier-players and controls, players who lived on campus had a higher mean number of roommates than those who lived in off-campus apartments (2.3 vs. 1.5, p = 0.046).
Potential risk factors were grouped into 3 categories: “sharing” (sharing soap/towels with teammates), “skin injury” (cuts, abrasions), and “close contact” (locker adjacent to case-players, living on-campus). Multivariate analysis including these categories indicated that sharing was a significant risk factor for CA-MRSA infection (OR 12.1, 95% CI 1.83–108, p = 0.006) and carriage (OR 17.4, 95% CI 1.03–undefined, p = 0.047).
Postintervention Surveillance
Daily hexachlorophene showers were in use from August 25 to September 19. No new infections were reported during the 4 weeks after the discontinuation of the hexachlorophene showers. From October 20 to November 9, MRSA SSTI developed in 4 players: a lineman with a chin abscess, a linebacker (player Y from 2002) with an elbow boil, a quarterback (player Z) with folliculitis on a leg, and a tight end with a gluteal boil. Three MRSA isolates (except from the tight end) were available for PFGE; all matched strain A. The lineman in this cluster shared bars of soap with his roommate, a case-player.
Because of ongoing disease transmission and to identify potential reservoirs of MRSA, all 28 staff and student trainers and managers were nasally cultured on November 3; 11 (39%) were positive for MSSA. None was positive for MRSA. On November 22, we observed an official game. Previously unidentified lapses in hygiene practices occurred on the sidelines. We observed that student trainers reused hand towels between players, and players shared towels among themselves. Subsequently, the team switched to single-use towels on the sidelines. No new infections were reported for the remainder of the 2003 season. In the following season (August–December 2004), no MRSA SSTI outbreak occurred on team A. However, player Z had a recurrence of MRSA pustules on the forearm and leg in October 2004. He responded to outpatient treatment with doxycycline, rifampin, and incision and drainage of the lesions. His MRSA isolate was not available for PFGE. Throughout the last 3 football seasons, we received no reports of SSTI outbreaks among opposing athletes after playing this team.
This report is the first of recurring CA-MRSA SSTIs in a football team during consecutive seasons. From 2 cases in 2002 to an outbreak involving 11 players in 2003 and then 1 case in 2004, we have shown that eradicating these infections is difficult once they become established in a football team. Infections were likely propagated year to year from previously infected players, and they appear to be susceptible to recurring colonization and infection themselves.
Consistent with other reports, our findings implicate sharing personal items and improper wound care as risk factors for CA-MRSA infections ( 17 , 18 ). While the concept is counterintuitive, soap sharing was also associated with MRSA infections in a prison outbreak ( 19 ). Therefore, teams should consider switching to liquid soaps in an outbreak situation and always provide prompt wound care.
Linemen were identified as a high-risk subgroup. They engage in frequent and aggressive skin-to-skin contact during games, similar to hand-to-hand combat maneuvers as reported in a military MRSA outbreak ( 20 ). In addition, linemen tend to be physically larger than their teammates. Increased body mass index and lineman position were risk factors for CA-MRSA infection in another football team outbreak ( 18 ).
Two recent reported CA-MRSA outbreaks in football teams detected no nasal carriage in their combined cohort of 182 football players ( 17 , 18 ). In contrast, we document a high MRSA nasal carriage rate (8%) among team A players even while hexachlorophene showers were provided. The actual carriage rate might be higher, since we obtained nasal cultures after all case-players had begun antimicrobial treatment. Additional case-players may have been carriers as well, but they may have been decolonized before culture. Further research is needed to study the association between nasal carriage of CA-MRSA and SSTI to develop decolonization guidelines. The data facilitated a carrier-control study. Similar to risk factors for infection, nasal acquisition of CA-MRSA is associated with sharing personal items, particularly in the locker room.
Crowded living conditions during training camp appear to facilitate the acquisition of CA-MRSA, which then propagates in on-campus housing. Investigators of an outbreak among military recruits found an association between having a roommate with an SSTI and MRSA infection ( 21 ). Consequently, players’ living arrangements should be as dispersed as possible.
Unique to our investigation are 1 confirmed and 2 presumed community-associated strains of MRSA. We presented laboratory results indicating that the outbreak strain was likely the USA300 genotype. Since we do not have PFGE results from 6 case-players, different strains could have caused those infections. However, a multiclonal outbreak is unlikely, since other MRSA SSTI outbreaks in Los Angeles County among soccer players, men who have sex with men, jail inmates, and newborns have been exclusively due to the USA300 strain ( 14,22 ; Los Angeles County Department of Health Services, unpub. data). In contrast, our limited data do not suggest a clonal spread of MSSA on this team. Multilocus sequence typing was not available locally, which prevented further characterization of the isolates.
Selection bias of case-players and controls is a limitation of this study. Enrollment of players with uncultured infections and those without PFGE results introduces the possibility of misdiagnosis and misclassification. Most football teams assign jersey numbers on the basis of field position. Therefore, our control selection method might not have captured a representative sample of the team. However, the distribution of field positions among controls and the entire team appears similar ( Table 2 ). The small sample size produces less precise (wide confidence intervals) results and prohibits more in-depth multivariate analyses. Reporting bias is possible, since players and the team fear negative publicity, and we do not have data on risk factors during the off-season. In order to maintain confidentiality, we were unable to interview several players because of high media scrutiny.
As CA-MRSA strains become more prevalent in the community ( 23 ), SSTIs will likely continue to afflict football players. Despite comprehensive infection control interventions, sporadic cases of MRSA SSTIs continue to occur on this team. However, a recurrent outbreak was averted in the latest season likely because of increased vigilance to proper hygiene practices and awareness of this disease among the staff and players.
Dr. Nguyen is a board-certified pediatrician and a medical officer in the United States Public Health Service. He is currently serving as an Epidemic Intelligence Service Officer of the Centers for Disease Control and Prevention and is stationed in Los Angeles, California. His research interests include investigating emerging infectious diseases, outbreaks, and public health issues.
Acknowledgment
The authors acknowledge Dan Jernigan, Arjun Srinivasan, Julie Magri, Nolan Lee, Sophia Kazakova, and Jon Rosenberg for their insight and assistance; the staff of the Acute Communicable Disease Control program and Public Health Laboratory of the Los Angeles County Department of Health Services; and the trainers, physicians, and players from team A for their hard work and participation.
- Yee D . Skin infection plaguing athletes [article on the Internet]. CBSNews.com. 2003 Oct 16 [cited 2004 Oct 16]. Available from http://www.cbsnews.com/stories/2003/10/16/health/main578473.shtml
- Mihoces G . Sports teams warned about unique skin infection. USAToday.com. 2003 Oct 14 [cited 2004 Dec 20]. Available from http://www.usatoday.com/sports/2003-10-14-sports-infections_x.htm
- National Collegiate Athletic Association . 1981-82 – 2003-04 NCAA sports sponsorship and participation rates report. 2004 Mar [cited 2004 Dec 27]. Available from http://www.ncaa.org/library/research/participation_rates/1982-2003/2003ParticipationReport.pdf
- Centers for Disease Control and Prevention . Methicillin-resistant Staphylococcus aureus infections among competitive sports participants—Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000–2003. MMWR Morb Mortal Wkly Rep . 2003 ; 52 : 793 – 5 . PubMed Google Scholar
- Stacey AR , Endersby KE , Chan PC , Marples RR . An outbreak of methicillin-resistant Staphylococcus aureus infection in a rugby football team. Br J Sports Med . 1998 ; 32 : 153 – 4 . DOI PubMed Google Scholar
- Goodman RA , Thacker SB , Solomon SL , Osterholm MT , Hughes JM . Infectious diseases in competitive sports. JAMA . 1994 ; 271 : 862 – 7 . DOI PubMed Google Scholar
- Lindenmayer JM , Schoenfeld S , O’Gracdy R , Carney JK . Methicillin-resistant Staphylococcus aureus in a high school wresting team and the surrounding community. Arch Intern Med . 1998 ; 158 : 895 – 9 . DOI PubMed Google Scholar
- Dominguez TJ . It’s not a spider bite, it’s community-acquired methicillin-resistant Staphylococcus aureus. J Am Board Fam Pract . 2004 ; 17 : 220 – 6 . DOI PubMed Google Scholar
- Naimi TS , LeDell KH , Como-Sabetti K , Borchardt SM , Boxrud DJ , Etienne J , Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA . 2003 ; 290 : 2976 – 84 . DOI PubMed Google Scholar
- Eady EA , Cove JH . Staphylococcal resistance revisited: community-acquired methicillin-resistant Staphylococcus aureus —an emerging problem for the management of skin and soft tissue infections. Curr Opin Infect Dis . 2003 ; 16 : 103 – 24 . DOI PubMed Google Scholar
- Vandenesch F , Naimi T , Enright MC , Lina G , Nimmo GR , Heffernan H , Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis . 2003 ; 9 : 978 – 84 . PubMed Google Scholar
- McDougal LK , Steward CD , Killgore GE , Chaitram JM , McAllister SK , Tenover FC . Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol . 2003 ; 41 : 5113 – 20 . DOI PubMed Google Scholar
- Tenover FC , Arbeit RD , Goering RV , Mickelsen PA , Murray BE , Persing DH , Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol . 1995 ; 33 : 2233 – 9 . PubMed Google Scholar
- Centers for Disease Control and Prevention . Public health dispatch: outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002–2003. MMWR Morb Mortal Wkly Rep . 2003 ; 52 : 88 .
- Laupland KB , Conly JM . Treatment of Staphylococcus aureus colonization and prophylaxis for infection with topical intranasal mupirocin: an evidence-based review. Clin Infect Dis . 2003 ; 37 : 933 – 8 . DOI PubMed Google Scholar
- Los Angeles County Department of Health Services . Community-associated methicillin-resistant Staphylococcus aureus information page [homepage on the Internet]. [cited 2004 Dec 23]. Available from http://lapublichealth.org/acd/MRSA.htm
- Begier EM , Frenette K , Barrett NL , Mshar P , Petit S , Boxrud DJ , A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis . 2004 ; 39 : 1446 – 53 . DOI PubMed Google Scholar
- Srinivasan A , Kazakova S . The bigger they are, the harder they fall: methicillin-resistant Staphylococcus aureus skin and soft tissue infections among professional football players—2003 [abstract #383]. Presented at the 14th Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; 2004 Apr 17–20; Philadelphia, Pennsylvania.
- Tobin-D’Angelo M , Lance-Parker S , Arnold K , LaMarre M , Taussig J , Lane ME . MRSA outbreak in a state prison: implications for prevention and control [abstract]. Presented at the 41st Annual Meeting of Infectious Diseases Society of America; 2003 Oct 8–12; San Diego, California.
- Zinderman CE , Conner B , Malakooti MA , LaMar JE , Armstrong A , Bohnkert BK . Community-acquired methicillin-resistant Staphylococcus aureus among military recruits. Emerg Infect Dis . 2004 ; 10 : 941 – 4 . PubMed Google Scholar
- Campbell KM , Vaughn AF , Russell KL , Smith B , Jimenez DL , Barrozo CP , Risk factors for community-associated methicillin-resistant Staphylococcus aureus infections in an outbreak of disease among military trainees in San Diego, California, in 2002. J Clin Microbiol . 2004 ; 42 : 4050 – 3 . DOI PubMed Google Scholar
- Centers for Disease Control and Prevention . Methicillin-resistant Staphylococcus aureus infections in correctional facilities—Georgia, California, and Texas, 2001–2003. MMWR Morb Mortal Wkly Rep . 2003 ; 52 : 992 – 6 . PubMed Google Scholar
- Kuehnert MJ , Hill H , McQuillan G , McAllister S , Kruszon-Moran D , Fosheim G , Prevalence of Staphylococcus aureus colonization in the United States—2001–2002 [abstract]. Presented at the 2004 Annual Meeting of the Infectious Diseases Society of America; 2004 Sep 29–Oct 3; Boston, Massachusetts.
- Figure 1 . Epidemic curve of clinical and methicillin-resistant Staphylococcus aureus skin and soft tissue infections among players on a college football team by date of diagnosis, Los Angeles County, August–September 2003.
- Figure 2 . Football field positions; see Table 2 for position-specific attack rates. S, safety; LB, linebacker; CB, cornerback; L, lineman; WR, wide receiver; TE, tight end; QB, quarterback; TB, tailback, FB, fullback.
- Figure 3 . Distribution of locker locations for case-players, methicillin-resistant Staphylococcus aureus (MRSA) nasal carriers, and methicillin-susceptible S. aureus (MSSA) nasal carriers.
- Table 1 . Characteristics of case-players (N = 11)
- Table 2 . Position-specific attack rates of clinical and methicillin-resistant Staphylococcus aureus skin and soft tissue infections among players on a college football team
- Table 3 . Comparison of antimicrobial susceptibility patterns for Staphylococcus aureus isolates from case-players, carriers, and players X and Y
- Table 4 . Comparison of selected potential risk factors and characteristics of case-players and carriers, versus controls
DOI: 10.3201/eid1104.041094
Table of Contents – Volume 11, Number 4—April 2005
| EID Search Options |
|---|
| – Search articles by author and/or keyword. |
| – Search articles by the topic country. |
| – Search articles by article type and issue. |
Please use the form below to submit correspondence to the authors or contact them at the following address:
Dao M. Nguyen, Los Angeles County Department of Health Services, 313 N. Figueroa St, Room 212, Los Angeles, CA 90012, USA; fax: 213-482-4856
Comment submitted successfully, thank you for your feedback.
There was an unexpected error. Message not sent.
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Article Citations
Highlight and copy the desired format.
| EID | Nguyen DM, Mascola L, Bancroft E. Recurring Methicillin-resistant Staphylococcus aureus Infections in a Football Team. Emerg Infect Dis. 2005;11(4):526-532. https://doi.org/10.3201/eid1104.041094 |
|---|---|
| AMA | Nguyen DM, Mascola L, Bancroft E. Recurring Methicillin-resistant Staphylococcus aureus Infections in a Football Team. . 2005;11(4):526-532. doi:10.3201/eid1104.041094. |
| APA | Nguyen, D. M., Mascola, L., & Bancroft, E. (2005). Recurring Methicillin-resistant Staphylococcus aureus Infections in a Football Team. , (4), 526-532. https://doi.org/10.3201/eid1104.041094. |
Metric Details
Article views: 1341.
Data is collected weekly and does not include downloads and attachments. View data is from .
Citations: 161
What is the altmetric attention score.
The Altmetric Attention Score for a research output provides an indicator of the amount of attention that it has received. The score is derived from an automated algorithm, and represents a weighted count of the amount of attention Altmetric picked up for a research output.
Microbiology with Diseases by Body System (4th Edition)
By bauman, robert w,, phd, chapter 6 - microbial nutrition and growth - clinical case study: boils in the locker room - questions - page 184: 3, work step by step, update this answer.
You can help us out by revising, improving and updating this answer.
After you claim an answer you’ll have 24 hours to send in a draft. An editor will review the submission and either publish your submission or provide feedback.

