Advertisement

Genetically modified crops: current status and future prospects
- Published: 31 March 2020
- Volume 251 , article number 91 , ( 2020 )
Cite this article

- Krishan Kumar 1 ,
- Geetika Gambhir 1 ,
- Abhishek Dass 1 ,
- Amit Kumar Tripathi 2 ,
- Alla Singh 3 ,
- Abhishek Kumar Jha 1 ,
- Pranjal Yadava 1 ,
- Mukesh Choudhary 3 &
- Sujay Rakshit 3
34k Accesses
250 Citations
15 Altmetric
Explore all metrics
Main conclusion
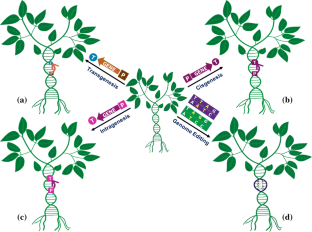
While transgenic technology has heralded a new era in crop improvement, several concerns have precluded their widespread acceptance. Alternative technologies, such as cisgenesis and genome-editing may address many of such issues and facilitate the development of genetically engineered crop varieties with multiple favourable traits.
Genetic engineering and plant transformation have played a pivotal role in crop improvement via introducing beneficial foreign gene(s) or silencing the expression of endogenous gene(s) in crop plants. Genetically modified crops possess one or more useful traits, such as, herbicide tolerance, insect resistance, abiotic stress tolerance, disease resistance, and nutritional improvement. To date, nearly 525 different transgenic events in 32 crops have been approved for cultivation in different parts of the world. The adoption of transgenic technology has been shown to increase crop yields, reduce pesticide and insecticide use, reduce CO 2 emissions, and decrease the cost of crop production. However, widespread adoption of transgenic crops carrying foreign genes faces roadblocks due to concerns of potential toxicity and allergenicity to human beings, potential environmental risks, such as chances of gene flow, adverse effects on non-target organisms, evolution of resistance in weeds and insects etc. These concerns have prompted the adoption of alternative technologies like cisgenesis, intragenesis, and most recently, genome editing. Some of these alternative technologies can be utilized to develop crop plants that are free from any foreign gene hence, it is expected that such crops might achieve higher consumer acceptance as compared to the transgenic crops and would get faster regulatory approvals. In this review, we present a comprehensive update on the current status of the genetically modified (GM) crops under cultivation. We also discuss the issues affecting widespread adoption of transgenic GM crops and comment upon the recent tools and techniques developed to address some of these concerns.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Genetically modified (gm) crops: milestones and new advances in crop improvement.

Alternative to Transgenesis: Cisgenesis and Intragenesis

Genetic Engineering: A Powerful Tool for Crop Improvement
Acharjee S, Sarmah BK, Kumar PA, Olsen K, Mahon R, Moar WJ, Moore A, Higgins TJV (2010) Transgenic chickpeas ( Cicer arietinum L.) expressing a sequence-modified cry2Aa gene. Plant Sci 178:333–339. https://doi.org/10.1016/j.plantsci.2010.02.001
Article CAS Google Scholar
Adang MJ, Brody MS, Cardineau G, Eagan N, Roush RT, Shewmaker CK, Jones A, Oakes JE, McBride KE (1993) The reconstruction and expression of a Bacillus thuringiensis cryIIIA gene in protoplasts and potato plants. Plant Mol Biol 21:1131–1145. https://doi.org/10.1007/bf00023609
Article CAS PubMed Google Scholar
Agarwal A, Yadava P, Kumar K, Singh I, Kaul T, Pattanayak A, Agrawal PA (2018) Insights into maize genome editing via CRISPR/Cas9. Physiol Mol Biol Plants 24:175–183. https://doi.org/10.1007/s12298-017-0502-3
Article CAS PubMed PubMed Central Google Scholar
Al-Babili S, Beyer P (2005) Golden Rice—Five years on the road—five years to go? Trends Plant Sci 10:565–573. https://doi.org/10.1016/j.tplants.2005.10.006
Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, Chen PJ, Wilson C, Newby GA, Raguram A, Liu DR (2019) Search and replace genome editing without double strand breaks or donor DNA. Nature 576:149–157. https://doi.org/10.1038/s41586-019-1711-4
Ariel FD, Manavella PA, Dezar CA, Chan RL (2007) The true story of the HD-Zip family. Trends Plant Sci 12:419–426. https://doi.org/10.1016/j.tplants.2007.08.003
Azevedo RA, Lea PJ (2001) Lysine metabolism in higher plants. Amino Acids 20:261–279. https://doi.org/10.1007/s007260170043
Bagla P (2010) Hardy cotton-munching pests are latest blow to GM crops. Sci 327:1439. https://doi.org/10.1126/science.327.5972.1439
Barry GF, Kishore GM, Padgette SR, Stallings WC (1997) Glyphosate-tolerant 5-enolpyruvylshikimate-3-phosphate synthases. US Patent 5633435. https://patents.google.com/patent/US5633435A/en
Bawa AS, Anilakumar KR (2013) Genetically modified foods: safety, risks and public concerns—a review. J Food Sci Technol 50:1035–1046. https://doi.org/10.1007/s13197-012-0899-1
Belhaj K, Chaparro-Garcia A, Kamoun S, Nekrasov V (2013) Plant genome editing made easy: targeted mutagenesis in model and crop plants using the CRISPR/Cas system. Plant Methods 9:39. https://doi.org/10.1186/1746-4811-9-39
Bevan MW, Flavell RB, Chilton MD (1983) A chimaeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature 304:184–187. https://doi.org/10.1038/304184a0
Bicar EH, Woodman CW, Sangtong V, Peterson JM, Yang SS, Lee M, Scott MP (2008) Transgenic maize endosperm containing a milk protein has improved amino acid balance. Transgenic Res 17:59–71. https://doi.org/10.1007/s11248-007-9081-3
Broadway RM, Duffey SS (1986) Plant proteinase inhibitors: Mechanism of action and effect on the growth and digestive physiology of larval Heliothis zea and Spodoptera exiqua . J Insect Physiol 32:827–833. https://doi.org/10.1016/0022-1910(86)90097-1
Brookes G, Barfoot P (2017) Environmental impacts of genetically modified (GM) crop use 1996–2015: Impacts on pesticide use and carbon emissions. GM Crops Food 8:117–147. https://doi.org/10.1080/21645698.2017.1309490
Article PubMed PubMed Central Google Scholar
Brookes G, Barfoot P (2018) Farm income and production impacts of using GM crop technology 1996–2016. GM Crops Food 9:59–89. https://doi.org/10.1080/21645698.2018.1464866
Brower LP, Taylor OR, Williams EH, Slayback DA, Zubieta RR, Ramirez MI (2012) Decline of monarch butterflies overwintering in Mexico: is the migratory phenomenon at risk? Insect Conserv Diver 5:95–100. https://doi.org/10.1111/j.1752-4598.2011.00142.x
Article Google Scholar
Bucchini L, Goldman L (2002) Starlink Corn: A Risk analysis. Environ Health Perspect 110:5–13. https://doi.org/10.1289/ehp.021105
Business Wire (2016) Global Genetically Modified Seeds Market to Witness Growth Through 2020 Due to Rise in Adoption of Bio-fuels: Reports Technavio. https://www.businesswire.com/news/home/20160830005089/en/Global-Genetically-Modified-Seeds-Market-Witness-Growth
Cai Y, Chen L, Liu X, Guo C, Sun S, Wu C, Jiang B, Han T, Hou W (2017) CRISPR/Cas9-mediated targeted mutagenesis of GmFT2a delays flowering time in soya bean. Plant Biotechnol J 16:176–185. https://doi.org/10.1111/pbi.12758
Callaway E (2018) CRISPR plants now subject to tough GM laws in European Union. Nature 560:16. https://doi.org/10.1038/d41586-018-05814-6
Carrière Y, Crowder DW, Tabashnik BE (2010) Evolutionary ecology of insect adaptation to Bt crops. Evol Appl 3:561–573. https://doi.org/10.1111/j.1752-4571.2010.00129.x
Castiglioni P, Warner D, Bensen RJ, Anstrom DC, Harrison J et al (2008) Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in Maize under water-limited conditions. Plant Physiol 147:446–455. https://doi.org/10.1104/pp.108.118828
Chaikam V (2012) In vivo maternal haploid induction in maize. In: B.M. Prasanna, V Chaikam, and G Mahuku, editors, Doubled haploid technology in maize breeding: Theory and practice. CIMMYT, D F, Mexico pp 9–13. https://hdl.handle.net/10883/1351
Chan RL, Cabello JV, Giacomelli JI (2013) HaHB11 provides improved plant yield and tolerance to abiotic stress. WO2013116750A1. https://patents.google.com/patent/WO2013116750A1
Chawla R, Shakya R, Rommens CM (2012) Tuber-specific silencing of asparagine synthetase-1 reduces the acrylamide-forming potential of potatoes grown in the field without affecting tuber shape and yield. Plant Biotechnol J 10:913–924. https://doi.org/10.1111/j.1467-7652.2012.00720.x
Chen JS, Dagdas YS, Kleinstiver BP, Welch MM, Sousa AA, Harrington LB, Sternberg SH, Joung JK, Yildiz A, Doudna JA (2017) Enhanced proofreading governs CRISPR–Cas9 targeting accuracy. Nature 550:407–410. https://doi.org/10.1038/nature24268
Chen LJ, Lee DS, Song ZP, Suh HS, Lu B (2004) Gene flow from cultivated rice ( Oryza sativa ) to its weedy and wild relatives. Ann Bot 93:67–73. https://doi.org/10.1093/aob/mch006
Chen TH, Murata N (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol 5:250–257. https://doi.org/10.1016/s1369-5266(02)00255-8
Chilton MD, Drummond MH, Merlo DJ, Sciaky D, Montoya AL, Gordon MP et al (1977) Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell 11:263–271. https://doi.org/10.1016/0092-8674(77)90043-5
Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ, Voytas DF (2010) Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 186:757–761. https://doi.org/10.1534/genetics.110.120717
Christian M, Qi Y, Zhang Y, Voytas DF (2013) Targeted mutagenesis of Arabidopsis thaliana using engineered TAL effector nucleases. G3 3:1697–1705. https://doi.org/10.1534/g3.113.007104
Chan RL, Cabello JV, Giacomelli JI (2013) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol 5(3):250–257. https://doi.org/10.1016/S1369-5266(02)00255-8
Clasen BM, Stoddard TJ, Luo S, Demorest ZL, Li J, Cedrone F, Tibebu R, Davison S, Ray EE, Daulhac A, Coffman A, Yabandith A, Retterath A, Haun W, Baltes NJ, Mathis L, Voytas DF, Zhang F (2016) Improving cold storage and processing traits in potato through targeted gene knockout. Plant Biotechnol J 14:169–176. https://doi.org/10.1111/pbi.12370
Cremer J, Treptow C, Eggeling L, Sahm H (1988) Regulation of enzymes of lysine biosynthesis in Corynebacterium glutamicum . Appl Environ Microbiol 134:3221–3229. https://doi.org/10.1099/00221287-134-12-3221
Curtin SJ, Xiong Y, Michno JM et al (2018) CRISPR/Cas9 and TALENs generate heritable mutations for genes involved in small RNA processing of Glycine max and Medicago truncatula . Plant Biotechnol J 16:1125–1137. https://doi.org/10.1111/pbi.12857
Dang W, Wei ZM (2007) An optimized Agrobacterium -mediated transformation for soybean for expression of binary insect resistance genes. Plant Sci 173:381–389. https://doi.org/10.1016/j.plantsci.2007.06.010
Davison J (2010) GM plants: Science, politics and EC regulations. Plant Sci 178:94–98. https://doi.org/10.1016/j.plantsci.2009.12.005
De Vos CJ, Swanenburg M (2018) Health effects of feeding genetically modified (GM) crops to livestock animals: A review. Food Chem Toxicol 117:3–12. https://doi.org/10.1016/j.fct.2017.08.031
Dezar CA, Gago GM, González DH, Chan RL (2005) Hahb-4 , a sunflower homeobox-leucine zipper gene, is a developmental regulator and confers drought tolerance to Arabidopsis thaliana plants. Transgenic Res 14:429–440. https://doi.org/10.1007/s11248-005-5076-0
Dill GM, CaJacob CA, Padgette SR (2008) Glyphosate-resistant crops: adoption, use and future considerations. Pest Manag Sci 64:326–331. https://doi.org/10.1002/ps.1501
Dively GP, Rose R, Sears MK, Hellmich RL, Stanley-Horn DE, Calvin DD, Russo JM, Anderson PL (2004) Effects on monarch butterfly larvae (Lepidoptera: Danaidae) after continuous exposure to Cry1Ab-expression corn during anthesis. Envn Entomol 33:1116–1125. https://doi.org/10.1603/0046-225x-33.4.1116
Domingo JL (2016) Safety assessment of GM plants: an updated review of the scientific literature. Food Chem Toxicol 95:12–18. https://doi.org/10.1016/j.fct.2016.06.013
Dong L, Li L, Liu C, Liu C, Geng S, Li X, Huang C, Mao L, Chen S, Xie C (2018) Genome editing and double-fluorescence proteins enable robust maternal haploid induction and identification in maize. Mol Plant 11:1214–1217. https://doi.org/10.1016/j.molp.2018.06.011
Doudna JA, Charpentier E (2014) The new frontier of genome engineering with CRISPR–Cas9. Science 346:1258096. https://doi.org/10.1126/science.1258096
Du H, Zeng X, Zhao M, Cui X, Wang Q, Yang H, Cheng H, Yu D (2016) Efficient targeted mutagenesis in soybean by TALENs and CRISPR/Cas9. J Biotechnol 217:90–97. https://doi.org/10.1016/j.jbiotec.2015.11.005
Duan X, Li X, Xue Q, Abo-El-Saad M, Xu D, Wu R (1996) Transgenic rice plants harboring an introduced potato Proteinase inhibitor II gene are insect resistant. Nat Biotechnol 14:494–498. https://doi.org/10.1038/nbt0496-494
Dufourmantel N, Tissot G, Goutorbe F, Garcon F, Jansens S, Pelissier B, Peltier G, Dubald M (2005) Generation and analysis of soybean plastid transformants expressing Bacillus thuringiensis Cry1Ab protoxin. Plant Mol Biol 58:659–668. https://doi.org/10.1007/s11103-005-7405-3
EFSA Panel on Genetically Modified Organisms (GMO) (2012) Scientific opinion addressing the safety assessment of plants developed through cisgenesis and intragenesis. EFSA J 10(2561):33. https://doi.org/10.2903/j.efsa.2012.2561
FAO (2010) Fats and fatty acids in human nutrition. Report of an expert consultation. Rome: Food and Agricultural Organisation of the United Nations. 10–14 November 2008, Geneva. FAO Food and Nutrition Paper 91. https://agris.fao.org/agris-search/search.do?recordID=XF2016049106
Fang J, Xu X, Wang P, Zhao JZ, Shelton AM, Cheng J, Shen Z (2007) Characterization of chimeric Bacillus thuringiensis Vip3 toxins. Appl Environ Microbiol 73:956–961. https://doi.org/10.1128/aem.02079-06
Faria JC, Albino MMC, Dias BBA, Cancado LJ, Cunha NB, Silva LM, Vianna GR, Aragão FJL (2006) Partial resistance to Bean golden mosaic virus in a transgenic common bean ( Phaseolus vulgaris L.) line expressing a mutated rep gene. Plant Sci 171:565–571. https://doi.org/10.1016/j.plantsci.2006.06.010
Ferreira SA, Pitz KY, Manshardt R, Zee F, Fitch M, Gonsalves D (2002) Virus coat protein transgenic papaya provides practical control of papaya ringspot virus in hawaii. Plant Dis 86:101–105. https://doi.org/10.1094/pdis.2002.86.2.101
Article PubMed Google Scholar
Ford CS, Allainguillaume J, Grilli-Chantler P, Cuccato J, Allender CJ, Wilkinson MJ (2006) Spontaneous gene flow from rapeseed ( Brassica napus ) to wild Brassica oleracea . Proc Biol Sci 273:3111–3115. https://doi.org/10.1098/rspb.2006.3686
Foster SJ, Park TH, Pel M, Brigneti G, Sliwka J, Jagger L, Vossen EVD, Jones JD (2009) Rpi-vnt1.1, a Tm-2 2 homolog from Solanum venturii , confers resistance to potato late blight. Mol Plant Microbe Interact 22:589–600. https://doi.org/10.1094/MPMI-22-5-0589
Fraley RT, Rogers SG, Horsch RB, Sanders PR, Flick JS et al (1983) Expression of bacterial genes in plant cells. Proc Natl Acad Sci USA 80:4803–4807. https://doi.org/10.1073/pnas.80.15.4803
Fujimoto H, Itoh K, Yamamoto M, Kyozuka J, Shimamoto K (1993) Insect resistant rice generated by introduction of a modifed δ-endotoxin gene of Bacillus thuringiensis . Nat Biotechnol 11:1151–1155. https://doi.org/10.1038/nbt1093-1151
Gago GM, Almoguera C, Jordano J, Gonzalez DH, Chan RL (2002) Hahb-4, a homeobox leucine zipper gene potentially involved in abscisic acid-dependent responses to water stress in sunflower. Plant Cell Environ 25:633–640. https://doi.org/10.1046/j.1365-3040.2002.00853.x
Galili G (1995) Regulation of lysine and threonine synthesis. Plant Cell 7:899–906. https://doi.org/10.1105/tpc.7.7.899
Gao JP, Chao DY, Lin HX (2007) Understanding abiotic stress tolerance mechanisms: recent studies on stress response in rice. J Integr Plant Biol 49:742–750. https://doi.org/10.1111/j.1744-7909.2007.00495.x
Gassmann AJ, Petzold-Maxwell JL, Keweshan RS, Dunbar MW (2011) Field-evolved resistance to Bt maize by western corn rootworm. PLoS ONE 6:e22629. https://doi.org/10.1371/journal.pone.0022629
Geiger HH (2009) Doubled haploids. In: Bennetzen JL, Hake S (eds) Maize handbook-volume II: genetics and genomics. Springer Verlag, Heidelberg, pp 641–657
Chapter Google Scholar
Geliebter A, Torbay N, Bracco EF, Hashim SA, Van Itallie TB (1983) Overfeeding with medium-chain triglyceride diet results in diminished deposition of fat. Am J Clin Nutr 37:1–4. https://doi.org/10.1093/ajcn/37.1.1
Gilbert N (2013) A hard look at GM crops. Nature 497:24–26. https://doi.org/10.1038/497024a
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gocal G (2015a) Non-transgenic trait development in crop plants using oligo-directed mutagenesis: Cibus’ Rapid Trait Development System. In: NABC Report 26. New DNA-Editing Approaches: Methods, Applications and Policy for Agriculture. pp 97–105. North American Agricultural Biotechnology Council, Ithaca, NY. https://ecommons.cornell.edu/bitstream/handle/1813/51428/nabc26_10_Gocal.pdf?sequence=1&isAllowed=y .
Gocal GFW, Schopke C, Beetham PR (2015) Oligomediated targeting gene editing. In: Puchta H, Thompson JG (eds) Advances in New Technology for Targeted Modification of Plant Genomes (Zhang F, Puchta H and Thompson JG Eds). Springer, New York, pp 73–89
Google Scholar
Green JM (2012) The benefits of herbicide-resistant crops. Pest Manag Sci 68:1323–1331. https://doi.org/10.1002/ps.3374
Griffiths AJF, Wessler SR, Lewontin RC, Gelbart WM, Suzuki DT, Miller JH (2005) Introduction to genetic analysis. 8th (ed.) FreemanWH, New York. https://www.bio.bg.ac.rs/materijali_predmeta/med-eng-griffiths-an-introduction-to-genetic-analysis.pdf
Guilinger JP, Pattanayak V, Reyon D, Tsai SQ, Sander JD, Joung JK, Liu DR (2014) Broad specificity profiling of TALENs results in engineered nucleases with improved DNA-cleavage specificity. Nat Methods 11:429–435. https://doi.org/10.1038/nmeth.2845
Guilinger JP, Thompson DB, Liu DR (2014) Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat Biotechnol 32:577–582. https://doi.org/10.1038/nbt.2909
Guo J, Litao Y, Xin L, Xiaoyan G, Lingxi J, Dabing Z (2009) Characterization of the exogenous insert and development of event-specific PCR detection methods for genetically modified Huanong No. 1 Papaya. J Agric Food Chem 57:7205–7212. https://doi.org/10.1021/jf901198x
Guo Y, Xu Q, Canzio D, Shou J, Li J, Gorkin DU, Jung I et al (2015) CRISPR inversion of CTCF sites alters genome topology and enhancer/promoter function. Cell 162:900–910. https://doi.org/10.1016/j.cell.2015.07.038
Gupta B, Tripathi AK, Joshi R, Pareek A, Singla-Pareek SL (2015) Designing climate smart future crops employing signal transduction components. In: Pandey GK (ed) Elucidation of abiotic stress signaling in plants: functional genomics perspectives. Springer, New York, pp 393–414
Halfill MD, Richards HA, Mabon SA, Stewart CN (2001) Expression of GFP and Bt transgenes in Brassica napus and hybridization with Brassica rapa . Theor Appl Genet 103:659–667. https://doi.org/10.1007/s001220100613
Han SM, Lee B, Won OJ, Hwang KS, Suh SJ, Kim C, Park KW (2015) Gene flow from herbicide resistant genetically modified rice to conventional rice ( Oryza sativa L.) cultivars. J Ecol Environ 38:397–403. https://doi.org/10.5141/ecoenv.2015.042
Haun W, Coffman A, Clasen BM, Demorest ZL, Lowy A, Ray E, Retterath A, Stoddard T, Juillerat A, Cedrone F, Mathis L, Voytas DF, Zhang F (2014) Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol J 12:934–940. https://doi.org/10.1111/pbi.12201
Haverkort J, Struik PC, Visser RGF, Jacobsen E (2009) Applied Biotechnology to Combat Late Blight in Potato Caused by Phytophthora Infestans . Potato Res 52:249–264. https://doi.org/10.1007/s11540-009-9136-3
Heap I (2014) Global perspective of herbicide-resistant weeds. Pest Manag Sci 70:1306–1315. https://doi.org/10.1002/ps.3696
Heap I, Duke SO (2018) Overview of glyphosate-resistant weeds worldwide. Pest Manag Sci 74:1040–1049. https://doi.org/10.1002/ps.4760
Heritage J (2004) The fate of transgenes in the human gut. Nat Biotechnol 22:170–172. https://doi.org/10.1038/nbt0204-170
Herrera-Estrella L, Block MD, Messens E, Hernalsteens JP, Montagu MV, Schell J (1983) Chimeric genes as dominant selectable markers in plant cells. EMBO J 2:987–995. https://doi.org/10.1002/j.1460-2075.1983.tb01532.x
Herrera-Estrella L, Depicker A, Montagu MV, Schell J (1983) Expression of chimaeric genes transfered into plant cells using a Ti-plasmid-derived vector. Nature 303:209–213. https://doi.org/10.1038/303209a0
Hilder VA, Gatehouse AMR, Sheerman SE, Barker RF, Boulter D (1987) A novel mechanism of insect resistance engineered into tobacco. Nature 330:160–163. https://doi.org/10.1038/330160a0
Hilton IB, D’Ippolito AM, Vockley CM, Thakore PI, Crawford GE, Reddy TE, Gersbach CA (2015) Epigenome editing by a CRISPR–Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol 33:510–517. https://doi.org/10.1038/nbt.3199
Holme IB, Dionisio G, Brinch-Pedersen H, Wendt T, Madsen CK, Vincze E, Holm PB (2012) Cisgenic barley with improved phytase activity. Plant Biotechnol J 10:237–247. https://doi.org/10.1111/j.1467-7652.2011.00660.x
Indurker S, Misra HS, Eapen S (2007) Genetic transformation of chickpea ( Cicer arietinum L.) with insecticidal crystal protein gene using particle gun bombardment. Plant Cell Rep 26:755–763. https://doi.org/10.1007/s00299-006-0283-6
ISAAA (2017) Global Status of Commercialized Biotech/GM Crops in 2017: Biotech Crop Adoption Surges as Economic Benefits Accumulate in 22 Years. ISAAA Brief No. 53. ISAAA: Ithaca, NY. https://www.isaaa.org/resources/publications/briefs/53/
ISAAA (2018) Global Status of Commercialized Biotech/GM Crops in 2018: Biotech Crops Continue to Help Meet the Challenges of Increased Population and Climate Change. ISAAA Brief No . 54. ISAAA: Ithaca, NY. https://www.isaaa.org/resources/publications/briefs/54/executivesummary/pdf/B54-ExecSum-English.pdf
ISAAA database (2019) GM Approval Database retrieved on 17 Nov 2019. https://www.isaaa.org/gmapprovaldatabase/default.asp
James C (1997) Global Status of Transgenic Crops in 1997. ISAAA Brief No. 5. ISAAA: Ithaca, NY. pp 31. https://www.isaaa.org/purchasepublications/itemdescription.asp?ItemType=BRIEFS&Control=IB005-1997
James C (2013) Global Status of Commercialized Biotech/GM Crops: 2013. ISAAA Brief No.46. ISAAA: Ithaca, NY. https://www.isaaa.org/resources/publications/briefs/46/
James C (2015) 20th Anniversary (1996 to 2015) of the Global Commercialization of Biotech Crops and Biotech Crop Highlights in 2015. ISAAA Brief No. 51. ISAAA: Ithaca, NY. https://isaaa.org/resources/publications/briefs/51/default.asp
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821. https://doi.org/10.1126/science.1225829
Jones HD (2015) Regulatory uncertainty over genome editing. Nature Plants 1:14011. https://doi.org/10.1038/nplants.2014.11
Joung JK, Sander JD (2013) TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol 14:49–55. https://doi.org/10.1038/nrm3486
Kalla R, Shimamoto K, Potter R, Nielsen PS, Linnestad C, Olsen OA (1994) The promoter of the barley aleurone-specific gene encoding a putative 7 kDa lipid transfer protein confers aleurone cell specific expression in transgenic rice. Plant J 6:849–860. https://doi.org/10.1046/j.1365-313X.1994.6060849.x
Kaniewski W, Lawson C, Sammons B, Haley L, Hart J, Delannay X, Tumer NE (1990) Field resistance of transgenic russet burbank potato to effects of infection by potato virus X and potato virus Y. Nat Biotechnol 8:750–754. https://doi.org/10.1038/nbt0890-750
Karlson D, Nakaminami K, Toyomasu T, Imai R (2002) A cold-regulated nucleic acid-binding protein of winter wheat shares a domain with bacterial cold shock proteins. J Biol Chem 277:35248–35256. https://doi.org/10.1074/jbc.m205774200
Keese P (2008) Risks from GMOs due to horizontal gene transfer. Environ Biosaf Res 7:123–149. https://doi.org/10.1051/ebr:2008014
Kelliher T, Starr D, Richbourg L, Chintamanani S, Delzer B, Nuccio ML, Green J, Chen Z, McCuiston J, Wang W, Liebler T, Bullock P, Martin B (2017) MATRILINEAL, a sperm-specific phospholipase, triggers maize haploid induction. Nature 542:105–109. https://doi.org/10.1038/nature20827
Kereša S, Grdiša M, Barić M, Barčić J, Marchetti S (2008) Transgenic plants expressing insect resistance genes. Sjemenarstvo 25:139–153. https://hrcak.srce.hr/file/43734
Khan MS, Yu X, Kikuchi A, Asahina M, Watanabe KN (2009) Genetic engineering of glycinebetaine biosynthesis to enhance abiotic stress tolerance in plants. Plant Biotechnol 26:125–134. https://doi.org/10.5511/plantbiotechnology.26.125
Kim JS, Park SJ, Kwak KJ, Kim YO, Song J, Jang B, Jung CH, Kang H (2007) Cold shock domain proteins and glycine-rich RNA-binding proteins from Arabidopsis thaliana can promote cold adaptation process in Escherichia Coli . Nucleic Acids Res 35:506–516. https://doi.org/10.1093/nar/gkl1076
Kim MH, Sato S, Sasaki K, Saburi W, Matsui H, Imai R (2013) Cold shock domain protein 3 is involved in salt and drought stress tolerance in Arabidopsis . FEBS Open Biol 3:438–442. https://doi.org/10.1016/j.fob.2013.10.003
Kim H, Kim JS (2014) A guide to genome engineering with programmable nucleases. Nat Rev Genet 15:321–334. https://doi.org/10.1038/nrg3686
Kim Y, Kweon J, Kim JS (2013) TALENs and ZFNs are associated with different mutation signatures. Nat Methods 10:185. https://doi.org/10.1038/nmeth.2364
Kim YG, Cha J, Chandrasegaran S (1996) Hybrid restriction enzymes: zinc finger fusions to FokI cleavage domain. Proc Natl Acad Sci USA 93:1156–1160. https://doi.org/10.1073/pnas.93.3.1156
Klümper W, Qaim M (2014) A Meta-Analysis of the Impacts of Genetically Modified Crops. PLoS ONE 9:e111629. https://doi.org/10.1371/journal.pone.0111629
Koul B, Srivastava S, Sanya I, Tripathi B, Sharma V, Amla DV (2014) Transgenic tomato line expressing modified Bacillus thuringiensis cry1Ab gene showing complete resistance to two lepidopteran pests. Springer Plus 3:84. https://doi.org/10.1186/2193-1801-3-84
Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W, Cortes D, Shulaev V, Mittler R (2008) Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J Biol Chem 283:34197–34203. https://doi.org/10.1074/jbc.M806337200
Koziel MG, Beland GL, Bowman C, Carozzi NB, Crenshaw C, Crossland L, Dawson J, Desai N, Hill M, Kadwell S et al (1993) Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringiensis . Nat Biotechnol 11:194–200. https://doi.org/10.1038/nbt0293-194
Kumar H, Kumar V (2004) Tomato expressing Cry1A(b) insecticidal protein from Bacillus thuringiensis protected against tomato fruit borer, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) damage in the laboratory, greenhouse and field. Crop Prot 23:135–139. https://doi.org/10.1016/j.cropro.2003.08.006
Kumar K, Aggarwal C, Sapna B, Singh I, Yadava P (2018) Microbial genes in crop improvement. Crop improvement through microbial biotechnology. Elsevier, Amsterdam, Netherlands, pp 39–56
Lawrenson T, Shorinola O, Stacey N, Li C, Østergaard L, Patron N, Uauy C, Harwood W (2015) Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease. Genome Biol 16:258. https://doi.org/10.1186/s13059-015-0826-7
Lea PJ, Joy KW, Ramos JL, Guerrero MG (1984) The action of 2-amino-4-(methylphosphinyl)-butanoic acid (phosphinothricin) and its 2-oxo-derivative on the metabolism of cyanobacteria and higher plants. Phytochemistry 23:1–6. https://doi.org/10.1016/0031-9422(84)83066-6
Li C, Zong Y, Wang Y, Jin S, Zhang D, Song Q, Zhang R, Gao C (2018) Expanded base editing in rice and wheat using a Cas9–adenosine deaminase fusion. Genome Biol 19:59. https://doi.org/10.1186/s13059-018-1443-z
Li T, Liu B, Spalding MH, Weeks DP, Yang B (2012) High-efficiency TALEN-based gene editing produces disease-resistance rice. Nat Biotechnol 30:390–392. https://doi.org/10.1038/nbt.2199
Li Z, Zhang D, Xiong X, Yan B, Xie W, Sheen J, Li JF (2017) A potent Cas9-derived gene activator for plant and mammalian cells. Nat Plants 3:930–936. https://doi.org/10.1038/s41477-017-0046-0
Liang Z, Chen K, Li T, Zhang Y, Wang Y, Zhao Q, Liu J et al (2017) ARTICLE Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat Commun 8:1–5. https://doi.org/10.1038/ncomms14261
Liu C, Li X, Meng D, Zhong Y, Chen C, Dong X, Xu X, Chen B, Li W, Li L et al (2017) A 4-bp insertion at ZmPLA1 encoding a putative phospholipase A generates haploid induction in maize. Mol Plant 10:520–522. https://doi.org/10.1016/j.molp.2017.01.011
Losey JE, Rayor LS, Carter ME (1999) Transgenic pollen harms monarch larvae. Nature 399:214. https://doi.org/10.1038/20338
Lowder LG, Paul JW, Qi Y (2017) Multiplexed transcriptional activation or repression in plants using CRISPR–dCas9-based Systems. In: Mueller-Roeber B, Kaufmann K (eds) Plant gene regulatory networks. Methods in molecular biology. Humana Press, New York, pp 167–184
Lu Y, Wu K, Jiang Y, Xia B, Li P, Feng H, Wyckhuys KAG, Guo Y (2010) Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 328:1151–1154. https://doi.org/10.1126/science.1187881
Luttrell RG, Ali I, Allen KC, Young III SY, Szalanski A, Williams K, Lorenz G, Parker Jr CD, Blanco C, (2004) Resistance to Bt in Arkansas populations of cotton bollworm, pp. 1373–1383. In Richter DA [ed.], Proceedings, 2004 Beltwide Cotton Conferences, 5–9 January 2004, San Antonio, TX, National Cotton Council of America, Memphis, TN. https://naldc.nal.usda.gov/download/12012/PDF
Lovei GL, Bøhn T, Hilbeck A (2010) Biodiversity, Ecosystem Services and Genetically Modified Organisms Third World Network, 131 Macalister Road 10400 Penang, Malaysia. ISBN: 978-967-5412-13-4. https://www.twn.my/title2/biosafety/pdf/bio10.pdf
Ma L, Zhu F, Li Z, Zhang J, Li X, Dong J, Wang T (2015) TALEN-Based mutagenesis of lipoxygenase LOX3 enhances the storage tolerance of rice ( Oryza sativa ) seeds. PLoS ONE 10:e0143877. https://doi.org/10.1371/journal.pone.0143877
Ma L, Zhang D, Miao Q, Yang J, Xuan Y, Hu Y (2017) Essential role of sugar transporter OsSWEET11 during the early stage of rice grain filling. Plant Cell Physiol 58:863–873. https://doi.org/10.1093/pcp/pcx040
Mahfouz MM, Li L, Piatek M et al (2012) Targeted transcriptional repression using a chimeric TALE-SRDX repressor protein. Plant Molecular Biol 78:311–321. https://doi.org/10.1007/s11103-011-9866-x
Majeed A, Makhdoom R, Husnain T, Riazuddin S (2011) Assessment of potato proteinase inhibitor-II gene as an antifungal and insecticidal agent. Acta Agric Scand Sect 61:92–96. https://doi.org/10.1080/09064710903433777
Malinovski T, Cambra M, Capote N, Zawadska B, Gorris T, Scorza R, Ravelonandro M (2006) Field trials of plum clones transformed with Plum pox virus coat protein (PPV CP) gene. Plant Dis 90:1012–1018. https://doi.org/10.1094/pd-90-1012
Mandaokar AD, Goyal RK, Shukla A, Bisaria S, Bhalla R, Reddy VS, Chaurasia A, Sharma RP, Altosaar I, Ananda Kumar P (2000) Transgenic tomato plants resistant to fruit borer ( Helicoverpa armigera Hübner). Crop Prot 19:307–312. https://doi.org/10.1016/j.cropro.2012.01.010
Marc F, Dennis G (1995) Resistance of transgenic hybrid squash zw-20 expressing the coat protein genes of zucchini yellow mosaic virus and watermelon mosaic virus 2 to mixed infections by both potyviruses. Nat Biotechnol 13:1466–1473. https://doi.org/10.1038/nbt1295-1466
McDougall P (2011) The Cost and Time Involved in the Discovery, Development and Authorisation of a New Plant Biotechnology Derived Trait. In: A Consultancy Study for Crop Life International, Pathhead, Midlothin, UK. https://croplife.org/wp-content/uploads/2014/04/Getting-a-Biotech-Crop-to-Market-Phillips-McDougall-Study.pdf
McPherson SA, Perlak FJ, Fuchs RL, Marrone PG, Lavrik PB, Fischho DA (1988) Characterization of the coleopteran specific protein gene of Bacillus thuringiensis var. tenebrionis . Nat Biotechnol 6:61–66. https://doi.org/10.1038/nbt0188-61
Mehrotra M, Singh AK, Sanyal I, Altosaar I, Amla DV (2011) Pyramiding of modified cry1Ab and cry1Ac genes of Bacillus thuringiensis in transgenic chickpea ( Cicer arietinum L.) for improved resistance to pod borer insect Helicoverpa armigera . Euphytica 182:87–102. https://doi.org/10.1007/s10681-011-0501-3
Mertens M (2008) Assessment of Environmental Impacts of Genetically Modified Plants. BfN- Skripten 217. Federal Agency for Nature Conservation, New York, USA. https://www.bfn.de/fileadmin/MDB/documents/service/skript217.pdf
Miller JC, Holmes MC, Wang J, Guschin DY, Lee YL et al (2007) An improved zinc-finger nuclease architecture for highly specific genome editing. Nat Biotechnol 25:778–785. https://doi.org/10.1038/nbt1319
Miller JC, Tan S, Qiao G, Barlow KA, Wang J et al (2011) A TALE nuclease architecture for efficient genome editing. Nat Biotechnol 29:143–148. https://doi.org/10.1038/nbt.1755
Miller JK, Bradford KJ (2010) The regulatory bottleneck for biotech specialty crops. Nat Biotechnol 28:1012–1014. https://doi.org/10.1038/nbt1010-1012
Mikami M, Toki S, Endo M (2016) Precision targeted mutagenesis via Cas9 paired nickases in Rice. Plant Cell Physiol 57(5):1058–1068. https://doi.org/10.1093/pcp/pcw049
Moradpour M, Abdulah SNA (2019) CRISPR/dCas9 platforms in plants: strategies and applications beyond genome editing. Plant Biotechnol J. https://doi.org/10.1111/pbi.13232
Morineau C, Bellec Y, Tellier F, Gissot L, Kelemen Z, Nogue F, Faure JD (2017) Selective gene dosage by CRISPR–Cas9 genome editing in hexaploid Camelina sativa . Plant Biotechnol J 15:729–739. https://doi.org/10.1111/pbi.12671
Murai N, Sutton DW, Murray MG, Slightom JL, Merlo DJ, Reichert NA, Sengupta-Gopalan C, Stock CA, Barker RF, Kemp JD, Hall TC (1983) Phaseolin gene from bean is expressed after transfer to sunflower via tumor-inducing plasmid vectors. Science 222:476–482. https://doi.org/10.1126/science.222.4623.476
Nahar K, Hasanuzzaman M, Fujita M (2016) Roles of osmolytes in plant adaptation to drought and salinity. In: Iqbal N, Nazar R, Khan AN (eds) Osmolytes and plants acclimation to changing environment: emerging omics technologies. Springer, New Delhi pp: 37–68
Netherwood T, Martín-Orúe SM, O'Donnell AG, Gockling S, Graham J, Mathers JC, Gilbert HJ (2004) Assessing the survival of transgenic plant DNA in the human gastrointestinal tract. Nat Biotechnol 22:204–209. https://doi.org/10.1038/nbt934
Oliva R, Ji C, Atienza-Grande G et al (2019) Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat Biotechnol. https://doi.org/10.1038/s41587-019-0267-z
Onaga G, Wydra K (2016) Advances in plant tolerance to biotic stresses, plant genomics, Ibrokhim Y. Abdurakhmonov, Intech Open. https://doi.org/10.5772/64351 . https://www.intechopen.com/books/plant-genomics/advances-in-plant-tolerance-to-biotic-stresses
Osakabe K, Osakabe Y, Toki S (2010) Site-directed mutagenesis in Arabidopsis using custom-designed zinc finger nucleases. Proc Natl Acad Sci USA 107:12034–12039. https://doi.org/10.1073/pnas.1000234107
Osakabe Y, Watanabe T, Sugano SS et al (2016) Optimization of CRISPR/Cas9 genome editing to modify abiotic stress responses in plants. Sci Rep. https://doi.org/10.1038/srep26685
Padgette SR, Kolacz KH, Delannay X, Re DB, LaVallee BJ, Tinius CN, Rhodes WK, Otero YI, Barry GF, Eichholz DA et al (1995) Development, identification and characterization of a glyphosate-tolerant soybean line. Crop Sci 35:1451–1461. https://doi.org/10.2135/cropsci1995.0011183x003500050032x
Paine JA, Shipton CA, Chaggar S et al (2005) Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat Biotechnol 223:482–487. https://doi.org/10.1038/nbt1082
Parrott WA, All JN, Adang MJ, Bailey MA, Boerma HR, Stewart CN Jr (1994) Recovery and evaluation of soybean plants transgenic for a Bacillus thuringiensis var. kurstaki insecticidal gene. Vitro Cell Dev Biol-Plant 30:144–149. https://doi.org/10.1007/bf02632204
Perlak FJ, Fuchs RL, Dean DA, McPherson SL, Fischho DA (1991) Modifcation of the coding sequence enhances plant expression of insect control protein genes. Proc Natl Acad Sci USA 88:3324–3328. https://doi.org/10.1073/pnas.88.8.3324
Perotti MF, Ribone PA, Chan RL (2017) Plant transcription factors from the homeodomain-leucine zipper family I. Role in development and stress responses. IUBMB Life 69:280–289. https://doi.org/10.1002/iub.1619
Rahman M, Hussain K, Khan MA, Bakhsh A, Rao AQ (2012) An insight of cotton leaf curl virus: a devastating plant pathogenic begomovirus. Pure Appl Bio 1:52–58. https://doi.org/10.19045/bspab.2012.13001
Ramachandran S, Buntin GD, All JN, Tabashnik BE, Raymer PL, Adang MJ, Pulliam DA, Stewart CN Jr (1998) Survival, development, and oviposition of resistant diamondback moth (Lepidoptera: Plutellidae ) on transgenic canola producing a Bacillus thuringiensis toxin. J Econ Entomol 91:1239–1244. https://doi.org/10.1093/jee/91.6.1239
Rascón-Cruz Q, Sinagawa-García S, Osuna-Castro JA, Bohorova N, ParedesLopez O (2004) Accumulation, assembly, and digestibility of amarantin expressed in transgenic tropical maize. Theor Appl Genet 108:335–342. https://doi.org/10.1007/s00122-003-1430-x
Rasmussen S, Barah P, Suarez-Rodriguez MC, Bressendorff S, Friis P, Costantino P, Bones AM, Nielsen HB, Mundy J (2013) Transcriptome responses to combinations of stresses in Arabidopsis . Plant Physiol 161:1783–1794. https://doi.org/10.1104/pp.112.210773
Ravelonandro M, Scorza R, Bachelier JC, Labonne G, Levy L, Damsteegt V, Callahan AM, Dunez J (1997) Resistance of transgenic Prunus domestica to Plum pox virus infection. Plant Dis 81:1231–1235. https://doi.org/10.1094/pdis.1997.81.11.1231
Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, Zhang F (2013) Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154(6):1380–1389. https://doi.org/10.1016/j.cell.2013.08.021
Raza A, Razzaq A, Mehmood SS, Zou X, Zhang X, Lv Y, Xu J (2019) Impact of climate change on crops adaptation and strategies to tackle its outcome: a review. Plants 8:34. https://doi.org/10.3390/plants8020034
Article CAS PubMed Central Google Scholar
Rensburg JBJ (2007) First report of field resistance by the stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. S Afr J Plant Soil 27:147–151. https://doi.org/10.1080/02571862.2007.10634798
Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134:1683–1696. https://doi.org/10.1104/pp.103.033431
Rommens CM, Haring MA, Swords K, Davies HV (2007) The intragenic approach as a new extension to traditional plant breeding. Trends Plant Sci 12:397–403. https://doi.org/10.1016/j.tplants.2007.08.001
Ruiz-Lopez N, Haslam RP, Napier JA, Sayanova O (2014) Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J 77:198–208. https://doi.org/10.1111/tpj.12378
Sander JD, Dahlborg EJ, Goodwin MJ, Cade L, Zhang F, Cifuentes D, Curtin SJ, Blackburn JS, Thibodeau-Beganny S, Qi Y et al (2011) Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA). Nat Methods 8:67–69. https://doi.org/10.1038/nmeth.1542
Sanyal I, Singh AK, Kaushik M, Amla DV (2005) Agrobacterium mediated transformation of chickpea ( Cicer arietinum L.) with Bacillus thuringiensis cry1Ac gene for resistance against pod borer insect Helicoverpa armigera . Plant Sci 168:1135–1146. https://doi.org/10.1016/j.plantsci.2004.12.015
Sauer H, Wild A, Ruhle W (1987) The effect of phosphinothricin (glufosinate) on photosynthesis II. The cause of inhibition of photosynthesis. Zeitschrift für Naturforschung C 42:270–278. https://doi.org/10.1515/znc-1987-0317
Sauer NJ, Mozoruk J, Miller RB, Warburg ZJ, Walker KA, Beetham PR et al (2016) Oligonucleotide-directed mutagenesis for precision gene editing. Plant Biotechnol J 14:496–502. https://doi.org/10.1111/pbi.12496
Schiml S, Fauser F, Puchta H (2014) The CRISPR/Cas system can be used as nuclease for in planta gene targeting and as paired nickases for directed mutagenesis in Arabidopsis resulting in heritable progeny. Plant J 80:1139–1150. https://doi.org/10.1111/tpj.12704
Schouten HJ, Krens FA, Jacobsen E (2006) Cisgenic plants are similar to traditionally bred plants. EMBO Rep 7:750–753. https://doi.org/10.1038/sj.embor.7400769
Sears MK, Hellmich RL, Stanley-Horn DE, Oberhauser KS, Pleasants JM, Mattila HR, Siegfried BD, Dively GP (2001) Impact of Bt corn pollen on monarch butterfly populations: a risk assessment. Proc Natl Acad Sci USA 98:11937–11942. https://doi.org/10.1073/pnas.211329998
Seralini GE, Clair E, Mesnage R, Gress S, Defarge N, Malatesta M, Hennequin D, de Vendômois JS (2012) Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Food Chem Toxicol 50:4221–4231. https://doi.org/10.1016/j.fct.2013.11.047
Seralini GE, Clair E, Mesnage R, Gress S, Defarge N, Malatesta M, Hennequin D, de Vendômois JS (2014) Republished study: long-term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Environ Sci Eur 26:14. https://doi.org/10.1186/s12302-014-0014-5
Shan Q, Zhang Y, Chen K, Zhang K, Gao C (2015) Creation of fragrant rice by targeted knockout of the OsBADH2 gene using TALEN technology. Plant Biotechnol J 13:791–800. https://doi.org/10.1111/pbi.12312
Shen L, Hua Y, Fu Y, Li J, Liu Q, Jiao X et al (2017) Rapid generation of genetic diversity by multiplex CRISPR/Cas9 genome editing in rice. Sci China Life Sci 60:506–515. https://doi.org/10.1007/s11427-017-9008-8
Shen L, Wang C, Fu Y, Wang J, Liu Q, Zhang X et al (2018) QTL editing confers opposing yield performance in different rice varieties. J Integr Plant Biol 60:89–93. https://doi.org/10.1111/jipb.12501
Sherkow JS (2018) The CRISPR patent landscape: past, present and future. CRISPR J 1:5–9. https://doi.org/10.1089/crispr.2017.0013
Shukla VK, Doyon Y, Miller JC, DeKelver RC, Moehle EA, Worden SE, Mitchell JC, Arnold NL, Gopalan S, Meng X, Choi VM, Rock JM, Wu YY, Katibah GE, Zhifang G, McCaskill D, Simpson MA, Blakeslee B, Greenwalt SA, Butler HJ, Hinkley SJ, Zhang L, Rebar EJ, Gregory PD, Urnov FD (2009) Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature 459:437–441. https://doi.org/10.1038/nature07992
Sindhu AS, Zheng ZW, Murai N (1997) The pea seed storage protein legumin was synthesized, processed and accumulated stably in transgenic rice endosperm. Plant Sci 130:189–196. https://doi.org/10.1016/S0168-9452(97)00219-7
Singh M, Kumar J, Singh S, Singh VP, Prasad SM (2015) Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev Environ Sci Biotechnol 14:407–426. https://doi.org/10.1007/s11157-015-9372-8
Smart RD, Blum M, Wesseler J (2017) Trends in approval times for genetically engineered crops in the United States and the European Union. J Agricul Econ 68:182–198. https://doi.org/10.1111/1477-9552.12171
Songstad DD, Petolino JF, Voytas DF, Reichert NA (2017) Genome Editing of Plants. Crit Rev Plant Sci 36:1–23. https://doi.org/10.1080/07352689.2017.1281663
Stewart CN Jr, Adang MJ, All JA, Raymer PL, Ramachandran S, Parrott WA (1996) Insect control and dosage effects in transgenic canola containing a synthetic Bacillus thuringiensis cryIAC gene. Plant Physiol 112:115–120. https://doi.org/10.1104/pp.112.1.115
St-Onge M, Jones P (2003) Greater rise in fat oxidation with medium-chain triglyceride consumption relative to long-chain triglyceride is associated with lower initial body weight and greater loss of subcutaneous adipose tissue. Int J Obes 27:1565–1571. https://doi.org/10.1038/sj.ijo.0802467
Stöger E, Parker M, Christou P, Casey R (2001) Pea legumin overexpressed in wheat endosperm assembles into an ordered para Crystalline matrix. Plant Physiol 125:1732–1742. https://doi.org/10.1104/pp.125.4.1732
Storer NP, Babcock JM, Schlenz M, Meade T, Thompson GD, Bing JW, Huckaba RM (2010) Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J Econ Entomol 103:1031–1038. https://doi.org/10.1603/ec10040
Suzie K, Ma JKC, Drake PMW (2008) Genetically modified plants and human health. J R Soc Med 101:290–298. https://doi.org/10.1258/jrsm.2008.070372
Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R (2014) Abiotic and biotic stress combinations. New Phytol 203:32–43. https://doi.org/10.1111/nph.12797
Swarts DC, Jinek M (2018) Cas9 versus Cas12a/Cpf1: Structure–function comparisons and implications for genome editing. Wiley Interdiscip Rev RNA 9:e1481. https://doi.org/10.1002/wrna.1481
Tabashnik BE, Gassman AJ, Crowder DW, Carriere Y (2008) Insect resistance to Bt crops: evidence versus theory. Nat Biotechnol 26:199–202. https://doi.org/10.1038/nbt1382
Tabashnik BE, Finson N, Johnson MW, Moar WJ (1993) Resistance to toxins from Bacillus thuringiensis subsp. kurstaki causes minimal cross-resistance to B. thuringiensis subsp. aizawai in diamondback moth (Lepidoptera: Plutellidae). Appl Environ Microbiol 59:1332–1335. https://aem.asm.org/content/59/5/1332
Tabashnik BE, Carrière Y (2010) Field-evolved resistance to Bt cotton: Bollworm in the US and pink bollworm in India. Southwest Entomologist 35:417–424. https://doi.org/10.3958/059.035.0326
Tabashnik BE, Brévault T, Carrière Y (2013) Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotechnol 31:510–521. https://doi.org/10.1038/nbt.2597
Tachibana K, Watanabe T, Sekizawa Y, Takematsu T (1986) Action mechanism of bialaphos 2. Accumulation of ammonia in plants treated with bialaphos. J Pestic Sci 1:33–37. https://doi.org/10.1584/jpestics.11.33
Takabe T, Nakamura T, Nomura M, Hayashi Y, Ishitani M, Muramoto Y, Tanaka A, Takabe T (1998) 8-Glycinebetaine and the genetic engineering of salinity tolerance in plants. Stress responses of photosynthetic organisms. Elsevier Science, Amsterdam, pp 115–131. https://doi.org/10.1016/b978-0-444-82884-2.50011-x
Thomas PE, Lawson EC, Zalewski JC, Reed GL, Kaniewski WK (2000) Extreme resistance to Potato leafroll virus in potato cv. Russet Burbank mediated by the viral replicase gene. Virus Res 71:49–62. https://doi.org/10.1016/s0168-1702(00)00187-8
Tohidfar M, Zare N, Jouzani GS, Efekhari SM (2013) Agrobacterium mediated transformation of alfalfa ( Medicago sativa ) using a synthetic cry3a gene to enhance resistance against alfalfa weevil. Plant Cell Tiss Org 113:227–235. https://doi.org/10.1007/s11240-012-0262-2
Tricoli DM, Carney KJ, Russell PF, McMaster JR, Groff DW et al (1995) Field evaluation of transgenic squash containing single or multiple virus coat protein gene constructs for resistance to cucumber mosaic virus, watermelon mosaic virus 2 and zucchini yellow mosaic virus. Nat Biotechnol 13:1458–1465. https://doi.org/10.1038/nbt1295-1458
Tsai SQ, Wyvekens N, Khayter C, Foden JA, Thapar V, Reyon D, Goodwin MJ, Aryee MJ, Joung JK (2014) Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat Biotechnol 32:569–576. https://doi.org/10.1038/nbt.2908
Tsatsakis AM, Nawaz MA, Kouretas D, Balias G, Savolainen K, Tutelyan VA, Golokhvast KS, Lee JD, Yang SH, Chung G (2017) Environmental impacts of genetically modified plants: a review. Food Chem Toxicol 156:818–833. https://doi.org/10.1016/j.envres.2017.03.011
Tuteja N, Gill SS (2014) Climate change and plant abiotic stress tolerance. Wiley-Blackwell, Amsterdam p 1208. ISBN: 978-3-527-33491-9. https://www.wiley.com/en-us/Climate+Change+and+Plant+Abiotic+Stress+Tolerance-p-9783527334919
Tuteja N, Verma S, Sahoo R, Raveendar S, Reddy I (2012) Recent advances in development of marker free transgenic plants: regulation and biosafety concern. J Biosci 37:167–197. https://doi.org/10.1007/s12038-012-9187-5
US EPA (2017) Starlink™ corn regulatory information. Pesticides. US EPA. https://www3.epa.gov/pesticides/chem_search/reg_actions/pip/starlink_corn.htm#proposal
USDA APHIS (2020) https://www.aphis.usda.gov/aphis/ourfocus/biotechnology/am-i-regulated
Usher S, Han L, Haslam RP, Michaelson LV, Sturtevant D, Aziz M, Chapman KD, Sayanova O, Napier JA (2017) Tailoring seed oil composition in the real world: optimising omega-3 long chain polyunsaturated fatty acid accumulation in transgenic Camelina sativa . Sci Rep 7:6570. https://doi.org/10.1038/s41598-017-06838-0
Vanblaere T, Szankowski I, Schaart J, Schouten H, Flachowsky H, Broggini GA, Gessler C (2011) The development of a cisgenic apple plant. J Biotechnol 154:304–311. https://doi.org/10.1016/j.jbiotec.2011.05.013
Vaughn T, Cavato T, Brar G, Coombe T, DeGooyer T, Ford S, Groth M, Howe A, Johnson S, Kolacz K et al (2005) A method of controlling corn rootworm feeding using a Bacillus thuringiensis protein expressed in transgenic maize. Crop Sci 45:931–938. https://doi.org/10.2135/cropsci2004.0304
Vauterin M, Frankard V, Jacobs M (2000) Functional rescue of a bacterial dapA auxotroph with a plant cDNA library selects for mutant clones encoding a feedback-insensitive dihydrodipicolinate synthase. Plant J 21:239–248. https://doi.org/10.1046/j.1365-313x.2000.00668.x
Veillet F, Perrot L, Chauvin L, Kermarrec MP, Guyon-Debast A, Chauvin JE, Nogue F, Mazier M (2019) Transgene-free genome editing in tomato and potato plants using agrobacterium-mediated delivery of a CRISPR/Cas9 cytidine base editor. Int J Mol Sci 20:402. https://doi.org/10.3390/ijms20020402
Wang B, Zhu L, Zhao B, Zhao Y, Xie Y, Zheng Z, Li Y, Sun J, Wang H (2019) Development of a haploid-inducer mediated genome editing (IMGE) system for accelerating maize breeding. Mol Plant 12:597–602. https://doi.org/10.1016/j.molp.2019.03.006
Wang Y, Cheng X, Shan Q, Zhang Y, Liu J, Gao C et al (2014) Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotechnol 32:947–951. https://doi.org/10.1038/nbt.2969
Waltz E (2014) Beating the heat. Nat Biotechnol 32:610–613. https://doi.org/10.1038/nbt.2948
Waltz E (2015) USDA approves next-generation GM potato. Nat Biotechnol 33:12–13. https://doi.org/10.1038/nbt0115-12
Waltz E (2016) CRISPR-edited crops free to enter market, skip regulation. Nat Biotechnol 34:582. https://doi.org/10.1038/nbt0616-582
Waltz E (2018) With a free pass, CRISPR-edited plants reach market in record time. Nat Biotechnol 36:6–7. https://doi.org/10.1038/nbt0118-6b
Waltz E (2019) Appetite grows for biotech foods with health benefits. Nat Biotechnol 37:573–580. https://doi.org/10.1038/d41587-019-00012-9
Watrud LS, Lee EH, Fairbrother A, Burdick C, Reichman JR, Bollman M, Storm M, King G, van de Water PK (2004) Evidence for landscape-level, pollen-mediated gene flow from genetically modified creeping bentgrass with CP4 EPSPS as a marker. Proc Natl Acad Sci USA 101:14533–14538. https://doi.org/10.1073/pnas.0405154101
Whelan AI, Lema MA (2015) Regulatory framework for gene editing and other new breeding techniques (NBTs) in Argentina. GM Crops Food 6:253–265. https://doi.org/10.1080/21645698.2015.1114698
WHO (2008) Interim summary of conclusions and dietary recommendations on total fat and fatty acids, from the Joint FAO/WHO Expert Consultation on fats and fatty acids in human nutrition. 10–14 November 2008, WHO: Geneva. https://www.who.int/nutrition/topics/FFA_summary_rec_conclusion.pdf
WHO (2009) Global prevalence of vitamin A deficiency in populations at risk 1995–2005. WHO global database on vitamin A deficiency. Geneva, World Health Organization. https://www.who.int/nutrition/publications/micronutrients/vitamin_a_deficiency/9789241598019/en/
Wolt JD, Wang K, Yang B (2016) The regulatory status of genome-edited crops. Plant Biotechnol J 14:510–518. https://doi.org/10.1111/pbi.12444
Wunn J, Kloti A, Burkhardt PK, Biswas GCG, Launis K, Iglesias VA, Potrykus I (1996) Transgenic Indica rice breeding line IR58 expressing a synthetic cryIA(b) gene from Bacillus thuringiensis provides effective insect pest control. BioTechnol 14:171–176. https://doi.org/10.1038/nbt0296-171
Yamamoto T, McLaughlin RE (1981) Isolation of a protein from the parasporal crystal of Bacillus thuringiensis var. kurstaki toxic to the mosquito larva Aedes taeniorhynchus . Biochem Biophys Res Commun 103:414–421. https://doi.org/10.1016/0006-291x(81)90468-x
Yan F, Zhang WW, Xiao H, Li SF, Cheng ZM (2007) Transgenic wheat expressing virus-derived hairpin RNA is resistant to Barley yellow dwarf virus. Yi Chuan 29:97–102. https://doi.org/10.1360/yc-007-0097 . https://www.ncbi.nlm.nih.gov/pubmed/17284432
Yan S, Zhu J, Zhu W, Li Z, Shelton AM, Luo J, Cui J, Zhang Q, Liu X (2015) Pollen-mediated gene flow from transgenic cotton under greenhouse conditions is dependent on different pollinators. Sci Rep 5:15917. https://doi.org/10.1038/srep15917
Yang RC, Xu HL, Yu WG, Lu CG, Long MS, Liu CQ, Pan NS, Chen ZL (1995) Transgenic tomato plants expressing cucumber mosaic virus coat protein (CMV-cp) and their resistance to cucumber mosaic virus (CMV). Acta Agric Jiangsu 11:42–46
Ye X, Al-Babili S, Klöti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287:303–305. https://doi.org/10.1126/science.287.5451.303
Zhang C, Wohlhueter R, Zhang H (2016) Genetically modified foods: a critical review of their promise and problems. Food Sci Hum Wellness 5:116–123. https://doi.org/10.1016/j.fshw.2016.04.002
Zhou J, Xin X, He Y, Chen H, Li Q, Tang X et al (2018) Multiplex QTL editing of grain-related genes improves yield in elite rice varieties. Plant Cell Rep 38:475–485. https://doi.org/10.1007/s00299-018-2340-3
Zhu YX, Ou-Yang WJ, Zhang YF, Chen ZL (1996) Transgenic sweet pepper plants from Agrobacterium mediated transformation. Plant Cell Rep 16:71–75. https://doi.org/10.1007/s002990050179
Zong Y, Wang Y, Li C, Zhang R, Chen K, Ran Y, Qiu JL, Wang D, Gao C (2017) Precise base editing in rice, wheat and maize with a Cas9–cytidine deaminase fusion. Nat Biotechnol 35:438–440. https://doi.org/10.1038/nbt.3811
Download references
Acknowledgements
The maize transformation and genome editing work in the laboratory of the corresponding author is funded by National Agricultural Science Fund (NASF; competitive Grant no. NASF/GTR-5004/2015-16/204). The funds from the Indian Council of Agricultural Research (ICAR) are gratefully acknowledged. GG, AD and AKJ acknowledge NASF support in the form of SRF, RA and LA fellowships, respectively.
Author information
Authors and affiliations.
ICAR-Indian Institute of Maize Research, Pusa Campus, New Delhi, 110012, India
Krishan Kumar, Geetika Gambhir, Abhishek Dass, Abhishek Kumar Jha & Pranjal Yadava
National Institute for Research in Environmental Health, Bhopal, 462001, India
Amit Kumar Tripathi
ICAR-Indian Institute of Maize Research, PAU Campus, Ludhiana, 141004, India
Alla Singh, Mukesh Choudhary & Sujay Rakshit
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Krishan Kumar .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 460 kb)
Rights and permissions.
Reprints and permissions
About this article
Kumar, K., Gambhir, G., Dass, A. et al. Genetically modified crops: current status and future prospects. Planta 251 , 91 (2020). https://doi.org/10.1007/s00425-020-03372-8
Download citation
Received : 15 June 2019
Accepted : 28 February 2020
Published : 31 March 2020
DOI : https://doi.org/10.1007/s00425-020-03372-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Transgenics
- Public concerns
- Intragenesis
- Genome editing
- Find a journal
- Publish with us
- Track your research
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Resources for You (Food)
- Agricultural Biotechnology
Science and History of GMOs and Other Food Modification Processes
Feed Your Mind Main Page
en Español (Spanish)
How has genetic engineering changed plant and animal breeding?
Did you know.
Genetic engineering is often used in combination with traditional breeding to produce the genetically engineered plant varieties on the market today.
For thousands of years, humans have been using traditional modification methods like selective breeding and cross-breeding to breed plants and animals with more desirable traits. For example, early farmers developed cross-breeding methods to grow corn with a range of colors, sizes, and uses. Today’s strawberries are a cross between a strawberry species native to North America and a strawberry species native to South America.
Most of the foods we eat today were created through traditional breeding methods. But changing plants and animals through traditional breeding can take a long time, and it is difficult to make very specific changes. After scientists developed genetic engineering in the 1970s, they were able to make similar changes in a more specific way and in a shorter amount of time.
A Timeline of Genetic Modification in Agriculture
A Timeline of Genetic Modification in Modern Agriculture

