

SSC Chemistry Assignment 2021 & 2022
SSC Chemistry Assignment 2021 & 2022 question and answer is available here. SSC 2021 exam candidates and SSC 2022 exam candidates, both Chemistry 2nd week, 3rd week, 5th week, 6th week, 8th week, 9th week assignment has been published. So we give all the solutions to the SSC Chemistry Assignment .
SSC Chemistry Assignment 2021 is for SSC Exam 2021 Candidates.
SSC Chemistry Assignment 2022 is for SSC Exam 2022, Class 10 Candidates.
Both Rosayon SSC Assignment questions and answers are below.
SSC Chemistry 9th Week Assignment 2022

অ্যাসাইনমেন্টঃ প্রকৃতিতে প্রাপ্ত কপারের দুটি আইসােটোপ ভর সংখ্যা ৬৩ ও ৬৫। প্রতিটি আসােটোপের মুল কণিকার সংখ্যা, ইলেকট্রন বিন্যাসের সাহায্যে মৌলটির পর্যায় সারণিতে অবস্থান, মৌলটির বিভিন্ন শক্তিস্তর ও উপশক্তিস্তর এবং তাতে বিদ্যমান। ইলেকট্রন সংখ্যা 2n2এবং 2(21+1) সূত্রের সাহায্যে বিশ্লেষণ কর
SSC Chemistry 9th Week Assignment Answer 2022

SSC Chemistry 8th Week Assignment 2021

অ্যাসাইনমেন্ট : যৌগ গঠনের সময় অষ্টক নিয়ম ও দুই-এর নিয়ম অনুসরণ, এদের গঠন প্রক্রিয়া, পনিতে দ্রাব্যতা এবং বিদ্যুৎ পরিবাহিতা।
SSC Chemistry 8th Week Assignment 2021 Answer

SSC Chemistry 6th Week Assignment 2021

অ্যাসাইনমেন্ট: বিভিন্ন যৌগ পর্যালােচনা করে পরমাণুসমূহের যােজনী, পরিবর্তনশীল যােজনী ও সুপ্ত যােজনী এবং যৌগগুলাের মধ্যে বিদ্যমান মৌলের তেজস্ক্রিয় আসােটোপের ব্যবহার
এটি এসএসসি ২০২১ ৬ষ্ট সপ্তাহের রসায়ন এসাইনমেন্টম আপনি যদি এসএসসি ২০২২ (বর্তমান নতুন দশম শ্রেণী) এর ৬ষ্ট সপ্তাহের রসায়ন এসাইনমেন্ট উত্তর পেতে চান তাহলে নিচে দেখুন। সমাধানের সাথে সাথে উত্তরটি পেতে আমাদের গ্রুপে যোগ দিন।
SSC Chemistry 6th Week Assignment 2021 Answer

SSC Chemistry 6th Week Assignment 2022

Assignment: ল্যাবরেটরীতে নিরাপদ উপকরণের বর্ণনা, দ্রব্যের বিভিন্ন সাংকেতিক চিহ্ন, নিরাপদ উপায় সাজানোর ধারণা ও দুর্ঘটনা রোধের উপায়
এটি এসএসসি ২০২২ (বর্তমান দশম শ্রেণী) শিক্ষার্থীদের জন্য এসাইনমেন্ট। আপনি যদি এসএসসি ২০২১ পরীক্ষার্থী হয়ে থাকেন, তাহলে আপনাদের এসএসসি ২০২১ রসায়ন এসাইনমেন্ট সমাধান নিচে রয়েছে।
SSC Chemistry 6th Week Assignment 2022 Answer

SSC Chemistry 5th Week Assignment 2021

অ্যাসাইনমেন্ট : রাসায়নিক বিক্রিয়া পর্যবেক্ষণ, বিক্রিয়ার সমীকরণ ও উৎপন্ন গ্যাসের সনাক্তকরণ পদ্ধতি বর্ণনা এবং কাপড় কাচা সোডা অথবা বেকিং সোডার আনবিক ভর নির্ণয়।
SSC Chemistry 5th Week Assignment 2021 Answer

SSC Chemistry 2nd Week Assignment 2021
SSC candidates have their first assignment in the Chemistry subject in the 2nd Week. Chemistry is determined from the third chapter of the first assignment. The title of the third chapter is the structure of matter. How a substance is formed and the different states of matter are discussed in this chapter. Thus, from the 2nd-week chemistry assignment of SSC, we will learn about the composition of matter. In order to solve the SSC 2nd week chemistry assignment correctly, one has to acquire detailed knowledge about substances and the basics of matter. Otherwise, it is not possible to give the answer to SSC 2nd week chemistry assignment correctly. So before writing the solution or answer of the SSC 2nd week chemistry assignment one must acquire knowledge about the content of the assignment.

Answer: প্রতীকের পাশে উল্লেখিত ভরসংখ্যাবিশিষ্ট মৌলের নিউট্রন সংখ্যা, বাের মডেল
Chemistry has been asked to prepare a report on electron configuration as the work of the 2nd Week assignment. Some symbols and their mass numbers are mentioned as signals to create the report. These must be used to create the report. A report must be prepared on the number of neutrons in the element with the mass number mentioned next to the symbol, the atomic structure according to the model, the electron configuration at the energy level, and the electron configuration at the substratum. The numbers mentioned in the chemistry assignment are given with the help of the figure below.
Here the structure of the atom according to the Bohr model should be mentioned along with the diagram and also the electron configuration of energy level and sub-energy level should be discussed. So those examinees who are worried about SSC 2nd Week chemistry assignment should follow our instructions. We will provide guidance on the solution of the 2nd Week assignment of chemistry or writing the answer sheet. Also for the convenience of students, we will upload the solution or answer of SSC 2nd Week chemistry assignment in PDF format. If anyone wants to download the solution or answer of 2nd Week chemistry assignment then visit our website.
Students need to follow a number of guidelines for writing the solution or answer of the 2nd Week chemistry assignment. Creating a solution or answer to the assignment by following the instructions below will definitely yield excellent results. As a result, it will be helpful to make the results of SSC 2021 better. Now I will give the instructions so that the students can create chemistry assignments in a good way by following them. The answer must be the number of neutrons of 4 elements. Also the structure of the atom according to the model of 4 elements has to be described with diagrams. Electron arrangement should be shown in the energy level of 4 elements. It is also necessary to show the electron configuration of the sub-layer of 4 elements. If you follow the above guidelines and create a solution or answer sheet for the chemistry assignment, you will definitely get very good results.
We can learn a lot from the chemistry assignment of SSC 2nd Week. For example, we can calculate the number of electrons, protons and neutrons in an atom. Rutherford and Bohr will also be able to describe the atomic model of atomic structure. I can arrange the electrons of the atom in different orbits of the atom and in different layers of the orbit.
SSC Chemistry 3rd Week Assignment 2021
Chapter Four has been selected as the SSC 3rd Week chemistry assignment. That is, all the questions of the 3rd Week chemistry assignment will be asked from the fourth chapter. And if you understand the fourth chapter well, it will be easier to give the solution or answer of the 3rd Week chemistry assignment. So before making a solution or answer of the SSC 3rd Week chemistry assignment one must acquire sufficient knowledge about the fourth chapter stage table. Because if you don’t have any knowledge about the periodic table, you can’t answer any questions about the periodic tables. So if you want to give a solution or answer to SSC 3rd Week chemistry assignment, you must follow the chapter.

Answer: Li, Be, Na, Mg মৌল চারটির ইলেকট্রন বিন্যাসের আলােকে পর্যায় সারণিতে অবস্থান
A report will have to be prepared as the work of the 3rd Week chemistry assignment. Students of SSC 2021 are asked to prepare a report from the periodic table to provide solutions or answers to the 3rd Week chemistry assignment. The task of the assignment is to make a report on the position in the periodic table in the light of the electron configuration of the element, the relative ionization energy and the properties of the group or class corresponding to the element. Here is a list of the four elements that make up the report. Assignment work should be completed based on this list. That is, the report has to be prepared and submitted.
What can we learn from SSC 3rd Week chemistry assignment?
There is a lot to learn about periodic tables and different elements from the SSC 3rd Week chemistry assignment. For example, it will be able to determine the relationship of the major groups in a periodic table with the electron configuration at the external energy level of the element. Students will be able to identify the stage of an element if they can complete the 3rd Week chemistry assignment properly. Also, by knowing the position of an element in the periodic table, one can get an idea about its physical and chemical properties. So you can tell the reason for the special naming of the elements. Also be able to show interest in estimating the religion of the elements by following the periodic table.
How to prepare solution or answer sheet of SSC 3rd Week chemistry assignment? In order to solve the 3rd Week SSC 2021 Chemistry Assignment or to prepare the answer sheet, the answer sheet must present some significant issues. The issues are- to determine the stages of 4 elements including electron configuration. It is not possible to get the full number if the stages are not determined correctly. Also 4 elements including electron configuration have to be determined correctly. The ionization energy of different elements of class and stage has to be compared properly. Features must also be specified with the special names of the two groups or classes. The above issues must be mentioned in the answer sheet. Besides, it is not possible to get very good marks through assignments. So if you want to get the best results they must follow our instructions.
SSC Chemistry Assignment 2021 & 2022 Answer & Solution PDF/Image

Facebook Page
Facebook Group
Earn Money By Writing Article
Recent Posts

রাজপাল যাদবের বাড়িতে তালা ঝুলিয়ে দিল ব্যাংক

গাজীপুরে এনআরবিসি ব্যাংকের ২৯ লাখ টাকা ছিনতাই

ওয়ালটনে নিয়োগ বিজ্ঞপ্তি, ৪০ বছরেও আবেদন

ঢাকা থেকে আন্তঃনগর ট্রেন চলবে দুপুরে

গণহত্যাকারীদের বিচারের মুখোমুখি কেন নয়, জানতে রুল

হেলিকপ্টার থেকে গুলিতে শিশুদের মৃত্যুর ঘটনায় হাইকোর্টের রুল

এখন যৌবন যার, যুদ্ধে যাবার তার শ্রেষ্ঠ সময়!

বিদ্যুৎ উন্নয়ন বোর্ডে নিয়োগ বিজ্ঞপ্তি

Dhaka Stock Exchange Latest Share Prices – ঢাকা স্টক এক্সচেঞ্জ

ছাত্র আন্দোলনে গুলিতে মারা গেছেন ৪ রোভার স্কাউট, আহত ৫২
Privacy Policy
- FREE: CAT Mock
- Free CAT Preparation
- CAT Study Material
- 500 Free CAT Questions
- Free CAT Preparation Videos
- Ask a Doubt
- CAT Online Coaching
- CAT Previous Papers PDF
- CAT Formulas PDF
- CAT Syllabus PDF
- CAT Study Plan PDF
- General Knowledge
- General Science
- Banking Daily Tests
- Banking Maths Fromulas PDF
- 10,000+ Banking Practice Questions
- Download Banking Previous Papers
Chemistry Notes PDF for RRB & SSC Exams – General Science