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Athl Train
- v.45(4); Jul-Aug 2010
National Athletic Trainers' Association Position Statement: Skin Diseases
Steven m. zinder.
* The University of North Carolina at Chapel Hill
Rodney S. W. Basler
† Fremont Dermatology, NE
‡ Lehigh University, Bethlehem, PA
Chris Scarlata
§ Cornell University, Ithaca, NY
David B. Vasily
‖ Aesthetica Cosmetic & Laser Center, Bethlehem, PA
To present recommendations for the prevention, education, and management of skin infections in athletes.
Background:
Trauma, environmental factors, and infectious agents act together to continually attack the integrity of the skin. Close quarters combined with general poor hygiene practices make athletes particularly vulnerable to contracting skin diseases. An understanding of basic prophylactic measures, clinical features, and swift management of common skin diseases is essential for certified athletic trainers to aid in preventing the spread of infectious agents.
Recommendations:
These guidelines are intended to provide relevant information on skin infections and to give specific recommendations for certified athletic trainers and others participating in athletic health care.
The nature of athletics exposes the skin of its participants to a wide variety of stresses. Trauma, environmental factors, and infectious agents act together to continually attack the integrity of the skin. Combined with the close quarters shared by athletes and generally poor hygiene practices, it is not difficult to see why skin infections cause considerable disruption to individual and team activities. 1 Skin infections in athletes are extremely common. Authors 2 of a recent literature review investigating outbreaks of infectious diseases in competitive sports from 1922 through 2005 reported that more than half (56%) of all infectious diseases occurred cutaneously. Recognition of these diseases by certified athletic trainers (ATs), who represent the first line of defense against spread of these infections to other team members, is absolutely essential. Prophylactic measures and swift management of common skin infections are integral to preventing the spread of infectious agents. The following position statement and recommendations provide relevant information on skin infections and specific guidelines for ATs working with the athletes who contract them.
RECOMMENDATIONS
Based on the current research and literature, the National Athletic Trainers' Association (NATA) suggests the following guidelines for prevention, recognition, and management of athletes with skin infections. The recommendations are categorized using the Strength of Recommendation Taxonomy criterion scale proposed by the American Academy of Family Physicians 3 on the basis of the level of scientific data found in the literature. Each recommendation is followed by a letter describing the level of evidence found in the literature supporting the recommendation: A means there are well-designed experimental, clinical, or epidemiologic studies to support the recommendation; B means there are experimental, clinical, or epidemiologic studies that provide a strong theoretical rationale for the recommendation; and C means the recommendation is based largely on anecdotal evidence at this time.
The recommendations have been organized into the following categories: prevention, education, and management of the skin infections. The clinical features of the most common skin lesions are presented in Table 1 .
Clinical Features of Common Skin Infections

