IEEE Account
- Change Username/Password
- Update Address

Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Artificial Intelligence in Health Care: Current Applications and Issues
Affiliations.
- 1 Department of Orthopedic Surgery, Samsung Medical Center, School of Medicine, Sungkyunkwan University, Seoul, Korea.
- 2 Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, School of Medicine, Sungkyunkwan University, Seoul, Korea.
- 3 Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4 Department of Radiology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 5 Department of Radiology, Seoul National University College of Medicine, Seoul, Korea.
- 6 Division of Gastroenterology, Department of Medicine, Samsung Medical Center, School of Medicine, Sungkyunkwan University, Seoul, Korea.
- 7 Department of R&D Planning, Korea Health Industry Development Institute (KHIDI), Cheongju, Korea.
- 8 Health Innovation Big Data Center, Asan Institute for Life Science, Asan Medical Center, Seoul, Korea.
- 9 Department of Radiology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 10 Department of Bio and Brain Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea.
- 11 Protocol Engineering Center, Electronics and Telecommunications Research Institute (ETRI), Daejeon, Korea.
- 12 Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 13 Division of Geriatrics, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 14 VUNO Inc., Seoul, Korea.
- 15 Department of Convergence Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 16 Lunit Inc., Seoul, Korea.
- 17 Big Data Research Center, Samsung Medical Center, School of Medicine, Sungkyunkwan University, Seoul, Korea.
- 18 Digital Healthcare Partners, Seoul, Korea.
- 19 Center for Bionics, Korea Institute of Science and Technology (KIST), Seoul, Korea.
- 20 Department of Biomedical Engineering, Seoul National University College of Medicine, Seoul, Korea. [email protected].
- PMID: 33140591
- PMCID: PMC7606883
- DOI: 10.3346/jkms.2020.35.e379
- Erratum: Correction of Author Name and Affiliation in the Article "Artificial Intelligence in Health Care: Current Applications and Issues". Park CW, Seo SW, Kang N, Ko B, Choi BW, Park CM, Chang DK, Kim H, Kim H, Lee H, Jang J, Ye JC, Jeon JH, Seo JB, Kim KJ, Jung KH, Kim N, Paek S, Shin SY, Yoo S, Choi YS, Kim Y, Yoon HJ. Park CW, et al. J Korean Med Sci. 2020 Dec 14;35(48):e425. doi: 10.3346/jkms.2020.35.e425. J Korean Med Sci. 2020. PMID: 33316861 Free PMC article. No abstract available.
In recent years, artificial intelligence (AI) technologies have greatly advanced and become a reality in many areas of our daily lives. In the health care field, numerous efforts are being made to implement the AI technology for practical medical treatments. With the rapid developments in machine learning algorithms and improvements in hardware performances, the AI technology is expected to play an important role in effectively analyzing and utilizing extensive amounts of health and medical data. However, the AI technology has various unique characteristics that are different from the existing health care technologies. Subsequently, there are a number of areas that need to be supplemented within the current health care system for the AI to be utilized more effectively and frequently in health care. In addition, the number of medical practitioners and public that accept AI in the health care is still low; moreover, there are various concerns regarding the safety and reliability of AI technology implementations. Therefore, this paper aims to introduce the current research and application status of AI technology in health care and discuss the issues that need to be resolved.
Keywords: Application; Artificial Intelligence; Health Care; Issue; Machine Learning.
© 2020 The Korean Academy of Medical Sciences.
PubMed Disclaimer
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Fig. 1. Research and development strategic plan…
Fig. 1. Research and development strategic plan of artificial intelligence.
AI = artificial intelligence.
Similar articles
- ARTIFICIAL INTELLIGENCE IN MEDICAL PRACTICE: REGULATIVE ISSUES AND PERSPECTIVES. Pashkov VM, Harkusha AO, Harkusha YO. Pashkov VM, et al. Wiad Lek. 2020;73(12 cz 2):2722-2727. Wiad Lek. 2020. PMID: 33611272 Review.
- Fulfilling the Promise of Artificial Intelligence in the Health Sector: Let's Get Real. Hashiguchi TCO, Oderkirk J, Slawomirski L. Hashiguchi TCO, et al. Value Health. 2022 Mar;25(3):368-373. doi: 10.1016/j.jval.2021.11.1369. Epub 2022 Jan 14. Value Health. 2022. PMID: 35227447
- A governance model for the application of AI in health care. Reddy S, Allan S, Coghlan S, Cooper P. Reddy S, et al. J Am Med Inform Assoc. 2020 Mar 1;27(3):491-497. doi: 10.1093/jamia/ocz192. J Am Med Inform Assoc. 2020. PMID: 31682262 Free PMC article.
- Application Scenarios for Artificial Intelligence in Nursing Care: Rapid Review. Seibert K, Domhoff D, Bruch D, Schulte-Althoff M, Fürstenau D, Biessmann F, Wolf-Ostermann K. Seibert K, et al. J Med Internet Res. 2021 Nov 29;23(11):e26522. doi: 10.2196/26522. J Med Internet Res. 2021. PMID: 34847057 Free PMC article. Review.
- Open Data and transparency in artificial intelligence and machine learning: A new era of research. Rodgers CM, Ellingson SR, Chatterjee P. Rodgers CM, et al. F1000Res. 2023 Apr 12;12:387. doi: 10.12688/f1000research.133019.1. eCollection 2023. F1000Res. 2023. PMID: 37065505 Free PMC article.
- Suitability of the Current Health Technology Assessment of Innovative Artificial Intelligence-Based Medical Devices: Scoping Literature Review. Farah L, Borget I, Martelli N, Vallee A. Farah L, et al. J Med Internet Res. 2024 May 13;26:e51514. doi: 10.2196/51514. J Med Internet Res. 2024. PMID: 38739911 Free PMC article. Review.
- The Structural and Molecular Mechanisms of Mycobacterium tuberculosis Translational Elongation Factor Proteins. Fang N, Wu L, Duan S, Li J. Fang N, et al. Molecules. 2024 Apr 29;29(9):2058. doi: 10.3390/molecules29092058. Molecules. 2024. PMID: 38731549 Free PMC article. Review.
- Exploring the matrix: knowledge, perceptions and prospects of artificial intelligence and machine learning in Nigerian healthcare. Adigwe OP, Onavbavba G, Sanyaolu SE. Adigwe OP, et al. Front Artif Intell. 2024 Jan 19;6:1293297. doi: 10.3389/frai.2023.1293297. eCollection 2023. Front Artif Intell. 2024. PMID: 38314120 Free PMC article.
- Novel Artificial Intelligence-Based Technology to Diagnose Asthma Using Methacholine Challenge Tests. Kang N, Lee K, Byun S, Lee JY, Choi DC, Lee BJ. Kang N, et al. Allergy Asthma Immunol Res. 2024 Jan;16(1):42-54. doi: 10.4168/aair.2024.16.1.42. Allergy Asthma Immunol Res. 2024. PMID: 38262390 Free PMC article.
- [Application and prospect of machine learning in orthopaedic trauma]. Tian C, Chen X, Zhu H, Qin S, Shi L, Rui Y. Tian C, et al. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2023 Dec 15;37(12):1562-1568. doi: 10.7507/1002-1892.202308064. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2023. PMID: 38130202 Free PMC article. Chinese.
- Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380(14):1347–1358. - PubMed
- Shimizu H, Nakayama KI. Artificial intelligence in oncology. Cancer Sci. 2020;111(5):1452–1460. - PMC - PubMed
- Obermeyer Z, Emanuel EJ. Predicting the future - big data, machine learning, and clinical medicine. N Engl J Med. 2016;375(13):1216–1219. - PMC - PubMed
- Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230–243. - PMC - PubMed
- Cruz JA, Wishart DS. Applications of machine learning in cancer prediction and prognosis. Cancer Inform. 2007;2:59–77. - PMC - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- Korean Academy of Medical Sciences
- PubMed Central
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
AI in Medicine— JAMA ’s Focus on Clinical Outcomes, Patient-Centered Care, Quality, and Equity
- 1 Associate Editor, JAMA ; and Yale School of Medicine, New Haven, Connecticut
- 2 Editorial Board member, JAMA ; and University of California, San Francisco
- 3 Electronic Editor, JAMA and JAMA Network
- 4 Associate Editor, JAMA ; and University of California, San Francisco
- 5 Executive Managing Editor, JAMA and JAMA Network
- 6 Managing Director of Strategy and Planning, JAMA and JAMA Network
- 7 Executive Editor, JAMA and JAMA Network
- 8 Editor in Chief, JAMA and JAMA Network
- Editorial Guidance on Reporting Use of AI in Research and Scholarly Publication Annette Flanagin, RN, MA; Romain Pirracchio, MD, MPH, PhD; Rohan Khera, MD, MS; Michael Berkwits, MD, MSCE; Yulin Hswen, ScD, MPH; Kirsten Bibbins-Domingo, PhD, MD, MAS JAMA
- Special Communication Creation and Adoption of Large Language Models in Medicine Nigam H. Shah, MBBS, PhD; David Entwistle, BS, MHSA; Michael A. Pfeffer, MD JAMA
- Medical News & Perspectives Can AI Solve Clinician Burnout? Yulin Hswen, ScD, MPH; Rebecca Voelker, MSJ JAMA
- Medical News & Perspectives Building Health Care Equity Into AI Tools Yulin Hswen, ScD, MPH; Rebecca Voelker, MSJ JAMA
- Medical News & Perspectives How Kaiser Permanente Is Testing AI in the Clinic Rebecca Voelker, MSJ; Yulin Hswen, ScD, MPH JAMA
- Medical News & Perspectives Can Predictive AI Improve Early Sepsis Detection? Rebecca Voelker, MSJ; Yulin Hswen, ScD, MPH JAMA
- Medical News & Perspectives Feeding AI the Right Diet for Health Care Success Rebecca Voelker, MSJ; Yulin Hswen, ScD, MPH JAMA
The transformative role of artificial intelligence (AI) in health care has been forecast for decades, 1 but only recently have technological advances appeared to capture some of the complexity of health and disease and how health care is delivered. 2 Recent emergence of large language models (LLMs) in highly visible and interactive applications 3 has ignited interest in how new AI technologies can improve medicine and health for patients, the public, clinicians, health systems, and more. The rapidity of these developments, their potential impact on health care, and JAMA ’s mission to publish the best science that advances medicine and public health compel the journal to renew its commitment to facilitating the rigorous scientific development, evaluation, and implementation of AI in health care.
JAMA editors are committed to promoting discoveries in AI science, rigorously evaluating new advances for their impact on the health of patients and populations, assessing the value such advances bring to health systems and society nationally and globally, and examining progress toward equity, fairness, and the reduction of historical medical bias. Moreover, JAMA ’s mission is to ensure that these scientific advances are clearly communicated in a manner that enhances the collective understanding of the domain for all stakeholders in medicine and public health. 4 For scientific development of AI to be most effective for improving medicine and public health requires a platform that recognizes and supports the vision of rapid cycle innovation and is also fundamentally grounded in the principles of reliable and reproducible clinical research that is ethically sound, respectful of rights to privacy, and representative of diverse populations. 2 , 3 , 5
The scientific development in AI can be viewed through the framework used to describe other health-related sciences. 6 In these domains, scientific discoveries begin with identifying biological mechanisms of disease. Then inventions that target these mechanisms are tested in progressively larger groups of people with and without diseases to assess the effectiveness and safety of these interventions. These are then scaled to large studies evaluating outcomes for individuals and populations with the disease. This well-established scientific development framework can work for research in AI as well, with reportable stages as inventions and findings move from one stage to the next.
The editors seek original science that focuses on developing, testing, and deploying AI in studies that improve understanding of its effects on the health outcomes of patients and populations. The starting point is original research rigorously examining the challenges and potential solutions to optimizing clinical care with AI. In addition, to ensure our readers remain abreast of major scientific development across the entire continuum of scientific innovation, we invite reviews, special communications, and opinion articles that summarize the potential health care applications of emerging technology written for our journal’s broad readership.
While highlighting new developments, JAMA will focus on these essential areas ( Figure ):
Clinical care and outcomes: JAMA ’s key interest is in clinically impactful science, and we will be most interested in studies demonstrating the effective translation of novel AI technologies to improve clinical care and outcomes. The potential for clinical impact will represent an important yardstick in our evaluation of all AI studies.
Patient-centered care: Early phases of scientific development have focused on directly measurable outcomes, reflecting the broader availability of data on these outcomes. However, how algorithmic care may shape the care experience of individuals and outcomes of interest to patients remains an understudied domain. 7 Implementing novel technology to enhance patient care and experience can only achieve its intended effect when patients believe that it offers them an advance—either through more time with their clinicians, more accessible information on their care decisions, or personalized interventions that target the outcomes of interest to them. We encourage studies that consider domains of autonomy, mobility, comfort, education, or other aspects of health not measured in traditional outcome assessments.
Health care quality: Advances in modern medicine are often stymied by the inability to translate evidence-based care to all patients. As clinicians increasingly provide care for more complex patient conditions in an ever-expanding therapeutic landscape, AI can play a crucial role in alleviating current challenges in optimizing clinical care, 8 if stewarded appropriately when positioned in the medical enterprise. 9 We are interested in studies that assess the potential for AI technologies to improve access to high-quality health care for all patients.
Fairness in AI algorithms: We encourage the explicit assessment of the fairness of algorithms and their potential effect on health inequities. Through development on biased data sources or restricted deployment in privileged health care settings, algorithms can potentially exacerbate health outcome gaps across socioeconomic and sociocultural axes. 9 We are interested in studies that assess the fairness of algorithms, their potential impact on health disparities, and strategies to mitigate biases.
Medical education and clinician experience: In addition to patient-facing science, we seek investigations into the role of AI in addressing the challenges clinicians face in medical training and in the practice of medicine. The information overload through digital health technologies has posed an increasing burden on clinicians, with unintended consequences for their health and well-being. This remains a central area to target for AI in health. The investigations in this domain will evaluate the use of AI to enable a health care team and its members to function to the highest and best use of their expertise.
Global solutions: To advance health care beyond well-resourced countries, critical technologies would need to adapt to the infrastructural, technological, and health care milieu across the globe. We invite investigations to submit science that demonstrates and evaluates AI applications that enhance care within the limitations of low-resource settings. AI-driven method development that enables low-cost tools to be even more effective at diagnosis and treatment, and those that guide the fair and appropriate allocation of limited resources, may move the needle on bridging the health disparities across societies across the globe.
JAMA is one of the most widely circulated general medicine journals in the world and the flagship journal of the JAMA Network, which includes 11 specialty journals and JAMA Network Open . Submissions are welcome to all the JAMA Network journals. The Network also offers the advantage of coordinated publications, as well as amplification of findings to specific audiences of interest. With a mission to reach clinicians, scientists, patients, policymakers, and the general public globally, the value of JAMA and the JAMA Network for authors and readers interested in AI in medicine is clear.
We seek to engage scientists and other thought leaders advancing AI and medicine across clinical, computational, health policy, and public health domains. We invite authors to communicate directly with the editors about topics they believe can impact health care delivery and to connect with the editors to discuss further the development of your science and our approach to its evaluation; such engagement is critical in this rapidly evolving field. We are committed to including diverse opinions and voices in the journal and urge experts from across the career spectrum and the globe to participate in the discourse. The editors are committed to communicating science effectively to a broad range of stakeholders across our digital, multimedia, and social media avenues. As AI promises to enable major health care transformation, JAMA and the JAMA Network are positioned to serve as a platform for the publication of this transformative work.
Corresponding Author: Kirsten Bibbins-Domingo, PhD, MD, MAS, JAMA ( [email protected] ).
Published Online: August 11, 2023. doi:10.1001/jama.2023.15481
Conflict of Interest Disclosures: Dr Khera reported receiving grants from NHLBI, Doris Duke Charitable Foundation, Bristol Myers Squibb, and Novo Nordisk, and serving as cofounder of Evidence2Health, outside the submitted work. Dr Butte reported being a cofounder and consultant to Personalis and NuMedii; consultant to Mango Tree Corporation, Samsung, 10x Genomics, Helix, Pathway Genomics, and Verinata (Illumina); has served on paid advisory panels or boards for Geisinger Health, Regenstrief Institute, Gerson Lehman Group, AlphaSights, Covance, Novartis, Genentech, Merck, and Roche; is a shareholder in Personalis and NuMedii; is a minor shareholder in Apple, Meta (Facebook), Alphabet (Google), Microsoft, Amazon, Snap, 10x Genomics, Illumina, Regeneron, Sanofi, Pfizer, Royalty Pharma, Moderna, Sutro, Doximity, BioNtech, Invitae, Pacific Biosciences, Editas Medicine, Nuna Health, Assay Depot, and Vet24seven, and several other nonhealth-related companies and mutual funds; and has received honoraria and travel reimbursement for invited talks from Johnson & Johnson, Roche, Genentech, Pfizer, Merck, Lilly, Takeda, Varian, Mars, Siemens, Optum, Abbott, Celgene, AstraZeneca, AbbVie, Westat, and many academic institutions, medical, or disease-specific foundations and associations, and health systems; receives royalty payments through Stanford University for several patents and other disclosures licensed to NuMedii and Personalis; and has had research funded by NIH, Peraton, Genentech, Johnson & Johnson, FDA, Robert Wood Johnson Foundation, Leon Lowenstein Foundation, Intervalien Foundation, Chan Zuckerberg Initiative, the Barbara and Gerson Bakar Foundation, and in the recent past, the March of Dimes, Juvenile Diabetes Research Foundation, California Governor’s Office of Planning and Research, California Institute for Regenerative Medicine, L’Oreal, and Progenity. No other disclosures were reported.
See More About
Khera R , Butte AJ , Berkwits M, et al. AI in Medicine— JAMA ’s Focus on Clinical Outcomes, Patient-Centered Care, Quality, and Equity. JAMA. 2023;330(9):818–820. doi:10.1001/jama.2023.15481
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Integrating artificial intelligence into biomedical science curricula: advancing healthcare education.