General Science – Chemistry PDF Notes for SSC CGL & RRB Exams (NTPC, JE, ALP and Group D):
Following is the Chemistry Important One-liner Notes for RRB Railway exams. You can download this Chemistry PDF useful for all competitive exams of UPSC (Civil services including IAS), SSC CGL, TNPSC etc.,
Download Static GK Capsule PDF
General Science Chemistry Notes in PDF
Download General Science – Chemistry Notes PDF
Last Day – 200 SSC Mocks for just Rs. 249
Download Chemistry Notes Set-2 PDF
Download Chemistry Notes Set-3 PDF
Basic Chemistry up to Class X level is asked in Competitive Exams such as Indian Railways –ALP and Group D exams and SSC exams. Cracku brings to you the capsule – One Liners covering exam specific topics in Chemistry.
Matter and its Nature
- Matter exists in three different states in Physical Form
- Solids – Molecules are closely packed. It is structural rigidity to changes of shape or volume
- Liquids – Molecular Bonds in a liquid are weaker than those in a solid but stronger than those in gases
- Gases – Molecular Bonds in a gas are loosely held and weaker than those in solids and liquids
| v Boiling Point | The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the Atmospheric Pressure |
| v Melting Point | The temperature at which the solid exists in equilibrium with its liquid under an external pressure of one atmosphere. |
| v Evaporation | Evaporation is the process of a substance in a liquid state changing to a gaseous state due to an increase in temperature and/or pressure |
| v Freezing Point | Freezing point is the temperature at which a liquid becomes a solid at normal atmospheric pressure |
| v Surface Tension | Surface tension is the elastic tendency of a fluid surface which makes it acquire the least surface area possible |
| v Decantation | Is a process to separate mixtures by removing a liquid layer that is free of a precipitate. The purpose may be to obtain the liquid free from particulates or to recover the precipitate |
| v Specific Gravity | The ratio of the mass of a substance to the mass of a reference substance for the same given volume |
| v Filtration | Filtration is process that separate solids from fluids by adding a medium through which only the fluid can pass through |
| v Sublimation | Sublimation is the transition of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase. |
| v Diffusion | Diffusion is defined as the movement of Gas into open space or diffusion into another gas |
| v Effusion | Movement of gas through a tiny hole is defined as effusion |
| v Emulsion | A fine dispersion of minute droplets of one liquid into another in which it is not soluble or miscible |
| v Viscosity | The state of being thick, sticky, and semi-fluid in consistency, due to internal friction |
| v Liquids at high altitudes boil at lower temperature due to low atmospheric pressure at high altitudes | |
| v Evaporation takes place only on the surface of Liquids | |
| v The melting point of Ice decreases with Increase in Pressure | |
| v Pure water has a maximum density of 1 gm/cm at 4 Degrees Celsius | |
| v Surface Tension decreases with Increase in temperature | |
| v Spherical Shape of Liquid Droplets is due to the property of Surface Tension in Liquids | |
| v Boiling Point and Evaporation of a liquid differ in a basic point that Evaporation occurs at all temperatures whereas Boiling Point of a liquid occurs at specific Temperature | |
- All matter is made up of Atoms which is the smallest particle of the element that consists of three fundamental units – Protons, electrons and neutrons.
| v Discovery of Atomic Nucleus | v Ernest Rutherford based on Geiger–Marsden Gold Foil Experiment |
| v Discovery of Protons | v E Goldstein |
| v Discovery of Electrons | v J J Thomson |
| v Discovery of Neutrons | v James Chadwick |
- Nucleus is the center of the Atom contains the neutral charges Neutrons and Positively charged Protons, the electrons revolve around the nucleus of an atom
- Protons, electrons and Neutrons are called sub-atomic particle.
- Each sub-atomic particle has an anti-particle with an opposite electric Charge
- “Positron” is not a sub-atomic particle but an anti-particle of electron which has same mass as electron – 9.10 ×10 -31 Kg with opposite charge, whereas Proton is a sub atomic particle with mass 1.6726219 × 10 -27 Kg and positive Charge
- Atoms combine with each other to form compound atoms called Molecules
- John Dalton was the first scientist to use symbols for elements in a very specific sense.
- The most commonly used measurement for atomic radius is Nanometer – 0 x 10 – 9 Metre and Angstrom A – 1.0 x 10 -10 Metre
- The relative Atomic Masses of all units have been measured w.r.t an atom of Carbon-12 which is equal to 1.66 × 10 -24 g
- Hydrogen has the smallest atom and is considered to have an atomic mass – 1
Current Affairs February 2018
Modern Indian History PDF Notes
- Compound formed between Atoms of Metals and Non-Metals have charged ions
| v Charge on the Ion – Negative | v Anion |
| v Charged on the Ion – Positive | v Cation |
- The combining capacity of an atom of an element with atoms of same element or different elements is called Valency of the element
| v Atomic Number | v Sum total of all protons present in the nucleus of an atom |
| v Atomic Mass | v Sum total of Protons and neutrons present in the nucleus of an Atom |
- The electrons present in the outermost shell of an atom are known as its valence electrons.
- Isotopes are atoms of same element having same Atomic Number but different Mass Numbers – (Hydrogen element has three Isotopes 1H1-Hydrogen, 2H1-Deuterium,3H1 – Tritium)
- Atoms of different elements with different Atomic Number but Same Mass Numbers are called Isobars. (Argon, Potassium, Calcium all have same Mass Numbers but different Atomic Number)
- Atoms of different elements, which have same number of neutrons but different atomic numbers, are called isotones.
- Avagadro Number: The number of Atoms present in 12g of Carbon of C-12 Isotope is 6.023 × 10 23 Atoms
- 1 Mole of any substance will contain Avagadro number of Molecules or 6.023 × 10 23 Atoms
- One Mole of any Gas at standard Atmospheric Pressure (STP) will have a volume of 22.4 Litres
- The electric neutrality of the atom is due to the presence of Equal number of Electrons and Protons in the atom
- The Spontaneous emission of radiation from the nucleus of an atom is a nuclear Phenomenon termed as Radioactivity
- Henry Becquerel first discovered radioactivity in 1896. The SI units to measure
- Radioactivity is Becquerel and unit of Becquerel is Second ‑1 (Second Inverse)
- The radiation dose absorbed by the human body is measured using the SI unit Gray or conventional unit RAD
- Carbon dating is a method for determining the age of an object containing organic material by using the properties of radiocarbon, a radioactive isotope of carbon.
- The most essential particle to continue the chain reaction in the fission of Uranium is Neutron.
SSC CGL Previous Papers Download PDF
Indian Polity PDF Notes
SSC CHSL PREVIOUS PAPERS DOWNLOAD
PERIODIC CLASSIFICATION OF ELEMENTS
- Elements, the purest form of substance can also be classified as
| v Metal – 91/118 in the Periodic Table
| v A material that is typically hard when in solid state, opaque, shiny, and has good electrical and thermal conductivity |
| v Non- Metals – 17/118 in the Periodic Table
| v Is a chemical element that tend to be highly volatile, have low elasticity, and are good insulators of heat and electricity |
| v Metalloids – 10/118 in the Periodic Table | v A metalloid is any chemical element which has properties in between those of metals and nonmetals |
- Eminent scientist suggested the classification of elements as Mendeleev’s Periodic Law which states that the Chemical and Physical Properties of elements are the periodic functions of their atomic weights.
- Important points to remember for competitive exams
| v Most Abundant element in the earth’s crust | v Oxygen |
| v Lightest element in the Universe | v Hydrogen |
| v The Only Liquid metallic Element | v Mercury |
| v Element which is the best conductor of Electricity | v Silver |
| v Highest electo-Negative Element | v Fluorine |
| v Most Malleable Element | v Gold |
| v The Most abundantly found element in the Human Body | v Oxygen |
ACIDS BASES AND SALTS
| ACIDS | BASES |
| Acids are compounds that form hydrogen ions when dissolved in water, and whose aqueous solutions react with bases and certain metals to form salts | Bases are compounds that, in aqueous solution, are slippery to the touch, taste astringent and react with acids to form salts |
| Acid is a Proton Donor | Base is a Proton Acceptor |
| An acid, which dissociates completely or almost completely in water. An acid that dissociates only partially when dissolved in water. | A base that dissociates completely or almost completely in water A base that dissociates partially when dissolved in water
|
| Acids are sour to taste | Bases are bitter to taste |
| The acidic property of an acid is due to the presence of hydrogen ions (H+) | Property of Base is due to the presence of hydroxyl (OH–) ions |
| A Salt results when an acid reacts with a base | |
| The P in pH stands for “Potenz” –meaning – Power | |
Medieval Indian History PDF Notes
Ancient Indian History PDF Notes
CHEMICAL BONDING, REACTIONS AND EQUATIONS
- The binding force of the constituent atoms of a molecule to maintain a mutual atomic order and definite shape is called Chemical Bonding
- There are three types of Chemical Bonding
| Electrovalent Bond | Covalent Bond | Metallic Bond |
| Chemical bond formed between two atoms due to transfer of electron(s) from one atom to the other. | A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. | Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons and positively charged metal ions. |
- Redox Reaction: Any reaction involves both a reduction process and a complementary oxidation process the two key reactions involved with electron transfer processes is called redox reaction
- An oxidation- reduction involves many parameters
| Oxidation Reaction | Reduction Reaction |
| Addition of Oxygen | Addition of Hydrogen |
| Removal of Hydrogen | Removal of Oxygen |
| Loss of electron | Gain of Electron |
| Increase in Valency | Decrease in Valency |
ORGANIC CHEMISTRY
Organic chemistry is the chemistry of carbon compounds, carbon which is an essential constituent of all Organic Compounds discovered till today.
The Simplest of all organic compounds are Hydrocarbons which contain only Hydrogen and Carbon
- CARBON – HYDROGEN – ORGANIC COMPOUNDS The three types of Hydro Carbons are Alkanes-CH 4 (Methane), Alkenes-C 2 H 4 (Ethene) and Alkynes- C 3 H 4 (Propyne)
- CARBON-HYDROGEN-OXYGEN Alcohols have (OH) Hydroxyl groups – Methanol (CH 3 OH) Most common compounds containing Carbon, Hydrogen and Oxygen is “Carbohydrates” (C 12 H 22 O 11 ) Some other examples are: Ketones, Aldehydes, Fatty Acids
- CARBON-NITROGEN-HYDROGEN Many important Organic Compounds are obtained in this combination Amines – NH 3 Cyanides etc…
RRB Asst Loco Pilot Cutoff Marks 2018
Oscar Award Winners 2018 PDF
IMPORTANT SCIENTISTS ASSOCIATED WITH CHEMISTRY
| v The first person to discover the evidence of Radio- Activity, The SI unit of Radio-activity is named after him. He is often considered as the Father of Radio-Activity | Antoine Henri Becquerel |
| v Scientist who did Pioneering research in the field of Radio- Activity Discovered important radio-active elements Radium and Polonium Won the Nobel Prize in chemistry in 1911 and Nobel Prize in Physics 1903 along with Antoine Henri Becquerel, Pierre Curie | Marie Curie |
| v A Swedish chemist, engineer, inventor, businessman, and philanthropist. Known for inventing dynamite and the founder of Nobel Prize instituted in 1895 | Alfred Nobel |
| v A Russian chemist and inventor who has formulated the Periodic Law, created a farsighted version of the periodic table of elements. | Dmitri Ivanovich Mendeleev |
| v A noted Scottish physician and chemist, known for his discoveries of Magnesium, latent heat, specific heat, and carbon dioxide | Joseph Black |
| v A Scottish physician, chemist and botanist who is credited with the discovery of nitrogen in 1772 | Daniel Rutherford |
| v A Cornish chemist and inventor, who is best remembered today for the discovery of multiple important elements essential for Humans – Pottasium, Calicium, Barium, Boron, Sodium | Sir Humphry Davy |
| v A British chemist who received the Nobel Prize in Chemistry in 1904 for the discovery of noble gases. | Sir William Ramsay |
| v English chemist and meteorologist who pioneered studies of Atomic Theory and who is credited with the discovery of color Blindness and is often considered as one of the founders of Modern Chemistry | John Dalton |
| v A Swedish chemist often considered as the founders of Modern Chemistry and is known for discovery of important elements – Silicon, Thorium | Baron Jons Jacob Berzelius |
| v A German Chemist considered as the father of Nuclear Chemistry and winner of Noble Prize in Chemistry in 1944 for discovery of Nuclear Fission | Otto Hann |
| v A Jewish chemist who received the Nobel Prize in Chemistry in 1918 for his invention of the Haber–Bosch process. | Fritz Haber |
| v The Most important element to Humans – Discovery of Oxygen is often credit with an English Scientist | Joseph Priestley |
| v A French physicist – a pioneer in magnetism, piezoelectricity and radioactivity, recipient of Nobel Prize in Physics for joint research on radiation Phenomenon | Pierre Curie |
| v A Mexican chemist known for his pivotal role in the discovery of the Antarctic ozone hole and Noble Prize winner for discovery of theory developed on depletion of Ozone due to CFCs | Mario Jose Molina |
| v A British biochemist one of only two people to have won twice the Nobel Prize in Chemistry for discovery of Amino acid sequence of insulin | Frederick Sanger |
| v An Italian scientist, most noted for his contribution to molecular theory now known as Avogadro’s law | Lorenzo Romano Amedeo Carlo Avogadro |
| v British Chemist known for development of Protein crystallography; determining the structure of Insulin | Dorothy Hodgkin |
| v Scientist credited with the discovery of the covalent bond | Gilbert N. Lewis |
| v Scientist credited with the discovery of 3 law of Thermodynamics | Walther Nernst |
| v Dutch Scientist and The first recipient of the Nobel Prize in Chemistry | Jacobus Henricus van ‘t Hoff |
| v Scientist credited with the discovery of Fluorine | Henri Moissan |
| v Current President of the Royal Society and recipient of Nobel Prize in Chemistry in 2009 for studying the structure of Ribosome | V Ramakrishnan |
| v A British scientist, and an important experimental and theoretical chemist noted for his discovery of hydrogen | James Chadwick |
IMPORTANT CHEMICALS AND COMPOUNDS
| Name of the Compound | Application |
| Hydrogen Chloride (HCL) | Found in stomach as Gastric Juice for Digestion of food |
| Sulphuric Acid | Often referred to as the “King of Chemicals”, has applications mainly in Car Batteries, Detergents, Fertilizers |
| Acetic Acid ( CH COOH) | Chemical name of Vinegar |
| Citric Acid | Present in Lemons and Citrus fruit |
| Sodium Chloride | Chemical Name of Common Salt |
| Benzoic Acid | Mainly used as a preservative for Food |
| Sodium Carbonate | Chemical Name of Washing Soda |
| Nitric Acid | Commonly used in Manufacturing of Fertilizers like Ammonium Nitrate |
| Sodium Hydrogen Carbonate | Chemical name of Baking Soda |
| Formic Acid | Used as Food Preservative, found mainly in ants, low concentration is useful to Humans. High Concentration is dangerous |
| Potassium Hydroxide | Chemical name of Caustic Pottash |
| Calcium Hydroxide | Chemical name of Lime Water |
| Boric Acid – Hydrogen Borate | Commonly used antiseptic, Flame retardant |
| Magnesium Hydroxide | Chemical name of Milk of Magnesia, commonly used an antacid |
| Aluminum Hydroxide | Most commonly used foaming agent in Fire Extinguishers |
| Potassium Nitrate | Commonly used in manufacturing of Match Sticks and Gunpowder |
| Calcium Carbonate | Very Important Compound in Cement Industry |
| Calcium Sulphate | Chemical name of Plaster of Paris |
| Calcium Hypochlorite | Chemical name of Bleaching Powder |
| 2-Acetoxybenzoic acid | Chemical name of Aspirin |
| Ethanol | Chemical name of Alcohol |
| Copper Sulphate | Chemical name of Blue Vitriol – Hydrated Salt |
| Ferrous Sulphate | Chemical name of Green Vitriol – Hydrated Salt |
| Magnesium Sulphate | Chemical name of Epsom Salt – Hydrated Salt |
| Borax | Chemical name of Sodium Borate – Hydrated Salt |
| Trichloromethane | Chemical name of Chloroform |
| Carbon Dioxide | Chemical Name of Dry Ice, primarily used as a cooling agent |
| Detergents are generally ammonium or sulphonate salts of long chain carboxylic acids Soaps are sodium or potassium salts of long chain carboxylic acids | |
| Composed of about 75% saturated hydrocarbons and 25% aromatic hydrocarbons | Diesel – Derived from Petroleum |
| Organic compound generally derived from a carboxylic acid and an alcohol | with characteristic odors are commonly used in synthetic flavors, perfumes, and cosmetics. |
| Second Isotope of Hydrogen –Water | Often called as Deuterium Oxide or Heavy Water, used in the nuclear reactor to slow down the speed of neutrons |
| Hydrated Iron Oxides | Rust – Red Oxide |
| Dinitrogen Monoxide | Chemical name of Laughing Gas |
| Hydroxy Propanoic Acid | Chemical name of Lactic Acid, a commonly used food preservative |
| Calcium Carbonate | Chemical name of Marble |
| Fuming Sulphuric Acid | Chemical name of Oleum, which is commonly used in Oil refining process |
| Ethanedioic Acid | Chemical name of Oxalic Acid- commonly used as a bleach for wood and |
| Trinitrophenol | Chemical name of Picric acid which is more acidic than phenol and is commonly used in military explosives, as an yellow dye. |
| Calcium Oxide | Chemical name of Quicklime |
| Chlorobenzalmalononitrile | Chemical name of Tear Gas |
| Zinc Sulphate | Chemical name of White Vitriol |
| Carbon Monoxide | Chemical name of Water Gas |
| Aluminum Hydroxide | Chemical name for Window Cleaner, has its application in the cleaning of windows |
| Silver Nitrate solution is poured into a solution of Sodium Chloride | White Precipitate |
| Potassium Iodide solution reacts with Lead Nitrate solution | Yellow Precipitate of Lead Iodide |
| Lead sulphide mineral | “Galena” primarily found ore of lead and is mined from large number of deposits from many countries |
Railways Group D Previous Year Papers
MAIN ORES OF IMPORTANT ELEMENTS
| Main ore of Iron | Hematite |
| Main ore of Aluminum | Bauxite |
| Main ore of Copper | Chalcopyrite |
| Main ore of Zinc | Sphalerite |
| Main ore of Lead | Galena |
| Main ore of Mercury | Cinnabar |
| Main source of Sodium | Rock Salt |
| Main ore of Tin | Cassiterite |
| Main Ore of Magnesium | Dolomite |
| Main ore of Phosphorous | Fluorapatite |
IMPORTANT DEFINITIONS, ABBREVIATIONS AND POINTS TO REMEMBER IN CHEMISTRY
| Element that is common to all acids | Hydrogen |
| Most Abundant element in the earth’s crust | Aluminum |
| First scientist to use symbols for elements in a very specific sense | John Dalton |
| Oxides of metals which show characteristics of both acidic and basic nature | Aluminum Oxide and Zinc Oxide – known as atmospheric oxides |
| Metals kept in kerosene to avoid combustion in open air due to their high reactivity | Potassium and Sodium |
| The relation of two or more compound that are composed of the same kind and number of atoms but differ from each other in structural arrangement | Isomers – Phenomenon is called Isomerism |
| The temperature at which a given mass of gas does not occupy any volume or does not exert pressure | Absolute Zero temperature |
| Celsius Scale Kelvin Scale Fahrenheit Scale
| Three scales to measure Temparature |
| Diamond Graphite Amorphous | Three states of Carbon |
| Substance that can exist in all the three states of matter – Solids, Liquids and Gases | Water |
| Acid that decomposes at ordinary room temperature | Nitric Acid |
| Strongest Oxidizing Agent | Fluorine |
| Strongest Reducing Agent | Lithium |
| Element found in maximum percentage in Human Body | Oxygen |
| The purest form of coal | Anthracite |
| Main element used in the conversion of Solar Energy | Silicon |
| PH values of important compounds: Hydrochloric Acid Vinegar Tomatoes Milk Pure Water Human Blood Milk of Magnesia Sodium Hydroxide | 0 2.2 4.5 6.6 7.0 7.4 10.5 14 |
| Element which has highest Melting Point | Tungsten |
| Element which has highest electron affinity | Chlorine |
| Element with highest Boiling Point | Tungsten |
| Element with lowest Boiling Point | Helium |
| Element which has the highest Density | Osmium |
| Element with the lowest Boiling Point | Helium |
| Element with the lowest Density | Hydrogen |
| Most commonly used chemical in photography | Silver Bromide |
| Most commonly used chemical for artificial rain or cloud seeding | Silver Iodide |
| Most common chemical used in toothpaste | Fluoride |
| Most common chemical used Voting Ink | Silver Nitrate |
| Chemical commonly used in Artificial ripening of Fruits | Calcium Carbide |
| Commonly used chemical used in Airbag | Sodium Azide |
| Commonly used chemical in Blood Bank | CPD : Citrate-Phosphate- Dextrose |
| Commonly used Chemical in Mouth Wash | Hydrogen peroxide |
| Most commonly used semiconductors | Germanium and Silicon |
Free SSC Online Coaching
RELATED ARTICLES MORE FROM AUTHOR