- The administration must provide the necessary fiscal and human resources to maintain infection control. 30 , 31 Evidence Category: B
- Custodial staffing must be increased to provide the enhanced vigilance required for a comprehensive infection-control plan. Evidence Category: C
- Adequate hygiene materials must be provided to the athletes, including antimicrobial liquid (not bar) soap in the shower and by all sinks. 7 , 32 – , 35 Evidence Category: B
- Infection-control policies should be included in an institution's policies and procedures manuals. 22 , 31 , 36 – , 38 Evidence Category: C
- Institutional leadership must hold employees accountable for adherence to recommended infection-control practices. 8 , 30 , 39 – , 43 Evidence Category: B
- Athletic departments should contract with a team dermatologist to assist with diagnosis, treatment, and implementation of infection control. 44 Evidence Category: C
- Cleaning and disinfection is primarily important for frequently touched surfaces such as wrestling mats, treatment tables, locker room benches, and floors. 9 , 10 , 45 , 46 Evidence Category: A
- A detailed, documented cleaning schedule must be implemented for all areas within the infection-control program, and procedures should be reviewed regularly. Evidence Category: C
- The type of disinfectant or detergent selected for routine cleaning should be registered with the Environmental Protection Agency, and the manufacturer's recommendations for amount, dilution, and contact time should be followed. 10 , 31 , 47 Evidence Category: B
Correct hand-washing technique must be used, including wetting the hands first, applying the manufacturer's recommended amount of antimicrobial soap, rubbing the hands together vigorously for at least 15 seconds, rinsing the hands with water, and then drying them thoroughly with a disposable towel. 48 Evidence Category: A
- If hands are not visibly dirty, they can be decontaminated with an alcohol-based hand rub. 17 , 18 , 41 , 50 , 51 Evidence Category: B
- Hands should be decontaminated before and after touching the exposed skin of an athlete and after removing gloves. 52 – , 56 Evidence Category: B
- Athletes must shower after every practice and game with an antimicrobial soap and water over the entire body. It is preferable for the athletes to shower in the locker rooms provided by the athletic department. 57 Evidence Category: B
- Athletes should refrain from cosmetic body shaving. 25 Evidence Category: B
- Soiled clothing, including practice gear, undergarments, outerwear, and uniforms, must be laundered on a daily basis. 10 Evidence Category: B
- Equipment, including knee sleeves and braces, ankle braces, etc, should be disinfected in the manufacturer's recommended manner on a daily basis. 58 Evidence Category: C
- Athletes must be discouraged from sharing towels, athletic gear, water bottles, disposable razors, and hair clippers. 57 , 59 Evidence Category: A
- Athletes with open wounds, scrapes, or scratches must avoid whirlpools and common tubs. Evidence Category: C
- Athletes are encouraged to report all abrasions, cuts, and skin lesions to and to seek attention from an AT for proper cleansing, treatment, and dressing. Evidence Category: C All acute, uninfected wounds (eg, abrasions, blisters, lacerations) should be covered with a semiocclusive or occlusive dressing (eg, film, foam, hydrogel, or hydrocolloid) until healing is complete to prevent contamination from infected lesions, items, or surfaces. Evidence Category: C
The sports medicine staff must educate everyone involved regarding infection-control policies and procedures. 7 , 32 – , 35 , 60
- Administrators must be informed of the importance of institutional support to maintaining proper infection-control policies. 7 , 32 – , 35 , 60 Evidence Category: B
- Coaches must be informed of the importance of being vigilant with their athletes about following infection-control policies to minimize the transmission of infectious agents. 7 , 32 – , 35 , 60 Evidence Category: B
- Follow good hygiene practices, including showering with antimicrobial soap and water after practices and games and frequent hand washing. 57 – , 59 Evidence Category: B
- Have all practice and game gear laundered daily. 10 , 17 Evidence Category: B
- Avoid sharing of towels, athletic gear, water bottles, disposable razors, and hair clippers. 57 , 59 Evidence Category: B
- Perform daily surveillance and report all abrasions, cuts, and skin lesions to and seek attention from the athletic training staff for proper cleansing, treatment, and wound dressing. Evidence Category: C
- The custodial staff must be included in the educational programs about infectious agents to be able to adequately help in daily disinfection of the facilities. 10 Evidence Category: C
Fungal Infections

Skin diseases. A, Tinea capitis. B, Tinea corporis. C, Herpes simplex. D, Molluscum contagiosum. E, Impetigo. F, Folliculitis. G, Furuncle/carbuncle. H and I, Methicillin-resistant Staphylococcus aureus . All photos used with permission from www.dermnet.com .
- Diagnosis : A culture of lesion scrapings is the most definitive test, but a potassium hydroxide (KOH) preparation gives more immediate results. 61 Evidence Category: B
Recommended Pharmacologic Treatment Regimens for Common Skin Infections

Return-to-Play Guidelines for Contact-Sport Athletes With Infectious Lesions a

- Diagnosis : A culture of lesion scrapings is the most definitive test, but a KOH preparation gives more immediate results. 61 Evidence Category: B
- Treatment : Topical treatment with a cidal antifungal agent, such as terbinafine, naftifine, ciclopirox, or oxiconazole (or more than one of these), twice a day, is effective for localized lesions. More diffuse inflammatory conditions should be treated with systemic antifungal medication ( Table 2 ). 11 , 57 , 61 , 62 , 65 Evidence Category: B
- Criteria for return to competition : Athletes must have used the topical fungicide for at least 72 hours, and lesions must be adequately covered with a gas-permeable membrane ( Table 3 ). 63 , 64 Evidence Category: B
Viral Infections
- Diagnosis : A culture of lesion scrapings is the most definitive test but may take days. A Tzanck smear that identifies herpes-infected giant cells may give more rapid, accurate results. 1 , 57 , 61 , 66 Evidence Category: B
Fully formed, ruptured, and crusted-over lesions are unaffected by antiviral medication. Evidence Category: B
- i. Athlete must be free of systemic symptoms, such as fever, malaise, etc. Evidence Category: B
- ii. Athlete must have developed no new blisters for 72 hours. Evidence Category: B
- iii. All lesions must be surmounted by a firm adherent crust. Evidence Category: B
- iv. Athlete must have completed a minimum of 120 hours of systemic antiviral therapy. Evidence Category: B
- v. Active lesions cannot be covered to allow participation. Evidence Category: B
- Diagnosis : Clinical findings and microscopic inspection are the basis for diagnosis. 73 Evidence Category: C
- Treatment : Many anecdotal therapies have been suggested, but physical destruction of the lesions with a sharp curette is recommended. 26 , 64 , 73 – , 81 Evidence Category: B
- Criteria for return to competition : Lesions should be curetted and covered with a gas-permeable membrane ( Table 3 ). 64 Evidence Category: B
Bacterial Infections
Specimens for culture and antimicrobial susceptibility should be obtained from any questionable lesions. 57 Evidence Category: B
Topical mupirocin (Bactroban; GlaxoSmithKline, Middlesex, United Kingdom), fusidic acid (Fucidin H; Leo Pharma, Ballerup, Denmark), and retapamulin (Altabax; GlaxoSmithKline, Middlesex, United Kingdom) have been shown effective in treating impetigo. 1 , 57 , 82 , 83 Evidence Category: B
- i. No new skin lesions for at least 48 hours. Evidence Category: B
- ii. Completion of a 72-hour course of directed antibiotic therapy. Evidence Category: B
- iii. No further drainage or exudate from the wound. Evidence Category: B
- iv. Active infections may not be covered for competition.
- i. Athlete must be referred to physician for incision, drainage, and culture. Evidence Category: B
- ii. Antibiotic therapy must be initiated to control local cellulitis. Evidence Category: B
- iv. Active infections may not be covered for competition. Evidence Category: B
- i. The differential diagnosis for any potential Staphylococcus lesion must include MRSA. 27 , 84 , 86 , 87 Evidence Category: B
- ii. Reports of “spider bites” should be considered a possible sign for community-associated MRSA (CA-MRSA). 84 Evidence Category: B
- iii. Specimens for culture and antimicrobial susceptibility should be obtained from any questionable lesions. 84 , 86 Evidence Category: B
- i.Athletes with suspicious lesions must be isolated from other team members. Evidence Category: B
- ii.Antibiotic treatment must be guided by local susceptibility data and be determined on a case-by-case basis. 23 , 84 , 86 , 88 – , 93 Evidence Category: A
- ii.Completion of a 72-hour course of directed antibiotic therapy. Evidence Category: B
Clinical Dermatology: A Color Guide to Diagnosis and Therapy by Habif 94 and Skin Disease: Diagnosis and Treatment by Habif et al 95 are excellent references for the recognition, diagnosis, and treatment of skin diseases, as is www.dermnet.com , a Web site that contains more than 23 000 images of skin diseases.