1. Introduction
2. methods and search strategy, 3. ai in healthcare, 4. impact of ai on laboratory settings during covid-19, 5. ai in biomedical sciences, 6. how to reform biomedical education, 7. challenges in incorporating ai into biomedical science curricula, 8. ethics and ai, 9. discussion, 10. limitations, 11. conclusions, author contributions, conflicts of interest.
- Booth, R.G.; Strudwick, G.; McBride, S.; O’Connor, S.; Solano López, A.L. How the Nursing Profession Should Adapt for a Digital Future. BMJ 2021 , 373 , n1190. [ Google Scholar ] [ CrossRef ]
- Barbour, A.B.; Frush, J.M.; Gatta, L.A.; McManigle, W.C.; Keah, N.M.; Bejarano-Pineda, L.; Guerrero, E.M. Artificial Intelligence in Health Care: Insights from an Educational Forum. J. Med. Educ. Curric. Dev. 2019 , 6 , 2382120519889348. [ Google Scholar ] [ CrossRef ]
- Sammut, C.; Webb, G.I. Encyclopedia of Machine Learning. In Encyclopedia of Machine Learning ; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; p. 881. ISBN 978-0-387-30768-8. [ Google Scholar ]
- Laï, M.-C.; Brian, M.; Mamzer, M.-F. Perceptions of Artificial Intelligence in Healthcare: Findings from a Qualitative Survey Study among Actors in France. J. Transl. Med. 2020 , 18 , 14. [ Google Scholar ] [ CrossRef ]
- Paton, C.; Kobayashi, S. An Open Science Approach to Artificial Intelligence in Healthcare. Yearb. Med. Inform. 2019 , 28 , 047–051. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wiljer, D.; Hakim, Z. Developing an Artificial Intelligence-Enabled Health Care Practice: Rewiring Health Care Professions for Better Care. J. Med. Imaging Radiat. Sci. 2019 , 50 , S8–S14. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Acemoglu, D.; Restrepo, P. The Wrong Kind of AI? Artificial Intelligence and the Future of Labour Demand. Camb. J. Reg. Econ. Soc. 2020 , 13 , 25–35. [ Google Scholar ] [ CrossRef ]
- Yu, K.-H.; Beam, A.L.; Kohane, I.S. Artificial Intelligence in Healthcare. Nat. Biomed. Eng. 2018 , 2 , 719–731. [ Google Scholar ] [ CrossRef ]
- Zare Harofte, S.; Soltani, M.; Siavashy, S.; Raahemifar, K. Recent Advances of Utilizing Artificial Intelligence in Lab on a Chip for Diagnosis and Treatment. Small 2022 , 18 , 2203169. [ Google Scholar ] [ CrossRef ]
- Sapci, A.H.; Sapci, H.A. Artificial Intelligence Education and Tools for Medical and Health Informatics Students: Systematic Review. JMIR Med. Educ. 2020 , 6 , e19285. [ Google Scholar ] [ CrossRef ]
- Lomis, K.; Jeffries, P.; Palatta, A.; Sage, M.; Sheikh, J.; Sheperis, C.; Whelan, A. Artificial Intelligence for Health Professions Educators. NAM Perspect. 2021 , 2021 . [ Google Scholar ] [ CrossRef ]
- Aung, Y.; Wong, D.; Ting, D. The Promise of Artificial Intelligence: A Review of the Opportunities and Challenges of Artificial Intelligence in Healthcare. Br. Med. Bull. 2021 , 139 , 4–15. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Musen, M.A.; Middleton, B.; Greenes, R.A. Clinical Decision-Support Systems. In Biomedical Informatics ; Shortliffe, E.H., Cimino, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2014. [ Google Scholar ]
- Meskó, B.; Hetényi, G.; Győrffy, Z. Will Artificial Intelligence Solve the Human Resource Crisis in Healthcare? BMC Health Serv. Res. 2018 , 18 , 1–4. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ting, D.S.W.; Cheung, C.Y.-L.; Lim, G.; Tan, G.S.W.; Quang, N.D.; Gan, A.; Hamzah, H.; Garcia-Franco, R.; San Yeo, I.Y.; Lee, S.Y. Development and Validation of a Deep Learning System for Diabetic Retinopathy and Related Eye Diseases Using Retinal Images from Multiethnic Populations with Diabetes. JAMA 2017 , 318 , 2211–2223. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- McKinney, S.M.; Sieniek, M.; Godbole, V.; Godwin, J.; Antropova, N.; Ashrafian, H.; Back, T.; Chesus, M.; Corrado, G.S.; Darzi, A. International Evaluation of an AI System for Breast Cancer Screening. Nature 2020 , 577 , 89–94. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J. An Artificial Intelligence-Enabled ECG Algorithm for the Identification of Patients with Atrial Fibrillation during Sinus Rhythm: A Retrospective Analysis of Outcome Prediction. Lancet 2019 , 394 , 861–867. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lakhani, P.; Sundaram, B. Deep Learning at Chest Radiography: Automated Classification of Pulmonary Tuberculosis by Using Convolutional Neural Networks. Radiology 2017 , 284 , 574–582. [ Google Scholar ] [ CrossRef ]
- Spencer, M. Brittleness and Bureaucracy: Software as a Material for Science. Perspect. Sci. 2015 , 23 , 466–484. [ Google Scholar ] [ CrossRef ]
- Dilsizian, S.E.; Siegel, E.L. Artificial Intelligence in Medicine and Cardiac Imaging: Harnessing Big Data and Advanced Computing to Provide Personalized Medical Diagnosis and Treatment. Curr. Cardiol. Rep. 2014 , 16 , 441. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kahn, C.E., Jr. From Images to Actions: Opportunities for Artificial Intelligence in Radiology. Radiology 2017 , 285 , 719–720. [ Google Scholar ] [ CrossRef ]
- Polónia, A.; Campelos, S.; Ribeiro, A.; Aymore, I.; Pinto, D.; Biskup-Fruzynska, M.; Veiga, R.S.; Canas-Marques, R.; Aresta, G.; Araújo, T. Artificial Intelligence Improves the Accuracy in Histologic Classification of Breast Lesions. Am. J. Clin. Pathol. 2021 , 155 , 527–536. [ Google Scholar ] [ CrossRef ]
- Cheng, J.-Z.; Ni, D.; Chou, Y.-H.; Qin, J.; Tiu, C.-M.; Chang, Y.-C.; Huang, C.-S.; Shen, D.; Chen, C.-M. Computer-Aided Diagnosis with Deep Learning Architecture: Applications to Breast Lesions in US Images and Pulmonary Nodules in CT Scans. Sci. Rep. 2016 , 6 , 24454. [ Google Scholar ] [ CrossRef ]
- Eggers, W.D.; Schatsky, D.; Viechnicki, P. AI-Augmented Government. Using Cognitive Technologies to Redesign Public Sector Work. Deloitte Cent. Gov. Insights 2017 , 1 , 24. [ Google Scholar ]
- Chen, J.; See, K.C. Artificial Intelligence for COVID-19: Rapid Review. J. Med. Internet Res. 2020 , 22 , e21476. [ Google Scholar ] [ CrossRef ]
- Stead, W.W.; Searle, J.R.; Fessler, H.E.; Smith, J.W.; Shortliffe, E.H. Biomedical Informatics: Changing What Physicians Need to Know and How They Learn. Acad. Med. 2011 , 86 , 429–434. [ Google Scholar ] [ CrossRef ]
- Wartman, S.A.; Combs, C.D. Medical Education Must Move From the Information Age to the Age of Artificial Intelligence. Acad. Med. 2018 , 93 , 1107–1109. [ Google Scholar ] [ CrossRef ]
- Durant, T.J.S.; Peaper, D.R.; Ferguson, D.; Schulz, W.L. Impact of COVID-19 Pandemic on Laboratory Utilization. J. Appl. Lab. Med. 2020 , 5 , 1194–1205. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Considerations for Development and Use of AI in Response to COVID-19 | Elsevier Enhanced Reader. Available online: https://www.sciencedirect.com/science/article/pii/S026840122030949X (accessed on 13 March 2023).
- Hirani, R.; Noruzi, K.; Khuram, H.; Hussaini, A.S.; Aifuwa, E.I.; Ely, K.E.; Lewis, J.M.; Gabr, A.E.; Smiley, A.; Tiwari, R.K.; et al. Artificial Intelligence and Healthcare: A Journey through History, Present Innovations, and Future Possibilities. Life 2024 , 14 , 557. [ Google Scholar ] [ CrossRef ]
- Rubí, J.N.S.; Gondim, P.R.d.L. Interoperable Internet of Medical Things Platform for E-Health Applications. Int. J. Distrib. Sens. Netw. 2020 , 16 , 1550147719889591. [ Google Scholar ] [ CrossRef ]
- Introduction to the Practice of Telemedicine—John Craig, Victor Petterson. 2005. Available online: https://journals.sagepub.com/doi/abs/10.1177/1357633X0501100102 (accessed on 28 February 2024).
- Tran, B.X.; Vu, G.T.; Ha, G.H.; Vuong, Q.-H.; Ho, M.-T.; Vuong, T.-T.; La, V.-P.; Ho, M.-T.; Nghiem, K.-C.P.; Nguyen, H.L.T.; et al. Global Evolution of Research in Artificial Intelligence in Health and Medicine: A Bibliometric Study. J. Clin. Med. 2019 , 8 , 360. [ Google Scholar ] [ CrossRef ]
- Mueller, J.M. The ABCs of Assured Autonomy. In Proceedings of the 2019 IEEE International Symposium on Technology and Society (ISTAS), Medford, MA, USA, 15–16 November 2019; pp. 1–5. [ Google Scholar ]
- Yapo, A.; Weiss, J. Ethical Implications of Bias in Machine Learning. In Proceedings of the 51st Hawaii International Conference on System Sciences (HICSS-51), Hilton Waikoloa Village, HI, USA, 3–6 January 2018. [ Google Scholar ]
- Sarwar, S.; Dent, A.; Faust, K.; Richer, M.; Djuric, U.; Van Ommeren, R.; Diamandis, P. Physician Perspectives on Integration of Artificial Intelligence into Diagnostic Pathology. NPJ Digit. Med. 2019 , 2 , 28. [ Google Scholar ] [ CrossRef ]
- Paranjape, K.; Schinkel, M.; Nannan Panday, R.; Car, J.; Nanayakkara, P. Introducing Artificial Intelligence Training in Medical Education. JMIR Med. Educ. 2019 , 5 , e16048. [ Google Scholar ] [ CrossRef ]
- Weng, S.F.; Vaz, L.; Qureshi, N.; Kai, J. Prediction of Premature All-Cause Mortality: A Prospective General Population Cohort Study Comparing Machine-Learning and Standard Epidemiological Approaches. PLoS ONE 2019 , 14 , e0214365. [ Google Scholar ] [ CrossRef ]
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019 , 25 , 44–56. [ Google Scholar ] [ CrossRef ]
- Sharma, A.; Abunada, T.; Said, S.S.; Kurdi, R.M.; Abdallah, A.M.; Abu-Madi, M. Clinical Practicum Assessment for Biomedical Science Program from Graduates’ Perspective. Int. J. Environ. Res. Public Health 2022 , 19 , 12420. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nurses Say Distractions Cut Bedside Time by 25% | HealthLeaders Media. Available online: https://www.healthleadersmedia.com/nursing/nurses-say-distractions-cut-bedside-time-25 (accessed on 5 March 2023).
- Goel, A.K.; Polepeddi, L. Jill Watson: A Virtual Teaching Assistant for Online Education. In Learning Engineering for Online Education ; Routledge: London, UK, 2018. [ Google Scholar ]
- Ching, T.; Himmelstein, D.S.; Beaulieu-Jones, B.K.; Kalinin, A.A.; Do, B.T.; Way, G.P.; Ferrero, E.; Agapow, P.-M.; Zietz, M.; Hoffman, M.M.; et al. Opportunities and Obstacles for Deep Learning in Biology and Medicine. J. R. Soc. Interface 2018 , 15 , 20170387. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- McCoy, L.G.; Nagaraj, S.; Morgado, F.; Harish, V.; Das, S.; Celi, L.A. What Do Medical Students Actually Need to Know about Artificial Intelligence? NPJ Digit. Med. 2020 , 3 , 86. [ Google Scholar ] [ CrossRef ]
- Price, W.N.; Cohen, I.G. Privacy in the Age of Medical Big Data. Nat. Med. 2019 , 25 , 37–43. [ Google Scholar ] [ CrossRef ]
- Stahnisch, F.W.; Verhoef, M. The Flexner Report of 1910 and Its Impact on Complementary and Alternative Medicine and Psychiatry in North America in the 20th Century. Evid. Based Complement Altern. Med 2012 , 2012 , 647896. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Prober, C.G.; Khan, S. Medical Education Reimagined: A Call to Action. Acad. Med. 2013 , 88 , 1407–1410. [ Google Scholar ] [ CrossRef ]
- NAACLS—National Accrediting Agency for Clinical Laboratory Science—Starting a NAACLS Accredited Program. Available online: https://www.naacls.org/Program-Directors/Fees/Procedures-for-Review-Initial-and-Continuing-Accre.aspx (accessed on 5 June 2022).
- Scanlan, P.M. A Review of Bachelor’s Degree Medical Laboratory Scientist Education and Entry Level Practice in the United States. EJIFCC 2013 , 24 , 5–13. [ Google Scholar ]
- Board of Certification. Available online: https://www.ascp.org/content/board-of-certification# (accessed on 22 May 2022).
- Alexander, G.L.; Powell, K.R.; Deroche, C.B. An Evaluation of Telehealth Expansion in U.S. Nursing Homes. J. Am. Med. Inform. Assoc. 2021 , 28 , 342–348. [ Google Scholar ] [ CrossRef ]
- Meskó, B.; Görög, M. A Short Guide for Medical Professionals in the Era of Artificial Intelligence. NPJ Digit. Med. 2020 , 3 , 126. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Davenport, T.; Kalakota, R. The Potential for Artificial Intelligence in Healthcare. Future Health J. 2019 , 6 , 94–98. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Grunhut, J.; Marques, O.; Wyatt, A.T.M. Needs, Challenges, and Applications of Artificial Intelligence in Medical Education Curriculum. JMIR Med. Educ. 2022 , 8 , e35587. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Erickson, B.J.; Korfiatis, P.; Akkus, Z.; Kline, T.L. Machine Learning for Medical Imaging. Radiographics 2017 , 37 , 505. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, J. Playing to Our Human Strengths to Prepare Medical Students for the Future. Korean J. Med. Educ. 2017 , 29 , 193–197. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Teng, M.; Singla, R.; Yau, O.; Lamoureux, D.; Gupta, A.; Hu, Z.; Hu, R.; Aissiou, A.; Eaton, S.; Hamm, C.; et al. Health Care Students’ Perspectives on Artificial Intelligence: Countrywide Survey in Canada. JMIR Med. Educ. 2022 , 8 , e33390. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mennella, C.; Maniscalco, U.; De Pietro, G.; Esposito, M. Ethical and Regulatory Challenges of AI Technologies in Healthcare: A Narrative Review. Heliyon 2024 , 10 , e26297. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, X.-W.; Lin, X. Big Data Deep Learning: Challenges and Perspectives. IEEE Access 2014 , 2 , 514–525. [ Google Scholar ] [ CrossRef ]
- Noorbakhsh-Sabet, N.; Zand, R.; Zhang, Y.; Abedi, V. Artificial Intelligence Transforms the Future of Health Care. Am. J. Med. 2019 , 132 , 795–801. [ Google Scholar ] [ CrossRef ]
- Kluge, E.-H.W. Artificial Intelligence in Healthcare: Ethical Considerations. In Proceedings of the Healthcare Management Forum; SAGE Publications Sage CA: Los Angeles, CA, USA, 2020; Volume 33, pp. 47–49. [ Google Scholar ]
- Kassam, A.; Kassam, N. Artificial Intelligence in Healthcare: A Canadian Context. Health Manag. Forum 2020 , 33 , 5–9. [ Google Scholar ] [ CrossRef ]
- Borenstein, J.; Howard, A. Emerging Challenges in AI and the Need for AI Ethics Education. AI Ethics 2021 , 1 , 61–65. [ Google Scholar ] [ CrossRef ]
- Azencott, C.-A. Machine Learning and Genomics: Precision Medicine versus Patient Privacy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018 , 376 , 20170350. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Taylor, Z.W.; Charran, C.; Childs, J. Using Big Data for Educational Decisions: Lessons from the Literature for Developing Nations. Educ. Sci. 2023 , 13 , 439. [ Google Scholar ] [ CrossRef ]
- Kingston, J.K. Artificial Intelligence and Legal Liability. In Proceedings of the International Conference on Innovative Techniques and Applications of Artificial Intelligence; Springer: Berlin/Heidelberg, Germany, 2016; pp. 269–279. [ Google Scholar ]
- Lee, T.T.; Kesselheim, A.S.U.S. Food and Drug Administration Precertification Pilot Program for Digital Health Software: Weighing the Benefits and Risks. Ann. Intern. Med. 2018 , 168 , 730–732. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hussey, P.; Adams, E.; Shaffer, F.A. Nursing Informatics and Leadership, an Essential Competency for a Global Priority: eHealth. Nurse Lead. 2015 , 13 , 52–57. [ Google Scholar ] [ CrossRef ]
- Atique, S.; Bautista, J.R.; Block, L.J.; Lee, J.J.; Lozada-Perezmitre, E.; Nibber, R.; O’Connor, S.; Peltonen, L.-M.; Ronquillo, C.; Tayaben, J.; et al. A Nursing Informatics Response to COVID-19: Perspectives from Five Regions of the World. J. Adv. Nurs. 2020 , 76 , 2462–2468. [ Google Scholar ] [ CrossRef ]
- Ahmad, M.N.; Abdallah, S.A.; Abbasi, S.A.; Abdallah, A.M. Student Perspectives on the Integration of Artificial Intelligence into Healthcare Services. Digit. Health 2023 , 9 , 205520762311740. [ Google Scholar ] [ CrossRef ]
- Sit, C.; Srinivasan, R.; Amlani, A.; Muthuswamy, K.; Azam, A.; Monzon, L.; Poon, D.S. Attitudes and Perceptions of UK Medical Students towards Artificial Intelligence and Radiology: A Multicentre Survey. Insights Imaging 2020 , 11 , 14. [ Google Scholar ] [ CrossRef ]
- Abdullah, R.; Fakieh, B. Health Care Employees’ Perceptions of the Use of Artificial Intelligence Applications: Survey Study. J. Med. Internet Res. 2020 , 22 , e17620. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Diagnostic accuracy | The incorporation of AI in diagnostics during COVID-19 increased the accuracy of diagnosis through faster and accurate image analysis |
| Laboratory workflow support | AI reduced the workload for healthcare providers by providing automation support for sample analysis and, later, speedy data analysis |
| Research and development | The incorporation of AI into healthcare served as the most efficient tool to discover and apply antiviral drugs and vaccines by the interpretation of big patient data |
| Patient triage optimization | AI supported patient triage by prioritizing critical cases depending on the available data |
| Quality assurance | The accuracy and reliability of testing were monitored by AI, resulting in reduced system error and delivering high quality work in laboratories |
| Radiology imaging and diagnostic approach | AI and machine learning training can support biomedical science students in using algorithms to read images of X-rays and CT and MRI scans to reach an accurate diagnosis |
| Precision medicine | Biomedical science students can support precision medicine by analyzing big datasets and providing tailored medicinal or genetic approaches to patients |
| Reducing workload | Efficient AI incorporation in healthcare can help future biomedical science students to reduce their workload as they will be trained in the automation of databases, imaging, etc. |
| Research and innovation | AI-trained biomedical science students can be an integral part of new innovations related to research or drug discovery |
| Career opportunities | AI-trained biomedical science students can explore more career and job opportunities in research and administrative healthcare settings |
| Strategy planning and disease controls | AI-trained healthcare professionals with a biomedical background can detect diseases at their early stages and estimate their potential to spread. This AI approach supports healthcare in planning strategies to control diseases |
| Ethical considerations | Ethical considerations are the biggest challenge in applying AI to patients and can be resolved by training biomedical students on the ethics related to AI in patient care |
| Curriculum Additions | Recommendations to Achieve AI Expertise |
|---|---|
| Biomedical engineering and computational data certification | Collaboration with computer/software engineering college and work on certification. Theoretical knowledge can be supplemented with practical experience with AI. |
| AI learning groups and open journal clubs | Interested students can receive hands-on training and learning from computer or data science students by sharing common learning groups. Students can exchange views and answer each other’s questions. |
| AI: fundamental concepts | Students should enhance their basic AI skills such as data handling, analysis, data visualization, and understanding. |
| AI: research opportunities | Interested students should be exposed to private–public AI services and should be involved in AI research projects. These projects would help students to apply new approaches to their work. |
| Field of Challenge | Challenge | Mitigation |
|---|---|---|
| Faculty expertise | The lack of faculty expertise represents the biggest challenge to incorporating AI into existing biomedical coursework. Many have only limited knowledge on AI methodologies and their applications. This lack of expertise acts as a barrier to teaching AI to biomedical students | Training the trainers is the solution to this challenge. CPD on AI should be offered through workshops, seminars, and short-term interdisciplinary AI courses to enhance their AI skills |
| Curriculum restructuring and AI integration | Amending the current curriculum with additional AI coursework is a challenge, as it might be overwhelming for biomedical students and preceptors | The curriculum should be reviewed thoroughly to find potential areas to incorporate AI into existing biomedical coursework. This can be achieved by adding foundational elective AI courses for students, which might require interdisciplinary faculty review |
| Variable student backgrounds | Biomedical students have different levels of prior knowledge on AI, posing a challenge to offering different levels of AI coursework to students to match their existing knowledge | Introductory AI modules should be provided in the coursework, but for “deep divers” with more extensive knowledge or interest in AI, more extensive AI coursework and research opportunities could be provided |
| Practical training in AI | Hands-on training for students on AI requires well-equipped labs with specialized equipment and software | AI training requires a new laboratory infrastructure with the latest software and tools for training. This will provide students with hands-on training on algorithms, data analysis, image processing, etc. |
| Limited resources | Limited financial resources pose a challenge to equip students with training in the latest AI technologies | Allocated funding and budgeting will support in planning new infrastructure and laboratories. In addition, partnership with different institutions will minimize the cost of AI incorporation |
| Ethical considerations | Ethical considerations: data mining, data privacy, and the responsible application of AI are a challenge | Case studies and real-world examples related to ethics will encourage critical thinking among students in relation to AI |
| Assessment methods | Effective and standardized assessment methods for assessing students’ knowledge on AI concepts are lacking | Designing exams, projects, presentations, hands-on assignments, and group projects related to the real-world biomedical application of AI will develop understanding and support student assessment |
| Interprofessional education (IPE) | Interdisciplinary collaboration between computer sciences, engineering departments, and biomedical sciences is challenging, considering the different objectives and priorities of coursework | IPE can be encouraged by structuring interdisciplinary teams with faculties from both computer science and biomedical backgrounds. In addition, faculty should participate in cross-disciplinary seminars to understand and incorporate AI more effectively in coursework. Interdisciplinary student mentors can also promote IPE by assigning joint AI projects to students |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Sharma, A.; Al-Haidose, A.; Al-Asmakh, M.; Abdallah, A.M. Integrating Artificial Intelligence into Biomedical Science Curricula: Advancing Healthcare Education. Clin. Pract. 2024 , 14 , 1391-1403. https://doi.org/10.3390/clinpract14040112
Sharma A, Al-Haidose A, Al-Asmakh M, Abdallah AM. Integrating Artificial Intelligence into Biomedical Science Curricula: Advancing Healthcare Education. Clinics and Practice . 2024; 14(4):1391-1403. https://doi.org/10.3390/clinpract14040112
Sharma, Aarti, Amal Al-Haidose, Maha Al-Asmakh, and Atiyeh M. Abdallah. 2024. "Integrating Artificial Intelligence into Biomedical Science Curricula: Advancing Healthcare Education" Clinics and Practice 14, no. 4: 1391-1403. https://doi.org/10.3390/clinpract14040112
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
How AI — and ASU — will advance the health care sector
As technology improves, it has the potential to accelerate diagnoses and enable earlier and possibly life-saving treatment.