Circa 8000 BCE: Humans use traditional modification methods like selective breeding and cross-breeding to breed plants and animals with more desirable traits.
1866: Gregor Mendel, an Austrian monk, breeds two different types of peas and identifies the basic process of genetics.
1922: The first hybrid corn is produced and sold commercially.
1940: Plant breeders learn to use radiation or chemicals to randomly change an organism’s DNA.
1953: Building on the discoveries of chemist Rosalind Franklin, scientists James Watson and Francis Crick identify the structure of DNA.
1973: Biochemists Herbert Boyer and Stanley Cohen develop genetic engineering by inserting DNA from one bacteria into another.
1982: FDA approves the first consumer GMO product developed through genetic engineering: human insulin to treat diabetes.
1986: The federal government establishes the Coordinated Framework for the Regulation of Biotechnology. This policy describes how the U.S. Food and Drug Administration (FDA), U.S. Environmental Protection Agency (EPA), and U.S. Department of Agriculture (USDA) work together to regulate the safety of GMOs.
1992: FDA policy states that foods from GMO plants must meet the same requirements, including the same safety standards, as foods derived from traditionally bred plants.
1994: The first GMO produce created through genetic engineering—a GMO tomato—becomes available for sale after studies evaluated by federal agencies proved it to be as safe as traditionally bred tomatoes.
1990s: The first wave of GMO produce created through genetic engineering becomes available to consumers: summer squash, soybeans, cotton, corn, papayas, tomatoes, potatoes, and canola. Not all are still available for sale.
2003: The World Health Organization (WHO) and the Food and Agriculture Organization (FAO) of the United Nations develop international guidelines and standards to determine the safety of GMO foods.
2005: GMO alfalfa and sugar beets are available for sale in the United States.
2015: FDA approves an application for the first genetic modification in an animal for use as food, a genetically engineered salmon.
2016: Congress passes a law requiring labeling for some foods produced through genetic engineering and uses the term “bioengineered,” which will start to appear on some foods.

2017: GMO apples are available for sale in the U.S.
2019: FDA completes consultation on first food from a genome edited plant.
2020 : GMO pink pineapple is available to U.S. consumers.
2020 : Application for GalSafe pig was approved.
How are GMOs made?
“GMO” (genetically modified organism) has become the common term consumers and popular media use to describe foods that have been created through genetic engineering. Genetic engineering is a process that involves:
- Identifying the genetic information—or “gene”—that gives an organism (plant, animal, or microorganism) a desired trait
- Copying that information from the organism that has the trait
- Inserting that information into the DNA of another organism
- Then growing the new organism
How Are GMOs Made? Fact Sheet
Making a GMO Plant, Step by Step
The following example gives a general idea of the steps it takes to create a GMO plant. This example uses a type of insect-resistant corn called “Bt corn.” Keep in mind that the processes for creating a GMO plant, animal, or microorganism may be different.

To produce a GMO plant, scientists first identify what trait they want that plant to have, such as resistance to drought, herbicides, or insects. Then, they find an organism (plant, animal, or microorganism) that already has that trait within its genes. In this example, scientists wanted to create insect-resistant corn to reduce the need to spray pesticides. They identified a gene in a soil bacterium called Bacillus thuringiensis (Bt) , which produces a natural insecticide that has been in use for many years in traditional and organic agriculture.

After scientists find the gene with the desired trait, they copy that gene.
For Bt corn, they copied the gene in Bt that would provide the insect-resistance trait.

Next, scientists use tools to insert the gene into the DNA of the plant. By inserting the Bt gene into the DNA of the corn plant, scientists gave it the insect resistance trait.
This new trait does not change the other existing traits.

In the laboratory, scientists grow the new corn plant to ensure it has adopted the desired trait (insect resistance). If successful, scientists first grow and monitor the new corn plant (now called Bt corn because it contains a gene from Bacillus thuringiensis) in greenhouses and then in small field tests before moving it into larger field tests. GMO plants go through in-depth review and tests before they are ready to be sold to farmers.
The entire process of bringing a GMO plant to the marketplace takes several years.
Learn more about the process for creating genetically engineered microbes and genetically engineered animals .
What are the latest scientific advances in plant and animal breeding?
Scientists are developing new ways to create new varieties of crops and animals using a process called genome editing . These techniques can make changes more quickly and precisely than traditional breeding methods.
There are several genome editing tools, such as CRISPR . Scientists can use these newer genome editing tools to make crops more nutritious, drought tolerant, and resistant to insect pests and diseases.
Learn more about Genome Editing in Agricultural Biotechnology .
How GMOs Are Regulated in the United States
GMO Crops, Animal Food, and Beyond
How GMO Crops Impact Our World
www.fda.gov/feedyourmind
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
A Meta-Analysis of the Impacts of Genetically Modified Crops
Affiliation Department of Agricultural Economics and Rural Development, Georg-August-University of Goettingen, Goettingen, Germany
* E-mail: [email protected]
- Wilhelm Klümper,

- Published: November 3, 2014
- https://doi.org/10.1371/journal.pone.0111629
- Reader Comments
Despite the rapid adoption of genetically modified (GM) crops by farmers in many countries, controversies about this technology continue. Uncertainty about GM crop impacts is one reason for widespread public suspicion.
We carry out a meta-analysis of the agronomic and economic impacts of GM crops to consolidate the evidence.
Data Sources
Original studies for inclusion were identified through keyword searches in ISI Web of Knowledge, Google Scholar, EconLit, and AgEcon Search.
Study Eligibility Criteria
Studies were included when they build on primary data from farm surveys or field trials anywhere in the world, and when they report impacts of GM soybean, maize, or cotton on crop yields, pesticide use, and/or farmer profits. In total, 147 original studies were included.
Synthesis Methods
Analysis of mean impacts and meta-regressions to examine factors that influence outcomes.
On average, GM technology adoption has reduced chemical pesticide use by 37%, increased crop yields by 22%, and increased farmer profits by 68%. Yield gains and pesticide reductions are larger for insect-resistant crops than for herbicide-tolerant crops. Yield and profit gains are higher in developing countries than in developed countries.
Limitations
Several of the original studies did not report sample sizes and measures of variance.
The meta-analysis reveals robust evidence of GM crop benefits for farmers in developed and developing countries. Such evidence may help to gradually increase public trust in this technology.
Citation: Klümper W, Qaim M (2014) A Meta-Analysis of the Impacts of Genetically Modified Crops. PLoS ONE 9(11): e111629. https://doi.org/10.1371/journal.pone.0111629
Editor: emidio albertini, University of Perugia, Italy
Received: June 23, 2014; Accepted: October 3, 2014; Published: November 3, 2014
Copyright: © 2014 Klümper, Qaim. This is an open-access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding: This research was financially supported by the German Federal Ministry of Economic Cooperation and Development (BMZ) and the European Union’s Seventh Framework Programme (FP7/2007-2011) under Grant Agreement 290693 FOODSECURE. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Neither BMZ nor FOODSECURE and any of its partner organizations, any organization of the European Union or the European Commission are accountable for the content of this article.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Despite the rapid adoption of genetically modified (GM) crops by farmers in many countries, public controversies about the risks and benefits continue [1] – [4] . Numerous independent science academies and regulatory bodies have reviewed the evidence about risks, concluding that commercialized GM crops are safe for human consumption and the environment [5] – [7] . There are also plenty of studies showing that GM crops cause benefits in terms of higher yields and cost savings in agricultural production [8] – [12] , and welfare gains among adopting farm households [13] – [15] . However, some argue that the evidence about impacts is mixed and that studies showing large benefits may have problems with the data and methods used [16] – [18] . Uncertainty about GM crop impacts is one reason for the widespread public suspicion towards this technology. We have carried out a meta-analysis that may help to consolidate the evidence.
While earlier reviews of GM crop impacts exist [19] – [22] , our approach adds to the knowledge in two important ways. First, we include more recent studies into the meta-analysis. In the emerging literature on GM crop impacts, new studies are published continuously, broadening the geographical area covered, the methods used, and the type of outcome variables considered. For instance, in addition to other impacts we analyze effects of GM crop adoption on pesticide quantity, which previous meta-analyses could not because of the limited number of observations for this particular outcome variable. Second, we go beyond average impacts and use meta-regressions to explain impact heterogeneity and test for possible biases.
Our meta-analysis concentrates on the most important GM crops, including herbicide-tolerant (HT) soybean, maize, and cotton, as well as insect-resistant (IR) maize and cotton. For these crops, a sufficiently large number of original impact studies have been published to estimate meaningful average effect sizes. We estimate mean impacts of GM crop adoption on crop yield, pesticide quantity, pesticide cost, total production cost, and farmer profit. Furthermore, we analyze several factors that may influence outcomes, such as geographic location, modified crop trait, and type of data and methods used in the original studies.
Materials and Methods
Literature search.
Original studies for inclusion in this meta-analysis were identified through keyword searches in relevant literature databanks. Studies were searched in the ISI Web of Knowledge, Google Scholar, EconLit, and AgEcon Search. We searched for studies in the English language that were published after 1995. We did not extend the review to earlier years, because the commercial adoption of GM crops started only in the mid-1990s [23] . The search was performed for combinations of keywords related to GM technology and related to the outcome of interest. Concrete keywords used related to GM technology were (an asterisk is a replacement for any ending of the respective term; quotation marks indicate that the term was used as a whole, not each word alone): GM*, “genetically engineered”, “genetically modified”, transgenic, “agricultural biotechnology”, HT, “herbicide tolerant”, Roundup, Bt, “insect resistant”. Concrete keywords used related to outcome variables were: impact*, effect*, benefit*, yield*, economic*, income*, cost*, soci*, pesticide*, herbicide*, insecticide*, productivity*, margin*, profit*. The search was completed in March 2014.
Most of the publications in the ISI Web of Knowledge are articles in academic journals, while Google Scholar, EconLit, and AgEcon Search also comprise book chapters and grey literature such as conference papers, working papers, and reports in institutional series. Articles published in academic journals have usually passed a rigorous peer-review process. Most papers presented at academic conferences have also passed a peer-review process, which is often less strict than that of good journals though. Some of the other publications are peer reviewed, while many are not. Some of the working papers and reports are published by research institutes or government organizations, while others are NGO publications. Unlike previous reviews of GM crop impacts, we did not limit the sample to peer-reviewed studies but included all publications for two reasons. First, a clear-cut distinction between studies with and without peer review is not always possible, especially when dealing with papers that were not published in a journal or presented at an academic conference [24] . Second, studies without peer review also influence the public and policy debate on GM crops; ignoring them completely would be short-sighted.
Of the studies identified through the keyword searches, not all reported original impact results. We classified studies by screening titles, abstracts, and full texts. Studies had to fulfill the following criteria to be included:
- The study is an empirical investigation of the agronomic and/or economic impacts of GM soybean, GM maize, or GM cotton using micro-level data from individual plots and/or farms. Other GM crops such as GM rapeseed, GM sugarbeet, and GM papaya were commercialized in selected countries [23] , but the number of impact studies available for these other crops is very small.
- The study reports GM crop impacts in terms of one or more of the following outcome variables: yield, pesticide quantity (especially insecticides and herbicides), pesticide costs, total variable costs, gross margins, farmer profits. If only the number of pesticide sprays was reported, this was used as a proxy for pesticide quantity.
- The study analyzes the performance of GM crops by either reporting mean outcomes for GM and non-GM, absolute or percentage differences, or estimated coefficients of regression models that can be used to calculate percentage differences between GM and non-GM crops.
- The study contains original results and is not only a review of previous studies.
In some cases, the same results were reported in different publications; in these cases, only one of the publications was included to avoid double counting. On the other hand, several publications involve more than one impact observation, even for a single outcome variable, for instance when reporting results for different geographical regions or derived with different methods (e.g., comparison of mean outcomes of GM and non-GM crops plus regression model estimates). In those cases, all observations were included. Moreover, the same primary dataset was sometimes used for different publications without reporting identical results (e.g., analysis of different outcome variables, different waves of panel data, use of different methods). Hence, the number of impact observations in our sample is larger than the number of publications and primary datasets ( Data S1 ). The number of studies selected at various stages is shown in the flow diagram in Figure 1 . The number of publications finally included in the meta-analysis is 147 ( Table S1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0111629.g001
Effect sizes and influencing factors
Effect sizes are measures of outcome variables. We chose the percentage difference between GM and non-GM crops for five different outcome variables, namely yield, pesticide quantity, pesticide cost, total production cost, and farmer profits per unit area. Most studies that analyze production costs focus on variable costs, which are the costs primarily affected through GM technology adoption. Accordingly, profits are calculated as revenues minus variable production costs (profits calculated in this way are also referred to as gross margins). These production costs also take into account the higher prices charged by private companies for GM seeds. Hence, the percentage differences in profits considered here are net economic benefits for farmers using GM technology. Percentage differences, when not reported in the original studies, were calculated from mean value comparisons between GM and non-GM or from estimated regression coefficients.
Since we look at different types of GM technologies (different modified traits) that are used in different countries and regions, we do not expect that effect sizes are homogenous across studies. Hence, our approach of combining effect sizes corresponds to a random-effects model in meta-analysis [25] . To explain impact heterogeneity and test for possible biases, we also compiled data on a number of study descriptors that may influence the reported effect sizes. These influencing factors include information on the type of GM technology (modified trait), the region studied, the type of data and method used, the source of funding, and the type of publication. All influencing factors are defined as dummy variables. The exact definition of these dummy variables is given in Table 1 . Variable distributions of the study descriptors are shown in Table S2 .
https://doi.org/10.1371/journal.pone.0111629.t001
Statistical analysis
In a first step, we estimate average effect sizes for each outcome variable. To test whether these mean impacts are significantly different from zero, we regress each outcome variable on a constant with cluster correction of standard errors by primary dataset. Thus, the test for significance is valid also when observations from the same dataset are correlated. We estimate average effect sizes for all GM crops combined. However, we expect that the results may differ by modified trait, so that we also analyze mean effects for HT crops and IR crops separately.
Meta-analyses often weight impact estimates by their variances; estimates with low variance are considered more reliable and receive a higher weight [26] . In our case, several of the original studies do not report measures of variance, so that weighting by variance is not possible. Alternatively, weighting by sample size is common, but sample sizes are also not reported in all studies considered, especially not in some of the grey literature publications. To test the robustness of the results, we employ a different weighting procedure, using the inverse of the number of impact observations per dataset as weights. This procedure avoids that individual datasets that were used in several publications dominate the calculation of average effect sizes.
Results and Discussion
Average effect sizes.
Distributions of all five outcome variables are shown in Figure S1 . Table 2 presents unweighted mean impacts. As a robustness check, we weighted by the inverse of the number of impact observations per dataset. Comparing unweighted results ( Table 2 ) with weighted results ( Table S3 ) we find only very small differences. This comparison suggests that the unweighted results are robust.
https://doi.org/10.1371/journal.pone.0111629.t002
On average, GM technology has increased crop yields by 21% ( Figure 2 ). These yield increases are not due to higher genetic yield potential, but to more effective pest control and thus lower crop damage [27] . At the same time, GM crops have reduced pesticide quantity by 37% and pesticide cost by 39%. The effect on the cost of production is not significant. GM seeds are more expensive than non-GM seeds, but the additional seed costs are compensated through savings in chemical and mechanical pest control. Average profit gains for GM-adopting farmers are 69%.
Average percentage differences between GM and non-GM crops are shown. Results refer to all GM crops, including herbicide-tolerant and insect-resistant traits. The number of observations varies by outcome variable; yield: 451; pesticide quantity: 121; pesticide cost: 193; total production cost: 115; farmer profit: 136. *** indicates statistical significance at the 1% level.
https://doi.org/10.1371/journal.pone.0111629.g002
Results of Cochran’s test [25] , which are reported in Figure S1 , confirm that there is significant heterogeneity across study observations for all five outcome variables. Hence it is useful to further disaggregate the results. Table 2 shows a breakdown by modified crop trait. While significant reductions in pesticide costs are observed for both HT and IR crops, only IR crops cause a consistent reduction in pesticide quantity. Such disparities are expected, because the two technologies are quite different. IR crops protect themselves against certain insect pests, so that spraying can be reduced. HT crops, on the other hand, are not protected against pests but against a broad-spectrum chemical herbicide (mostly glyphosate), use of which facilitates weed control. While HT crops have reduced herbicide quantity in some situations, they have contributed to increases in the use of broad-spectrum herbicides elsewhere [2] , [11] , [19] . The savings in pesticide costs for HT crops in spite of higher quantities can be explained by the fact that broad-spectrum herbicides are often much cheaper than the selective herbicides that were used before. The average farmer profit effect for HT crops is large and positive, but not statistically significant because of considerable variation and a relatively small number of observations for this outcome variable.
Impact heterogeneity and possible biases
Table 3 shows the estimation results from the meta-regressions that explain how different factors influence impact heterogeneity. Controlling for other factors, yield gains of IR crops are almost 7 percentage points higher than those of HT crops (column 1). Furthermore, yield gains of GM crops are 14 percentage points higher in developing countries than in developed countries. Especially smallholder farmers in the tropics and subtropics suffer from considerable pest damage that can be reduced through GM crop adoption [27] .
https://doi.org/10.1371/journal.pone.0111629.t003
Most original studies in this meta-analysis build on farm surveys, although some are based on field-trial data. Field-trial results are often criticized to overestimate impacts, because farmers may not be able to replicate experimental conditions. However, results in Table 3 (column 1) show that field-trial data do not overestimate the yield effects of GM crops. Reported yield gains from field trials are even lower than those from farm surveys. This is plausible, because pest damage in non-GM crops is often more severe in farmers’ fields than on well-managed experimental plots.
Another concern often voiced in the public debate is that studies funded by industry money might report inflated benefits. Our results show that the source of funding does not significantly influence the impact estimates. We also analyzed whether the statistical method plays a role. Many of the earlier studies just compared yields of GM and non-GM crops without considering possible differences in other inputs and conditions that may also affect the outcome. Net impacts of GM technology can be estimated with regression-based production function models that control for other factors. Interestingly, results derived from regression analysis report higher average yield effects.
Finally, we examined whether the type of publication matters. Controlling for other factors, the regression coefficient for journal publications in column (1) of Table 3 implies that studies published in peer-reviewed journals show 12 percentage points higher yield gains than studies published elsewhere. Indeed, when only including observations from studies that were published in journals, the mean effect size is larger than if all observations are included ( Figure S2 ). On first sight, one might suspect publication bias, meaning that only studies that report substantial effects are accepted for publication in a journal. A common way to assess possible publication bias in meta-analysis is through funnel plots [25] , which we show in Figure S3 . However, in our case these funnel plots should not be over-interpreted. First, only studies that report variance measures can be included in the funnel plots, which holds true only for a subset of the original studies used here. Second, even if there were publication bias, our mean results would be estimated correctly, because we do include studies that were not published in peer-reviewed journals.
Further analysis suggests that the journal review process does not systematically filter out studies with small effect sizes. The journal articles in the sample report a wide range of yield effects, even including negative estimates in some cases. Moreover, when combining journal articles with papers presented at academic conferences, average yield gains are even higher ( Table 3 , column 2). Studies that were neither published in a journal nor presented at an academic conference encompass a diverse set of papers, including reports by NGOs and outspoken biotechnology critics. These reports show lower GM yield effects on average, but not all meet common scientific standards. Hence, rather than indicating publication bias, the positive and significant journal coefficient may be the result of a negative NGO bias in some of the grey literature.
Concerning other outcome variables, IR crops have much stronger reducing effects on pesticide quantity than HT crops ( Table 3 , column 3), as already discussed above. In terms of pesticide costs, the difference between IR and HT is less pronounced and not statistically significant (column 4). The profit gains of GM crops are 60 percentage points higher in developing countries than in developed countries (column 6). This large difference is due to higher GM yield gains and stronger pesticide cost savings in developing countries. Moreover, most GM crops are not patented in developing countries, so that GM seed prices are lower [19] . Like for yields, studies published in peer-reviewed journals report higher profit gains than studies published elsewhere, but again we do not find evidence of publication bias (column 7).
This meta-analysis confirms that – in spite of impact heterogeneity – the average agronomic and economic benefits of GM crops are large and significant. Impacts vary especially by modified crop trait and geographic region. Yield gains and pesticide reductions are larger for IR crops than for HT crops. Yield and farmer profit gains are higher in developing countries than in developed countries. Recent impact studies used better data and methods than earlier studies, but these improvements in study design did not reduce the estimates of GM crop advantages. Rather, NGO reports and other publications without scientific peer review seem to bias the impact estimates downward. But even with such biased estimates included, mean effects remain sizeable.
One limitation is that not all of the original studies included in this meta-analysis reported sample sizes and measures of variance. This is not untypical for analyses in the social sciences, especially when studies from the grey literature are also included. Future impact studies with primary data should follow more standardized reporting procedures. Nevertheless, our findings reveal that there is robust evidence of GM crop benefits. Such evidence may help to gradually increase public trust in this promising technology.
Supporting Information
Histograms of effect sizes for the five outcome variables.
https://doi.org/10.1371/journal.pone.0111629.s001
Impacts of GM crop adoption including only studies published in journals.
https://doi.org/10.1371/journal.pone.0111629.s002
Funnel plots for the five outcome variables.
https://doi.org/10.1371/journal.pone.0111629.s003
List of publications included in the meta-analysis.
https://doi.org/10.1371/journal.pone.0111629.s004
Distribution of study descriptor dummy variables for different outcomes.
https://doi.org/10.1371/journal.pone.0111629.s005
Weighted mean impacts of GM crop adoption.
https://doi.org/10.1371/journal.pone.0111629.s006
Data used for the meta-analysis.
https://doi.org/10.1371/journal.pone.0111629.s007
Acknowledgments
We thank Sinja Buri and Tingting Xu for assistance in compiling the dataset. We also thank Joachim von Braun and three reviewers of this journal for useful comments.
Author Contributions
Conceived and designed the research: WK MQ. Analyzed the data: WK MQ. Contributed to the writing of the manuscript: WK MQ. Compiled the data: WK.
- View Article
- Google Scholar
- 2. Fernandez-Cornejo J, Wechsler JJ, Livingston M, Mitchell L (2014) Genetically Engineered Crops in the United States. Economic Research Report ERR-162 (United Sates Department of Agriculture, Washington, DC).
- 6. European Academies Science Advisory Council (2013) Planting the Future: Opportunities and Challenges for Using Crop Genetic Improvement Technologies for Sustainable Agriculture (EASAC, Halle, Germany).
- 7. European Commission (2010) A Decade of EU-Funded GMO Research 2001–2010 (European Commission, Brussels).
- 12. Sexton S, Zilberman D (2012) Land for food and fuel production: the role of agricultural biotechnology. In: The Intended and Unintended Effects of US Agricultural and Biotechnology Policies (eds. Zivin, G. & Perloff, J.M.), 269–288 (University of Chicago Press, Chicago).
- 13. Ali A, Abdulai A (2010) The adoption of genetically modified cotton and poverty reduction in Pakistan. Journal of Agricultural Economics 61, 175–192.
- 17. Smale M, Zambrano P, Gruere G, Falck-Zepeda J, Matuschke I, et al.. (2009) Measuring the Economic Impacts of Transgenic Crops in Developing Agriculture During the First Decade: Approaches, Findings, and Future Directions (International Food Policy Research Institute, Washington, DC).
- 23. James C (2013) Global Status of Commercialized Biotech/GM Crops: 2013. ISAAA Briefs No.46 (International Service for the Acquisition of Agri-biotech Applications, Ithaca, NY).
- 24. Rothstein HR, Hopewell S (2009) Grey literature. In: Handbook of Research Synthesis and Meta-Analysis, Second Edition (eds. Cooper, H., Hegdes, L.V. & Valentine, J.C.), 103–125 (Russell Sage Foundation, New York).
- 25. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Introduction to Meta-Analysis (John Wiley and Sons, Chichester, UK).
- 26. Shadish WR, Haddock CK (2009) Combining estimates of effect size. In: Handbook of Research Synthesis and Meta-Analysis, Second Edition (eds. Cooper, H., Hegdes, L.V. & Valentine, J.C.), 257–277 (Russell Sage Foundation, New York).
This page has been archived and is no longer updated
Genetically Modified Organisms (GMOs): Transgenic Crops and Recombinant DNA Technology
People have been altering the genomes of plants and animals for many years using traditional breeding techniques. Artificial selection for specific, desired traits has resulted in a variety of different organisms, ranging from sweet corn to hairless cats. But this artificial selection , in which organisms that exhibit specific traits are chosen to breed subsequent generations, has been limited to naturally occurring variations. In recent decades, however, advances in the field of genetic engineering have allowed for precise control over the genetic changes introduced into an organism . Today, we can incorporate new genes from one species into a completely unrelated species through genetic engineering, optimizing agricultural performance or facilitating the production of valuable pharmaceutical substances. Crop plants, farm animals, and soil bacteria are some of the more prominent examples of organisms that have been subject to genetic engineering.
Current Use of Genetically Modified Organisms
Table 1: Examples of GMOs Resulting from Agricultural Biotechnology
The pharmaceutical industry is another frontier for the use of GMOs. In 1986, human growth hormone was the first protein pharmaceutical made in plants (Barta et al ., 1986), and in 1989, the first antibody was produced (Hiatt et al ., 1989). Both research groups used tobacco, which has since dominated the industry as the most intensively studied and utilized plant species for the expression of foreign genes (Ma et al ., 2003). As of 2003, several types of antibodies produced in plants had made it to clinical trials. The use of genetically modified animals has also been indispensible in medical research. Transgenic animals are routinely bred to carry human genes, or mutations in specific genes, thus allowing the study of the progression and genetic determinants of various diseases.
Potential GMO Applications
Many industries stand to benefit from additional GMO research. For instance, a number of microorganisms are being considered as future clean fuel producers and biodegraders. In addition, genetically modified plants may someday be used to produce recombinant vaccines. In fact, the concept of an oral vaccine expressed in plants (fruits and vegetables) for direct consumption by individuals is being examined as a possible solution to the spread of disease in underdeveloped countries, one that would greatly reduce the costs associated with conducting large-scale vaccination campaigns. Work is currently underway to develop plant-derived vaccine candidates in potatoes and lettuce for hepatitis B virus (HBV), enterotoxigenic Escherichia coli (ETEC), and Norwalk virus. Scientists are also looking into the production of other commercially valuable proteins in plants, such as spider silk protein and polymers that are used in surgery or tissue replacement (Ma et al ., 2003). Genetically modified animals have even been used to grow transplant tissues and human transplant organs, a concept called xenotransplantation. The rich variety of uses for GMOs provides a number of valuable benefits to humans, but many people also worry about potential risks.
Risks and Controversies Surrounding the Use of GMOs
Despite the fact that the genes being transferred occur naturally in other species, there are unknown consequences to altering the natural state of an organism through foreign gene expression . After all, such alterations can change the organism's metabolism , growth rate, and/or response to external environmental factors. These consequences influence not only the GMO itself, but also the natural environment in which that organism is allowed to proliferate. Potential health risks to humans include the possibility of exposure to new allergens in genetically modified foods, as well as the transfer of antibiotic-resistant genes to gut flora.
Horizontal gene transfer of pesticide, herbicide, or antibiotic resistance to other organisms would not only put humans at risk , but it would also cause ecological imbalances, allowing previously innocuous plants to grow uncontrolled, thus promoting the spread of disease among both plants and animals. Although the possibility of horizontal gene transfer between GMOs and other organisms cannot be denied, in reality, this risk is considered to be quite low. Horizontal gene transfer occurs naturally at a very low rate and, in most cases, cannot be simulated in an optimized laboratory environment without active modification of the target genome to increase susceptibility (Ma et al ., 2003).
In contrast, the alarming consequences of vertical gene transfer between GMOs and their wild-type counterparts have been highlighted by studying transgenic fish released into wild populations of the same species (Muir & Howard, 1999). The enhanced mating advantages of the genetically modified fish led to a reduction in the viability of their offspring . Thus, when a new transgene is introduced into a wild fish population, it propagates and may eventually threaten the viability of both the wild-type and the genetically modified organisms.
Unintended Impacts on Other Species: The Bt Corn Controversy
One example of public debate over the use of a genetically modified plant involves the case of Bt corn. Bt corn expresses a protein from the bacterium Bacillus thuringiensis . Prior to construction of the recombinant corn, the protein had long been known to be toxic to a number of pestiferous insects, including the monarch caterpillar, and it had been successfully used as an environmentally friendly insecticide for several years. The benefit of the expression of this protein by corn plants is a reduction in the amount of insecticide that farmers must apply to their crops. Unfortunately, seeds containing genes for recombinant proteins can cause unintentional spread of recombinant genes or exposure of non-target organisms to new toxic compounds in the environment.
The now-famous Bt corn controversy started with a laboratory study by Losey et al . (1999) in which the mortality of monarch larvae was reportedly higher when fed with milkweed (their natural food supply) covered in pollen from transgenic corn than when fed milkweed covered with pollen from regular corn. The report by Losey et al . was followed by another publication (Jesse & Obrycki, 2000) suggesting that natural levels of Bt corn pollen in the field were harmful to monarchs.
Debate ensued when scientists from other laboratories disputed the study, citing the extremely high concentration of pollen used in the laboratory study as unrealistic, and concluding that migratory patterns of monarchs do not place them in the vicinity of corn during the time it sheds pollen. For the next two years, six teams of researchers from government, academia, and industry investigated the issue and concluded that the risk of Bt corn to monarchs was "very low" (Sears et al ., 2001), providing the basis for the U.S. Environmental Protection Agency to approve Bt corn for an additional seven years.
Unintended Economic Consequences
Another concern associated with GMOs is that private companies will claim ownership of the organisms they create and not share them at a reasonable cost with the public. If these claims are correct, it is argued that use of genetically modified crops will hurt the economy and environment, because monoculture practices by large-scale farm production centers (who can afford the costly seeds) will dominate over the diversity contributed by small farmers who can't afford the technology. However, a recent meta-analysis of 15 studies reveals that, on average, two-thirds of the benefits of first-generation genetically modified crops are shared downstream, whereas only one-third accrues upstream (Demont et al ., 2007). These benefit shares are exhibited in both industrial and developing countries. Therefore, the argument that private companies will not share ownership of GMOs is not supported by evidence from first-generation genetically modified crops.
GMOs and the General Public: Philosophical and Religious Concerns
In a 2007 survey of 1,000 American adults conducted by the International Food Information Council (IFIC), 33% of respondents believed that biotech food products would benefit them or their families, but 23% of respondents did not know biotech foods had already reached the market. In addition, only 5% of those polled said they would take action by altering their purchasing habits as a result of concerns associated with using biotech products.
According to the Food and Agriculture Organization of the United Nations, public acceptance trends in Europe and Asia are mixed depending on the country and current mood at the time of the survey (Hoban, 2004). Attitudes toward cloning, biotechnology, and genetically modified products differ depending upon people's level of education and interpretations of what each of these terms mean. Support varies for different types of biotechnology; however, it is consistently lower when animals are mentioned.
Furthermore, even if the technologies are shared fairly, there are people who would still resist consumable GMOs, even with thorough testing for safety, because of personal or religious beliefs. The ethical issues surrounding GMOs include debate over our right to "play God," as well as the introduction of foreign material into foods that are abstained from for religious reasons. Some people believe that tampering with nature is intrinsically wrong, and others maintain that inserting plant genes in animals, or vice versa, is immoral. When it comes to genetically modified foods, those who feel strongly that the development of GMOs is against nature or religion have called for clear labeling rules so they can make informed selections when choosing which items to purchase. Respect for consumer choice and assumed risk is as important as having safeguards to prevent mixing of genetically modified products with non-genetically modified foods. In order to determine the requirements for such safeguards, there must be a definitive assessment of what constitutes a GMO and universal agreement on how products should be labeled.
These issues are increasingly important to consider as the number of GMOs continues to increase due to improved laboratory techniques and tools for sequencing whole genomes, better processes for cloning and transferring genes, and improved understanding of gene expression systems. Thus, legislative practices that regulate this research have to keep pace. Prior to permitting commercial use of GMOs, governments perform risk assessments to determine the possible consequences of their use, but difficulties in estimating the impact of commercial GMO use makes regulation of these organisms a challenge.
History of International Regulations for GMO Research and Development
In 1971, the first debate over the risks to humans of exposure to GMOs began when a common intestinal microorganism, E. coli , was infected with DNA from a tumor-inducing virus (Devos et al ., 2007). Initially, safety issues were a concern to individuals working in laboratories with GMOs, as well as nearby residents. However, later debate arose over concerns that recombinant organisms might be used as weapons. The growing debate, initially restricted to scientists, eventually spread to the public, and in 1974, the National Institutes of Health (NIH) established the Recombinant DNA Advisory Committee to begin to address some of these issues.
In the 1980s, when deliberate releases of GMOs to the environment were beginning to occur, the U.S. had very few regulations in place. Adherence to the guidelines provided by the NIH was voluntary for industry. Also during the 1980s, the use of transgenic plants was becoming a valuable endeavor for production of new pharmaceuticals, and individual companies, institutions, and whole countries were beginning to view biotechnology as a lucrative means of making money (Devos et al ., 2007). Worldwide commercialization of biotech products sparked new debate over the patentability of living organisms, the adverse effects of exposure to recombinant proteins, confidentiality issues, the morality and credibility of scientists, the role of government in regulating science, and other issues. In the U.S., the Congressional Office of Technology Assessment initiatives were developed, and they were eventually adopted worldwide as a top-down approach to advising policymakers by forecasting the societal impacts of GMOs.
Then, in 1986, a publication by the Organization for Economic Cooperation and Development (OECD), called "Recombinant DNA Safety Considerations," became the first intergovernmental document to address issues surrounding the use of GMOs. This document recommended that risk assessments be performed on a case-by-case basis. Since then, the case-by-case approach to risk assessment for genetically modified products has been widely accepted; however, the U.S. has generally taken a product-based approach to assessment, whereas the European approach is more process based (Devos et al ., 2007). Although in the past, thorough regulation was lacking in many countries, governments worldwide are now meeting the demands of the public and implementing stricter testing and labeling requirements for genetically modified crops.
Increased Research and Improved Safety Go Hand in Hand
Proponents of the use of GMOs believe that, with adequate research, these organisms can be safely commercialized. There are many experimental variations for expression and control of engineered genes that can be applied to minimize potential risks. Some of these practices are already necessary as a result of new legislation, such as avoiding superfluous DNA transfer (vector sequences) and replacing selectable marker genes commonly used in the lab (antibiotic resistance) with innocuous plant-derived markers (Ma et al ., 2003). Issues such as the risk of vaccine-expressing plants being mixed in with normal foodstuffs might be overcome by having built-in identification factors, such as pigmentation, that facilitate monitoring and separation of genetically modified products from non-GMOs. Other built-in control techniques include having inducible promoters (e.g., induced by stress, chemicals, etc.), geographic isolation, using male-sterile plants, and separate growing seasons.
GMOs benefit mankind when used for purposes such as increasing the availability and quality of food and medical care, and contributing to a cleaner environment. If used wisely, they could result in an improved economy without doing more harm than good, and they could also make the most of their potential to alleviate hunger and disease worldwide. However, the full potential of GMOs cannot be realized without due diligence and thorough attention to the risks associated with each new GMO on a case-by-case basis.
References and Recommended Reading
Barta, A., et al . The expression of a nopaline synthase-human growth hormone chimaeric gene in transformed tobacco and sunflower callus tissue. Plant Molecular Biology 6 , 347–357 (1986)
Beyer, P., et al . Golden rice: Introducing the β-carotene biosynthesis pathway into rice endosperm by genetic engineering to defeat vitamin A deficiency. Journal of Nutrition 132 , 506S–510S (2002)
Demont, M., et al . GM crops in Europe: How much value and for whom? EuroChoices 6 , 46–53 (2007)
Devlin, R., et al . Extraordinary salmon growth. Nature 371 , 209–210 (1994) ( link to article )
Devos, Y., et al . Ethics in the societal debate on genetically modified organisms: A (re)quest for sense and sensibility. Journal of Agricultural and Environmental Ethics 21 , 29–61 (2007) doi:10.1007/s10806-007-9057-6
Guerrero-Andrade, O., et al . Expression of the Newcastle disease virus fusion protein in transgenic maize and immunological studies. Transgenic Research 15 , 455–463(2006) doi:10.1007/s11248-006-0017-0
Hiatt, A., et al . Production of antibodies in transgenic plants. Nature 342 , 76–79 (1989) ( link to article )
Hoban, T. Public attitudes towards agricultural biotechnology. ESA working papers nos. 4-9. Agricultural and Development Economics Division, Food and Agricultural Organization of the United Nations (2004)
Jesse, H., & Obrycki, J. Field deposition of Bt transgenic corn pollen: Lethal effects on the monarch butterfly. Oecologia 125 , 241–248 (2000)
Losey, J., et al . Transgenic pollen harms monarch larvae. Nature 399 , 214 (1999) doi:10.1038/20338 ( link to article )
Ma, J., et al . The production of recombinant pharmaceutical proteins in plants. Nature Reviews Genetics 4 , 794–805 (2003) doi:10.1038/nrg1177 ( link to article )
Muir, W., & Howard, R. Possible ecological risks of transgenic organism release when transgenes affect mating success: Sexual selection and the Trojan gene hypothesis. Proceedings of the National Academy of Sciences 96 , 13853–13856 (1999)
Sears, M., et al . Impact of Bt corn on monarch butterfly populations: A risk assessment. Proceedings of the National Academy of Sciences 98 , 11937–11942 (2001)
Spurgeon, D. Call for tighter controls on transgenic foods. Nature 409 , 749 (2001) ( link to article )
Takeda, S., & Matsuoka, M. Genetic approaches to crop improvement: Responding to environmental and population changes. Nature Reviews Genetics 9 , 444–457 (2008) doi:10.1038/nrg2342 ( link to article )
United States Department of Energy, Office of Biological and Environmental Research, Human Genome Program. Human Genome Project information: Genetically modified foods and organisms, (2007)
- Add Content to Group
Article History
Flag inappropriate.


Email your Friend

- | Lead Editor: Bob Moss

Within this Subject (34)
- Applications in Biotechnology (4)
- Discovery of Genetic Material (4)
- DNA Replication (6)
- Gene Copies (5)
- Jumping Genes (4)
- RNA (7)
- Transcription & Translation (4)
Other Topic Rooms
- Gene Inheritance and Transmission
- Gene Expression and Regulation
- Nucleic Acid Structure and Function
- Chromosomes and Cytogenetics
- Evolutionary Genetics
- Population and Quantitative Genetics
- Genes and Disease
- Genetics and Society
- Cell Origins and Metabolism
- Proteins and Gene Expression
- Subcellular Compartments
- Cell Communication
- Cell Cycle and Cell Division
© 2014 Nature Education
- Press Room |
- Terms of Use |
- Privacy Notice |

Visual Browse
This website uses cookies.
By clicking the "Accept" button or continuing to browse our site, you agree to first-party and session-only cookies being stored on your device to enhance site navigation and analyze site performance and traffic. For more information on our use of cookies, please see our Privacy Policy .
American Economic Review: Insights
National and global impacts of genetically modified crops.
ISSN 2640-205X (Print) | ISSN 2640-2068 (Online)
- Editorial Policy
- Annual Report of the Editor
- Editorial Process: Discussions with the Editors
- Research Highlights
- Contact Information
- Current Issue
- Forthcoming Articles
- Submission Guidelines
- Accepted Article Guidelines
- Style Guide
- Reviewer Guidelines
- Casper Worm Hansen
- Asger Mose Wingender
- Article Information
Additional Materials
- Replication Package
- Online Appendix (1.20 MB)
- Author Disclosure Statement(s) (176.72 KB)
JEL Classification
- Q18 Agricultural Policy; Food Policy; Animal Welfare Policy
Largest-Ever Study Reveals Environmental Impact of Genetically Modified Crops
- By Caroline Newman , [email protected]

According to new research from University of Virginia economist Federico Ciliberto, widespread adoption of genetically modified crops has decreased the use of insecticides, but increased the use of weed-killing herbicides as weeds become more resistant.
Ciliberto led the largest study of genetically modified crops and pesticide use to date, alongside Edward D. Perry of Kansas State University, David A. Hennessy of Michigan State University and GianCarlo Moschini of Iowa State University. The four economists studied annual data from more than 5,000 soybean and 5,000 maize farmers in the U.S. from 1998 to 2011, far exceeding previous studies that have been limited to one or two years of data.
“The fact that we have 14 years of farm-level data from farmers all over the U.S. makes this study very special,” Ciliberto said. “We have repeated observations of the same farmers and can see when they adopted genetically modified seeds and how that changed their use of chemicals.”