General Knowledge Questions and Answers for Competitive exams GK PDF

How to Crack CMAT 2024: A Comprehensive Guide

National Parks and Wildlife Sanctuaries (Geography) for SSC MTS
[…] Download Chemistry Notes PDF […]
nice notes send ne questions of chemistry.Please……
[…] Chemistry Notes […]
Please can I get Physics notes?
CHEMISTRY BY MY MURDA SIR TOPPER OF MURDAGHAR GHAZIPUR IS BETTER THAN THIS PDF FILE
LEAVE A REPLY Cancel reply
Save my name, email, and website in this browser for the next time I comment.
- Terms and Conditions
- Privacy Policy
- Get Cracku App
Get Exam Updates
Verify Email Address
Submit Code
skip →
verify phone
Enter verification code:
Send verification text to phone:
Gave the wrong phone number? Edit Number
Send Your phone no has been successfully verified.
Select Your Exam
MBA – CAT,IIFT,XAT,CMAT
Set a different course as default
Select Course
SSC Chemistry Assignment Answer 2022 pdf Download
SSC Chemistry Assignment Answer 2022 pdf Download has been published today on my daily result bd com website. The Department of Secondary and Higher Education has released a three-week assignment in the first phase as part of the preparation for this year’s secondary examination. Students have to do a total of 24 assignments in 12 weeks for SSC. These assignments need to be done on three group-based elective subjects. The assignment was published on the department’s website on Sunday night.
Assignment distribution and evaluation guidelines have also been published. Due to the corona virus, only three issues will be taken in the group-based election this time. The short syllabus of these subjects will be completed on the basis of the assignment. In a virtual press conference last Thursday, Education Minister Dipu Moni said that if the situation in Corona is favorable, secondary examinations will be held.
SSC Chemistry Assignment 2022

SSC Chemistry 5th Week Assignment 2021

SSC Chemistry Assignment Answer 2021 pdf Download

You have to complete the short syllabus through this. However, students do not have to do any assignments in the required subjects including Bengali and English and in the fourth subject. The number of required subjects will be determined by mapping the subjects of JSC, SSC and equivalent examinations. That means only three elective subjects will be evaluated.
Class 9 – 10 Chemistry Subject Assignment Solution 2021 pdf Download
Class 10 Assignment Subject: Chemistry Class: 10 Week: 4th Type: Answer SSC 2k21 Chemistry-2nd part solution

The probable time of examination is SSC and equivalent in the second week of November this year and HSC and equivalent examination is the first week of December. Students must submit assignments for this test. SSC and equivalent assignments on the basis of a short syllabus will be given from 16th July. Which was released on Sunday night. A total of 24 assignments will be given in 12 weeks.
Students will submit assignments twice a week on three elective subjects. A total of eight assignments have to be done in each elective subject.

Test Result BD

6th Week SSC 2022 Chemistry Assignment Answer
In this post about 6th Week, SSC 2022 Chemistry Assignment question and Answer Related. Now we were given by this post your subject assignment answer. Many students want to know when will be given the 6th Week SSC 2022 Chemistry Assignment Answer. Dear students this post is for you. By this post we have given the 6th Week SSC Chemistry Assignment Answer 2022 PDF And Jpg You can download both by this post.
SSC 2022 Chemistry Assignment is one of the subjects of the SSC education of Bangladesh. The SSC SSC 2022 Chemistry Assignment is quite different from that of other papers. You can know clearly about the pattern of this paper and the SSC 2022 Chemistry Assignment that can be expected by reading this post completely. We have given many 6th Week SSC 2022 Chemistry Assignment answer posts on other subjects for the SSC contending students and similarly, we have given the SSC 2022 Chemistry Assignment Answer which you can know through the information available here.
According to the new 6th week SSC 2022 Chemistry Assignment question released by all education boards, the Chemistry studies will be only 2022 exam other subjects. However, this paper is also separated into two different parts. subject part and objective questions part. 6th Week Assignment only Subject which candidates have to answer the questions related to the subject theory questions. Now we have given you SSC 2022 Chemistry Assignment 6th week answer by this page.

To easily qualify the chemistry studies paper Assignment answer info, candidates can concentrate more on written through which they can get more marks. You can also check out the model questions of both the parts available here and this will be helpful for you.

How to Download 6th Week SSC 2022 Chemistry Assignment
The SSC 2022 6th Week Chemistry Assignment questions Answer in the PDFs accessible here are only for reference purposes and gives you just an idea of the model and type of questions you can expect in the paper. They are not sure that you can get the same questions in the examinations. So, just consider them for reference and prepare similar questions during the preparation.
A final word of SSC 2022 6th Week Chemistry Answer
For 6th Week SSC Chemistry Assignment 2022 of other subjects, keep looking at our website regularly. Bookmark this site for all SSC 2022 assignment answer updates. Dear students if you see any answers are wrong please informal our comment box as soon as we can try your question solve.

SSC Chemistry 2022 Assignment Solution PDF File – 14th Week
Secondary School Certificate had to be submitted for SSC Chemistry Assignment Answer 2022 for 14th weeks. There website has been published chemistry assignment ssc full question solution 14th week. We also posted and you can get here your SSC ten class Chemistry assignment solution pictures and PDF files at chakrirkhobor.net. You want to get SSC Assignment questions and answers to keep reading below. You can get the ssc chemistry Assignment 2022 all Board.
Chemistry assignment atoms and particles: their organization, structure, properties. We try a full assignment solution for class SSC. Most of the Assignment finder many time fine Chemistry Assignment ten Class pdf download. Like different classes, the position will uncover ninth week Chemistry Assignment Answer.
SSC Chemistry Assignment Answer 2022- 14th Week
All Education Board start all subject Assignment and will end on maybe December 2022. SSC Chemistry assignments start in June and end in December 2022. Students can also check their SSC Chemistry 1st paper assignments question solve on these websites. This year newly start Assignment answer 2022 all board on their website.
How to do Chemistry Assignment Answer 2022?
It would be ideal if you’re Assignment and ensure you are following the procedure appropriately. Here you go. On the off chance that you don’t think about the SSC Class Chemistry Assignment process, this area will improve your insight in a matter of seconds.
Chemistry Assignment Solution Download 2022
All candidates who are filled the ssc Assignment application form can download your Assignment 2022 . SSC Chemistry Assignment 2022 PDF Download. SSC Education Board has recently released the notification regarding the ssc Assignment Exam solution 2022 exam dates check on the official Website. I realize who lost his employment because of lockdown. Nonetheless, since the understudies need to offer their own input, they need to reply.