AI-generated images by Alex Davis/ASU
Editor's note: This feature article is part of our “AI is everywhere ... now what?” special project exploring the potential (and potential pitfalls) of artificial intelligence in our lives. Explore more topics and takes on the project page .
A patient walks into a hospital complaining of stomach pain.
After a scan is performed, the doctor confirms the diagnosis of appendicitis, surgery is scheduled and the patient leaves the hospital the following day, his appendix out and his pain gone.
It’s a situation that happens every day, in hospitals across the world. But what if, using an artificial intelligence learning model, that scan — along with past medical history, lifestyle choices and other relevant data — also showed the patient is at risk for a heart attack or stroke?
How many lives might be saved?
“AI can see things that humans cannot,” said Dr. Bhavik Patel , the chief AI officer at Mayo Clinic in Arizona. “AI can take that scan and tell us what your heart risk and stroke factors are even though we didn’t image the heart or brain. AI can help us impact things to where we’re making a difference in people’s lives.”
It would be a misnomer to say AI is the next frontier in health care. AI has been part of medical care for more than 20 years, used to analyze medical imaging data such as X-rays or MRIs, transcribe medical documents and streamline administrative tasks.
But as AI’s technology improves, it has the potential to process information faster, thus accelerating diagnoses and enabling earlier and possibly life-saving treatment for patients.
- Algorithms that spot malignant tumors.
- A smartphone app that can alert a caregiver before someone falls.
- Learning models that recognize changes in speech patterns, which could indicate neurological conditions.
The possibilities, medical professionals say, are endless.
“I think AI represents the fourth industrial revolution, and health care is just one of the many verticals it’s revolutionizing,” Patel said. “You shouldn’t say health care anymore without saying AI. It should be an integral part of it.”