Associate economics professor Federico Ciliberto co-led the largest research study to date examining how genetically modified soybeans and maize have impacted pesticide use in the U.S. (Photo by Dan Addison, University Communications)
Since 2008, genetically engineered crops have accounted for more than 80 percent of maize and soybean crops planted in the U.S. Maize seeds are modified with two genes: one kills insects that eat the seed and one allows the seed to tolerate glyphosate, a herbicide commonly used in weed killers like Roundup. Soybeans are modified with just one glyphosate-resistant gene.
Unsurprisingly, maize farmers who used the insect-resistant seeds used significantly less insecticide – about 11.2 percent less – than farmers who did not use genetically modified maize. The maize farmers also used 1.3 percent less herbicide over the 13-year period.
Soybean crops, on the other hand, saw a significant increase in herbicide use, with adopters of genetically modified crops using 28 percent more herbicides than non-adopters.
Ciliberto attributes this increase to the proliferation of glyphosate-resistant weeds.
“In the beginning, there was a reduction in herbicide use, but over time the use of chemicals increased because farmers were having to add new chemicals as weeds developed a resistance to glyphosate,” Ciliberto said.
Maize farmers, he said, have not yet had to address the same level of resistance, in part because they did not adopt genetically modified crops as quickly as their counterparts in the soy industry. However, the study did find evidence that both maize and soybean farmers increased herbicide use during the last five years of the study, indicating that weed resistance is a growing problem for both groups.
From 2006 to 2011, the percentage of hectares sprayed with only glyphosate shrunk from more than 70 percent to 41 percent for soybean farmers and from more than 40 percent to 19 percent for maize farmers. The decrease resulted from farmers having to resort to other chemicals as glyphosate-resistant weeds became more common.
“Evidence suggests that weeds are becoming more resistant and farmers are having to use additional chemicals, and more of them,” Ciliberto said.
Insects do not appear to have developed a similar resistance, in part because federal regulations require farmers to have a “safe haven” in their fields that is free of genetically modified crops. Insects and worms in those safe havens have no need to develop resistance, and because they interact and breed with insects in other parts of the field, they help prevent the development of resistant genes.
Despite the decrease in insecticide use, continued growth in herbicide use poses a significant environmental problem as large doses of the chemicals can harm biodiversity and increase water and air pollution.
Ciliberto and his colleagues measured the overall environmental impact of the changes in chemical use that have resulted from the adoption of genetically modified crops, using a measure called the environmental impact quotient, or EIQ, to account for chemicals’ impact on farmworkers, consumers and the environment. Comparing adopters to non-adopters, they found little change in the impact on farmworkers and consumers. However, the adoption of genetically modified soybeans correlated with a negative impact on the environment as increased herbicide use also increased contamination of local ecosystems.
Overall, Ciliberto said he was surprised by the extent to which herbicide use had increased and concerned about the potential environmental impact.
“I did not expect to see such a strong pattern,” he said.
Media Contact
Caroline Newman
Article Information
September 14, 2016
/content/largest-ever-study-reveals-environmental-impact-genetically-modified-crops
- Tools and Resources
- Customer Services
- Agriculture and the Environment
- Case Studies
- Chemistry and Toxicology
- Environment and Human Health
- Environmental Biology
- Environmental Economics
- Environmental Engineering
- Environmental Ethics and Philosophy
- Environmental History
- Environmental Issues and Problems
- Environmental Processes and Systems
- Environmental Sociology and Psychology
- Environments
- Framing Concepts in Environmental Science
- Management and Planning
- Policy, Governance, and Law
- Quantitative Analysis and Tools
- Sustainability and Solutions
- Share This Facebook LinkedIn Twitter
Article contents
Pros and cons of gmo crop farming.
- Rene Van Acker , Rene Van Acker University of Guelph
- M. Motior Rahman M. Motior Rahman University of Guelph
- and S. Zahra H. Cici S. Zahra H. Cici University of Guelph
- https://doi.org/10.1093/acrefore/9780199389414.013.217
- Published online: 26 October 2017
The global area sown to genetically modified (GM) varieties of leading commercial crops (soybean, maize, canola, and cotton) has expanded over 100-fold over two decades. Thirty countries are producing GM crops and just five countries (United States, Brazil, Argentina, Canada, and India) account for almost 90% of the GM production. Only four crops account for 99% of worldwide GM crop area. Almost 100% of GM crops on the market are genetically engineered with herbicide tolerance (HT), and insect resistance (IR) traits. Approximately 70% of cultivated GM crops are HT, and GM HT crops have been credited with facilitating no-tillage and conservation tillage practices that conserve soil moisture and control soil erosion, and that also support carbon sequestration and reduced greenhouse gas emissions. Crop production and productivity increased significantly during the era of the adoption of GM crops; some of this increase can be attributed to GM technology and the yield protection traits that it has made possible even if the GM traits implemented to-date are not yield traits per se . GM crops have also been credited with helping to improve farm incomes and reduce pesticide use. Practical concerns around GM crops include the rise of insect pests and weeds that are resistant to pesticides. Other concerns around GM crops include broad seed variety access for farmers and rising seed costs as well as increased dependency on multinational seed companies. Citizens in many countries and especially in European countries are opposed to GM crops and have voiced concerns about possible impacts on human and environmental health. Nonetheless, proponents of GM crops argue that they are needed to enhance worldwide food production. The novelty of the technology and its potential to bring almost any trait into crops mean that there needs to remain dedicated diligence on the part of regulators to ensure that no GM crops are deregulated that may in fact pose risks to human health or the environment. The same will be true for the next wave of new breeding technologies, which include gene editing technologies.
- genetically modified
- herbicide tolerance
- insect resistance
Introduction
Genetically modified organisms (GMOs) result from recombinant DNA technology that allows for DNA to be transferred from one organism to another (transgenesis) without the genetic transfer limits of species to species barriers and with successful expression of transferred genes in the receiving organism (Gray, 2001 ). Four crops, maize, canola, soybean, and cotton, constitute the vast majority of GM crop production (James, 2015a ), and GM crops have been grown commercially since 1995 (Bagavathiannan, Julier, Barre, Gulden, & Van Acker, 2010 ). The acceptance of GM crops by farmers has been rapid, with the global GM production area growing from 1.7 million hectares in 1996 (International Service for the Acquisition of Agri-biotech Applications [ISAAA], 2015 ) to 182 million hectares in 2014 (James, 2014 ). Just 10 countries represent almost 98% of the GM hectares worldwide. The top GM producing countries are the United States (73.1 million ha), Brazil (42.2 million ha), Argentina (24.3 million ha), Canada (11.6 million ha), and India (11.6 million ha) (James, 2014 ). GM soybean is the most popular GM crop and almost 50% of global soybean acres are now GM soybean (James, 2015b ). For corn and cotton the global proportion of GM is 30% and 14%, respectively (James, 2015b ). GM canola occupies only 5% of the global canola hectares (James, 2015b ). GM crops are grown on only 3.7% of the world’s total agricultural land, by less than one percent of the world’s farmers. Almost 100% of GM crops on the market are either herbicide tolerant (HT) or insect resistant or have both of these two traits (Dill, CaJacob, & Padgette, 2008 ).
The production of GM crops is not equal across the world and in some jurisdictions there is little or no production. Countries in the European Union (EU) are a notable example in this regard. The near complete moratorium on the production of GM crops in the EU is based on common public view and political decisions rather than GM food safety assessment (Fischer, Ekener-Petersen, Rydhmer, & Edvardsson Björnberg, 2015 ). This is also true for Switzerland, where, for example, since 2005 GM foods and crops have been banned because of strong negative views on the part of both Swiss farmers and citizens (Mann, 2015 ). Five EU countries (Spain, Portugal, the Czech Republic, Slovakia and Romania) accounted for 116,870 hectares of Bt maize cultivation in 2015 , down 18% from the 143,016 hectares in 2014 . The leading EU producer is Spain, with 107,749 hectares of Bt maize in 2015 , down 18% from the 131,538 hectares in 2014 (James, 2015a ). Russia is the world's largest GM-free zone (James, 2015a ). Despite the claimed benefits over risks, and the wide adoption of biotech-improved crop varieties in many parts of the world, Europe and Africa still remain largely GM-free in terms of production (Paarlberg, 2008 ). This may be due in part to the relative absence of reliable public scientific studies on the long-term risks of GM crops and foods and the seed monopoly that is linked to GM technology development (Paarlberg, 2008 ). In Asia, four countries, including Turkey, have banned GM crops. The GM concerns in Europe have also slowed down the approval of GM crops in many developing countries because of impacts on agricultural exports (Inghelbrecht, Dessein, & Huylenbroeck, 2014 ). Many African governments have been slow to approve, or have sometimes even banned GM crops, in order not to lose export markets and to maintain positive relations with the EU, especially given implications for development aid (Wafula, Waithaka, Komen, & Karembu, 2012 ). In addition, a few African nations have banned GM cultivation over fears of losing European markets (ISAAA, 2015 ). Public concerns over GM crops and foods have also had an impact on production of GM crops in North America. The withdrawal of the GM Bt potato (NewLeaf™) varieties from the North American market due to the concerns of two of the largest buyers of processing potatoes (Frito-Lay and McDonalds) was the result of feared consumer rejection (Kynda & Moeltner, 2006 ).
The extensive adaptation of GM crops does, however, also have some drawbacks. The occurrence of outcrossing with non-GM crops, gene flow, and the adventitious presence of GM crops on organic farms has sparked concerns among various stakeholders, including farmers who are growing GM crops (Ellstrand, 2003 ; Marvier & Van Acker, 2005 ). Public concern over GM crops is centered in three areas: human health, environmental safety, and trade impacts (Van Acker, Cici, Michael, Ryan, & Sachs, 2015 ). GM biosafety is also forcing both agriculture and food companies to appreciate GM safety in their marketing decisions (Blaine & Powell, 2001 ; Rotolo et al., 2015 ). The adoption of GM crops in a given jurisdiction is a function of public GM acceptance as well as the level of public trust of regulatory authorities (Vigani & Olper, 2013 ). Examples of these include feeding the world, consumer choice, and seed ownership (Van Acker & Cici, 2014 ). Opponents of GM crops have questioned their necessity in terms of agricultural productivity to feed the world (Gilbert, 2013 ). They point to studies that have shown that current agricultural output far exceeds global calorie needs and that distribution, access, and waste are the key limitations to feeding those who are hungry and not gross production per se (Altieri, 2005 ).
The novelty of GM technology has been both an asset and a challenge for those companies producing GM seeds. Supporters of GM crops have asserted that GM is merely an evolution of conventional breeding approaches (Herdt, 2006 ). They have insisted that humans have been genetically modifying crops for millennia and that GM technology has been an extension and facilitation of natural breeding. At the same time, however, GM crops are patentable, emphasizing that the process is truly novel and different from the natural breeding (Boucher, 1999 ). In addition, expert technical assessments acknowledge the unique and novel nature of GM crops (Taylor, 2007 ). This situation highlights the conundrum and challenge of not only introducing disruptive new technologies into society but having such technologies accepted by society (Van Acker et al., 2015 ). The socioeconomic nature of most risks along with the continuing farm income crisis in North America has led some to argue for the adoption of a more comprehensive approach to risk assessment of GM crops and all new agricultural technologies (Mauro et al., 2009 ).
The Green Revolution was driven by global hunger, and some argue that the next agricultural production revolution, which is perhaps being sparked by the introduction of GM crops, would be driven by other global needs including sustainability and the needs of individuals (Lipton & Longhurst, 2011 ). The green revolution of the 1960s and 1970s depended on the use of fertilizers, pesticides, and irrigation methods to initiate favorable conditions in which high-yielding modern varieties could thrive. Between 1970 and 1990 , fertilizer use in developing countries rose by 360% while pesticide use increased by 7 to 8% annually. The environmental impacts, of the adoption of these technologies did in some cases override their benefits. These impacts included polluted land, water, and air, and the development of resistant strains of pests. GM crops could be used to sustain or grow production levels while diminishing environmental impacts yet despite the rapid adoption of GM crops many of the problems associated with the green revolution remain (Macnaghten & Carro-Ripalda, 2015 ). The pros and cons of GM crops are many and diverse but there is little argument over the ambiguous consequences of this comparatively new technology, and numerous critics noted the potential pros and cons of GM crops as soon as they were launched in the early 1990s (Mannion, 1995a , 1995b , 1995c ).
Pros of GMO Crop Farming
The world population has exceeded 7 billion people and is forecasted to reach beyond 11 billion by 2100 (United Nations, 2017 ). The provision of an adequate food supply for this booming population is an ongoing and tremendous challenge. The companies that develop GM seeds point to this challenge as the key rationale for their need, and they explain that GM seeds will help to meet the “feeding the world” challenge in a number of ways.
Productivity of GM Crops
GM seed companies promised to raise productivity and profitability levels for farmers around the world (Pinstrup-Andersen, 1999 ). GM seed companies had expected GM crops to be adopted by farmers because the traits they were incorporating provided direct operational benefits for farmers that could be linked to increased profits for farmers (Hatfield et al., 2014 ). The proponents of GM crops have argued that the application of GM technology would fundamentally improve the efficiency, resiliency, and profitability of farming (Apel, 2010 ). In addition GM seed companies argue that the adoption of GM crops helps to reduce the application of pesticides, which has a direct impact on the sustainability of the cropping systems (Lal, 2004 ) as well as profitability for farmers (Morse, Mannion, & Evans, 2011 ). Some have even suggested that the production of GM crops creates a halo effect for nearby non-GM crops by reducing pest pressures within regions that are primarily sown to GM crops (Mannion & Morse, 2013 ).
There is an expectation widely held by those in agriculture that GM seeds increase yields, or at least protect yield potential. GM crops with resistance to insects and herbicides can substantially simplify crop management and reduce crop losses, leading to increased yields (Pray, Jikun Huang, Hu, & Rozelle, 2002 ; Pray, Nagarajan, Huang, Hu, & Ramaswami, 2011 ; Nickson, 2005 ). GM varieties of soybean, cotton, and maize produced 20%, 15%, and 7% higher yield, respectively, than non-GM varieties (Mannion & Morse, 2013 ). The Economic Research Service (ERS) of the United States Department of Agriculture (USDA) noticed a significant relationship between increased crop yields and increased adoption of herbicide- and pesticide-tolerant GM crop seeds, and the USDA reported significantly increased yields when farmers adopted herbicide-tolerant cotton and Bt cotton (USDA, 2009 ). India cultivated a record 11.6 million hectares of Bt cotton planted by 7.7 million small farmers in 2014 , with an adoption rate of 95%, up from 11.0 million hectares in 2013 . The increase from 50,000 hectares in 2002 to 11.6 million hectares in 2014 represents an unprecedented 230-fold increase in 13 years (James, 2014 ). This rapid adoption has been attributed to the increased yields farmers in this region experienced because of the efficacy of the GM seeds on cotton bollworm and the additional income farmers received as a result (James, 2014 ; Morse & Mannion, 2009 ). Similarly, the benefits that were obtained by resource-poor cotton farmers in South Africa have led many smallholders in South Africa and elsewhere in sub-Saharan Africa to accept GM cotton (Hillocks, 2009 ). Similar benefits were also obtained by resource-poor farmers growing Bt maize in the Philippines (James, 2010 ).
Tillage Systems
The adoption of no tillage and minimum tillage practices in agriculture started in the 1980s. In fact, the largest extension of both no tillage and conservation tillage and the concomitant declines in soil erosion significantly predates the release of the first HT varieties of maize and soybean in 1996 (National Research Council [NRC], 2010 ). However, farmers in the United States who adopted HT crops were more likely to practice conservation tillage and vice versa (NRC, 2010 ). There was an increase in HT crops and conservation tillage in the United States during the period of rapid GM crop adoption from 1997–2002 (Fernandez-Cornejo, Hallahan, Nehring, Wechsler, & Grube, 2012 ). Soybeans genetically engineered with HT traits have been the most widely and rapidly adopted GM crop in the United States, followed by HT cotton. Adoption of HT soybeans increased from 17% of U.S. soybean acreage in 1997 to 68% in 2001 and 93% in 2010 . Plantings of HT cotton expanded from about 10% of U.S. acreage in 1997 to 56% in 2001 and 78% in 2010 (Fernandez-Cornejo et al., 2012 ). Some argue that the adoption of GM HT varieties resulted in farmers’ deciding to use conservation tillage, or farmers who were practicing conservation tillage may have adopted GM HT crops more readily (Mauro & McLachlan, 2008 ). Adoption of HT soybean has a positive and highly significant impact on the adoption of conservation tillage in the United States (Carpenter, 2010 ). Technologies that promote conservation tillage practices decrease soil erosion in the long term and fundamentally promote soil conservation (Montogomery, 2007 ), while reducing nutrient and carbon loss (Brookes & Barfoot, 2014 ; Giller, Witter, Corbeels, & Pablo, 2009 ; Mannion & Morse, 2013 ; Powlson et al., 2014 ). Adopting HT soybean has decreased the number of tillage operations between 25% and 58% in the United States and in Argentina (Carpenter, 2010 ). The introduction of HT soybean has been cited as an important factor in the rapid increase of no tillage practices in Argentina, and the adoption of no tillage practices in this region has allowed for wheat to be double cropped with soybean which has led to a fundamental increase in farm productivity (Trigo, Cap, Malach, & Villareal, 2009 ). Substantial growth in no tillage production linked to the adoption of GM HT crops has also been noted in Canada. Several authors have reported a positive correlation between the adoption of GM HT canola and the adoption of zero-tillage systems in western Canada (Phillips, 2003 ; Beckie et al., 2006 ; Kleter et al., 2007 ). The no tillage canola production area in western Canada increased from 0.8 million hectares to 2.6 million hectares from 1996 to 2005 . This area covers about half the total canola area in Canada (Qaim & Traxler, 2005 ). In addition, tillage passes among farmers growing HT canola in Canada dropped by more than 70% in this same period (Smyth, Gusta, Belcher, Phillips, & Castle, 2011 ). Fields planted with HT crops in this region require less tillage between crops to manage weeds (Fawcett & Towery, 2003 ; Nickson, 2005 ).
Reductions in tillage and pesticide application have great benefits because they minimize inputs of fossil fuels in farming systems and in doing so, they reduce the carbon footprint of crop production (Baker, Ochsner, Venterea, & Griffis, 2007 ). The mitigation of soil erosion is important with respect to environmental conservation and the conservation of productivity potential. The adoption of no tillage practices would also save on the use of diesel fuel, and it enriches carbon sequestration in soils (Brookes & Barfoot, 2014 ). Brookes and Barfoot ( 2008 ) suggested that the fuel reduction because of GM crop cultivation resulted in a carbon dioxide emissions savings of 1215 × 10 6 Kg. This corresponds to taking more than 500,000 cars off the road. In addition, a further 13.5 × 10 9 Kg of carbon dioxide could be saved through carbon sequestration, which is equivalent to taking 6 million cars off the road. The impact of GM crops on the carbon flows in agriculture may be considered as a positive impact of GM crops on the environment (Knox et al., 2006 ).
Herbicide Tolerance and Pest Management
Herbicide tolerance in GM crops is achieved by the introduction of novel genes. The control of weeds by physical means or by using selective herbicides is time-consuming and expensive (Roller & Harlander, 1998 ). The most widely adopted HT crops are glyphosate tolerant (Dill, CaJabob, & Padgette, 2008 ) colloquially (and commercially for Monsanto) known as “Roundup Ready” crops. Herbicide tolerant GM crops have provided farmers with operational benefits. The main benefits associated with HT canola, for example, were easier and better weed control (Mauro & McLachlan, 2008 ). The development of GM HT canola varieties has also been linked to incremental gains in weed control and canola yield (Harker, Blackshaw, Kirkland, Derksen, & Wall, 2000 ). Despite all of the weed management options available in traditional canola, significant incentives remained for the development of HT canola. The most apparent incentives were special weed problems such as false cleavers ( Galium aparine ) and stork’s bill ( Erodium cicutarium ), and the lack of low-cost herbicide treatments for perennials such as quackgrass ( Agropyron repens ) and Canada thistle ( Cirsium arvense ). Mixtures of herbicides can control many of the common annual and perennial weeds in western Canada but they are expensive and not necessarily reliable (Blackshaw & Harker, 1992 ). In addition, some tank-mixtures led to significant canola injury and yield loss (Harker, Blackshaw, & Kirkland, 1995 ). Thus, canola producers welcomed the prospect of applying a single nonselective herbicide for all weed problems with little concern for specific weed spectrums, growth stages, tank mixture interactions (i.e., antagonism or crop injury) and/or extensive consultations. Two major GM HT canola options are widely used in western Canada. Canola tolerant to glufosinate was the first transgenic crop to be registered in Canada (Oelck et al., 1995 ). Canola tolerant to glyphosate (Roundup Ready) followed shortly thereafter. The GM HT canola offers the possibility of improved weed management in canola via a broader spectrum of weed control and/or greater efficacy on specific weeds (Harker et al., 2000 ). The greatest gains in yield attributed to the adoption of GM HT crops has been for soybean in the United States and Argentina and for GM HT canola in Canada (Brookes & Barfoot, 2008 ).
The reduction of pesticide applications is a major direct benefit of GM crop cultivation: reducing farmers’ exposure to chemicals (Hossain et al., 2004 ; Huang, Hu, Rozelle, & Pray, 2005 ) and lowering pesticide residues in food and feed crops, while also releasing fewer chemicals into the environment and potentially increasing on-farm diversity in insects and pollinators (Nickson, 2005 ). Additionally, improved pest management can reduce the level of mycotoxins in food and feed crops (Wu, 2006 ). Insect resistance in GM crops has been conferred by transferring the gene for toxin creation from the bacterium Bacillus thuringiensis (Bt) into crops like maize. This toxin is naturally occurring in Bt and is presently used as a traditional insecticide in agriculture, including certified organic agriculture, and is considered safe to use on food and feed crops (Roh, Choi, Li, Jin, & Je, 2007 ). GM crops that produce this toxin have been shown to require little or no additional pesticide application even when pest pressure is high (Bawa & Anilakumar, 2013 ). As of the end of the 21st century , insect resistant GM crops were available via three systems (Bt variants). Monsanto and Dow Agrosciences have developed SmartStax maize, which has three pest management attributes, including protection against both above-ground and below-ground insect pests, and herbicide tolerance, which facilitates weed control (Monsanto, 2009 ). SmartStax maize GM varieties were first approved for release in the United States in 2009 and combine traits that were originally intended to be used individually in GM crops (Mannion & Morse, 2013 ). Significant reductions in pesticide use is reported by adoption of Bt maize in Canada, South Africa, and Spain, as well as Bt cotton, notably in China (Pemsl, Waibel, & Gutierrez, 2005 ), India (Qiam, 2003 ), Australia, and the United States (Mannion & Morse, 2013 ).
Human Health
GM crops may have a positive influence on human health by reducing exposure to insecticides (Brimner, Gallivan, & Stephenson, 2005 ; Knox, Vadakuttu, Gordon, Lardner, & Hicks, 2006 ) and by substantially altering herbicide use patterns toward glyphosate, which is considered to be a relatively benign herbicide in this respect (Munkvold, Hellmich, & Rice, 1999 ). However these claims are mostly based on assumption rather than real experimental data. There is generally a lack of public studies on the potential human health impacts of the consumption of food or feed derived from GM crops (Domingo, 2016 ; Wolt et al., 2010 ) and any public work that has been done to date has garnered skepticism and criticism, including, for example, the work by Seralini et al. ( 2013 ). However, the GM crops that are commercialized pass regulatory approval as being safe for human consumption by august competent authorities including the Food and Drug Administration in the United States and the European Food Safety Authority in Europe. Improvement of GM crops that will have a direct influence on health such as decreased allergens (Chu et al., 2008 ), superior levels of protein and carbohydrates (Newell-McGloughlin, 2008 ), greater levels of essential amino acids, essential fatty acids, vitamins and minerals including, multivitamin corn (Naqvi et al., 2009 ; Zhu et al., 2008 ), and maximum zeaxanthin corn (Naqvi et al., 2011 ) hold much promise but have yet to be commercialized. Malnutrition is very common in developing countries where poor people rely heavily on single food sources such as rice for their diet (Gómez-Galera et al., 2010 ). Rice does not contain sufficient quantities of all essential nutrients to prevent malnutrition and GM crops may offer means for supplying more nutritional benefits through single food sources such as rice (White & Broadley, 2009 ). This not only supports people to get the nutrition they require, but also plays a potential role in fighting malnutrition in developing nations (Sakakibara & Saito, 2006 ; Sauter, Poletti, Zhang, & Gruissem, 2006 ). Golden rice is one the most known examples of a bio-fortified GM crop (Potrykus, 2010 ). Vitamin A deficiency renders susceptibility to blindness and affects between 250,000 and 500,000 children annually and is very common in parts of Africa and Asia (Golden Rice Project, 2009 ). A crop like Golden rice could help to overcome the problem of vitamin A deficiency by at least 50% at moderate expense (Stein, Sachdev, & Qaim, 2008 ), yet its adoption has been hampered by activist campaigns (Potrykus, 2012 ).
Environmental Benefits
For currently commercialized GM crops the environmental benefits as previously pointed out are primarily linked to reductions in pesticide use and to reductions in tillage (Christou & Twyman, 2004 ; Wesseler, Scatasta, & El Hadji, 2011 ). Reductions in pesticide use can lead to a greater conservation of beneficial insects and help to protect other non-target species (Aktar, Sengupta, & Chowdhury, 2009 ). Reduced tillage helps to mitigate soil erosion and environmental pollution (Wesseler et al., 2011 ; Brookes & Barfoot, 2008 ) and can lead to indirect environmental benefits including reductions in water pollution via pesticide and fertilizer runoff (Christos & Ilias, 2011 ). It has been claimed that growing Bt maize could help to significantly reduce the use of chemical pesticides and lower the cost of production to some extent (Gewin, 2003 ). The deregulation process for GM crops includes the assessment of potential environmental risks including unintentional effects that could result from the insertion of the new gene (Prakash, Sonika, Ranjana, & Tiwary, 2011 ). Development of GM technology to introduce genes conferring tolerance to abiotic stresses such as drought or inundation, extremes of heat or cold, salinity, aluminum, and heavy metals are likely to enable marginal land to become more productive and may facilitate the remediation of polluted soils (Czako, Feng, He, Liang, & Marton, 2005 ; Uchida et al., 2005 ). The multiplication of GM crop varieties carrying such traits may increase farmers’ capacities to cope with these and other environmental problems (Dunwell & Ford, 2005 ; Sexton & Zilberman, 2011 ). Therefore, GM technology may hold out further hope of increasing the productivity of agricultural land with even less environmental impact (Food and Agriculture Organization [FAO], 2004 ).
Some proponents of GM crops have argued that because they increase productivity they facilitate more sustainable farming practices and can lead to “greener” agriculture. Mannion and Morse ( 2013 ), for example, argue that GM crops require less energy investment in farming because the reduced application of insecticide lowers energy input levels, thereby reducing the carbon footprint. It has been suggested by other authors that the adoption of GM crops may have the potential to reduce inputs such as chemical fertilizers and pesticides (Bennett, Ismael, Morse, & Shankar, 2004 ; Bennett, Phipps, Strange, & Grey, 2004 ). Others note that higher crop yields facilitated by GM crops could offset greenhouse gas emissions at scales similar to those attributed to wind and solar energy (Wise et al., 2009 ). Greenhouse gas emissions from intensive agriculture are also offset by the conservation of non-farmed lands. While untilled forest soils and savannas, for example, act as carbon stores, farmed land is often a carbon source (Burney, Davis, & Lobell, 2010 ).
The Economy
GM crops are sold into a market and are subject to the market in terms of providing a realized value proposition for farmers and value through the food chain in terms of reduced costs of production (Lucht, 2015 ). Currently the GM crops on the market are targeted to farmers and have a value proposition based on economic benefits to farmers via operational benefits (Mauro, McLachlan, & Van Acker, 2009 ). Due to higher yield and lower production cost of GM crops, farmers will get more economic return and produce more food at affordable prices, which can potentially provide benefits to consumers including the poor (Lucht, 2015 ; Lemaux, 2009 ). The most significant economic benefits attributed to GM crop cultivation have been higher gross margins due to lower costs of pest management for farmers (Klümper & Qaim, 2014 ; Qaim, 2010 ). GM varieties have provided a financial benefit for many farmers (Andreasen, 2014 ). In some regions, GM crops have led to reduced labor costs for farmers (Bennett et al., 2005 ). Whether GM crops have helped to better feed the poor and alleviate global poverty is not yet proven (Yuan et al., 2011 ).
Cons of GMO Crop Farming
The intensive cultivation of GM crops has raised a wide range of concerns with respect to food safety, environmental effects, and socioeconomic issues. The major cons are explored for cross-pollination, pest resistance, human health, the environment, the economy, and productivity.
Cross-Pollination
The out crossing of GM crops to non-GM crops or related wild type species and the adventitious mixing of GM and non-GM crops has led to a variety of issues. Because of the asynchrony of the deregulation of GM crops around the world, the unintended presence of GM crops in food and feed trade channels can cause serious trade and economic issues. One example is “LibertyLink” rice, a GM variety of rice developed by Bayer Crop Science, traces of which were found in commercial food streams even before it was deregulated for production in the United States. The economic impact on U.S. rice farmers and millers when rice exports from the United States were halted amounted to hundreds of millions of dollars (Bloomberg News, 2011 ). A more recent example is Agrisure Viptera corn, which was approved for cultivation in the United States in 2009 but had not yet been deregulated in China. Exports of U.S. corn to China contained levels of Viptera corn, and China closed its borders to U.S. corn imports for a period. The National Grain and Feed Association (NGFA) had encouraged Syngenta to stop selling Viptera because of losses U.S. farmers were facing, and there is an ongoing class-action lawsuit in the United States against Syngenta (U.S. District Court, 2017 ). Concerns over the safety of GM food have played a role in decisions by Chinese officials to move away from GM production. Cross-pollination can result in difficulty in maintaining the GM-free status of organic crops and threaten markets for organic farmers (Ellstrand, Prentice, & Hancock, 1999 ; Van Acker, McLean, & Martin, 2007 ). The EU has adopted a GM and non-GM crop coexistence directive that has allowed nation-states to enact coexistence legislation that aims to mitigate economic issues related to adventitious presence of GM crops in non-GM crops (Van Acker et al., 2007 ).
GM crops have also been criticized for promoting the development of pesticide-resistant pests (Dale, Clarke, & Fontes, 2002 ). The development of resistant pests is most due to the overuse of a limited range of pesticides and overreliance on one pesticide. This would be especially true for glyphosate because prior to the development of Roundup Ready crops glyphosate use was very limited and since the advent of Roundup Ready crops there has been an explosion of glyphosate-resistant weed species (Owen, 2009 ). The development of resistant pests via cross-pollination to wild types (weeds) is often cited as a major issue (Friedrich & Kassam, 2012 ) but it is much less of a concern because it is very unlikely (Owen et al., 2011 ; Ellstrand, 2003 ). There are, however, issues when genes transfer from GM to non-GM crops creating unexpected herbicide resistant volunteer crops, which can create challenges and costs for farmers (Van Acker, Brule-Babel, & Friesen, 2004 ; Owen, 2008 ; Mallory-Smith & Zapiola, 2008 ).
Some critics of GM crops express concerns about how certain GM traits may provide substantive advantages to wild type species if the traits are successfully transferred to these wild types. This is not the case for GM HT traits, which would offer no advantage in non-cropped areas where the herbicides are not used, but could be an issue for traits such as drought tolerance (Buiatti, Christou, & Pastore, 2013 ). This situation would be detrimental because the GM crops would grow faster and reproduce more often, allowing them to become invasive (FAO, 2015 ). This has sometime been referred to as genetic pollution (Reichman et al., 2006 ). There are also some concerns that insects may develop resistance to the pesticides after ingesting GM pollen (Christou, Capell, Kohli, Gatehouse, & Gatehouse, 2006 ). The potential impact of genetic pollution of this type is unclear but could have dramatic effects on the ecosystem (Stewart et al., 2003 ).
Pest Resistance
Repeated use of a single pesticide over time leads to the development of resistance in populations of the target species. The extensive use of a limited number of pesticides facilitated by GM crops does accelerate the evolution of resistant pest populations (Bawa & Anilakumar, 2013 ). Resistance evolution is a function of selection pressure from use of the pesticide and as such it is not directly a function of GM HT crops for example, but GM HT crops have accelerated the development of glyphosate resistant weeds because they have promoted a tremendous increase in the use of glyphosate (Owen, 2009 ). Farmers have had to adjust to this new problem and in some cases this had added costs for farmers (Mauro, McLachlan, & Van Acker, 2009 ; Mannion & Morse, 2013 ). The management of GM HT volunteers has also produced challenges for some farmers. These are not resistant weeds as they are not wild type species, but for farmers they are herbicide-resistant weeds in an operational sense (Knispel, McLachlan, & Van Acker, 2008 ; Liu et al., 2015 ). Pink bollworm has become resistant to the first generation GM Bt cotton in India (Bagla, 2010 ). Similar pest resistance was also later identified in Australia, China, Spain, and the United States (Tabashnik et al., 2013 ). In 2012 , army worms were found resistant to Dupont-Dow’s Bt corn in Florida (Kaskey, 2012 ), and the European corn borer is also capable of developing resistance to Bt maize (Christou et al., 2006 ).
Although the deregulation of GM crops includes extensive assessments of possible human health impacts by competent authorities there are still many who hold concerns about the potential risks to human health of GM crops. For some this is related to whether transgenesis itself causes unintended consequences (Domingo, 2016 ), while for others it is concerns around the traits that are possible using GM (Herman, 2003 ). Some criticize the use of antibiotic resistance as markers in the transgenesis procedure and that this can facilitate antibiotic resistance development in pathogens that are a threat to human health (Key, Ma, & Drake, 2008 ). Many critics of GM crops express concerns about allergenicity (Lehrer & Bannon, 2005 ). Genetic modification often adds or mixes proteins that were not native to the original plant, which might cause new allergic reactions in the human body (Lehrer & Bannon, 2005 ). Gene transfer from GM foods to cells of the body or to bacteria in the gastrointestinal tract would cause concern if the transferred genetic material unfavorably influences human health, but the probability of this occurring is remote. Other concerns include the possibility of GM crops somehow inducing mutations in human genes (Ezeonu, Tagbo, Anike, Oje, & Onwurah, 2012 ) or other unintended consequences (Yanagisawa, 2004 ; Lemaux, 2009 ; Gay & Gillespie, 2005 ; Wesseler, Scatasta, & El Hadji, 2011 ) but commentary by these authors is speculative and is not based on experimentation with current GM crops.

Environment
For currently commercialized GM crops the potential environmental impacts are mostly related to how these crops impact farming systems. Some argue that because crops like Roundup Ready soybean greatly simplify weed management they facilitate simple farming systems including monocultures (Dunwell & Ford, 2005 ). The negative impact of monocultures on the environment is well documented and so this might be considered an indirect environmental effect of GM crops (Nazarko, Van Acker, & Entz, 2005 ; Buiatti, Christou, & Pastore, 2013 ). Other concerns that have been raised regarding GM crops include the effects of transgenic on the natural landscape, significance of gene flow, impact on non-target organisms, progression of pest resistance, and impacts on biodiversity (Prakash et al., 2011 ). Again, many of these concerns may be more a function of the impacts of simple and broad-scale farming practices facilitated by GM crops rather than GM crops per se. However, there has been considerable concern over the environmental impact of Bt GM crops highlighted by studies that showed the potential impact on monarch butterfly populations (Dively et al., 2004 ). This begged questions then about what other broader effects there may be on nontarget organisms both direct and indirect (Daniell, 2002 ). In addition, there may be indirect effects associated with how GM crops facilitate the evolution of pesticide resistant pests in that the follow-on control of these pest populations may require the use of more pesticides and often older chemistries that may be more toxic to the environment in the end (Nazarko et al., 2005 ).
Bringing a GM crop to market can be both expensive and time consuming, and agricultural bio-technology companies can only develop products that will provide a return on their investment (Ramaswami, Pray, & Lalitha, 2012 ). For these companies, patent infringement is a big issue. The price of GM seeds is high and it may not be affordable to small farmers (Ramaswami et al., 2012 ; Qaim, 2009 ). A considerable range of problems has been associated with GM crops, including debt and increased dependence on multinational seed companies, but these can also be combined with other agricultural technologies to some extent (Kloppenburg, 1990 ; Finger et al., 2011 ). The majority of seed sales for the world’s major crops are controlled by a few seed companies. The issues of private industry control and their intellectual property rights over seeds have been considered problematic for many farmers and in particular small farmers and vulnerable farmers (Fischer, Ekener-Petersen, Rydhmer, & Edvardsson Björnberg, 2015 ; Mosher & Hurburgh, 2010 ). In addition, efforts by GM seed companies to protect their patented seeds through court actions have created financial and social challenges for many farmers (Marvier & Van Acker, 2005 ; Semal, 2007 ). There is considerable debate about the extent to which GM crops bring additional value to small and vulnerable farmers with strong opinions on both sides (Park, McFarlane, Phipps, & Ceddia, 2011 ; Brookes & Barfoot, 2010 ; James, 2010 ; Smale et al., 2009 ; Subramanian & Qaim, 2010 ). As the reliance on GM seeds extends, concerns grow about control over the food supply via seed ownership and the impacts on the diversity of seed sources, which can impact the resilience of farming systems across a region (Key et al., 2008 ). The risk of GM crops to the world economy can be significant. Global food production is dominated by a few seed companies, and they have increased the dependence of developing countries on industrialized nations (Van Acker, Cici, Michael, Ryan, & Sachs, 2015 ).
Productivity
Justification for GM crops on the basis of the need to feed the world is often used by proponents of the technology, but the connection between GM crops and feeding the world is not direct. GM crops are used by farmers and are sold primarily on the basis of their direct operational benefits to farmers, including the facilitation of production and/or more production (Mauro et al., 2009 ). Farmers realize these benefits in terms of cost savings or increased production or both and are looking to increase their margins by using the technology. Companies producing GM seeds can be very successful if they are able to capture a greater share of a seed market because they supply farmers with operational benefits such as simplified weed management (Blackshaw & Harker, 1992 ) even if there are no productivity gains. In addition, the traits in GM crops on the market as of the early part of the 21st century are not yield traits per se but are yield potential protection traits that may or may not result in greater productivity.
Conclusions
Genetic modification via recombinant DNA technology is compelling because it does provide a means for bringing truly novel traits into crops and the adoption of GM crops has been rapid in a range of countries around the world. Only a very limited number of traits have been incorporated to date into GM crops, the two primary traits being herbicide tolerance (HT) and insect resistance. Nonetheless, farmers who have adopted GM crops have benefited from the operational benefits they provide, and current GM crops have facilitated the adoption of more sustainable farming practices, in particular, reduced tillage. The ongoing asynchronous approvals of GM crops around the world mean that there will always be issues related to the adventitious presence of GM crops in crop shipments and trade disruptions. Pollen mediated gene flow from crop to crop, and seed admixtures are challenges of GM crop farming and agricultural marketing as a result. The adoption of GM HT crops has also accelerated the evolution of herbicide resistant weeds, which has created additional operational challenges and costs for farmers. The GM crops commercialized to date have all been deregulated and deemed to be safe to the environment and safe in terms of human health by competent authorities around the world, including the European Food Safety Association. There remain, however, critics of the technology who point to a lack of public research on the potential risks of GM and GM crops. GM crops will continue to be developed because they provide real operational benefits for farmers, who are the ones who purchase the seeds. The novelty of the technology and its potential to bring almost any trait into crops mean that there needs to remain dedicated diligence on the part of regulators to ensure that no GM crops are deregulated that may in fact pose risks to human health or the environment, but there will also remain the promise of the value of novel inventions that bring benefits to consumers and the environment. The same will be true for the next wave of new breeding technologies, which include gene editing technologies such as CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) (Cong et al., 2013 ). These new technologies have even greater potential for modifying crops than GM technology and they avoid some of the characteristics of GM technology that have underpinned criticisms including, for example, the presence of foreign DNA.
- Aktar, W. M. , Sengupta, D. , & Chowdhury, A. (2009). Impact of pesticides use in agriculture: Their benefits and hazards. Interdisciplinary Toxicology , 2 (1), 1–12.
- Altieri, M. A. (2005). The myth of coexistence: Why transgenic crops are not compatible with agroecologically based systems of production. Bulletin of Science Technology & Society , 25 , 1–11.
- Andreasen, M. (2014). GM food in the public mind—facts are not what they used to be. Nature Biotechnology , 32 , 25.
- Apel, A. (2010). The costly benefits of opposing agricultural biotechnology. New Biotechnology , 27 , 635–640.
- Bagavathiannan, M. V. , Julier, B. , Barre, P. , Gulden, R. H. , & Van Acker, R. C. (2010). Genetic diversity of feral alfalfa ( Medicago sativa L.) populations occurring in Manitoba, Canada, and comparison with alfalfa cultivars: An analysis using SSR markers and phenotypic traits. Euphytica , 173 , 419–432.
- Bagla, P. (2010). Hardy cotton-munching pests are latest blow to GM crops. Science , 327 , 1439.
- Baker, J. M. , Ochsner, T. E. , Venterea, R. T. , & Griffis, T. J. (2007). Tillage and soil carbon sequestration—What do we really know? Agriculture, Ecosystems and Environment , 118 , 1–5.
- Bawa, A. S. , & Anilakumar, K. R. (2013). Genetically modified foods: Safety, risks and public concerns—a review. Journal of Food Science and Technology , 50 (6), 1035–1046.
- Beckie, H. J. , Harker, K. N. , Hall, L. M. , Warwick, S. I. , Légère, A. , Sikkema, P. H. , . . . Simard, M. J. (2006). A decade of herbicide-resistant crops in Canada. Canadian Journal of Plant Science , 86 , 1243–1264.
- Bennett, R. M. , Ismael, Y. , & Morse, S. (2005). Explaining contradictory evidence regarding impacts of genetically modified crops in developing countries: Varietal performance of transgenic cotton in India. Journal of Agricultural Science , 143 , 35–41.
- Bennett, R. , Ismael, Y. , Morse, S. , & Shankar, B. (2004). Reductions in insecticide use from adoption of Bt cotton in South Africa: Impacts on economic performances and toxic load to the environment. Journal of Agricultural Sciences , 142 , 665–674.
- Bennett, R. , Phipps, R. , Strange, A. , & Grey, P. (2004). Environmental and human health impacts of growing genetically modified herbicide tolerant sugar beet: A life-cycle assessment. Plant Biotechnology Journal , 2 , 273–278.
- Blackshaw, R. E. , & Harker, K. N. (1992). Combined postemergence grass and broadleaf weed control in canola ( Brassica napus ). Weed Technology , 6 , 892–897.
- Blaine, K. , & Powell, D. (2001). Communication of food-related risks. AgBioForum , 4 , 179–185.
- Bloomberg News . (2011). Bayer Settles With Farmers Over Modified Rice Seeds . New York Times .
- Boucher, D. H. (1999). The paradox of plenty: Hunger in bountiful world . Oakland, CA: Food First Books.
- Brimner, T. A. , Gallivan, G. J. , & Stephenson, G. R. (2005). Influence of herbicide-resistant canola on the environmental impact of weed management . Pest Management Science , 61 , 47–52.
- Brookes, G. , & Barfoot, P. (2008). Global impact of biotech crops: Socio-economic and environmental effects, 1996–2006. AgBioForum , 11 , 21–38.
- Brookes, G. , & Barfoot, P. (2010). GM crops: Global socio-economic and environmental impacts 1996–2008 . Dorchester, U.K.: PG Economics.
- Brookes, G. , & Barfoot, P. (2014). Key global economic and environmental impacts of genetically modified (GM) crop use 1996–2012. GM Crops and Food: Biotechnology in Agricultural and the Food Chain , 5 , 149–160.
- Buiatti, M. , Christou, P. , & Pastore, G. (2013). The application of GMOs in agriculture and in food production for a better nutrition: Two different scientific points of view. Genes Nutrition , 8 (3), 255–270.
- Burney, J. A. , Davis, S. J. , & Lobell, D. B. (2010). Greenhouse gas mitigation by agricultural intensification . Proceedings of the National Academy of Sciences of the United States of America , 107 (26), 12052–12057.
- Carpenter, J. E. (2010). Peer-reviewed surveys indicate positive impact of commercialized GM crops. Nature Biotechnology , 28 , 219–221.
- Chikelu, M. B. A. , Elcio, P. G. , & Kakoli, G. (2012). Re-orienting crop improvement for the changing climatic conditions of the 21st century . Agriculture and Food Security , 1 (1), 1–17.
- Christos A. D. , & Ilias, G. E. (2011). Pesticide exposure, safety issues, and risk assessment indicators. International Journal of Environmental Research and Public Health , 8 (5), 1402–1419.
- Christou, P. , Capell, T. , Kohli, A. , Gatehouse, J. A. , & Gatehouse, A. M. R. (2006). Recent developments and future prospects in insect pest control in transgenic crops . Trends Plant Science , 11 , 302–308.
- Christou, P. , & Twyman, R. M. (2004) The potential of genetically enhanced plants to address food insecurity . Nutrition Research Reviews , 17 , 23–42.
- Chu, Y. , Faustinelli, P. , Ramos, M. L. , Hajduch, M. , Stevenson, S. , Thelen, J. J. , et al. (2008). Reduction of IgE binding and nonpromotion of Aspergillus flavus fungal growth by simultaneously silencing Ara h 2 and Ara h 6 in peanut . Journal of Agricultural and Food Chemistry , 56 , 11225–11233.
- Cong, L. , Ran, F. A. , Cox, D. , Lin, S. , Barretto, R. , Habib, N. , . . . Zhang, F. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science , 339 , 819–823.
- Czako, M. , Feng, X. , He, Y. , Liang, D. , & Marton, L. (2005). Genetic modification of wetland grasses for phytoremediation. Zeitschrift fu¨r Naturforschung , 60c , 285–291.
- Dale, P. J. , Clarke, B. , & Fontes, E. M. G. (2002). Potential for the environmental impact of transgenic crops. Nature Biotechnology , 20 (6), 567–574.
- Daniell, H. (2002). Molecular strategies for gene containment in transgenic crops. Nature Biotechnology , 20 , 581–586.
- Dill, G. M. , CaJacob, C. A. , & Padgette, S. R. (2008). Glyphosate-resistant crops: Adoption, use and future considerations. Pest Management Science , 64 , 326–331.
- Dively, G. P. , Rose, R. , Sears, M. K. , Hellmich, R. L. , Stanley-Horn, D. E. , Calvin, D. D. , . . . Anderson, P. L. (2004). Effects on monarch butterfly larvae (Lepidoptera: Danaidae) after continuous exposure to Cry1Ab-expressing corn during anthesis. Environmental Entomology , 33 (4), 1116–1125.
- Domingo J. L. (2016). Safety assessment of GM plants: An updated review of the scientific literature. Food and Chemical Toxicology , 95 , 12–18.
- Dunwell, J. M. , & Ford, C. S. (2005). Technologies for biological containment of GM and non-GM crops . Defra Contract CPEC 47. London: DEFRA.
- Ellstrand, N. (2003). Current knowledge of gene flow in plants: Implications for transgenic flow. Philosophical Transactions of the Royal Society B: Biological Science , 358 (1434), 1163–1170.
- Ellstrand, N. C. , Prentice, H. C. , & Hancock, J. F. (1999). Gene flow and introgression from domesticated plants into their wild relatives. Annual Review of Ecology and Systematics , 30 , 539–563.
- Ezeonu, C. S. , Tagbo, R. , Anike, E. N. , Oje, O. A. , & Onwurah, I. N. E. O. (2012). Biotechnological tools for environmental sustainability: Prospects and challenges for environments in Nigeria—a standard review . Biotechnology Research International , 1 , 1–26.
- Fawcett, R. , & Towery, D. (2003). Conservation tillage and plant biotechnology: How new technologies can improve the environment by reducing the need to plow . West Lafayette, IN: Conservation Technology Information Center (CTIC), Purdue University.
- Fernandez-Cornejo, J. , Hallahan, C. , Nehring, R. , Wechsler, S. , & Grube, A. (2012). Conservation tillage, herbicide use, and genetically engineered crops in the United States: The case of soybeans. AgBioForum , 15 , 231–241.
- Fernandez-Cornejo, J. , Wechsler, S. J. , Livingston, M. , & Mitchell, L. (2014). Genetically engineered crops in the United States. Washington, DC: United States Department of Agriculture—Economic Research Service.
- Finger, R. , El Benni, N. , Kaphengst, T. , Evans, C. , Herbert, S. , Lehmann, B. , . . . Stupak, N. (2011). A meta-analysis on farm-level costs and benefits of GM crops. Sustainability , 3 (5), 743–762.
- Fischer, K. , Ekener-Petersen, E. , Rydhmer, L. , & Edvardsson Björnberg, K. (2015). Social impacts of GM crops in agriculture: A systematic literature review . Sustainability , 7 , 8598–8620.
- Food and Agriculture Organization . (2004). Agricultural biotechnology: Meeting the needs of the poor? The state of food and agriculture 2003–04 . Rome: Food and Agriculture Organization of the United Nations.
- Food and Agriculture Organization . (2015). FAO statistical pocketbook 2015: World food and agriculture . Rome: Food and Agriculture Organization.
- Friedrich, T. , & Kassam, A. (2012). No-till farming and the environment: Do no-till systems require more chemicals? Outlooks on Pest Management , 23 (4), 153–157.
- Gay, P. B. , & Gillespie, S. H. (2005). Antibiotic resistance markers in genetically modified plants: A risk to human health? Lancet Infect Disease , 5 (10), 637–646.
- Gewin, V. (2003). Genetically modified corn—environmental benefits and risks . PLoS Biology , 1 (1), e8.
- Gilbert, N. (2013). A hard look at GM crops. Nature , 497 , 24–26.
- Giller, K. E. , Witter, E. , Corbeels, M. , & Pablo, T. (2009). Conservation agriculture and smallholder farming in Africa: The heretics’ view. Field Crops Research , 114 , 23–34.
- Golden Rice Project . (2009). Golden Rice is part of the solution ..
- Gómez-Galera, S. , Rojas, E. , Sudhakar, D. , Zhu, C. , Pelacho, A. M. , Capell, T. , & Christou, P. (2010). Critical evaluation of strategies for mineral fortification of staple food crops . Transgenic Research , 19 , 165–180.
- Gray, R. (2001). Introduction. In M. Fulton , H. Furtan , D. Gosnell , R. Gray , J. Hobbs , J. Holzman , et al. (Eds.), Transforming agriculture: The benefits and costs of genetically modified crops . Ottawa, ON: Canadian Biotechnology Advisory Committee.
- Gunther, M. (2007, July 2). Attack of the mutant rice . Fortune .
- Harker, K. N. , Blackshaw, R. E. , & Kirkland, K. J. (1995). Ethametsulfuron interactions with grass herbicides on canola ( Brassica napus , B. rapa ). Weed Technology , 9 , 91–98.
- Harker, K. N. , Blackshaw, R. E. , Kirkland, K. J. , Derksen, D. A. , & Wall, D. (2000). Herbicide-tolerant canola: Weed control and yield comparisons in western Canada. Canadian Journal of Plant Science , 80 (3), 647–654.
- Hatfield, J. , Takle, G. , Grotjahn, R. , Holden, P. , Izaurralde, R. C. , Mader, T. , (2014). Agriculture. In J. M. Melillo , T. C. R. Terese , & G. W. Yohe (Eds.), Climate change impacts in the United States: The Third National Climate Assessment (pp. 150–174). Washington, DC: U.S. Government Printing Office.
- Herdt, R. W. (2006). Biotechnology in agriculture. Annual Review of Environment and Resources , 31 , 265–295.
- Herman, E. M. (2003). Genetically modified soybeans and food allergies. Journal of Experimental Botany , 54 , 1317–1319.
- Hillocks, R. J. (2009). GM cotton for Africa. Outlook on Agriculture , 38 , 311–316.
- Hossain, F. , Pray, C. E. , Lu, Y. , Huang, J. , Fan, C. , & Hu, R. (2004). Genetically modified cotton and farmers’ health in China. International Journal of Occupational Environmental Health , 10 , 296–303.
- Huang, J. , Hu, R. , Rozelle, S. , & Pray, C. (2005). Insect-resistance GM rice in farmers’ fields: Assessing productivity and health effects in China. Science , 308 , 688–690.
- Inghelbrecht, L. , Dessein, J. , & Huylenbroeck, G. V. (2014). The non-GM crop regime in the EU: How do industries deal with this wicked problem? Wageningen Journal of Life Sciences , 70 , 103–112.
- International Service for the Acquisition of Agri-biotech Applications [ISAAA] . (2015). Annual report executive summary, 20th anniversary (1996 to 2015) of the global commercialization of biotech crops: Highlights in 2015 . ISAAA Brief No. 51. Ithaca, NY: ISAAA.
- James, C. (2010). Global status of commercialized biotech/GM crops: 2010 . ISAAA Brief No. 42. Ithaca, NY: ISAAA.
- James, C. (2014). Global status of commercialized biotech/GM crops: 2013 . ISAAA Brief No. 49. Ithaca, NY: ISAAA.
- James, C. (2015a). Global status of commercialized biotech/GM crops: 2014 . ISAAA Brief No. 49. Ithaca, NY: ISAAA.
- James, C. (2015b). 20th Anniversary (1996 to 2015) of the global commercialization of biotech crops: Highlights in 2015 . ISAAA Brief No. 51. Ithaca, NY: ISAAA.
- Kaskey, J. (2012, November 16). DuPont-Dow corn defeated by armyworms in Florida: Study. Bloomberg News .
- Key, S. , Ma, J. K.-C. , & Drake, P. M. W. (2008). Genetically modified plants and human health. Journal of the Royal Society of Medicine , 101 (6), 290–298.
- Kleter, G. A. , Bhula, R. , Bodnaruk, K. , Carazo, E. , Felsot, A. S. , Harris, C. A. , . . . Wong, S.-S. (2007). Altered pesticide use on transgenic crops and the associated general impact from an environmental perspective. Pest Management Science , 63 (11), 1107–1115.
- Kloppenburg, J. R. (1990). First the seed: The political economy of plant biotechnology . Cambridge, U.K.: Cambridge University Press.
- Klümper, W. , & Qaim, M. (2014). A meta-analysis of the impacts of genetically modified crops . PLoS ONE , 9 (11), e111629.
- Knispel, A. L. , McLachlan, S. M. , Van Acker, R. C. , & Friesen, L. F. (2008). Gene flow and multiple herbicide resistance in escaped canola populations. Weed Science , 56 , 72–80.
- Knox, O. G. G. , Vadakattu, G. V. S. R. , Gordon, K. , Lardner, R. , & Hicks, M. (2006). Environmental impact of conventional and Bt insecticidal cotton expressing one and two Cry genes in Australia . Australian Journal of Agricultural Research , 57 , 501–509.
- Kynda R. C. , & Moeltner, K. (2006). Genetically modified food market participation and consumer risk perceptions: A cross-country comparison. Canadian Journal of Agricultural Economics , 54 , 289–310.
- Lal, R. (2004). Soil carbon sequestration impacts on global climate change and food security. Science , 304 (5677), 1623–1627.
- Lehrer, S. B. , & Bannon, G. A. (2005). Risks of allergic reactions to biotech proteins in foods: Perception and reality. Allergy , 60 (5), 559–564.
- Lemaux, P. G. (2009). Genetically engineered plants and foods: A scientist’s analysis of the issues (Part II). Annual Review Plant Biology , 60 , 511–559.
- Lipton, M. , & Longhurst, V. (2011). New seeds and poor people . Abingdon, U.K.: Routledge.
- Liu, Y. B. , Darmency, H. , Stewart, C. N. , Wei, W., Jr. , Tang, Z. X. , & Ma, K. P. (2015). The effect of Bt-transgene introgression on plant growth and reproduction in wild Brassica juncea. Transgenic Research , 24 , 537–547.
- Lucht, J. M. (2015). Public acceptance of plant biotechnology and GM crops. Viruses , 7 (8), 4254–4281.
- Macnaghten, P. , & Carro-Ripalda, S. (2015). Governing agricultural sustainability: Global lessons from GM crops . London: Routledge.
- Mallory-Smith, C. , & Zapiola, M. (2008). Gene flow from glyphosate-resistant crops. Pest Management Science , 64 , 428–440.
- Mann, S. (2015). Is “GMO free” an additional “organic”? On the economics of chain segregation. AgBioForum , 18 , 26–33.
- Mannion, A. M. (1995a). Biotechnology and environmental quality. Progress in Physical Geography , 19 , 192–215.
- Mannion, A. M. (1995b). Agriculture and environmental change: Temporal and spatial dimensions . Chichester, U.K.: John Wiley.
- Mannion, A. M. (1995c). The three Bs: Biodiversity, biotechnology and business. Environmental Conservation , 22 , 201–210.
- Mannion, A. M. , & Morse, S. (2013). GM crops 1996–2012: A review of agronomic, environmental and socio-economic impacts . University of Reading, Geographical Paper No. 195. Retrieved from http://www.reading.ac.uk/geographyandenvironmentalscience/Research/ges-resGeogPapers.aspx
- Marvier, M. , & Van Acker, R. C. (2005). Can crop transgenes be kept on a leash? Frontier Ecology Environment , 3 , 99–106.
- Mauro, I. J. , & McLachlan, S. M. (2008). Farmer knowledge and risk analysis: Post release evaluation of herbicide-tolerant canola in Western Canada. Risk Analysis , 28 (2), 463–476.
- Mauro, I. J. , McLachlan, S. M. , & Van Acker, R. C. (2009). Farmer knowledge and a priori risk analysis: Pre-release evaluation of genetically modified Roundup Ready wheat across the Canadian prairies. Environmental Science and Pollution Research , 16 , 689–701.
- Monsanto . (2009). SmartStax corn receives Japanese import approval .
- Montgomery, D. R. (2007). Soil erosion and agricultural sustainability. Proceedings of the National Academy of Sciences of the United States , 104 , 13268–13272.
- Morse, S. , & Mannion, A. M. (2009). Can genetically-modified cotton contribute to sustainable development in Africa? Progress in Development Studies , 9 , 225–247.
- Morse, S. , Mannion, A. M. , & Evans, C. (2011). Location, location, location: Presenting evidence for genetically modified crops. Applied Geography , 34 (2), 274–280.
- Mosher, G. , & Hurburgh, C. (2010). Transgenic plant risk: Coexistence and economy. Encyclopedia of Biotechnology in Agriculture and Food , 1 , 639–642.
- Munkvold, G. P. , Hellmich, R. L. , & Rice, L. G. (1999). Comparison of fumonisin concentrations in kernels of transgenic Bt corn hybrids and non-transgenic hybrids . Plant Disease , 81 , 556–565.
- Naqvi, S. , Zhu, C. , Farre, G. , Ramessar, K. , Bassie, L. , Breitenbach, J. , . . . Christou, P. (2009). Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways . Proceedings National Academy of Sciences, USA , 106 , 7762–7767.
- Naqvi, S. , Zhu, C. , Farre, G. , Sandmann, G. , Capell, T. , & Christou, P. (2011). Synergistic metabolism in hybrid corn indicates bottlenecks in the carotenoid pathway and leads to the accumulation of extraordinary levels of the nutritionally important carotenoid zeaxanthin . Plant Biotechnology Journal , 9 , 384–393.
- National Research Council . (2010). The impact of genetically engineered crops on farm sustainability in the United States . Washington, DC: National Academies Press.
- Nazarko, O. M. , Van Acker, R. C. , & Entz, M. H. (2005). Strategies and tactics for herbicide use reduction in field crops in Canada: A review. Canadian Journal of Plant Science , 85 , 457–479.
- Newell-McGloughlin, M. (2008). Nutritionally improved agricultural crops . Plant Physiology , 147 , 939–953.
- Nickson, T. E. (2005). Crop biotechnology—the state of play. In G. M. Poppy & M. J. Wilkinson (Eds.), Gene flow from GM plants (pp. 12–42). Oxford: Blackwell.
- Oelck, M. M. , MacDonald, R. , Belyk, M. , Ripley, V. , Weston, B. , Bennett, C. , et al. (1995, July 4–7). Registration, safety assessment and agronomic performance of transgenic canola cv. “Innovator” in Canada. In D. J. Murphy (Ed.), Proceedings of the 9th International Rapeseed Congress (Vol. 4, pp. 1420–1432). Cambridge, U.K.: Organising Committee of the Ninth International Rapeseed Congress.
- Oerke, E. C. (2006). Crop losses to pests, centenary review. Journal of Agricultural Science , 144 , 31–43.
- Owen, M. D. K. (2008). Weed species shifts in glyphosate-resistant crops. Pest Management Science , 64 , 377–387.
- Owen, M. D. K. (2009). Herbicide-tolerant genetically modified crops: Resistance management. In N. Ferry & A. M. R. Gatehouse (Eds.), Environmental impact of genetically modified crops (pp. 115–164). Wallingford, U.K.: CABI.
- Owen, M. D. K. , Young, B. G. , Shaw, D. R. , Wilson, R. G. , Jordan, D. L. , Dixon, P. M. , & Weller, S. C. (2011). Benchmark study on glyphosate-resistant crop systems in the United States. Part 2: Perspectives. Pest Management Science , 67 , 747–757.
- Paarlberg, R. (2008). Starved for science: How biotechnology is being kept out of Africa . Cambridge, MA: Harvard University Press.
- Park, R. J. , McFarlane, I. , Phipps, R. H. , & Ceddia, G. (2011). The role of transgenic crops in sustainable development . Plant Biotechnology Journal , 9 , 2–21.
- Pemsl, D. , Waibel, H. , & Gutierrez, A. P. (2005). Why do some Bt-cotton farmers in China continue to use high levels of pesticide? International Journal of Agricultural Sustainability , 3 , 44–56.
- Phillips, P. W. B. (2003). The economic impact of herbicide tolerant canola in Canada. In N. Kalaitzandonakes (Ed.), The economic and environmental impacts of Agbiotech: A global perspective (pp. 119–140). New York: Kluwer Academic.
- Pimentel, D. , Hunter, M. S. , Lagro, J. A. , Efroymson, R. A. , Landers, J. C. , Mervis, F. T. , et al. (1989). Benefits and risks of genetic engineering in agriculture. BioScience , 39 (9), 606–614.
- Pinstrup-Andersen, P. (1999). Agricultural biotechnology, trade, and the developing countries. AgBioForum , 2 , 215–217.
- Potrykus, I. (2010). Lessons from the “Humanitarian Golden Rice” project: Regulation prevents development of public good genetically engineered crop products. Nature Biotechnology , 27 , 466–472.
- Potrykus, I. (2012). “Golden Rice,” a GMO-product for public good, and the consequences of GE-regulation. Journal of Plant Biochemistry and Biotechnology , 21 , 68–75.
- Powlson, D. S. , Stirling, C. M. , Jat, M. L. , Gerard, B. G. , Palm, C. A. , Sanchez, P. A. , & Cassman, K. G. (2014). Limited potential of no-till agriculture for climate change mitigation. Nature Climate Change , 4 , 678–683.
- Prakash, D. , Sonika, V. , Ranjana, B. , & Tiwary, B. N. (2011). Risks and precautions of genetically modified organisms . ISRN Ecology, ID 369573.
- Pray, C. E. , Huang, J. , Hu, R. , & Rozelle, S. (2002). Five years of Bt cotton in China—the benefits continue. Plant Journal , 31 (4), 423–430.
- Pray, C. E. , Nagarajan, L. , Huang, J. , Hu, R. , & Ramaswami, B. (2011). Impact of Bt cotton, the potential future benefits from biotechnology in China and India. In C. Carter , G. Moschini , & I. Sheldon (Eds.), Genetically modified food and global welfare (pp. 83–114). Bingley, U.K.: Emerald.
- Qaim, M. (2003). Bt cotton in India: Field trial results and economic projections. World Development , 31 , 2115–2127.
- Qaim, M. (2009). The economics of genetically modified crops. Annual Review Resource Economics , 1 , 665–693.
- Qaim, M. (2010). Benefits of genetically modified crops for the poor: Household income, nutrition and health. New Biotechnology , 27 , 552–557.
- Qaim, M. , & Traxler, G. (2005). Roundup ready soybeans in Argentina: Farm level and aggregate welfare effects. Agricultural Economics , 32 , 73–86.
- Ramaswami, B. , Pray, C. E. , & Lalitha, N. (2012). The spread of illegal transgenic cotton varieties in India: Biosafety regulation, monopoly, and enforcement. World Development , 40 (1), 177–188.
- Reichman, J. R. , Watrud, L. S. , Lee, E. H. , Burdick, C. A. , Bollman, M. A. , Storm, M. J. , . . . Mallory-Smith, C. (2006). Establishment of transgenic herbicide-resistant creeping bentgrass (Agrostis stolonifera L.) in nonagronomic habitats. Molecular Ecology , 15 , 4243–4255.
- Roh, J. Y. , Choi, J. Y. , Li, M. S. , Jin, B. R. , & Je, Y. H. (2007). Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. Journal of Microbial Biotechnology , 17 (4), 547–559.
- Roller, S. , & Harlander, S. (1998). Genetic modifications in the food industry: A strategy for food quality improvement . London: Blackie Academic & Professional.
- Rotolo, G. C. , Francis, C. , Craviotto, R. M. , Viglia, S. , Pereyra, A. , & Ulgiati, S. (2015). Time to rethink the GMO revolution in agriculture. Ecological Informatics , 26 , 35–49.
- Sakakibara, K. , & Saiko, K. (2006). Review: Genetically modified plants for the promotion of human health. Biotechnology Letters , 28 , 1983–1991.
- Sauter, C. , Poletti, S. , Zhang, P. , & Gruissem, W. (2006). Biofortification of essential natural compounds and trace elements in rice and cassava. Proceedings of the Nutrition Society , 65 , 153–159.
- Semal, J. (2007). Patentability of living organisms: From biopatent to bio-big-bang. Chairs Agricultures , 16 , 41–48.
- Seralini, G. E. , Mesnage, R. , Defarge, N. , Gress, S. , Hennequin, D. , Clair, E. , . . . de Vendômois, J. S. (2013). Answers to critics: Why there is a long term toxicity due to a Roundup-tolerant genetically modified maize and to a Roundup herbicide. Food Chemistry Toxicology , 53 , 476–483.
- Sexton, S. , & Zilberman, D. (2011). Biotechnology and biofuel. In C. Carter , G. Moschini , & I. Sheldon (Eds.), Genetically modified food and global welfare (pp. 225–242). Bingley, U.K.: Emerald.
- Smale, M. , Zambrano, P. , Gruère, G. , Falck-Zepeda, J. , Matuschke, I. , Horna, D. , . . . Jones, H. (2009). Measuring the economic impacts of transgenic crops in developing agriculture during the first decade . Washington, DC: International Food Policy Research Institute (IFPRI).
- Smyth, S. J. , Gusta, M. , Belcher, K. , Phillips, P. W. B. , & Castle, D. (2011). Environmental impacts from herbicide tolerant canola production in Western Canada. Agricultural Systems , 104 , 403–410.
- Stein, A. J. , Sachdev, H. P. S. , & Qaim, M. (2008). Genetic engineering for the poor: Golden Rice and public health in India. World Development , 36 , 144–158.
- Stewart Jr, C. N. , Halfhill, M. D. , & Warwick, S. I. (2003). Transgene introgression from genetically modified crops to their wild relatives. Nature Reviews Genetics , 4 , 806.
- Subramanian, A. , & Qaim, M. (2010). The impact of Bt cotton on poor households in rural India . Journal of Development Studies , 46 , 295–311..
- Tabashnik, B. E. , Brevault, T. , & Carriere, Y. (2013). Insect resistance in Bt crops: Lessons from the first billion acres. Nature Biotechnology , 31 , 510–521.
- Taylor I. E. P. (2007). Genetically engineered crops: Interim policies, uncertain legislation . New York: Haworth.
- Trigo, E. , Cap, E. , Malach, V. , & Villareal, F. (2009). The case of zero-tillage technology in Argentina . Washington, DC: International Food Policy Research Institute.
- Uchida, E. , Ouchi, T. , Suzuki, Y. , Yoshida, T. , Habe, H. , Yamaguchi, I. , … Nojiri, H. (2005). Secretion of bacterial xenobiotic degrading enzymes from transgenic plants by an apoplastic expressional system: An applicability for phytoremediation. Environmental Science and Technology , 39 , 7671–7677.
- United Nations Department of Economic and Social Affairs, Population Division . (2017). World population prospects: The 2017 revision, key findings and advance tables .
- United States Department of Agriculture . (2009). US Department of Agriculture GAIN report: EU-27 biotechnology: GE plants and animals . Washington, DC, USDA.
- U.S. District Court, Kansas . (2017). Syngenta AG MIR162 Corn Litigation , 14-md-2591. Retrieved from http://www.ksd.uscourts.gov/syngenta-ag-mir162-corn-litigation/
- Van Acker, R. C. , Brule-Babel, A. L. , & Friesen, L. F. (2004). Intraspecific gene movement can create environmental risk: The example of Roundup Ready® wheat in western Canada. In B. Breckling & R. Verhoeven (Eds.), Risk, hazard, damage—specification of criteria to assess environmental impact of genetically modified organisms (pp. 37–47). Bonn, Germany: Naturschutz und Biolische Viefalt.
- Van Acker, R. C. , & Cici, S. Z. H. (2014). Coexistence in the case of a perennial species complex: The potential challenges of coexistence between GM and non-GM Prunus species. AgBioForum , 17 , 70–74.
- Van Acker, R. C. , Cici, S. Z. H. , Michael, L. , Ryan, C. , & Sachs, E. (2015, November 17–20). Gaining societal acceptance of biotechnology: The case for societal engagement. In Seventh International Conference on Coexistence between Genetically Modified (GM) and Non-GM Based Agricultural Supply Chains (GMCC-15) . Amsterdam.
- Van Acker, R. C. , McLean, N. , & Martin, R. C. (2007). Development of quality assurance protocols to prevent GM-contamination of organic crops. In J. Cooper , U. Niggli , & C. Leifert (Eds.), Handbook of organic food safety and quality (pp. 466–489). Boca Raton, FL: CRC.
- Verma, S. R. (2013). Genetically modified plants: Public and scientific perceptions . ISRN Biotechnology , 2013 , 820671.
- Vigani, M. , & Olper, A. (2013). GMO standards, endogenous policy and the market for information. Food Policy , 43 , 32–43.
- Wafula, D. , Waithaka, M. , Komen, J. , & Karembu, M. (2012). Biosafety legislation and biotechnology development gains momentum in Africa . GM Crops Food , 3 (1), 72–77.
- Wesseler, J. , Scatasta, S. , & El Hadji, F. (2011). The environmental benefits and costs of genetically modified (GM) crops. In C. Carter , G. Moschini , & I. Sheldon (Eds.), Genetically modified food and global welfare (pp. 173–199). Bingley, U.K.: Emerald.
- White, P. J. , & Broadley, M. R. (2009). Biofortification of crops with seven mineral elements often lacking in human diets: Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytologist , 182 , 49–84.
- Wise, M. , Calvin, K. , Thomson, A. , Clarke, L. , Bond-Lamberty, B. , Sands, R. , … Edmonds, J. (2009). Implications of limiting CO 2 concentrations for land use and energy. Science , 324 , 1183–1186.
- Wolt, J. D. , Keese, P. , Raybould, A. , Fitzpatrick, J. W , Burachik, M. , Gray, A. , … Wu, F. (2010). Problem formulation in the environmental risk assessment for genetically modified plants. Transgenic Research , 19 , 425–436.
- Wu, F. (2006). Mycotoxin reduction in Bt corn: Potential economic, health, and regulatory impacts. Transgenic Research , 15 , 277–289.
- Yanagisawa, S. (2004). Improved nitrogen assimilation using transcription factors. Plant Research , 2004 , 1–4.
- You, C. B. , Song, W. , Lin, M. , Hai, W. L. , Li, P. , & Wang, Y. T. (2012). Allergens host plant interaction. In C. B. You , Z. I. Chen , & Y. Ding (Eds.), Biotechnology in agriculture: Proceedings of the First Asia-Pacific Conference on Agricultural Biotechnology, Beijing, China, 20–24 August 1992 (pp. 468–473). Dordrecht, The Netherlands: Kluwer.
- Yuan, D. , Bassie, L. , Sabalza, M. , Miralpeix, B. , Dashevskaya, S. , Farre, G. , … Christou, P. (2011). The potential impact of plant biotechnology on the millennium development goals . Plant Cell Reports , 30 , 249–265.
- Zhu, C. , Naqvi, S. , Breitenbach, J. , Sandmann, G. , Christou, P. , & Capell, T. (2008). Combinatorial genetic transformation generates a library of metabolic phenotypes for the carotenoid pathway in corn . Proceedings National Academy of Sciences USA , 105 , 18232–18237.
Printed from Oxford Research Encyclopedias, Environmental Science. Under the terms of the licence agreement, an individual user may print out a single article for personal use (for details see Privacy Policy and Legal Notice).
date: 18 August 2024
- Cookie Policy
- Privacy Policy
- Legal Notice
- Accessibility
- [81.177.182.154]
- 81.177.182.154
Character limit 500 /500
Are Genetically Modified Crops the Answer to World Hunger?
Hunger is a major world crisis for which a solution has not yet been found. Since their advent, genetically modified crops have been hailed as the key to solving world hunger.
Biology, Health, Conservation, Social Studies, Economics
Tearless GM Onion
GM crops may be modified to improve yield, enhance nutrition, or better adapt to environmental conditions. They can even be altered to resist pests or eliminate unwanted effects, like this type of onion that doesn't cause people to tear up when chopped.
Photograph by Redux Pictures LLC