How to install Chemistry Assignment for 2022?
So all People Keep read this article and know all details here. See here SSC Class Chemistry Assignment Answer, Pdf question solution; full off Marks all details given on this Page. Check also ssc Chemistry Assignment submit Date 2022 and Download link on this page. Check assignment Date Of School 2022. So all Applicants continue to read our article and know all details.
How to get Chemistry paper Assignment Answer?
Well, it’s quite obvious most of the students in Chemistry are belongs from all area, they may not have the facility of Computer, Laptop or Android MOBILE, if you are not able to check your Assignment, then you can get your schools. All education Board facilities get SSC Chemistry Answer.
When Chemistry Assignment will be Start 2022?
To whom it may concern & get bet Class Ten class Chemistry Assignment of All board and you try to see here. You cannot know to check and follow these rules.
14th Week Ten class Chemistry Assignment answer
In conclusion, Every Assignment seeker knows chakrirkhobor.net published all type of Assignment with an answer pdf file. This Notice also found on my website. Finally, you can understand get the total subject Chemistry Assignment all board 2022. So you have to connect for their address. In conclusion, for the next update about update assignment Notice. Finally 14th Week Class Chemistry Assignment etc. So stay with us.
6th Week Chemistry Assignment Answer SSC 2024

The SSC 2024 sixth week assignment has been published in the light of the short syllabus of the 2024 SSC candidates prepared by the National Curriculum and Textbook Board as per the instructions of the Ministry of Education due to the deadly coronavirus. On 23 August 2024, the authority published the sixth week assignment of students from all public and private educational institutions of the country to participate in the SSC examination in 2024 on the website of the department.
According to the grid of assignment publication, the Department of Secondary and Higher Education published the assignment for the fifth week of SSC 2024 on 16 August 2024, following which assignment homework was given for the scheduled subjects for the sixth week on 23 August. [ বাংলায় দেখুন ]
SSC 6th Week Assignment 2024
Assignments of the week prepared in the light of the short syllabus prescribed for the SSC candidates of 2024 have been assigned to the SSC candidates of all the Boards of Education for the sixth week from Geography and Environment, Chemistry, Accounting, Economics, Biology, Finance and Banking, Politics and Citizenship, Higher Mathematics text books.
Students will collect assignments from educational institutions in accordance with proper hygiene rules from 24th August 2024 and submit the relevant subject to the teacher by 29th August 2024 after completing the assignment writing guidelines given by the Department of Secondary and Higher Education.
SSC 2024 Science Department Examiner Friends, I have brought you an article on the use of Chemical Assignments in the sixth week of the 2024 SSC Examinations in Chemistry by reviewing various compounds, atomic valence, variable valence and latent valence and the use of existing elemental radioactive isotopes. Hopefully, by following this, you will be able to complete the chemistry assignment solution of the sixth week of 2024 SSC exam very nicely.
6th Week Chemistry Assignment SSC 2024

Preparation of a report on the use of radioactive isotopes of other elements by reviewing the following compounds as hydrogen atom 1 (one).
H2O, CO2, CCl4, PCl5, PCl3, PI5, SO2, SO3
Instructions (signal / step / circumference):
A) The calculation of atoms in 6 compounds has to be calculated; B) variable plan and latent plan should be calculated; C) In case of variable plan, large plan should be considered as maximum plan; D) write down the use of radioactive isotopes;

- 5th Week Assignment Chemistry Answer Hsc 2024
- 6th Week Biology Assignment Answer SSC 2024
Siam Shihab
The Site is down as we are performing important server maintenance, during which time the server will be unavailable for approximately 24 hours. Please hold off on any critical actions until we are finished. As always your feedback is appreciated.

- Study Packages
- NCERT Solutions
- Sample Papers
- Online Test

Notes for SSC Chemistry
Select chapter, recent notes, general organic chemistry, some important man made materials, general concepts of chemistry, environmental pollution, metals and non-metals, acids, bases and salts, classification of elements and periodicity in, structure of atom, nature of matter, कार्बन एवं इसके यौगिक, अधातुएँ एवं उपधातुएं, दैनिक जीवन में रसायन, पर्यावरणीय रसायन, कार्बनिक रसायन, हाइड्रोकार्बन एवं ईंधन, हाइड्रोजन एवं इसके यौगिक, धातुएं एवं मिश्र धातुएं, रेडॉक्स अभिक्रियाएं एवं विद्युत रसायन, साम्यावस्था, अम्ल, क्षार एवं लवण.

Reset Password.
OTP has been sent to your mobile number and is valid for one hour
Mobile Number Verified
Your mobile number is verified.

- প্রবিধানমালা
- স্থাবর অস্থাবর সম্পত্তির তালিকা
- প্রতিষ্ঠানের লক্ষ্য ও উদ্দেশ্য
- ভিশন ও মিশন
- সিটিজেন চার্টার
- অর্গানোগ্রাম
- কর্মকর্তাবৃন্দ
- প্রাক প্রাথমিক
- উচ্চ মাধ্যমিক
- জাতীয় শিক্ষাক্রম রূপরেখা
- সম্পূরক পঠনসামগ্রী প্রণয়ন ও নির্বাচন নীতিমালা
সিলেবাস ও মান বন্টন
- এসএসসি ও এইচএসসি পরীক্ষার পুনর্বিন্যাসকৃত পাঠ্যসূচি
- জেএসসি পরীক্ষার মান বন্টন ও নমুনা প্রশ্ন
- নীতিমালা ও নোটিশ
- শিখন ঘাটতি চিহ্নিতকরণ ও নিরাময়যোগ্য পাঠ পরিকল্পনা

শিক্ষাক্রম মূল্যায়ন
- প্রাথমিক শিক্ষাক্রম মূল্যায়ন
নতুন বছরের পাঠ্যপুস্তক
- ২০২৪ শিক্ষাবর্ষের সকল স্তরের পাঠ্যপুস্তক
- English for Today Listening Text
- পাঠ্যপুস্তক সংক্রান্ত মতামত
- ২০২৩ শিক্ষাবর্ষের সকল স্তরের পাঠ্যপুস্তক
- ২০২২ শিক্ষাবর্ষের সকল স্তরের পাঠ্যপুস্তক
- ২০২১ শিক্ষাবর্ষের সকল স্তরের পাঠ্যপুস্তক
- ২০২০ শিক্ষাবর্ষের সকল স্তরের পাঠ্যপুস্তক
- ২০১৯ শিক্ষাবর্ষের সকল স্তরের পাঠ্যপুস্তক
- ২০১৮ শিক্ষাবর্ষের সকল স্তরের পাঠ্যপুস্তকের তালিকা
- ২০১৭ শিক্ষাবর্ষের সকল স্তরের পাঠ্যপুস্তকের তালিকা
নতুন বছরের শিক্ষক সহায়িকা
- ২০২৪ শিক্ষাবর্ষের শিক্ষক সহায়িকা
শিক্ষক সহায়িকা আর্কাইভ
- ২০২৩ শিক্ষাবর্ষের শিক্ষক সহায়িকা
- প্রাথমিক স্তর
শিক্ষক ডায়েরি
- ২০২৩ শিক্ষাবর্ষের শিক্ষক ডায়েরি
আইডিটি ও ই-লার্নিং
- ই-লার্নিং ও ই-ম্যানুয়েল
- আইডিটি ষষ্ঠ শ্রেণি
ডিজিটাইজেশন
- পাঠ্যপুস্তকের চাহিদা গ্রহণ/বিতরণ সিস্টেম
- ডিজিটাল স্বাক্ষর
মাল্টিমিডিয়া টকিং বুক
- ২০২৩ শিক্ষাবর্ষের ব্রেইল এর সকল শ্রেণির মাল্টিমিডিয়া টকিং বুক
- উদ্ভাবনী কমিটি
- নতুন আইডিয়া
- প্রাথমিক স্তরের মূল্যায়ন নির্দেশিকা
- এনওসি ও বিদেশ ভ্রমন
- পদন্নোতি/বেতন নির্ধারণ
- বিদেশ ভ্রমন
- অন্যান্য অফিস আদেশ
- গুরুত্বপূর্ণ ডকুমেন্টসমূহ
- আর্কাইভ নোটিশ
- ঋণদান নীতিমালা ২০২০
বিভিন্ন ফরম
- এসিআর ফর্ম (ক্যাডার কর্মকর্তা)
- ফটোগ্যালারি
- ভিডিও গ্যালারি
কনটেন্টটি শেয়ার করতে ক্লিক করুন
National Portal Bangladesh
পোর্টাল সাবস্ক্রাইব করুন
Share with :

২০১৮ শিক্ষাবর্ষের নবম ও দশম শ্রেণির পাঠ্যপুস্তক
| ১। | বাংলা সাহিত্য | ||
| ২। | বাংলা সহপাঠ | ||
| ৩। | বাংলা ভাষার ব্যাকরণ | ||
| ৪। | English for Toady | ||
| ৫। | গণিত | ||
| ৬। | চারু ও কারুকলা | ||
| ৭। | তথ্য ও যোগাযোগ প্রযুক্তি | ||
| ৮। | ক্যারিয়ার এডুকেশন | ||
| ৯। | রচনাসম্ভার | ||
| ১০ | পদার্থবিজ্ঞান | ||
| ১১। | রসায়ন | ||
| ১২। | জীববিজ্ঞান | ||
| ১৩। | বাংলাদেশের ইতিহাস ও বিশ্বসভ্যতা | ||
| ১৪। | ভূগোল ও পরিবেশ | ||
| ১৫। | অর্থনীতি | ||
| ১৬। | কৃষিশিক্ষা | ||
| ১৭। | গার্হস্থ্য বিজ্ঞান | ||
| ১৮। | পৌরনীতি ও নাগরিকতা | ||
| ১৯। | হিসাববিজ্ঞান | ||
| ২০। | ফিন্যান্স ও ব্যাংকিং | ||
| ২১। | ব্যবসায় উদ্যোগ | ||
| ২২। | ইসলাম ও নৈতিক শিক্ষা | ||
| ২৩। | হিন্দু ধর্ম ও নৈতিক শিক্ষা | ||
| ২৪। | বৌদ্ধধর্ম ও নৈতিক শিক্ষা | ||
| ২৫। | খ্রিষ্টধর্ম ও নৈতিক শিক্ষা | ||
| ২৬। | বিজ্ঞান | ||
| ২৭। | বাংলাদেশ ও বিশ্বপরিচয় | ||
| ২৮। | শারীরিক শিক্ষা, স্বাস্থ্য বিজ্ঞান ও খেলাধুলা | ||
| ২৯। | উচ্চতর গণিত | ||
| ৩০। | Enlish Grammer and Composition |
পরিকল্পনা ও বাস্তবায়নে: মন্ত্রিপরিষদ বিভাগ , এটুআই , বিসিসি , ডিওআইসিটি ও বেসিস ।
কারিগরি সহায়তায়:


- University Admission
- School Admission
- Admission Result
- 18th NTRCA Result 2024
- NU Honours Admission Result 2024
- Nursing Admission Result 2024
- Primary Result 3rd Phase 2024 PDF Download
- SSC Result 2024
- HSC Routine 2024
SSC Chemistry Assignment Answer 2021 All weeks
There is currently no content classified with this term.
SSC 2022 Chemistry Assignment Answer (9th Week)
SSC 2022 Chemistry Assignment Answer: Directorate of Secondary and Higher Secondary Education is published the SSC 2022 Assignment Answer for the Chemistry Subjects. The Assignment work, Questions with solutions are available at dshe.gov.bd.
DSHE is published the SSC Chemistry 9th Week Assignment on 25th January 2022 . Students now can check the assignment question and answer from the below assignment section.
SSC Chemistry Assignment Answer 2022
The Directorate of Secondary and Higher Secondary Education is release the 9th Week chemistry assignments for the SSC Exam 2022.
According to the SSC Assignment Routine, The Chemistry assignment will be available in the Sixth, Ninth, Fourteen and Twenty weeks of the SSC Assignment Program 2022.
SSC Chemistry Assignment 2022 is now available on the website of the Directorate of Secondary and Higher Secondary Education (DSHE). Students now can check the week-based solution in the answer section.
The DSHE is released the 9th Week Assignments on 25th January 2022. The Assignment Question and answers of the 9th Week will be posted shortly.
The answers to the SSC Chemistry Assignment are now worth Marks. As a result, students will not be able to ignore the Assignment Work. In order to receive the highest possible grade, they must write the best assignment solutions. They will get marks based on their performance in the Chemistry Assignment Answer.
The primary goal of this assignment is to prepare students for the final exam of the Bangladesh Secondary School Certificate Examination. The SSC Chemistry Assignment carries a mark for students from science group.
The SSC Chemistry syllabus has shortened to benefit students taking the exams because of the COVID-19 Corona Virus.
Furthermore, DSHE will publish the assignment work on the website once a week in accordance with this syllabus.
The revised curriculum and chapters will be used in the preparation of the SSC Assignment 2022 for Chemistry Subjects in the Science Group by the Directorate of Secondary and Higher Education (DSHE).
They plan to distribute the Higher Mathematics assignment in these following weeks:
Read Also- SSC Physics Assignment Answer 2022
DSHE is published the SSC Chemistry Assignment Answer 2022 for the 9th on dshe.gov.bd. Students now can check the assignment work with solutions for the first week.
Because of the COVID-19 Corona Virus, the syllabus for SSC Chemistry subjects has been shortened for this year’s students. Further, according to the syllabus, DSHE will publish the assignment work every week on their website.
It has been decided to keep only Eights chapters for the Accounting curriculum. The Eights chapters are- First (রসায়নের ধারণা), Second (পদার্থের অবস্থা), Third (পদার্থের গঠন), Fourth (পর্যায় সারণী), Fifth (রাসায়নিক বন্ধন), Sixth (মৌলের ধারণা ও রাসায়নিক গণনা), Seventh (রাসায়নিক বিক্রিয়া), Eleventh (খনিজ সম্পদ- জীবাশ্ম)
SSC 9th Week Chemistry Assignment Answer 2022
The Ninth Week assignment for SSC batch 2022 Chemistry Subjects is published on 25th January 2022. DSHE has assigned the 9th Week Assignment from the Third Chapter (পদার্থের গঠন) of the main text book.
Students now should download the question from dshe.gov.bd. To help the students from science group, We have published the assignment question with answer for the 9th Week.
Students can view the question here in image file or download the assignment work in pdf file. They just need to follow this article for assignment question with answer. DSHE will publish the SSC Chemistry 9th Week Assignment on 25th January 2022 in pdf file on dshe.gov.bd .
Class: Ten Exam Year: SSC 2022 Subject: Chemistry Group: Science Week: 9th Week Assignment Work: প্রকৃতিতে প্রাপ্ত কপারের দুটি আইসােটোপ। ভর সংখ্যা ৬৩ ও ৬৫। প্রতিটি আসােটোপের মূল কণিকার সংখ্যা, ইলেকট্রন বিন্যাসের সাহায্যে মৌলটির পর্যায় সারণিতে অবস্থান, মৌলটির বিভিন্ন উপশক্তিস্তর এবং তাতে বিদ্যমান। ইলেকট্রন সংখ্যা 2nএবং 2(21+1) সূত্রের সাহায্যে বিশ্লেষণ কর।