Sherine Gabriel , executive vice president of ASU Health , stressed that AI will not replace health care professionals or dramatically alter the doctor-patient relationship.
Instead, Gabriel said, AI is a tool that will ultimately result in better patient care.
“It’s not going to make the diagnosis for you, but it’s going to make the diagnosis easier, simpler and way quicker,” Gabriel said. “It’s going to identify diagnostic targets more quickly than we have been able to in the past. The thinking isn’t really that different. It’s just putting everything on overdrive.”
One example:
Bradley Greger , an associate professor in ASU’s School of Biological and Health Systems Engineering, part of the Ira A. Fulton Schools of Engineering, is using AI and machine learning to read and analyze signals from the brain.
The technology could have multiple applications: Enabling paralyzed people to control a robotic limb; helping blind people to see with the aid of a camera connected to the visual processing areas of the brain; aiding patients with seizure disorder.
“There’s unhealthy electrical activity in the brain that causes seizures. It’s very complicated datasets,” Greger said. “We can use AI to look for patterns, and then we can tell people when they’re going to have a seizure or they need to have more medication or something like that.
“AI is really good at searching through very complex datasets and looking for patterns that correlate or give you some knowledge about what’s happening with the pathology itself. A human can do it, but it just takes a human a long time.”
AI can also help with administrative tasks, thus allowing doctors to have more face time with their patients.
DeepScribe, an AI-powered medical scribe, uses machine learning and language processing to extract medical information from a phone conversation between a provider and patient and almost immediately produce a finished medical note that goes into a patient’s record. Typically, experts say, doctors spend more than three hours a day documenting conversations with their patients.
“AI is a really great tool for doctors to have at their disposal,” Greger said.

AI will be a central tenet of ASU Health, which includes a new medical school called the School of Medicine and Advanced Medical Engineering, the School of Technology for Public Health, the Health Observatory at ASU and the Medical Master’s Institute — as well as the existing College of Health Solutions and Edson College of Nursing and Health Innovation.
“AI will be used in almost every conceivable way imaginable,” Gabriel said. “It’s going to be interwoven into everything we teach. We want to be certain that the next generation of providers have all of those skills at hand in order to optimally improve health care.”
Gabriel said ASU Health will approach AI with a humanistic perspective, thus the creation of the AI + Center of Patient Stories. ASU Health will use reporters and students in the Walter Cronkite School of Journalism and Mass Communication to engage with patients and write their stories.
The center will then use AI applications like virtual reality, augmented reality or real-time mixed reality to digitally enhance the stories.
“We’re trying to create not just a video for them to watch, but an immersive educational experience for students to help build empathy, help build understanding and help them build the connection I think they need to really be able to care for those patients and populations,” Gabriel said. “We really want to use technology to enhance the humanistic aspects of health care.”
ASU Health also will create an AI + Medical Suite of the Future. Gabriel described it as a patient care setting where AI will be used to create information that is packaged in a way that patients or their loved ones can access it and “understand what’s going on in a much deeper level.” The suite, Gabriel said, could include an “AI coach” that can answer questions.
“There are lots and lots of studies that show a patient will have a conversation in a doctor’s office, but even if that conversation is taped, the patient will only retain a small part of it, either because the doctor didn’t explain it well or it’s an emotionally charged situation,” Gabriel said. “Or they’ll go home, and a loved one will say, ‘So, what did the doctor say?’
“That happens every day of the week. But these (AI) tools give us the opportunity to change that structure. It’s about bringing all of the curated information together from that patient encounter in a way that optimizes their health outcome.”

Across ASU, faculty is working with AI to improve patient care.
Thurmon Lockhart , a professor in the School of Biological and Health Systems Engineering, has developed a wearable device that goes across a patient’s sternum and measures body posture as well as arm and leg movements in real time.
“Traditional fall-risk assessments for seniors don’t always target specific types of risk, like muscle weakness or gait stability,” Lockhart said in a previously published ASU Thrive story .
When the risk of falling is deemed high, a smartphone app called the Lockhart Monitor can alert the user or caregiver.
Visar Berisha , the associate dean of research and commercialization in the Ira A. Fulton Schools of Engineering, is working with Julie Liss , an associate dean and professor in the College of Health Solutions, to develop AI models that analyze a person’s words and speech patterns.
Those patterns, Berisha said, can help determine whether a patient may suffer from neurological conditions like Alzheimer’s, Parkinson’s or amyotrophic lateral sclerosis, also known as Lou Gehrig's disease.
“A lot of that analysis has been done manually in the sense that patients would come to a lab, record samples, and then clinicians would listen for different types of changes in the speed signal that were indicative of the underlying condition,” Berisha said. “You needed to be an expert, have lots of training and a lot of time.”
Berisha said he and Liss have been working for more than a decade on an AI model that would automate the process by having patients download a mobile app on their personal devices and then provide speech samples for analysis.
“It scales it in a way that wasn’t possible before,” he said.
In addition, Berisha said, AI might be able to notice subtle changes in a person’s speech pattern that a clinician might not spot.
Patel said the AI “revolution” in health care isn’t something that will happen in five, 10 or even 20 years.
“Just because of the way the technology has improved, I think we’re very near to getting those exponential benefits,” he said.
Greger predicted AI will be omnipresent in the medical field.
“It’s going to be everywhere,” he said. “It’s just going to be this thing around that everybody uses. And it will have very specific applications. The radiologists will use it for image processing. The neurologists will use it to process the signals that come out of the brain and help them identify diseases. It’ll be a tool that’s very widely used. Just like the computer or car is today.”
Not all health care experts are as certain of AI’s immediate impact, though.
“I don’t know that the rate of change is going to be very fast,” Berisha said. “There are some structural complexities in the American health care system that make it really difficult to introduce new tools in everyday care.”
A 2019 paper in the National Library of Medicine amplified those difficulties. The paper noted that health care decisions have been made almost exclusively by humans, and the use of smart machines could raise issues of accountability, permission, transparency and privacy.
The paper, titled “The Potential for Artificial Intelligence in Healthcare,” also questioned whether health care providers will be able to adequately explain to a patient how deep learning algorithms led to, say, a cancer diagnosis. In turn, that could impact the doctor-patient relationship.
There’s also a question of accuracy within AI diagnoses, according to the paper. Machine learning systems could be subject to algorithmic bias and predict a greater likelihood of disease on the basis of gender or race when those aren’t causal factors.
Berisha said health care professionals may be reluctant to turn to AI for simpler reasons: time and money. He said overburdened care providers who only have “seven minutes to spend” with a patient may decide they don’t have the time to evaluate information they’re not certain will help with diagnosis or treatment.
In addition, he said, the cost of AI, plus the hundreds of applications that will be available, may dissuade providers.
“I agree it’s going to be transformational,” he said. “I just think it’ll take a while.”
AI is everywhere ... now what?