Hunger is one of the greatest global challenges of the 21st century. Despite some improvements within the last two decades, global hunger is again on the rise, with 2016 data indicating that more than 800 million people around the world suffer from malnutrition . Children under five years of age represent 150 million of those affected, and for roughly three million of these children every year, the struggle ends in death. When faced with such staggering statistics, it is natural to wish for one simple solution to prevent these deaths and rid the world of hunger . Use of genetically modified (GM) crops is among the proposed solutions—but is it truly a viable solution? GM crops are plants that have been modified, using genetic engineering, to alter their DNA sequences to provide some beneficial trait. For example, genetic engineering can improve crop yield , resulting in greater production of the target crop. Scientists can also engineer pest-resistant crops, helping local farmers better withstand environmental challenges that might otherwise wipe out a whole season of produce . Crops can even be engineered to be more nutritious, providing critical vitamins to populations that struggle to get specific nutrients needed for healthy living. However, GM seeds are produced primarily by only a few large companies who own the intellectual property for the genetic variations. A transition to GM crops would closely align global food production with the activities of a few key companies. From an economic standpoint, that poses a risk to long-term food security by creating the potential for a single-point failure. If that company failed, then the crop it provides would not be available to the people who depend on that crop. Moreover, a large proportion of those affected by malnutrition are small farmers in sub-Saharan Africa, where use of GM crops is less common. Since attitudes toward GM crops tend to correlate with education levels and access to information about the technology, there is a concern that sub-Saharan African farmers may be hesitant to adopt GM crops. More generally, public perception of GM foods is plagued by concerns of safety, from the potential for allergic response to the possible transfer of foreign DNA to non-GM plants in the area. None of these concerns are backed by evidence, but they persist nonetheless. Whether based on legitimate concerns or lack of scientific information and understanding, local rejection of GM crops has the potential to derail efforts to use these crops as a tool against malnutrition . However, there are case stories for success: Adoption of GM cotton in India has improved family income and, as a result, reduced hunger . While there are these controversies and complexities that pose challenges for the use of GM foods, these are secondary to a larger issue. We already live in a world that produces enough food to feed everyone. Thus, hunger results from inequity, not food shortage. Unequal distribution of quality food among communities suffering from poverty is the primary culprit in today’s world hunger , not abundance or quantity of food stocks. For those suffering from malnutrition , access to quality food depends on a variety of political, environmental, and socioeconomic factors—most notably, armed conflict and natural disasters . When viewed through this lens, GM crops may have a role to play in combatting global hunger , but merely increasing crop production or nutritional value (via any method) will not solve the larger problem of inequity in access to food. For example, farmers whose livelihoods depend on production of commercial crops rather than food staples may be able to increase their income by growing GM crops, affording them the financial resources to purchase more or higher-quality food. Moreover, GM crops might better withstand certain natural disasters , such as drought. However, since data shows that political unrest is the primary driver of hunger , it is unclear whether these farmers would be able to sell their products or use their income on nutritional food sources within a country plagued by conflict. Unfortunately, GM foods are not the cure-all to hunger the world needs. The path to eradicating global hunger is more complex than any one solution and is in fact far more complex than only addressing food quantity or quality. The United Nations Global Goals for Sustainable Development address world hunger in Goal 2: Zero Hunger , which aims to “end hunger , achieve food security and improved nutrition and promote sustainable agriculture.” This goal lays the foundation to combatting world hunger via a multipronged approach, including political action and reduction of violence, agricultural and technical innovations, efforts to end poverty , and educational initiatives. Luckily, with allies such as the United Nations Children’s Fund (UNICEF) and the World Food Programme, this grand challenge may be achievable—and maybe GM foods will play a role, but they cannot be relied upon as a magical solution.
Articles & Profiles
Media credits.
The audio, illustrations, photos, and videos are credited beneath the media asset, except for promotional images, which generally link to another page that contains the media credit. The Rights Holder for media is the person or group credited.
Production Managers
Program specialists, last updated.
January 3, 2024
User Permissions
For information on user permissions, please read our Terms of Service. If you have questions about how to cite anything on our website in your project or classroom presentation, please contact your teacher. They will best know the preferred format. When you reach out to them, you will need the page title, URL, and the date you accessed the resource.
If a media asset is downloadable, a download button appears in the corner of the media viewer. If no button appears, you cannot download or save the media.
Text on this page is printable and can be used according to our Terms of Service .
Interactives
Any interactives on this page can only be played while you are visiting our website. You cannot download interactives.
Related Resources
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Genetically modified crops: current status and future prospects
Affiliations.
- 1 ICAR-Indian Institute of Maize Research, Pusa Campus, New Delhi, 110012, India. [email protected].
- 2 ICAR-Indian Institute of Maize Research, Pusa Campus, New Delhi, 110012, India.
- 3 National Institute for Research in Environmental Health, Bhopal, 462001, India.
- 4 ICAR-Indian Institute of Maize Research, PAU Campus, Ludhiana, 141004, India.
- PMID: 32236850
- DOI: 10.1007/s00425-020-03372-8
While transgenic technology has heralded a new era in crop improvement, several concerns have precluded their widespread acceptance. Alternative technologies, such as cisgenesis and genome-editing may address many of such issues and facilitate the development of genetically engineered crop varieties with multiple favourable traits. Genetic engineering and plant transformation have played a pivotal role in crop improvement via introducing beneficial foreign gene(s) or silencing the expression of endogenous gene(s) in crop plants. Genetically modified crops possess one or more useful traits, such as, herbicide tolerance, insect resistance, abiotic stress tolerance, disease resistance, and nutritional improvement. To date, nearly 525 different transgenic events in 32 crops have been approved for cultivation in different parts of the world. The adoption of transgenic technology has been shown to increase crop yields, reduce pesticide and insecticide use, reduce CO 2 emissions, and decrease the cost of crop production. However, widespread adoption of transgenic crops carrying foreign genes faces roadblocks due to concerns of potential toxicity and allergenicity to human beings, potential environmental risks, such as chances of gene flow, adverse effects on non-target organisms, evolution of resistance in weeds and insects etc. These concerns have prompted the adoption of alternative technologies like cisgenesis, intragenesis, and most recently, genome editing. Some of these alternative technologies can be utilized to develop crop plants that are free from any foreign gene hence, it is expected that such crops might achieve higher consumer acceptance as compared to the transgenic crops and would get faster regulatory approvals. In this review, we present a comprehensive update on the current status of the genetically modified (GM) crops under cultivation. We also discuss the issues affecting widespread adoption of transgenic GM crops and comment upon the recent tools and techniques developed to address some of these concerns.
Keywords: Cisgenesis; GM crops; Genome editing; Intragenesis; Public concerns; Transgenics.
PubMed Disclaimer
Similar articles
- Genetically modified (GM) crops: milestones and new advances in crop improvement. Kamthan A, Chaudhuri A, Kamthan M, Datta A. Kamthan A, et al. Theor Appl Genet. 2016 Sep;129(9):1639-55. doi: 10.1007/s00122-016-2747-6. Epub 2016 Jul 5. Theor Appl Genet. 2016. PMID: 27381849 Review.
- Intragenesis and cisgenesis as alternatives to transgenic crop development. Holme IB, Wendt T, Holm PB. Holme IB, et al. Plant Biotechnol J. 2013 May;11(4):395-407. doi: 10.1111/pbi.12055. Epub 2013 Feb 20. Plant Biotechnol J. 2013. PMID: 23421562 Review.
- Cisgenesis and intragenesis: new tools for improving crops. Espinoza C, Schlechter R, Herrera D, Torres E, Serrano A, Medina C, Arce-Johnson P. Espinoza C, et al. Biol Res. 2013;46(4):323-31. doi: 10.4067/S0716-97602013000400003. Biol Res. 2013. PMID: 24510134 Review.
- Genome editing for crop improvement: Challenges and opportunities. Abdallah NA, Prakash CS, McHughen AG. Abdallah NA, et al. GM Crops Food. 2015;6(4):183-205. doi: 10.1080/21645698.2015.1129937. GM Crops Food. 2015. PMID: 26930114 Free PMC article.
- Dealing with transgene flow of crop protection traits from crops to their relatives. Gressel J. Gressel J. Pest Manag Sci. 2015 May;71(5):658-67. doi: 10.1002/ps.3850. Epub 2014 Aug 15. Pest Manag Sci. 2015. PMID: 24977384 Review.
- Revitalising Riboflavin: Unveiling Its Timeless Significance in Human Physiology and Health. Aragão MÂ, Pires L, Santos-Buelga C, Barros L, Calhelha RC. Aragão MÂ, et al. Foods. 2024 Jul 17;13(14):2255. doi: 10.3390/foods13142255. Foods. 2024. PMID: 39063339 Free PMC article. Review.
- Robust soybean leaf agroinfiltration. Trull BN, Sultana MS, Pfotenhauer AC, Stockdale JN, Pantalone V, Zhang B, Stewart CN Jr. Trull BN, et al. Plant Cell Rep. 2024 Jun 5;43(6):162. doi: 10.1007/s00299-024-03245-4. Plant Cell Rep. 2024. PMID: 38837057
- Alternative protein sources: science powered startups to fuel food innovation. Lurie-Luke E. Lurie-Luke E. Nat Commun. 2024 May 28;15(1):4425. doi: 10.1038/s41467-024-47091-0. Nat Commun. 2024. PMID: 38806477 Free PMC article. Review.
- Viral Threats to Fruit and Vegetable Crops in the Caribbean. Tennant P, Rampersad S, Alleyne A, Johnson L, Tai D, Amarakoon I, Roye M, Pitter P, Chang PG, Myers Morgan L. Tennant P, et al. Viruses. 2024 Apr 13;16(4):603. doi: 10.3390/v16040603. Viruses. 2024. PMID: 38675944 Free PMC article. Review.
- GMOIT: a tool for effective screening of genetically modified crops. Zhou P, Liu X, Liang J, Zhao J, Zhang Y, Xu D, Li X, Chen Z, Shi Z, Gao J. Zhou P, et al. BMC Plant Biol. 2024 Apr 25;24(1):329. doi: 10.1186/s12870-024-05035-2. BMC Plant Biol. 2024. PMID: 38664610 Free PMC article.
- Science. 1983 Nov 4;222(4623):476-82 - PubMed
- Theor Appl Genet. 2004 Jan;108(2):335-42 - PubMed
- Biotechnology (N Y). 1996 Feb;14(2):171-6 - PubMed
- Amino Acids. 2001;20(3):261-79 - PubMed
- Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):12034-9 - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources, other literature sources.
- The Lens - Patent Citations
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
September 1, 2013
13 min read
The Truth about Genetically Modified Food
Proponents of genetically modified crops say the technology is the only way to feed a warming, increasingly populous world. Critics say we tamper with nature at our peril. Who is right?
By David H. Freedman
Robert Goldberg sags into his desk chair and gestures at the air. “Frankenstein monsters, things crawling out of the lab,” he says. “This the most depressing thing I've ever dealt with.”
Goldberg, a plant molecular biologist at the University of California, Los Angeles, is not battling psychosis. He is expressing despair at the relentless need to confront what he sees as bogus fears over the health risks of genetically modified (GM) crops. Particularly frustrating to him, he says, is that this debate should have ended decades ago, when researchers produced a stream of exonerating evidence: “Today we're facing the same objections we faced 40 years ago.”
Across campus, David Williams, a cellular biologist who specializes in vision, has the opposite complaint. “A lot of naive science has been involved in pushing this technology,” he says. “Thirty years ago we didn't know that when you throw any gene into a different genome, the genome reacts to it. But now anyone in this field knows the genome is not a static environment. Inserted genes can be transformed by several different means, and it can happen generations later.” The result, he insists, could very well be potentially toxic plants slipping through testing.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Williams concedes that he is among a tiny minority of biologists raising sharp questions about the safety of GM crops. But he says this is only because the field of plant molecular biology is protecting its interests. Funding, much of it from the companies that sell GM seeds, heavily favors researchers who are exploring ways to further the use of genetic modification in agriculture. He says that biologists who point out health or other risks associated with GM crops—who merely report or defend experimental findings that imply there may be risks—find themselves the focus of vicious attacks on their credibility, which leads scientists who see problems with GM foods to keep quiet.
Whether Williams is right or wrong, one thing is undeniable: despite overwhelming evidence that GM crops are safe to eat, the debate over their use continues to rage, and in some parts of the world, it is growing ever louder. Skeptics would argue that this contentiousness is a good thing—that we cannot be too cautious when tinkering with the genetic basis of the world's food supply. To researchers such as Goldberg, however, the persistence of fears about GM foods is nothing short of exasperating. “In spite of hundreds of millions of genetic experiments involving every type of organism on earth,” he says, “and people eating billions of meals without a problem, we've gone back to being ignorant.”
So who is right: advocates of GM or critics? When we look carefully at the evidence for both sides and weigh the risks and benefits, we find a surprisingly clear path out of this dilemma.
Benefits and worries
The bulk of the science on GM safety points in one direction. Take it from David Zilberman, a U.C. Berkeley agricultural and environmental economist and one of the few researchers considered credible by both agricultural chemical companies and their critics. He argues that the benefits of GM crops greatly outweigh the health risks, which so far remain theoretical. The use of GM crops “has lowered the price of food,” Zilberman says. “It has increased farmer safety by allowing them to use less pesticide. It has raised the output of corn, cotton and soy by 20 to 30 percent, allowing some people to survive who would not have without it. If it were more widely adopted around the world, the price [of food] would go lower, and fewer people would die of hunger.”
In the future, Zilberman says, those advantages will become all the more significant. The United Nations Food and Agriculture Organization estimates that the world will have to grow 70 percent more food by 2050 just to keep up with population growth. Climate change will make much of the world's arable land more difficult to farm. GM crops, Zilberman says, could produce higher yields, grow in dry and salty land, withstand high and low temperatures, and tolerate insects, disease and herbicides.
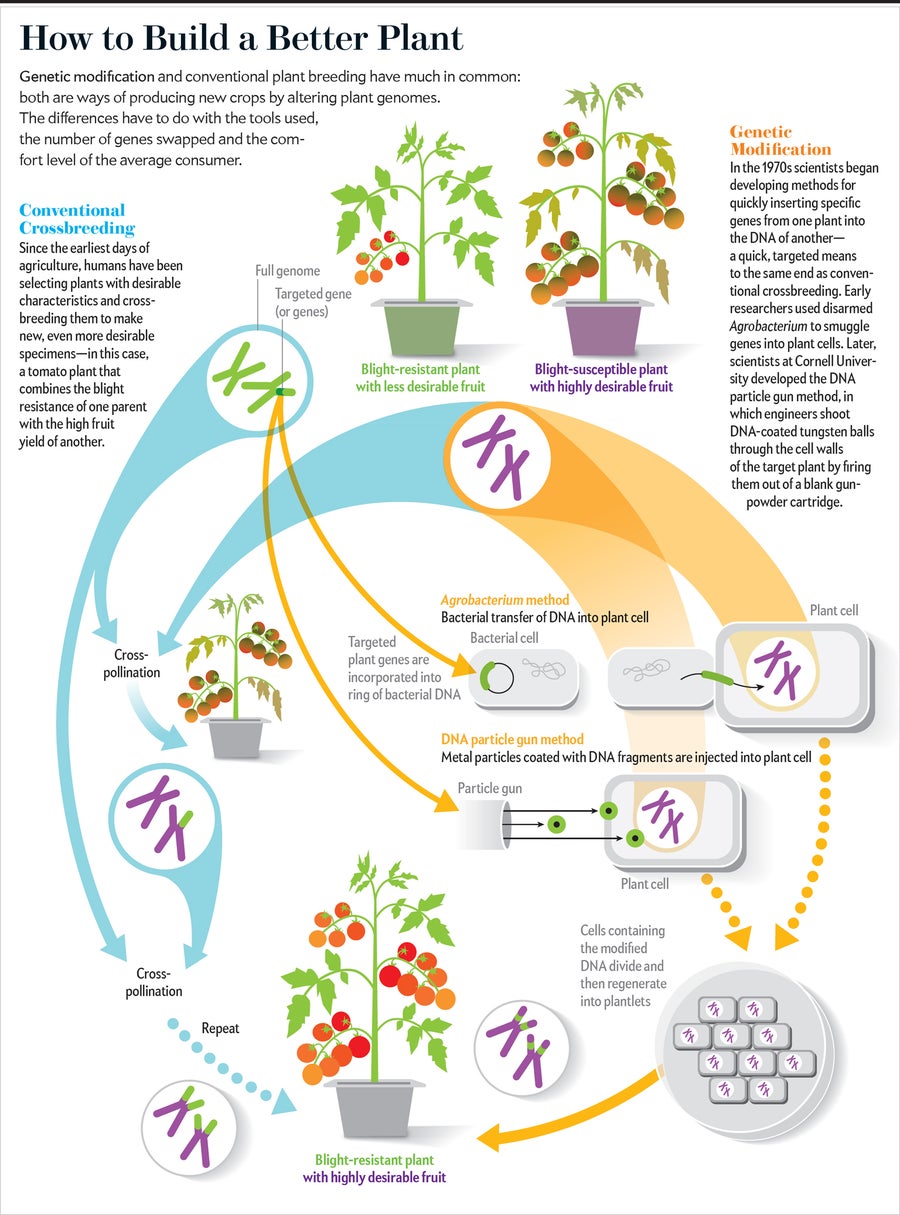
Credit: Jen Christiansen
Despite such promise, much of the world has been busy banning, restricting and otherwise shunning GM foods. Nearly all the corn and soybeans grown in the U.S. are genetically modified, but only two GM crops, Monsanto's MON810 maize and BASF's Amflora potato, are accepted in the European Union. Ten E.U. nations have banned MON810, and although BASF withdrew Amflora from the market in 2012, four E.U. nations have taken the trouble to ban that, too. Approval of a few new GM corn strains has been proposed there, but so far it has been repeatedly and soundly voted down. Throughout Asia, including in India and China, governments have yet to approve most GM crops, including an insect-resistant rice that produces higher yields with less pesticide. In Africa, where millions go hungry, several nations have refused to import GM foods in spite of their lower costs (the result of higher yields and a reduced need for water and pesticides). Kenya has banned them altogether amid widespread malnutrition. No country has definite plans to grow Golden Rice, a crop engineered to deliver more vitamin A than spinach (rice normally has no vitamin A), even though vitamin A deficiency causes more than one million deaths annually and half a million cases of irreversible blindness in the developing world.
Globally, only a tenth of the world's cropland includes GM plants. Four countries—the U.S., Canada, Brazil and Argentina—grow 90 percent of the planet's GM crops. Other Latin American countries are pushing away from the plants. And even in the U.S., voices decrying genetically modified foods are becoming louder. In 2016 the U.S. federal government passed a law requiring labeling of GM ingredients in food products, replacing GM-labeling laws in force or proposed in several dozen states.
The fear fueling all this activity has a long history. The public has been worried about the safety of GM foods since scientists at the University of Washington developed the first genetically modified tobacco plants in the 1970s. In the mid-1990s, when the first GM crops reached the market, Greenpeace, the Sierra Club, Ralph Nader, Prince Charles and a number of celebrity chefs took highly visible stands against them. Consumers in Europe became particularly alarmed: a survey conducted in 1997, for example, found that 69 percent of the Austrian public saw serious risks in GM foods, compared with only 14 percent of Americans.
In Europe, skepticism about GM foods has long been bundled with other concerns, such as a resentment of American agribusiness. Whatever it is based on, however, the European attitude reverberates across the world, influencing policy in countries where GM crops could have tremendous benefits. “In Africa, they don't care what us savages in America are doing,” Zilberman says. “They look to Europe and see countries there rejecting GM, so they don't use it.” Forces fighting genetic modification in Europe have rallied support for “the precautionary principle,” which holds that given the kind of catastrophe that would emerge from loosing a toxic, invasive GM crop on the world, GM efforts should be shut down until the technology is proved absolutely safe.
But as medical researchers know, nothing can really be “proved safe.” One can only fail to turn up significant risk after trying hard to find it—as is the case with GM crops.
A clean record
The human race has been selectively breeding crops, thus altering plants' genomes, for millennia. Ordinary wheat has long been strictly a human-engineered plant; it could not exist outside of farms, because its seeds do not scatter. For some 60 years scientists have been using “mutagenic” techniques to scramble the DNA of plants with radiation and chemicals, creating strains of wheat, rice, peanuts and pears that have become agricultural mainstays. The practice has inspired little objection from scientists or the public and has caused no known health problems.
The difference is that selective breeding or mutagenic techniques tend to result in large swaths of genes being swapped or altered. GM technology, in contrast, enables scientists to insert into a plant's genome a single gene (or a few of them) from another species of plant or even from a bacterium, virus or animal. Supporters argue that this precision makes the technology much less likely to produce surprises. Most plant molecular biologists also say that in the highly unlikely case that an unexpected health threat emerged from a new GM plant, scientists would quickly identify and eliminate it. “We know where the gene goes and can measure the activity of every single gene around it,” Goldberg says. “We can show exactly which changes occur and which don't.”
And although it might seem creepy to add virus DNA to a plant, doing so is, in fact, no big deal, proponents say. Viruses have been inserting their DNA into the genomes of crops, as well as humans and all other organisms, for millions of years. They often deliver the genes of other species while they are at it, which is why our own genome is loaded with genetic sequences that originated in viruses and nonhuman species. “When GM critics say that genes don't cross the species barrier in nature, that's just simple ignorance,” says Alan McHughen, a plant molecular geneticist at U.C. Riverside. Pea aphids contain fungi genes. Triticale is a century-plus-old hybrid of wheat and rye found in some flours and breakfast cereals. Wheat itself, for that matter, is a cross-species hybrid. “Mother Nature does it all the time, and so do conventional plant breeders,” McHughen says.
Could eating plants with altered genes allow new DNA to work its way into our own? It is possible but hugely improbable. Scientists have never found genetic material that could survive a trip through the human gut and make it into cells. Besides, we are routinely exposed to—and even consume—the viruses and bacteria whose genes end up in GM foods. The bacterium Bacillus thuringiensis , for example, which produces proteins fatal to insects, is sometimes enlisted as a natural pesticide in organic farming. “We've been eating this stuff for thousands of years,” Goldberg says.
In any case, proponents say, people have consumed as many as trillions of meals containing genetically modified ingredients over the past few decades. Not a single verified case of illness has ever been attributed to the genetic alterations. Mark Lynas, a prominent anti-GM activist who in 2013 publicly switched to strongly supporting the technology, has pointed out that every single news-making food disaster on record has been attributed to non-GM crops, such as the Escherichia coli –infected organic bean sprouts that killed 53 people in Europe in 2011.
Critics often disparage U.S. research on the safety of genetically modified foods, which is often funded or even conducted by GM companies, such as Monsanto. But much research on the subject comes from the European Commission, the administrative body of the E.U., which cannot be so easily dismissed as an industry tool. The European Commission has funded 130 research projects, carried out by more than 500 independent teams, on the safety of GM crops. None of those studies found any special risks from GM crops.
Plenty of other credible groups have arrived at the same conclusion. Gregory Jaffe, director of biotechnology at the Center for Science in the Public Interest, a science-based consumer-watchdog group in Washington, D.C., takes pains to note that the center has no official stance, pro or con, with regard to genetically modifying food plants. Yet Jaffe insists the scientific record is clear. “Current GM crops are safe to eat and can be grown safely in the environment,” he says. The American Association for the Advancement of Science, the American Medical Association and the National Academy of Sciences have all unreservedly backed GM crops. The U.S. Food and Drug Administration, along with its counterparts in several other countries, has repeatedly reviewed large bodies of research and concluded that GM crops pose no unique health threats. Dozens of review studies carried out by academic researchers have backed that view.
Opponents of genetically modified foods point to a handful of studies indicating possible safety problems. But reviewers have dismantled almost all of those reports. For example, a 1998 study by plant biochemist Árpád Pusztai, then at the Rowett Institute in Scotland, found that rats fed a GM potato suffered from stunted growth and immune system–related changes. But the potato was not intended for human consumption—it was, in fact, designed to be toxic for research purposes. The Rowett Institute later deemed the experiment so sloppy that it refuted the findings and charged Pusztai with misconduct.
Similar stories abound. Most recently, a team led by Gilles-Éric Séralini, a researcher at the University of Caen Lower Normandy in France, found that rats eating a common type of GM corn contracted cancer at an alarmingly high rate. But Séralini has long been an anti-GM campaigner, and critics charged that in his study, he relied on a strain of rat that too easily develops tumors, did not use enough rats, did not include proper control groups and failed to report many details of the experiment, including how the analysis was performed. After a review, the European Food Safety Authority dismissed the study's findings. Several other European agencies came to the same conclusion. “If GM corn were that toxic, someone would have noticed by now,” McHughen says. “Séralini has been refuted by everyone who has cared to comment.”
Some scientists say the objections to GM food stem from politics rather than science—that they are motivated by an objection to large multinational corporations having enormous influence over the food supply; invoking risks from genetic modification just provides a convenient way of whipping up the masses against industrial agriculture. “This has nothing to do with science,” Goldberg says. “It's about ideology.” Former anti-GM activist Lynas agrees. He has gone as far as labeling the anti-GM crowd “explicitly an antiscience movement.”
Persistent doubts
Not all objections to genetically modified foods are so easily dismissed, however. Long-term health effects can be subtle and nearly impossible to link to specific changes in the environment. Scientists have long believed that Alzheimer's disease and many cancers have environmental components, but few would argue we have identified all of them.
And opponents say that it is not true that the GM process is less likely to cause problems simply because fewer, more clearly identified genes are replaced. David Schubert, an Alzheimer's researcher who heads the Cellular Neurobiology Laboratory at the Salk Institute for Biological Studies in La Jolla, Calif., asserts that a single, well-characterized gene can still settle in the target plant's genome in many different ways. “It can go in forward, backward, at different locations, in multiple copies, and they all do different things,” he says. And as U.C.L.A.'s Williams notes, a genome often continues to change in the successive generations after the insertion, leaving it with a different arrangement than the one intended and initially tested. There is also the phenomenon of “insertional mutagenesis,” Williams adds, in which the insertion of a gene ends up quieting the activity of nearby genes.
True, the number of genes affected in a GM plant most likely will be far, far smaller than in conventional breeding techniques. Yet opponents maintain that because the wholesale swapping or alteration of entire packages of genes is a natural process that has been happening in plants for half a billion years, it tends to produce few scary surprises today. Changing a single gene, on the other hand, might turn out to be a more subversive action, with unexpected ripple effects, including the production of new proteins that might be toxins or allergens.
Opponents also point out that the kinds of alterations caused by the insertion of genes from other species might be more impactful, more complex or more subtle than those caused by the intraspecies gene swapping of conventional breeding. And just because there is no evidence to date that genetic material from an altered crop can make it into the genome of people who eat it does not mean such a transfer will never happen—or that it has not already happened and we have yet to spot it. These changes might be difficult to catch; their impact on the production of proteins might not even turn up in testing. “You'd certainly find out if the result is that the plant doesn't grow very well,” Williams says. “But will you find the change if it results in the production of proteins with long-term effects on the health of the people eating it?”
It is also true that many pro-GM scientists in the field are unduly harsh—even unscientific—in their treatment of critics. GM proponents sometimes lump every scientist who raises safety questions together with activists and discredited researchers. And even Séralini, the scientist behind the study that found high cancer rates for GM-fed rats, has his defenders. Most of them are nonscientists, or retired researchers from obscure institutions, or nonbiologist scientists, but the Salk Institute's Schubert also insists the study was unfairly dismissed. He says that as someone who runs drug-safety studies, he is well versed on what constitutes a good-quality animal toxicology study and that Séralini's makes the grade. He insists that the breed of rat in the study is commonly used in respected drug studies, typically in numbers no greater than in Séralini's study; that the methodology was standard; and that the details of the data analysis are irrelevant because the results were so striking.
Schubert joins Williams as one of a handful of biologists from respected institutions who are willing to sharply challenge the GM-foods-are-safe majority. Both charge that more scientists would speak up against genetic modification if doing so did not invariably lead to being excoriated in journals and the media. These attacks, they argue, are motivated by the fear that airing doubts could lead to less funding for the field. Says Williams: “Whether it's conscious or not, it's in their interest to promote this field, and they're not objective.”
Both scientists say that after publishing comments in respected journals questioning the safety of GM foods, they became the victims of coordinated attacks on their reputations. Schubert even charges that researchers who turn up results that might raise safety questions avoid publishing their findings out of fear of repercussions. “If it doesn't come out the right way,” he says, “you're going to get trashed.”
There is evidence to support that charge. In 2009 Nature detailed the backlash to a reasonably solid study published in the Proceedings of the National Academy of Sciences USA by researchers from Loyola University Chicago and the University of Notre Dame. The paper showed that GM corn seemed to be finding its way from farms into nearby streams and that it might pose a risk to some insects there because, according to the researchers' lab studies, caddis flies appeared to suffer on diets of pollen from GM corn. Many scientists immediately attacked the study, some of them suggesting the researchers were sloppy to the point of misconduct.
A way forward
There is a middle ground in this debate. Many moderate voices call for continuing the distribution of GM foods while maintaining or even stepping up safety testing on new GM crops. They advocate keeping a close eye on the health and environmental impact of existing ones. But they do not single out GM crops for special scrutiny, the Center for Science in the Public Interest's Jaffe notes: all crops could use more testing. “We should be doing a better job with food oversight altogether,” he says.
Even Schubert agrees. In spite of his concerns, he believes future GM crops can be introduced safely if testing is improved. “Ninety percent of the scientists I talk to assume that new GM plants are safety-tested the same way new drugs are by the FDA,” he says. “They absolutely aren't, and they absolutely should be.”
Stepped-up testing would pose a burden for GM researchers, and it could slow down the introduction of new crops. “Even under the current testing standards for GM crops, most conventionally bred crops wouldn't have made it to market,” McHughen says. “What's going to happen if we become even more strict?”
That is a fair question. But with governments and consumers increasingly coming down against GM crops altogether, additional testing may be the compromise that enables the human race to benefit from those crops' significant advantages.
David H. Freedman is a journalist who has been covering science, business and technology for more than 30 years.

Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Questions and answers /
Food, genetically modified
These questions and answers have been prepared by WHO in response to questions and concerns from WHO Member State Governments with regard to the nature and safety of genetically modified food.
Genetically modified organisms (GMOs) can be defined as organisms (i.e. plants, animals or microorganisms) in which the genetic material (DNA) has been altered in a way that does not occur naturally by mating and/or natural recombination. The technology is often called “modern biotechnology” or “gene technology”, sometimes also “recombinant DNA technology” or “genetic engineering”. It allows selected individual genes to be transferred from one organism into another, also between nonrelated species. Foods produced from or using GM organisms are often referred to as GM foods.
GM foods are developed – and marketed – because there is some perceived advantage either to the producer or consumer of these foods. This is meant to translate into a product with a lower price, greater benefit (in terms of durability or nutritional value) or both. Initially GM seed developers wanted their products to be accepted by producers and have concentrated on innovations that bring direct benefit to farmers (and the food industry generally).
One of the objectives for developing plants based on GM organisms is to improve crop protection. The GM crops currently on the market are mainly aimed at an increased level of crop protection through the introduction of resistance against plant diseases caused by insects or viruses or through increased tolerance towards herbicides.
Resistance against insects is achieved by incorporating into the food plant the gene for toxin production from the bacterium Bacillus thuringiensis (Bt). This toxin is currently used as a conventional insecticide in agriculture and is safe for human consumption. GM crops that inherently produce this toxin have been shown to require lower quantities of insecticides in specific situations, e.g. where pest pressure is high. Virus resistance is achieved through the introduction of a gene from certain viruses which cause disease in plants. Virus resistance makes plants less susceptible to diseases caused by such viruses, resulting in higher crop yields.
Herbicide tolerance is achieved through the introduction of a gene from a bacterium conveying resistance to some herbicides. In situations where weed pressure is high, the use of such crops has resulted in a reduction in the quantity of the herbicides used.
Generally consumers consider that conventional foods (that have an established record of safe consumption over the history) are safe. Whenever novel varieties of organisms for food use are developed using the traditional breeding methods that had existed before the introduction of gene technology, some of the characteristics of organisms may be altered, either in a positive or a negative way. National food authorities may be called upon to examine the safety of such conventional foods obtained from novel varieties of organisms, but this is not always the case.
In contrast, most national authorities consider that specific assessments are necessary for GM foods. Specific systems have been set up for the rigorous evaluation of GM organisms and GM foods relative to both human health and the environment. Similar evaluations are generally not performed for conventional foods. Hence there currently exists a significant difference in the evaluation process prior to marketing for these two groups of food.
The WHO Department of Food Safety and Zoonoses aims at assisting national authorities in the identification of foods that should be subject to risk assessment and to recommend appropriate approaches to safety assessment. Should national authorities decide to conduct safety assessment of GM organisms, WHO recommends the use of Codex Alimentarius guidelines (See the answer to Question 11 below).
The safety assessment of GM foods generally focuses on: (a) direct health effects (toxicity), (b) potential to provoke allergic reaction (allergenicity); (c) specific components thought to have nutritional or toxic properties; (d) the stability of the inserted gene; (e) nutritional effects associated with genetic modification; and (f) any unintended effects which could result from the gene insertion.
While theoretical discussions have covered a broad range of aspects, the three main issues debated are the potentials to provoke allergic reaction (allergenicity), gene transfer and outcrossing.
Allergenicity
As a matter of principle, the transfer of genes from commonly allergenic organisms to non-allergic organisms is discouraged unless it can be demonstrated that the protein product of the transferred gene is not allergenic. While foods developed using traditional breeding methods are not generally tested for allergenicity, protocols for the testing of GM foods have been evaluated by the Food and Agriculture Organization of the United Nations (FAO) and WHO. No allergic effects have been found relative to GM foods currently on the market.
Gene transfer
Gene transfer from GM foods to cells of the body or to bacteria in the gastrointestinal tract would cause concern if the transferred genetic material adversely affects human health. This would be particularly relevant if antibiotic resistance genes, used as markers when creating GMOs, were to be transferred. Although the probability of transfer is low, the use of gene transfer technology that does not involve antibiotic resistance genes is encouraged.
Outcrossing
The migration of genes from GM plants into conventional crops or related species in the wild (referred to as “outcrossing”), as well as the mixing of crops derived from conventional seeds with GM crops, may have an indirect effect on food safety and food security. Cases have been reported where GM crops approved for animal feed or industrial use were detected at low levels in the products intended for human consumption. Several countries have adopted strategies to reduce mixing, including a clear separation of the fields within which GM crops and conventional crops are grown.
Environmental risk assessments cover both the GMO concerned and the potential receiving environment. The assessment process includes evaluation of the characteristics of the GMO and its effect and stability in the environment, combined with ecological characteristics of the environment in which the introduction will take place. The assessment also includes unintended effects which could result from the insertion of the new gene.
Issues of concern include: the capability of the GMO to escape and potentially introduce the engineered genes into wild populations; the persistence of the gene after the GMO has been harvested; the susceptibility of non-target organisms (e.g. insects which are not pests) to the gene product; the stability of the gene; the reduction in the spectrum of other plants including loss of biodiversity; and increased use of chemicals in agriculture. The environmental safety aspects of GM crops vary considerably according to local conditions.
Different GM organisms include different genes inserted in different ways. This means that individual GM foods and their safety should be assessed on a case-by-case basis and that it is not possible to make general statements on the safety of all GM foods.
GM foods currently available on the international market have passed safety assessments and are not likely to present risks for human health. In addition, no effects on human health have been shown as a result of the consumption of such foods by the general population in the countries where they have been approved. Continuous application of safety assessments based on the Codex Alimentarius principles and, where appropriate, adequate post market monitoring, should form the basis for ensuring the safety of GM foods.
The way governments have regulated GM foods varies. In some countries GM foods are not yet regulated. Countries which have legislation in place focus primarily on assessment of risks for consumer health. Countries which have regulatory provisions for GM foods usually also regulate GMOs in general, taking into account health and environmental risks, as well as control- and trade-related issues (such as potential testing and labelling regimes). In view of the dynamics of the debate on GM foods, legislation is likely to continue to evolve.
GM crops available on the international market today have been designed using one of three basic traits: resistance to insect damage; resistance to viral infections; and tolerance towards certain herbicides. GM crops with higher nutrient content (e.g. soybeans increased oleic acid) have been also studied recently.
The Codex Alimentarius Commission (Codex) is the joint FAO/WHO intergovernmental body responsible for developing the standards, codes of practice, guidelines and recommendations that constitute the Codex Alimentarius, meaning the international food code. Codex developed principles for the human health risk analysis of GM foods in 2003.
Principles for the risk analysis of foods derived from modern biotechnology
The premise of these principles sets out a premarket assessment, performed on a caseby- case basis and including an evaluation of both direct effects (from the inserted gene) and unintended effects (that may arise as a consequence of insertion of the new gene) Codex also developed three Guidelines:
Guideline for the conduct of food safety assessment of foods derived from recombinant-DNA plants
Guideline for the conduct of food safety assessment of foods produced using recombinant-DNA microorganisms
Guideline for the conduct of food safety assessment of foods derived from recombinant-DNA animals
Codex principles do not have a binding effect on national legislation, but are referred to specifically in the Agreement on the Application of Sanitary and Phytosanitary Measures of the World Trade Organization (SPS Agreement), and WTO Members are encouraged to harmonize national standards with Codex standards. If trading partners have the same or similar mechanisms for the safety assessment of GM foods, the possibility that one product is approved in one country but rejected in another becomes smaller.
The Cartagena Protocol on Biosafety, an environmental treaty legally binding for its Parties which took effect in 2003, regulates transboundary movements of Living Modified Organisms (LMOs). GM foods are within the scope of the Protocol only if they contain LMOs that are capable of transferring or replicating genetic material. The cornerstone of the Protocol is a requirement that exporters seek consent from importers before the first shipment of LMOs intended for release into the environment.
The GM products that are currently on the international market have all passed safety assessments conducted by national authorities. These different assessments in general follow the same basic principles, including an assessment of environmental and human health risk. The food safety assessment is usually based on Codex documents.
Since the first introduction on the market in the mid-1990s of a major GM food (herbicide-resistant soybeans), there has been concern about such food among politicians, activists and consumers, especially in Europe. Several factors are involved. In the late 1980s – early 1990s, the results of decades of molecular research reached the public domain. Until that time, consumers were generally not very aware of the potential of this research. In the case of food, consumers started to wonder about safety because they perceive that modern biotechnology is leading to the creation of new species.
Consumers frequently ask, “what is in it for me?”. Where medicines are concerned, many consumers more readily accept biotechnology as beneficial for their health (e.g. vaccines, medicines with improved treatment potential or increased safety). In the case of the first GM foods introduced onto the European market, the products were of no apparent direct benefit to consumers (not significantly cheaper, no increased shelflife, no better taste). The potential for GM seeds to result in bigger yields per cultivated area should lead to lower prices. However, public attention has focused on the risk side of the risk-benefit equation, often without distinguishing between potential environmental impacts and public health effects of GMOs.
Consumer confidence in the safety of food supplies in Europe has decreased significantly as a result of a number of food scares that took place in the second half of the 1990s that are unrelated to GM foods. This has also had an impact on discussions about the acceptability of GM foods. Consumers have questioned the validity of risk assessments, both with regard to consumer health and environmental risks, focusing in particular on long-term effects. Other topics debated by consumer organizations have included allergenicity and antimicrobial resistance. Consumer concerns have triggered a discussion on the desirability of labelling GM foods, allowing for an informed choice of consumers.
The release of GMOs into the environment and the marketing of GM foods have resulted in a public debate in many parts of the world. This debate is likely to continue, probably in the broader context of other uses of biotechnology (e.g. in human medicine) and their consequences for human societies. Even though the issues under debate are usually very similar (costs and benefits, safety issues), the outcome of the debate differs from country to country. On issues such as labelling and traceability of GM foods as a way to address consumer preferences, there is no worldwide consensus to date. Despite the lack of consensus on these topics, the Codex Alimentarius Commission has made significant progress and developed Codex texts relevant to labelling of foods derived from modern biotechnology in 2011 to ensure consistency on any approach on labelling implemented by Codex members with already adopted Codex provisions.
Depending on the region of the world, people often have different attitudes to food. In addition to nutritional value, food often has societal and historical connotations, and in some instances may have religious importance. Technological modification of food and food production may evoke a negative response among consumers, especially in the absence of sound risk communication on risk assessment efforts and cost/benefit evaluations.
Yes, intellectual property rights are likely to be an element in the debate on GM foods, with an impact on the rights of farmers. In the FAO/WHO expert consultation in 2003 , WHO and FAO have considered potential problems of the technological divide and the unbalanced distribution of benefits and risks between developed and developing countries and the problem often becomes even more acute through the existence of intellectual property rights and patenting that places an advantage on the strongholds of scientific and technological expertise. Such considerations are likely to also affect the debate on GM foods.
Certain groups are concerned about what they consider to be an undesirable level of control of seed markets by a few chemical companies. Sustainable agriculture and biodiversity benefit most from the use of a rich variety of crops, both in terms of good crop protection practices as well as from the perspective of society at large and the values attached to food. These groups fear that as a result of the interest of the chemical industry in seed markets, the range of varieties used by farmers may be reduced mainly to GM crops. This would impact on the food basket of a society as well as in the long run on crop protection (for example, with the development of resistance against insect pests and tolerance of certain herbicides). The exclusive use of herbicide-tolerant GM crops would also make the farmer dependent on these chemicals. These groups fear a dominant position of the chemical industry in agricultural development, a trend which they do not consider to be sustainable.
Future GM organisms are likely to include plants with improved resistance against plant disease or drought, crops with increased nutrient levels, fish species with enhanced growth characteristics. For non-food use, they may include plants or animals producing pharmaceutically important proteins such as new vaccines.
WHO has been taking an active role in relation to GM foods, primarily for two reasons:
on the grounds that public health could benefit from the potential of biotechnology, for example, from an increase in the nutrient content of foods, decreased allergenicity and more efficient and/or sustainable food production; and
based on the need to examine the potential negative effects on human health of the consumption of food produced through genetic modification in order to protect public health. Modern technologies should be thoroughly evaluated if they are to constitute a true improvement in the way food is produced.
WHO, together with FAO, has convened several expert consultations on the evaluation of GM foods and provided technical advice for the Codex Alimentarius Commission which was fed into the Codex Guidelines on safety assessment of GM foods. WHO will keep paying due attention to the safety of GM foods from the view of public health protection, in close collaboration with FAO and other international bodies.
Food, Genetically modified
- Agriculture ›
Genetically modified crops - statistics & facts
Acreage of gm crops, gm crops in the u.s., gm crops in canada, consumer attitudes and understanding, key insights.
Detailed statistics
Acreage of genetically modified crops worldwide 2003-2019
Acreage of genetically modified crops by species 2003-2019
Acreage of genetically modified crops 2015-2019, by country
Editor’s Picks Current statistics on this topic
Crop Production
Global genetically modified crops by countries 2019, based on acreage
Global adoption rate for major biotech crops worldwide 2019, by type
Biotech and non-biotech corn areas in the U.S. 2022
Further recommended statistics
Global overview.
- Premium Statistic Acreage of genetically modified crops worldwide 2003-2019
- Premium Statistic Acreage of genetically modified crops 2015-2019, by country
- Premium Statistic Global genetically modified crops by countries 2019, based on acreage
- Premium Statistic Acreage of genetically modified crops by species 2003-2019
- Basic Statistic Global adoption rate for major biotech crops worldwide 2019, by type
Acreage of genetically modified crops worldwide from 2003 to 2019 (in million hectares)
Acreage of genetically modified crops worldwide from 2015 to 2019, by leading country (in million hectares)*
Area of genetically modified (GM) crops worldwide in 2019, by country (in million hectares)
Acreage of genetically modified crops from 2003 to 2019, by species (in million hectares)
Adoption of GM technology among selected major crops worldwide in 2019, by type*
- Premium Statistic Acreage of major genetically engineered crops in the U.S. by type 2019
- Premium Statistic Percentage of genetically modified crops in the U.S. by type 1997, 2018, 2019 & 2020
- Premium Statistic U.S. acreage of genetically modified corn 2014-2019
- Premium Statistic Biotech and non-biotech corn areas in the U.S. 2022
- Basic Statistic Distribution of U.S. biotech corn acres 2006-2023
- Premium Statistic U.S. acreage of genetically modified soybeans 2014-2019
- Premium Statistic U.S. acreage of genetically modified cotton 2014-2019
Acreage of major genetically engineered crops in the U.S. by type 2019
Major genetically modified (GM) crops in the United States in 2019, by crop type (in million hectares)*
Percentage of genetically modified crops in the U.S. by type 1997, 2018, 2019 & 2020
Percentage of genetically modified crops in the U.S. in 1997, 2018, 2019, and 2020, by type (as percent of total acreage)
U.S. acreage of genetically modified corn 2014-2019
Acreage of genetically modified (GM) corn in the United States from 2014 to 2019 (in million hectares)
Biotech and non-biotech corn areas in the U.S. in 2022 (in 1,000 acres)*
Distribution of U.S. biotech corn acres 2006-2023
Distribution of U.S. biotech and non-biotech corn acreage from 2006 to 2023, by type
U.S. acreage of genetically modified soybeans 2014-2019
Acreage of genetically modified (GM) soybeans in the United States from 2014 to 2019 (in million hectares)
U.S. acreage of genetically modified cotton 2014-2019
Acreage of genetically modified (GM) cotton in the United States from 2014 to 2019 (in million acres hectares)
- Premium Statistic Seeded area of genetically engineered crops in Canada 2012-2023
- Premium Statistic Seeded area of genetically engineered canola in Canada 2012-2023
- Premium Statistic Genetically engineered canola share of seeded canola area in Canada 2012-2023
- Premium Statistic Canada's seeded area of genetically engineered soybeans 2012-2023
- Premium Statistic Genetically engineered soybeans share of seeded soybeans area in Canada 2012-2023
- Premium Statistic Seeded area of genetically engineered corn in Canada 2011-2023
- Premium Statistic Genetically engineered corn share of seeded corn area in Canada 2012-2023
- Premium Statistic Seeded area of genetically modified soybeans in Ontario 2012-2024
- Premium Statistic Seeded area of genetically modified soybeans in Quebec 2012-2024
- Premium Statistic Seeded area of genetically modified corn for grains in Ontario 2012-2024
- Premium Statistic Seeded area of genetically modified corn for grains in Quebec 2012-2024
Seeded area of genetically engineered crops in Canada 2012-2023
Seeded area of genetically engineered crops in Canada from 2012 to 2023 (in million hectares)
Seeded area of genetically engineered canola in Canada 2012-2023
Seeded area of genetically engineered canola in Canada from 2012 to 2023 (in million hectares)
Genetically engineered canola share of seeded canola area in Canada 2012-2023
Genetically engineered canola share of seeded canola area in Canada from 2012 to 2023
Canada's seeded area of genetically engineered soybeans 2012-2023
Seeded area of genetically engineered soybeans in Canada from 2012 to 2023 (in million hectares)
Genetically engineered soybeans share of seeded soybeans area in Canada 2012-2023
Genetically engineered soybeans share of seeded soybeans area in Canada from 2012 to 2023
Seeded area of genetically engineered corn in Canada 2011-2023
Seeded area of genetically engineered corn in Canada from 2011 to 2023 (in million hectares)
Genetically engineered corn share of seeded corn area in Canada 2012-2023
Genetically engineered corn share of seeded corn area in Canada from 2012 to 2023
Seeded area of genetically modified soybeans in Ontario 2012-2024
Seeded area of genetically modified soybeans in Ontario from 2012 to 2024 (in 1,000 hectares)
Seeded area of genetically modified soybeans in Quebec 2012-2024
Seeded area of genetically modified soybeans in Quebec from 2012 to 2024 (in 1,000 hectares)
Seeded area of genetically modified corn for grains in Ontario 2012-2024
Seeded area of genetically modified corn for grains in Ontario from 2012 to 2024 (in 1,000 hectares)
Seeded area of genetically modified corn for grains in Quebec 2012-2024
Seeded area of genetically modified corn for grains in Quebec from 2012 to 2024 (in 1,000 hectares)
Mon - Fri, 9am - 6pm (EST)
Mon - Fri, 9am - 5pm (SGT)
Mon - Fri, 10:00am - 6:00pm (JST)
Mon - Fri, 9:30am - 5pm (GMT)