Assignment Answer:

Source: DSHE
2 thoughts on “SSC 2022 Chemistry Assignment Answer (9th Week)”
Leave a comment cancel reply.
Save my name, email, and website in this browser for the next time I comment.
Exam Result Hub
Examresulthub.com is an educational portal where Students, parents, teachers and educational institute can get jobs and education news in Bangladesh.
Individual Result
- NU AC BD Results
- Nu ac bd honours result
- nu ac bd degree result
- NU Ac bd Masters Reslt
- SSC Exam Pattern
SSC Exam Pattern & Syllabus
SSC Syllabus – The Staff Selection Commission sets the SSC exam pattern and syllabus of all the exams. The aim is to give candidates a clear idea about the Staff Selection Commission syllabus and exam pattern for all exams and provide them with SSC study materials.
The SSC conducts exams annually to recruit candidates in various departments, organizations, offices under Govt. of India. The SSC Syllabus for most of the exams keeps changing at an interval of a few years. Candidates need to have updated data on the SSC Exams before going ahead with the application form filling.
Hence, this article will help the candidates understand the SSC syllabus and the latest SSC exam pattern properly. Candidates appearing for exams conducted by the Staff Selection Commission can download the latest SSC Syllabus PDF provided in the article.
The SSC Syllabus and exam pattern for the following Exams are discussed below:
- SSC Stenographer
SSC Syllabus PDF – Download Here
Candidates should check the SSC official Notification to have a clear idea of the exam-specific details.

SSC CGL Syllabus
Before getting into the SSC Syllabus for the CGL exam, the Exam Pattern needs to be understood.
The SSC CGL Exam will be conducted in four tiers as indicated below:
- Tier I- Computer Based Examination
- Tier II- Computer Based Examination
- Tier III- Pen and Paper Mode (Descriptive paper)
- Tier IV- Computer Proficiency Test/ Skill Test (wherever applicable)/ Document Verification.
The table below gives an idea about the SSC CGL Exam Pattern:
| General Intelligence and Reasoning | 25 | 50 | 60 Minutes (Total) For VH/ OH (afflicted with Cerebral Palsy/ deformity in writing hand- 80 Minutes. | |
| General Awareness | 25 | 50 | ||
| Quantitative Aptitude | 25 | 50 | ||
| English Comprehension | 25 | 50 | ||
| Paper 1- Quantitative abilities | 100 | 200 | 120 Minutes (for each Paper) For VH/ OH (afflicted with Cerebral Palsy/ deformity in writing hand- 160 Minutes | |
| Paper 2- English language & Comprehension | 200 | 200 | ||
| Paper 3- Statistics | 100 | 200 | ||
| Paper 4- General Studies(Finance & Economics) | 100 | 200 |
Aspirants preparing for any exam conducted by the Selection Commission can check the SSC General Awareness for relevant topics for preparing the General Awareness Section of the exams.
Tier III SSC CGL Exam Pattern:
| Tier III | Pen and Paper mode | Descriptive Paper in Hindi/English (Writing of Essays, Precis, Applications, etc.) | 100 | 60 Minutes For VH/ OH (afflicted with Cerebral Palsy/ deformity in writing hand- 80 Minutes |
The SSC CGL syllabus covers major topics related to various subjects like – Reasoning, Aptitude, Current Affairs, English Language and Comprehension.
SSC CGL Syllabus for Tier I
Tier I comprises four sections-
- General Intelligence & Reasoning
- General Awareness
- Quantitative Aptitude/ SSC maths syllabus
- English Comprehension skills
| , , Vocab and grammar skills, Spotting the errors, , | Symbolic/Number Analogy, Semantic Classification, Symbolic/Number Classification, Space Visualization, Venn Diagrams, Drawing inferences | History- Harappa Civilization, Vedic culture, Medieval India and their important systems; India’s freedom movement and their leaders, Geography- about countries and their geographical details, Famous seaports and airports and their location. General Knowledge, current affairs | Number System Problems Percentage. Ratio & Proportion, Square roots, Averages, Interest, Complementary angles, Heights and Distances, Histogram, Data analysis |
Candidates can check important tips to master error spotting to attempt the question in exams with relative ease.
Aspirants can go through a variety of Sentence Correction Questions to understand how questions on sentence corrections are framed in the exam.
SSC CGL Syllabus for Tier II
The SSC CGL syllabus for Tier II will have 4 papers-
- Quantitative Aptitude
- English Language & Comprehension
- General Studies- Finance & Economics-
The detailed SSC CGL Syllabus for Tier-II Exam is given below:
| Number System Problems Percentage, Time and distance, Time & Work, Basic algebraic identities of School Algebra | conversion and narration, Shuffling of sentence parts in passages, Comprehension passage. | Data distribution, calculation, a diagrammatic representation of data, Moments and relationships- skewness, kurtosis | Economics-Macro & Micro Economics, Growth & Development Market analysis- production, demand, supply, Indian Economy, Economic reforms, Money and banking, Role of Technology in reforming the Economic domain |
Given in the link are Important Maths Tricks and shortcuts , check for assistance.
Candidates can go through SSC CGL Previous Year Question Papers to understand the scope of the syllabus the exam pattern of CGL well.
SSC CGL Syllabus for Tier III
The main aim of this Tier III exam is to evaluate the candidates’ language proficiency, grammar knowledge, vocabulary usage and writing skills in English/Hindi.
The topics for this exam can be related to current affairs and sensitive matters like- Demonetization, women empowerment, Terrorism, Right to equality, freedom of women in a country like India, etc.
Candidates need to write precis, application, letters, essays, passages as a part of the qualification for this exam.
Staff Selection Commission Syllabus for CGL Tier-IV
The syllabus for the SSC CGL Tier-IV exam is as follows:
| Data Entry Speed test | 8,000 (eight thousand) Key Depression per hour on the Computer | Tax Assistants( Central & Excise) |
| A passage of about 2000 (two thousand) key depressions for a duration of 15 (fifteen) minutes. | ||
| Computer Proficiency Test | Word Processing Spreadsheet Generation of slides | Assistant Section Officer of Central Secretariat Service (CSS), Assistant Section Officer (MEA), Assistant in Serious Fraud Investigation Office (SFIO) under the Ministry of Corporate Affairs, and Assistant (GSI) in the Ministry of Mines. |
Candidates should check the SSC CGL syllabus to get a clear idea of the exam topics and patterns for better preparation.
Important Pointers for SSC CGL Exam Pattern:
- The Commission reserves the right to make changes in the scheme of examination.
- As per the SSC exam pattern for the CGL exam, i n Tier-I, there will be a negative marking of 0.50 for each wrong answer
- In Tier-II, there will be a negative marking of 0.25 for each wrong answer in Paper-II (English Language and Comprehension) and of 0.50 marks for each wrong answer in Paper-I, Paper-III, and Paper-IV.
- In Tier-II, Paper-I and Paper-II are compulsory for all the posts.
- As per the SSC CGL Exam Pattern, in Tier-II, Paper-III will be for only those candidates who apply for the post of Junior Statistical Officer (JSO) and who are shortlisted in Tier-I for this Post/ Paper.
- In Tier-II, Paper-IV will be for only those candidates who are shortlisted in Tier-I for Paper-IV i.e. for the posts of Assistant Audit Officer/ Assistant Accounts Officer.
- The SSC CGL Exam Pattern has Computer Proficiency Test/ Skill Test (wherever applicable)/ Document Verification that is conducted as per the provisions of the notice of examination.
- Candidates should check SSC CGL official notification from time to time for more details on the syllabus, exam pattern changes.
- Get free SSC CGL mock tests based on the latest exam pattern in the linked article.
SSC CPO Syllabus
The SSC exam syllabus and paper pattern information for the Central Police Organization (CPO) exam is covered in this section.
The SSC CPO Exam has a pattern which consists of :
- Physical Standard Test (PST)/ Physical Endurance Test (PET)
- Detailed Medical Examination (DME)
All these stages of the examination are mandatory.
The detailed SSC CPO Exam Pattern is as follows:
| Paper 1 | General Intelligence & Reasoning | 50 | 50 | 02 hours |
| General Knowledge & General Awareness | 50 | 50 | ||
| Quantitative Aptitude | 50 | 50 | ||
| English Comprehension | 50 | 50 | ||
| Paper 2 | English language & Comprehension | 200 | 200 | 02 hours |
Prepare for the SSC CPO exam by solving the SSC CPO mock test series and analyze your performance based on the marks scored in each test.
The SSC exam syllabus-related information regarding the SSC CPO Exam is given below.
SSC CPO Syllabus for Paper I covers the same topics as in SSC Syllabus for the CGL examination.
The SSC Exam syllabus for CPO Paper-II covers English Language & Comprehension. The topics that need to be focused while preparing for Paper II are as mentioned below.
SSC CPO Syllabus for Paper-II
| Error recognition, filling in the blanks (using verbs, , , , etc), Vocabulary, Spellings, Grammar, Sentence Structure, Synonyms, Antonyms, Sentence Completion, Phrases, and Idiomatic use of Words, comprehension etc. |
Check important Idioms and Phrases Questions and Answers in the given link. Candidates can check the General English for competitive exams to get the relevant list, rules, and concept-based topics covered in the English language section.
Checking the SSC CPO syllabus will help the candidates to get a clear idea of the exam topics and patterns for better preparation.
SSC CPO Exam Pattern- Salient Points:
- As per the SSC CPO Exam Pattern, the questions in both papers will be of Objective Multiple Choice Type.
- Questions will be set in Hindi and English in Parts-I, II and III of Paper-I.
- As per the SSC Exam Pattern, there will be a negative marking of 0.25 marks for each wrong answer in Paper-I & Paper-II.
- The Commission at its discretion may fix qualifying marks in Paper-I, Paper-II and any part(s) of Paper-I.
- The Commission reserves the right to alter/ modify the scheme of examination.
- Check SSC CPO Previous Year Question Papers for assistance in preparation and a better understanding of the exam pattern and syllabus.
SSC CHSL Syllabus
The SSC exam syllabus and paper pattern for the Combined Higher Secondary Level (CHSL) is mentioned in this section.
The SSC CHSL Exam will consist of the following Pattern:
- Computer-Based Examination (Tier-I)
- Descriptive Paper (Tier-II)
- Skill Test/ Typing Test (Tier-III)
The SSC Exam pattern for CHSL- Tier-I
| Part I | English Language (Basic Knowledge) | 50 | 50 | 60 Minutes (80 Minutes for PwD candidates |
| Part II | General Intelligence | 50 | 50 | |
| Part III | Quantitative Aptitude (Basic Arithmetic Skill)/ SSC maths syllabus | 50 | 50 | |
| Part IV | General Awareness | 50 | 50 |
The SSC Exam pattern for CHSL- Tier II
| Tier II | Pen and Paper mode | Descriptive Paper in Hindi/English (Writing of Essays, Precis, Applications, etc.) | 100 | 60 Minutes /Reserved candidates- 80 Minutes |
The SSC Exam pattern for CHSL- Tier III
| Data Entry Skill test | Skill Test for Data Entry Operator | Data Entry Speed of 8,000 (eight thousand) Key Depressions on Computer. | 60 minutes/80 minutes for reserved candidates |
| Data Entry Operator in the Office of the Comptroller and Auditor General of India (C&AG): | Speed of 15000 key depressions on Computer‟ | ||
| Typing Test | LDC/ JSA and Postal Assistant/ Sorting Assistant | English typing speed- 35 words per minute Hindi typing speed- 30 words per minute | 60 minutes/80 minutes for reserved candidates |
The information regarding the SSC syllabus for the CHSL exam is given below.
The Staff Selection Commission Syllabus for the CHSL exam is again similar to the one in SSC CGL and CPO exam. Given below is the brief of the SSC exam syllabus covered in the CHSL examination.
SSC CHSL Syllabus for Tier I
| Logical Reasoning | Computation of Whole Number, Decimal and Fractions, Relationship between numbers. | Reading Comprehension | History |
| Profit & Loss | Culture | ||
| Ranking/ /Alphabet Test | Simple Interest & Compound Interest & Surds & Indices | Para Jumbles | Geography |
| Permutation, Combination & Probability |
Candidates can check important Alpha-Numeric Series Questions on the given page for preparation and practice.
Also, get the list of important Logical reasoning topics and important SSC study material on the given page that explains the concepts and tricks to solve the questions in the exam.
SSC CHSL Syllabus for Tier II
| Essay Writing | Letter Writing |
| Application | Comprehension |
Go through the formats of the following to increase the chances of fetching good marks in the descriptive tests of various SSC examinations.
- Letter writing format
- Essay Writing – Overview
- Precis writing format
It is important for candidates to know the difference between precis and summary writing , check the given link.
SSC CHSL Syllabus for Tier III
| Data Entry Operator | Data Entry Speed of 8000 key depressions per hour on the computer. The speed will be adjudged on the basis of the correct entry of words/key depressions as per the given passage | The duration of the test will be for 15 minutes and printed matter in English containing about 2000-2200 key depressions would be given to each candidate who would enter the same in the test computer |
| Data Entry Operator in the Office of the Comptroller and Auditor General of India (C&AG) | The speed of 15000 key depressions per hour will be adjudged on the basis of the correct entry of words/key depressions as per the given passage | The duration of the test will be for 15 minutes and printed matter in English containing about 3700-4000 key depressions would be given to each candidate who would enter the same in the test computer |
| Lower Division Clerk/ Junior Secretariat Assistant (LDS/JSA) and Postal Assistants/ Sorting Assistants (PA/SA) | The speed of 10500 key depressions per hour will be adjudged on the basis of the correct entry of words/key depressions as per the given passage | The duration of the test will be for 15 minutes and printed matter in English containing about 9000 key-depressions/hour would be given to each candidate who would enter the same in the test computer. |
Important Pointers for SSC CHSL Exam Pattern:
- The Tier-I Examination will consist of Objective Type, Multiple choice questions only & will be set in English & Hindi for Part-II, III & IV.
- As per the SSC exam pattern for CHSL, there will be a negative marking of 0.50 marks for each wrong answer.
- The minimum qualifying marks in Tier-II would be 33 percent.
- The paper will have to be written either in Hindi or in English & a combination of both languages while answering will be rewarded with zero marks.
- The SSC CHSL syllabus reference in detail will help understand the SSC CHSL exam better in terms of preparation strategy, books and study material.
- Aspirants can get free SSC CHSL Mock tests as per the latest exam pattern in the linked article.
Related Links:
SSC JE Syllabus
The SSC Syllabus and exam pattern for the Junior Engineer (JE) are covered in brief in this section.
The exam pattern of Junior Engineer is divided into two papers i.e. Paper-I (Computer-based Test) and Paper-II (Written Test).
The SSC JE Exam Pattern is given below:
| Paper-I: Objective Type | Computer-based Test | General Intelligence and Reasoning | 50 | 50 | 02 hours |
| General Awareness | 50 | 50 | |||
| Part-A General Engineering (Civil & Structural) Or Part-B General Engineering (Electrical) Or Part-C General Engineering (Mechanical) | 100 | 100 | |||
| Paper-II: Descriptive Type | Written Test | Part-A General Engineering (Civil & Structural) Or Part- B General Engineering (Electrical) Or Part-C General Engineering (Mechanical) | 300 | 02 hours |
Check the following links for assistance in preparation:
- Reasoning puzzles
- Blood relation
- Seating Arrangements
- Machine Input Output
The SSC JE Syllabus is given below. The subjects covered in SSC JE Paper I syllabus and the important topics of each subject are given below:
| Relationship concepts Arithmetical reasoning Problem-solving Space visualization Visual memory Analysis and judgment
| Geography Culture History Economic Scene Scientific Research General Polity | Building Materials Surveying Estimating Soil Mechanics Costing and Valuation Concrete Technology Irrigation Engineering, |
| Basic concepts Circuit law AC Fundamentals Magnetic Circuit Electrical Machines Measurement and Measuring instruments Synchronous Machines
Theory of Machines and Machine Design 1st Law of Thermodynamics 2nd Law of Thermodynamics IC Engine Performance Air standard Cycles for IC Engines IC Engines Combustion Boilers IC Engine Cooling & Lubrication Classification Rankine cycle of System |
The SSC JE Paper-II syllabus is given below:
Paper-II is a written test of 300 marks for a duration of 2 hours on any of the following parts chosen by the respective candidate:
- Part-A (Civil Engineering & Structural Engineering)
- Part-B (Electrical Engineering)
- Part-C (Mechanical Engineering)
Candidates need to select any one of the parts which they wish to take during the exam.
Basically, Paper-II of the SSC JE syllabus includes a detailed explanation of each of the topics under each part as discussed in the General Engineering section of Paper I.
Important Pointers for SSC JE Exam Pattern:
- As per the SSC JE Exam Pattern, Paper-I and Paper-II for General Engineering, the candidate will be required to attempt only the part as selected in the application form filled by the candidate.
- There will be a negative marking of 0.25 marks for each wrong answer in Paper-I . Candidates are, therefore, advised to keep this in mind while answering the questions.
- For more details on the SSC JE syllabus and exam pattern, refer to the SSC JE syllabus article linked here.