LITERATURE REVIEW
Transmission of the infectious agent.
For the transmission of infectious agents, 3 basic elements are required: a source of the agent, an adequate susceptible host, and a mode of transmission for the agent to the host. 31 , 96 Infectious agents in health care settings have been shown to come from many sources, including other patients, 97 – , 100 roommates, and visitors. 99 , 101 These agents are also present in the athletic setting. The infected source may show active lesions or may be completely asymptomatic while in the incubation period of an infectious disease. It is, therefore, important to always assume that individuals are carriers of pathogenic microorganisms.
A very complex relationship exists between an infectious agent and a potential host patient. 31 Many factors, including the immune state of the patient at the time of exposure, virulence of the infectious agent, quantity of the infectious innoculum, and medications taken by the patient (eg, corticosteroids) can affect the outcome after exposure to an infectious agent. 31 , 102 Outcomes can range from no effect at all to asymptomatic colonization of the host to full symptomatic disease states. 31 Athletes have unique characteristics that make them particularly susceptible hosts. They participate in high-risk activities 103 and have constant assaults to the integrity of their skin, 57 making transmission that much easier.
Transmission of infectious agents to the host can occur in a myriad of ways: through direct or indirect contact, droplets, airborne routes, or percutaneous or mucous membrane exposure. 31 Direct transmission occurs when one infected person transfers the infectious agent to another through direct skin-to-skin contact. 31 Indirect transmission refers to situations in which a susceptible person is infected by contact with a contaminated surface, such as a wrestling mat or contaminated clothing. Many cases of indirect transmission in the health care setting are found in the literature, including patient care devices, 104 – , 106 shared toys in pediatric wards, 98 inadequately cleaned instruments, 6 , 107 – , 109 and poor hand hygiene, 9 , 45 the latter of which is possibly the most common method of indirect transmission. Inadequate vigilance about hand washing is thought to be largely responsible for transferring infectious agents from one surface to another in health care settings, dramatically increasing disease transmission. Also, clothing has been shown to be contaminated with potential pathogens after coming in contact with infectious agents. 110 , 111 Although supporting literature on indirect transmission in the athletic setting is lacking, it is not difficult to imagine the potential harm.
Droplet transmission occurs when infected droplets from sneezing, coughing, or talking make contact with the eyes, nose, or mouth of the host subject. 112 Airborne transmission occurs when residue from evaporated droplets or dust particles stays suspended in the air for long periods of time and becomes inhaled by a susceptible host. 113 , 114 In the athletic setting, the most common mode of transmission of skin diseases is direct or indirect contact from the source to the host. Other modes of transmission are beyond the scope of this review.
First and foremost, for a prevention plan to be effective, the organization (university, high school, corporation, etc) should be committed to preventing disease transmission. 31 This commitment should be manifested by including disease-transmission prevention in existing safety programs and policies and procedures manuals. 22 , 36 – , 38 These manuals should describe how the prevention principles will be applied, how infected persons will be identified, and how to communicate information about potentially infected persons to the proper personnel. 31 Skin diseases, especially CA-MRSA, are reaching pandemic proportions, so organizations should be prepared to provide fiscal and human resources for controlling infection in an ever-changing environment. 31
Furthermore, a culture of institutional safety shared by administrators, staff, and, in this case, athletes is essential to controlling infectious disease. 30 Standard precautions and preventive measures must become the norm in athletic facilities for these programs to be implemented. Hospital-based studies have shown a direct correlation between high levels of “safety culture” and adherence to safe practices. Institutions that have seamlessly integrated these programs into their daily routine have had a high degree of success in keeping their stakeholders accountable for disease-prevention measures. 39 , 40 , 43 This adherence to recommended practices can significantly minimize the transmission of infectious disease. 8 , 41 , 42
Education about infectious-disease transmission and the recommended practices to minimize it should be an essential component to any infectious disease-prevention program. Understanding the science behind the recommended practices allows the health care team to more readily apply the standard precautions and modify them to their specific setting. 7 , 32 – , 35 Adherence to safety precautions is higher in groups that have received education in infectious-disease control. 60
Hand hygiene is the single most important practice in reducing the transmission of infectious agents. 31 , 48 Because of the significance of this issue, the Centers for Disease Control and Prevention assembled the Hand Hygiene Task Force, which wrote a 56-page document, “Guideline for Hand Hygiene in Health-Care Settings.” 48 The guidelines 48 include recommendations to wash hands with antimicrobial soap when the hands are visibly dirty 49 or with an alcohol-based hand rub in the absence of visible soiling of the hands. 17 , 18 , 41 , 50 – , 52 , 115 Hands should always be decontaminated before 54 and after contacting a patient's skin, 52 , 53 after removing gloves, 55 , 56 and after using the restroom. 116 – , 118 Trivial as it may seem, properly decontaminating the hands is of utmost importance. The correct technique for hand washing includes wetting the hands first, applying an appropriate amount of product, rubbing the hands together vigorously for at least 15 seconds, rinsing the hands with water, and then drying thoroughly with a disposable towel. 48
The nature of athletic competition necessitates overall good personal hygiene practices. Close personal contact in both locker and dormitory rooms is a significant risk factor in disease transmission. 57 – , 59 Athletes are encouraged to shower with antimicrobial soap and water over the entire body immediately after each practice and game. 57 Athletes should also be discouraged from cosmetic body shaving (ie, shaving a body area other than the face or legs), which has been shown to increase the risk of CA-MRSA more than 6-fold. 25 Good personal hygiene decreases the colonization of bacteria 58 and can be a first line of defense against transmission of infectious agents.
It is also important to maintain a clean environment in the athletic training room, locker rooms, and athletic venues. Cleaning and disinfection is primarily important for frequently touched surfaces, such as wrestling mats, treatment tables, and locker room benches and floors. 9 , 10 , 45 , 46 An example of a cleaning schedule for a National Collegiate Athletic Association (NCAA) Division I wrestling program is provided in Table 4 . Maintaining a properly cleaned and disinfected facility requires a team approach, including contributions from ATs, athletic administration, coaches, athletes, and custodial staff. Education of all involved parties is essential to minimizing transmission of infectious agents, and regular review of the cleaning procedures should be performed. 48 The type of disinfectant or detergent selected for routine cleaning and disinfection is relatively unimportant, as long as it is registered by the Environmental Protection Agency and the manufacturer's recommendations for amount, dilution, and contact time are all followed. 10 , 31 , 47 Some authors have suggested using a 1∶10 ratio of household bleach to tap water for routine environmental disinfection. 119 Facility-based pathogen reservoirs most often result from a failure to follow the instructions rather than from the cleaning agent itself. 24 , 120
A Sample Cleaning Schedule for a National Collegiate Athletic Association Division I Wrestling Program a