Genetically Engineered Crops: Experiences and Prospects (2016)
Chapter: 5 human health effects of genetically engineered crops, 5 human health effects of genetically engineered crops.
In this chapter, the committee examines the evidence that substantiates or negates specific hypotheses and claims about the health risks and benefits associated with foods derived from genetically engineered (GE) crops. There are many reviews and official statements about the safety of foods from GE crops (for example, see Box 5-1 ), but to conduct a fresh examination of the evidence, the committee read through a large number of articles with original data so that the rigor of the evidence could be assessed.
Some of the evidence available to the committee came from documents that were part of the U.S. regulatory process for GE crops conducted by the U.S. Environmental Protection Agency (EPA), the U.S. Department of Agriculture (USDA), and the U.S. Food and Drug Administration (FDA). Other evidence came from studies published by regulatory agencies in other countries or by companies, nongovernmental organizations (NGOs), and academic institutions. The committee also sought evidence from the public and from the speakers at its public meetings and webinars. 1
The committee thinks that it is important to make clear that there are limits to what can be known about the health effects of any food, whether non-GE or GE. If the question asked is “Is it likely that eating this food today will make me sick tomorrow?” researchers have methods of getting quantitative answers. However, if the question is “Is it likely that eating
__________________
1 The committee has compiled publicly available information on funding sources and first-author affiliation for the references cited in this chapter; the information is available at https://www.nationalacademies.org/ge-crops .
this food for many years will make me live one or a few years less than if I never eat it?” the answer will be much less definitive. Researchers can provide probabilistic predictions that are based on the available information about the chemical composition of the food, epidemiological data, genetic variability across populations, and studies conducted with animals, but absolute answers are rarely available. Furthermore, most current toxicity studies are based on testing individual chemicals rather than chemical mixtures or whole foods because testing of the diverse mixtures of chemicals experienced by humans is so challenging ( Feron and Groten, 2002 ; NRC, 2007 ; Boobis et al., 2008 ; Hernández et al., 2013 ).
With regard to the issue of uncertainty, it is useful to note that many of the favorable institutional statements about safety of foods from GE crops in Box 5-1 contain caveats, for example: “no overt consequences,” “no effects on human health have been shown,” “are not per se more risky,” and “are not likely to present risks for human health.” Scientific research can answer many questions, but absolute safety of eating specific foods and the safety of other human activities is uncertain.
The review in this chapter begins with an examination of what is known about the safety of foods from non-GE plants and how they are used as counterparts to those from GE crops in food-safety testing. U.S. food-safety regulatory testing for GE products and GE food-safety studies conducted outside the agency structure are then assessed. A variety of hypothesized health risks posed by and benefits of GE crops are examined, and the chapter concludes with a short discussion of the challenges that society will face in assessing the safety of GE foods that are likely to be developed with emerging genetic-engineering technologies.
COMPARING GENETICALLY ENGINEERED CROPS WITH THEIR COUNTERPARTS
An oft-cited risk of GE crops is that the genetic-engineering process could cause “unnatural” changes in a plant’s own naturally occurring proteins or metabolic pathways and result in the unexpected production of toxins or allergens in food ( Fagan et al., 2014 ). Because analysis of risks of the product of the introduced transgene itself is required during risk assessment, the argument for unpredicted toxic chemicals in GE foods is based on the assumption that a plant’s endogenous metabolism is more likely to be disrupted through introduction of new genetic elements via genetic engineering than via conventional breeding or normal environmental stresses on the plant. The review below begins by discussing natural chemical constituents of plants in the context of food safety to provide a background on what the natural plant toxins are and how they vary in non-GE plants. The review then goes on to explain the premise used by regulatory agencies to compare GE crops with their non-GE counterparts.
Endogenous Toxins in Plants
Most chemicals of primary metabolism (for example, those involved in the formation of carbohydrates, proteins, fats, and nucleic acids) are shared between animals and plants and are therefore unlikely to be toxic. Perceived risks associated with alterations of plant compounds arise mainly from alterations of plant-specific molecules, popularly known as plant natural products and technically named secondary metabolites. Collec-
tively, there are more than 200,000 secondary metabolites in the plant kingdom ( Springob and Kutchan, 2009 ). Crop species vary in the number of secondary metabolites that they produce. For example, potato ( Solanum tuberosum ) is known for its high diversity of secondary metabolites and can have more than 20 sesquiterpenes (a single group of related compounds), some of which are thought to confer resistance to diseases ( Kuc, 1982 ). The concentrations of these secondary metabolites within some tissues in a particular plant species may vary from high—for example, chlorogenic acids alone make up about 12 percent of the dry matter of green coffee beans ( Ferruzzi, 2010 )—to trace amounts (many minor saponins in legumes) and may be associated with particular stages of plant development (some found only in seeds) or may increase in response to external stimuli, such as pathogen or herbivore attack, drought, or altered mineral nutrition ( Small, 1996 ; Pecetti et al., 2006 ; Nakabayashi et al., 2014 ). Many secondary metabolites function as protective agents, for example, by absorbing damaging ultraviolet radiation ( Treutter, 2006 ), acting as antinutrients ( Small, 1996 ), or killing or halting insects and pathogens that damage crops ( Dixon, 2001 ). Plant secondary metabolites that protect against pathogen attack have been classified as either phytoanticipins (if they exist in a preformed state in a plant before exposure to a pathogen) or phytoalexins (if their synthesis and accumulation are triggered by pathogen attack) ( VanEtten et al., 1994 ; Ahuja et al., 2012 ). The toxic properties of some plant compounds are understood, but most of these compounds have not been studied. Some secondary metabolites and other products (such as proteins and peptides) in commonly consumed plant materials can be toxic to humans when consumed in large amounts, and examples are listed below:
- Steroidal glycoalkaloids in green potato skin, which can cause gastrointestinal discomfort or, more severely, vomiting and diarrhea.
- Oxalic acid in rhubarb, which can cause symptoms ranging from breathing difficulty to coma.
- Gossypol in cottonseed oil and cake, which can cause respiratory distress, anorexia, impairment of reproductive systems, and interference with immune function in monogastric animals.
- Nonprotein amino acid canavanine in alfalfa sprouts, which can be neurotoxic.
- Hemolytic triterpene saponins in many legume species, which can increase the permeability of red blood cell membranes.
- Cyanogenic glycosides in almonds and cassava, which can cause cyanide poisoning.
- Phototoxic psoralens in celery, which are activated by ultraviolet sunlight and can cause dermatitis and sunburn and increase the risk of skin cancer.
Friedman (2006) provided information that demonstrated that some glycoalkaloids in potato can have both harmful and beneficial effects. The Food and Agriculture Organization has recognized that foods often contain naturally occurring food toxins or antinutrients but that at naturally occurring concentrations in common diets they can be safely consumed by humans ( Novak and Haslberger, 2000 ; OECD, 2000 ). The health risks associated with some secondary metabolites in common foodstuffs are generally well understood, and the plants are either harvested at times when the concentrations of the compounds are low, the tissues with the highest concentrations of toxins are discarded, or, as in the case of cassava ( Manihot esculenta ), the food is prepared with special methods to remove the toxic compounds. In other cases, food preparation may be the cause of the presence of a toxic compound (for example, the formation of the probable carcinogen acrylamide when potatoes are fried at high temperatures or when bread is toasted). Plant breeders have generally screened for toxins that are typical of the plant group from which a crop was domesticated and have excluded plants that have high concentrations of the compounds.
Unintended changes in the concentrations of secondary metabolites can result from conventional breeding ( Sinden and Webb, 1972 ; Hellenas et al., 1995 ). In some cases, conventionally bred varieties have been taken off the market because of unusually high concentrations of a toxic compound, as in the case of a Swedish potato variety that was banned from sale in the 1980s because of high concentrations of glycoalkaloids ( Hellenas et al., 1995 ).
Rather than being a cause of worry, many secondary metabolites are perceived as having potential health benefits for humans and are consumed in increasingly large quantities ( Murthy et al., 2015 ). Examples include the isoflavone phytoestrogens found in a number of leguminous plants, such as soybean ( Glycine max ) and clover ( Trifolium spp.), which have been ascribed beneficial activities, including chemoprevention of breast and prostate cancers, cardiovascular disease, and post-menopausal ailments ( Dixon, 2004 ; Patisaul and Jefferson, 2010 ). Also, various perceived antioxidants, such as anthocyanins ( Martin et al., 2013 ), and some saponins may have anticancer activity ( Joshi et al., 2002 ). There is, however, disagreement as to whether many of the compounds are beneficial or toxic at the concentrations consumed in herbal medicines or dietary supplements (see, for example, Patisaul and Jefferson, 2010 ).
FINDING: Crop plants naturally produce an array of chemicals that protect against herbivores and pathogens. Some of these chemicals can be toxic to humans when consumed in large amounts.
Substantial Equivalence of Genetically Engineered and Non–Genetically Engineered Crops
A major question addressed in the regulation of GE crops is whether the concentrations of the toxic secondary metabolites are affected by genetic engineering. In addition to the plant toxins, nutrients, introduced genes, and proteins and their metabolic products in specific GE crops are assessed with a comparative approach that is generally encompassed by the concept of substantial equivalence.
The concept of substantial equivalence has a long history in safety testing of GE foods. The term and concept were “borrowed from the [U.S. FDA’s] definition of a class of new medical devices that do not differ materially from their predecessors, and thus, do not raise new regulatory concerns” ( Miller, 1999:1042 ). No simple definition of substantial equivalence is found in the regulatory literature on GE foods. In 1993, the Organisation for Economic Co-operation and Development (OECD) explained that the “concept of substantial equivalence embodies the idea that existing organisms used as food, or as a source of food, can be used as the basis for comparison when assessing the safety of human consumption of a food or food component that has been modified or is new” ( OECD, 1993:14 ).
The Codex Alimentarius Commission’s Guideline for the Conduct of Food Safety Assessment of Foods Derived from Recombinant-DNA Plants is careful to state that “the concept of substantial equivalence is a key step in the safety assessment process. However, it is not a safety assessment in itself; rather it represents the starting point which is used to structure the safety assessment of a new food relative to its conventional counterpart” ( CAC, 2003:2 ). The Codex guideline also makes clear that a safety assessment of a new food based on the concept of substantial equivalence “does not imply absolute safety of the new product; rather, it focuses on assessing the safety of any identified differences so that the safety of the new product can be considered relative to its conventional counterpart” ( CAC, 2003:2 ). The OECD (2006) came to a similar conclusion. Conflict among stakeholders often comes into play during the determination of what constitutes evidence of differences from substantial equivalence sufficient to justify a detailed food-safety assessment.
The Codex Alimentarius Commission concluded that the concept of substantial equivalence “aids in the identification of potential safety and nutritional issues and is considered the most appropriate strategy to date for safety assessment of foods derived from recombinant-DNA plants” ( CAC, 2003:2 ). Despite some criticism of the substantial-equivalence concept itself (for example, Millstone et al., 1999 ) and operational problems (for example, Novak and Haslberger, 2000 ), it remains the cornerstone for
GE food-safety assessment by regulatory agencies. The present committee examined its use in practice and its empirical limitations.
The precautionary principle, which is described in more detail in Chapter 9 (see Box 9-2 ) is a deliberative principle related to the regulation of health, safety, and the environment and typically involves taking measures to avoid uncertain risks. The precautionary principle has been interpreted in a number of ways, but it is not necessarily incompatible with use of the concept of substantial equivalence. In the case of foods, including GE foods, it can be reasonably argued that even a small adverse chronic effect should be guarded against, given that billions of people could be consuming the foods. However, the degree of precaution taken in the face of uncertainty is a policy decision that varies among countries and according to the specific uncertainty being considered. For example, many European countries and the European Union (EU) as a whole generally take a more precautionary approach with GE foods and climate change whereas the United States has historically taken a more precautionary approach with tobacco products and ozone depletion ( Wiener et al., 2011 ). The reader is directed to Chapter 9 for further discussion of how different regulatory frameworks address uncertainty in the safety of GE foods.
Some differences between a GE food and its non-GE counterpart are intentional and identifiable (for example, the presence of a Bt toxin in maize kernels) or are due to practices directly associated with the use of the GE crops (for example, increased use of glyphosate). Some of the risks posed by the intended changes can be anticipated on the basis of the physiological and biochemical characteristics of the engineered change. There are often established protocols for assessing such risks, especially when a change involves exposure to a known toxin. However, other risks have been hypothesized for GE crops because previous uses of a trait (for example, Bt as an insecticidal spray) did not have consumption of the GE plant products as the route of exposure. New routes of exposure could result in unanticipated effects.
In contrast with such intended differences, some potential differences between GE crops and their non-GE counterparts are unintentional and can be difficult to anticipate and discern ( NRC, 2004 ). Two general sources of unintended differences could affect food safety:
- Unintended effects of the targeted genetic changes on other characteristics of the food (for example, the intended presence of or increase in one compound in plant cells could result in changes in plant metabolism that affect the abundance of other compounds).
- Unintended effects associated with the genetic-engineering process (for example, DNA changes resulting from plant tissue culture).
Much of the concern voiced by some citizens and scientists about the safety of GE foods is focused on potential risks posed by unintended differences. Some of the biochemical and animal testing done by or for government agencies is aimed at assessing the toxicity of such unintended differences, but what is adequate and appropriate testing for assessing specific toxicities is often difficult to determine. In some cases, the unintended effects are somewhat predictable or can be determined; in such cases, tests can be designed. In other cases, the change or risk could be something that has not even been considered, so the only effective testing is of the whole food itself. As discussed in Chapter 6 , there is a tradeoff between costs of such testing and societal benefits of reduction in risks.
The approach of comparing new varieties to existing varieties is just as applicable to crops developed by conventional plant breeding as it is to GE crops (see Chapter 9 ). The discussion above on endogenous toxins (see section “Endogenous Toxins in Plants”) shows that such crops pose some risks. The 2000 National Research Council report Genetically Modified Pest-Protected Plants found that “there appears to be no strict dichotomy between the risks to health and the environment that might be posed by conventional and transgenic pest-protected plants” ( NRC, 2000:4 ). Similarly, the 2004 National Research Council report Safety of Genetically Engineered Foods found that all forms of conventional breeding and genetic engineering may have unintended effects and that the probability of unintended effects of genetic engineering falls within the range of unintended effects of diverse conventional-breeding methods. The 2002 National Research Council report Environmental Effects of Transgenic Plants found that “the transgenic process presents no new categories of risk compared to conventional methods of crop improvement but that specific traits introduced by both approaches can pose unique risks” ( NRC, 2002:5 ). That finding remains valid with respect to food safety and supports the conclusion that novel varieties derived from conventional-breeding methods could be assessed with the substantial-equivalence concept.
FINDING: The concept of substantial equivalence can aid in the identification of potential safety and nutritional issues related to intended and unintended changes in GE crops and conventionally bred crops.
FINDING: Conventional breeding and genetic engineering can cause unintended changes in the presence and concentrations of secondary metabolites.
OVERVIEW OF U.S. REGULATORY TESTING OF RISKS TO HUMAN HEALTH
Although the committee agrees that crops developed through conventional breeding could result in food-safety risks, its statement of task focuses on GE crops. Furthermore, there have been claims and counterclaims about the relative safety of GE crops and their associated technologies compared with conventionally bred crops and their associated technologies. Therefore, the remainder of this chapter examines possible risks and benefits associated with GE crops and assesses the methods used to test them in and beyond government regulatory systems.
Whether testing is done for regulatory purposes or beyond the regulatory realm, it typically involves three categories of testing: acute or chronic animal toxicity tests, chemical compositional analysis, and allergenicity testing or prediction. Although the precision, transparency, specific procedures, and interpretation of results vary among countries, criticisms about the adequacy of testing are not so much country-specific as they are method- and category-specific. For example, there may be arguments about whether a 90-day whole-food animal test is more appropriate than a 28-day test, but the bigger issue is about whether whole-food testing is appropriate. The committee uses a description of the U.S. testing methods as an example, but it mostly examines the criticism of food-safety testing more broadly.
The structure of the U.S. regulatory process for GE crops based on the Coordinated Framework for the Regulation of Biotechnology is briefly reviewed in Chapter 3 and is examined in more detail in Chapter 9 . The focus in this chapter is on the testing itself. The present section provides insight into U.S. procedures by describing the risk-testing methods used for two examples of traits in commercialized GE crops: Bt toxins and crop resistance to the herbicides glyphosate and 2,4-D.
Regulatory Testing of Crops Containing Bt Toxins
EPA considers plant-produced Bt toxins to be “plant-incorporated protectants,” a class of products generally defined as “a pesticidal substance that is intended to be produced and used in a living plant, or in the produce thereof, and the genetic material necessary for the production of that pesticidal substance” (40 CFR §174.3). EPA specifically exempts plant-incorporated protectants whose genetic material codes for a pesticidal substance that is derived from plants that are sexually compatible. Bt toxin genes are not exempted because they come from bacteria (see Chapter 9 for regulatory details).
For Bt toxins produced by GE crops, EPA took into consideration that there was already toxicity testing of Bt toxins in microbial pesticides and
that the toxins were proteins that, if toxic, typically show almost immediate toxicity at low doses ( EPA, 2001a ; also see Box 5-2 ). The pesticidal safety tests mostly involved acute toxicity testing in mice and digestibility studies in simulated gastric fluids because one characteristic of food allergens is that they are not rapidly digested by such fluids.
Box 5-2 provides a verbatim example of the procedures used for testing as reported in EPA fact sheets for the Cry1F Bt toxin so that readers can see what is involved in the testing. The actual research is not typically done by EPA itself. The registrant is usually responsible for testing. Results of the tests of Cry1F show no clinical signs of any toxicity even when Cry1F protein was fed at 576 mg/kg body weight, which would be the equivalent of about ¼ cup of pure Cry1F for a 90.7-kilogram (200-pound) person. Another part of the testing described in Box 5-2 is allergenicity testing. Concerns about the EPA testing methods are discussed in sections below on each category of testing.
Regulatory Testing of Crops Resistant to Glyphosate and 2,4-D and of the New Uses of the Herbicides Themselves
The regulatory actions taken for herbicide-resistant (HR) crops are different from regulatory actions taken to assess Bt crops. With Bt crops, regulatory actions are related to the crop itself. With HR crops, there are regulatory processes for the plant itself and separate regulatory processes for the new kind of exposure that can accompany spraying of a herbicide on a crop or on a growth stage of a crop that has never been sprayed prior to availability of the GE variety.
EPA governs the registration of herbicides such as glyphosate and 2,4-D. Both glyphosate and 2,4-D were registered well before the commercialization of GE crops. However, EPA has authority to re-examine herbicides if their uses or exposure characteristics change.
A good example of such re-examination was the 2014 EPA registration of the Dow AgroSciences Enlist Duo® herbicide, which contains both glyphosate and 2,4-D for use on GE maize ( Zea mays ) and soybean. Because the glyphosate component of Enlist Duo had already been in use on GE maize and soybean, EPA did not conduct further testing of glyphosate alone. However, 2,4-D was registered previously only for applications to maize up to 20 centimeters tall and for preplant applications to soybean. The proposed use of 2,4-D on GE crops was expected to change use patterns and exposure and thereby triggered a safety assessment of the new use 2,4-D. Additionally, EPA compared the toxicity of the formulation that contained both herbicides to the toxicity of the individual herbicides and concluded the formulation did not show greater toxicity or risk compared to either herbicide alone.
In the human health risk assessment portion of the EPA Enlist Duo registration document, the following tests and results with 2,4-D were considered ( EPA, 2014a ):
- An acute dietary test in rats that found a lowest observed-adverse-effect level (LOAEL) of 225 mg/kg (about 1 ounce per 200-pound person).
- A chronic-dietary-endpoint, extended one-generation reproduction toxicity study in rats that found a LOAEL of 46.7 mg/kg-day in females and higher in males.
- Inhalation tests involving data from a 28-day inhalation toxicity study in rats that found a LOAEL of 0.05 mg/L-day.
- Dermal tests that showed no dermal or systemic toxicity after repeated applications to rabbits at the limit dose of 1000 mg/kg-day.
- Reviews of epidemiological and animal studies, which did not support a linkage between human cancer and 2,4-D exposure.
Analysis of the results of those tests and agronomic and environmental assessments resulted in the product’s registration.
EPA received over 400,000 comments in response to the initial proposal to register the new use of 2,4-D. Some of the concerns submitted to EPA were similar to ones some members of the public expressed in public comments to the committee, including questions about whether EPA had considered toxicity of only the active ingredient or of the formulated herbicide and whether it had tested for synergistic effects of 2,4-D and glyphosate. EPA (2014b:7) responded that
acute oral, dermal, and inhalation data, skin and eye irritation data, and skin sensitization data are available for the 2,4-D choline salt and glyphosate formulation for comparison with the 2,4-D parent compound and glyphosate parent compound data, and these test results show similar profiles. The mixture does not show a greater toxicity compared to either parent compound alone. Although no longer duration toxicity studies are available, toxic effects would not be expected as the maximum allowed 2,4-D exposure is at least 100-fold below levels where toxicity to individual chemicals might occur, and exposure to people is far below even that level.
The committee did not have access to the actual data from the registrant. 2
EPA does not regulate the commercialization of the GE herbicide-resistant crops themselves. That is the role of USDA’s Animal and Plant Health Inspection Service (APHIS) under the Plant Protection Act. Under its
2 In November 2015, EPA took steps to withdraw the product’s registration in light of new information that indicated there could be synergistic effects of the two herbicides, which could possibly result in greater toxicity to nontarget plants ( Taylor, 2015 ). A court ruling in January 2016 allowed the herbicide to remain on the market while EPA considered other administrative actions ( Callahan, 2016 ).
statutory authority, APHIS controls and prevents the spread of plant pests (see Box 3-5 ). On the basis of a plant-pest risk assessment ( USDA–APHIS, 2014a ), APHIS concluded that Enlist™ GE herbicide-resistant maize and soybean engineered to be treated with the Enlist Duo herbicide (containing glyphosate and 2,4-D) were unlikely to become plant pests and deregulated them on September 18, 2014 ( USDA–APHIS, 2014b ). In its document on the decision to deregulate Enlist GE herbicide-resistant maize and soybean ( USDA–APHIS, 2014a:ii ), APHIS states a general policy that “if APHIS concludes that the GE organism is unlikely to pose a plant pest risk, APHIS must then issue a regulatory determination of nonregulated status, since the agency does not have regulatory authority to regulate organisms that are not plant pests. When a determination of nonregulated status has been issued, the GE organism may be introduced into the environment without APHIS’ regulatory oversight.”
FDA did not identify any safety or regulatory issues in its consultation with Dow AgroSciences on the Enlist maize and soybean varieties ( FDA, 2013 ). FDA also explained the basis of Dow’s conclusion that Enlist soybean is not “materially different in composition” from other soybean varieties ( FDA, 2013 ):
Dow reports the results of compositional analysis for 62 components in soybean grain, including crude protein, crude fat, ash, moisture, carbohydrates, [acid detergent fiber] ADF, [neutral detergent fiber] NDF, total dietary fiber (TDF), lectin, phytic acid, raffinose, stachyose, trypsin inhibitor, soy isoflavones (i.e., total daidzein, total genistein, total glycitein), minerals, amino acids, fatty acids, and vitamins. No statistically significant differences in the overall treatment effect and the paired contrasts between each of the DAS-44406-6 soybean treatment groups and the control were observed for 29 of the components. A statistically significant difference in the overall treatment effect was observed for 16 components (crude protein, carbohydrates (by difference), NDF, calcium, potassium, cystine, palmitic acid, oleic acid, linoleic acid, linolenic acid, behenic acid, folic acid, γ-tocopherol, total tocopherol, lectin, and trypsin inhibitor). However, differences between the control and the DAS-44406-6 treatment groups were small in magnitude. Differences between DAS-44406-6 soybean and the control were considered not biologically relevant because the mean values were either within the ranges generated using the reference lines, consistent with the ranges of values in the published literature, or both.
FINDING: U.S. regulatory assessment of GE herbicide-resistant crops is conducted by USDA, and by FDA when the crop can be consumed, while the herbicides are assessed by EPA when there are new potential exposures.
FINDING: When mixtures of herbicides are used on a new GE crop, EPA assesses the interaction of the mixture as compared to the individual herbicidal compounds.
Technical Assessment of Human Health Risks Posed by Genetically Engineered Crops
As explained in Chapter 2 , the development and use of GE crops is governed by more than national and regional regulatory standards. In the cases of the GE crops commercially available in the United States and some other countries in 2015, inputs from many public and private institutions regarding their specific concerns have influenced the type and extent of GE crop food-safety tests conducted by companies, agencies, and other researchers. Many stakeholders have criticized the testing used by U.S. and other national regulatory agencies for lacking rigor (for example, Hilbeck et al., 2015 ). Researchers in companies, NGOs, and universities have sometimes conducted more extensive safety tests than are required by national agencies or have reanalyzed existing data, as described below. All testing as of 2015 fell into three categories: animal testing, compositional analysis, and allergenicity testing and prediction.
Animal Testing
Short-Term and Long-Term Rodent Testing with Compounds and Whole Foods . One common criticism of the animal testing conducted by or for regulatory agencies in the United States and elsewhere is related to its short duration (for example, Séralini et al., 2014 ; Smith, 2014 ). Indeed, there is a range in the duration and doses within the test protocols used by regulatory agencies that depends in part on the product. Doses for subchronic and chronic toxicity studies are such that the lowest dose (exposure level), which is many times higher than expected for human exposure, is set to ensure that it does not elicit acute adverse effects that would interfere with examining the potential chronic-effect endpoints. As can be seen in the discussion above, EPA conducted an extended one-generation reproduction toxicity study in male and female rats in its assessment of 2,4-D, and it relied on previous long-term studies for the assessment of cancer risk associated with it. For assessment of the Bt toxin Cry1F and for the bacterially derived proteins in 2,4-D-resistant maize and soybean, company testing submitted to EPA, FDA, and USDA relied on acute toxicity testing. In all the cases above, the experiments were conducted by adding large amounts of a single test chemical to an animal’s diet. Tests with high concentrations of a chemical are typical of EPA testing protocols for pesticides.
What is different between GE crop evaluation and that of general agri-
cultural chemicals is the use of “whole food” tests. These tests are aimed at assessing potential hazards due to the combined intentional and unintentional changes that might have been caused by the genetic engineering of the crop. In such tests, it is not possible to use concentrations higher than what is in the crop itself because potential unintended effects are not typically known. Thus, it is impossible for a researcher to know what compounds should be increased in concentration in a fabricated diet, and the only way to assess such unintended effects is to feed the actual GE crop to test animals. For testing GE maize, soybean, and rice ( Oryza sativa ), 3 flour from kernels or seed is added to an animal’s diet and constitutes between about 10–60 percent of the diet. The high percentages can be used because the crop products are nutritious for the animal. In the case of whole foods that are not typically part of a rodent’s diet, whether GE or non-GE, it is impossible to achieve very high concentrations of the test food because it would cause nutritional imbalance. The whole-food tests done for regulatory agencies are generally conducted for 28 or 90 days with rats, but some researchers have run tests for multiple generations.
The utility of the whole-food tests has been questioned by a number of government agencies and by industry and academic researchers (for example, Ricroch et al., 2014 ), and they are not an automatic part of the regulatory requirements of most countries that have specific GE food-testing requirements ( CAC, 2008 ; Bartholomaeus et al., 2013 ). However, in its 2010 report A Decade of EU-Funded GMO Research (2001–2010) , the European Directorate-General for Research and Innovation concluded that “the data from a well-designed 90-day rodent feeding study, together with data covering the gene insert, the compositional analysis, and the toxicity of the novel gene product, form the optimal basis for a comparative assessment of the safety of [genetically engineered] food and its conventional counterpart in the pre-market situation” ( EC, 2010a:157 ). The European Food Safety Authority (EFSA) developed principles and guidance for establishing protocols for 90-day whole-food studies in rodents at the European Commission’s request ( EFSA, 2011b ), and 90-day, whole-food studies were made mandatory by the European Commission ( EC, 2013 ). Most studies reported in the peer-reviewed literature have concluded that there was a lack of adverse effects of biological or toxicological significance (see, for example, Knudsen and Poulsen, 2007 ; MacKenzie et al., 2007 ; He et al., 2008 , 2009 ; Onose et al., 2008 ; Liu et al., 2012 ), even though some of the studies found statistically significant differences between the GE and non-GE comparator in toxicity.
The criticisms of whole-food tests come from two perspectives. One perspective is that whole-food studies cannot provide useful tests of food
3 GE rice was not commercialized in 2015, but GE varieties in development have been tested.
safety because they are not sensitive enough to detect differences (see, for example, Bartholomaeus et al., 2013 ; Kuiper et al., 2013 ; Ricroch et al., 2013a , 2014 ) and that animal testing is not needed because other types of required testing ensure safety ( Bartholomaeus et al., 2013 ; Ricroch et al., 2014 ). Ricroch et al. (2014) pointed to the costs of the 90-day tests, which they reported as being €250,000 (in 2013 money). The second perspective is that whole-food tests could be useful, but there is concern about their design and conduct or about the parties who conduct them (the companies commercializing the GE crops). That perspective is evident in Séralini et al. (2007) , Domingo and Bordonaba (2011) , Hilbeck et al. (2015) , and Krimsky (2015) . Boxes 5-3 and 5-4 describe some of the specific procedures and practices involved in doing these tests.
The committee heard from invited speakers ( Entine, 2014 ; Jaffe, 2014 ) and members of the public who provided comments at meetings and it received a number of written public comments highlighting the work of one research group ( Séralini et al., 2012 , 2014 ) that has conducted a number of whole-food studies of GE herbicide-resistant and insect-resistant crops and
of direct consumption of glyphosate. Some comments made to the committee pointed to the publications of that research group as evidence that GE crops and foods derived from GE crops were deleterious to human health; other comments questioned the robustness and accuracy of the research. The committee also heard from the lead researcher himself at one of its meetings ( Séralini, 2014 ). Because of the attention garnered by this specific research group, the committee examined the primary research paper from the group and many articles related to it ( Box 5-5 ).
A general question that remains for all whole-food studies using animals is, How many animals, tested for how long, are needed to assess food safety when a whole food is tested? That question is related to the question of how large an effect the tested food would have to have on the animal for it to be detected with the experiment. The statistical procedure called power analysis can answer the first question, but the committee did not find such analyses in articles related to GE crop whole-food studies. The EFSA scientific committee ( EFSA, 2011b ) provided general guidance on power analysis. Figure 5-2 , from the EFSA report, shows the relationship between the number of experimental units (cages with two animals) per treatment group and the power of an experiment in standard-deviation units. Standard deviations quantify how much the measurement of a trait or effect varies among animals that have been given the same diet. The report concluded that, if researchers follow OECD Test No. 408 of 10 males and 10 females per treatment ( OECD, 1998a ), a test should be able to detect a difference equal to about 1 standard deviation (with 90-percent confidence) unless the food has a different effect on males and females, in which case, the smallest difference that could be detected would be about 1.5 standard deviations from the experimental mean.
Because the relationship is quite abstract for the nonstatistician, the committee examined the size of the standard deviations in a number of whole-food safety articles. It found that the sizes of the standard deviations compared with the mean value of a measured trait depended heavily on the trait being measured and on the specific research article. For example, in the Hammond et al. (2004) study, the average white blood cell count for the four treatments, each with 9 or 10 female Sprague-Dawley rats, is 6.84 10 3 /µl, and the average standard deviation is 1.89 10 3 /µl. On the basis of rough calculations, this test would have the power to discern statistically whether the GE food caused an increase in white blood cell count of about 35 percent with about 90-percent confidence. If the male white blood cell count effects and standard deviations were similar to those in females, the test could have found about a 25-percent increase.
OECD (1998a) made general recommendations, such as those used in
Hammond et al. (2004) , for the number of units (cages with two animals) per treatment. Following these guidelines leads to the assumption that less than a 25-percent change in the white blood cell count was not biologically relevant. The EU Standing Committee on the Food Chain and Animal Health adopted the mandatory use of 90-day whole-food testing of GE crops, and its protocols generally follow OECD guidelines for the testing of chemicals ( EC, 2013 ).
EFSA also published a document ( EFSA, 2011c ) that focused specifically on the questions, What is statistical significance? and What is biological relevance? The accessibly written document makes clear that the two are very different and that it is important to decide how large a difference is biologically relevant before designing an experiment to test a null hypothesis of no difference. The problem in most whole-food animal studies is in determining how large a biological difference is relevant. Most of the statistically significant differences observed in the literature on the animal-testing data were around a 10- to 30-percent change, but the authors do not give detailed explanations of why they conclude that a statistically significant difference is not biologically relevant. A general statement is sometimes made that the difference is within the range for the species, but because the range of values for the species typically come from multiple laboratories, such a statement is not useful unless the laboratories, instrumentation, and health of the animals were known to be comparable.
Clearly, the European Commission relied on both expert judgment and citizen concerns in making its assessment of biological relevance of the effects of GE foods in requiring 90-day testing. It is reasonable to ask what balance of the two is the basis for this judgment. As pointed out by the 2002 National Research Council report, “risk analysis of transgenic plants must continue to fulfill two distinct roles: (1) technical support for regulatory decision making and (2) establishment and maintenance of regulatory legitimacy” ( NRC, 2002:6 ). Fulfilling the two roles can lead to different country-specific and region-specific decisions. This issue is discussed further in Chapter 9 .
One specific criticism of the 90-day whole-food studies revolves around an EU-funded project conducted by Poulsen et al. (2007) in which rice was genetically engineered to produce the kidney bean lectin, agglutinin E-form, which is known to have toxic properties. In a 90-day test, rats were fed diets of 60-percent rice with the lectin gene or 60-percent rice without the lectin gene. The researchers concluded that they did not find any meaningful differences between the two treatments. However, in a treatment in which the diets were spiked with 0.1-percent recombinant lectin (a high dose), biological effects including significant differences in weight of small intestines, stomach, and pancreas and in plasma biochemistry were found. Poulsen et al. included results from a preceding 28-day feeding study and
compositional analyses of the rice diets. The criticism involves the question, If a whole-food study with a known toxin does not demonstrate effects, how can the test be considered useful? ( Bartholomaeus et al., 2013 ). If a whole-food study with an animal finds statistically significant effects, there is obviously a need for further safety testing, but when there is a negative result, there is uncertainty as to whether there is an adverse effect on health. In the specific case of lectin gene in rice, one could argue that the statistical power of the whole-food test was insufficient or that, when the toxin is in the structure of the food, it is no longer toxic so the food is safe.
Other Long-Term Studies with Rodents . In addition to the work of Séralini et al. (2012 , 2014 ), there have been other long-term rodent studies, some of which included multiple generations. Magana-Gomez and de la Barca (2009) , Domingo and Bordonaba (2011) , Snell et al. (2012) , and Ricroch et al. (2013b) reviewed the studies. Some found no statistically significant differences, but quite a few found statistically significant differences that the authors generally did not consider biologically relevant, typically without providing data on what was the normal range. In the multigeneration studies, the sire and dam are dosed via the diet before conception, and the parent generation and pups are dosed via the diet throughout the duration of the study to determine multiple generational outcomes, including growth, behavior, and phenotypic characteristics. Some studies have looked at three or four generations. For example, Kiliç and Akay (2008) conducted a three-generation rat study in which 20 percent of the diet was Bt maize or a non- Bt maize that otherwise was genetically similar. All generations of female and male rats were fed the assigned diets, and the third-generation offspring that were fed the diets were sacrificed after 3.5 months for analysis. The authors found statistical differences in kidney and liver weights and long kidney glomerular diameter between the GE and non-GE treatments but considered them not biologically relevant. Similarly, statistically significant differences were observed in amounts of globulin and total protein between the two groups. There was no presentation of standards used for judging what would be a biologically relevant difference or for what the normal range was in the measurements.
The standard deviations in measurements of the traits (that is, effects) of individual animals in a treatment in the long-term studies were similar to those of studies of shorter duration. Therefore, the power of the tests to detect statistically significant differences was in the range of 10–30 percent. The committee could not find justification for considering this statistical power sufficient. It can be argued that the number of replicates (number of units of two animals per treatment) in the studies should be substantially increased, but one argument against an increase in numbers is related to the ethics of subjecting more animals to testing ( EC, 2010b ). One could
also argue that it is unethical to conduct an underpowered study. However, most if not all of the rodent studies are based on widely accepted safety evaluation protocols with fixed numbers of animals per treatment. Cultural values regarding precaution for human safety and those regarding the number of animals subjected to testing are in conflict in this case. As pointed out by Snell et al. (2012) , a close examination of the long-term and multigenerational studies reveals that some have problems with experimental design, the most common being that the GE and non-GE sources were not isogenic and were grown in different locations (or unknown locations). Those problems in design make it difficult to determine whether differences are due to the genetic-engineering process or GE trait or to other sources of variation in the nutritional quality of the crops.
In cases in which testing produces equivocal results or tests are found to lack rigor, follow-up experimentation with trusted research protocols, personnel, and publication outlets is needed to decrease uncertainty and increase the legitimacy of regulatory decisions. There is a precedent of such follow-up studies in the literature on GE crop environmental effects that could serve as a general model for follow-up food-safety testing (see Chapter 4 section “Genetically Engineered Crops, Milkweed, and Monarch Butterflies”). The USDA Biotechnology Risk Assessment Research Grants Program has enabled this approach in a few cases.
Beyond Rodent Studies. Mice and rats are typically used in toxicity studies because of their general physiological similarities to humans and their small size, but some farm animals are considered to be better models of human physiology than rodents. The best example is the pig, which is considered to be better than rodents as a model, especially with respect to nutritional evaluations ( Miller and Ullrey, 1987 ; Patterson et al., 2008 ; Litten-Brown et al., 2010 ). Porcine insulin has been used for decades to control blood sugar in patients who have childhood-onset diabetes mellitus (type I diabetes). Pig heart valves are used for human mitral valve replacement, and pig skin has been investigated as a possible donor tissue. The pig is monogastric as is the human, and its gastrointestinal tract absorbs and metabolizes nutrients (lipids and micronutrients) in the same manner as in humans.
Reviews of studies with animals fed GE foods have included studies using both rodents and farm animals ( Bartholomaeus et al., 2013 ; DeFrancesco, 2013 ; Ricroch et al., 2013a , b , 2014 ; Swiatkiewicz et al., 2014 ; Van Eenennaam and Young, 2014 ). Those animal studies have taken advantage of the fact that maize and soybean are major components of the diets of many farm animals. Some of the reported studies that used farm animals have designs similar to those of rodent studies and have variation in duration and replicates similar to that of the rodent experiments. Some of the tests were run for 28 days (for example, Brouk et al.,
2011 ; Singhal et al., 2011 ), others for a long term ( Steinke et al., 2010 ) or in multiple generations ( Trabalza-Marinucci et al., 2008 ; Buzoianu et al., 2013b ).
The experiments with pigs are especially relevant. Most of them were conducted in one prolific laboratory ( Walsh et al., 2011 , 2012a , b , 2013 ; Buzoianu et al., 2012a , b , c , d , 2013a , b ). The studies range from examination of short-term growth of piglets to multigenerational studies of sows and piglets, with mixed designs having either generation or both exposed to Bt maize and non- Bt maize. Characteristics measured included food consumption and growth, assessment of organ size and health, immunological markers, and microbial communities. The authors of the studies generally concluded that Bt maize does not affect health of the pigs, but they reported a number of statistically significant differences between Bt maize treatment and control maize treatment. In one experiment ( Walsh et al., 2012a ), the weaned piglets that were fed Bt maize had lower feed-conversion efficiency during days 14–30 (P > 0.007) but no significant effect over the full span of the experiment. In another experiment ( Buzoianu et al., 2013b ), there was lower efficiency in the Bt treatment during days 71–100 (P > 0.01) but again no effect over the full span of the experiment.
In those experiments with pigs and experiments with other farm animals and rodents, there was apparently one source of the GE food and one source of the non-GE food per study, and it is generally not clear that the food sources were isogenic or grown in the same location. That makes it difficult to determine whether any statistical differences found were due to the engineered trait or to the batches of food used, which in at least some experiments varied in nutrient content and may have differed in bioactive compounds (produced in response to plant stressors), which may have a profound effect on outcomes of nutritional studies. Another issue is that many statistical tests were performed in most studies. That could result in accumulation of false-positive results ( Panchin and Tuzhikov, 2016 ). Although this is not a situation in which a stringent correction for doing multiple tests is called for ( Dunn, 1961 ), there is reason to be cautious in interpretation of statistical significance of individual results because multiple tests can lead to artifactual positive results. The issue of multiple test results is common in many fields, and one approach used in genetics is to use the initial tests for hypothesis generation with follow-up experiments that test an a priori hypothesis (for example, Belknap et al., 1996 ). If a straightforward application of Bonferonni correction is used, each animal study that measures multiple outcomes, whether for GE crops or any other potential toxicant, could require over 1,000 animals to obtain reasonable statistical power ( Dunn, 1961 ).
In addition to the literature on controlled experiments with livestock, Van Eenennaam and Young (2014 ) reviewed the history of livestock health
and feed-conversion ratios as the U.S. livestock industry shifted from non-GE to GE feed. Producers of cattle, milk cows, pigs, chickens, and other livestock are concerned about the efficiency of conversion of animal feed into animal biomass because it affects profit margins. The data examined start as early as 1983 and run through 2011. Therefore, livestock diets shifted from all non-GE feed to mostly GE feed within the duration of the study. Van Eenennaam and Young found that, if anything, the health and feed-conversion efficiencies of livestock had increased since the introduction of GE crops but that the increase was a steady rise, most likely because of more efficient practices not associated with use of GE feed. In the studies that they reviewed, the number of animals examined was large (thousands). Of course, most livestock are slaughtered at a young age, so that data cannot address the issue of longevity directly. However, given the general relationship between general health and longevity, the data are useful.
FINDING: The current animal-testing protocols based on OECD guidelines for the testing of chemicals use small samples and have limited statistical power; therefore, they may not detect existing differences between GE and non-GE crops or may produce statistically significant results that are not biologically meaningful.
FINDING: In addition to experimental data, long-term data on the health and feed-conversion efficiency of livestock that span a period before and after introduction of GE crops show no adverse effects on these measures associated with introduction of GE feed. Such data test for correlations that are relevant to assessment of human health effects, but they do not examine cause and effect.
RECOMMENDATION: Before an animal test is conducted, it is important to justify the size of a difference between treatments in each measurement that will be considered biologically relevant.
RECOMMENDATION: A power analysis for each characteristic based on standard deviations in treatments in previous tests with the animal species should be done whenever possible to increase the probability of detecting differences that would be considered biologically relevant.
RECOMMENDATION: In cases in which early published studies produced equivocal results regarding health effects of a GE crop, followup experimentation using trusted research protocols, personnel, and publication outlets should be used to decrease uncertainty and increase the legitimacy of regulatory decisions.
RECOMMENDATION: Public funding in the United States should be provided for independent follow-up studies when equivocal results are found in reasonably designed initial or preliminary experimental tests.
Compositional Analysis
Compositional Analysis of Genetically Engineered Crops. As part of the regulatory process of establishing substantial equivalence, GE crop developers submit data comparing the nutrient and chemical composition of their GE plant with a similar (isoline) variety of the crop. In the United States, submitting such data to FDA is voluntary, although as of 2015 this seems to always be done by developers. Developers and regulators compare key components of the GE variety with published reference guides that list the concentrations and variabilities of nutrients, antinutrients, and toxicants that occur in crops already in the food supply. 4 The section “Regulatory Testing of Crops with Resistance to Glyphosate and 2,4-D and the New Uses of the Herbicides Themselves” earlier in this chapter gives an example of the types of nutrients and chemicals that are generally measured. In the specific case of the soybean resistant to 2,4-D and glyphosate, measurements of 62 components in the soybean were submitted by Dow AgroSciences. There were statistically significant differences between the GE and comparison varieties in 16 of the 62. The differences were considered to be small and within the range of published values for other soybean varieties. They were therefore “considered not biologically relevant.” In compositional analysis, as in some of the whole-food animal testing, it is difficult to know how much of the variance and range in values for the components is due to the crop variety, the growing conditions, and the specific laboratory experimental equipment. In the United States, regulatory agencies require that the comparison be between the GE crop and its isogenic conventionally bred counterpart grown in side-by-side plots. In those cases, it is hard to attribute differences to anything but the genetic-engineering process.
FINDING: Statistically significant differences in nutrient and chemical composition have been found between GE and non-GE plants by using traditional methods of compositional analysis, but the differences have been considered to fall within the range of naturally occurring variation found in currently available non-GE crops.
4 OECD develops consensus documents that provide reference values for existing food crops ( OECD, 2015 ). These are publicly available online at http://www.oecd.org/science/biotrack/consensusdocumentsfortheworkonthesafetyofnovelfoodsandfeedsplants.htm (accessed May 9, 2016). The International Life Science Institute (ILSI) also maintains a crop composition database at www.cropcomposition.org (accessed May 9, 2016). ILSI reports that in 2013 the database contained more than 843,000 data points representing 3,150 compositional components.
Composition of Processed Genetically Engineered Foods . General compositional analysis and the specific content of the introduced proteins are typically conducted on raw products, such as maize kernels or soybean seed. However, much of the human consumption of these products occurs after substantial exposure to heat or other processing. If in processing of foods the amounts of GE proteins substantially increase, consumers are potentially exposed to a risk that is different from that anticipated from testing the raw material. In the production of oil, for example, the goal is to separate the oil from other compounds in the raw crop, such as proteins and carbohydrates. Crude oils can contain plant proteins ( Martín-Hernández et al., 2008 ), but in highly purified oils even sophisticated approaches have failed to find any nondegraded proteins ( Hidalgo and Zamora, 2006 ; Martín-Hernández et al., 2008 ). Those results are reflected in the fact that people who are allergic to soybean are not affected by purified oils ( Bush et al., 1985 ; Verhoeckx et al., 2015 ).
A few studies have searched for a means of finding DNA in plant-derived oils to identify the origin of the oil as GE or non-GE for labeling purposes ( Costa et al., 2010a , b ) or to identify the origin of olive oil ( Muzzalupo et al., 2015 ). It is possible to detect DNA, but the amounts are typically diminished in purified oils to 1 percent or less of the original content. Similarly, Oguchi et al. (2009) were not able to find any DNA in purified beet sugar. Some countries exempt products from labeling if GE protein or DNA is not detectable. For example, in Japan, where foods with GE ingredients typically require labeling, oil, soy sauce, and beet sugar are excluded because of degradation of GE proteins and DNA ( Oguchi et al., 2009 ). Australia and New Zealand have similar exemptions from labeling for such highly refined foods as sugars and oils ( FSANZ, 2013 ).
The detection of GE protein and DNA in other processed foods depends on the type of processing. For example, the amount of the Bt protein Cry1Ab detected by immunoassay in tortillas depends on cooking time ( de Luis et al., 2009 ). The detected amount of Cry9C protein remaining in samples of corn bread, muffins, and polenta was about 13, 5, and 3 percent of the amount in the whole-grain maize ( Diaz et al., 2002 ). For Cry1Ab in rice, Wang et al. (2015) found that baking was more effective in lowering the detection using polyclonal antibodies of the Cry1Ab protein than microwaving, but 20 minutes of baking at 180ºC left almost 40 percent of the protein intact. Heat denaturation of proteins can lower antibody binding to epitopes and cause lower detection of GE proteins.
FINDING: The amount of GE protein and DNA in food ingredients can depend on the specific type of processing; some foods contain no detectable protein and little DNA. In a few countries that have manda-
tory labeling of GE foods, that is taken into account, and food without detectable GE DNA or GE protein is not labeled.
Newer Methods for Assessing Substantial Equivalence . As explained in Chapter 2 , governance of GE crops includes regulatory governance. Although not required to by governing bodies, companies and academic researchers have moved beyond the typical measurements of food composition to newer technologies that involve transcriptomics, proteomics, and metabolomics. The new methods provide a broad, nontargeted assessment of thousands of plant characteristics, including the concentrations of most of the messenger RNAs, proteins, and small molecules in a plant or food. These methods are more likely to detect changes in a GE crop than the current regulatory approaches. If a GE crop has been changed only as intended, any changes observed in these -omics measurements theoretically should be predictable in a given environment. The science behind the methods, including the current limitations of their interpretation, is discussed in Chapter 7 . The discussion here focuses on how the methods have already been applied in the assessment of risk of health effects of currently commercialized GE crops.
Ricroch et al. (2011) reviewed -omics data from 44 studies of crops and detailed studies of the model plant Arabidopsis thaliana . Of those studies, 17 used transcriptomics, 12 used proteomics, and 26 used metabolomic methods. Ricroch (2013) updated the number of studies to 60. The committee found that many more studies had been done since those reviews were published, and many of them have used multiple -omics approaches. The sophistication of the studies has increased ( Ibáñez et al., 2015 ) and is likely to increase further. As recommended in Chapter 7 , there is a need to develop further and share databases that contain detailed -omics data ( Fukushima et al., 2014 ; Simó et al., 2014 ).
In some studies of GE plants in which simple marker genes were added, there were almost no changes in the transcriptome ( El Ouakfaoui and Miki, 2005 ), but use of other -omics methods has revealed changes ( Ren et al., 2009 ). For example, in a comparison of glyphosate-resistant soybean and non-GE soybean, García-Villalba et al. (2008) found that three free amino acids, an amino acid precursor, and flavonoid-derived secondary metabolites (liquiritigenin, naringenin, and taxifolin) had greater amounts in the GE soybean and 4-hydroxy-l-threonine was present in the non-GE soybean, but not in the GE variety. They hypothesized that the change in the flavonoids may have been because the modified EPSPS enzyme (a key enzyme of the shikimate pathway leading to aromatic amino acids) introduced to achieve glyphosate resistance could have different enzymatic properties that influenced the amounts of aromatic amino acids. The committee was not aware of such a hypothesis before this metabolomic study. (A concern was expressed in a comment submitted to the committee that
the EPSPS transgene would cause endocrine disruption. The committee found no evidence to suggest that the changes found by García-Villalba et al. would have such an effect.)
On the basis of previous experimentation, it is predicted that, when a gene for a nonenzymatic protein (such as a Bt toxin gene) is added to a plant, there will be very few changes in the plant’s metabolism ( Herman and Price, 2013 ). However, when a gene has been added specifically to alter one metabolic pathway of a plant, a number of predicted and unpredicted changes have been found. For example, Shepherd et al. (2015) found that, when they downregulated enzymes (that is, decreased expression or activity) involved in the production of either of two toxic glycoalkaloids (alpha-chaconine and alpha-solanine) in a GE potato with RNA-interfering transgenes that regulated synthesis of one toxic glycoalkaloid, the other compound usually increased. When they downregulated production of both compounds, beta-sitosterol and fucosterol increased. Neither of these compounds has the degree of toxicity associated with alpha-chaconine and alpha-solanine. Other compounds also differed from controls in concentration, but some of the changes may have been due to products generated during the tissue-culture process used in these experiments and not to the transgenes.
Many of the studies have found differences between the GE plants and the isogenic conventionally bred counterparts, but for many components there is more variation among the diverse conventionally bred varieties than between the GE and non-GE lines ( Ricroch et al., 2011 , Ricroch, 2013 ). Furthermore, the environmental conditions and the stage of the fruit or seed affect the finding. Chapter 7 addresses the future utility of the -omics approaches in assessing the biological effects of genetic engineering.
FINDING: In most cases examined, the differences found in comparisons of transcriptomes, proteomes, and metabolomes in GE and non-GE plants have been small relative to the naturally occurring variation found in conventionally bred crop varieties due to genetics and environment.
FINDING: If an unexpected change in composition beyond the natural range of variation in conventionally bred crop varieties were present in a GE crop, -omics approaches would be more likely to find the difference than current methods.
FINDING: Differences in composition found by using -omics methods do not, on their own, indicate a safety problem.
Food Allergenicity Testing and Prediction
Allergenicity is a widespread adverse effect of foods, several plants, tree and grass pollens, industrial chemicals, cosmetics, and drugs. Self-reporting of lifetime allergic responses to each of the most common food allergens (milk, egg, wheat, soy, peanut, tree nuts, fish, and shellfish) ranges from 1 to 6 percent of the population ( Nwaru et al., 2014 ). Allergies are induced in a two-step process: sensitization from an initial exposure to a foreign protein or peptide followed by elicitation of the allergic response on a second exposure to the same or similar agent. Sensitization and elicitation are generally mediated by immunoglobulins, primarily IgE, and the responses may range from minor palatal or skin itching and rhinitis to severe bronchial spasms and wheezing, anaphylaxis, and death. In addition to IgE responses to food allergens, IgA has been identified as an inducible immune mediator primarily in the gastrointestinal mucosa in response to foods, foreign proteins, pathogenic microorganisms, and toxins. The role of IgA in classical allergy has been investigated ( Macpherson et al., 2008 ).
Assessment of the potential allergenicity of a food or food product from a GE crop is a special case of food-toxicity testing and is based on two scenarios: transfer of any protein from a plant known to have food-allergy properties and transfer of a protein that could be a de novo allergen. Predictive animal testing for allergens in foods (GE and non-GE) is not sufficient for allergy assessment ( Wal, 2015 ). Research efforts are ongoing to discover or develop an animal model that predicts sensitization to allergy ( Ladics and Selgrade, 2009 ), but so far none has proved predictive ( Goodman, 2015 ). Therefore, researchers have relied on multiple indirect methods for predicting whether an allergic response could be caused by a protein that is either added to a food by genetic engineering or appears in the food as an unintended effect of genetic engineering. Endogenous protein concentrations with known allergic properties also have to be monitored because it is possible that their concentration could increase due to genetic engineering.
A flow diagram of the interactive approach to allergen testing recommended by the Codex Alimentarius Commission ( CAC, 2009 ) and EFSA (2010 , 2011a ) is presented in Figure 5-3 ( Wal, 2015 ); Box 5-2 describes the EPA testing of the Bt toxin Cry1F that generally follows this approach. The logic behind the approach starts with the fact that any gene for a protein that comes from a plant that is known to cause food allergies has a higher likelihood of causing allergenicity than any gene from a plant that does not cause an allergic response. If the introduced protein is similar to a protein already known to be an allergen, it becomes suspect and should be tested in people who have an allergy to the related protein. Finally, if a protein fits none of the above characteristics but is not digested by simulated gastric fluid, it could be a novel food allergen. The latter factor comes from
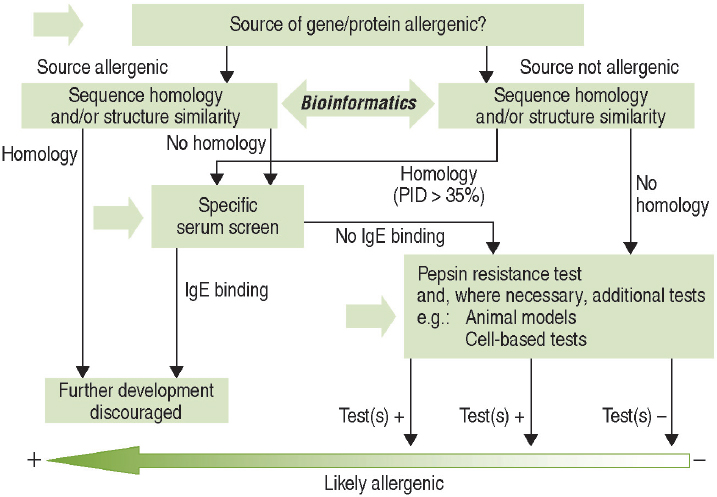
research demonstrating that some, but not all, proteins already known to be food allergens are resistant to digestion by gut fluid.
There is one case in which that approach was used and a GE crop with allergenicity issues was detected early and prevented from being commercialized, and a second case in which a GE crop was withdrawn from the market based on the possibly that it included a food allergen. In the first case, research was conducted on a soybean line genetically engineered to produce a Brazil nut ( Bertholletia excelsa ) protein, which was a known allergen. Sera from patients allergic to Brazil nut protein were available and tested positive for activity against the GE soybean protein. Because the segregation from the human food supply of GE soybean with that protein could not be guaranteed, the project was halted ( Nordlee et al., 1996 ). The soybean variety was never commercialized.
In the second case, EPA allowed a Bt maize variety developed by Aventis CropScience with a potential for allergenicity (due to decreased digestion of the protein Cry9c in simulated gastric fluid) to be sold as cattle feed under the name StarLink™; because of the potential for allergenicity, the variety was not approved for direct human consumption. However, the Bt protein was found in human food, so the maize variety was removed from all markets. After that incident, EPA no longer distinguished between Bt proteins in human food versus in animal feed ( EPA, 2001b ). Bt crop varieties are approved in the United States for all markets or none.
The interactive approach for testing should work for GE crops when the testing is for a transgene that is expressed by the plant as a protein that does not affect its metabolism (for example, Bt toxins). The testing does not cover endogenous allergens whose concentrations have been increased by unintended effects of genetic engineering. In 2013, the European Commission set a requirement for assessing endogenous allergens in GE crops ( EC, 2013 ). A number of articles since then have supported the approach ( Fernandez et al., 2013 ) or have found it unnecessary and impractical ( Goodman et al., 2013 ; Graf et al., 2014 ). Soybean is an example of a crop that has endogenous allergens. A paper on endogenous soybean allergens concluded that there is enough knowledge of only some soybean allergens for proper testing ( Ladics et al., 2014 ). As emphasized by Wal (2015) , there is considerable variation among conventionally bred varieties in the concentrations of endogenous allergens, especially when they are grown under different conditions. Therefore, the existing variation must be taken into consideration in assessing a GE variety. Of course, the issue is not only the magnitude of variation but the potential change in the overall exposure of the global human population to the allergen.
One example of an existing potential allergen of concern is gamma-zein, one of the storage proteins produced in the maize kernel that is a comparably hard-to-digest protein ( Lee and Hamaker, 2006 ). Concern was expressed to the committee that GE maize may have higher amounts of gamma-zein, which could be allergenic ( Smith, 2014 ). Krishnan et al. (2010) found that young pigs consuming maize generate antibodies against gamma-zein. That observation and the fact that the protein withstands pepsin digestion suggest that gamma-zein could be an allergen. In a comparison of the Bt maize line MON810 with non- Bt maize, known maize allergens, including the 27-kDa and 50-kDa gamma-zein proteins, were not found to be in significantly different amounts ( Fonseca et al., 2012 ). On the other hand, conventionally bred Quality Protein Maize is reported to have a 2 to 3 fold higher
concentration of the 27-kDa gamma-zein protein ( Wu et al., 2010 ). There is one patent for decreasing gamma-zein through genetic engineering. 5
There can be a connection between immune response and allergenicity. One well-cited study brought up in the public comment period was that by Finamore et al. (2008) , who assessed the effect of Bt maize ingestion on the mouse gut and peripheral immune system. They found that Bt maize produced small but statistically significant changes in percentage of T and B cells and of CD4+, CD8+, γδT, and αβT subpopulations at gut and peripheral sites and alterations of serum cytokines in weanlings fed for 30 days and in aged mice. However, there was no significant response in weaning mice that were fed for 90 days, which they related to further maturation of the immune system. They concluded that there was no evidence that the Bt toxin in maize caused substantial immune dysfunction. Similarly, Walsh et al. (2012a) did not find immune function changes in a long-term pig feeding study (80 or 110 days) on Bt MON810 maize compared with non-GE maize. Overall, no changes of concern regarding Bt maize feeding and altered immune response have been found.
At a public meeting that the committee held on health effects of GE foods, a question was raised about whether current testing for allergenicity is insufficient because some people do not have acidic conditions in their stomachs. Regarding that issue, digestibility of the proteins is assessed with simulated gastric fluid (0.32 percent pepsin, pH 1.2, 37ºC), under the premise that an undigested protein may lead to the absorption of a novel allergenic fragment ( Astwood et al., 1996 ; Herman et al., 2006 ). Stomach fluid is typically acidic, with a pH of 1.5–3.5, which is the range at which pepsin (the digestive enzyme of the stomach) is active, and the volume of stomach fluid is 20–200 mL (about 1–3 ounces). Simulated gastric fluid was developed to represent human gastric conditions in the stomach and is used in bioavailability studies of drugs and foods ( U.S. Pharmacopeia, 2000 ).
In general, if the pH of the stomach is greater than 5, pepsin will not be active, and less breakdown of large proteins will take place. Hence, the usefulness of simulated gastric fluid in the case of a less acidic (higher pH) stomach is questionable, whether used for non-GE foods or GE foods. Untersmayr and Jensen-Jarolim (2008:1301) concluded that “alterations in the gastric milieu are frequently experienced during a lifetime either physiologically in the very young and the elderly or as a result of gastrointestinal pathologies. Additionally, acid-suppression medications are frequently used for treatment of dyspeptic disorders.” Trikha et al. (2013) used a group of 4,724 children (under 18 years old) who had received a
5 Jung, R., W.-N. Hu, R.B. Meeley, V.J.H. Sewalt, and R. Nair. Grain quality through altered expression of seed proteins. U.S. Patent 8,546,646, filed September 14, 2012, and issued October 1, 2013.
diagnosis of gastroesophageal reflux disease (GERD) and who were treated with gastric acid-suppressive medication and matched with 4,724 children who had GERD but were not so treated. Those treated with acid-reducing medicine were more than 1.5 times as likely to have a diagnosis of food allergy as those who were not so treated. The difference between the two GERD groups was statistically significant (hazard ratio, 1.68; 95-percent confidence interval, 1.15–2.46).
The National Research Council report Safety of Genetically Engineered Foods pointed out that there were important limitations in allergenicity predictions that could be done before commercialization ( NRC, 2004 ). Since that report was published, there have been improvements in the allergen database, and research has been funded to improve precommercialization prediction. However, as the committee heard from an invited speaker, “no new methods have been demonstrated to predict sensitization and allergy in the absence of proven exposure” ( Goodman, 2015 ). Before commercialization, the general population will probably not have been exposed to an allergen similar enough to an allergen in a GE plant to cause cross-reactivity, so it would be useful to use the precommercialization tests only as a rough predictor. To ensure that allergens did not remain in the food system, the Safety of Genetically Engineered Foods report called for a two-step process of precommercialization testing and post-commercialization testing. Even though progress has been made on allergenicity prediction since that report was published in 2004, the committee found that post-commercialization testing would be useful in ensuring that no new allergens are introduced. There have been no steps toward post-commercialization testing since 2004. The committee recognized that such testing would be logistically challenging, as described in a scientific report to EFSA ( ADAS, 2015 ). Post-commercialization surveillance of such specific agents as drugs and medical devices is difficult, but there is generally a well-defined endpoint to look for in patients. In the case of food, the detection of an allergic response to a particular protein would be confounded by multiple exposures in the diet. However, several region-wide human populations have been exposed to GE foods for many years whereas others have not; this could enable an a priori hypothesis to be tested that populations that have been exposed to foods from specific GE crops will not show a higher rate of allergic response to such foods.
FINDING: For crops with endogenous allergens, knowing the range of allergen concentrations in a broad set of crop varieties grown in a variety of environments is helpful, but it is most important to know whether adding a GE crop to the food supply will change the general exposure of humans to the allergens.
FINDING: Because testing for allergenicity before commercialization could miss allergens to which the population had not previously been exposed, post-commercialization allergen testing would be useful in ensuring that consumers are not exposed to allergens, but such testing would be difficult to conduct.
FINDING: There is a substantial population of persons who have higher than usual stomach pH, so tests of digestibility of proteins in simulated acidic gastric fluid may not be relevant to this population.
GENETICALLY ENGINEERED CROPS AND OCCURRENCE OF DISEASES AND CHRONIC CONDITIONS
The overall results of short-term and long-term animal studies with rodents and other animals and other data on GE-food nutrient and secondary compound composition convinces many (for example, Bartholomaeus et al., 2013 ; Ricroch et al., 2013a , b ; Van Eenennaam and Young, 2014 ) but not all involved researchers (for example, Dona and Arvanitoyannis, 2009 ; Domingo and Bordonaba, 2011 ; Hilbeck et al., 2015 ; also see DeFrancesco, 2013 ) that currently marketed GE foods are as safe as foods from conventionally bred crops. The committee received comments from an invited speaker ( Smith, 2014 ) and from the public regarding the possible relationship between increases in the incidence of specific chronic diseases and the introduction of GE foods into human diets. Appendix F includes a representative list of the comments about GE food safety that were sent to the committee through the study’s website. The comments mentioned concerns about such chronic diseases as cancers, diabetes, and Parkinson’s; possible organ-specific injuries (liver and kidney toxicity); and such disorders as autism and allergies. Smith (2003:39) made the claim that “diabetes rose by 33 percent from 1990 to 1998, lymphatic cancers are up, and many other illnesses are on the rise. Is there a connection to [genetically modified] foods? We have no way of knowing because no one has looked for one.”
As part of the committee’s effort to respond to its task to “assess the evidence for purported negative effects of GE crops and their accompanying technologies,” it used available peer-reviewed data and government reports to assess whether any health problems may have increased in frequency in association with commercialization of GE crops or were expected to do so on the basis of the results of toxicity studies. The committee presents additional biochemical data from animal experiments but relies mostly on epidemiological studies that used time-series data. The epidemiological data for some specific health problems are generally robust over time (for example, cancers) but are less reliable for others. The committee presents the available data knowing that they include a number of sources of bias,
including changes over time in survey methods and in the tools for detection of specific chronic diseases. As imperfect as the data may be, they are in some cases the only information available beyond animal experiments for formulating or testing hypotheses about possible connections between a GE food and a specific disease. The committee points out that the lack of rigorous data on incidence of disease is not only a problem for assessing effects of GE foods on health. More rigorous data on time, location, and sociocultural trends in disease would enable better assessment of potential health problems caused by environmental factors and other products from new technologies.
Cancer Incidence
A review of the American Cancer Society’s database indicates that mortality from cancers in the United States and Canada has continued to decrease or stabilized in all categories except cancers of the lung and bronchus attributable to smoking. The decreases in mortality are due in part to early detection and improved treatment, so mortality data can mask the rate at which cancers occur. For that reason, the committee sought data on cancer incidence rather than cancer mortality. Figures 5-4 and 5-5 show
changes in cancer incidence in U.S. women and men, respectively, from 1975 to 2011 ( NCI, 2014 ). If GE foods were causing a substantial number of specific cancers, the incidence of those cancers would be expected to show a change in slope in the time series after 1996, when GE traits were first available in commercial varieties of soybean and maize. The figures show that some cancers have increased and others decreased, but there is no obvious change in the patterns since GE crops were introduced into the U.S. food system. Figures 5-6 and 5-7 show cancer incidence in women and men in the United Kingdom, where GE foods are not generally being consumed. For the specific types of cancers that are reported in both the United States and the United Kingdom, there is no obvious difference in the patterns that could be attributed to the increase in consumption of GE foods in the United States. (The absolute numbers cannot be compared because of differences in methodology.)
Forouzanfar et al. (2011) published data on breast and cervical cancer incidence worldwide from 1980 to 2010. As can be seen in Figure 5-8 , the global incidence of those two cancers has increased. An examination of the plots for North America (high income) (Canada and the United States), where GE foods are eaten, compared with the plots for western
Europe, where GE foods generally are not eaten, shows similar increases in incidence of breast cancer and no increase in cervical cancer. The data do not support the hypothesis that GE-food consumption has substantially increased breast and cervical cancer. (The data for North America [high
income] and western Europe are different from those in the studies above on the incidence of cancer in the United States and the United Kingdom.)
Taken together, Figure 5 through Figure 8 do not support the hypothesis that GE foods have resulted in a substantial increase in the incidence of cancer. However, they do not establish that there is no relationship between cancer and GE foods because there can be a delay in the onset of cancer that would obscure a trend, and one could hypothesize that something else has occurred with GE foods in the United States that has lowered cancer incidence and thus obscured a relationship. The committee had limited evidence on which to make its judgments, but the evidence does not support claims that the incidence of cancers has increased because of consumption of GE foods.
There is ongoing debate about potential carcinogenicity of glyphosate in humans. Assessment of glyphosate is relevant to the committee’s report because it is the principal herbicide used on HR crops ( Livingston, et al. 2015 ), and it has been shown that there are higher residues of glyphosate in HR soybean treated with glyphosate than in non-GE soybean ( Duke et al., 2003 ; Bøhn et al., 2014 ). Box 5-5 provides details about a study by Séralini et al. (2012 , 2014) that concluded that glyphosate causes tumors in rats. The committee found that this study was not conclusive and used incorrect statistical analysis. The most detailed epidemiological study that tested for a relationship between cancer and glyphosate as well as other agricultural chemicals found “no consistent pattern of positive associations indicating a causal relationship between total cancer (in adults or children) or any site-specific cancer and exposure to glyphosate” ( Mink et al., 2012:440 ; also see section below “Health Effects of Farmer Exposure to Insecticides and Herbicides”).
In 1985, EPA classified glyphosate as Group C (possibly carcinogenic to humans) on the basis of tumor formation in mice. However, in 1991, after reassessment of the mouse data, EPA changed the classification to Group E (evidence of noncarcinogenicity in humans) and in 2013 reaffirmed that “based on the lack of evidence of carcinogenicity in two adequate rodent carcinogenicity studies, glyphosate is not expected to pose a cancer risk to humans” ( EPA, 2013:25399 ).
In 2015, the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) issued a monograph on glyphosate as part of its volume on some organophosphate insecticides and herbicides ( IARC, 2015 ). In the monograph, IARC classified glyphosate in Group 2A (probably carcinogenic to humans). A summary and reasons for the classification were published in Lancet Oncology ( Guyton et al., 2015 ).
The 2015 IARC Working Group found that, although there is “ limited evidence in humans for the carcinogenicity of glyphosate,” there is “ sufficient evidence in experimental animals for the carcinogenicity of glyphosate”
( IARC, 2015:78 ). Furthermore, IARC noted that there is mechanistic support in that glyphosate induces oxidative stress, which could cause DNA damage, and some epidemiological data that support the classification.
EFSA (2015) evaluated glyphosate after the IARC report was released and concluded that glyphosate is unlikely to pose a carcinogenic risk to humans. Canada’s health agency concluded that “the level of human exposure, which determines the actual risk, was not taken into account by WHO (IARC)” ( Health Canada, 2015 ). The Canadian agency found that current food and dermal exposure to glyphosate even by those who work directly with glyphosate is not a health concern as long as it is used as directed on product labels ( Health Canada, 2015 ). EPA (2015) found that glyphosate does not interact with estrogen, androgen, or thyroid systems.
A comment to the committee expressed concern that glyphosate breaks down to formaldehyde, which was classified as a known human carcinogen by IARC (2006) . However, this hypothesis was not supported; Franz et al. (1997) used radiolabeled glyphosate and failed to show formation of formaldehyde in the normal environmental degradation of glyphosate.
FINDING: The incidence of a variety of cancer types in the United States has changed over time, but the changes do not appear to be associated with the switch to consumption of GE foods. Furthermore, patterns of change in cancer incidence in the United States are generally similar to those in the United Kingdom and Europe, where diets contain much lower amounts of food derived from GE crops. The data do not support the assertion that cancer rates have increased because of consumption of products of GE crops.
FINDING: There is significant disagreement among expert committees on the potential harm that could be caused by the use of glyphosate on GE crops and in other applications. In determining the risk from glyphosate and formulations that include glyphosate, analyses must take into account both marginal exposure and potential harm.
Kidney Disease
It has been hypothesized that kidney disease may have increased because GE proteins reached the kidney. The committee examined epidemiological data to determine whether there was a correlation between the consumption of GE foods and the prevalence of chronic kidney disease (CKD).
The total prevalence of all stages of CKD in the United States increased 2 percent from about 12 percent in 1988–1994 to 14 percent in 1999–2004, but the total prevalence has not increased significantly since then.
Figure 5-9 presents prevalence data on the five progressively more serious, recognized stages of CKD ( USRDS, 2014 ). The greatest percent increase is seen in Stage 3, and based on the study ( USRDS, 2014 ), a large amount of the increase occurred in people with comorbidity of cardiovascular disease. Prevalence of CKD increases substantially with age ( Coresh et al., 2003 ), so the aging of the U.S. population may contribute to the overall increase ( U.S. Census Bureau, 2014 ), as does the increase in diabetes and hypertension ( Coresh et al., 2007 ).
FINDING: The available data on prevalence of chronic kidney disease in the United States show a 2 percent increase from 1988 to 2004, but the increase does not appear to be attributable to consumption of GE foods.
Obesity in humans is a complex condition associated with several genetic and environmental factors—including geography, ethnicity, socioeconomic status, lack of exercise, availability of fresh fruits and vegetables, and less nutritional meals ( Thayer et al., 2012 )—and an altered functioning microbiome ( Turnbaugh et al., 2009 ).
Studies of various species examined body-weight gain when animals were fed a GE crop, a non-GE isogenic comparator, or a non-GE, nonisogenic control. The authors concluded that there were no biologically relevant differences in body-weight gain regardless of the length of the studies ( Rhee et al. 2005 ; Hammond et al., 2006 ; Arjó et al., 2012 ; Buzoianu et al., 2012b ; Ricroch et al., 2013a , b ; Halle and Flachowsky, 2014 ; Nicolia et al. 2014 ).
Human population studies have shown that obesity has become more prevalent in the United States (for example, Fryar et al., 2014 ). An (2015) provided a graphic of the change in U.S. adults (sorted by education level) from 1984 to 2013 ( Figure 5-10 ). As can be seen in the figure, the percentage of obese U.S. adults increased until about 2009, at which time it appears to level off. Because there is no increase in the slope after commercialization of GE crops, these data do not support the hypothesis that GE crops have increased obesity. These time-series data do not prove that there is no association, but if one is present, it is not strong.
Those statistics on obesity coincide with those on the incidence of type II diabetes in the United States ( Abraham et al., 2015 ) and therefore do not support a relationship between GE crops and type II diabetes.
FINDING: The committee found no published evidence to support the hypothesis that the consumption of GE foods has caused higher U.S. rates of obesity or type II diabetes.
Gastrointestinal Tract Diseases
Although the gastrointestinal tract has evolved to digest dietary proteins in the stomach and small intestine effectively for absorption and use of amino acids, it is normal for some full proteins or their fragments to cross the gut barrier through a paracellular route (between cells) or damaged mucosa and for the immune system, which has a high presence at the interface of the gut wall and the internal circulation, to respond accordingly. It is also not unusual, given the high sensitivity of today’s analytical equipment, for proteins or fragments to be detected in minute amounts in different body fluids. Detection methods are not specific to transgene-produced proteins but can find any dietary protein or fragment that is able to pass from the gastrointestinal tract into the bloodstream and tissues. The presence
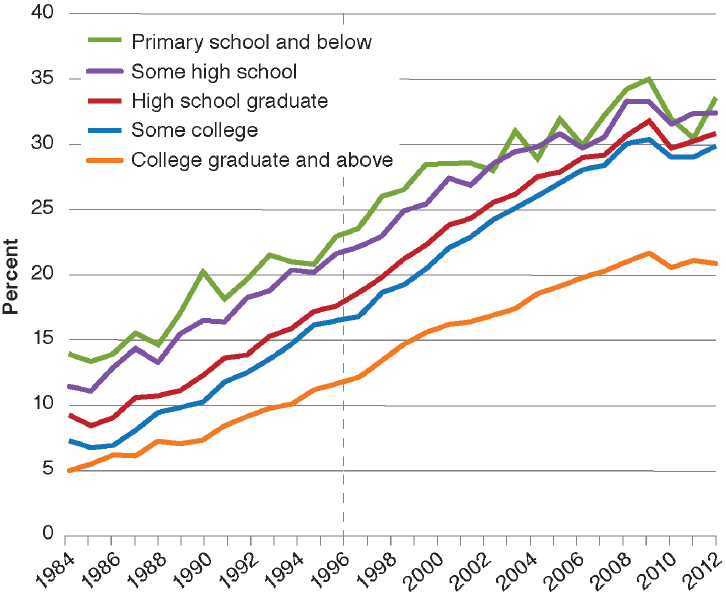
of a dietary protein or its fragment in the bloodstream or in tissues is not unusual or a cause for health concerns.
About 60–70 percent of the body’s immune system is in the gastrointestinal tract’s gut-associated lymphoid tissue, which has an interface with the gut luminal contents, including toxins, allergens, and the associated microbiota. For GE crops, a public concern has been that the immune system is compromised through ingested transgenic proteins. That possibility has been investigated in animal studies that examined immune system bio-markers and epithelial cell integrity (see section “Beyond Rodent Studies” above and Walsh et al., 2011 ).
It was suggested to the committee in presentations and public comments that fragments of transgenes may have some special properties that would result in human diseases if they were absorbed into the body through
the digestive tract. The mechanism by which such genes or proteins would affect the body is not clear, although Smith (2013) hypothesized that consuming GE foods increased gut permeability.
FINDING: The committee could find no published evidence supporting the hypothesis that GE foods generate unique gene or protein fragments that would affect the body.
Celiac Disease
Celiac disease is an autoimmune disorder that affects about 1 percent of the population of western countries. It is triggered in susceptible people by consumption of gluten-containing cereal grains ( Fasano et al., 2003 ; Catassi et al., 2010 ). Symptoms of celiac disease are the result of an immune reaction that causes marked gastrointestinal inflammation in persons susceptible to gliadin, a component of gluten protein found in wheat, rye ( Secale cereale ), and barley ( Hordeum vulgare ) ( Green and Cellier, 2007 ). In addition to exposure to gluten, the etiology of celiac disease is multifactorial and includes genetic predisposition, microbial infection of the gastrointestinal tract, antibiotic exposure, and gastrointestinal erosion ( Riddle et al., 2012 ). Diagnosis is based on detection of serum concentrations (serotypes) of IgA tissue transglutaminase and endomysial antibody IgA, the relief of symptoms upon gluten avoidance, and tissue biopsy. The genetic changes related to the serotyped IgAs are found in about 30 percent of the Caucasian population, but susceptibility to celiac disease is found in only 1 percent of this population ( Riddle et al., 2012 ).
The committee was able to find data on the incidence of celiac disease in the United Kingdom ( West et al., 2014 ; Figure 5-11 ) and a detailed study conducted by the Mayo Clinic in one county in Minnesota ( Murray et al., 2003 ; Ludvigsson et al., 2013 ). In the Minnesota and UK studies, there is a clear pattern of increase in celiac-disease incidence (or at least its detection or the extent of self-reports) that started before 1996 ( Catassi et al., 2010 ), when U.S. citizens began to consume more GE foods and the use of glyphosate increased in the United States but not in the United Kingdom. The increases are similar in magnitude to that found in U.S. military personnel, in whom prevalence increased from 1.3 per 100,000 in 1999 to 6.5 per 100,000 in 2008 ( Riddle et al., 2012 ). The authors cautioned that most cases of celiac disease are undiagnosed. Some of the observed increase may be related to improvements in diagnostic criteria, greater awareness of the disease in physicians and patients, better blood tests, and increases in the number of biopsies. However, recent observations point to an increase in incidence beyond those factors (J. A. Murray, Mayo Clinic, personal communication, February 1, 2016).
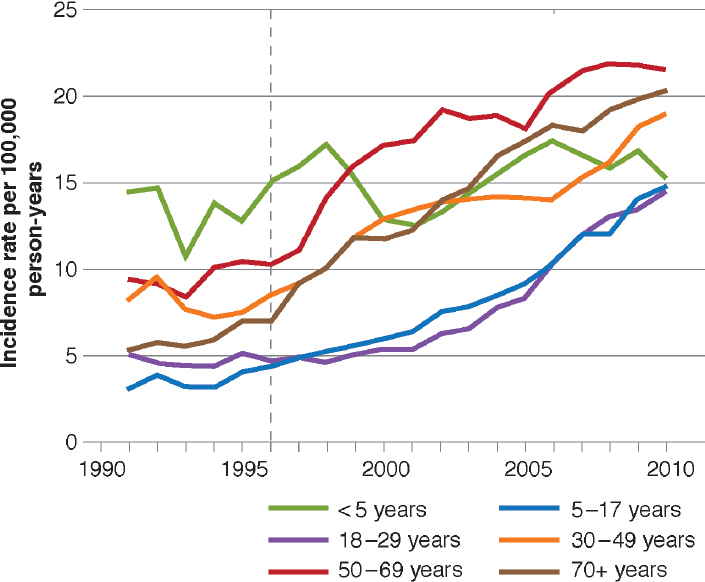
On the basis of data collected in the 2009–2010 National Health and Nutrition Examination Survey, Rubio-Tapia et al. (2012) reported a prevalence of celiac disease of 0.71 percent with 1.01 percent in non-Hispanic whites in a sample of 7,798 subjects. It should be noted that there has not been any commercial production of GE wheat, rye, or barley in the world. The committee found no evidence that the introduction of GE foods affected the incidence or prevalence of celiac disease worldwide.
FINDING: Celiac-disease detection began increasing in the United States before the introduction of GE crops and the increased use of glyphosate. It appears to have increased similarly in the United Kingdom, where GE foods are not typically consumed and glyphosate use did not increase. The data are not robust, but they do not show a major difference in the rate of increase in incidence of celiac disease between the two countries.
Food Allergies
Speakers and some members of the public suggested that the prevalence of food allergies has increased because of GE crops. The committee examined records on the prevalence of food allergies in the United States over time. As is clear from Figure 5-12 and Jackson et al. (2013) , the prevalence of food allergies in the United States is rising. For a rough comparator, the committee examined data on hospital admissions for food allergies in the United Kingdom over time ( Figure 5-13 ). UK citizens eat far less food derived from GE crops. The data ( Gupta et al., 2007 ) suggest that food allergies are increasing in the United Kingdom at about the same rate as in the United States (but the types of measurement are different).
FINDING: The committee did not find a relationship between consumption of GE foods and the increase in prevalence of food allergies.
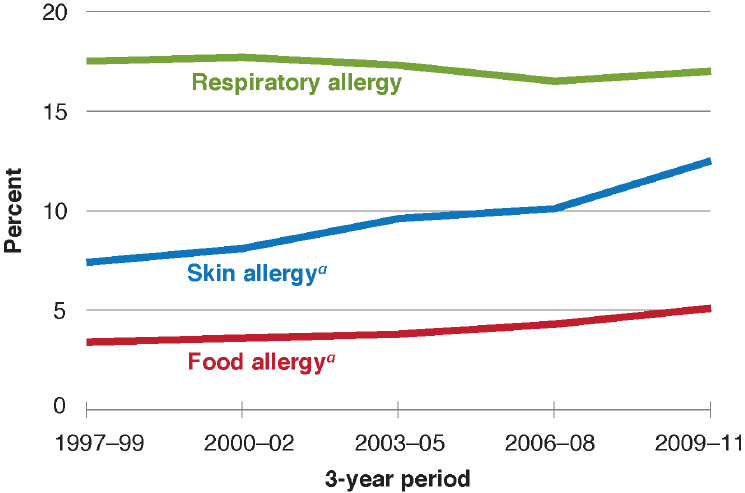
Autism Spectrum Disorder
Autism is often described by such symptoms as difficulty in communicating, forming personal relationships, and using language and abstract concepts. According to the American Psychiatric Association (2013) , autism spectrum disorder (ASD) encompasses the previous diagnoses of autism, Asperger syndrome, pervasive developmental disorder not otherwise specified, and childhood disintegrative disorder. Accurate diagnosis of ASD can be difficult, but efforts to identify children with ASD have increased in the United States over the last three decades ( CDC, 2014 ).
In the 2010 Centers for Disease Control and Prevention (CDC) survey of ASD in 11 regions of the United States ( CDC, 2014 ), the overall prevalence in children 8 years old was about 1 in 68 (1.47 percent), but there was wide variation among regions and sociocultural groupings of children. The CDC report stated that “the extent to which this variation might be
attributable to diagnostic practices, under-recognition of ASD symptoms in some racial/ethnic groups, socioeconomic disparities in access to services, and regional differences in clinical or school-based practices that might influence the findings in this report is unclear” ( CDC, 2014:1 ). The degree to which the increase in ASD prevalence since 1990 is due to improved diagnosis is also unclear.
Before 1990, few children in the United States or the United Kingdom had diagnoses of ASD ( Taylor et al., 2013 ), but the prevalence has increased dramatically in both countries. Researchers in the United States and United Kingdom wrote a report that examined prevalence of ASD in the United Kingdom over time and compared it with that in the United States ( Taylor et al., 2013 ). They concluded that “a continuous simultaneous extraordinary rise in the number of children diagnosed as autistic began in both countries in the early 1990s and lasted for a decade. The distribution of first time diagnosis according to age and gender was the same. These similarities between countries as well as within different locations in each country point to a common etiology for this extraordinary medical case” ( Taylor et al., 2013:5 ). There is a higher prevalence in the United States, but it is difficult to evaluate whether it is because of differences in efforts in and approaches to diagnosis and in sociocultural factors that seem to influence prevalence. The overall similarities in prevalence of ASD in the United Kingdom, where GE foods are rarely eaten, and in the United States, where GE foods are commonly eaten, suggest that the major rise in ASD is not associated with consumption of GE foods.
FINDING: The similarity in patterns of increase in autism spectrum disorder in children in the United States, where GE foods are commonly eaten, and the United Kingdom, where GE foods are rarely eaten, does not support the hypothesis of a link between eating GE foods and prevalence of autism spectrum disorder.
OTHER HUMAN HEALTH CONCERNS RELATED TO GENETICALLY ENGINEERED CROPS
The committee heard from some members of the public and some invited speakers that ailments of gastrointestinal origin could be caused by GE crops or their associated technologies or by foods derived from GE crops. The committee investigated the evidence available for that hypothesis.
Gastrointestinal Tract Microbiota
The committee received comments from the public that foods derived from GE crops could change the gut microbiota in an adverse way. Three
scenarios can be considered as related to the potential effects of GE crops on the gut microbiota: the effect of the transgene product (for example, Bt toxin), unintended alteration of profiles of GE plant secondary metabolites, and herbicide (and adjuvant) residue (for example, glyphosate) and its metabolites in HR crops.
Research on the human gut microbiota (the community of microorganisms that live in the digestive tract) is rapidly evolving with recent reports ( Dethlefsen and Relman, 2011 ; David et al., 2014 ) that suggest that microbiota perturbations occur fairly quickly owing to dietary components or antibiotic treatment. Microbiota composition and state are now well recognized to be linked to noncommunicable chronic diseases and other health problems, so factors that cause either beneficial or adverse changes in the microbiota are of interest to researchers and clinicians. However, the science has not reached the point of understanding how specific changes in microbiota composition affect health and what represents a “healthy” microbiota. The effect of different dietary patterns (for example, high-fat versus high-carbohydrate diets) on the gut microbiota has been linked to metabolic syndrome ( Ley, 2010 ; Zhang et al., 2015 ).
As discussed above, most proteins, including those in GE and conventionally bred crops, are at least partially digested in the stomach by the action of pepsin that is maintained by the acidic pH of the stomach in most people. Further digestion and absorption are a function of the small intestine, where amino acids and dipeptides and tripeptides are absorbed. Therefore, an effect of a dietary protein on the microbiota, whether from GE or non-GE foods, is unlikely. However, there is some evidence that Bt proteins can be toxic to microorganisms ( Yudina et al., 2007 ), and some nondegraded Bt protein is found within the lumen of the gut but not in the general circulation of pigs ( Walsh et al., 2011 ). Buzoianu et al. (2012c , 2013a ) studied the effect of Bt maize feeding on microbiota composition in pigs. In their 2012 study, 110-day feeding of Bt maize (variety MON810) and of isogenic non-GE maize diets led to no differences in cultured Enterobacteriaceae, Lactobacillus , and total anaerobes from the gut; 16S rRNA sequencing showed no differences in bacterial taxa, except the genus Holdemania with which no health effects are associated ( Buzoianu et al., 2012c ). In the follow-up study in which intestinal content of sows and their offspring were examined with 16S rRNA gene sequencing, the only observed difference for major bacterial phyla was that Proteobacteria were less abundant in sows fed Bt maize before farrowing and in offspring at weaning compared with the controls ( Buzoainu et al., 2013a ). Fecal Firmicutes were more abundant in offspring fed GE maize. There were other inconsistent differences in mostly low-abundance microorganisms. On the basis of the overall results from their studies, the authors concluded that none of the changes seen in the animals was expected to have biologically relevant health effects on the animals.
Relatively few studies have examined the influence of plant secondary metabolites from any crop on the gut microbiota. The review of Valdés et al. (2015) highlighted investigations on polyphenol-rich foods—such as red wine, tea, cocoa, and blueberries—on the microbiota. Effects were considered minor. As discussed above (see the section “Endogenous Toxins in Plants”), current commercialized GE crops do not have distinctly different secondary metabolite profiles that would lead one to think that they would affect the gut microbiota.
No studies have shown that there are perturbations of the gut microbiota of animals fed foods derived from GE crops that are of concern. However, the committee concluded that this topic has not been adequately explored. It will be important to conduct research that leads to an understanding of whether GE foods or GE foods coupled with other chemicals have biologically relevant effects on the gut microbiota.
FINDING: On the basis of available evidence, the committee determined that the small perturbations found in the gut microbiota of animals fed foods derived from GE crops are not expected to cause health problems. A better understanding of this subject is likely as the methods for identifying and quantifying gut microorganisms mature.
Horizontal Gene Transfer to Gut Microorganisms or Animal Somatic Cells
Horizontal (or lateral) gene transfer is “the stable transfer of genetic material from one organism to another without reproduction or human intervention” ( Keese, 2008:123 ). Since GE crops were commercialized, concern has been voiced by some scientists and some members of the public that foreign DNA introduced into plants through genetic-engineering technologies might, after ingestion, be transferred to the human gut microbiota and directly or indirectly (that is, from bacteria) into human somatic cells. Although most of the concern regarding horizontal gene transfer has been focused on antibiotic-resistance genes used as markers of the transgenic event, other transgenes, such as those with Bt toxins, have also been of concern.
A prerequisite for horizontal gene transfer is that the recombinant DNA must survive the adverse conditions of both food processing and passage through the gastrointestinal tract. Netherwood et al. (2004) showed in patients with a surgically implanted exiting tube placed at the end of the small intestine (an ileostomy) that a small amount of the GE soybean transgene EPSPS passed through the upper gastrointestinal tract to the point of the distal ileum; in subjects without an ileostomy, no transgene was recovered from their feces. In their review on stability and degradation of
DNA from foods in the gastrointestinal tract, Rizzi et al. (2012) noted that recombinant plant DNA fragments were detected in the gastrointestinal tracts of nonruminant animals but not detected in blood or other tissues, although some nonrecombinant plant DNA could be found. The authors concluded that some natural plant DNA fragments persist in the lumen of the gastrointestinal tract and in the bloodstream of animals and humans.
For an event to be considered horizontal gene transfer, DNA must be in the form of a functional (rather than fragmented) gene, enter into bacterial or somatic cells, and be incorporated into the genome with an appropriate promoter, and it must not adversely affect the competitiveness of the cells; otherwise, the effect would be short-lived.
Plant DNA has not been demonstrated to be incorporated into animal cells; however, it has been shown to be transferred in prokaryotes (bacteria). Indeed, molecular geneticists had to find genetic-engineering approaches for getting DNA to be taken into eukaryote cells and incorporated into a genome. The report A Decade of EU-Funded GMO Research (2001–2010) ( EC, 2010a ) described a study that shows that rumen ciliates (a type of microorganism) exposed to Bt 176 maize for 2 or 3 years did not incorporate the Bt 176 transgene. There are no reproducible examples of horizontal gene transfer of recombinant plant DNA into the human gastrointestinal microbiota or into human somatic cells. Three independent reviews of the literature on the topic ( van den Eede et al., 2004 ; Keese, 2008 ; Brigulla and Wackernagel, 2010 ) concluded that new gene acquisition by the gut bacteria through horizontal gene transfer would be rare and does not pose a health risk.
FINDING: On the basis of its understanding of the process required for horizontal gene transfer from plants to animals and data on GE organisms, the committee concludes that horizontal gene transfer from GE crops or conventionally bred crops to humans does not pose a substantial health risk.
Transfer of Transgenic Material Across the Gut Barrier into Animal Organs
Conflicting reports exist regarding the question of intact transgenes and transgenic proteins from foods crossing the gut barrier. Spisák et al. (2013) published results that indicate that complete genes in foods can pass into human blood. That is plausible, but Lusk (2014) examined the approach used by Spisák et al. and found it more likely that the findings were due to contaminants. Lusk emphasized the need for negative controls in such studies. Placental transfer of foreign DNA into mice was found by Schubbert et al. (1998) by detection in the mouse fetus, but a later report
from the same laboratory ( Hohlweg and Doerfler, 2001 ) did not find the transfer in an eight-generation study.
Studies with dairy cows and goats did not find transgenes or GE proteins in milk, although chloroplast DNA fragments were detected in milk ( Phipps et al., 2003 ; Nemeth et al., 2004 ; Calsamiglia, et al., 2007 ; Rizzi et al., 2008 ; Guertler et al., 2009 , Einspanier, 2013 ; Furgał-Dierżuk et al., 2015 ). That makes it clear that there is no apparent potential for trangenes or transgenic proteins to be present in dairy products. However, these animals are ruminants, and their digestive systems are different from that of humans.
Walsh et al. (2012a) studied the fate of a Bt gene and protein in pigs that have digestive systems that are more similar to that of humans. They found no evidence of the gene or protein in any organs or blood after 110 days of feeding on Bt maize, but they did find them in the digestive contents of the stomach, cecum, and colon. Fragments of Cry1Ab transgene (as well as other common maize gene fragments) but not the intact Bt gene were found in blood, liver, spleen, and kidney of pigs raised on Bt maize ( Mazza et al., 2005 ).
FINDING: Experiments have found that Cry1Ab fragments but not intact Bt genes can pass into organs and that these fragments present concerns no different than other genes that are in commonly consumed non-GE foods and that pass into organs as fragments.
FINDING: There is no evidence that Bt transgenes or proteins have been found in the milk of ruminants. Therefore, the committee finds that there should be no exposure to Bt transgenes or proteins from consuming dairy products.
OVERALL FINDING ON PURPORTED ADVERSE EFFECTS ON HUMAN HEALTH OF FOODS DERIVED FROM GE CROPS: On the basis of detailed examination of comparisons of currently commercialized GE and non-GE foods in compositional analysis, acute and chronic animal-toxicity tests, long-term data on health of livestock fed GE foods, and human epidemiological data, the committee found no differences that implicate a higher risk to human health from GE foods than from their non-GE counterparts.
ASSESSMENT OF HUMAN HEALTH BENEFITS FROM GENETICALLY ENGINEERED CROPS
There are now a number of examples of crops, either commercialized or in the pipeline toward commercialization, that have GE traits that could improve human health. Improvement of human health can be the sole moti-
vation for development of a specific crop trait, or it can be the secondary effect of a crop trait that is developed primarily for another reason. For example, the genetic engineering of rice to have higher beta-carotene has the specific goal of reducing vitamin A deficiency. GE maize that produces Bt toxins is engineered to decrease insect-pest damage, but a secondary effect could be a decrease in contamination of maize kernels by fungi that produce mycotoxins, such as fumonisins, that at high concentrations could impair human health. Beyond the direct effects of the crops on improvement of human health, there is also a potential indirect benefit associated with a decline in the exposure of insecticide applicators and their families to some insecticides because some GE plants decrease the need for insecticidal control.
Foods with Additional Nutrients or Other Healthful Qualities
Improved micronutrient content.
According to WHO, some 250 million preschool children are vitamin A–deficient. Each year, 250,000–500,000 vitamin A–deficient children become blind, and half of them die within 12 months of losing their sight. 6 Unlike children in wealthier societies, those children have diets that are restricted mostly to poor sources of nutrients, such as rice ( Hefferon, 2015 ). Overall improvement of the diets of the children and their parents is a goal that has not been reached; measures that improve the nutritional quality of their food sources are desirable although not optimal, as a diverse, healthy diet would be.
Crop breeders have used conventional breeding to improve the concentrations of beta-carotene in maize ( Gannon et al., 2014 ; Lividini and Fiedler, 2015 ), cassava, banana and plantain ( Musa spp.) ( Saltzman et al., 2013 ), and sweet potato ( Ipomoea batatas ) ( Hotz et al., 2012a , b ). There is some loss of beta-carotene during storage and cooking, but bioavailability is still good ( Sanahuja et al., 2013 ; De Moura et al., 2015 ). The most rigorous assessments of the effects of those high–beta-carotene varieties were conducted with orange-fleshed sweet potato (high in beta-carotene) in farming areas of Mozambique and Uganda. In both countries, there was increased beta-carotene intake. In Uganda, there was a positive relationship between consumption of high–beta-carotene sweet potato and positive vitamin A status ( Hotz et al., 2012a ). A more recent study in Mozambique found a decrease in diarrhea prevalence associated with consumption of the high–beta-carotene sweet potato ( Jones and DeBrauw, 2015 ).
6 Micronutrient deficiencies. Available at http://www.who.int/nutrition/topics/vad/en/ . Accessed October 30, 2015.
No reported experiments have tested any crop with high–beta-carotene for unintended effects. There has been concern about the potential for too high a concentration of beta-carotene in crops because of the hypervitaminosis A syndrome that can be caused by direct intake of too much vitamin A, but that is not a problem when the source is beta-carotene ( Gannon et al., 2014 ).
Golden Rice, which was produced through genetic engineering to increase beta-carotene content, is one of the most recognized examples of the use of genetic-engineering technology to improve a crop’s nutritional value. It is based on the understanding that rice possesses the entire machinery to synthesize beta-carotene in leaves but not in the grain. The breakthrough in the development of Golden Rice was the finding that only two genes are required to synthesize beta-carotene in the endosperm of the rice grain ( Ye et al., 2000 ). The first version of Golden Rice had a beta-carotene content of 6 µg/g. To raise the content to a point where it could alleviate vitamin A deficiency without consumption of very large amounts of rice, a second version of Golden Rice was produced by transforming the plant with the psy gene from maize. The carotene content was thereby raised above 30 µg/g ( Paine et al., 2005 ). Varieties that yield well, have good taste and cooking qualities, and cause no adverse health effects from unintended changes in the rice could have highly important health effects ( Demont and Stein, 2013 ; Birol et al., 2015 ). There have been claims that Golden Rice was ready for public release for well over a decade ( Hefferon, 2015 ), but this is not the case.
There is a publication on a field test of the first version of Golden Rice ( Datta et al., 2007 ), but the committee could not find information on the newer, higher–beta-carotene Golden Rice in the peer-reviewed literature. Therefore, it contacted the International Rice Research Institute (IRRI) Golden Rice project coordinator, Violeta Villegas, for an update on the status of the project. In discussions with Dr. Villegas (IRRI, personal communication, 2015), it was clear that the project is progressing with a new lead transgenic event, GR2-E, because of difficulties with the previous lead event, GR2-R. The GR2-E event has been backcrossed into varieties that have been requested by several countries including the Philippines, Bangladesh, and Indonesia. As of March 2016, Golden Rice GR2-E in PSBRc82 and BRRI dhan20 genetic backgrounds was being grown in confined field tests in the Philippines and Bangladesh, respectively. Both Golden Rice varieties underwent preliminary assessment inside the greenhouse prior to planting in confined field tests. If performance is good, the varieties will be moved to open field-testing on multiple locations. Once a food regulatory approval is received in one of the participating countries, IRRI will supply the rice with the GR2-E event to an independent third party to assess its efficacy at alleviating vitamin A deficiency.
Past issues with persons and organizations opposed to Golden Rice for a myriad of reasons may have affected IRRI’s work on the rice, but the overall project status 7 points out that development of Golden Rice varieties that meet the needs of farmers and consumers and that are in full compliance with the regulatory systems of the partnering countries remains the primary objective. IRRI’s summary statement on its Golden Rice project was that “Golden Rice will only be made available broadly to farmers and consumers if it is successfully developed into rice varieties suitable for Asia, approved by national regulators, and shown to improve vitamin A status in community conditions. If Golden Rice is found to be safe and efficacious, a sustainable delivery program will ensure that Golden Rice is acceptable and accessible to those most in need.” 8
Increasing concentrations of beta-carotene is only one goal of conventional crop breeding and genetic engineering. Projects for increasing iron and zinc in crops as different as wheat, pearl millet ( Pennisetum glaucum ), and lentil ( Lens culinaris ) are at varied stages of development ( Saltzman et al., 2013 ).
FINDING: Experimental results with non-GE crop varieties that have increased concentrations of micronutrients demonstrate that both GE and non-GE crops with these traits could have favorable effects on the health of millions of people, and projects aimed at providing these crops are at various stages of completion and testing.
Altering Oil Composition
Substantial efforts have been made to increase the oxidative stability of soybean oil, a major cooking oil all over the world, as a means of avoiding trans-fats generated through the hydrogenation process and enhancing omega-3 fatty acid content of the oil for use in both food and feed applications. Soybean oil is composed principally of five fatty acids: palmitic acid (16:0, carbon number:double bond number), stearic acid (18:0), oleic acid (18:1), linoleic acid (18:2), and linolenic acid (18:3) in approximate percentages of 10, 4, 18, 55, and 13. High content of unsaturated fats creates a disadvantage in industrial processing because they are susceptible to oxidation and trans-fat generation during hydrogenation, whereas oils with a high percentage of oleic acid (about 80 percent) require less processing and offer another route to decrease concentrations
7 What is the status of the Golden Rice project coordinated by IRRI? Available at http://irri.org/golden-rice/faqs/what-is-the-status-of-the-golden-rice-project-coordinated-by-irri . Accessed October 30, 2015.
of trans-fats in food products. High-oleic acid-containing soybean was produced by downregulating expression of the fatty acid desaturating enzymes FAD2-1A and -1B to decrease the concentration of trans-fats in soybean ( EFSA, 2013 ). In 2015, high-oleic acid soybean was commercially available in North America and was produced on a small area in the United States for specialty-product contracts (C. Hazel, DuPont Pioneer, personal communication, December 14, 2015).
Canola ( Brassica napus ), known in Europe as rapeseed, is the major oilseed crop in Canada. Canola was developed through conventional breeding at the University of Manitoba, Canada, by Downey and Stefansson in the early 1970s and had a good nutritional profile—58-percent oleic acid and 36-percent polyunsaturated fatty acids—in addition to low erucic acid and a moderate concentration of saturated fatty acid (6 percent). Because of demand for saturated functional oils for the trans - fat–free market, high-lauric acid GE canola was created in 1995 through an “ Agrobacterium mediated transformation in which the transfer-DNA (T-DNA) contained the gene encoding the enzyme 12:0 ACP thioesterase ( bay TE ) from the California Bay tree ( Umbellularia californica ). In addition, the T-DNA contained sequences that encoded the enzyme neomycin phosphotransferase II (NPTII). The expression of NPTII activity was used as a selectable trait to screen transformed plants for the presence of the bay TE gene. No other translatable DNA sequences were incorporated into the plant genome” ( Health Canada, 1999:1 ). The presence of lauric acid (12:0) in the oil allows it to be used as a replacement for other types of oils with lauric acid (for example, coconut and palm kernel oil) in such products as “confectionery coatings and fillings, margarines, spreads, shortenings, and commercial frying oils. It has also been used as a substitute for cocoa butter, lard, beef fats, palm oil, and partially or fully hydrogenated soybean, maize, cottonseed, peanut, safflower, and sunflower oils” ( Health Canada, 1999:2 ). However, low yield and comparably poor agronomic traits have removed high-lauric acid canola from the commercial market. The long-term use of crops with altered oil content is uncertain.
FINDING: Crops with altered oil composition might improve human health, but this will depend on the specific alterations, how the crops yield, and how the products of the crops are used.
Genetically Engineered Foods with Lower Concentrations of Toxins
Acrylamide is produced in starchy foods when they are cooked at high temperatures. Processing of potatoes for French fries and potato chips generates acrylamide. Toasting bread also produces acrylamide. That is viewed as a problem because the U.S. National Toxicology Program (2014)
concluded that acrylamide “is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals” and causes neurological damage at high exposure. Acrylamide is produced from a chemical reaction between asparagine and a reducing sugar, so decreasing the concentration of either is expected to decrease acrylamide. A potato line was genetically engineered to have low amounts of free asparagine and in early tests had as little as 5 percent of the acrylamide compared with non-GE potatoes when cooked at high temperatures ( Rommens et al., 2008 ).
In 2014, USDA deregulated a low-acrylamide potato produced by Simplot Plant Sciences ( USDA–APHIS, 2014c ) on the basis of nonplant pest status. The company also provided information to FDA. No problems were found by FDA with respect to the company’s assessment of composition or safety ( FDA, 2015 ). It should be noted that for many people reduced acrylamide in potatoes is expected to lower overall acrylamide intake substantially, but many foods contain acrylamide ( FDA, 2000b , revised 2006). An FDA survey of commonly consumed foods showed French fries at seven McDonald’s locations had an average acrylamide concentration of 288 parts per billion (ppb), whereas Gerber Finger Foods Biter Biscuits had 130 ppb and Wheatena Toasted Wheat Cereal had 1,057 ppb, which is much more than from fast-food French fries ( FDA, 2002 , revised 2006). 9 Any toasted bread is expected to be high in acrylamide. Therefore, how much low-acrylamide potato decreases total exposure depends on individual diets. Furthermore, EPA has established limits for exposure to acrylamide, and current actual exposures are generally below the limits.
Although the low-acrylamide potato is the only GE crop with a lower food-toxin concentration that has been deregulated in the United States, other GE crops with lower natural toxin concentrations are in the pipeline. Potatoes and other crops in the “deadly nightshade” family (Solanaceae, which includes tomato and eggplant) produce glycoalkaloids, some of which have human toxicity, as described above (see the section “Endogenous Toxins in Plants” in this chapter). Langkilde et al. (2012) conducted a compositional and toxicological analysis of the potatoes with lower solanine and higher chaconine. The study used Syrian golden hamsters instead of rats because the hamsters are very sensitive to the glycoalkaloids. There were some statistically significant differences, but they were considered not of biological relevance. At this point, the evidence is not sufficient to conclude that a low-glycoalkaloid potato would be healthier for humans.
Highly toxic chemicals (aflatoxins and fumonisins) are produced by Fusarium and Aspergillis fungi on the kernels of maize ( Bowers et al.,
9 Acrylamide concentrations reported by FDA were for individual purchased food products and were not adjusted for unit-to-unit variation.
2014 ). Aflatoxins are considered by the U.S. National Toxicology Program (2014) to be “human carcinogens based on sufficient evidence of carcinogenicity from studies in humans.” They are also associated with many other illnesses and considered a global health problem ( Wild and Gong, 2010 ). Fumonisins cause a number of physiological disorders and are considered possibly carcinogenic to humans ( IARC, 2002 ). Several investigators have reported a substantial decrease in fumonisins in Bt maize compared with conventionally bred varieties ( Munkvold and Desjardins, 1997 ; Bowers et al., 2014 ). However, there is no clear association between Bt maize and aflatoxin concentrations ( Wiatrak et al., 2005 ; Abbas et al., 2007 ; Bowen et al., 2014 ).
Research continues on how to use genetic engineering to develop varieties of maize and peanut ( Arachis hypogaea ) that inhibit aflatoxin production, but a GE solution has so far been elusive ( Bhatnagar-Mathur et al., 2015 ). A reduction in aflatoxin in both maize and peanut would have substantial health benefits in some developing countries ( Williams et al., 2004 ; Wild and Gong, 2010 ).
FINDING: It is possible that GE crops that would result in improved health by lowering exposure of humans to plant-produced toxins in foods could be developed, but there is insufficient information to assess the possibility. However, GE plants that indirectly or directly reduce fungal-toxin production and intake would offer substantial benefits to some of the world’s poorest populations, which have the highest dietary intake of food-associated fungal toxins.
Health Effects of Farmer Exposure to Insecticides and Herbicides
Chapter 4 presents data that demonstrate substantially lower use of insecticides in some Bt crops than in conventionally bred crops. There is a logical expectation that a decrease in the number of insecticide applications would lead to lower farm-worker exposure and therefore lower health burden, especially in countries where acute poisonings due to applicator exposure are common. Racovita et al. (2015) reviewed five studies of Bt cotton in China, India, Pakistan, and South Africa that ranged from one to four growing seasons. All reported a decline in the number of insecticide applications to Bt versus non- Bt cotton. In a study in China by Huang et al. (2002) , Bt cotton was treated with insecticides 6.6 times and non- Bt cotton was treated 19.8 times during the growing season. The frequency of Bt and non- Bt cotton farmers reporting poisonings were 5 percent and 22 percent, respectively in 1999, 7 percent and 29 percent in 2000, 8 percent and 12 percent in 2001. Kouser and Qaim (2011) found fewer overall insecticide treatments in a study conducted in India: 1.5 treatments
of Bt cotton and 2.2 treatments of non- Bt cotton. In this study, the farmers who used Bt cotton reported 0.19 poisonings per season while those with conventionally bred cotton reported 1.6 poisonings. Bennett et al. (2006) studied the same types of farmers in South Africa. Bt cotton was not yet widely available in the beginning of the experiment, but eventually some farmers adopted Bt cotton and decreased spraying. The study looked at overall poisonings according to hospital records over time; there were 20 poisonings in the year before common availability of Bt cotton and four in a later year, when there was 60 percent adoption of Bt cotton.
The findings of those and other studies (for example, Huang et al., 2005 ; Dev and Rao, 2007 ; Kouser and Qaim, 2013 ) are in line with an expectation of a decrease in poisonings when Bt cotton is grown instead of non- Bt cotton. However, Racovita et al. (2015:15) , who carefully assessed each of the studies, found many shortcomings that led them to conclude that “the link between [genetically modified] crop cultivation and a reduction in number of pesticide poisonings should be considered as still circumstantial.” The shortcomings include the fact that the number of poisonings is based on farmer recall of incidents sometimes more than a year after the field season or, in the Bennett et al. (2006) study, simply based on hospital cases. Another issue was that there may have been differences in risk–avoidance behavior between farmers who did and did not plant Bt cotton. Finally, the studies focused on farmers, not farm workers, who do not control farm operations. Racovita et al. (2015) called for more rigorous studies that would address the shortcomings of previous studies, given the politicized nature of the use of Bt crops.
Farm-worker exposure to insecticides and herbicides is lower in the United States and some other developed countries than is the case for farm workers on resource-poor farms. However, there is substantial exposure, and any effects seen in the United States would be of global concern. Prospective cohort studies of health are the high benchmark of epidemiology studies, and the Agricultural Health Study (AHS) funded by the U.S. National Institute of Environmental Health Sciences used this approach to evaluate private and commercial applicators in Iowa and North Carolina. The landmark study resulted in two peer-reviewed articles on glyphosate exposure and cancer incidence ( De Roos et al., 2005 ; Mink et al., 2012 ) and one on glyphosate exposure and non-cancer health outcomes ( Mink et al., 2011 ). De Roos et al. (2005:49) concluded that “glyphosate exposure was not associated with cancer incidence overall or with most cancer subtypes we studied.” The data suggested a weak association with multiple myeloma on the basis of a small number of cases, but that association was not found in a follow-up study ( DeRoos et al., 2005 ; Mink et al., 2012 ). Mink et al. (2012:440) reported on the continuation of the AHS cohort study and found “no consistent pattern of positive associations indicating a causal relationship between total
cancer (in adults or children) or any site-specific cancer and exposure to glyphosate.” Mink et al. (2011) reviewed noncancer health outcomes that included respiratory conditions, diabetes, myocardial infarction, reproductive and developmental outcomes, rheumatoid arthritis, thyroid disease, and Parkinson’s disease. They reviewed cohort, case–control, and cross-sectional studies within the AHS study and found “no evidence of a consistent pattern of positive associations indicating a causal relationship between any disease and exposure to glyphosate” ( Mink et al., 2011:172 ).
FINDING: There is evidence that use of Bt cotton in developing countries is associated with reduced insecticide poisonings. However, there is a need for more rigorous survey data addressing the shortcomings of existing studies.
FINDING: A major government-sponsored prospective study of farm-worker health in the United States does not show any significant increases in cancer or other health problems that are due to use of glyphosate.
ASSESSMENT OF FOOD SAFETY OF CROPS TRANSFORMED THROUGH EMERGING GENETIC-ENGINEERING TECHNOLOGIES
Increased precision and complexity of genetic-engineering alterations.
At the time that the committee wrote its report, major commercialized GE crops had been engineered by using Agrobacterium tumefaciens mediated or gene gun-mediated transformation, both of which result in semirandom insertion of the transgene into the genome. Variation in expression of the transgene was routinely observed because of the specific genomic characteristics of the insertion sites. Because of that variation, there was a need to screen large numbers of transgenic plants to identify the optimal transgenic individual. Regulations in the United States require approval of each transformation event regardless of whether the transgene itself was previously approved for release in that crop. That is at least in part because of the potential for unintended effects of each insertion.
Precision genome-editing technologies now permit insertion of single or multiple genes into one targeted location in the genome and thereby eliminate variation that is due to position effects (see Chapter 7 ). Such precision is expected to decrease unintended effects of gene insertion, although it will not eliminate the effects of somaclonal variation (discussed in Chapter 7 ).
Consider, for example, the engineering of completely new metabolic pathways into a plant for nutritional enhancement. The simplest example
would be a set of two genes, such as has been used to create Golden Rice to deliver precursors of vitamin A. A more complex example would be engineering of fish oils (very long-chain unsaturated fatty acids) to improve the health profile of plant oils; depending on the target species, this process has required introduction of at least of three and at most nine transgenes ( Abbadi et al., 2004 ; Wu et al., 2005 ; Ruiz-Lopez et al., 2014 ). If each of those transgenes is integrated into the genome on a different chromosome on the basis of separate insertion events, it will require a number of generations of crosses to put them all together in one plant. If, instead, all the transgenes could be targeted at the same site on a chromosome either simultaneously or one after another, they would not segregate from each other as they were moved into elite varieties. From a food-safety perspective, engineering transgenes into a single target locus also ensures that expression of the whole pathway is preserved so that the correct end product accumulates. Emerging genetic-engineering technologies currently enable insertion of a few genes in one construct, but in the future that number may increase dramatically.
In the future, the scale of genetic-engineering alterations may go much further than just manipulating oil profiles. The committee heard from speakers about projects aimed at changing the entire photosynthetic pathway of the rice plant ( Weber, 2014 ) to create an entirely novel crop ( Zhu et al., 2010 ; Ruan et al., 2012 ). The committee also heard from researchers interested in developing cereal crops with nitrogen fixation. Those projects are discussed further in Chapter 8 . Although the precision of future genetic-engineering alterations should decrease unintended effects of the process of engineering, the complexity of the changes in a plant may leave it not substantially equivalent to its non-GE counterpart.
It is also important to note that crops that use RNA interference (RNAi) were coming on the market when the committee was writing its report. EPA convened a science advisory panel to evaluate hazards that might arise from use of this genetic-engineering approach. The panel concluded that “dietary RNA is extensively degraded in the mammalian digestive system by a combination of ribonucleases (RNases) and acids that are likely to ensure that all structural forms of RNA are degraded throughout the digestive process. There is no convincing evidence that ingested [double-stranded] RNA is absorbed from the mammalian gut in a form that causes physiologically relevant adverse effects” ( EPA, 2014c:14 ). When the committee was writing its report, deployment of dietary RNAi was a new technology. EPA’s panel made a number of recommendations, including investigating factors that may affect absorption and effects of dietary double-stranded RNAs and investigating the stability of double-stranded RNA in people who manifest diseases.
FINDING: The precision of emerging genetic-engineering technologies should decrease some sources of unintended changes in the plants, thus simplifying food-safety testing. However, engineering involving major changes in metabolic pathways or insertion of multiple resistance genes will complicate the determination of food safety because changes in metabolic pathways are known to have unexpected effects on plant metabolites.
Increased Diversity of Crops To Be Engineered
The most far-ranging effects of emerging genetic-engineering technologies may be the diversity of crops that will be engineered and commercialized. Commercial GE crops at the time the committee conducted its review were mainly high-production commodity crops (maize, soybean, and cotton) engineered with trans-kingdom genes, but the applications of emerging genetic-engineering technologies are much broader: these technologies can be easily applied to any plant species that can be regenerated from tissue culture. Furthermore, the emerging technologies described in Chapter 7 can focus on any gene in which an altered nucleotide sequence results in a desired trait.
As a consequence, the committee expects a sizable increase in the number of food-producing crop species that are genetically altered. Examples of new target crops include forages (grasses and legumes), beans, pulses, a wide array of vegetables, herbs, and spices, and plants grown for flavor compounds. New traits will probably include fiber content (either increased to add more fiber or decreased to improve digestibility), altered oil profiles, decreased concentrations of antinutrients, increased or more consistent concentrations of such phytochemicals as antioxidants (for example, flavonoids) and phytoestrogens (for example, isoflavones or lignans), and increased mineral concentrations. Some of these are considered further in Chapter 8 .
From a food-safety perspective, the increase in crops and traits presents a number of challenges. First is the need to develop better and more detailed baseline data on the general chemical composition and probably the transcriptomic profiles of currently marketed conventionally bred varieties of the crops (see Chapter 7 ). Perhaps more problematic will be designing whole-food animal-testing regimens if the food from the crop cannot be used as a major component of the test animals’ diet. Maize, rice, soybean, and other grains can be added to diets at up to 30 percent without adverse effects on animal health. That is unlikely to be the case with new spices or some vegetables. It would be beneficial if new, publicly acceptable approaches for testing could be developed that do not require animal testing ( NRC, 2007 ; Liebsch et al., 2011 ; Marx-Stoelting et al., 2015 ). Chapter 9
addresses the potential need to move to an entirely product-based approach to regulation and testing based on the novelty of a new crop or food.
FINDING: Some future GE crops will be designed to be substantially different from current crops and may not be as amenable to animal testing as currently marketed GE crops.
RECOMMENDATION: There is an urgent need for publicly funded research on novel molecular approaches for testing future products of genetic engineering so that accurate testing methods will be available when the new products are ready for commercialization.
CONCLUSIONS
The committee’s objective in this chapter was to examine the evidence that supports or negates specific hypotheses and claims about the risks and benefits associated with foods derived from GE crops. As acknowledged at the beginning of the chapter, understanding the health effects of any food, whether non-GE or GE, can be difficult. The properties of most plant secondary metabolites are not understood, and isolating the effects of diet on animals, including humans, is challenging. Although there are well-developed methods for assessing potential allergenicity of novel foods, these methods could miss some allergens. However, the research that has been conducted in studies with animals and on chemical composition of GE foods reveals no differences that would implicate a higher risk to human health from eating GE foods than from eating their non-GE counterparts. Long-term epidemiological studies have not directly addressed GE food consumption, but available time-series epidemiological data do not show any disease or chronic conditions in populations that correlate with consumption of GE foods. The committee could not find persuasive evidence of adverse health effects directly attributable to consumption of GE foods.
New methods to measure food composition that involve transcriptomics, proteomics, and metabolomics provide a broad, nontargeted assessment of thousands of plant RNAs, proteins, and compounds. When the methods have been used, the differences found in comparisons of GE with non-GE plants have been small relative to the naturally occurring variation found in conventionally bred crop varieties. Differences that are detected by using -omics methods do not on their own indicate a safety problem.
There is some evidence that GE insect-resistant crops have had benefits to human health by reducing insecticide poisonings and decreasing exposure to fumonisins. Several crops had been developed or were in development with GE traits designed to benefit human health; however, when the committee was writing its report, commercialized crops with health benefits
had been only recently introduced and were not widely grown, so the committee could not evaluate whether they had had their intended effects.
New crops developed with the use of emerging genetic-engineering technologies were in the process of being commercialized. The precision associated with the technologies should decrease some sources of unintended changes that occur when plants are genetically engineered and thus simplify food-safety testing. However, engineering involving major changes in metabolic pathways or insertion of multiple resistance genes will complicate the determination of food safety because changes in metabolic pathways are known to have unexpected effects on plant metabolites. Therefore, publicly funded research on novel approaches for testing future products of genetic engineering is needed so that accurate testing methods will be available when the new products are ready for commercialization.
Abbadi, A., F. Domergue, J. Bauer, J.A. Napier, R. Welti, U. Zähringer, P. Cirpus, and E. Heinz. 2004. Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: Constraints on their accumulation. Plant Cell 16:2734–2748.
Abbas, H.K., W.T. Shier, and R.D. Cartwright. 2007. Effect of temperature, rainfall and planting date on aflatoxin and fumonisin contamination in commercial Bt and non-Bt maize hybrids in Arkansas. Phytoprotection 88:41–50.
Abraham, T.M., K.M. Pencina, M.J. Pecina, and C.S. Fox. 2015. Trends in diabetes incidence: The Framingham heart study. Diabetes Care 38:482–487.
ADAS, 2015. Strategy support for the post-market monitoring (PMM) of GM plants: Review of existing PPM strategies developed for the safety assessment of human and animal health. EFSA supporting publication 2014:EN-739.
Ahuja, I., R. Kissen, and A.M. Bones. 2012. Phytoalexins in defense against pathogens. Trends in Plant Science 17:73–90.
American Association for the Advancement of Science. 2012. Statement by the AAAS Board of Directors on Labeling of Genetically Modified Foods. October 20. Available at http://www.aaas.org/sites/default/files/AAAS_GM_statement.pdf . Accessed October 13, 2015.
American Psychiatric Association. 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Publishing.
Amos, J. September 19, 2012. French GM-fed Rat Study Triggers Furore. Online. BBC News. Available at http://www.bbc.com/news/science-environment-19654825 . Accessed December 13, 2015.
An, R. 2015. Educational disparity in obesity among U.S. adults, 1984–2013. Annals of Epidemiology 25:637–642.
Arjó, G., T. Capell, X. Matias-Guiu, C. Zhu, P. Christou, and C. Piñol. 2012. Mice fed on a diet enriched with genetically engineered multivitamin corn show no sub-acute toxic effects and no sub-chronic toxicity. Plant Biotechnology Journal 10:1026–1034.
Astwood, J.D., J.N. Leach, and R.L. Fuchs. 1996. Stability of food allergens to digestion in vitro. Nature Biotechnology 14:1269–1273.
Bartholomaeus, A., W. Parrott, G. Bondy, and K. Walker. 2013. The use of whole food animal studies in the safety assessment of genetically modified crops: Limitations and recommendations. Critical Reviews in Toxicology 43:1–24.
Belknap, J.K., S.R. Mitchell, L.A. O’Toole, M.L. Helms, and J.C. Crabbe. 1996. Type I and type II error rates for quantitative trait loci (QTL) mapping studies using recombinant inbred mouse strains. Behavior Genetics 26:149–160.
Bennett, R., S. Morse, and Y. Ismael. 2006. The economic impact of genetically modified cotton on South African smallholders: Yield, profit and health effects. Journal of Development Studies 42:662–677.
Berry, C. 2013. Letter to the Editor. Food and Chemical Toxicology 53:445–446.
Bhatnagar-Mathur, P., S. Sunkara, M. Bhatnagar-Panwar, F. Waliyar, and K.K. Sharma. 2015. Biotechnological advances for combating Aspergillus flavus and aflatoxin contamination in crops. Plant Science 234:119–132.
Birol, E., J.V. Meenakshi, A. Oparinde, S. Perez, and K. Tomlins. 2015. Developing country consumers’ acceptance of biofortified foods: A synthesis. Food Security 7:555–568.
Bøhn, T., M. Cuhra, T. Traavik, M. Sanden, J. Fagan, and R. Primicerio. 2014. Compositional differences in soybeans on the market: Glyphosate accumulates in Roundup Ready GM soybeans. Food Chemistry 153:207–215.
Boobis, A.R., B.C. Ossendorp, U. Banasiak, P.Y. Hamey, I. Sebestyen, and A. Moretto. 2008. Cumulative risk assessment of pesticide residues in food. Toxicology Letters 180:137–150.
Bowen, K.L., K.L. Flanders, A.K. Hagan, and B. Ortiz. 2014. Insect damage, aflatoxin content, and yield of Bt corn in Alabama. Journal of Economic Entomology 107:1818–1827.
Bowers, E., R. Hellmich, and G. Munkvold. 2014. Comparison of fumonisn contamination using HPLC and ELISA methods in Bt and near-isogenic maize hybrids infested with European corn borer or Western bean cutworm. Journal of Agricultural and Food Chemistry 62:6463–6472.
Brigulla, M., and W. Wackernagel. 2010. Molecular aspects of gene transfer and foreign DNA acquisition in prokaryotes with regard to safety issues. Applied Microbiology and Biotechnology 86:1027–1041.
Brix, A.E., A. Nyska, J.K. Haseman, D.M. Sells, M.P. Jokinen, and N.J. Walker. 2005. Incidences of selected lesions in control female Harlan Sprague–Dawley rates from two-year studies performed by the National Toxciology Program. Toxicologic Pathology 33:477–483.
Brouk, M.J., B. Cvetkovic, D.W. Rice, B.L. Smith, M.A. Hinds, F.N. Owens, C. Iiams, and T.E. Sauber. 2011. Performance of lactating dairy cows fed corn as whole plant silage and grain produced from genetically modified corn containing event DAS-59122–7 compared to a nontransgenic, near-isogenic control. Journal of Dairy Science 94:1961–1966.
Bush, R.K., S.L. Taylor, J.A. Nordlee, and W.W. Busse. 1985. Soybean oil is not allergenic to soybean-sensitive individuals. Journal of Allergy and Clinical Immunology 76:242–245.
Butler, D. October 11, 2012. Hyped GM Maize Study Faces Growing Scrutiny. Online. Nature. Available at http://www.nature.com/news/hyped-gm-maize-study-faces-growingscrutiny-1.11566 . Accessed December 13, 2015.
Buzoianu, S.G., M.C. Walsh, M.C. Rea, O. O’Donovan, E. Gelencsér, G. Ujhelyi, E. Szabó, A. Nagy, R.P. Ross, G.E. Gardiner, and P.G. Lawlor. 2012a. Effects of feeding Bt maize to sows during gestation and lactation on maternal and offspring immunity and fate of transgenic material. PLoS ONE 7:e47851.
Buzoianu, S.G., M.C. Walsh, M.C. Rea, J.P. Cassidy, R.P. Ross, G.E. Gardiner, and P.G. Lawlor. 2012b. Effect of feeding genetically modified Bt MON810 maize to approximately 40-day-old pigs for 110 days on growth and health indicators. Animal 6:1609–1619.
Buzoianu, S.G., M.C. Walsh, M.C. Rea, O. O’Sullivan, F. Crispie, P.D. Cotter, P.R. Ross, G.E. Gardiner, and P.G. Lawlor. 2012c. The effect of feeding Bt MON810 maize to pigs for 110 days on intestinal microbiota. PLoS One 7:e33668.
Buzoianu, S.G., M.C. Walsh, M.C. Rea, O. O’Sullivan, F. Crispie, P.D. Cotter, P.R. Ross, G.E. Gardiner, and P.G. Lawlor. 2012d. High-throughput sequence-based analysis of the intestinal microbiota of weanling pigs fed genetically modified MON810 maize expressing Bacillus thuringiensis Cry1Ab (Bt maize) for 31 days. Applied and Environmental Microbiology 78:4217–4224.
Buzoianu, S.G., M.C. Walsh, M.C. Rea, L. Quigley, O. O’Sullivan, P.D. Cotter, R.P. Ross, G.E. Gardiner, and P.G. Lawlor. 2013a. Sequence-based analysis of the intestinal Microbiota of sows and their offspring fed genetically modified maize expressing a truncated form of Bacillus thuringiensis Cry1Ab protein (Bt Maize). Applied and Environmental Microbiology 79:7735–7744.
Buzoianu, S.G., M.C. Walsh, M.C. Rea, J.P. Cassidy, T.P. Ryan, P.R. Ross, G.E. Gardiner, and P.G. Lawlor. 2013b. Transgenerational effects of feeding genetically modified maize to nulliparious sows and offspring on offspring growth and health. Journal of Animal Science 91:318–330.
CAC (Codex Alimentarius Commission). 2003. Guideline for the Conduct of Food Safety Assessment of Foods Using Recombinant DNA Plants. Doc CAC/GL 45-2003. Rome: World Health Organization and Food and Agriculture Organization.
CAC (Codex Alimentarius Commission). 2008. Annex 2: Food Safety Assessment of Foods Derived from Recombinant-DNA Plants Modified for Nutritional or Health Benefits in Guideline for the Conduct of Food Safety Assessment of Foods Using Recombinant DNA Plants. Doc CAC/GL 45-2003. Rome: World Health Organization and Food and Agriculture Organization.
CAC (Codex Alimentarius Commission). 2009. Foods Derived from Modern Biotechnology. Rome: World Health Organization and Food and Agriculture Organization.
Callahan, P. January 28, 2016. Court clears way for revival of worrisome weedkiller. EPA nixes approval of Enlist Duo weed killer. Online. Chicago Tribune. Available at http://www.chicagotribune.com/news/watchdog/ct-dow-enlist-duo-court-ruling-20160127- . Accessed March 21, 2016.
Calsamiglia, S., B. Hernandez, G.F. Hartnell, and R. Phipps. 2007. Effects of corn silage derived from a genetically modified variety containing two transgenes on feed intake, milk production, and composition, and the absence of detectable transgenic deoxyribonucleic acid in milk in Holstein dairy cows. Journal of Dairy Science 90:4718–4723.
Catassi, C., D. Kryszak, B. Bhatti, C. Strugeon, K. Helzlsouer, S.L. Clipp, D. Gelfond, E. Puppa, A. Sferruzza, and A. Fasano. 2010. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Annals of Medicine 42:530–538.
CDC (Centers for Disease Control and Prevention). 2014. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morbidity and Mortality Weekly Report 63:1–21.
Coresh, J., B.C. Astor, T. Greene, G. Eknoyan, and A.S. Levey. 2003. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third national health and nutrition examination survey. American Journal of Kidney Diseases 41:1–12.
Coresh, J., E. Selvin, L.A. Stevens, J. Manzi, J.W. Kusek, P. Eggers, F. Van Lente, and A.S. Levey. 2007. Prevalence of Chronic Kidney Disease in the United States. Journal of the American Medical Association 298:2038–2047.
Costa, J., I. Mafra, J.S. Amaral, and M.B.P.P. Oliveira. 2010a. Detection of genetically modified soybean DNA in refined vegetable oils. European Food Research and Technology 230:915–923.
Costa, J., I. Mafra, J.S. Amaral, and M.B.P.P. Oliveira. 2010b. Monitoring genetically modified soybean along the industrial soybean oil extraction and refining processes by polymerase chain reaction techniques. Food Research International 43:301–306.
Council on Science and Public Health of the American Medical Association House of Delegates. 2012. Report 2 (A-12). Labeling of Bioengineered Foods (Resolutions 508 and 509-A-11). Available at http://factsaboutgmos.org/sites/default/files/AMA%20Report.pdf . Accessed March 12, 2016.
Datta, S.K., K. Datta, V. Parkhi, M. Rai, N. Baisakh, G. Sahoo, S. Rehana, A. Bandyopadhyay, M. Alamgir, M.S. Ali, E. Abrigo, N. Oliva, and L. Torrizo. 2007. Golden Rice: Introgression, breeding, and field evaluation. Euphytica 154:271–278.
David, L.A., C.F. Maurice, R.N. Carmody, D.B. Gootenberg, J.E. Button, B.E. Wolfe, A.V. Ling, A.S. Devlin, Y. Varma, M.A. Fischbach, S.B. Biddinger, R.J. Dutton, and P.J. Turnbaugh. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563.
Davis, R.K., G.T. Stevenson, and K.A. Busch. 1956. Tumor incidence in normal Sprague-Dawley female rats. Cancer Research 16:194–197.
DeFrancesco, L. 2013. How safe does transgenic food need to be? Nature Biotechnology 31:794–802.
de Luis, R., M. Lavilla, L. Sanchez, M. Calvo, and M.D. Perez. 2009. Immunochemical detection of Cry1A(b) protein in model processed foods made with transgenic maize. European Food Research and Technology 229:15–19.
De Moura, F.F., A. Miloff, and E. Boy. 2015. Retention of provitamin A carotenoids in staple crops targeted for biofortification in Africa: Cassava, maize and sweet potato. Critical Reviews in Food Science and Nutrition 55:1246–1269.
De Roos, A.J., A. Blair, J.A. Rusiecki, J.A. Hoppin, M. Svec, M. Dosemeci, D.P. Sandler, and M.C. Alavanja. 2005. Cancer incidence among glyphosate-exposed pesticide applicators in the Agricultural Health Study. Environmental Health Perspective 113:49–54.
Demont, M., and A.J. Stein. 2013. Global value of GM rice: A review of expected agronomic and consumer benefits. New Biotechnology 30:426–436.
Dethlefsen, L., and D A. Relman. 2011. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proceedings of the National Academy of Sciences of the United States of America 108: 4554–4561.
Dev, M.S., and N.C. Rao. 2007. Socio-economic Impact of Bt Cotton. Monograph No. 3. Hyderabad: Centre for Economic and Social Studies.
Diaz, C., C. Fernandez, R. McDonald, and J.M. Yeung. 2002. Determination of Cry9C protein in processed foods made with StarLink™ corn. Journal of AOAC International 85:1070–1076.
Dinse, G.E., S.D. Peddada, S.F. Harris, and S.A. Elmore. 2010. Comparison of NTP historical control tumor incidence rates in female Harlan Sprague–Dawley and Fischer 344/N rats. Toxicologic Pathology 38:765–775.
Dixon, R.A. 2001. Natural products and disease resistance. Nature 411:843–847.
Dixon, R.A. 2004. Phytoestrogens. Annual Review of Plant Biology 55:225–261.
Domingo, J.L., and J.G. Bordonaba. 2011. A literature review on the safety assessment of genetically modified plants. Environment International 37:734–742.
Dona, A., and I.S. Arvanitoyannis. 2009. Health risks of genetically modified foods. Critical Reviews in Food Science and Nutrition 49:164–175.
Duke, S.O., A.M. Rimando, P.F. Pace, K.N. Reddy, and R.J. Smeda. 2003. Isoflavone, glyphosate, and aminomethylphosphonic acid levels in seeds of glyphosate-treated, glyphosate resistant soybean. Journal of Agricultural and Food Chemistry 51:340–344.
Dung, L.T., and L.H. Ham. 2013. Comments on “Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize.” Food and Chemical Toxicology 53:443–444.
Dunn, O.J. 1961. Multiple comparisons among means. Journal of the American Statistical Association 56:52–64.
EC (European Commission). 2010a. A Decade of EU-funded GMO Research (2001–2010). Brussels: European Commission.
EC (European Commission). 2010b. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union 276:33–79.
EC (European Commission). 2013. Commission implementing regulation (EU) No 503/2013 of 3 April 2013 on applications for authorisation of genetically modified food and feed in accordance with Regulation (EC) No 1829/2003 of the European Parliament and of the Council and amending Commission Regulations (EC) No 641/2004 and (EC) No 1981/2006. Official Journal of the European Union 157:1–48.
EFSA (European Food Safety Authority). 2007. Statement of the Scientific Panel on Genetically Modified Organisms on the Analysis of Data from a 90-day Rat Feeding Study with MON 863 Maize. Available at http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/GMO_statement_MON863%2C0.pdf . Accessed December 13, 2015.
EFSA (European Food Safety Authority). 2010. Scientific opinion on the assessment of allergenicity of GM plants and microorganisms and derived food and feed. EFSA Journal 8:1700.
EFSA (European Food Safety Authority). 2011a. Guidance on risk assessment of food and feed from genetically modified plants. EFSA Journal 9:2150.
EFSA (European Food Safety Authority). 2011b. Scientific opinion on guidance on conducting repeated-dose 90-day oral toxicity study in rodents on whole food/feed. EFSA Journal 9:2438.
EFSA (European Food Safety Authority). 2011c. Statistical significance and biological relevance. EFSA Journal 9:2372.
EFSA (European Food Safety Authority). 2012. Review of the Séralini et al . (2012) publication on a 2-year rodent feeding study with glyphosate formulations and GM maize NK603 as published online on 19 September 2012 in Food and Chemical Toxicology. EFSA Journal 10:2910.
EFSA (European Food Safety Authority). 2013. Scientific Opinion on application EFSA-GMO-NL-2007-45 for the placing on the market of herbicide-tolerant, high-oleic acid, genetically modified soybean 305423 for food and feed uses, import and processing under Regulation (EC) No 1829/2003 from Pioneer. EFSA Journal 11:3499.
EFSA (European Food Safety Authority). 2015. Conclusion on the peer review of the pesticide risk assessment for the active substance glyphosate. EFSA Journal 13:4302.
Einspanier, R. 2013. The fate of transgenic DNA and newly expressed proteins. Pp. 130–139 in Animal Nutrition with Transgenic Plants, G. Flachowsky, ed. Oxfordshire: UK: CABI Biotechnology Series.
El Ouakfaoui, S., and B. Miki. 2005 The stability of the Arabidopsis transcriptome in transgenic plants expressing the marker genes nptII and uidA . Plant Journal 41:791–800.
Entine, J. 2014. A Science-based Look at Genetically Engineered Crops. Presentation to the National Academy of Sciences’ Committee on Genetically Engineered Crops: Past Experience and Future Prospects, September 16, Washington, DC.
EPA (U.S. Environmental Protection Agency). 1989. Good laboratory practice standards. Federal Register 54:34067.
EPA (U.S. Environmental Protection Agency). 2001a. Bacillus thuringiensis subspecies Cry1F Protein and the Genetic Material Necessary for Its Production (Plasmid Insert PHI 8999) in Corn. Available at http://ofmpub.epa.gov/apex/pesticides/f?p=chemicalsearch:3:0::no:1,3,31,7,12,25:p3_xchemical_id:1322 . Accessed October 10, 2015.
EPA (U.S. Environmental Protection Agency). 2001b. EPA Releases Draft Report on Starlink corn. Online. EPA Press Release. Available at https://yosemite.epa.gov/opa/admpress.nsf/blab9f485b098972852562e7004dc686/cd9013801973259885256a0800710574?OpenDocument . Accessed March 12, 2016.
EPA (U.S. Environmental Protection Agency). 2013. Glyphosate; Pesticide tolerances. Federal Register 78:25396–25401.
EPA (U.S. Environmental Protection Agency). 2014a. Final Registration of Enlist Duo™ Herbicide. October 15. Available at http://www2.epa.gov/sites/production/files/2014-10/documents/final_registration_-_enlist_duo.pdf . Accessed October 13, 2015.
EPA (U.S. Environmental Protection Agency). 2014b. Memorandum: Response to Public Comments Received Regarding New Uses of Enlist Duo™ on Corn and Soybeans. October 14. Available at http://www2.epa.gov/sites/production/files/2014-10/documents/response_to_comments.pdf . Accessed October 10, 2015.
EPA (U.S. Environmental Protection Agency). 2014c. SAP Minutes No. 2014-02, A Set of Scientific Issues Being Considered by the Environmental Protection Agency Regarding: RNAi Technology: Problem Formulation for Human Health and Ecological Risk Assessment. Available at https://www.epa.gov/sites/production/files/2015-06/documents/012814minutes.pdf . Accessed March 13, 2016.
EPA (U.S. Environmental Protection Agency). 2015. EDSP Weight of Evidence Analysis of Potential Interaction with the Estrogen, Androgen, or Thyroid Pathway; Chemical: Glyphosate. Available at https://www.epa.gov/sites/production/files/2015-06/documents/glyphosate-417300_2015-06-29_txr0057175.pdf . Accessed March 13, 2016.
Fagan, J., M. Antoniou, and C. Robinson. 2014. GMO Myths and Truths. London: Earth Open Source.
Fasano, A., I. Berti, T. Gerarduzzi, T. Not, R.B. Colletti, S. Drago, Y. Elitsur, P.H. Green, S. Guandalini, I.D. Hill, and M. Pietzak. 2003. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: A large multicenter study. Archives of Internal Medicine 163:286–292.
FDA (U.S. Food and Drug Administration). 1979. Good Laboratory Practice Regulations Management Briefings: Post Conference Report. Rockville, MD: FDA.
FDA (U.S. Food and Drug Administration). 2000a, revised 2007. Guidance for Industry and Other Stakeholders: Toxicological Principles for the Safety Assessment of Food Ingredients (Redbook 2000). Available at http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/IngredientsAdditivesGRASPackaging/ucm2006826.htm . Accessed October 29, 2015.
FDA (U.S. Food and Drug Administration). 2000b, revised 2006. Survey Data on Acrylamide in Food: Total Diet Study Results. Available at http://www.fda.gov/Food/FoodborneIllnessContaminants/ChemicalContaminants/ucm053566.htm . Accessed October 30, 2015.
FDA (U.S. Food and Drug Administration). 2002, revised 2006. Survey Data on Acrylamide in Food: Individual Food Products. Available at http://www.fda.gov/food/foodborneillnesscontaminants/chemicalcontaminants/ucm053549.htm . Accessed December 22, 2015.
FDA (U.S. Food and Drug Administration). 2013. Biotechnology Consultation Note to the File BNF No. 000133. December 16. Available at http://www.fda.gov/Food/FoodScienceResearch/GEPlants/Submissions/ucm382207.htm . Accessed October 29, 2015.
FDA (U.S. Food and Drug Administration). 2015. Biotechnology Consultation Agency Response Letter BNF No. 000141. March 20. Available at http://www.fda.gov/Food/FoodScienceResearch/GEPlants/Submissions/ucm436169.htm . Accessed October 30, 2015.
Fernandez, A., E.N.C. Mills, M. Lovik, A. Spök, A. Germini, A. Mikalsen, and J.M. Wal. 2013. Endogenous allergens and compositional analysis in the allergenicity assessment of genetically modified plants. Food and Chemical Toxicology 62:1–6.
Feron, V.J., and J.P. Groten. 2002. Toxicological evaluation of chemical mixtures. Food and Chemical Toxicology 40:825–839.
Ferruzzi, M.G. 2010. The influence of beverage composition on delivery of phenolic compounds from coffee and tea. Physiology & Behavior 100:33–41.
Finamore, A., M. Roselli, S. Britti, G. Monastra, R. Ambra, A. Turrini, and E. Mengheri. 2008. Intestinal and peripheral immune response to MON810 maize ingestion in weaning and old mice. Journal of Agricultural and Food Chemistry 56:11533–11539.
Folta, K. 2014. Letter to the Editor. Food and Chemical Toxicology 65:392.
Fonseca, C., S. Planchon, J. Renaut, M.M. Oliveira, and R. Batista. 2012. Characterization of maize allergens—MON810 vs. its non-transgenic counterpart. Journal of Proteomics 75:2027–2037.
Forouzanfar, M.H., K.J. Foreman, A.M. Delossantos, R. Lozano, A.D. Lopez, C.J.L. Murray, and M. Naghavi. 2011. Breast and cervical cancer in 187 countries between 1980 and 2010: A systematic analysis. Lancet 378:1461–1484.
Franz J.E., M.K. Mao, and J.A. Sikorski. 1997. Glyphosate: A Unique Global Herbicide. ACS Monograph 189. Washington, DC: American Chemical Society.
Friedman, M. 2006. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. Journal of Agricultural and Food Chemistry 54:8655–8681.
Fryar, C.D., M.D. Carroll, and C.L. Ogden. 2014. Prevalence of overweight, obesity, and extreme obesity among adults: United States, 1960–1962 through 2011–2012. Available at http://www.cdc.gov/nchs/data/hestat/obesity_adult_11_12/obesity_adult_11_12.pdf . Accessed October 13, 2015.
FSANZ (Food Standards Australia New Zealand). 2013. GM Food Labelling. Available at http://www.foodstandards.gov.au/consumer/gmfood/labelling/Pages/default.aspx . Accessed December 22, 2015.
Fukushima, A., M. Kusano, R.F. Mejia, M. Iwasa, M. Kobayashi, M. Hayashi, A. Watanabe-Takahashi, T. Narisawa, T. Tohge, M. Hur, E. Sykin Wurtele, B.J. Nikolau, and K. Saito. 2014. Metabolomic characterization of knockout mutants in Arabidopsis: Development of a metabolite profiling database for knockout mutants in Arabidopsis. Plant Physiology 165:948–961.
Furgał-Dierżuk, I., J. Strzetelski, M. Twardowska, K. Kwiatek, and M. Mazur. 2015. The effect of genetically modified feeds on productivity, milk composition, serum metabolite profiles and transfer of tDNA into milk of cows. Journal of Animal and Feed Sciences 24:19–30.
Gannon, B., C. Kaliwile, S.A. Arscott, S. Schmaelzle, J. Chileshe, N. Kalungwana, M. Mosonda, K. Pixley, C. Masi, and S.A. Tanumihardjo. 2014. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: A community-based, randomized placebo-controlled trial. American Journal of Clinical Nutrition 100:1541–1550.
García-Villalba, R., C. León, G. Dinelli, A. Segura-Carretero, A. Fernández-Gutiérrez, V. Garcia-Cañas, and A. Cifuentes. 2008. Comparative metabolomic study of transgenic versus conventional soybean using capillary electrophoresis-time-of-flight mass spectrometry. Journal of Chromatography A 1195:164–173.
Gasnier, C., C. Dumont, N. Benachour, E. Clair, M.C. Chagnon, and G.-É. Séralini. 2009. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 262:184–191.
Goodman, R. 2015. Evaluating GE Food Sources for Risks of Allergy: Methods, Gaps and Perspective. Presentation to the National Academy of Sciences’ Committee on Genetically Engineered Crops: Past Experience and Future Prospects, March 5, Washington, DC.
Goodman, R.E., R. Panda, and H. Ariyarathna. 2013. Evaluation of endogenous allergens for the safety evaluation of genetically engineered food crops: Review of potential risks, test methods, examples and relevance. Journal of Agricultural and Food Chemistry 61:8317–8332.
Graf, L., H. Hayder, and U. Mueller. 2014. Endogenous allergens in the regulatory assessment of genetically engineered crops. Food and Chemical Toxicology 73:17–20.
Green, P.H., and C. Cellier. 2007. Celiac disease. New England Journal of Medicine 357:1731–1743.
Guertler, P., V. Paul, C. Albrecht, and H.H.D. Meyer. 2009. Sensitive and highly specific quantitative real-time PCR and ELISA for recording a potential transfer of novel DNA and Cry1Ab protein from feed into bovine milk. Analytical and Bioanalytical Chemistry 393:1629–1638.
Gupta. R., A. Sheikh, D.P. Strachan, and H.R. Anderson. 2007. Time trends in allergic disorders in the UK. Thorax 62:91–96.
Guyton, K.Z., D. Loomis, Y. Grosse, F.E. Ghissassi, L. Benbrahim-Tallaa, N. Guha, C. Scoccianti, H. Mattock, and K. Straif. 2015. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncology 16:490–491.
Halle, I., and G. Flachowsky. 2014. A four-generation feeding study with genetically modified (Bt) maize in laying hens. Journal of Animal and Feed Sciences 23:58–63.
Hammond, B., R. Dudek, J. Lemen, and M. Nemeth. 2004. Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food and Chemical Toxicology 42:1003–1014.
Hammond, B.G., R. Dudek, J.K. Lemen, and M.A. Nemeth. 2006. Results of a 90-day safety assurance study with rats fed grain from corn borer-protected corn. Food and Chemical Toxicology 44:1092–1099.
Hammond, B., D.A. Goldstein, and D. Saltmiras. 2013. Response to original research article, ‘Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize.’ Food and Chemical Toxicology 53:459–464.
Hayes, A.W. 2014. Retraction notice to “Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize.” Food and Chemical Toxicology 63:244.
He, X.Y., K.L. Huang, X. Li, W. Qin, B. Delaney, and Y.B. Luo. 2008. Comparison of grain from corn rootworm resistant transgenic DAS-59122-7 maize with non-transgenic maize grain in a 90-day feeding study in Sprague-Dawley rats. Food and Chemical Toxicology 46:1994–2002.
He, X.Y., Z. Mao, Y.B. Tang, X. Luo, S.S. Li, J.Y. Cao, B. Delaney, and L.H. Kun. 2009. A 90-day toxicology study of transgenic lysine-rich maize grain (Y642) in Sprague-Dawley rats. Food and Chemical Toxicology 47:425–432.
Health Canada. 1999. Novel Food Information—Food Biotechnology, High Lauric Acid Canola Lines 23-198, 23-18-17. Available at http://www.hc-sc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/gmf-agm/ofb-096-100-a-eng.pdf . Accessed May 11, 2016.
Health Canada. 2015. Proposed Re-evaluation Decision PRVD2015-01, Glyphosate. Available at http://www.hc-sc.gc.ca/cps-spc/pest/part/consultations/_prvd2015-01/prvd201501-eng.php . Accessed March 13, 2016.
Hefferon, K.L. 2015. Nutritionally enhanced food crops; Progress and perspectives. International Journal of Molecular Sciences 16:3895–3914.
Hellenas, K.E., C. Branzell, H. Johnsson, and P. Slanina. 1995. High levels of glycoalkaloids in the established Swedish potato variety Magnum Bonum. Journal of the Science of Food and Agriculture 23:520–523.
Herman, R.A. and W.D. Price. 2013. Unintended compositional changes in genetically modified (GM) crops: 20 years of research. Journal of Agricultural and Food Chemistry 61:11695–11701.
Herman, R.A., N.P. Storer, and Y. Gao. 2006. Digestion assays in allergenicity assessment of transgenic proteins. Environmental Health Perspectives 114:1154–1157.
Hernández, A.F., T. Parrón, A.M. Tsatsakis, M. Requena, R. Alarcón, and O. López-Guarnido. 2013. Toxic effects of pesticide mixtures at a molecular level: Their relevance to human health. Toxicology 307:136–145.
Hidalgo, F.J., and R. Zamora. 2006. Peptides and proteins in edible oils: Stability, allergenicity, and new processing trends. Trends in Food Science & Technology 17:56–63.
Hilbeck, A., R. Binimelis, N. Defarge, R. Steinbrecher, A. Székács, F. Wickson, M. Antoniou, P.L. Bereano, E.A. Clark, M. Hansen, E. Novotny, J. Heinemann, H. Meyer, V. Shiva, and B. Wynne. 2015. No scientific consensus on GMO safety. Environmental Sciences Europe 27:4.
Hohlweg, U., and W. Doerfler. 2001. On the fate of plant or other foreign genes upon the uptake in food or after intramuscular injection in mice. Molecular Genetics and Genomics 265:225–233.
Hotz, C., C. Loechl, A. Lubowa, J.K. Tumwine, G. Ndeezi, A. Nandutu Masawi, R. Baingana, A. Carriquiry, A. de Brauw, J.V. Meenakshi, and D.O. Gilligan. 2012a. Introduction of beta-carotene-rich orange sweet potato in rural Uganda resulted in increased vitamin A intakes among children and women and improved vitamin A status among children. Journal of Nutrition 142:1871–1880.
Hotz, C., C. Loechl, A. de Brauw, P. Eozenou, D. Gilligan, M. Moursi, B. Munhaua, P. van Jaarsveld, A. Carriquiry, and J.V. Meenakshi. 2012b. A large-scale intervention to introduce orange sweet potato in rural Mozambique increases vitamin A intakes among children and women. British Journal of Nutrition 108:163–176.
Huang, J., R. Hu, C. Fan, C.E. Pray, and S. Rozelle. 2002. Bt cotton benefits, costs, and impacts in China. AgBioForum 5:153–166.
Huang, J., R. Hu, S. Rozelle, and C. Pray. 2005. Insect-resistant GM rice in farmers’ fields: Assessing productivity and health effects in China. Science 308:688–690.
IARC (International Agency for Research on Cancer). 2002. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 82: Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene, and Styrene. Lyon, France: IARC.
IARC (International Agency for Research on Cancer). 2006. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 88: Formaldehyde, 2-Butoxyethanol, and 1- tert -Butoxypropan-2-ol. Lyon, France: IARC.
IARC (International Agency for Research on Cancer). 2015. Glyphosate. Part of Volume 112 in International Agency for Research on Cancer Monographs on the Evaulation of Carcinogenic Risks to Humans: Some Organophosphate Insecticides and Herbicides: Diazinon, Glyphosate, Malathion, Parathion, and Tetrachlorvinphos. Available at http://monographs.iarc.fr/ENG/Monographs/vol112/mono112-09.pdf . Accessed December 20, 2015.
Ibáñez, C., C. Simó, V. García-Cañas, T. Acunha, and A. Cifuentes. 2015. The role of direct high-resolution mass spectrometry in foodomics. Analytical and Bioanalytical Chemistry 407:6275–6287.
Jackson, K.D., L.D. Howie, and L.J. Akinbami. 2013. Trends in allergenic conditions among children: United States 1997–2011. NCHS Data Brief 121:1–8.
Jaffe, G. 2014. Issues for the Committee on Genetically Engineered Crops to Consider. Presentation to the National Academy of Sciences’ Committee on Genetically Engineered Crops: Past Experience and Future Prospects, September 16, Washington, DC.
John, B. 2014. Letter to the Editor. Food and Chemical Toxicology 65:391.
Johnson, N. July 1, 2014. Retracted Roundup-fed Rat Research Republished. Online. The Grist. Available at http://grist.org/food/retracted-roundup-fed-rat-research-republished/ . Accessed December 13, 2015.
Jones, Y. M., and A. De Brauw. 2015. Using Agriculture to Improve Child Health: Promoting Orange Sweet Potatoes Reduces Diarrhea. World Development 74:15–24.
Joshi, L., J.M. van Eck, K. Mayo, R.G. Silvestro, M.E. Blake, T. Ganapathi, V. Haridas, J.U. Gutterman, and C.J. Arntzen. 2002. Metabolomics of plant saponins: Bioprospecting triterpene glycoside diversity with respect to mammalian cell targets. OMICS A Journal of Integrative Biology 6:235–246.
Keese, P. 2008. Risks from GMOs due to horizontal gene transfer. Environmental Biosafety Research 7:123–149.
Kiliç, A., and M.T. Akay. 2008. A three-generation study with genetically modified Bt corn in rats: Biochemical and histopathological investigation. Food and Chemical Toxicology 46:1164–1170.
Knudsen, I., and M. Poulsen. 2007. Comparative safety testing of genetically modified foods in a 90-day rat feeding study design allowing the distinction between primary and secondary effects of the new genetic event. Regulatory Toxicology and Pharmacology 49:53–62.
Kouser, S., and M. Qaim. 2011. Impact of Bt cotton on pesticide poisoning in smallholder agriculture: A panel data analysis. Ecological Economics 70:2105–2113.
Kouser, S., and M. Qaim. 2013. Valuing financial, health, and environmental benefits of Bt cotton in Pakistan. Agricultural Economics 44:323–335.
Krimsky, S. 2015. An illusory consensus behind GMO health assessment. Science, Technology, & Human Values 40:883–914.
Krishnan, H.B., M.S. Kerley, G.L. Allee, S. Jang, W.S. Kim, and C.J. Fu. 2010. Maize 27 kDa gamma-zein is a potential allergen for early weaned pigs. Journal of Agricultural and Food Chemistry 58:7323–7328.
Kuc, J. 1982. Phytoalexins from the Solanaceae. Pp. 81–105 in Phytoalexins, J.A. Bailey and J.W. Mansfield, eds. New York: Wiley.
Kuiper, H.A., E.J. Kok, and H.V. Davies. 2013. New EU legislation for risk assessment of GM food: No scientific justification for mandatory animal feeding trials. Plant Biotechnology Journal 11:781–784.
Ladics, G.S., and M.K. Selgrade. 2009. Identifying food proteins with allergenic potential: Evolution of approaches to safety assessment and research to provide additional tools. Regulatory Toxicology and Pharmacology 54:S2-S6.
Ladics, G.S., G.J. Budziszewski, R.A. Herman, C. Herouet-Guicheney, S. Joshi, E.A. Lipscomb, S. McClain, and J.M. Ward. 2014. Measurement of endogenous allergens in genetically modified soybeans—Short communication. Regulatory Toxicology and Pharmacology 70:75–79.
Langkilde, S., M. Schrøder, T. Frank, L.V.T. Shepherd, S. Conner, H.V. Davies, O. Meyer, J. Danier, M. Rychlik, W.R. Belknap, K.F. McCue, K.-H. Engel, D. Stewart, I. Knudsen, and M Poulsen. 2012. Compositional and toxicological analysis of a GM potato line with reduced α-solanine content—A 90-day feeding study in the Syrian Golden hamster. Regulatory Toxicology and Pharmacology 64:177–185.
Lee, S.H., and B.R. Hamaker. 2006. Cys 155 of 27 kDa maize γ-zein is a key amino acid to improve its in vitro digestibility. FEBS Letters 580:5803–5806.
Ley, R.E. 2010. Obesity and the human microbiome. Current Opinion in Gastroenterology 26:5–11.
Liebsch, M., B. Grune, A. Seiler, D. Butzke, M. Oelgeschläger, R. Pirow, S. Adler, C. Riebeling, and A. Luch. 2011. Alternatives to animal testing: Current status and future perspectives. Archives of Toxicology 85:841–858.
Litten-Brown, J.C., A.M. Corson, and L. Clarke. 2010. Porcine models for the metabolic syndrome digestive and bone disorders: A general overview. Animal 4:899–920.
Liu, P., X. He, D. Chen, Y. Luo, S. Cao, H. Song, T. Liu, K. Huang, and W. Xu. 2012. A 90-day subchronic feeding study of genetically modified maize expressing Cry1Ac-M protein in Sprague–Dawley rats. Food and Chemical Toxicology 50:3215–3221.
Lividini, K., and J.L. Fielder. 2015. Assessing the promise of biofortification: A case study of high provitamin A maize in Zambia. Food Policy 54:65–77.
Livingston, M, J. Fernandez-Cornejo, J. Unger, C. Osteen, D. Schimmelpfennig, T. Park, and D. Lambert. 2015. The Economics of Glyphosate Resistance Management in Corn and Soybean Production. Washington, DC: U.S. Department of Agriculture–Economic Research Service.
Ludvigsson, J.F., A. Rubio-Tapia, C.T. van Dyke, L.J. Lelton, A.R. Zinsmeister, B.D. Lahr, and J.A. Murray. 2013. Increasing incidence of celiac disease in a North American population. American Journal of Gastroenterology 108:818–824.
Lusk, R.W. 2014. Diverse and widespread contamination evident in the unmapped depths of high throughput sequencing data. PLoS ONE 9:e110808.
MacKenzie, S.A., I. Lamb, J. Schmidt, L. Deege, M.J. Morrisey, M. Harper, R.J. Layton, L.M. Prochaska, C. Sanders, M. Locke, J.L. Mattsson, A. Fuentes, and B. Delaney. 2007. Thirteen week feeding study with transgenic maize grain containing event DAS-01507-1 in Sprague-Dawley rats. Food and Chemical Toxicology 45:551–562.
Macpherson, A.J., K.D. McCoy, F-.E. Johansen, and P. Brandtzaeg. 2008. The immune geography of IgA induction and function. Mucosal Immunology 1:11–22.
Magana-Gomez, J.A., and A.M.C. de la Barca. 2009. Risk assessment of genetically modified crops for nutrition and health. Nutrition Reviews 67:1–16.
Martin, C., Y. Zhang, C. Tonelli, and K. Petroni. 2013. Plants, diet, and health. Annual Review of Plant Biology 64:19–46.
Martín-Hernández, C., S. Bénet, and L. Obert. 2008. Determination of proteins in refined and nonrefined oils. Journal of Agricultural and Food Chemistry 56:4348–4351.
Marx-Stoelting, P., A. Braeuning, T. Buhrke, A. Lampen, L. Niemann, M. Oelgeschlaeger, S. Rieke, F. Schmidt, T. Heise, R. Pfeil, and R. Solecki. 2015. Application of omics data in regulatory toxicology: Report of an international BfR expert workshop. Archives in Toxicology 89:2177–2184.
Mazza, R., M. Soave, M. Morlacchini, G. Piva, and A. Marocco. 2005. Assessing the transfer of genetically modified DNA from feed to animal tissues. Transgenic Research 14:775–784.
Miller, E.R., and D.E. Ullrey. 1987. The pig as a model for human nutrition. Annual Review of Nutrition 7:361–382.
Miller, H.I. 1999. Substantial equivalence: Its uses and abuses. Nature Biotechnology 17:1042–1043.
Millstone, E., E. Brunner, and S. Mayer. 1999. Beyond “substantial equivalence.” Nature 401:525–526.
Mink, P.J., J.S. Mandel, J.I. Lundin, and B.K. Sceurman. 2011. Epidemiologic studies of glyphosate and non-cancer health outcomes: A review. Regulatory Toxicology and Pharmacology 61:172–184.
Mink, P.J., J.S. Mandel, B.K. Sceurman, and J.I. Lundin. 2012. Epidemiologic studies of glyphosate and cancer: A review. Regulatory Toxicology and Pharmacology 63:440–452.
Munkvold, G.P., and A.E. Desjardins. 1997. Fumonisins in maize: Can we reduce their occurrence? Plant Disease 81:556–565.
Murray, J.A., C. Van Dyke, M.F. Plevak, R.A. Dierkhising, A.R. Zinsmeister, and L.J. Melton. 2003. Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clinical Gastroenterology and Hepatology 1:19–27.
Murthy, H.N., M.I. Georgiev, S.-Y. Park, V.S. Dandin, and K.-Y. Paek. 2015. The safety assessment of food ingredients derived from plant cell, tissue and organ cultures: A review. Food Chemistry 176:426–432.
Muzzalupo, I., F. Pisani, F. Greco, and A. Chiappetta. 2015. Direct DNA amplification from virgin olive oil for traceability and authenticity. European Food Research and Technology 241:151–155.
Nakabayashi, R., K. Yonekura-Sakakibara, K. Urano, M. Suzuki, Y. Yamada, T. Nishizawa, F. Matsuda, M. Kojima, H. Sakakibara, K. Shinozaki, A.J. Michael, T. Tohge, M. Yamazaki, and K. Saito. 2014. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant Journal 77:367–379.
National Toxicology Program. 2014. Report on Carcinogens, Thirteenth Edition. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service.
NCI (National Cancer Institute). 2014. Surveillance, Epidemology and End Results (SEER) Program. Available at http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index . Accessed October 29, 2015.
Nemeth, A., A. Wurz, L. Artim, S. Charlton, G. Dana, K. Glenn, P. Hunst, J. Jennings, R. Shilito, and P. Song. 2004. Sensitive PCR analysis of animal tissue samples for fragments of endogenous and transgenic plant DNA. Journal of Agricultural and Food Chemistry 52:6129–6135.
Netherwood, T., S.M. Martin-Orue, A.G. O’Donnell, S. Gockling, J. Graham, J.C. Mathers, and H.J. Gilbert. 2004. Assessing the survival of transgenic plant DNA in the human gastrointestinal tract. Nature Biotechnology 22:204–209.
Nicolia, A., A. Manzo, F. Veronesi, and D. Rosellini. 2014. An overview of the last 20 years of genetically engineered crop safety research. Critical Reviews in Biotechnology 34:77–88.
Nordlee, J.A., S.L. Taylor, J.A. Townsend, L.A. Thomas, and R.K. Bush. 1996. Identification of a Brazil-nut allergen in transgenic soybean. New England Journal of Medicine 334:688–692.
Novak, W.K., and A.G. Haslberger. 2000. Substantial equivalence of antinutritional and inherent plant toxins in genetically modified novel foods. Food and Chemical Toxicology 38:473–483.
NRC (National Research Council). 2000. Genetically Modified Pest-Protected Plants: Science and Regulation. Washington, DC: National Academy Press.
NRC (National Research Council). 2002. Environmental Effects of Transgenic Plants: The Scope and Adequacy of Regulation. Washington, DC: National Academy Press.
NRC (National Research Council). 2004. Safety of Genetically Engineered Foods: Approaches to Assessing Unintended Health Effects. Washington, DC: National Academies Press.
NRC (National Research Council). 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, DC: National Academies Press.
Nwaru, B.I., L. Hickstein, S.S. Panesar, G. Roberts, A. Muraro, and A. Sheikh. 2014. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 69:992–1007.
OECD (Organisation for Economic Co-operation and Development). 1993. Safety Evaluation of Foods Derived by Modern Biotechnology: Concepts and Principles. Paris: OECD.
OECD (Organisation for Economic Co-operation and Development). 1998a. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents in OECD Guidelines for the Testing of Chemicals. Paris: OECD.
OECD (Organisation for Economic Co-Operation and Development). 1998b. Principles of Good Laboratory Practice and Compliance Monitoring. Available at http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/mc/chem(98)17&doclanguage=en . Accessed October 13, 2015.
OECD (Organisation for Economic Co-Operation and Development). 2000. Report of the Task Force for the Safety of Novel Foods and Feeds. Available at http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/mc/chem(98)17&doclanguage=enhttp://www.biosafety.be/ARGMO/Docments/report_taskforce.pdf . Accessed October 12, 2015.
OECD (Organisation for Economic Co-Operation and Development). 2006. An Introduction to the Food/Feed Safety Consensus Documents of the Task Force. Series on the Safety of Novel Foods and Feeds, No 14. Paris: OECD.
OECD (Organisation for Economic Co-Operation and Development). 2015. Safety Assessment of Foods and Feeds Derived from Transgenic Crops, Volume 2, Novel Food and Feed Safety. Paris: OECD.
Oguchi, T., M. Onishi, Y. Chikagawa, T. Kodama, E. Suzuki, M. Kasahara, H. Akiyama, R. Teshima, S. Futo, A. Hino, S. Furui, and K. Kitta. 2009. Investigation of residual DNAs in sugar from sugar beet ( Beta vulgaris L.). Journal of the Food Hygenic Society of Japan 50:41–46.
Onose, J., T. Imai, M. Hasumura, M. Ueda, Y. Ozeki, and M. Hirose. 2008. Evaluation of subchronic toxicity of dietary administered Cry1Ab protein from Bacillus thuringiensis var. Kurustaki HD-1 in F344 male rats with chemically induced gastrointestinal impairment. Food and Chemical Toxicology 46:2184–2189.
Paine, J.A., C.A. Shipton, S. Chaggar, R.M. Howells, M.J. Kennedy, G. Vernon, S.Y. Wright, E. Hinchliffe, J.L. Adams, A.L. Silverstone, and R. Drake. 2005. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nature Biotechnology 23:482–487.
Panchin, A.Y., and A.I. Tuzhikov. 2016. Published GMO studies find no evidence of harm when corrected for multiple comparisons. Critical Reviews in Biotechnology, Early Online:1–5.
Patisaul, H.B., and W. Jefferson. 2010. The pros and cons of phytoestrogens. Frontiers in Neuroendocrinology 31:400–419.
Patterson, J.K., X.G. Lei, and D.D. Miller. 2008. The pig as an experimental model for elucidating the mechanisms governing dietary influence on mineral absorption. Experimental Biology and Medicine 233:651–664.
Pecetti, L., A. Tava, A. Romani, M.G. De Benedetto, and P. Corsi. 2006. Variety and environment effects on the dynamics of saponins in lucerne ( Medicago sativa L.). European Journal of Agronomy 25:187–192.
Phipps, R.H., E.R. Deaville, and B.C. Maddison. 2003. Detection of transgenic DNA and endogenous plant DNA in rumen fluid, duodenal digesta, milk, blood and feces of lactating dairy cows. Journal of Dairy Science 86:4070–4078.
Poulsen, M., M. Schrøder, A. Wilcks, S. Kroghsbo, R.H. Lindecrona, A. Miller, T. Frenzel, J. Danier, M. Rychlik, Q. Shu, K. Emami, M. Taylor, A. Gatehouse, K.H. Engel, and I. Knudsen. 2007. Safety testing of GM-rice expressing PHA-E lectin using a new animal test design. Food Chemistry and Toxicology 45:364–377.
Racovita, M., D.N. Oboryo, W. Craig, and R. Ripandelli. 2015. What are the non-food impacts of GM crop cultivation on farmers’ health. Environmental Evidence 4:17.
Ren, Y., T. Wang, Y. Peng, B. Xia, and L.J. Qu. 2009. Distinguishing transgenic from nontransgenic Arabidopsis plants by (1)H NMR-based metabolic fingerprinting. Journal of Genetics and Genomics 36:621–628.
Rhee, G.S., D.H. Cho, Y.H. Won, J.H. Seok, S.S. Kim, S.J. Kwack, R.D. Lee, S.Y. Chae, J.W. Kim, B.M. Lee, K.L. Park, and K.S. Choi. 2005. Multigenerational reproductive and developmental toxicity study of bar gene inserted into genetically modified potato on rats. Journal of Toxicology and Environmental Health, Part A: Current Issues 68:2263–2276.
Ricroch, A.E. 2013. Assessment of GE food safety using “-omics” techniques and long-term animal feeding studies. New Biotechnology 30:349–354.
Ricroch, A., J.B. Bergé, and M. Kuntz. 2011. Evaluation of genetically engineered crops using transcriptomic, proteomic and metabolomic profiling techniques. Plant Physiology 155:1752–1761.
Ricroch, A.E., A. Berheim, C. Snell, G. Pascal, A. Paris, and M. Kuntz. 2013a. Long-term and multi-generational animal feeding studies. Pp. 112–127 in Animal Nutrition with Transgenic Plants, G. Flachowsky, ed. Oxfordshire, UK: CABI Biotechnology Series.
Ricroch, A., A. Berheim, G. Pascal, A. Paris, and M. Kuntz. 2013b. Assessment of the health impact of GE plant diets in long term and multigenerational animal feeding trials. P. 234 in Animal Nutrition with Transgenic Plants, G. Flachowsky, ed. Oxfordshire, UK: CABI Biotechnology Series.
Ricroch, A.E., A. Boisron, and M. Kuntz. 2014. Looking back at safety assessment of GM food/feed: An exhaustive review of 90-day animal feeding studies. International Journal of Biotechnology 13:230–256.
Riddle, M.S., J.A. Murray, and C.K. Porter. 2012. The incidence and risk of celiac disease in a healthy US population. American Journal of Gastroenterology 107:1248–1255.
Rizzi, A., L. Brusetti, S. Arioli, K.M. Nielsen, I. Tamagnini, A. Tamburini, C. Sorlini, and D. Daffonchio. 2008. Detection of feed-derived maize DNA in goat milk and evaluation of the potential of horizontal transfer to bacteria. European Food Research and Technology 227:1699–1709.
Rizzi, A., N. Raddadi, C. Sorlini, L. Nordgård, K.M. Nielsen, and D. Daffonchio. 2012. The stability and degradation of dietary DNA in the gastrointestinal tract of mammals: Implications for horizontal gene transfer and the biosafety of GMOs. Critical Reviews in Food Science and Nutrition 52:142–161.
Roberfroid, M. 2014. Letter to the Editor. Food and Chemical Toxicology 65:390.
Rommens, C.M., H. Yan, K. Swords, C. Richael, and J. Ye. 2008. Low-acrylamide French fries and potato chips. Plant Biotechnology Journal 6:843–853.
Ruan, C.J., H.B. Shao, and J.A. Teixeira da Silva. 2012. A critical review on the improvement of photosynthetic carbon assimilation in C3 plants using genetic engineering. Critical Reviews in Biotechnology 32:1–21.
Rubio-Tapia, A., J.F. Ludvigsson, T.L. Brantner, J.A. Murray, and J.E. Everhart. 2012. The prevalence of celiac disease in the United States. American Journal of Gastroenterology 107:1538–1544.
Ruiz-Lopez, N., R.P. Haslam, J.A. Napier, and O. Sayanova. 2014. Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant Journal 77:198–208.
Saltzman, A., E. Birol, H.E. Bouis, E. Boy, F.F. De Moura, Y. Islam, and W.H. Pfeiffer. 2013. Biofortification: Progress toward a more nourishing future. Global Food Security 2:9–17.
Sanahuja, G., G. Farré, J. Berman, U. Zorrilla-López, R.M. Twyman, T. Capell, P. Christou, and C. Zhu. 2013. A question of balance: Achieving appropriate nutrient levels in biofortified staple crops. Nutritional Research Reviews 26:235–245.
Sanders, D. 2013. Letter to the Editor. Food and Chemical Toxicology 53:450–453.
Schubbert, R., U. Hohlweg, D. Renz, and W. Doerfler. 1998. On the fate of orally ingested foreign DNA in mice: Chromosomal association and placental transmission to the fetus. Molecular Genetics and Genomics 259:569–576.
Séralini, G.E. 2014. Presentation to the National Academy of Sciences’ Committee on Genetically Engineered Crops: Past Experience and Future Prospects, September 16, Washington, DC.
Séralini, G.E., D. Cellier, and J.S. de Vendomois. 2007. New analysis of a rat feeding study with a genetically modified maize reveals signs of hepatorenal toxicity. Archives of Environmental Contamination and Toxicology 52:596–602.
Séralini, G.E., E. Clair, R. Mesnage, S. Gress, N. Defarge, M. Malatesta, D. Hennequin, and J.S. de Vendômois. 2012. Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Food and Chemical Toxicology 50:4221–4231.
Séralini, G.E., E. Clair, R. Mesnage, S. Gress, N. Defarge, M. Malatesta, D. Hennequin, and J.S. de Vendômois. 2014. Republished study: Long-term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Environmental Sciences Europe 26:14.
Shepherd, L.V.T., C.A. Hackett, C.J. Alexander, J.W. McNicol, J.A. Sungurtas, D. Stewart, K.F. McCue, W.R. Belknap, and H.V. Davies. 2015. Modifying glycoalkaloid content in transgenic potato—Metabolome impacts. Food Chemistry 187:437–443.
Simó, C., C. Ibáñez, A. Valdés, A. Cifuentes, and V. García-Cañas. 2014. Metabolomics of genetically modified crops. International Journal of Molecular Sciences 15:18941–18966.
Sinden, S.L., and R.E. Webb. 1972. Effect of variety and location on the glycoalkaloid content of potatoes. American Potato Journal 49:334–338.
Singhal, K.K., A.K. Tyagi, Y.S. Rajput, M. Singh, H. Kaur, T. Perez, and G.F. Hartnell. 2011. Feed intake, milk production and composition of crossbred cows fed with insect-protected Bollgard II® cottonseed containing Cry1Ac and Cry2Ab proteins. Animal 5:1769–1773.
Small, E. 1996. Adaptations to herbivory in alfalfa ( Medicago sativa ). Canadian Journal of Botany 74:807–822.
Smith, J.M. 2003. Seeds of Deception: Exposing Industry and Government Lies about the Safety of the Genetically Engineered Foods You’re Eating. Fairfield, IA: Yes! Books.
Smith, J.M. 2013. Are genetically modified foods a gut-wrenching combination? Institute for Responsible Technology. Available at http://responsibletechnology.org/glutenintroduction/ . Accessed October 12, 2015.
Smith, J.M. 2014. Recommendations for the Committee on Genetically Engineered Crops. Presentation to the National Academy of Sciences’ Committee on Genetically Engineered Crops: Past Experience and Future Prospects, September 16, Washington, DC.
Snell, C., A. Bernheim, J.B. Berge, M. Kuntz, G. Pascal, A. Paris, and A.E. Ricroch. 2012. Assessment of the health impact of GM plant diets in long-term and multigenerational animal feeding trials: A literature review. Food and Chemical Toxicology 50:1134–1148.
Spisák, S., N. Solymosi, P. Ittzés, A. Bodor, D. Kondor, G. Vattay, B. Barták, F. Sipos, O. Galamb, Z. Tulassay, Z. Szállási, S. Rasmussen, T. Sicheritz-Ponten, S. Brunak, B. Molnár, and I. Csabai. 2013. Complete genes may pass from food to human blood. PLoS ONE 8:e69805.
Springob, K., and T.M. Kutchan. 2009. Introduction to the different classes of natural products. Pp. 3–50 in Plant-Derived Natural Products, A.E. Osbourn and V. Lanzotti, eds. New York: Springer-Verlag.
Steinke, K., P. Guertler, V. Paul, S. Wiedemann, T. Ettle, C. Albrecht, H.H.D. Meyer, H. Spiekers, and F.J. Schwarz. 2010. Effects of long-term feeding of genetically modified corn (event MON810) on the performance of lactating dairy cows. Journal of Animal Physiology and Animal Nutrition 94:e185–e193.
Swiatkiewicz, S., M. Swiatkiewicz, A. Arczewska-Wlosek, and D. Jozefiak. 2014. Genetically modified feeds and their effect on the metabolic parameters of food-producing animals: A review of recent studies. Animal Feed Science and Technology 198:1–19.
Taylor, A. November 25, 2015. EPA nixes approval of Enlist Duo weed killer. Online. The Des Moines Register. Available at http://www.desmoinesregister.com/story/money/agriculture/2015/11/25/epa-nixes-approval-enlist-duo-weed-killer/76386952/ . Accessed December 13, 2015.
Taylor, B., H. Jick, and D. MacLaughlin. 2013. Prevalence and incidence rates of autism in the UK: Time trend from 2004–2010 in children aged 8 years. BMJ 3:e003219.
Thayer, K.A., J.J. Heindel, J.R. Bucher, and M.A. Gallo. 2012. Role of environmental chemicals in diabetes and obesity: A National Toxicology Program workshop review. Environmental Health Perspectives 120:779–789.
Trabalza-Marinucci, M., G. Brandi, C. Rondini, L. Avellini, C. Giammarini, S. Costarelli, G. Acuti, C. Orlandi, G. Filippini, E. Chiaradia, M. Malatesta, S. Crotti, C. Antonini, G. Amagliani, E. Manuali, A.R. Mastrogiacomo, L. Moscati, M.N. Haouet, A. Gaiti, and M. Magnani. 2008. A three-year longitudinal study on the effects of a diet containing genetically modified Bt176 maize on the health status and performance of sheep. Livestock Science 113:178–190.
Treutter, D. 2006. Significance of flavonoids in plant resistance: A review. Environmental Chemistry Letters 4:147–157.
Trikha, A., J.A. Baillargeon, Y.F. Kuo, A. Tan, K. Pierson, G. Sharma, G. Wilkinson, and R.S. Bonds. 2013. Development of food allergies in patients with Gastroesophageal Reflux Disease treated with gastric acid suppressive medications. Pediatric Allergy and Immunology 24:582–588.
Turnbaugh, P.J., M. Hamady, T. Yatsunenko, B.L. Cantarel, A. Duncan, R.E. Ley, M.L. Sogin, W.J. Jones, B.A. Roe, J.P. Affourtit, M. Egholm, B. Henrissat, A.C. Heath, R. Knight, and J.I. Gordon. 2009. A core gut microbiome in obese and lean twins. Nature 457:480–484.
Untersmayr, E., and E. Jensen-Jarolim. 2008. The role of protein digestibility and antacids on food allergy outcomes. Journal of Allergy and Clinical Immunology 121:1301–1308.
U.S. Census Bureau. 2014. 65+ in the United States: 2010. Washington, DC: U.S. Government Printing Office.
U.S. Pharmacopeia. 2000. Pharmacopeia, simulated gastric fluid, TS, simulated intestinal fluid, TS. United States Pharmacopeial Convention, v. 24. The National Formulary 9 (US Pharmacopiea Board of Trustees), Rockville, MD, 2235.
USDA–APHIS (U.S. Department of Agriculture–Animal and Plant Health Inspection Service). 2014a. Dow AgroSciences Petitions (09-233-01p, 09-349-01p, and 11-234-01p) for Determinations of Nonregulated Status for 2,4-D-Resistant Corn and Soybean Varieties. Final Environmental Impact Statement—August 2014. Available at https://www.regulations.gov/document?D=APHIS-2013-0042-10218 . Accessed May 9, 2016.
USDA–APHIS (U.S. Department of Agriculture–Animal and Plant Health Inspection Service). 2014b. Record of Decision: Dow AgroSciences Petitions (09-233-01p, 09-349-01p, and 11-234-01p) for Determination of Nonregulated Status for 2,4-D-Resistant Corn and Soybean Varieties. Available at https://www.aphis.usda.gov/brs/aphisdocs/24d_rod.pdf . Accessed December 13, 2015.
USDA–APHIS (U.S. Department of Agriculture–Animal and Plant Health Inspection Service). 2014c. Determinations of Nonregulated Status: J.R. Simplot Co.; Potato Genetically Engineered for Low Acrylamide Potential and Reduced Black Spot Bruise. Available at http://www.regulations.gov/#!documentDetail;D=APHIS-2012-0067-0384 . Accessed December 22, 2015.
USRDS (United States Renal Data System). 2014. CKD in the general population. Pp. 12–22 in 2014 USRDS Annual Data Report Volume 1. Available at http://www.usrds.org/2014/view/Default.aspx . Accessed October 13, 2015.
Valdés, L., A. Cuervo, N. Salazr, P. Ruas-Madiedo, M. Gueimondea, and S. González. 2015. The relationship between phenolic compounds from diet and microbiota: Impact on human health. Food & Function 6:2424–2439.
van den Eede, G., H. Aarts, H.J. Buhk, G. Corthier, H.J. Flint, W. Hammes, B. Jacobsen, T. Midtvedt, J. van der Vossen, A. von Wright, W. Wackernagel, and A. Wilcks. 2004. The relevance of gene transfer to the safety of food and feed derived from genetically modified (GM) plants. Food and Chemical Toxicology 42:1127–1156.
Van Eenennaam, A.L., and A.E. Young. 2014. Prevalence and impacts of genetically engineered feedstuffs on livestock populations. Journal of Animal Science 92:4255–4278.
VanEtten, H., J.W. Mansfield, J.A. Bailey, and E.E. Farmer. 1994. Two classes of plant antibiotics: Phytoalexins versus “phytoanticipins.” The Plant Cell 6:1191–1192.
Verhoeckx, K.C.M., Y.M. Vissers, J.L. Baumert, R. Faludi, M. Feys, S. Flanagan, C. Herouet-Guicheney, T. Holzhauser, R. Shimojo, N. van der Bolt, H. Wichers, and I. Kimber. 2015. Food processing and allergenicity. Food and Chemical Toxicology 80:223–240.
Wal, J.M. 2015. Assessing and managing allergenicity of genetically modified (GM) foods. Pp. 161–178 in Handbook of Food Allergen Detection and Control, S. Flanagan, ed. Cambridge, UK: Woodhead Publishing.
Walsh, M.C., S.G. Buzoianu, G.E. Gardiner, M.C. Rea, E. Gelencsér, A. Jánosi, M.M. Epstein, R.P. Ross, and P.G. Lawlor. 2011. Fate of transgenic DNA from orally administered Bt MON810 maize and effects on immune response and growth in pigs. PLoS ONE 6:e27177.
Walsh, M.C., S.G. Buzoianu, M.C. Rea, O. O’Donovan, E. Gelencsér, G. Ujhelyi, R.G. Ross, G.E. Gardiner, and P.G. Lawlor. 2012a. Effects of feeding Bt MON810 maize to pigs for 110 days on peripheral immune response and digestive fate of the Cry1Ab gene and truncated Bt toxin. PLoS ONE 7:e36141.
Walsh, M.C., S.G. Buzoianu, G.E. Gardiner, M.C. Rea, R.P. Ross, J.P. Cassidy, and P.G. Lawlor. 2012b. Effects of short-term feeding of Bt MON810 maize on growth performance, organ morphology and function in pigs. British Journal of Nutrition 107:364–371.
Walsh, M.C., S.G. Buzoianu, G.E. Gardiner, M.C. Rea, O. O’Donovan, R.P. Ross, and P.G. Lawlor. 2013. Effects of feeding Bt MON810 maize to sows during first gestation and lactation on maternal and offspring health indicators. British Journal of Nutrition 109:873–881.
Wang, X., X. Chen, J. Xu, C. Dai, and W. Shen. 2015. Degradation and detection of transgenic Bacillus thuringiensis DNA and proteins in flour of three genetically modified rice events submitted to a set of thermal processes. Food and Chemical Toxicology 84:89–98.
Weber, A. 2014. C 4 Photosynthesis—A Target for Genome Engineering. Presentation to the National Academy of Sciences’ Committee on Genetically Engineered Crops: Past Experience and Future Prospects, December 10, Washington, DC.
West, J., K.M. Fleming, L.J. Tata, T.R. Card, and C.J. Crooks. 2014. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: Population-based study. American Journal of Gastroenterology 109:757–768.
Wiatrak, P.J., D.L. Wright, J.J. Marois, and D. Wilson. 2005. Influence of planting date on aflatoxin accumulation in Bt, non-Bt, and tropical non-Bt hybrids. Agronomy Journal 97:440–445.
Wiener, J.B., M.D. Rogers, J.K. Hammitt, and P.H. Sand, eds. 2011. The Reality of Precaution: Comparing Risk Regulation in the United States and Europe. New York: RFF Press.
Wild, C.P., and Y.Y. Gong. 2010. Mycotoxins and human disease: A largely ignored global health issue. Carcinogensis 31:71–82.
Williams, J.H., T.D. Phillips, P.E. Jolly, J.K. Stiles, C.M. Jolly, and D. Aggarwal. 2004. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. American Journal of Clinical Nutrition 80:1106–1122.
World Health Organization. 2014. Frequently Asked Questions on Genetically Modified Foods. Available at http://www.who.int/foodsafety/areas_work/food-technology/Frequently_asked_questions_on_gm_foods.pdf . Accessed March 12, 2016.
Wu, G., M. Truksa, N. Datla, P. Vrinten, J. Bauer, T. Zank, P. Cirpus, E. Heinz, and X. Qiu. 2005. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nature Biotechnology 23:1013–1017.
Wu, Y., D.R. Holding, and J. Messing. 2010. γ-Zeins are essential for endosperm medication in quality protein maize. Proceedings of the National Academy of Sciences of the United States of America 107:12810–12815.
Ye, X., S. Al-Babili, A. Klöti, J. Zhang, P. Lucca, P. Beyer, and I. Potrykus. 2000. Engineering the provitamin A (β-Carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287:303–305.
Yudina, T.G., A.L. Brioukhanov, I.A. Zalunin, L.P. Revina, A.I. Shestakov, N.E. Voyushina, G.G. Chestukhina, and A.I. Netrusov. 2007. Antimicrobial activity of different proteins and their fragments from Bacillus thuringiensis parasporal crystals against clostridia and archaea. Anaerobe 13:6–13.
Zhang, C., A. Yin, H. Li, R. Wang, G. Wu, J. Shen, M. Zhang, L. Wang, Y. Houb, H. Ouyang, Y. Zhang, Y. Zheng, J. Wang, X. Lv, Y. Wang, F. Zhang, B. Zeng, W. Li, F. Yan, Y. Zhao, X. Pang, X. Zhang, H. Fu, F. Chen, N. Zhao, B.R. Hamaker, L.C. Bridgewater, D. Weinkove, K. Clement, J. Dore, E. Holmes, H. Xiao, G. Zhao, S. Yang, P. Bork, J.K. Nicholson, H. Wei, H. Tang, X. Zhang, and L. Zhao. 2015. Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine 2:968–984.
Zhu, X.-G., L. Shan, Y. Wang, and W.P. Quick. 2010. C4 rice—an ideal arena for systems biology research. Journal of Integrative Plant Biology 52:762–770.
Genetically engineered (GE) crops were first introduced commercially in the 1990s. After two decades of production, some groups and individuals remain critical of the technology based on their concerns about possible adverse effects on human health, the environment, and ethical considerations. At the same time, others are concerned that the technology is not reaching its potential to improve human health and the environment because of stringent regulations and reduced public funding to develop products offering more benefits to society. While the debate about these and other questions related to the genetic engineering techniques of the first 20 years goes on, emerging genetic-engineering technologies are adding new complexities to the conversation.
Genetically Engineered Crops builds on previous related Academies reports published between 1987 and 2010 by undertaking a retrospective examination of the purported positive and adverse effects of GE crops and to anticipate what emerging genetic-engineering technologies hold for the future. This report indicates where there are uncertainties about the economic, agronomic, health, safety, or other impacts of GE crops and food, and makes recommendations to fill gaps in safety assessments, increase regulatory clarity, and improve innovations in and access to GE technology.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Genetically Modified Crops and Food Security
1 Department of Agricultural Economics and Rural Development, Georg-August-University of Goettingen, Goettingen, Germany
Shahzad Kouser
2 Institute of Agricultural and Resource Economics, University of Agriculture, Faisalabad, Pakistan
Analyzed the data: MQ SK. Wrote the paper: MQ SK. Conceived and designed the survey: MQ.
Associated Data
The role of genetically modified (GM) crops for food security is the subject of public controversy. GM crops could contribute to food production increases and higher food availability. There may also be impacts on food quality and nutrient composition. Finally, growing GM crops may influence farmers’ income and thus their economic access to food. Smallholder farmers make up a large proportion of the undernourished people worldwide. Our study focuses on this latter aspect and provides the first ex post analysis of food security impacts of GM crops at the micro level. We use comprehensive panel data collected over several years from farm households in India, where insect-resistant GM cotton has been widely adopted. Controlling for other factors, the adoption of GM cotton has significantly improved calorie consumption and dietary quality, resulting from increased family incomes. This technology has reduced food insecurity by 15–20% among cotton-producing households. GM crops alone will not solve the hunger problem, but they can be an important component in a broader food security strategy.
Introduction
Food security exists when all people have physical and economic access to sufficient, safe, and nutritious food. Unfortunately, food security does not exist for a significant proportion of the world population. Around 900 million people are undernourished, meaning that they are undersupplied with calories [1] . Many more suffer from specific nutritional deficiencies, often related to insufficient intake of micronutrients. Eradicating hunger is a central part of the United Nations’ Millennium Development Goals [2] . But how to achieve this goal is debated controversially. Genetically modified (GM) crops are sometimes mentioned in this connection. Some see the development and use of GM crops as key to reduce hunger [3] , [4] , while others consider this technology as a further risk to food security [5] , [6] . Solid empirical evidence to support either of these views is thin.
There are three possible pathways how GM crops could impact food security. First, GM crops could contribute to food production increases and thus improve the availability of food at global and local levels. Second, GM crops could affect food safety and food quality. Third, GM crops could influence the economic and social situation of farmers, thus improving or worsening their economic access to food. This latter aspect is of particular importance given that an estimated 50% of all undernourished people worldwide are small-scale farmers in developing countries [7] .
In regard to the first pathway, GM technologies could make food crops higher yielding and more robust to biotic and abiotic stresses [8] , [9] . This could stabilize and increase food supplies, which is important against the background of increasing food demand, climate change, and land and water scarcity. In 2012, 170 million hectares (ha) – around 12% of the global arable land – were planted with GM crops, such as soybean, corn, cotton, and canola [10] , but most of these crops were not grown primarily for direct food use. While agricultural commodity prices would be higher without the productivity gains from GM technology [11] , impacts on food availability could be bigger if more GM food crops were commercialized. Lack of public acceptance is one of the main reasons why this has not yet happened more widely [12] .
Concerning the second pathway, crops with new traits can be associated with food safety risks, which have to be assessed and managed case by case. But such risks are not specific to GM crops. Long-term research confirms that GM technology is not per se more risky than conventional plant breeding technologies [13] . On the other hand, GM technology can help to breed food crops with higher contents of micronutrients; a case in point is Golden Rice with provitamin A in the grain [14] . Such GM crops have not yet been commercialized. Projections show that they could reduce nutritional deficiencies among the poor, entailing sizeable positive health effects [15] , [16] .
The third pathway relates to GM crop use by smallholder farmers in developing countries. Half of the global GM crop area is located in developing countries, but much of this refers to large farms in countries of South America. One notable exception is Bacillus thuringiensis (Bt) cotton, which is grown by around 15 million smallholders in India, China, Pakistan, and a few other developing countries [10] . Bt cotton provides resistance to important insect pests, especially cotton bollworms. Several studies have shown that Bt cotton adoption reduces chemical pesticide use and increases yields in farmers’ fields [17] – [20] . There are also a few studies that have shown that these benefits are associated with increases in farm household income and living standard [21] – [23] . Higher incomes are generally expected to cause increases in food consumption in poor farm households. On the other hand, cotton is a non-food cash crop, so that the nutrition impact is uncertain.
Here we address this question and analyze the impact of Bt cotton adoption on calorie consumption and dietary quality in India. Bt cotton was first commercialized in India in 2002. In 2012, over 7 million farmers had adopted this technology on 10.8 million ha – equivalent to 93% of the country’s total cotton area [10] . For the analysis, we carried out a household survey and collected comprehensive data over a period of several years. This is the first ex post study that analyzes food security effects of Bt cotton or any other GM crop with micro level data.
Materials and Methods
Ethics statement.
Our study builds on data from a socioeconomic survey of farm households in India. Details of this survey are explained further below. The institutional review board of the University of Goettingen only reviews clinical research; our study cannot be classified as clinical research. We consulted with the Head of the Research Department of the University of Goettingen, who confirmed that there is no institutional review board at our University that would require a review of such survey-based socioeconomic research.
Farm Household Survey
We carried out a panel survey of Indian cotton farm households in four rounds between 2002 and 2008. We used a multistage sampling procedure. Four states were purposively selected, namely Maharashtra, Karnataka, Andhra Pradesh, and Tamil Nadu. These four states cover a wide variety of different cotton-growing situations, and they produce 60% of all cotton in central and southern India [23] . In these four states, we randomly selected 10 cotton-growing districts and 58 villages, using a combination of census data and agricultural production statistics [18] , [19] , [23] . Within each village, we randomly selected farm households from complete lists of cotton producers. Sample households were visited individually, and the household head was taken through a face-to-face interview, for which we used a structured questionnaire. The questionnaire covered a wide array of agricultural and socioeconomic information, such as input-output details in cotton production, technology adoption, other income sources, and household living standards. The interviews were carried out in local languages by a small team of enumerators, who were trained and supervised by the researchers.
Prior to starting each interview, the study objective was explained. We also clarified that the data collected would be treated confidentially, analyzed anonymously, and be used for research purposes only. Based on this, the interviewees were asked for their verbal informed consent to participate. We decided not ask for written consent, because the interviews were not associated with any risk for participants. Furthermore, many of the sample farmers had relatively low educational backgrounds and were not used to formal paperwork. Very few households did not agree to participate; they were replaced with other randomly selected households in the same villages.
The first-round survey interviews took place in early 2003, shortly after the cotton harvest for the 2002 season was completed. The same survey was repeated at two-year intervals in early 2005 (referring to the 2004 cotton season), early 2007 (referring to the 2006 season), and early 2009 (referring to the 2008 season). In total, 533 households were interviewed during the 7-year period. Most of these households were visited in several rounds. The total sample consists of 1431 household observations ( Table 1 ). In 2002, the proportion of Bt adopters was still relatively small, but it increased rapidly in the following years. By 2008, 99% of the sample households had adopted this technology. To our knowledge, this is the only longer-term panel survey of Bt cotton farm households in a developing country (the data set with the variables used in this article is available as Data S1 ).
| Farm households | 2002 | 2004 | 2006 | 2008 | Total |
| Adopters of Bt | 131 | 246 | 333 | 375 | 1085 |
| Non-adopters of Bt | 210 | 117 | 14 | 5 | 346 |
| Total | 341 | 363 | 347 | 380 | 1431 |
Calorie Consumption Data
The survey questionnaire included a detailed food consumption recall, which is a common tool to assess food security at the household level [24] . For a 30-day recall period, households were asked about the quantity consumed of different food items and the corresponding monetary value. The questions covered food consumed from own production, market purchases, gifts, and transfers.
The quantity data for the different food items were converted to calories consumed by using calorie conversion factors for India [25] , [26] . The total household calorie consumption from the 30-day recall was then divided by 30 to obtain a calorie value per day. Taking into account the age and gender structure of households, as well as physical activity levels of household members, the number of adult equivalents (AE) was calculated for each household. Male adults involved in farming count as 1.0 AE, female adults involved in farming as 0.8 AE. Male and female adults with lower physical activity levels count as 0.8 and 0.7, respectively. For children and adolescents, appropriate adjustments were made [25] – [27] . The daily household calorie consumption was divided by the number of AE in a household to obtain the calories consumed per AE and day.
Values for minimum dietary energy requirements found in the literature vary, which is due to several reasons [24] . Values stated per capita are lower than those stated per AE, because children have lower calorie requirements than adults. Moreover, not all studies take physical activity levels into account already in the AE calculations, as we do. The average daily calorie requirement for a moderately active AE in India is 2875 kcal/day [25] . According to the World Health Organization, a safe minimum daily intake should not fall below 80% of the calorie requirement, meaning 2300 kcal per AE. Minimum values around 2300 kcal per day for adult men are also found in other studies [28] . Based on this, we take 2300 kcal per AE as the threshold, that is, households with daily calorie consumption below 2300 kcal per AE are considered food insecure.
Most of the calories consumed in rural India are from cereals such as wheat, rice, millet, and sorghum that are rich in carbohydrates but less nutritious in terms of protein and micronutrient contents. Hence, in addition to total calories consumed we calculated the number of calories consumed from more nutritious foods to assess dietary quality. In the category “more nutritious foods”, we include pulses, fruits, vegetables, and all animal products (i.e., milk, milk products, meat, fish, and eggs). Recent research suggests that the share of calories consumed from higher value, non-staple foods can also be used as an indicator of nutritional sufficiency [29] . The reason is that poor and undernourished households will largely choose foods that are the cheapest available sources of calories, namely cereals in the context of rural India. Only when they have surpassed subsistence, consumers will begin to substitute towards foods that are more expensive sources of calories [29] .
It should be mentioned that food consumption data from household surveys may not provide very accurate data to measure nutritional status [24] , [30] . Sometimes, consumption data overestimate calorie intakes, because food losses, waste, and other uses within the household cannot be properly accounted for. However, this limitation applies to both adopters and non-adopters of Bt, so that the comparison between Bt and non-Bt, which is relevant for the impact assessment, is unaffected.
Regression Models
To estimate the impact of Bt cotton adoption on calorie consumption, we regress total daily calorie consumption per AE on Bt adoption, measured as the number of hectares of Bt cotton grown by a household in a particular year. Since Bt adoption increases farm profits and household incomes [23] , we expect a positive and significant treatment effect. However, calorie consumption is also influenced by other factors that need to be controlled for. We control for education of the household head (measured in terms of the number of years of schooling); education plays an important role for both income generation and consumption behavior. We also include a variable for household size (measured in terms of AE). Moreover, we control for farm size in terms of area owned, which is a proxy for agricultural asset ownership more generally. Farm income is not included in the model, as this is directly influenced by Bt adoption. However, off-farm income, measured in US$ per year, is controlled for. We also include state dummies for Karnataka, Andhra Pradesh, and Tamil Nadu (Maharashtra is the reference state), capturing climatic and agroecological differences. Given the panel structure of the data with four survey rounds, we use year dummies for 2004, 2006, and 2008 (2002 is the reference year).
Panel data models are often estimated with a random effects estimator [31] . However, a random effects estimator can lead to biased impact estimates when there is unobserved heterogeneity between Bt adopting and non-adopting households. Such bias resulting from endogeneity of the treatment variable is referred to as selection bias in the impact assessment literature [23] , [31] . Unobserved heterogeneity may potentially result from differences in household characteristics (e.g., Bt adopting farmers may have higher motivation, better management skills, or better access to information) or farm characteristics (e.g., differences in soil quality, or water access). Our panel data allow us to control for such unobserved heterogeneity. Since we surveyed the same households repeatedly over a 7-year period when Bt adoption increased, for many households we have observations with and without Bt adoption. Hence, we rely on a within household estimator, which is also called a fixed effects estimator. Differencing within households with the fixed effects estimator eliminates time-invariant unobserved factors, so that they can no longer bias the impact estimates [31] . A Hausman test is used to confirm the appropriateness of the fixed effects specification [19] , [31] .
We estimate an additional model using calories from more nutritious foods (i.e., pulses, fruits, vegetables, and animal products) instead of total calorie consumption as dependent variable. This additional model helps to analyze impacts of Bt cotton adoption on dietary quality. A positive coefficient for the treatment variable would indicate that Bt adoption increases the consumption of more nutritious foods, thus not only contributing to more calories but also to better dietary quality.
Results and Discussion
Descriptive statistics are shown in Table 2 . The average farm household owns 5 ha of land, without a significant difference between Bt adopters and non-adopters. Around half of this area is grown with cotton. Other crops cultivated include wheat, millet, sorghum, pulses, and in some locations rice, among others. Households are relatively poor; average annual per capita consumption expenditures range between 300 and 500 US$.
| Variables | Adopters of Bt (N = 1085) | Non-adopters of Bt (N = 346) |
| Farm size (ha) | 5.11 (5.85) | 4.85 (5.51) |
| Cotton area cultivated (ha) | 2.35 (2.35) | 2.79 (19.67) |
| Area cultivated with Bt cotton (ha) | 1.97 (2.08) | 0.00 (0.00) |
| Age of farmer (years) | 45.58 (12.86) | 45.94 (12.36) |
| Education of farmer (years) | 7.58 (4.94) | 6.69 (5.03) |
| Per capita consumption expenditure (US$/year) | 490.31 (430.18) | 311.72 (355.58) |
| Off-farm income (US$/year) | 560.70 (1455.44) | 504.27 (2289.87) |
| Calorie consumption per AE (kcal/day) | 3329.41 (719.38) | 2829.88 (598.99) |
| Calories consumed from more nutritious foods per AE (kcal/day) | 703.89 (374.90) | 638.89 (345.41) |
| Household size (AE) | 5.01 (2.42) | 5.14 (2.24) |
| Food insecure households (%) | 7.93 | 19.94 |
Mean values are shown with standard deviations in parentheses. N: Number of observations; AE: adult equivalent.
Bt adopting households consume significantly more calories than non-adopting households, and a smaller proportion of them is food insecure ( Figure 1 , Table 2 ). This suggests that the cash income gains through Bt adoption may have improved food security among cotton-producing households. Yet, this simple comparison does not yet prove a causal relationship.