SSC GD Syllabus
The SSC Syllabus and exam pattern for the Constable in General Duty (GD) exam are discussed in this section.
The SSC GD Exam consists of a Computer-based mode which consists of one objective type paper containing 100 questions carrying 100 marks.
SSC GD Exam Pattern
| Part A- General Intelligence and Reasoning Part B- General Knowledge and General Awareness Part C- Elementary Mathematics Part D- English/ Hindi | 25 25 25 25 | 25 25 25 25 | 90 minutes |
The SSC Syllabus for SSC GD Constable examination is aligned to the syllabus of CGL, CPO and CHSL exams. Candidates preparing for SSC GD can check SSC Syllabus mentioned above in this article.
For more details on the syllabus and SSC exam pattern of Constable GD Exam, refer to the SSC GD syllabus.
Aspirants can also refer to the SSC GD Mock test page to get free mock tests based on the latest exam pattern.
Important Pointers of SSC exam pattern and syllabus for General Duty Exam:
- The computer-based examination will be conducted in English and Hindi only.
- Based on the performance in CBE, candidates will be shortlisted for PET/ PST by the Staff Selection Commission.
- Physical Efficiency Test (PET) and Physical Standard Test (PST) will be conducted at various centers finalized by the CAPFs.
SSC MTS Syllabus
The SSC MTS Exam Pattern and syllabus are discussed in this section.
The SSC MTS exam consists of two papers: SSC MTS Paper I and SSC MTS Paper-II.
SSC MTS Paper I is an objective type online mode examination consisting of 100 questions in total. It consists of four sections i.e. General Awareness, General Reasoning and Intelligence, English, and Numerical Aptitude.
Each section in the paper I is of 25 marks carrying 25 questions (1 mark for each question). There is a negative marking of 0.25 marks on each wrong attempt. Candidates are required to obtain the minimum qualifying marks to be called for the next round.
SSC MTS Paper-II is a descriptive type offline examination of 50 marks. Candidates have to write a short essay or letter to pass the elementary language test.
The Staff selection commission releases the SSC MTS syllabus along with the notification every year. SSC MTS syllabus is largely the same every year.
To check the topic-wise SSC Syllabus for the MTS examination for all the four sections mentioned above, kindly check the SSC MTS Syllabus page.
SSC Stenographer Syllabus
The SSC Stenographer exam pattern and syllabus are important to understand before starting with the preparation.
The SSC Stenographer exam consists of two stages:
- Computer Based Test
The exam pattern of the SSC Stenographer CBT exam is as mentioned below.
| General Awareness | 50 | 50 |
| General Intelligence & Reasoning | 50 | 50 |
| English Language & Comprehension | 100 | 100 |
| Total | 200 | 200 |
The computer-based test is an online objective type test. Candidates have to complete the CBT exam in 2 hours. One mark is awarded for each right answer and 0.25 marks are deducted for every wrong answer.
The exam is divided into three sections English Language, General Awareness and General Reasoning & Intelligence. Candidates can check the detailed topic-wise SSC Stenographer syllabus and important booklist for preparation in the given link.
Keep referring to the SSC official notification for any changes in the SSC syllabus or SSC Exam Pattern.
Those who are preparing for either SSC exams or any other government exams have to prepare for Reasoning Ability as it is an important part of the syllabus of most of the examinations. Check 3 sutras to prepare for reasoning ability for competitive exams.
Candidates preparing for various government exams can check the detailed syllabus of respective exams on the given links:

Frequently Asked Question – SSC Syllabus & Exam Pattern
Q.1. what are the subjects covered in the ssc syllabus of various ssc exams, q.2. is there a negative marking in the ssc exam, q.3. what is the minimum education qualification for all ssc exams.
Ans. All the SSC Exams have different requirements for the educational qualification of the candidates. The academic qualification for the various posts in SSC are as follows:
- SSC CGL – A Graduation Degree in any discipline with a minimum of 50% marks.
- SSC JE – A degree or Diploma in Engineering relevant to the post applied for (no minimum percentage specified)
- SSC CHSL – A 12th pass certificate from any board or university (no minimum percentage specified)
- SSC GD – A 10th Pass certificate from a recognized board (no minimum percentage specified)
- SSC CPO – A Graduation Degree in any discipline from a recognized university (no minimum percentage specified)
| SSC Exams Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
Connect with us for Free Preparation
Get access to free crash courses & video lectures for all government exams., register with byju's & download free pdfs, register with byju's & watch live videos.


SSC Chemistry Assignment 3rd Week Answer 2021 – SSC assignment 2021 chemistry 3rd week – SSC assignment chemistry 3rd week answer

SSC Chemistry 3rd Week Assignment 2021. SSC 2021 Chemistry Assignment Answers have been assigned for SSC Candidates 2021 3rd, 3rd, 5th, 6th, 8th, 9th, 11th, and 12th week’s assignment. A total of 8 Assignment answers have to be submitted for SSC Chemistry Assignment 2021. The full Marks for these eight assignments will be 50. SSC 2021 Chemistry Assignment for 2nd and 3rd week has already been published. The next week’s assignment will be published after the submission of these two weeks’ solutions.
SSC Chemistry 3rd Week Assignment 2021
Chemistry assignment for 3rd week has been published for the candidates of SSC science branch of 2021. The “Chapter Three: The Structure of Matter” section is scheduled for the second week. Read the scheduled chapter well and practice and write the answer to the assigned question for the assignment. If you want, you can take a look at the chemistry assignment solution we have created.
SC Chemistry Assignment 3rd Week Answer 2021, Dear Examiners of the SSC 2021 Science Department, The SSC Exam 2021 Chemistry 1st Week Assignment for you has been selected. SSC Chemistry Assignment 2021 & 2022 question and answer is available here. SSC 2021 exam candidates and SSC 2022 exam candidates, both Chemistry 2nd week, the 3rd-week assignment has been published. So we give all the solutions to the SSC Chemistry Assignment.
SSC candidates have their first assignment in the Chemistry subject in the 3rd Week. Chemistry is determined from the third chapter of the first assignment. The title of the third chapter is the structure of matter. SSC chemistry assignment 3rd week. How a substance is formed and the different states of matter are discussed in this chapter. Thus, from the 3rd-week chemistry assignment of SSC, we will learn about the composition of matter. In order to solve the SSC 3rd week chemistry assignment correctly, one has to acquire detailed knowledge about substances and the basics of matter. chemistry assignment SSC 2021 1st week answer
Otherwise, it is not possible to give the answer to SSC 3rd week chemistry assignment correctly. So before writing the solution or answer of the SSC 3rd week chemistry assignment one must acquire knowledge about the content of the assignment.SSC assignment 2021 3rd week answer
SSC Chemistry Assignment
Here the structure of the atom according to the Bohr model should be mentioned along with the diagram and also the electron configuration of energy level and sub-energy level should be discussed. So those examinees who are worried about SSC 3rd Week chemistry assignment should follow our instructions. We will provide guidance on the solution of the 3rd Week assignment of chemistry or writing the answer sheet. Also for the convenience of students, we will upload the solution or answer of SSC 3rd Week chemistry assignment in PDF format. If anyone wants to download the solution or answer to 3rd Week chemistry assignment then visit our website. SSC assignment 2021 chemistry 3rd week
chemistry assignment SSC 2021
Students need to follow a number of guidelines for writing the solution or answer of the 3rd Week chemistry assignment. Creating a solution or answer to the assignment by following the instructions below will definitely yield excellent results. As a result, it will be helpful to make the results of SSC 2021 better. Now I will give the instructions so that the students can create chemistry assignments in a good way by following them. The answer must be the number of neutrons of 4 elements. SSC assignment chemistry 1st week
Chemistry 3rd Week Assignment
Also the structure of the atom according to the model of 4 elements has to be described with diagrams. Electron arrangement should be shown in the energy level of 4 elements. It is also necessary to show the electron configuration of the sub-layer of 4 elements. If you follow the above guidelines and create a solution or answer sheet for the chemistry assignment, you will definitely get very good results. SSC chemistry assignment 2021 answer SSC assignment 2021 3rd week answer
SSC 3rd week assignment answer
We can learn a lot from the chemistry assignment of SSC 3rd Week. For example, we can calculate the number of electrons, protons and neutrons in an atom. Rutherford and Bohr will also be able to describe the atomic model of atomic structure. I can arrange the electrons of the atom in different orbits of the atom and in different layers of the orbit.
SSC 2021 Chemistry Assignment 3rd week Answer
এসএসসি ২০২১ অ্যাসাইনমেন্টের ৩য় সপ্তাহে রসায়ন বিষয়নি নির্ধারণ করা হয়েছে। রসায়ন বিষয়ে এটি দ্বিতীয় অ্যাসাইনমেন্ট। তৃতীয় সপ্তাহের অ্যাসাইনেমেন্ট চতুর্থ অধ্যায়েল পর্যা সারণি নির্ধারণ করা হয়েছে। অধ্যায়টি ভালোভাবে পড়ো এবং অনুশীলন করে তোমার সমাধান তৈরি করো। তোমার রসায়ন ৩য় সপ্তাহের অ্যাসাইনমেন্ট সমাধান করার সময় অবশ্যই ইলেক্ট্রন বিন্যাস করে মৌল চারটির পর্যায় সারণির পর্যায় নির্ণয় করতে হবে, ইলেকট্রন বিন্যাস করে মৌল চারটর পর্যায় সারণির গ্রুপ বা শ্রেণি নির্ণয় করতে হবে, পর্যায় সারণির একই পর্যায় এবং একই গ্রুপ বা শ্রেণিতে পাশাপাশি অবস্থিত আয়নিকরণ শক্তির তুলনা করবে এবং মৌল সংশ্লিষ্ট গ্রুপ বা শ্রেণির বৈশিষ্ট উল্লেখ করবে। তুমি চাইলে আাদের তৈরি করা রসায়ন ৩য় সপ্তাহের অ্যাসাইনমেন্ট দেখে নিতে পারো।