Artificial intelligence isn't just handy for creating images like the above — it has implications in an increasingly broad range of fields, from health to education to saving the planet.
Explore the ways in which ASU faculty are thinking about and using AI in their work:
- Enhancing education: ASU experts explain how artificial intelligence can help teachers — but training and access is key .
- A more sustainable future: Artificial intelligence is helping researchers find solutions to urgent worldwide issues .
- Advancing health care: AI has the potential to accelerate diagnoses and enable earlier and possibly life-saving treatment.
- The ethical costs: Issues around privacy, bias, surveillance and even extinction are raised with advancements in AI.
- Expert Q&As: Read smart minds' thoughts on everything from food security to the power grid to the legal system on our special project page .
More Health and medicine

Pilot program to address HIV care, intimate partner violence in Uganda
Editor's note: This is the second in a five-part series about ASU faculty conducting summer research abroad. Read about carbon collection in the Namib Desert.Uganda has one of the highest rates of…

Hot and bothered: ASU event to discuss heat and health
Arizona State University's upcoming Health Talks event on July 18 will address how vulnerable populations are being impacted by heat across the world and how state governments are planning to deal…

ASU students redesign 'Workstation on Wheels' for HonorHealth nurses
Going into this summer, Sheetal Jha, a biomedical engineering student, had specific expectations for the internship she would be participating in. “I was looking to gain hands-on experience in…
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Medical Devices
- Digital Health Center of Excellence
- Software as a Medical Device (SaMD)
Artificial Intelligence and Machine Learning in Software as a Medical Device

Update: March 15, 2024
Artificial Intelligence and Medical Products: How CBER, CDER, CDRH, and OCP are Working Together
The U.S. Food and Drug Administration (FDA) issued " Artificial Intelligence and Medical Products: How CBER, CDER, CDRH, and OCP are Working Together ," which outlines the agency's commitment and cross-center collaboration to protect public health while fostering responsible and ethical medical product innovation through Artificial Intelligence.
Download the Paper (PDF - 1.2 MB)
Artificial intelligence (AI) and machine learning (ML) technologies have the potential to transform health care by deriving new and important insights from the vast amount of data generated during the delivery of health care every day. Medical device manufacturers are using these technologies to innovate their products to better assist health care providers and improve patient care. The complex and dynamic processes involved in the development, deployment, use, and maintenance of AI technologies benefit from careful management throughout the medical product life cycle.
On this page:
What is artificial intelligence and machine learning, how are artificial intelligence and machine learning (ai/ml) transforming medical devices, how is the fda considering regulation of artificial intelligence and machine learning medical devices, additional resources.
Artificial Intelligence is a machine-based system that can, for a given set of human-defined objectives, make predictions, recommendations, or decisions influencing real or virtual environments. Artificial intelligence systems use machine- and human-based inputs to perceive real and virtual environments; abstract such perceptions into models through analysis in an automated manner; and use model inference to formulate options for information or action.
Machine Learning is a set of techniques that can be used to train AI algorithms to improve performance at a task based on data.
Some real-world examples of artificial intelligence and machine learning technologies include:
- An imaging system that uses algorithms to give diagnostic information for skin cancer in patients.
- A smart sensor device that estimates the probability of a heart attack.
Additional information can be found at Safe, Secure, and Trustworthy Development and Use of Artificial Intelligence .
AI/ML technologies have the potential to transform health care by deriving new and important insights from the vast amount of data generated during the delivery of health care every day. Medical device manufacturers are using these technologies to innovate their products to better assist health care providers and improve patient care. One of the greatest benefits of AI/ML in software resides in its ability to learn from real-world use and experience, and its capability to improve its performance.
The FDA reviews medical devices through an appropriate premarket pathway, such as premarket clearance (510(k)), De Novo classification , or premarket approval . The FDA may also review and clear modifications to medical devices, including software as a medical device, depending on the significance or risk posed to patients of that modification. Learn the current FDA guidance for risk-based approach for 510(k) software modifications .
The FDA's traditional paradigm of medical device regulation was not designed for adaptive artificial intelligence and machine learning technologies. Many changes to artificial intelligence and machine learning-driven devices may need a premarket review.
On April 2, 2019, the FDA published a discussion paper " Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) - Discussion Paper and Request for Feedback " that describes a potential approach to premarket review for artificial intelligence and machine learning-driven software modifications.
In January 2021, the FDA published the " Artificial Intelligence and Machine Learning Software as a Medical Device Action Plan " or "AI/ML SaMD Action Plan." Consistent with the action plan, the FDA later issued the following documents:
- October 2021 - Good Machine Learning Practice for Medical Device Development: Guiding Principles
- April 2023 - Draft Guidance: Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence/Machine Learning (AI/ML)-Enabled Device Software Functions
- October 2023 - Predetermined Change Control Plans for Machine Learning-Enabled Medical Devices: Guiding Principles
- June 2024 - Transparency for Machine Learning-Enabled Medical Devices: Guiding Principles
On March 15, 2024 the FDA published the " Artificial Intelligence and Medical Products: How CBER, CDER, CDRH, and OCP are Working Together ," which represents the FDA's coordinated approach to AI. This paper is intended to complement the " AI/ML SaMD Action Plan " and represents a commitment between the FDA's Center for Biologics Evaluation and Research (CBER), the Center for Drug Evaluation and Research (CDER), and the Center for Devices and Radiological Health (CDRH), and the Office of Combination Products (OCP), to drive alignment and share learnings applicable to AI in medical products more broadly.
If you have questions about artificial intelligence, machine learning, or other digital health topics, ask a question about digital health regulatory policies .
- Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices
- Artificial Intelligence Program: Research on AI/ML-Based Medical Devices
- Digital Health Research and Partnerships
- Artificial Intelligence and Medical Products
Subscribe to Digital Health
Sign up to receive email updates on Digital Health.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 June 2023
Bias in AI-based models for medical applications: challenges and mitigation strategies
- Mirja Mittermaier ORCID: orcid.org/0000-0003-0678-6676 1 , 2 ,
- Marium M. Raza 3 &
- Joseph C. Kvedar ORCID: orcid.org/0000-0002-7517-2291 3
npj Digital Medicine volume 6 , Article number: 113 ( 2023 ) Cite this article
19k Accesses
35 Citations
61 Altmetric
Metrics details
- Computational models
- Health policy
Artificial intelligence systems are increasingly being applied to healthcare. In surgery, AI applications hold promise as tools to predict surgical outcomes, assess technical skills, or guide surgeons intraoperatively via computer vision. On the other hand, AI systems can also suffer from bias, compounding existing inequities in socioeconomic status, race, ethnicity, religion, gender, disability, or sexual orientation. Bias particularly impacts disadvantaged populations, which can be subject to algorithmic predictions that are less accurate or underestimate the need for care. Thus, strategies for detecting and mitigating bias are pivotal for creating AI technology that is generalizable and fair. Here, we discuss a recent study that developed a new strategy to mitigate bias in surgical AI systems.
Bias in medical AI algorithms
Artificial intelligence (AI) technology is increasingly applied to healthcare, from AI-augmented clinical research to algorithms for image analysis or disease prediction. Specifically, within the field of surgery, AI applications hold promise as tools to predict surgical outcomes 1 , aid surgeons via computer vision for intraoperative surgical navigation 2 , and even as algorithms to assess technical skills and surgical performance 1 , 3 , 4 , 5 .
Kiyasseh et al. 4 highlight this potential application in their work deploying surgical AI systems (SAIS) on videos of robotic surgeries from three hospitals. They used SAIS to assess the skill level of surgeons completing multiple different surgical activities, including needle handling and needle driving. In applying this AI model, Kiyasseh et al. 4 found that it could reliably assess surgical performance but exhibited bias. The SAIS model showed an underskilling or overskilling bias at different rates across surgeon sub-cohort. Underskilling was the AI model downgrading surgical performance erroneously, predicting a particular skill to be lower quality than it actually was. Overskilling was the reverse—the AI model upgraded surgical performance erroneously, predicting a specific skill to be of higher quality than it was. Underskilling and overskilling were measured based on the AI-based predictions’ negative and positive predictive values negative, respectively.
Strategies to mitigate bias
The issue of bias being exhibited, perpetuated, or even amplified by AI algorithms is an increasing concern within healthcare. Bias is usually defined as a difference in performance between subgroups for a predictive task 6 , 7 . For example, an AI algorithm used for predicting future risk of breast cancer may suffer from a performance gap wherein black patients are more likely to be assigned as “low risk” incorrectly. Further, an algorithm trained on hospital data from German patients might not perform well in the USA, as patient population, treatment strategies or medications might differ. Similar cases have already been seen in healthcare systems 8 . There could be many different reasons for this performance gap. Bias can be generated across AI model development steps, including data collection/preparation, model development, model evaluation, and deployment in clinical settings 9 . With this particular example, the algorithm may have been trained on data predominantly from white patients, or health records from Black patients may be less accessible. Additionally, there are likely underlying social inequalities in healthcare access and expenditures that impact how a model might be trained to predict risk 6 , 10 . Regardless of the cause, the impact of an algorithm disproportionately assigning false negatives would include fewer follow-up scans, and potentially more undiagnosed/untreated cancer cases, worsening health inequity for an already disadvantaged population. Thus, strategies to detect and mitigate bias will be pivotal to improving healthcare outcomes. Bias mitigation strategies may involve interventions such as pre-processing data through sampling before a model is built, in-processing by implementing mathematical approaches to incentivize a model to learn balanced predictions, and post-processing 11 . Further, as experts can be aware of biases specific to datasets, “keeping the human in the loop” can be another important strategy to mitigate bias.
With their SAIS model, Kiyasseh et al. 4 developed a strategy called TWIX to mitigate bias. TWIX is an add-on application that taught the SAIS model to add a prediction of the importance of video clips that was used to assess surgical skill. They hypothesized that the SAIS model’s bias might be due to the system latching onto unreliable video frames for assessment. TWIX requiring model predictions of video clip importance served a similar role to human assessors explaining the rationale for assessments. Kiyasseh et al. 4 found that TWIX mitigated SAIS model bias, improving model performance both for the disadvantaged surgeon sub-cohorts and for surgical skill assessments overall. This accomplishment is beneficial not only for this particular use case but also implies that this type of bias mitigation strategy could be used to continue to improve AI applications in the future.
A look into the future—challenges with continuously learning AI models
Bias within AI algorithms must continue to be studied and mitigated as AI technology develops. Looking into the future, one question that will most definitely arise is what level of bias is acceptable for an AI algorithm 4 . This is analogous to the question of what accuracy threshold is acceptable for a particular AI system 4 . Previous groups suggested that any performance discrepancy is indicative of algorithmic bias, but expecting completely bias-free systems before implementation is unrealistic 12 . Performance discrepancy may also differ based on the data and population an AI algorithm is trained on and then subsequently applied to. Currently, there is significant heterogeneity in terms of the datasets AI algorithms are trained with within algorithm types themselves 13 , 14 . The question of whether AI algorithms may need to be more generalizable, trained on larger and more diverse datasets to be applied to broader populations, or more localized and applied narrowly remains to be addressed. In any case, AI models will have to be explainable 15 with transparent methodologies so that these questions can be studied and debated in the coming years.
Another issue for the future is whether AI algorithms will be able to be changed/edited, just as Kiyasseh et al. 4 added TWIX to their existing SAIS algorithm. An AI algorithm can either be locked—once the algorithm is trained, the model provides the same result when the same input is applied—or adaptive 16 . In this case, the AI model could be updated continuously as it learns from new data over time rather than becoming outdated within a few years. However, continuous learning also possesses the risk of increasing or adding new bias if the new data are biased 17 . Thus, methodologies for regular bias detection and continual bias mitigation will be key to AI implementation.
From a regulatory standpoint, new initiatives also aim to tackle the issue of biased data in AI systems. The STANDING Together initiative (standards for data diversity, inclusivity, and generalizability), launched in September 2022, aims to develop recommendations for the composition (who is represented) and reporting (how they are represented) of datasets underpinning medical AI systems 18 . Further, the FDA has recognized challenges due to bias in AI and ML algorithms and released an action plan (“Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) Action Plan”) in January 2021 9 , 19 , emphasizing the importance of identifying and mitigating bias in AI systems 9 . As part of the FDA Action Plan, the FDA intends to support the piloting of real-world performance monitoring 19 , allowing for the detection of bias after deployment. Further, to meet regulatory challenges that come with continuously adopting AI models, the FDA recently released a draft guidance to develop a less burdensome regulatory approach supporting the iterative improvement of, e.g., AI models while continuing to assure their safety and effectiveness 20 . These types of regulatory steps should be encouraged, as they will become increasingly necessary to ensure the minimization of bias without the blockade of AI innovation.
The integration of AI into medical technology and healthcare systems is only going to increase in the coming years. Key to AI model integration and usability will be bias mitigation. Kiyasseh et al. describe an innovative approach to bias mitigation with their TWIX system. As technology continues to develop, the push toward bias mitigation occurs at all levels—from model development and over training to deployment and implementation. This effort will require checks and balances from innovators, healthcare institutions, and regulatory entities.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Ma, R. et al. Surgical gestures as a method to quantify surgical performance and predict patient outcomes. NPJ Digital Med. 5 , 187 (2022).
Article Google Scholar
Chadebecq, F., Vasconcelos, F., Mazomenos, E. & Stoyanov, D. Computer vision in the surgical operating room. Visc. Med. 36 , 456–462 (2020).
Article PubMed PubMed Central Google Scholar
Kiyasseh, D. et al. A multi-institutional study using artificial intelligence to provide reliable and fair feedback to surgeons. Commun. Med. 3 , 42 (2023).
Kiyasseh, D. et al. Human visual explanations mitigate bias in AI-based assessment of surgeon skills. NPJ Digital Med. 6 , 54 (2023).
Kiyasseh, D. et al. A vision transformer for decoding surgeon activity from surgical videos. Nat. Biomed. Eng . https://doi.org/10.1038/s41551-023-01010-8 (2023).
Seyyed-Kalantari, L., Zhang, H., McDermott, M. B. A., Chen, I. Y. & Ghassemi, M. Underdiagnosis bias of artificial intelligence algorithms applied to chest radiographs in under-served patient populations. Nat. Med. 27 , 2176–2182 (2021).
Article CAS PubMed PubMed Central Google Scholar
Yang, J., Soltan, A. A. S., Eyre, D. W., Yang, Y. & Clifton, D. A. An adversarial training framework for mitigating algorithmic biases in clinical machine learning. NPJ Digital Med. 6 , 55 (2023).
Obermeyer, Z., Powers, B., Vogeli, C. & Mullainathan, S. Dissecting racial bias in an algorithm used to manage the health of populations. Science 366 , 447–453 (2019).
Article CAS PubMed Google Scholar
Vokinger, K. N., Feuerriegel, S. & Kesselheim, A. S. Mitigating bias in machine learning for medicine. Commun. Med. 1 , 25 (2021).
Panch, T., Mattie, H. & Atun, R. Artificial intelligence and algorithmic bias: implications for health systems. J. Glob. Health 9 , 010318 (2019).
Article PubMed Google Scholar
Xu, J. et al. Algorithmic fairness in computational medicine. EBioMedicine 84 , 104250 (2022).
Townson, S. Manage AI Bias Instead of Trying to Eliminate It. https://sloanreview.mit.edu/article/manage-ai-bias-instead-of-trying-to-eliminate-it/2023 (MIT Sloan Management Review, 2023).
Gubatan, J. et al. Artificial intelligence applications in inflammatory bowel disease: emerging technologies and future directions. World J. Gastroenterol. 27 , 1920–1935 (2021).
Moglia, A., Georgiou, K., Georgiou, E., Satava, R. M. & Cuschieri, A. A systematic review on artificial intelligence in robot-assisted surgery. Int. J. Surg. 95 , 106151 (2021).
Theunissen, M. & Browning, J. Putting explainable AI in context: institutional explanations for medical AI. Ethics Inf. Technol. 24 , 23 (2022).
Benjamens, S., Dhunnoo, P. & Mesko, B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digital Med. 3 , 118 (2020).
DeCamp, M. & Lindvall, C. Latent bias and the implementation of artificial intelligence in medicine. J. Am. Med. Inform. Assoc. 27 , 2020–2023 (2020).
Ganapathi, S. et al. Tackling bias in AI health datasets through the STANDING Together initiative. Nat. Med. 28 , 2232–2233 (2022).
FDA. Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) Action Plan. www.fda.gov/media/145022/download (2021).
FDA. Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence/Machine Learning (AI/ML)-Enabled Device Software Functions. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/marketing-submission-recommendations-predetermined-change-control-plan-artificial (2023).
Download references
Acknowledgements
M.M. is a fellow of the BIH—Charité Digital Clinician Scientist Program funded by the Charité—Universitätsmedizin Berlin, the Berlin Institute of Health at Charité, and the German Research Foundation (DFG).
Author information
Authors and affiliations.
Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Department of Infectious Diseases, Respiratory Medicine and Critical Care, Berlin, Germany
Mirja Mittermaier
Berlin Institute of Health at Charité—Universitätsmedizin Berlin, Charitéplatz 1, 10117, Berlin, Germany
Harvard Medical School, Boston, MA, USA
Marium M. Raza & Joseph C. Kvedar
You can also search for this author in PubMed Google Scholar
Contributions
M.M. wrote the first draft. M.M.R. contributed to the first draft and provided critical revisions. J.C.K. provided critical revisions. All authors critically reviewed and revised the manuscript and approved the final manuscript.
Corresponding author
Correspondence to Mirja Mittermaier .
Ethics declarations
Competing interests.
J.C.K. is the Editor-in-Chief of npj Digital Medicine . M.M. and M.M.R. declare no competing interests.
Supplementary information
Reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Mittermaier, M., Raza, M.M. & Kvedar, J.C. Bias in AI-based models for medical applications: challenges and mitigation strategies. npj Digit. Med. 6 , 113 (2023). https://doi.org/10.1038/s41746-023-00858-z
Download citation
Received : 27 April 2023
Accepted : 06 June 2023
Published : 14 June 2023
DOI : https://doi.org/10.1038/s41746-023-00858-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
New regulatory thinking is needed for ai-based personalised drug and cell therapies in precision oncology.
- Bouchra Derraz
- Gabriele Breda
- Stephen Gilbert
npj Precision Oncology (2024)
Levels of autonomy in FDA-cleared surgical robots: a systematic review
- Turner S. Baker
- Benjamin I. Rapoport
npj Digital Medicine (2024)
Artificial intelligence and robotic surgical education
- Riley Brian
- Alyssa Murillo
- Adnan Alseidi
Global Surgical Education - Journal of the Association for Surgical Education (2024)
Transformer Models in Healthcare: A Survey and Thematic Analysis of Potentials, Shortcomings and Risks
- Kerstin Denecke
- Richard May
- Octavio Rivera-Romero
Journal of Medical Systems (2024)
The ethics of using artificial intelligence in scientific research: new guidance needed for a new tool
- David B. Resnik
- Mohammad Hosseini
AI and Ethics (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Pathways to Governing AI Technologies in Healthcare
Leading policymakers, academics, healthcare providers, AI developers, and patient advocates discuss the path forward for healthcare AI policy at closed-door workshop.