Functions were estimated non-parametrically using the Epanechnikov kernel with 1085 and 346 observations for adopting and non-adopting households, respectively. AE: adult equivalent.
Impact of Bt Cotton Adoption on Food Security
To further analyze the relationship between Bt adoption and calorie consumption, we use panel regression models, as explained above. The main explanatory variable of interest is the Bt cotton area of a farm household, for which descriptive statistics are shown in Table 3 . The average Bt area among technology adopters in the sample is close to 2 ha, which is equivalent to 85% of the total cotton area of these farms. A breakdown by survey year shows that the average Bt area increased from less than 1.0 ha in 2002 to 2.4 ha in 2008. Hence, not only the number of Bt adopters but also the Bt area per adopting household increased considerably over time.
| 2002 | 2004 | 2006 | 2008 | Total | |
| Mean Bt area (ha) | 0.94 | 1.64 | 2.15 | 2.37 | 1.97 |
| Standard deviation | 1.32 | 1.87 | 2.14 | 2.22 | 2.08 |
| Number of observations | 131 | 246 | 333 | 375 | 1085 |
The regression results are shown in Table 4 . Each ha of Bt cotton has increased total calorie consumption by 74 kcal per AE and day. For the average adopting household, the net effect is 145 kcal per AE ( Figure 2 ), implying a 5% increase over mean calorie consumption in non-adopting households. Most of the calories consumed in rural India stem from cereals that are rich in carbohydrates but less nutritious in terms of protein and micronutrients. Yet the results show that Bt adoption has significantly increased the consumption of calories from more nutritious foods, thus also contributing to improved dietary quality.