Soiled textiles, including towels, athletic clothing, elastic wraps, etc, can be reservoirs for infectious agents. Although these items can be significant contributors to infectious-disease transmission, if handled, transported, and laundered properly, the risk of transmission to a susceptible host is negligible. 10 Another suggested potential risk factor for acquiring an infectious disease, sharing personal items such as bar soap, towels, water bottles, and protective equipment (eg, wrestling head gear), should be prohibited at all times. 57 , 59 Athletic clothing and towels need to be laundered every day after practice, and equipment such as neoprene sleeves, knee braces, and other protective equipment should be disinfected with a 1∶10 bleach solution daily 58 despite the fact that some authors 121 – , 123 have reported cases of contact dermatitis at this concentration.
The following sections provide literature support for fungal, viral, and bacterial infections. Background information, clinical features, diagnosis, treatment, prevention, and guidelines for return to competition will be presented for each of the infectious agents.
FUNGAL INFECTIONS
Dermatophytes (fungal organisms living in soil, on animals, or on humans 124 ) include a group of fungi that infect and survive mostly on dead keratin cells in the stratum corneum of the epidermis. The infectious organisms responsible for fungal infections are typically from the Trichophyton genus. 61 Specifically, Trichophyton tonsurans and Trichophyton rubrum are most often associated with tinea capitis and tinea corporis, respectively. 61 , 125 , 126
Chronic perspiration and the macerating affect of abrasive trauma contribute to the successful penetration of ubiquitous fungal elements, particularly in warm, moist areas such as the toe webs, inguinal creases, and axillary folds. In contact sports, the skin-on-skin contact of the participants and abrasions, both clinical and subclinical, also lend themselves to the passage of fungal infections from one athlete to the next. Dermatophyte infection can be manifested in many ways. Infections on the face and head are called tinea capitis , infections on the body are termed tinea corporis , infections in the groin are called tinea cruris , and infections of the feet are called tinea pedis . 57 , 124 A number of authors 107 , 125 , 127 – , 130 have researched the epidemiologic considerations of this widespread cutaneous problem among athletes.
The most common dermatophyte infection is tinea pedis, with prevalence rates ranging from 25% to 70% over the life span. 127 , 129 A review of 10 recent reports has presented information on athletic teams infected with tinea corporis. 125 As would be expected, the rates varied greatly from one group to another, depending in part upon the method of fungal identification and the fact that a certain level of penetration of infection into the team was necessary to identify the team for study. Certainly athletic teams at all levels of competition have no evidence of fungal infection. The rates of incidence of infection in the reported studies ranged from 20% to 77%. One overview 128 performed in the mid-1980s indicated that 60% of college wrestlers and 52% of high school wrestlers demonstrated tinea infections at some time during the course of the season. Other investigators have reported that 84.7% of high school wrestling teams had at least 1 wrestler with tinea corporis 131 and 95% of a Swedish club wrestling team exhibited cutaneous findings consistent with tinea infection, with 75% of those demonstrating positive cultures for T tonsurans . 130
From information gathered in these studies, it is obvious that fungal infection rates among athletes vary widely. However, we can assume that any athletic team in which the problem has been identified can expect active infections in one-half to as many as three-fourths of its members, underlying the importance of aggressive treatment of isolated cases once they have been identified.
Clinical Features
The clinical presentation of cutaneous fungal infections is diverse. Tinea capitis often presents as gray scaly patches accompanied by mild hair loss. 57 , 61 Tinea corporis, commonly known as ringworm, is characterized by a well-defined round, erythematous, scaly plaque with raised borders. 57 , 61 Tinea corporis gladiatorum (tinea corporis among athletes) 63 , 125 many times presents with a more irregular lesion, however. 120 Tinea corporis is most commonly found on the head, neck, trunk, and upper extremities and only rarely affects the lower extremities. 125 , 131 Tinea cruris presents with a well-defined erythematous plaque in the pubic and inguinal areas. 66 Finally, tinea pedis presents in the toe webs, where macerated skin is usually accompanied by thick scaling or desquamation. Marginated erythema with advancing scales will often progress from the toe web to the entire sole of the foot and extend over the lateral margins in the “moccasin” distribution. 62 Although early infection tends to be unilateral, bilateral involvement of the feet is common by the time the athlete seeks attention for the problem. Vesicle formation may appear near the advancing border, and the underside of the epithelium covering these vesicles is a rich source of fungal elements for diagnosis by both KOH preparation 57 and fungal culture. 57 , 61
Although fungal cultures are more definitive than a KOH test, especially for specifically diagnosing the exact causative organism, 3 weeks may be required to determine that a culture is negative. Positive growth, of course, occurs more rapidly. The culture should be taken for ultimate confirmation, but a KOH preparation provides a more immediate determination of infection. 61 In the hands of an experienced practitioner, these simple tests are invaluable for instituting immediate therapy, even though some KOH preparations lead to equivocal results. Very simply, scale obtained from a suspicious lesion is applied to a glass microscope slide, a 10% KOH solution is added, and a coverslip is applied. The slide is warmed, usually with a match, to degrade the keratin and expose the fungal elements.
Athletes in noncontact sports or with localized cases may initially be treated with topical preparations as a conservative first-line approach. 57 , 61 Topical treatments, including the cidal imidazoles, allylamines, and napthiomates, tend to be well tolerated by patients. 57 , 61 More widespread, inflammatory, or otherwise difficult-to-treat cases may require the use of systemic antifungals, such as fluconazole or terbinafine, 65 which can have substantial side effects. 61
The topical cidal antifungals terbinafine, naftifine, ciclopirox, and oxiconazole are suggested 11 with 2 to 4 times daily applications. Although this regimen may be effective in the off season, athletes in the midst of a competitive season should probably be treated immediately with oral terbinafine, itraconazole, or fluconazole. Topical treatment is typically required through the entire course of the season or at least 2 weeks in the off season. Typically, systemic treatment for common fungal infections should last 2 to 4 weeks. Scalp lesions can be particularly difficult to eradicate, so systemic therapy with medications such as terbinafine, ketoconazole, itraconazole, or fluconazole may be prescribed for up to 6 weeks; daily use of an antifungal shampoo such as ketoconazole or ciclopirox may be required in particularly virulent scalp infections in athletes. 4 , 62 A summary of common treatment regimens for dermatophyte infections is presented in Table 2 .
In athletes who are prone to tinea pedis, careful attention to drying the feet is a necessity, including careful towel drying, particularly of the toe webs. The regular application of foot powder or 20% aluminum chloride (Drysol; Person & Covey, Inc, Glendale, CA) is also valuable. Wearing shower shoes in the locker room may be beneficial. Daily changing of athletic socks and even blow drying of the feet and athletic shoes have also been recommended. 62 Immediately showering after each training session and thoroughly drying all areas, especially intertriginous areas, is recommended, as well as the use of absorbent sports briefs and the application of bacteriostatic powder, such as Zeasorb-AF (Stiefel Laboratories, Inc, Research Triangle Park, NC), to the axillae and groin.
Wrestlers represent a particularly difficult and crucial subset in terms of preventing fungal infections. Wrestlers with extensive active lesions, which can be identified on visual inspection by ATs and coaches, must be withheld from all contact. Wrestlers who have demonstrated a particular susceptibility for tinea corporis in the course of the competitive season have been successfully treated prophylactically throughout the entire season with a low dosage of fluconazole (150 mg every other week or 200 mg per month). 1 , 62 , 132 Wrestlers with particularly recalcitrant (persistent or recurrent) infections should have their family members and animals (eg, dogs, cats, and farm animals) examined as well, because these may be reservoirs for reinfecting the athletes. 133
Careful attention is also required to disinfecting wrestling mats. Although several investigators 134 were not able to isolate fungal organisms from mats, T tonsurans has been cultured from a mat immediately after use. 135 The cleaning of mats is probably not as significant as the concern about skin-to-skin contact, but careful disinfecting greatly reduces and may even eliminate tinea infections in some wrestling teams. 130 Daily cleaning of the mats with chlorine-containing disinfectant sprays, at least during the course of the competitive season, is recommended ( Table 4 ).
Return to Competition
Athletes with tinea may return to sport only after they are cleared by the examining physician or AT. 64 Clearance to compete can only be given if the lesions have adequately responded to treatment, which generally requires 3 days of topical treatment in minor cases or 2 weeks of systemic treatment in more severe cases. 63 Athletes with solitary or closely clustered, localized lesions will not be disqualified if the lesions are in a body location that can be covered securely. The barrier preparation should be a dressing, such as Opsite (Smith & Nephew, London, United Kingdom) or Bioclusive (Johnson & Johnson, Langhorne, PA) followed by Pro Wrap (Fabrifoam, Exton, PA) and stretch tape. Dressings should be changed after each match so that the lesion can air dry 64 ( Table 3 ).
VIRAL INFECTIONS
Two primary viral infections are prevalent in athletic populations: herpes simplex and MC. Herpes simplex infection is common among athletes, especially those engaged in activities with full skin-on-skin contact, such as wrestling 57 , 136 , 137 and rugby. 1 , 57 , 137 Molluscum contagiosum is a highly infectious pox virus skin infection caused by the MC virus, which is classified within the family of poxviruses (Poxviridae). 75
Herpes Simplex
Herpes infection is caused by the herpes simplex virus (HSV), and outbreaks in athletes that spread throughout the entire team have been widely reported. 128 , 136 In a study of high school wrestlers at one summer wrestling camp, 60 of 175 wrestlers at the camp developed herpes lesions. 136 In the general population, up to 60% of college students possessed antibodies for HSV. 138 Herpes infections specifically contracted by athletes were first studied in 1964, 139 but then there was a 24-year hiatus in the literature between the earlier clinical publications and the flurry of clinical reports between 1988 and 1992. 128 , 136 , 140 , 141
Clinical features of HSV have been well described in the medical literature since 1964. 139 After an incubation period of 3 to 10 days, patients develop a variety of systemic signs or symptoms depending on their preexisting immunity to HSV. Symptoms can range in severity from a mild viral prodromal illness to an almost influenza-like illness with symptoms of fever, severe malaise, prostration, polyarthralgias, polymyalgias, pharyngitis, and conjunctivitis. Physical signs of the infection in athletes can include disseminated skin lesions and complications of conjunctivitis, keratitis, stomatitis, meningitis, arthritis, and hepatitis as well as marked lymphadenopathy and hepatosplenomegaly. Secondary infection with S aureus is common and often simulates bacterial folliculitis.
It is important for ATs to recognize the unique clinical features of HSV infection because an innocuous-appearing HSV lesion on the lip of an athlete can infect many other athletes who lack immunity against the virus. Recurrent HSV infections typically appear as a localized cluster of tense vesicles on the lip; however, it is important to note that particles from the virus reside latently in the dorsal root ganglia of the host's sensory nerves. Thus, recurrent HSV infections can appear in areas other than the lip and oftentimes in areas of previous outbreaks. 57 , 142 Typically, HSV lesions are located on the head, face, neck, or upper extremities. 19 , 46 , 68 , 128 The outbreak is usually preceded by symptoms that can include irritability, headache, tingling, and burning or itching of the skin at the site of recurrence. 61 Whether athletes are contagious during the prodromal period is unclear. However, we know that individuals with recurrent HSV labialis (fever blisters or cold sores) can shed the virus intermittently between episodes and in the absence of lesions, 143 and these individuals may represent a reservoir of virus for infecting previously uninfected athletes. The presence of HSV in the secretions of uninfected athletes is a significant one that needs to be investigated. If proven, a strong case could be made for season-long daily prophylaxis of all individuals on a team.
After the prodrome described above, a primary HSV outbreak often includes widespread clustered vesicles on an erythematous base in areas of contact of the head and neck, trunk, and arms in infected athletes. 61 , 144 Many times, numerous clustered perifollicular vesicles crust rapidly, giving the false impression of folliculitis. The vesicles may continue to erupt for a period of 7 to 10 days and eventually evolve into dry, crusted lesions.
The diagnosis of HSV is often delayed for days and misdiagnosed as occlusion, bacterial folliculitis, or other pyoderma because HSV clinically can simulate these conditions very closely. A high index of suspicion and clinical expertise is critical in evaluating athletes and diagnosing HSV. Viral culture of vesicle scrapings is the most definitive diagnostic tool, but results can take days. 1 , 57 , 61 , 66 A Tzanck smear that identifies herpes-infected giant cells is invaluable in making the correct diagnosis while awaiting the culture results. 1 , 57 , 61
The AT plays a very important and proactive role in the epidemiologic control of skin infections in athletes. This role begins with daily skin examinations before practices and games or matches. Any athletes with suspicious lesions should be immediately triaged to the team physician for disposition the same day. Whenever possible, ATs should establish relationships with local dermatologists to handle all their skin evaluation needs. An individual suspected of having a contagious skin disease should be immediately isolated from other team members until he or she is examined by the team dermatologist and the skin infection is properly managed. Implementation of these stringent epidemiologic-based concepts can result in a significant reduction in the incidence of skin infections among members of athletic teams.
Treatment of primary HSV is most effective with antiviral drugs such as acyclovir or valacyclovir. 69 Acyclovir represented the original therapy for HSV, 70 , 71 but the unwieldy dosing pattern of 5 times a day made compliance an issue. 67 The typical dosing regimen for valacyclovir, however, is 500 mg twice daily for 7 days. 67 , 68 Once the lesions are fully formed, ruptured, and crusted over, antiviral medications are no longer effective. 57 Topical antiviral creams have proven to be ineffective. 72
Retrospectively, Anderson 44 evaluated HSV outbreaks in Minnesota high school wrestlers during the 1999 season. Statistical analysis of these data confirmed the importance of properly screening and triaging all athletes with suspicious skin lesions for diagnosis and treatment before allowing further contact with other wrestlers. The average time from exposure to outbreak was 6.8 ± 1.70 days, with a 32.7% probability of transmission to sparring partners in a group.
In an evidence-based study, Anderson 68 reported on the prophylactic use of valacyclovir and concluded that wrestlers with a history of HSV for more than 2 years were adequately treated with valacyclovir 500 mg/d, and those with a history of lesions for less than 2 years showed reductions in HSV infections with 1 g/d of valacyclovir. 145
According to NCAA guidelines, 64 the athlete may not return to participation until he or she has received 5 days of oral antiviral therapy and all lesions have a dried, adherent crust ( Table 3 ).
Molluscum Contagiosum
In the United States between 1990 and 1999, 280 000 physician visits per year for MC were estimated. 146 The prevalence of MC in children has been reported to be as high as 7.4% 147 and considerably higher 148 in more confined communities. Several authors 149 , 150 have found no sex differences in the incidence of MC, whereas others 147 showed boys to be affected more often. The infection is commonly seen in younger children; however, because of skin-to-skin transmission, it is not uncommon for athletes, including swimmers, 147 , 151 cross-country runners, 5 and wrestlers, 152 to demonstrate MC infection in areas of direct contact with bodily secretions from other athletes. In addition to contact exposure, certain predisposing factors, such as atopic dermatitis, increase the likelihood of developing MC. 153 Many times in these individuals, a small, particularly itchy patch of eczema can develop around the lesions a month or more after their onset. 154 In addition, immunocompromised individuals and those on systemic steroids are at increased risk of developing extensive MC infections. 28 , 155 – , 157 Paradoxical immunosuppression in young, conditioned athletes has been described as a predisposing factor to explain the prevalence of infection in this population. 96