স্তরঃ এস.এস.সি পরীক্ষা ২০২১
বিভাগঃ বিজ্ঞান
বিষয়ঃ রসায়ন
বিষয় কোডঃ ১৩৭
মোট নম্বরঃ ১৬
অ্যাসাইনমেন্ট নম্বর-০২
অধ্যায় ও শিরােনামঃ চতুর্থ অধ্যায়: পর্যায় সারণি
অ্যাসাইনমেন্টঃ Li, Be, Na, Mg মৌল চারটির ইলেকট্রন বিন্যাসের আলােকে পর্যায় সারণিতে অবস্থান, তুলনামূলক আয়নিকরণ শক্তি এবং মৌল সংশ্লিষ্ট গ্রুপ বা শ্রেণির বৈশিষ্ট্য সম্পর্কিত একটি প্রতিবেদন প্রণয়ন;
শিখনফল/বিষয়বস্তুঃ
১. মৌলের সর্ববহিঃস্তর শক্তিস্তরের ইলেকট্রন বিন্যাসের সাথে পর্যায় সারণির প্রধান গ্রুপগুলাের সম্পর্ক নির্ণয় করতে পারব (প্রথম ৩০ টি মৌল)
২. একটি মৌলের পর্যায় শনাক্ত করতে পারব।
৩. পর্যায় সারণিতে কোনাে মৌলের অবস্থান জেনে এর ভৌত ও রাসায়নিক ধর্ম সম্পর্কে ধারণা করতে পারব।
৪. মৌলসমূহের বিশেষ নামকরণের কারণ বলতে পারব।
৪. পর্যায় সারণি অনুসরণ করে মৌলসমূহের ধর্ম অনুমানে আগ্রহ প্রদর্শন করতে পারব।
নির্দেশনা (সংকেত/ধাপ/পরিধি):
১. ইলেকট্রন বিন্যাস করে মৌল চারটির পর্যায় সারণির পর্যায় নির্ণয় করতে হবে ইলেকট্রন বিন্যাস করে মৌল চারটির পর্যায় সারণির গ্রুপ বা শ্রেণি নির্ণয় করতে হবে।
২. পর্যায় সারণির একই পর্যায় এবং একই গ্রুপ বা শ্রেণিতে পাশাপাশি অবস্থিত মৌলের আয়নিকরণ শক্তির তুলনা করতে হবে।
৩. মৌল সংশ্লিষ্ট গ্রুপ বা শ্রেণির বৈশিষ্ট্য উল্লেখ করতে হবে।
অধ্যায় ০৪ পর্যায় সারণি অ্যাসাইনমেন্ট :
Li Be Na Mg
মৌল চারটির ইলেকট্রন বিন্যাসের আলোকে পর্যায় সারণিতে অবস্থান, তুলনামূলক আয়নিকরণ শক্তি এবং মৌল সংশ্লিষ্ট গ্রুপ বা শ্রেণির বৈশিষ্ট্যদ সম্পর্কিত একটি প্রতিবেদন প্রণয়ন।
নমুনা সমাধান
(ক) মৌলগুলাের ইলেকট্রন বিন্যাস লক্ষ্য করলে দেখা যাবে যে, লিথিয়াম (Li) এর ক্ষেত্রে সর্বশেষ ইলেকট্রন দ্বিতীয় শক্তি স্তরে প্রবেশ করেছে। তাই আমরা বলতে পারি, Li(3) এর পর্যায় হচ্ছে 2।
অনুরূপভাবে, FT বেরিলিয়াম (Be) এর ক্ষেত্রে সর্বশেষ ইলেকট্রন দ্বিতীয় শক্তি স্তরে প্রবেশ করেছে। তাই আমরা বলতে পারি, Mg(12) এরপর যায় হচ্ছে 3।
(খ) গ্রুপ বা শ্রেণি নির্ণয় :
প্রশ্নে উল্লেখিত মৌলগুলাের ইলেকট্রন বিন্যাস :

মৌলগুলাের ইলেকট্রন বিন্যাস লক্ষ্য করলে দেখা যাবে যে, লিথিয়াম (Li) এর ক্ষেত্রে সর্ববহিঃস্থ শক্তিস্তরে একটিমাত্র ইলেকট্রন রয়েছে। তাই আমরা বলতে পারি,Li(3) এর গ্রুপ হচ্ছে 1।
অনুরূপভাবে, বেরিলিয়াম (Be) এর ক্ষেত্রে সর্ববহিঃস্থ শক্তিস্তরে দুইটি ইলেকট্রন রয়েছে।
তাই আমরা বলতে পারি, Be(4) এর গ্রুপ হচ্ছে 2। সােডিয়াম (Na) এর ক্ষেত্রে সর্ববহিঃস্থ শক্তিস্তরে একটিমাত্র ইলেকট্রন রয়েছে। তাই আমরা বলতে পারি, Na(11) এর গ্রুপ হচ্ছে 1। ম্যাগনেসিয়াম (Mg) এর ক্ষেত্রে সর্ববহিঃস্থ শক্তিস্তরে দুইটি ইলেকট্রন রয়েছে। তাই আমরা বলতে পারি, Mg(12) এর গ্রুপ হচ্ছে 2।
(গ) তুলনামূলক আয়নীকরণ শক্তি :
আমরা জানি, একই পর্যায়ে যত বাম দিক থেকে ডান দিকে যাওয়া যায়, অর্থাৎ পারমাণবিক সংখ্যা যত বাড়তে থাকে পরমাণুর আকার ততােই হ্রাস পেতে থাকে। আর পারমাণবিক আকার হ্রাস পেলে পরমাণুর আয়নীকরণ শক্তি বাড়ে। কারণ, এতে একটি করে ইলেকট্রন যুক্ত হয় এবং নিউক্লিয়াসের সাথে আকর্ষণ বেড়ে যায়।
আবার, একই গ্রুপে উপর থেকে নিচে আসলে একটি করে নতুন শক্তিস্তর যুক্ত হয়। এর ফলে পারমাণবিক ব্যাসার্ধ বেড়ে যায়। ফলে আয়নিকরণ শক্তি কমে যায়। অর্থাৎ এখানে বেরিলিয়ামের (Be) আয়নীকরণ শক্তির মান সবচেয়ে বেশি।
আয়নিকরণ শক্তির ক্রম হবে নিম্নরূপ : 𝐵𝑒>𝐿𝑖>𝑀𝑔>𝑁𝑎 ।
(ঘ) মৌল সংশ্লিষ্ট গ্রুপ বা শ্রেণির বৈশিষ্ট্য :
প্রশ্নে উল্লেখিত মৌলগুলাের ইলেকট্রন বিন্যাস লক্ষ্য করলে আমরা দেখতে পাই যে, Li(3) এর গ্রুপ হচ্ছে 1। Be(4) এর গ্রুপ হচ্ছে 2। Na(11) এর গ্রুপ হচ্ছে 1। Mg(12) এর গ্রুপ হচ্ছে 2।
অর্থাৎ লিথিয়াম (Li) এবং সােডিয়াম (Na) একই গ্রুপে অবস্থিত এবং তাদের গ্রুপ হচ্ছে 1। আবার বেরিলিয়াম (Be) এবং ম্যাগনেসিয়াম (Mg) একই গ্রুপে অবস্থিত এবং তাদের গ্রুপ হচ্ছে 2.
গ্রুপ -1 (ক্ষার ধাতু)
পর্যায় সারণির ১নং গ্রুপে ৭টি মৌল আছে। এদের মধ্যে হাইড্রোজেন ছাড়া বাকি ৬ টি মৌলকে (লিথিয়াম, সােডিয়াম, পটাশিয়াম, রুবিডিয়াম, সিজিয়াম এবং ফ্রানসিয়াম ) ফ্রানসিয়াম) ক্ষারধাতু বলে।
এই ছয়টি মৌলের প্রত্যেকটি পানিতে দ্রবীভূত হয়ে এই ছয়টি মৌলের প্রত্যেকটি পানিতে দ্রবীভূত হয়ে হাইড্রোজেন গ্যাস এবং ক্ষার তৈরি করে বলে এদেরকে ক্ষারধাতু (Alkali Metals) বলা হয়।
এ ধাতু গুলাে খুবই সক্রিয়। এরা আয়নিক বন্ধন গঠন করে। এরা নরম ও চকচকে হয়।
গ্রুপ- 2 (মৃৎক্ষার ধাতু)
পর্যায় সারণির ২নং গ্রুপে বেরিলিয়াম, ম্যাগনেসিয়াম, ক্যালসিয়াম, স্ট্রনসিয়াম, বেরিয়াম এবং রেডিয়াম এই ৫ টি মৌল আছে। এই মৌলগুলােকে মৃৎক্ষার ধাতু বলে।
এই ধাতুগুলােকে মাটিতে বিভিন্ন যৌগ হিসেবে পাওয়া যায়।
আবার, এরা ক্ষার তৈরি করে। এজন্য সামগ্রিকভাবে এদের মৃৎক্ষার ধাতু (Alkaline Earth Metals)।
এদের হাইড্রোক্সাইড গুলাে এসিডের সাথে বিক্রিয়া করে লবণ ও পানি উৎপন্ন করে। এরা আয়নিক বন্ধন গঠন করে।
SSC Chemistry 2nd Week Assignment 2021
SSC candidates have their first assignment in the Chemistry subject in the 2nd Week. Chemistry is determined from the third chapter of the first assignment. The title of the third chapter is the structure of matter. SSC chemistry assignment 2nd week. How a substance is formed and the different states of matter are discussed in this chapter. Thus, from the 2nd-week chemistry assignment of SSC, we will learn about the composition of matter. In order to solve the SSC 2nd week chemistry assignment correctly, one has to acquire detailed knowledge about substances and the basics of matter. chemistry assignment SSC 2021 1st week answer
Otherwise, it is not possible to give the answer to SSC 2nd week chemistry assignment correctly. So before writing the solution or answer of the SSC 2nd week chemistry assignment one must acquire knowledge about the content of the assignment.SSC assignment 2021 2nd week answer
SSC 2nd week assignment answer
We can learn a lot from the chemistry assignment of SSC 2nd Week. For example, we can calculate the number of electrons, protons and neutrons in an atom. Rutherford and Bohr will also be able to describe the atomic model of atomic structure. I can arrange the electrons of the atom in different orbits of the atom and in different layers of the orbit.
assignment SSC 2021 2nd week answer chemistry