Results based on calorie consumption regression models estimated with panel data and household fixed effects (within estimator). Full model results are shown in Table 4 . Calories from more nutritious foods include pulses, fruits, vegetables, and animal products. Effects for the average adopting household take into account the number of ha of Bt cotton actually grown. **Significant at the 5% level. ***Significant at the 1% level.
| Model (1) | Model (2) | Model (3) | |
| Variables | Total calories (RE model) | Total calories (FE model) | Calories from more nutritious foods (FE model) |
| Bt area (ha) | 79.08*** (18.85) | 73.71*** (21.40) | 23.17** (10.05) |
| Farm size (ha) | 9.27** (4.22) | −0.69 (7.80) | 1.97 (3.56) |
| Education of farmer (years) | 9.41** (4.40) | – | – |
| Off-farm income (US$/year) | 0.07*** (0.02) | 0.05*** (0.02) | 0.01 (0.007) |
| Household size (AE) | −62.48*** (10.71) | −89.46*** (14.43) | −29.33*** (6.89) |
| Karnataka (dummy) | 88.36 (57.97) | – | – |
| Andhra Pradesh (dummy) | 21.46 (58.00) | – | – |
| Tamil Nadu (dummy) | 212.86** (84.56) | – | – |
| 2004 (dummy) | −34.35 (48.97) | −5.98 (51.60) | −45.25 (25.33) |
| 2006 (dummy) | 13.68 (54.48) | 30.09 (61.12) | −112.87*** (29.41) |
| 2008 (dummy) | −92.92 (60.51) | −74.59 (69.51) | −72.70** (30.20) |
| Constant | 3229.31*** (90.46) | 3537.08*** (78.16) | 843.23*** (41.42) |
| Number of observations | 1431 | 1431 | 1431 |
| R | 0.13 | 0.09 | 0.10 |
| Hausman test (chi-square statistic) | 16.82** | ||
The dependent variable in models (1) and (2) is the total number of kcal consumed per AE and day. The dependent variable in model (3) is the number of kcal consumed from more nutritious foods (i.e., pulses, fruits, vegetables, and animal products) per AE and day. All coefficient estimates can be interpreted as marginal effects; robust standard errors are shown in parentheses. AE: adult equivalent; RE: random effects; FE: fixed effects.
We applied the total calorie consumption effect of Bt to the subsample of non-adopters to simulate the food security impact of adoption: if all non-adopters switched to Bt, the proportion of food insecure households would drop by 15–20% ( Table 5 ). Most of these nutritional benefits have materialized already, as over 90% of all cotton farm households in India have adopted Bt technology by now.
| Food insecure households (%) | Change in food insecurity relative to status quo (%) | |
| Non-adopters of Bt cotton (status quo) | 19.94 | |
| If non-adopters adopted Bt on their total cotton area | 15.90 | −20.26 |
| If non-adopters adopted Bt on 85% of their cotton area | 16.76 | −15.95 |
The proportion of food insecure households in the status quo refers to the subsample of 346 non-adopters. For these households, changes in calorie consumption through Bt adoption were simulated, assuming full Bt adoption (on 100% of their cotton area) and partial Bt adoption (on 85% of their cotton area, as observed in the subsample of Bt adopters). For the simulations, the net effect of Bt on total calorie consumption per ha was used ( Figure 2 ).
Robustness Checks
We tested the robustness of the Bt effects by estimating calorie consumption models with alternative specifications. These additional estimates are shown in Table 6 . We first look at possible changes in impact over time. In model (1), the Bt area variable is split into two periods, namely 2002–04 and 2006–08. In both periods, the Bt impact on calorie consumption was positive and significant, but the effect was bigger in 2002–04 than in 2006–08. The reason for this change is not that income effects of Bt adoption would shrink; recent research showed that the profit gains of Bt cotton in India were constant or even increased over time [23] . The change in the calorie effect per ha of Bt is rather due to the fact that the Bt area per farm increased considerably in the later period, as was shown above. Measured per farm household, the calorie consumption effect of Bt was actually very similar in 2002–04 and 2006–08.
| Model (1) | Model (2) | Model (3) | Model (4) | |
| Variables | Total calories | Calories from more nutritious foods | Total calories | Total calories |
| Bt area 2002–04 (ha) | 135.25*** (28.95) | 17.94 (13.24) | – | – |
| Bt area 2006–08 (ha) | 54.67** (23.33) | 24.79** (10.46) | – | – |
| Cumulative Bt area (ha) | – | – | 17.20 (12.20) | −28.08** (13.21) |
| Bt area (ha) | – | – | – | 105.63*** (26.82) |
| Number of observations | 1431 | 1431 | 1431 | 1431 |
| Bt area (ha) | 73.71*** (21.40) | 76.19*** (27.62) | 110.01*** (27.48) | 53.40 (30.99) |
| Bt (dummy) | – | – | – | 599.84*** (70.29) |
| Number of observations | 1431 | 1016 | 852 | 852 |
All models are estimated with household fixed effects. Other explanatory variables were included in estimation, as in Table 4 , but are not shown here for brevity. The dependent variable in all models is calorie consumption measured in kcal per AE and day. Coefficient estimates can be interpreted as marginal effects; robust standard errors are shown in parentheses.
The smaller calorie consumption effect per ha of Bt with an increasing Bt area on a farm is consistent with Engel’s law, which states that the proportion of the household budget spent on food decreases as income rises [32] . Unsurprisingly, the same trend is not observed when we focus on higher value, non-staple foods. The results of model (2) in Table 6 suggest that the Bt effect on calories from more nutritious foods has been increasing over time. Hence, Bt cotton adoption leads to a lower staple calorie share, implying higher nutritional sufficiency and better dietary quality [29] .
In model (3) of Table 6 , we analyze whether the Bt effect is cumulative, meaning that households that have adopted Bt earlier or on larger areas benefit over-proportionally. This might be the case when profit gains from Bt adoption are reinvested, possibly entailing larger consumption benefits in subsequent periods. To test for this option, we constructed a cumulative Bt area variable, adding up the Bt area on a farm in a particular year and Bt areas on the same farm in previous survey rounds. The coefficient of this variable is insignificant; cumulative effects do not seem to be important. If we include this variable together with the standard Bt area variable, the cumulative coefficient turns negative while the actual treatment effect increases (model 4). Again, this is consistent with Engel’s law, implying that larger areas with Bt lead to lower proportions of the income gains being spent on calories.
In models (6) and (7), we analyze to what extent changes in the sample affect the estimation results. For easy comparison, results from the full-sample reference model, which were discussed above, are repeated in model (5). It is sometimes observed that early adopters of a new technology benefit more than late adopters. This may be due to cumulative effects, which we already tested for. In addition, general equilibrium adjustments may contribute to differential impacts between early and late adopters [33] . In model (6), we exclude all households that had adopted Bt already in the first survey round in 2002. The change in the Bt effect is very small, so we conclude that late adopters enjoy the same nutritional benefits per ha of Bt as early adopters.
This specification in model (6) with early adopters excluded is also an additional robustness check for possible issues of endogeneity and selection bias. The fixed effects panel estimator controls for time-invariant heterogeneity between adopters and non-adopters of Bt. But it cannot control for possible time-variant differences, which might play a role if early adopters are more innovative also with respect to other opportunities not captured in our data. The similarity of the results in models (5) and (6) substantiates that the estimated Bt impacts do not suffer from selection bias. In model (7), we exclude all observations of households that had adopted Bt in all survey rounds, so that the results are purely based on within household comparisons. The treatment effect remains highly significant. It even increases in magnitude, suggesting that the full-sample result is rather a cautious, lower-bound estimate. Finally, model (8) includes a dummy for Bt adoption in addition to the Bt area variable used before. The dummy produces a large coefficient, underlining the positive food security impact of Bt adoption. But the Bt area effect remains positive and significant, too, which confirms that using a continuous treatment variable is appropriate.
Overall, the additional results with alternative specifications strengthen the findings and show that the positive impacts of Bt cotton adoption on food security in India are very robust.
Conclusions
The results of this research confirm that the income gains through Bt cotton adoption among smallholder farm households in India have positive impacts on food security and dietary quality. GM crops are not a panacea for the problems of hunger and malnutrition. Complex problems require multi-pronged solutions. But the evidence suggests that GM crops can be an important component in a broader food security strategy. So far, food security impacts are still confined to only a few concrete examples. The nutritional benefits could further increase with more GM crops and traits becoming available in the future. Appropriate policy and regulatory frameworks are required to ensure that the needs of poor farmers and consumers are taken into account and that undesirable social consequences are avoided.
Supporting Information
Acknowledgments.
We thank Vijesh Krishna and two anonymous reviewers of this journal for very useful comments.
Funding Statement
This research was supported by the German Research Foundation (DFG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.