Artificial intelligence (AI) has the potential to transform healthcare delivery by enhancing diagnostic accuracy, streamlining administrative operations, and increasing patient engagement. From 2017-2021, the healthcare sector received more private AI investment globally than any other, attracting $28.9 billion .
Enthusiasm for these new healthcare technologies has long been accompanied by concerns about patient safety, harmful biases, and data security. Regulators face the challenge of fostering these innovative tools while preserving safe, fair, and secure machine learning algorithms within the constraints of regulatory frameworks created in an era of physical devices, paper records, and analog data. The rapid adoption of AI into healthcare processes creates an urgent need to review existing regulatory frameworks.
Recognizing this gap, the Stanford Institute for Human-Centered AI (HAI) convened a select group of 55 leading policymakers, scientists, healthcare providers, ethicists, AI developers, and patient advocates for a closed-door workshop in May 2024. The meeting was hosted by HAI’s Healthcare AI Policy Steering Committee—a multidisciplinary committee of Stanford faculty that works to advance policy and research in these areas—to highlight key AI policy gaps and to galvanize support for regulatory changes.
Read a related conversation with HAI Associate Director Curt Langlotz: How Can We Better Regulate Health AI?
Under the Chatham House Rule, participants discussed shortcomings in federal healthcare AI policy in three areas: AI software for clinical decision support, healthcare enterprise AI tools, and patient-facing AI applications. Below, we summarize the key themes, policy considerations, and participant opinions for each regulatory area.
Like Driving a 1976 Chevy Impala on 2024 Roads
Healthcare is one of the most highly regulated industries in the United States. And the industry’s wide-ranging regulatory frameworks are already being applied to AI.
The Food and Drug Administration (FDA) has regulatory responsibility for many software systems primarily through its 510(k) device clearance process, which considers software as a medical device (SaMD) . AI applications used in administrative and clinical enterprise contexts must adhere to rules from the Office of the National Coordinator for Health Information Technology that, for example, mandates algorithmic transparency . The governance of direct-to-consumer health AI tools falls under various consumer product frameworks, although little enforcement has yet occurred in this nascent area.
These regulatory frameworks are outdated. The FDA’s regulatory authority, established in 1976, was designed to regulate hardware devices, not software reliant on training data and requiring meticulous ongoing performance monitoring. Similarly, the Health Insurance Portability and Accountability Act (HIPAA)—a 1996 law that set national standards for the privacy and security of health data—predates the explosion of digital health information. Its provisions did not foresee the need for vast amounts of patient records in order to train machine learning algorithms.
Regulators are effectively driving a Chevy Impala on 2024 roads, struggling to adapt to today’s road conditions, one participant noted. The traditional regulatory paradigms in healthcare urgently need to adapt to a world of rapid AI development. The vast majority of workshop participants believe that a new or substantially changed regulatory framework is necessary for effective healthcare AI governance.
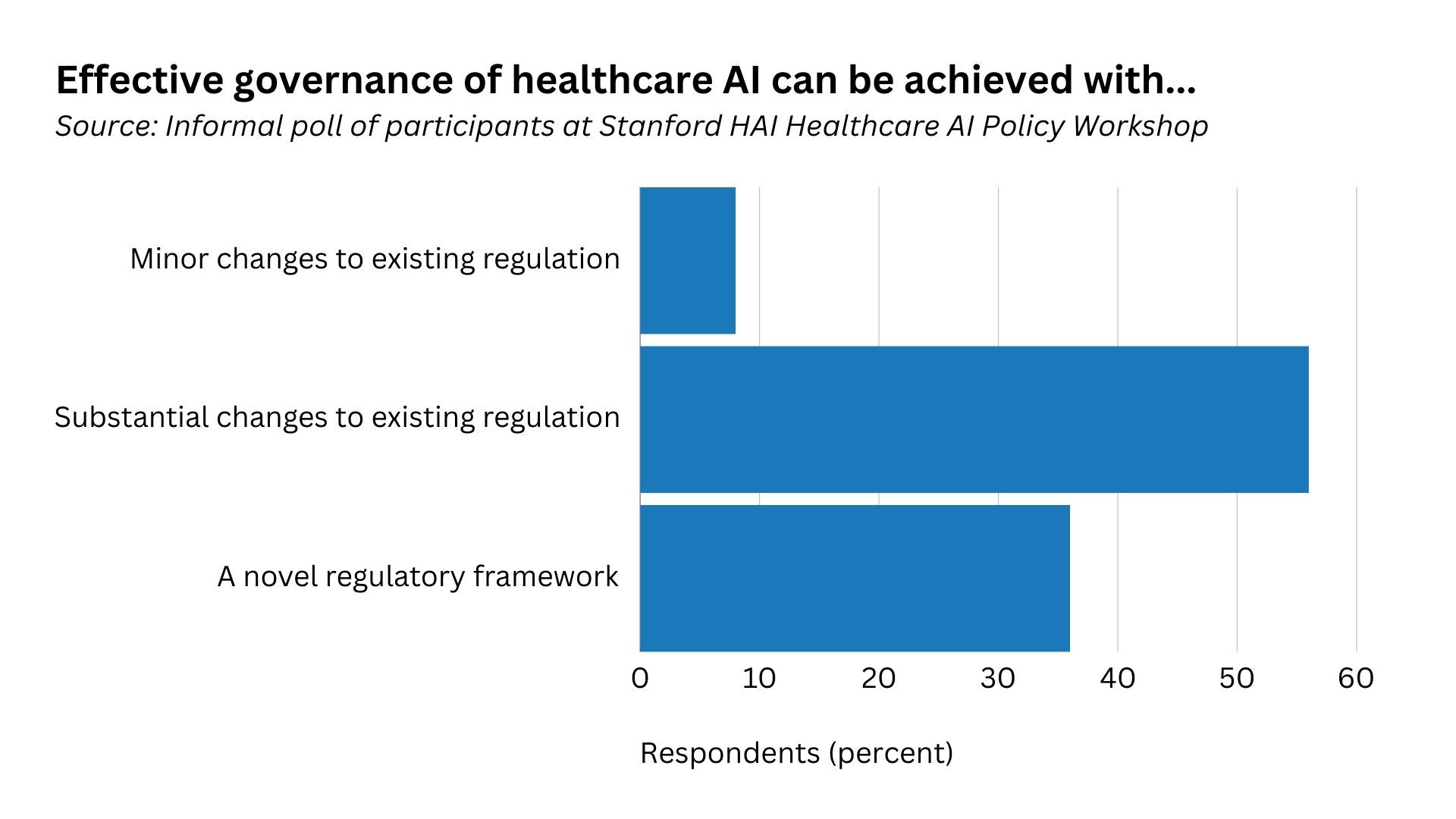
Use Case 1: AI in Software as a Medical Device
Developers of novel AI-powered medical devices with diagnostic capabilities currently face a major challenge: The FDA device clearance process requires them to submit evidence for each individual diagnostic capability. For AI products with hundreds of diagnostic capabilities, such as an algorithm that can detect substantially all abnormalities that might appear on a chest X-ray, submitting each one for regulatory clearance is not commercially feasible. This can result in global software companies bringing to market downgraded, less innovative products and hinder U.S. AI medical device innovation.
Workshop participants proposed new policy approaches to help streamline market approval for these multifunctional software systems while still ensuring clinical safety. First, public-private partnerships will be crucial to managing the evidentiary burden of such approval, with a potential focus on advancing post-market surveillance. Second, participants supported better information sharing during the device clearance process. Sharing details regarding test data and device performance during the clearance process could enable healthcare providers to better assess whether software tools will operate safely in their practices. Although close to 900 medical devices that incorporate AI or machine learning software have been cleared by the FDA, clinical adoption has been slow as healthcare organizations have limited information on which to base purchasing decisions.
Finally, some participants called for more fine-grained risk categories for AI-powered medical devices, the vast majority of which are currently classified as Class II devices with moderate risk. Clinical risk varies greatly between different types of AI/machine learning software devices, necessitating a more tailored approach. For example, an algorithm that measures the dimensions of a blood vessel for later human review is lower risk than an algorithm that triages mammograms to bypass human review.
Use Case 2: AI in Enterprise Clinical Operations and Administration
Should a human always be in the loop when autonomous AI tools are integrated in clinical settings? Fully autonomous AI technologies, such as ones that diagnose eye conditions or auto-report normal chest X-rays, promise to address grave doctor resource shortages. Other forms of automation, such as ambient intelligence technologies that draft responses to patient emails or capture progress notes during doctor-patient interactions, also greatly improve efficiencies in clinical settings.
Some participants argued for human oversight to ensure safety and reliability, while others warned that human-in-the-loop requirements could increase the administrative burden on doctors and make them feel less accountable for resulting clinical decisions. Some identified laboratory testing as a successful hybrid model, where a device is overseen by a physician and undergoes regular quality checks. Any out-of-range values are checked by a human.
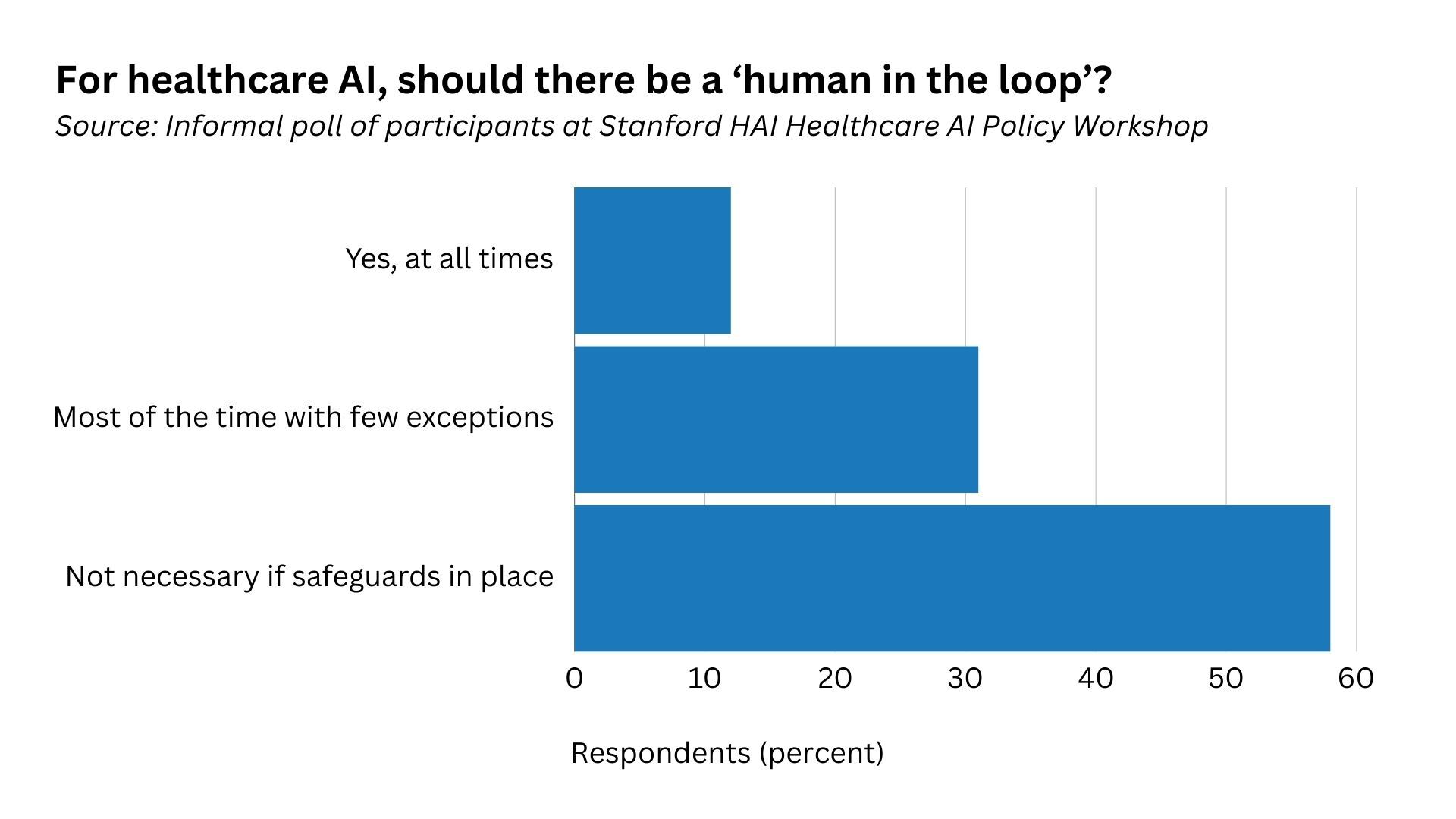
The integration of AI in clinical settings also begs the question of what levels of transparency healthcare providers and patients need to use AI tools safely. What responsibilities do developers have to communicate information about model design, functionality, and risks—for example, through model cards , which are akin to a “nutrition label” healthcare providers can use to make informed decisions about whether to use an AI tool?
Additionally, should patients be told when AI is being used in any stage of their treatment, and, if so, how and when? Patients often delegate decisions about what technology to use—from scalpels to decision support pop-up windows—to their caregivers and the healthcare organizations they work for. And less sophisticated forms of AI are already used throughout the healthcare system, such as rule-based systems that warn of drug-to-drug interactions. Yet many participants felt that in some circumstances, such as an email message that purports to come from a healthcare provider, the patient should be informed that AI played a role.
Use Case 3: Patient-Facing AI Applications
An increasing number of patient-facing applications, such as mental health chatbots based on LLMs , promise to democratize healthcare access or to offer new services to patients through mobile devices. And yet, no targeted guardrails have been put in place to ensure these patient-facing, LLM-powered applications are not giving out harmful or misleading medical information—even or especially when the chatbots claim they do not offer medical advice, despite sharing information in a manner that closely resembles medical advice.
Clarification of the regulatory status of these patient-facing products is urgently needed. Yet workshop participants disagreed over whether generative AI applications, for example, should be governed more like medical devices or medical professionals.
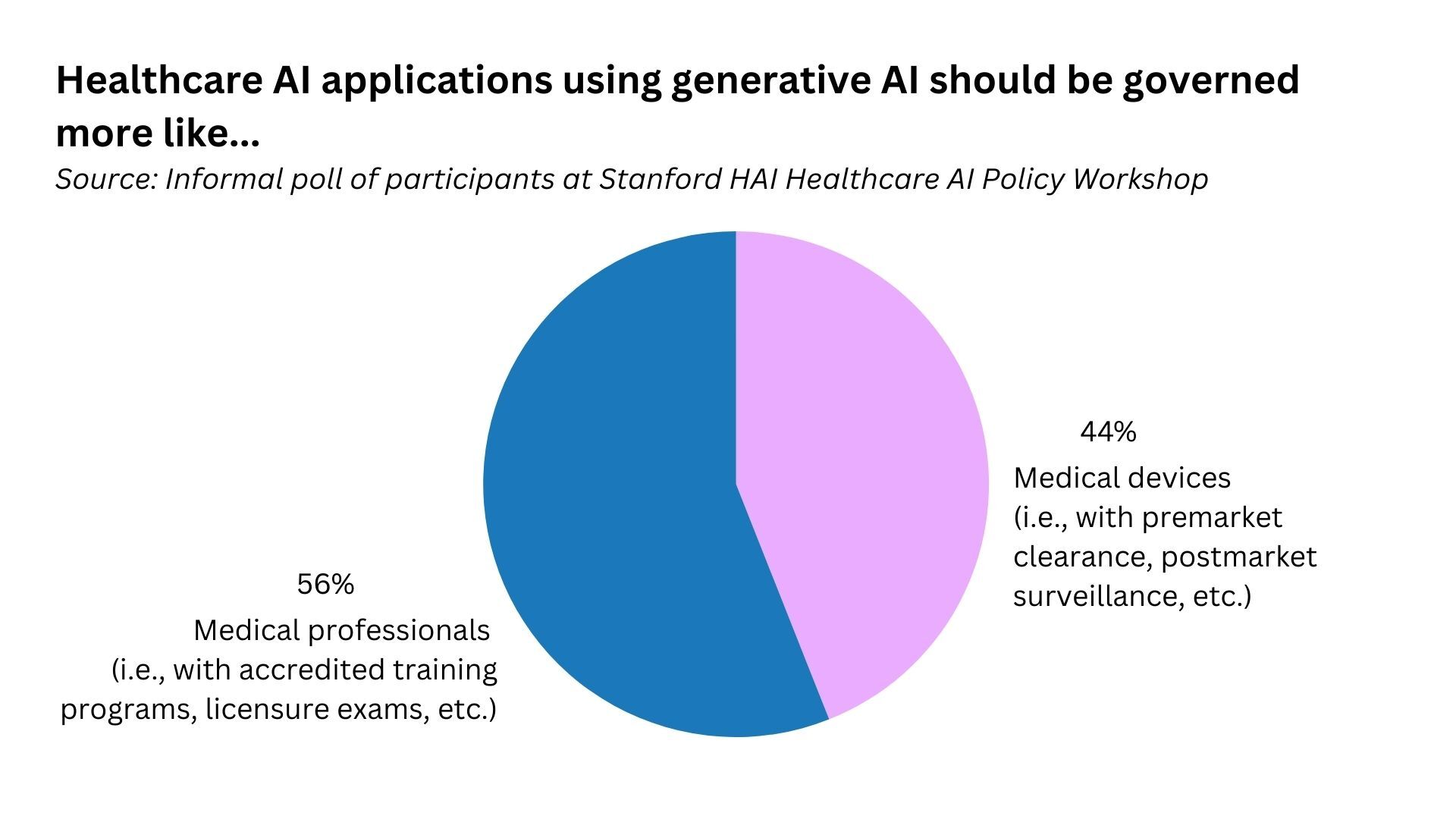
The patient perspective is crucial to ensuring the trustworthiness of healthcare AI applications and the healthcare system more broadly. Many participants noted that patients rarely participate in the development, deployment, or regulation of patient-facing AI applications. The needs and viewpoints of entire patient populations must be considered to ensure regulatory frameworks address health disparities caused or exacerbated by AI.
What Comes Next?
These are only a few of the many questions and concerns surrounding the future of healthcare AI regulation. Much more multidisciplinary research and multistakeholder discussions are needed to answer these questions and develop feasible policy solutions that assure safety while supporting a nimble approach that brings innovative, life-saving AI applications to market. HAI and its Healthcare AI Policy Steering Committee will continue to conduct research into these areas to support policy and regulatory frameworks that lead to the safe, equitable, and effective use of healthcare AI.
Stanford HAI’s mission is to advance AI research, education, policy and practice to improve the human condition. Learn more .
- Health Tech
- Health Insurance
- Medical Devices
- Gene Therapy
- Neuroscience
- H5N1 Bird Flu
- Health Disparities
- Infectious Disease
- Mental Health
- Cardiovascular Disease
- Chronic Disease
- Alzheimer's
- Coercive Care
- The Obesity Revolution
- The War on Recovery
- Adam Feuerstein
- Matthew Herper
- Jennifer Adaeze Okwerekwu
- Ed Silverman
- CRISPR Tracker
- Breakthrough Device Tracker
- Generative AI Tracker
- Obesity Drug Tracker
- 2024 STAT Summit
- Wunderkinds Nomination
- STAT Madness
- STAT Brand Studio
Don't miss out
Subscribe to STAT+ today, for the best life sciences journalism in the industry
Real-time vital signs data: a hidden source of value in medical AI
By Julio La Torre July 18, 2024