মোট নম্বরঃ ১০
অ্যাসাইনমেন্ট নম্বর-০১
অধ্যায় ও অধ্যায়ের শিরােনামঃ তৃতীয়, পদার্থের গঠন।
অ্যাসাইনমেন্টঃ প্রতীকের পাশে উল্লেখিত ভরসংখ্যাবিশিষ্ট মৌলের নিউট্রন সংখ্যা, বাের মডেল অনুসারে পরমাণুর গঠনের চিত্র, শক্তিস্তরে ইলেকট্রন বিন্যাস এবং উপশক্তিস্তরে (অরবিটালসমূহে) ইলেকট্রন বিন্যাস সংশ্লিষ্ট একটি প্রতিবেদন প্রণয়ন।
Na(11), ভরসংখ্যা -23 P(15), ভরসংখ্যা -31 K(19), ভরসংখ্যা -40 Cu(29), ভরসংখ্যা -63
১. পরমাণু ইলেকট্রন, প্রােটন ও নিউট্রন সংখ্যা হিসাব করতে পারব। ২. পরমাণুর গঠন সম্পর্কে রাদারফোর্ড ও বাের পরমাণু মডেলের বর্ণনা করতে পারব। ৩. পরমাণুর বিভিন্ন কক্ষপথ এবং কক্ষপথের বিভিন্ন উপস্তরে পরমাণুর ইলেকট্রনসমূহকে বিন্যাস করতে পারব।
৪টি মৌলের নিউট্রন সংখ্যার হিসাব বের করতে হবে। ৪টি মৌলের বাের মডেল অনুসারে পরমাণুর গঠনের চিত্র অংকন করতে হবে ৪টি মৌলের শক্তিস্তরে ইলেকট্রন বিন্যাস করতে হবে ৪টি মৌলের উপশক্তিস্তরে (অরবিটালসমূহে) ইলেকট্রন বিন্যাস করতে হবে।
অধ্যায় ৩ পদার্থের গঠন
অ্যাসাইনমেন্ট : প্রতীকের পাশে উল্লেখিত ভরসংখ্যাবিশিষ্ট মৌলের নিউট্রন সংখ্যা, বোর মডেল অনুসারে পরমাণুর গঠনের চিত্র, শক্তস্তরে ইলেকট্রন বিন্যাস এবং উপশক্তিস্তরে (অরবিটালসমূহে) ইলেকট্রন বিন্যাস সংশ্লিষ্ট একটি প্রতিবেদন প্রণয়ন।
(ক) নিউট্রন সংখ্যা হিসাব : Na মৌলের নিউট্রন সংখ্যা =23−11=12 P মৌলের নিউট্রন সংখ্যা =31−15=16 K মৌলের নিউট্রন সংখ্যা =40−19=21 Cu মৌলের নিউট্রন সংখ্যা =63−29=34
বোর মডেল অনুসারে পরমানুর গঠনের চিত্র অঙ্কন :
𝑁𝑎Na এর ইলেকট্রন বিন্যাস-
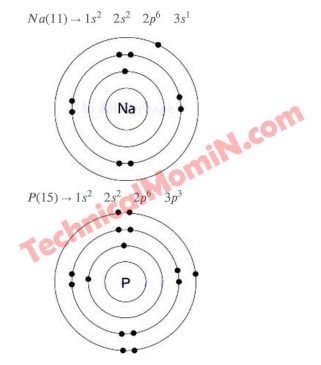
(গ) শক্তিস্তরে ইলেকট্রন বিন্যাস
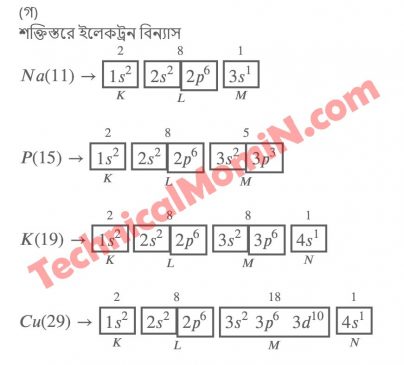
উপশক্তিস্তরে (অরবিটাল সমূহে) ইলেকট্রন বিন্যাস –
আমরা জানি, n=1 এর জন্য উপশক্তিস্তর ০ থেকে (n−1) পর্যন্ত। তাই প্রথম শক্তিস্তরে একটি উপশক্তিস্তর বিদ্যমান =1𝑠 𝑛=2 হলে, উপশক্তিস্তর হল = 2𝑠, 2p n=3 হলে, উপশক্তি স্তর হল = 3𝑠, 3p, 3d n=4 হলে, উপশক্তি স্তর হল = 4s, 4p, 4d, 4f
s উপশক্তিস্তরে থাকে সর্বোচ্চ ২টি ইলেকট্রন। 𝑝 উপশক্তিস্তরে থাকে সর্বোচ্চ ৬টি ইলেকট্রন। d উপশক্তিস্তরে থাকে সর্বোচ্চ ১০টি ইলেকট্রন। f উপশক্তিস্তরে থাকে সর্বোচ্চ ১৪টি ইলেকট্রন।
পরমাণুর অরবিটালের ক্রমবর্ধমান শক্তিগুলো : 1𝑠 <2𝑠 <2𝑝 <3𝑠< 3𝑝 <4𝑠 <3𝑑

https://www.youtube.com/watch?v=Mug_mFmVoJQ&pp=sAQA
এসএসসি প্রথম সপ্তাহের এসাইনমেন্ট ২০২১
১ম সপ্তাহের পদার্থ বিজ্ঞান
বাংলাদেশের ইতিহাস ও বিশ্বসভ্যতা
ফিন্যান্স ও ব্যাংকিং
ব্যবসায় উদ্যোগ
Finance and Banking 1st Week Assignment Answer
Biology 1st Week Assignment Answer
Business Entrepreneurship 1st Week Assignment Answer
৯ম শ্রেণীর ৯ম সপ্তাহের সকল অ্যাসাইনমেন্ট উত্তর
হিসাব বিজ্ঞান
ইতিহাস ও বিশ্বসভ্যতা
ফিন্যন্স ও ব্যাংকিং
পৌরনীতি ও নাগরিকতা
Join our YouTube channel
class 8 all subject
এস এস সি ( SSC ) দ্বিতীয় সপ্তাহের সকল এসাইনমেন্ট উত্তর
ইংরেজি এসাইনমেন্ট উত্তর
বিজ্ঞান এসাইনমেন্ট উত্তর
বাংলাদেশ ও বিশ্বপরিচয় এসাইনমেন্ট উত্তর
ইংরেজি এসাইনমেন্ট উত্তর পদার্থ বিজ্ঞান এসাইনমেন্ট উত্তর হিসাব বিজ্ঞান এসাইনমেন্ট উত্তর অর্থনীতি এসাইনমেন্ট উত্তর পৌরনীতি ও সুশাসন এসাইনমেন্ট উত্তর যুক্তিবিদ্যা এসাইনমেন্ট উত্তর
তাছাড়া আমাদের ওয়েবসাইটে ( Class 6 ) ষষ্ঠ শ্রেণীর অ্যাসাইনমেন্ট , ( Class 7 ) সপ্তম শ্রেণীর অ্যাসাইনমেন্ট ( Class 8 ) অষ্টম শ্রেণীর অ্যাসাইনমেন্ট , ( Class 9 ) নবম শ্রেণীর অ্যাসাইনমেন্ট , ( SSC ) দশম শ্রেণীর অ্যাসাইনমেন্ট ( HSC ) একাদশ শ্রেণীর অ্যাসাইনমেন্ট উত্তর দেওয়া হয়ে থাকে আপনারা চাইলে দেখতে পারেন ।
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.

IMAGES
COMMENTS
Download TOP 50 SSC CHEMISTRY QUESTIONS. FREE SSC EXAM YOUTUBE VIDEOS. Question 1: What is the common characteristic of the elements of the same group in the periodic table? a) Electrons in outer most shell. b) Total number of electrons. c) Total number of protons. d) Atomic weight.
CHEMISTRY ONE SHOT LECTURE FOR SSC CGL 2024 | SCIENCE FOR SSC EXAMS 2024 | PARMAR SSCSSC SCIENCE (CHEMISTRY) PREVIOUS YEARIn this video you will get complete...
SSC Chemistry Assignment 2021 & 2022 question and answer is available here. SSC 2021 exam candidates and SSC 2022 exam candidates, both Chemistry 2nd week, 3rd week, 5th week, 6th week, 8th week, 9th week assignment has been published. So we give all the solutions to the SSC Chemistry Assignment. SSC Chemistry Assignment 2021 is for SSC Exam ...
Assignment for SSC Examinees, 2021 Subject: Chemistry Subject Code: 137 Level: SSC Assignment Number, Chapter Number, Chapter Title Assignment Learning Outcomes Guidelines (cues/steps or stages) Assessment Criterion /Rubric Com'ts To follow the octet and duet rules d 05 a) Octet and
SSC 2021 Chemistry | English Version | Assignment-1🔴Don't forget to Subscribe & Click "🔔" https://bit.ly/3aY8fYY🔴Like, Comment & Share helps Support my wo...
SSC Chemistry Assignment Answer 2022. To the covid -19 situation, it has been decided that students need to submit assignments on different subjects and Chemistry is one of them. Especially for the students of the science group, this subject carries so much importance. The Answer of Chemistry Subject For SSC Has Been Published. you Can Download ...
Last Day - 200 SSC Mocks for just Rs. 249. Download Chemistry Notes Set-2 PDF. Download Chemistry Notes Set-3 PDF. Basic Chemistry up to Class X level is asked in Competitive Exams such as Indian Railways -ALP and Group D exams and SSC exams. Cracku brings to you the capsule - One Liners covering exam specific topics in Chemistry.
SSC 2k21 Chemistry-2nd part solution. The probable time of examination is SSC and equivalent in the second week of November this year and HSC and equivalent examination is the first week of December. Students must submit assignments for this test. SSC and equivalent assignments on the basis of a short syllabus will be given from 16th July.
According to the new 6th week SSC 2022 Chemistry Assignment question released by all education boards, the Chemistry studies will be only 2022 exam other subjects. However, this paper is also separated into two different parts. subject part and objective questions part. 6th Week Assignment only Subject which candidates have to answer the ...
SSC Chemistry 2022 Assignment Solution PDF File - 14th Week. March 3, 2022 by Desk Job. Secondary School Certificate had to be submitted for SSC Chemistry Assignment Answer 2022 for 14th weeks. There website has been published chemistry assignment ssc full question solution 14th week. We also posted and you can get here your SSC ten class ...
The SSC 2021 sixth week assignment has been published in the light of the short syllabus of the 2021 SSC candidates prepared by the National Curriculum and Textbook Board as per the instructions of the Ministry of Education due to the deadly coronavirus. On 23 August 2021, the authority published the sixth week assignment of students from all public and private educational institutions of the ...
SSC 2021 Assignment 1st week Answer Chemistry SolutionSSC 2021 Board Exam Assignment Answer : https://www.youtube.com/playlist?list=PLUem2r8EvSzNfxN7o1k4iSN...
Select Chapter. Some Basic Concepts of Chemistry / रसायन की कुछ मूलभूत अवधारणाएँ 1 Structure of Atom / परमाणु संरचना 2 Acids, Bases and Salts / अम्ल, क्षार एवं लवण 2 Metals and Non-metals / धातु और अधातु 3 Classification ...
ক্রমিক পাঠ্যপুস্তকের নাম বাংলা ভার্সন ইংরেজি ভার্সন ১। বাংলা ...
SSC Chemistry Assignment Answer 2021 has been Published. Solutions has been Published for SSC Science Group Students 2nd, 3rd, 5th, 6th, 8th, 9th, 11th and 12th Weeks. SSC Chemistry Assignment Answer 2021 All weeks
The SSC Chemistry Assignment carries a mark for students from science group. The SSC Chemistry syllabus has shortened to benefit students taking the exams because of the COVID-19 Corona Virus. Furthermore, DSHE will publish the assignment work on the website once a week in accordance with this syllabus.
The SSC Syllabus and exam pattern for the Junior Engineer (JE) are covered in brief in this section. The exam pattern of Junior Engineer is divided into two papers i.e. Paper-I (Computer-based Test) and Paper-II (Written Test). The SSC JE Exam Pattern is given below: Paper.
"BD Tutor" is a modern online teaching platform."BD Tutor" will always try to teach something new."BD Tutor" is one of the most popular educational based You...
Top 10 teachers for Chemistry (SSC) assignment help. WhatsApp, message & call private Chemistry (SSC) teachers from 125 countries JavaScript is turned off in your web browser.
In order to solve the SSC 2nd week chemistry assignment correctly, one has to acquire detailed knowledge about substances and the basics of matter. chemistry assignment SSC 2021 1st week answer Otherwise, it is not possible to give the answer to SSC 2nd week chemistry assignment correctly.
SSC 2021 Assignment 3rd Week Answer Chemistry Solution .SSC 2021 Board Exam Assignment Answer : https://www.youtube.com/playlist?list=PLUem2r8EvSzNfxN7o1k4...
Statistical analysis can detect when ChatGPT is used to cheat on multiple-choice chemistry exams by McKenzie Harris, Florida State University Credit: Journal of Chemical Education (2024).
SSC 2021 Chemistry Assignment Solution || SSC 2021 Chemistry Assignment Answer 2nd Weekhow to write ssc 2021 chemistry assignment answer, how to write ssc ch...
SSC 2021 Chemistry Assignment 3rd Week। SSC 2021 Assignment 3rd Week Answer || Chemistry Solution#takechemistryfunbd, #Rakibsirssc 2021 assignment 3rd week,s...