The clinical features of MC are fairly characteristic and usually do not present a diagnostic dilemma. The lesions typically are umbilicated, or delled, flesh-colored to light-pink pearly papules, measuring 1 to 10 mm in diameter. 73 Although usually a benign, self-resolving infection in nonimmunosuppressed people, 29 MC left untreated can persist for 2 to 4 years before clearing spontaneously. 158 Untreated MC can present a number of problems in athletes, including the development of secondary pyodermas with S aureus and an eczematous eruption surrounding individual lesions. 75 Rupture of molluscum papules can result in furuncle-like lesions that can heal with depressed varicelliform-type scars. 75 , 120 In fact, scarring after long-standing, untreated MC is not uncommon. 75
Because of the characteristic nature of MC lesions, the diagnosis of clinically suspicious lesions is routinely made on clinical examination. If the diagnosis is still uncertain, a Tzanck smear can be done on the crushed core contents of an individual molluscum papule to look for molluscum bodies, which appear on electron microscopic analysis as large, brick-shaped virus particles in positive samples. The MC lesions can occur as solitary lesions or be clustered (usually no more than 20) on body surfaces and, at times, be inoculated extensively into hair-growing areas, such as the beard or pubic area. 73
Numerous anecdotal therapies have been used for the treatment of MC, including agents such as cantharidin 81 and salicylic acid 79 and modalities such as cryotherapy 74 and pulsed-dye laser. 77 More recently, topical immunomodulators such as imiquimod have been used with varying degrees of success. 26 , 76 Evidence-based reviews 20 of reported anecdotal treatment modalities for molluscum show no definite statistical evidence of benefits to these therapies.
Physical destruction of scattered MC lesions with a sharp curette is recommended as the preferred method of treatment by many authors, 64 , 75 , 78 , 80 but little evidence-based research has been conducted using randomized controlled trials to evaluate its success. Physical destruction of the lesions is useful for rapidly clearing an athlete's skin and, thus, allowing participation in events and preventing both autoinoculation and spread to other athletes. 80 , 159 Curettage can be done easily with or without the use of topical anesthetic creams. When extensive, MC can be a reason for an athlete's disqualification from participation; however, solitary lesions can be appropriately covered or curetted before competition, according to the NCAA Wrestling Championships Handbook . 64 Although a recent evidence-based medicine review failed to determine any standard effective therapies, 160 the most efficient way to clear this infection rapidly and return the athlete to participation is simple curettage of lesions.
Prevention of the spread of this highly contagious infection is best accomplished by meticulous hygiene after exposure to another athlete's skin secretions or inanimate objects that have come in contact with secretions from other athletes, such as swimming pool benches, towels, gym equipment, and wrestling mats.
In most cases, the athlete must undergo some type of treatment before returning to competition. At this time, the NCAA requires athletes to have the lesions curetted or removed before return to play, although localized or solitary lesions may be covered with a gas-permeable dressing followed by stretch tape 64 ( Table 3 ).
BACTERIAL INFECTIONS
Bacterial infections are most commonly caused by various gram-positive strains of Streptococcus and Staphylococcus bacteria. 1 , 161 As much as 30% of the healthy population is colonized with Staphylococcus bacteria in the anterior nares. 162 Outbreaks of S aureus infections have been reported in football, basketball, and rugby players. 12 , 59 , 163
Impetigo is a contagious superficial bacterial infection, or pyoderma, of the skin caused by S aureus and group A β-hemolytic Streptococcus . 57 , 137 , 161 Impetigo is classified as bullous or nonbullous forms. 57
Bullous impetigo presents with superficial blisters (bullae) that rupture easily. The eruptions are typically moist and surrounded by a scaly rim. 164 Nonbullous impetigo, the most common form, initially presents with a thin-walled vesicle followed rapidly by rupture and desquamation to expose a raw, denuded surface covered with a yellowish-brown or honey-colored serous crusting in the perinasal and periorofacial areas. 1 , 57 , 164
Diagnosis of bacterial infections is primarily based on the history and characteristic appearance of the lesions, but with the increasing vigilance regarding antibiotic- resistant strains of Staphylococcus infections, scrapings or drainage samples of the lesions should be cultured. 57
Although impetigo has no standard therapy, the management guidelines include culture and sensitivity of suspicious lesions and treatment with appropriate topical or oral (or both) antibiotics. Good evidence shows that topical mupirocin, fusidic acid, or retapamulin is as effective or more effective and has fewer side effects than oral antibiotics. 83 Other authors 1 , 82 , 83 have recommended antibiotics such as dicloxacillin and cephalexin; or, if the athlete is allergic to penicillins, erythromycin may be used effectively. 1
Any suspicious lesions should be cultured and tested for antimicrobial sensitivity before return to competition. In general, return to competition after bacterial infections should not be allowed until the athlete has completed a 72-hour course of directed antibiotic therapy, has no further drainage or exudate from the wounds, and has developed no new lesions for at least 48 hours. 64 Also, because of the communicable nature of bacterial infections, active lesions should not be covered to allow for participation. 64
Folliculitis, Furuncles, and Carbuncles
Folliculitis, furuncles, and carbuncles are caused by follicular-based S aureus infections that arise in areas of high friction and perspiration. 57
Folliculitis presents as a myriad of perifollicular papules and pustules on hair-bearing areas, especially in areas that have been shaved, taped, or abraded. 94 Furuncles, or boils, are also follicular-based S aureus infections presenting as tender areas that, over a period of a few days, develop a reddened nodular swelling. 57 , 84 , 85 The lesions are essentially a perifollicular abscess that often progresses to spontaneous rupture and drainage. 57 Multiple furuncles that coalesce into a common, purulent mass, called a carbuncle, can be associated with surrounding cellulitis. 57 , 94
Diagnosis of folliculitis, furuncles, or carbuncles should follow the same progression as the diagnosis of impetigo. All diagnostic decisions should be based on the history and characteristic appearance of the lesions, with scraping or drainage samples of the lesions cultured to rule out antibiotic-resistant strains of Staphylococcus infections. 57
Athletes with folliculitis should be referred for culture of purulent perifollicular lesions and appropriate antibiotics. 57 Simple furuncles may be treated with warm compresses to promote drainage, but more fluctuant furuncles and carbuncles require incision and drainage. 57 , 84 , 165 After drainage, the athlete needs systemic antimicrobial therapy and close follow-up. 57 , 84 , 85 These lesions must be managed properly with incision, drainage, and antibiotics to control surrounding cellulitis. 84 As mentioned previously, furuncles and carbuncles may be caused by antibiotic-resistant strains of the Staphylococcus bacteria, so it is essential that this diagnosis be considered. Although ATs are not expected to manage these Staphylococcus abscesses, the athlete must be referred to a knowledgeable physician who will perform incision and drainage when necessary and treat with oral antibiotics.
Guidelines for return to competition after folliculitis, furuncles, or carbuncles are the same as for impetigo. Suspicious lesions should be cultured and tested for antimicrobial sensitivity, and return to participation should not be allowed until the athlete has completed at least 72 hours of directed antibiotic therapy, no drainage or exudate is visible from the wound, and no new lesions have developed in the previous 48 hours. Bacterial infections cannot be covered to allow for participation. 64
Methicillin-Resistant S aureus
In the early 1960s, an antibiotic-resistant strain of S aureus known as MRSA was described. 87 , 166 Methicillin-resistant S aureus has acquired the mecA gene 167 , 168 and is resistant to β-lactam antibiotics, including penicillins and cephalosporins, 13 , 86 , 88 although resistance to other classes of antibiotics, such as fluoroquinolones and tetracyclines, is increasing. 14 , 84 , 88 Until recently, MRSA was thought to be exclusively a hospital-acquired infection. 2 , 86 , 88 In the mid- to late 1990s, however, MRSA infections started to be detected in the community outside the typical health care settings, 2 , 86 , 168 , 169 being diagnosed in athletes participating in football, 25 , 170 , 171 wrestling, 21 and fencing, 171 where as many as 70% of team members required hospitalization and intravenous antibiotic therapy. 170 , 171 In one study, 172 the mortality attributable to MRSA infections was estimated to be as high as 22%. This new manifestation of MRSA, called CA-MRSA, is reported to be the most frequent cause of skin infections seen in emergency rooms across the country. 169 , 173 In one hospital in Texas, the number of CA-MRSA cases increased from 9 in 1999 to 459 in 2003. 174 Although the spectrum of MRSA appears to be similar in both types (furuncles, carbuncles, and abscesses are most commonly reported 27 , 84 , 86 , 88 ), CA-MRSA contains isolates that are distinct from those of MRSA acquired in the health care setting. 86 , 169 , 175 Risk factors associated with MRSA include recent hospitalization, outpatient visits, or close contact with a person with risk factors. 176 The risk factors for CA-MRSA are not as well defined, and it is not uncommon for patients with no identifiable risk factors to become infected. 177
An alarming increase in the prevalence of MRSA nasal colonization has been noted in both healthy children 15 and adults. 178 Nasal colonization of MRSA isolates in healthy children increased from 2.2% to 9.2% 15 and in healthy adults from 0.8% to 7.3% between 2001 and 2004. 86 , 179 Additionally, transmission of CA-MRSA is quite easy in close-contact settings, such as locker rooms and athletic fields, 175 so prevention, recognition, and proper management of MRSA are important responsibilities for the AT.
Prompt recognition of bacterial infections in athletes is vital to preventing both the spread of this highly contagious infection to other team members and contamination of athletic facilities where athletes congregate. Health care professionals should always consider CA-MRSA in the differential diagnosis of all patients presenting with symptoms associated with Staphylococcus disease.
Initially, CA-MRSA infections present similarly to other bacterial infections. 27 , 84 , 86 , 87 Furuncles, carbuncles, and abscesses are the most frequent clinical manifestations. 88 , 180 The lesions may begin as small pustules and develop into larger pustules or abscesses with areas of erythema and some tissue necrosis. 84 , 170 In several documented cases, 16 , 84 , 87 patients and their caregivers have confused CA-MRSA lesions with spider bites.
With any presentation of a skin and soft tissue infection compatible with that caused by S aureus or history of a “spider bite,” MRSA must be included in the differential diagnosis. 84 Any abscess or purulent skin lesion, particularly with signs of severe local or systemic infection, should be cultured for MRSA isolates and antimicrobial susceptibility. 84 , 86
It is critical for ATs to understand the proper recognition, dispensation, and management of MRSA infections. An athlete with a suspected MRSA infection must be immediately isolated from other team members and referred to a knowledgeable physician. The physician, who must maintain close contact with the AT in such cases, should abide by the evolving guidelines for the management of these infections. Individual treatment should be guided by local susceptibility data, because prevalence of resistance to antimicrobial agents varies geographically and is likely to change over time. 84 Although evidence from controlled clinical trials is presently insufficient to establish optimal treatment regimens for MRSA, several antimicrobial therapies have been proposed. 84 Mild to moderate cases in patients with no significant comorbidities still respond well to β-lactam agents. 84 Some experts 84 , 91 suggest that a prevalence of 10% to 15% of S aureus isolates, however, means that a change to alternative antimicrobial therapies might be needed. Alternative agents (both oral and parenteral) include vancomycin, 84 clindamycin, 92 daptomycin, 23 tigecycline, 93 minocycline, trimethoprim-sulfamethoxazole, 89 rifampin, 84 , 89 and linezolid. 84 , 86
Given their potential for rapid development of resistance, some antimicrobial agents are discouraged for the treatment of MRSA. Specifically, these agents include fluoroquinolones (ciprofloxacin and levofloxacin) 88 , 90 and macrolides/azalides (erythromycin, clarithromycin, and azithromycin). 88
Currently, no oral antibiotic prophylaxis is recommended for bacterial infections. Some authors 137 , 138 , 180 have discussed using agents such as mupirocin and antiseptic body washes to eliminate S aureus nasal colonization in healthy patients, although very limited data have examined the association between MRSA colonization and subsequent infection. 181 Prophylaxis is best accomplished by following standard infection-control precautions, good hand hygiene, and overall hygiene practices as recommended earlier.
Because of the prevalence and virulent nature of CA-MRSA, any suspicious lesions should be cultured and tested for antimicrobial sensitivity before the athlete returns to participation. In general, after a bacterial infection, return to play should not be allowed until the athlete has completed a 72-hour course of directed antibiotic therapy, has no further drainage or exudate from the wounds, and has developed no new lesions for at least 48 hours. 64 Also, because of the communicable nature of bacterial infections, active lesions cannot be covered to allow participation. 64
CONCLUSIONS
Certified ATs and other athletic team health care providers must be able to identify the signs and symptoms of common skin diseases in athletes. This position statement outlines the current recommendations to educate the stakeholders in their athletic programs about minimizing disease transmission, preventing the spread of infectious agents, and improving the recognition and management of common skin diseases in athletes.
Acknowledgments
We gratefully acknowledge the efforts of B. J. Anderson, MD; Wilma F. Bergfeld, MD, FAAD; Daniel Monthley, MS, ATC; Jeffrey Stoudt, MA, ATC; James Thornton, MA, ATC, PES; James Leyden, MD; and the Pronouncements Committee in the preparation of this document.
The NATA publishes its position statements as a service to promote the awareness of certain issues to its members. The information contained in the position statement is neither exhaustive nor exclusive to all circumstances or individuals. Variables such as institutional human resource guidelines, state or federal statutes, rules, or regulations, as well as regional environmental conditions, may impact the relevance and implementation of these recommendations. The NATA advises its members and others to carefully and independently consider each of the recommendations (including the applicability of same to any particular circumstance or individual). The position statement should not be relied upon as an independent basis for care, but rather as a resource available to NATA members or others. Moreover, no opinion is expressed herein regarding the quality of care that adheres to or differs from NATA's position statements. The NATA reserves the right to rescind or modify its position statements at any time.
Ensuring Your Athletes Are Safe in The Locker Room And Have Safe IAQ
Learn how to help your athletes stay safe and healthy by improving indoor air quality in school athletic facilities..
Sports season is upon us. This time of year, from game-winning goals to team celebrations, brings student-athletes together, and we encourage them to excel. Unfortunately, some unwanted intruders may be trying to join the team and sabotage the health of your players.
The locker room is an ideal setting for bacteria and infections to spread. The environment is often warm and humid, and players may share items such as towels, razors, athletic equipment, or uniforms. Bacteria, mold, and fungi can thrive in a moist environment and enter the skin or the lungs, causing sores, flu, boils, and more.
According to this study, the average gym locker room faucet handle had 8 times more bacteria than the school cafeteria water fountain spigot. And a locker room bench had 6 times more bacteria than an animal cage.
To protect your athletes from harmful bacteria, the best defense is a good offense. Taking proactive precautions can help reduce the risk of spreading the bacteria and taking down your team members. Read on to learn more about the pathogens your players may be exposed to and how to keep them safe.