I f data represents the next gold rush for health care, a vast treasure trove of it slips away every day. The increased enthusiasm for AI has led to significant investments in novel solutions for health care, with data coming from a variety of sources such as medical charts, imaging, literature, guidelines, and the like. A largely untapped source of valuable data is staring health care practitioners like myself right in the face: monitors that track vital signs.
As an entrepreneur working with the flow of vital sign data for the past couple years, I’m increasingly convinced of its importance. Captured every second by monitors in hospitals, vital signs have immense potential to improve patient care and provide value from AI that investors have been hoping for but have not yet seen. I believe this potential is why BD (Becton, Dickinson and Company) recently acquired Edwards Lifesciences’ critical care unit for $4.2 billion.
advertisement
As a hospitalist, I’ve observed how continuous monitoring, once reserved for patients in intensive care units, is now expanding to include broader swaths of patients. This shift is driven by advancements in hardware, making monitors smaller, more comfortable, and more affordable.
Rather than taking a blood pressure measurement once every four hours or checking oxygen saturation during rounds, continuous monitoring of vital signs offers immediate insights into a patient’s condition. When health starts to deteriorate and the body’s balance is interrupted, changes in vital signs reveal how the body is trying to compensate. Real-time physiological data from monitors recording blood pressure, oxygen saturation, heart rate, and temperature captures detailed patterns and trends that go beyond simple numerical readings: they include complex signals that need to be interpreted before clinical decisions are made. Depending on the setting, such as the operating room or the post-anesthesia care unit, other parameters may also be monitored.
Related: 6 tactics to make artificial intelligence work on the frontlines
These data are now abundant and free-flowing in hospitals, yet underused by practitioners. They are also largely overlooked by researchers and companies working on health care artificial intelligence and machine learning.
The sensors used in hospitals are much more accurate than consumer wearables like Oura or Apple Watch. While those devices have roles to play in personal health, hospital-grade monitors offer the depth of data necessary for clinical decision-making. Real-time physiological data capture the subtleties of patient conditions that no human could detect. With sophisticated modeling, they could identify major problems before they happen. Events such as infections, blood clots, and strokes occur frequently in hospitals, and early detection could make a significant difference.
Almost every outcome I care about as a physician can be correlated to a patient’s vital signs.
Where I think most of the value will be added is from the concept of “always on” clinical trials, a concept I recently heard about from Julie Yoo and Vijay Pande, both general partners at Andreessen Horowitz, on A16z’s excellent Raising Health podcast . “Always on” clinical trials refer to a continuous, real-time infrastructure that allows for on-demand analysis of patient data to identify outcomes retrospectively or prospectively. In every hospital, many organic clinical trials could be happening daily, but countless data points are flashing by unused. For these data to become meaningful, they not only need to be collected and stored appropriately, but also need to be tied to two things: precise timing on intervention and outcomes.
This is where vital sign monitors come in. Not only do they provide a solid source of information for determining outcomes, but the continuous nature of their data collection also makes them the perfect backbone for always-on clinical trials.
Creating value from continuous vital sign monitoring will come from tying real-time physiological data to relevant data points in the medical chart, tailored to specific models and desired outcomes. This approach can pave the way for always-on trials, continuously running and yielding valuable insights. Imagine being able to use AI to sift through vast amounts of data to instantly identify outcomes for specific subsets of patients who were given a particular drug in the hospital. This capability is within reach and represents an exciting frontier for AI in medicine.
Related: Artificial intelligence: crossing the border between health care and tech
The potential for using AI to continuously assess vital sign data is vast, and would represent a fundamental shift in patient care and medical research. Yet there are significant challenges to realizing this potential. Collecting, storing, standardizing, and effectively using this kind of data is a daunting task, as I am now learning through my own company and research. Robust data security measures would have to be put in place to protect patient information. Creating the necessary infrastructure would be one of the hardest challenges, requiring access to monitors and seamless integration with existing hospital systems. Developing a sustainable business model that incentivizes investment and addresses the costs of implementation and maintenance would also be crucial.
Despite these challenges, I believe that the integration of AI and real-time vital sign data in hospital settings holds great promise for creating significant value and improving patient outcomes. Much of this value will be created in hospital care, which accounts for more than 30% of health care expenditures, the largest contributor to costs.
Vital signs have been used to monitor health for more than 2,000 years . Taking advantage of advances in monitoring and data analysis can create new roles for them in predicting — and resolving — health problems.
Julio La Torre, M.D., M.B.A., is a practicing hospitalist physician, a co-founder and CEO of AiroSolve, and a recent graduate of the UCLA Biodesign Accelerator fellowship.
LETTER TO THE EDITOR
Have an opinion on this essay submit a letter to the editor here ., about the author reprints, julio la torre.
Artificial intelligence
STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect
To submit a correction request, please visit our Contact Us page .