IMAGES
COMMENTS
The global yearly net income increased by 34.3% in 2010-2012. 13,14 Furthermore, while increasing global yield by 22%, GM crops reduced pesticide (active ingredient) usage by 37% and environmental impact (insecticide and herbicide use) by 18%. 15 To achieve the same yield standards more than 300 million acres of conventional crops would have ...
Main conclusion While transgenic technology has heralded a new era in crop improvement, several concerns have precluded their widespread acceptance. Alternative technologies, such as cisgenesis and genome-editing may address many of such issues and facilitate the development of genetically engineered crop varieties with multiple favourable traits. Abstract Genetic engineering and plant ...
Genetically modified organisms (GMO) have been topics of hot debates over the last few decades. Some countries have been known to have a fierce regulatory framework over the genetically modified crops. The regulations of the European Union are the ones that have been subjects of continued criticism in this regard.
"GMO" (genetically modified organism) has become the common term consumers and popular media use to describe foods that have been created through genetic engineering. Genetic engineering is a ...
Most research effort has been devoted to assessing people's attitudes towards GM foods as a technology. ... Sahai S. Genetically modified crops: issues for India. Fin Agric. 2003; 35:7-11. [Google Scholar] Snell C, Bernheim A, Bergé J-B, Kuntz M, Pascal G, Paris A, Agnès ER. Assessment of the health impact of GM plant diets in long-term ...
Introduction. Despite the rapid adoption of genetically modified (GM) crops by farmers in many countries, public controversies about the risks and benefits continue -.Numerous independent science academies and regulatory bodies have reviewed the evidence about risks, concluding that commercialized GM crops are safe for human consumption and the environment -.
Almost 2 years ago, a group of 20 scientists began hashing out a consensus on the risks and benefits of genetically engineered (GE) crops. Since the launch of their study, sponsored by the National Academies of Science, Engineering, and Medicine, the public debate around the safety of genetically modified organisms (GMOs) and whether to label ...
In 1971, the first debate over the risks to humans of exposure to GMOs began when a common intestinal microorganism, E. coli, was infected with DNA from a tumor-inducing virus (Devos et al ., 2007 ...
(June 2023) - We estimate the impact of genetically modified (GM) crops on countrywide yields, harvested area, and trade using a triple-differences rollout design that exploits variation in the availability of GM seeds across crops, countries, and time. We find positive impacts on yields, especially in poor countries.
Associate economics professor Federico Ciliberto co-led the largest research study to date examining how genetically modified soybeans and maize have impacted pesticide use in the U.S. (Photo by Dan Addison, University Communications) Since 2008, genetically engineered crops have accounted for more than 80 percent of maize and soybean crops ...
Introduction. Genetically modified organisms (GMOs) result from recombinant DNA technology that allows for DNA to be transferred from one organism to another (transgenesis) without the genetic transfer limits of species to species barriers and with successful expression of transferred genes in the receiving organism (Gray, 2001).Four crops, maize, canola, soybean, and cotton, constitute the ...
Most of the genetically modified traits in these crops are input traits such as herbicide resistance or insect resistance (Stein and Rodríguez-Cerezo Citation 2010). But increasingly there is a move toward output traits that improve the quality and nutritive value of the plant.
Genetic modification in plants was first recorded 10,000 years ago in Southwest Asia where humans first bred plants through artificial selection and selective breeding. Since then, advancements in agriculture science and technology have brought about the current GM crop revolution. GM crops are promising to mitigate current and future problems ...
GM crops may be modified to improve yield, enhance nutrition, or better adapt to environmental conditions. They can even be altered to resist pests or eliminate unwanted effects, like this type of onion that doesn't cause people to tear up when chopped. Hunger is one of the greatest global challenges of the 21st century.
In this chapter, the committee examines the evidence that substantiates or negates specific hypotheses and claims about the health risks and benefits associated with foods derived from genetically engineered (GE) crops. There are many reviews and official statements about the safety of foods from GE crops (for example, see Box 5-1), but to conduct a fresh examination of the evidence, the ...
1 ICAR-Indian Institute of Maize Research, Pusa Campus, New Delhi, 110012, ... in crop plants. Genetically modified crops possess one or more useful traits, such as, herbicide tolerance, insect resistance, abiotic stress tolerance, disease resistance, and nutritional improvement. To date, nearly 525 different transgenic events in 32 crops have ...
Genetically modified crops (GM crops) are plants used in agriculture, the DNA of which has been modified using genetic engineering methods. Plant genomes can be engineered by physical methods or by use of Agrobacterium for the delivery of sequences hosted in T-DNA binary vectors.In most cases, the aim is to introduce a new trait to the plant which does not occur naturally in the species.
1. Introduction. In July 2011, a group of protesters from Greenpeace, a non-governmental, environmental organization, broke into an experimental farm of the Commonwealth Scientific and Industrial Research Organization (CSIRO), an Australian federal government agency for scientific research, and destroyed the entire crop of genetically modified wheat.
Nearly all the corn and soybeans grown in the U.S. are genetically modified, but only two GM crops, Monsanto's MON810 maize and BASF's Amflora potato, are accepted in the European Union. Ten E.U ...
Genetically modified organisms (GMOs) can be defined as organisms (i.e. plants, animals or microorganisms) in which the genetic material (DNA) has been altered in a way that does not occur naturally by mating and/or natural recombination. ... GM crops that inherently produce this toxin have been shown to require lower quantities of insecticides ...
The most widely accepted genetically modified traits in GM crops are herbicide tolerance and insect resistance. GM soybean, maize, canola, and cotton are the most common examples of these crops in the market (James 2014).Developing countries like India and China are the largest producers of genetically modified Bt cotton (Markoulatos et al. 2004; Trapero et al. 2016).
Percentage of genetically modified crops in the U.S. in 1997, 2018, 2019, and 2020, by type (as percent of total acreage) Premium Statistic U.S. acreage of genetically modified corn 2014-2019
The 2000 National Research Council report Genetically Modified Pest-Protected Plants found that "there appears to be no strict dichotomy between the risks to health and the environment ... Some comments made to the committee pointed to the publications of that research group as evidence that GE crops and foods derived from GE crops were ...
Genetically modified food controversies are disputes over the use of foods and other goods derived from genetically modified crops instead of conventional crops, and other uses of genetic engineering in food production. The disputes involve consumers, farmers, biotechnology companies, governmental regulators, non-governmental organizations, and scientists.
The role of genetically modified (GM) crops for food security is the subject of public controversy. GM crops could contribute to food production increases and higher food availability. There may also be impacts on food quality and nutrient composition. ... European Commission (2010) A Decade of EU-Funded GMO Research 2001-2010 (European ...