Common Contaminants Found in Locker Rooms
The most common culprit found in most locker rooms is Staph, but other illnesses such as the common cold, flu, athlete's foot, and ringworm can also be spread in the changing rooms.
Short for Staphylococcus, this bacterium causes minor infections but has the potential to develop into something more serious. It’s hazardous for those with compromised immune systems and can lead to sepsis or bloodstream infections.
A Staph infection typically begins on the skin and soft tissue. It often presents as a red swollen hair follicle or a skin boil that is red, warm, and painful. It’s very contagious and can develop quickly.
MRSA stands for Methicillin-resistant Staphylococcus aureus. It is a strain of Staph that is resistant to certain antibiotics. It’s much more challenging to treat and can lead to severe complications.
Because athletes often get scrapes, cuts, and other injuries, this creates the opportunity for a nasty infection to enter the player’s body. Although these infections are invisible to the naked eye, they have the potential to cause deep painful abscesses that require surgical draining.

Mold is a fungus that thrives in warm and wet environments, making the locker room shower a perfect place to grow. It can be tracked inside shoes and work its way into the tile and grout. There are many different types of molds, some of which are considered toxic and can cause breathing issues if not dealt with properly.
Mold can also cause problems for people who have allergies or asthma. They may experience sneezing, wheezing, itchy eyes, or irritated skin.
Athletes Foot
Athlete’s Foot, aka. Tinea Pedis is a fungal infection lurks in warm wet places like locker rooms, communal showers, and pool decks. It starts as a scaly rash on the skin between the toes and can spread across the foot, causing itching, stinging, and burning.
Plus, it’s highly contagious. If one athlete on the team has an active athlete's foot infection and they don’t take the proper precautions, it can spread quickly to other teammates.
How To Reduce Contaminants in Locker Rooms?
Practice good hygiene.
After every practice and game, athletes should shower and wash their entire bodies with antimicrobial soap. Also, athletes should wash their uniforms after every game, and shared sports equipment should be sanitized.
Keep Wounds Protected
If an athlete has a cut or a scrape, they must keep it covered with a bandage until it is fully healed. Use antibacterial cream to treat wounds, and avoid swimming with an open cut or scrape. Always wear flip-flops when taking a shower.
.jpeg)
Don’t Share Personal Care Items
Each athlete should have a clean towel, soap, razor, and washcloth. Discourage your athletes from sharing personal care items, including hairbrushes, razors, washcloths, or makeup.
Improve Air Quality
If you remember back to what your high school locker room was like, one of the first things to come to mind is probably the smell or what we would call bad indoor air quality. Many things likely contributed to that smell, including the heating, ventilation, and air conditioning design. Focusing on improving air quality can not only help improve that all-familiar smell but keep your players safe from harmful contaminants that could affect their health.
An excellent passive method of improving IAQ is contaminant separation by having a dedicated dirty changing vestibule or mud room. This mud room is where athletes go directly after a workout or event to remove soiled uniforms, cleats, and other gear. Ideally, the mud room is adjacent to the laundry area, so the dirty uniforms and equipment do not have to be transferred throughout the facility to be cleaned.

Additionally, placing showers between the mud and locker rooms would encourage athletes to shower before entering the clean locker room. The locker room IAQ benefits from this setup by preventing dirty uniforms and equipment from polluting the locker space, where athletes store their clean clothes and generally spend time between activities.
Improving IAQ Through School HVAC Upgrades
Another method is providing increased filtration is a well-known way of improving IAQ. Providing MERV 13 filters in air handling units has become the expectation in many facilities. Including MERV 8 filters upstream of the more expensive MERV 13 filters help prolong their life.
Add commercial grade-air purification for your locker rooms, such as air scrubbers, ActivePure® Induct Guardian , air purifiers, and dehumidifiers, which are excellent for improving and maintaining your indoor air quality. You will instantly feel the changes immediately after using these air-cleaning devices.

Choosing The Right IAQ Technology From The Start
Not all air purifiers work the same. Some manufacturers produce air purifiers, but not all follow standards related to air filtration, testing efficacy, and operational safety. First, it is essential to know what mechanical and electric air cleaning technologies they feature when looking at air purifiers.
Consider an air purifier featuring ActivePure Technology, which has undergone exhaustive testing in both laboratory and real-world settings. It has been proven to reduce dangerous pathogens including those that cause COVID-19, RNA virus, Avian-Bird Flu, Swine Flu, and much more. It also reduces bacteria, mold, and fungal colonies from forming on surfaces, and eliminates up to 99.99% of airborne Staphylococcus Epidermidis bacteria.

When choosing the best air filter for your business, you should also consider additional efficiency measures like air exchanges per hour (ACH) and cubic feet per minute (CFM) . These measures will help you find the right air purifier to provide optimal air exchanges for the spaces you seek to protect such as the locker room.
For more information on how you can keep your student-athletes safe and protected from illnesses so they can play to their full potential - follow us on LinkedIn .
Navigating Carbon Emission Regulations in Washington State and Washington D.C. - Part 2
Navigating carbon emission regulations for businesses in the u.s. - part 1: boston and california, safeguarding workspaces against wildfire smoke: an indoor air quality guide.

Simplified Energy Management & IAQ Solutions From E360

E360 Indoor Air Quality Monitoring

E360 Energy Efficiency Solutions

Advanced Air Purification Systems
Navigating the Future of Energy Efficiency: Key Takeaways from COP28
Epa grant funding: transforming indoor air quality in schools, tackling the energy cost crisis in schools, absenteeism in k-12 schools: how improving indoor air quality can help, unlocking efficiency: how smart buildings benefit businesses, how businesses can identify asbestos risk and improve air quality, the importance of indoor air quality during cold & flu season, prioritizing energy cost reduction and indoor air quality in senior care facilities, why we should still be concerned about covid, understanding covid-19 variants, happy holidays from sanalife celebrating our clients and team, paramount group shows significant iaq improvements from installing activepure, little rock school district reports positive impact on in-person learning after installation of air purifiers in schools, an overview of boma's commercial building classes, facility manager's guide to upgrading your hvac system, 10 data-driven approaches to make facilities management easier, breathing new life into our schools: lessons from the pandemic on indoor air quality, understanding mold in indoor spaces and its impact on health and air quality, the impact of iot & ai on the evolution of nec electrical codes & energy management, choosing a control-agnostic energy management system: maximizing efficiency and flexibility, automated demand response: a new way to save money on your energy bill, disinfecting facilities with ultraviolet germicidal irradiation uvgi and uv-c, uv-c disinfection for covid-19 variants, technological trends for cleaning & maintaining public restrooms during the pandemic, sign up for the sanalife monthly newsletter.
Our best stories on indoor air quality and facilities management delivered straight to your inbox.
By signing up, you agree to our Privacy Policy & Cookie Statement and to receive marketing and account-related emails from Sanalife. You can unsubscribe at any time.
Your Path To Net Zero Starts With E360
We're dedicated to providing advanced energy management and indoor air quality solutions to schools, businesses, and organizations across the United States.
Contact Our Team
Air purifiers, technologies.
©2024 InTech Energy, Inc. DBA Sanalife and E360. All rights reserved. Various trademarks held by their respective owners.
Question asked by Filo student
Boils in the Locker Room For several weeks, faculty, students, and staff at Rayburn High School have been dealing with an epidemic of unsightly, painful boils and skin infections. Over 40 athletes have been too sore to play sports, and 13 students have been hospitalized. Nurses take clinical samples of the drainage of the infections from hospitalized students, and medical laboratory scientists culture the samples. Isolated bacterial colonies are circular, convex, and golden. The bacterium is Gram-positive, mesophilic, and facultatively halophilic and can grow with or without oxygen. The cells are spherical and remain attached in clusters. 1. What color are the Gram-stained cells? 2. What does the term “facultatively halophilic” mean? 3. What is the scientific description of the bacterium's oxygen requirement? 4. If the bacterium divides every 30 minutes under laboratory conditions, how many cells would there be in a colony after 24 hours?
Views: 5,517 students
Text solution Verified

Students who ask this question also asked
Views: 5,488
View solution
Views: 5,154
Views: 5,236
Views: 5,510

Stuck on the question or explanation?
Connect with our Biology tutors online and get step by step solution of this question.
| Question Text | |
| Topic | All topics |
| Subject | Biology |
| Class | Class 9 |
| Answer Type | Text solution: |
Are you ready to take control of your learning?
Download Filo and start learning with your favourite tutors right away!

IMAGES
VIDEO
COMMENTS
What is a boil? A boil is a red lump in the skin that may be warm and painful to the touch . It is a localized accumulation of pus and tissue debris. (Boils often start in an infected hair follicleBacteria form an abscess or pocket of pus. With time, the pus may come to a head and drain out through the skin.)
A case-control study found that sharing bars of soap and having preexisting cuts or abrasions were associated with infection. ... On the basis of anecdotal reports of players sleeping in the locker room on used towels and delaying treatment of cuts and abrasions, we hypothesized that poor hygiene habits and compromised skin integrity might ...
A case of recurrent painful boils. hony Moussa, Rodney SinclairCASEA woman aged 39 years presented with a 12-year history of recurrent painful nodules and boi. s to the axillae and pubic areas. On examination of the axilla (Figure 1), she was noted to have multiple tender inflam. d nodules and extensive scarring. Pus was able to b.
Question: Boils in the Locker Room For several weeks, faculty, students, and staff at Rayburn High School have been dealing with an epidemic of unsightly, painful boils and skin infections. Over 40 athletes have been too sore to play sports, and 13 students have been hospitalized. Nurses take clinical samples of the drainage of the infections ...
Boils in the Locker Room. For several weeks, faculty, students, and staff at Rayburn High School have been dealing with an epidemic of unsightly, painful boils and skin infections. Over 40 athletes have been too sore to play sports, and 13 students have been hospitalized.
Clinical Case Study Boils in the Locker Room For several weeks, faculty, students, and staff at Rayburn High School have been dealing with an epidemic of unsightly, painful boils and skin infections. Over 40 athletes have been too sore to play sports, and 13 students have been hospitalized. Nurses take clinical samples of the drainage of the ...
Microbiology with Diseases by Body System (4th Edition) answers to Chapter 6 - Microbial Nutrition and Growth - Clinical Case Study: Boils in the Locker Room - Questions - Page 184 3 including work step by step written by community members like you. Textbook Authors: Bauman, Robert W,, PhD, ISBN-10: 032191855X, ISBN-13: 978--32191-855-0, Publisher: Benjamin Cummings
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-1062. Crossref
Question: Please Note: Several of the questions below are different than the ones in the text. For several weeks, faculty, students, and staff at Rayburn High School have been dealing with an epidemic of unsightly, painful boils and skin infections. Over 40 athletes have been too sore to play sports, and 13 students have been hospitalized.
Study with Quizlet and memorize flashcards containing terms like Who was Lewanhook and what did he develop?, Who was Linnaeus?, Prokaryotic Charactoristics and more. ... Case Study 2: Clinical Case: Boils in the Locker Room. 5 terms. leipau33. Preview. Microbiology 2300 Chapter 10. 49 terms. SWOOD201. Preview. Chapter 7 Questions. 68 terms ...
Cleaning and disinfection is primarily important for frequently touched surfaces, such as wrestling mats, treatment tables, and locker room benches and floors. 9, 10, 45, 46 An example of a cleaning schedule for a National Collegiate Athletic Association (NCAA) Division I wrestling program is provided in Table 4. Maintaining a properly cleaned ...
Clinical Case Study #1-Boils in the Locker Room For several weeks, faculty, students, and staff at Rayburn High School have been dealing with an epidemic of unsightly, painful boils and skin infections. Over 40 athletes have been too sore to play sports, and 13 students have been hospitalized.
Study with Quizlet and memorize flashcards containing terms like Which type of media has ingredients that foster the growth of certain bacteria while suppressing the growth of others?, 1. Learning Goal: This excerise will facilitate your understanding of the selective and differential media called MacConkey agar. Overview: The diagnostic media, MacConkey, is a selective and differential media ...
Bacteria, mold, and fungi can thrive in a moist environment and enter the skin or the lungs, causing sores, flu, boils, and more. According to this study, the average gym locker room faucet handle had 8 times more bacteria than the school cafeteria water fountain spigot. And a locker room bench had 6 times more bacteria than an animal cage.
Solution For Boils in the Locker Room For several weeks, faculty, students, and staff at Rayburn High School have been dealing with an ... and 13 students have been hospitalized. Nurses take clinical samples of the drainage of the infections from hospitalized students, and medical laboratory scientists culture the samples. Isolated bacterial ...
Boils in the locker room for several weeks, faculty, students, and staff at rayburn high school have been dealing with an epidemic of unsightly, painful boils and skin infections. over 40 athletes have been too sore to play sports, and 13 students have been hospitalized. nurses take clinical samples of the drainage of the infections from hospitalized students, and medical laboratory scientists ...