Recommended

Recommended Stories

Readers respond to essays on philanthropy and nursing schools, opioid overdoses, and more


The ‘Risky Research Review Act’ would do more harm than good

STAT Plus: Broad Institute, facing end of Microsoft cloud contract, shuffles data science leadership

STAT Plus: A cancer care startup is making a new bet with payers and providers: only pay if it works

STAT Plus: Top FDA officials weighing regulation of ultra-processed foods, internal documents show
- Research article
- Open access
- Published: 10 April 2021
The role of artificial intelligence in healthcare: a structured literature review
- Silvana Secinaro 1 ,
- Davide Calandra 1 ,
- Aurelio Secinaro 2 ,
- Vivek Muthurangu 3 &
- Paolo Biancone 1
BMC Medical Informatics and Decision Making volume 21 , Article number: 125 ( 2021 ) Cite this article
159k Accesses
292 Citations
25 Altmetric
Metrics details
Background/Introduction
Artificial intelligence (AI) in the healthcare sector is receiving attention from researchers and health professionals. Few previous studies have investigated this topic from a multi-disciplinary perspective, including accounting, business and management, decision sciences and health professions.
The structured literature review with its reliable and replicable research protocol allowed the researchers to extract 288 peer-reviewed papers from Scopus. The authors used qualitative and quantitative variables to analyse authors, journals, keywords, and collaboration networks among researchers. Additionally, the paper benefited from the Bibliometrix R software package.
The investigation showed that the literature in this field is emerging. It focuses on health services management, predictive medicine, patient data and diagnostics, and clinical decision-making. The United States, China, and the United Kingdom contributed the highest number of studies. Keyword analysis revealed that AI can support physicians in making a diagnosis, predicting the spread of diseases and customising treatment paths.
Conclusions
The literature reveals several AI applications for health services and a stream of research that has not fully been covered. For instance, AI projects require skills and data quality awareness for data-intensive analysis and knowledge-based management. Insights can help researchers and health professionals understand and address future research on AI in the healthcare field.
Peer Review reports
Artificial intelligence (AI) generally applies to computational technologies that emulate mechanisms assisted by human intelligence, such as thought, deep learning, adaptation, engagement, and sensory understanding [ 1 , 2 ]. Some devices can execute a role that typically involves human interpretation and decision-making [ 3 , 4 ]. These techniques have an interdisciplinary approach and can be applied to different fields, such as medicine and health. AI has been involved in medicine since as early as the 1950s, when physicians made the first attempts to improve their diagnoses using computer-aided programs [ 5 , 6 ]. Interest and advances in medical AI applications have surged in recent years due to the substantially enhanced computing power of modern computers and the vast amount of digital data available for collection and utilisation [ 7 ]. AI is gradually changing medical practice. There are several AI applications in medicine that can be used in a variety of medical fields, such as clinical, diagnostic, rehabilitative, surgical, and predictive practices. Another critical area of medicine where AI is making an impact is clinical decision-making and disease diagnosis. AI technologies can ingest, analyse, and report large volumes of data across different modalities to detect disease and guide clinical decisions [ 3 , 8 ]. AI applications can deal with the vast amount of data produced in medicine and find new information that would otherwise remain hidden in the mass of medical big data [ 9 , 10 , 11 ]. These technologies can also identify new drugs for health services management and patient care treatments [ 5 , 6 ].
Courage in the application of AI is visible through a search in the primary research databases. However, as Meskò et al. [ 7 ] find, the technology will potentially reduce care costs and repetitive operations by focusing the medical profession on critical thinking and clinical creativity. As Cho et al. and Doyle et al. [ 8 , 9 ] add, the AI perspective is exciting; however, new studies will be needed to establish the efficacy and applications of AI in the medical field [ 10 ].
Our paper will also concentrate on AI strategies for healthcare from the accounting, business, and management perspectives. The authors used the structured literature review (SLR) method for its reliable and replicable research protocol [ 11 ] and selected bibliometric variables as sources of investigation. Bibliometric usage enables the recognition of the main quantitative variables of the study stream [ 12 ]. This method facilitates the detection of the required details of a particular research subject, including field authors, number of publications, keywords for interaction between variables (policies, properties and governance) and country data [ 13 ]. It also allows the application of the science mapping technique [ 14 ]. Our paper adopted the Bibliometrix R package and the biblioshiny web interface as tools of analysis [ 14 ].
The investigation offers the following insights for future researchers and practitioners:
bibliometric information on 288 peer-reviewed English papers from the Scopus collection.
Identification of leading journals in this field, such as Journal of Medical Systems, Studies in Health Technology and Informatics, IEEE Journal of Biomedical and Health Informatics, and Decision Support Systems.
Qualitative and quantitative information on authors’ Lotka’s law, h-index, g-index, m-index, keyword, and citation data.
Research on specific countries to assess AI in the delivery and effectiveness of healthcare, quotes, and networks within each region.
A topic dendrogram study that identifies five research clusters: health services management, predictive medicine, patient data, diagnostics, and finally, clinical decision-making.
An in-depth discussion that develops theoretical and practical implications for future studies.
The paper is organised as follows. Section 2 lists the main bibliometric articles in this field. Section 3 elaborates on the methodology. Section 4 presents the findings of the bibliometric analysis. Section 5 discusses the main elements of AI in healthcare based on the study results. Section 6 concludes the article with future implications for research.
Related works and originality
As suggested by Zupic and Čater [ 15 ], a research stream can be evaluated with bibliometric methods that can introduce objectivity and mitigate researcher bias. For this reason, bibliometric methods are attracting increasing interest among researchers as a reliable and impersonal research analytical approach [ 16 , 17 ]. Recently, bibliometrics has been an essential method for analysing and predicting research trends [ 18 ]. Table 1 lists other research that has used a similar approach in the research stream investigated.
The scientific articles reported show substantial differences in keywords and research topics that have been previously studied. The bibliometric analysis of Huang et al. [ 19 ] describes rehabilitative medicine using virtual reality technology. According to the authors, the primary goal of rehabilitation is to enhance and restore functional ability and quality of life for patients with physical impairments or disabilities. In recent years, many healthcare disciplines have been privileged to access various technologies that provide tools for both research and clinical intervention.
Hao et al. [ 20 ] focus on text mining in medical research. As reported, text mining reveals new, previously unknown information by using a computer to automatically extract information from different text resources. Text mining methods can be regarded as an extension of data mining to text data. Text mining is playing an increasingly significant role in processing medical information. Similarly, the studies by dos Santos et al. [ 21 ] focus on applying data mining and machine learning (ML) techniques to public health problems. As stated in this research, public health may be defined as the art and science of preventing diseases, promoting health, and prolonging life. Using data mining and ML techniques, it is possible to discover new information that otherwise would be hidden. These two studies are related to another topic: medical big data. According to Liao et al. [ 22 ], big data is a typical “buzzword” in the business and research community, referring to a great mass of digital data collected from various sources. In the medical field, we can obtain a vast amount of data (i.e., medical big data). Data mining and ML techniques can help deal with this information and provide helpful insights for physicians and patients. More recently, Choudhury et al. [ 23 ] provide a systematic review on the use of ML to improve the care of elderly patients, demonstrating eligible studies primarily in psychological disorders and eye diseases.
Tran et al. [ 2 ] focus on the global evolution of AI research in medicine. Their bibliometric analysis highlights trends and topics related to AI applications and techniques. As stated in Connelly et al.’s [ 24 ] study, robot-assisted surgeries have rapidly increased in recent years. Their bibliometric analysis demonstrates how robotic-assisted surgery has gained acceptance in different medical fields, such as urological, colorectal, cardiothoracic, orthopaedic, maxillofacial and neurosurgery applications. Additionally, the bibliometric analysis of Guo et al. [ 25 ] provides an in-depth study of AI publications through December 2019. The paper focuses on tangible AI health applications, giving researchers an idea of how algorithms can help doctors and nurses. A new stream of research related to AI is also emerging. In this sense, Choudhury and Asan’s [ 26 ] scientific contribution provides a systematic review of the AI literature to identify health risks for patients. They report on 53 studies involving technology for clinical alerts, clinical reports, and drug safety. Considering the considerable interest within this research stream, this analysis differs from the current literature for several reasons. It aims to provide in-depth discussion, considering mainly the business, management, and accounting fields and not dealing only with medical and health profession publications.
Additionally, our analysis aims to provide a bibliometric analysis of variables such as authors, countries, citations and keywords to guide future research perspectives for researchers and practitioners, as similar analyses have done for several publications in other research streams [ 15 , 16 , 27 ]. In doing so, we use a different database, Scopus, that is typically adopted in social sciences fields. Finally, our analysis will propose and discuss a dominant framework of variables in this field, and our analysis will not be limited to AI application descriptions.
Methodology
This paper evaluated AI in healthcare research streams using the SLR method [ 11 ]. As suggested by Massaro et al. [ 11 ], an SLR enables the study of the scientific corpus of a research field, including the scientific rigour, reliability and replicability of operations carried out by researchers. As suggested by many scholars, the methodology allows qualitative and quantitative variables to highlight the best authors, journals and keywords and combine a systematic literature review and bibliometric analysis [ 27 , 28 , 29 , 30 ]. Despite its widespread use in business and management [ 16 , 31 ], the SLR is also used in the health sector based on the same philosophy through which it was originally conceived [ 32 , 33 ]. A methodological analysis of previously published articles reveals that the most frequently used steps are as follows [ 28 , 31 , 34 ]:
defining research questions;
writing the research protocol;
defining the research sample to be analysed;
developing codes for analysis; and
critically analysing, discussing, and identifying a future research agenda.
Considering the above premises, the authors believe that an SLR is the best method because it combines scientific validity, replicability of the research protocol and connection between multiple inputs.
As stated by the methodological paper, the first step is research question identification. For this purpose, we benefit from the analysis of Zupic and Čater [ 15 ], who provide several research questions for future researchers to link the study of authors, journals, keywords and citations. Therefore, RQ1 is “What are the most prominent authors, journal keywords and citations in the field of the research study?” Additionally, as suggested by Haleem et al. [ 35 ], new technologies, including AI, are changing the medical field in unexpected timeframes, requiring studies in multiple areas. Therefore, RQ2 is “How does artificial intelligence relate to healthcare, and what is the focus of the literature?” Then, as discussed by Massaro et al. [ 36 ], RQ3 is “What are the research applications of artificial intelligence for healthcare?”.
The first research question aims to define the qualitative and quantitative variables of the knowledge flow under investigation. The second research question seeks to determine the state of the art and applications of AI in healthcare. Finally, the third research question aims to help researchers identify practical and theoretical implications and future research ideas in this field.
The second fundamental step of the SLR is writing the research protocol [ 11 ]. Table 2 indicates the currently known literature elements, uniquely identifying the research focus, motivations and research strategy adopted and the results providing a link with the following points. Additionally, to strengthen the analysis, our investigation benefits from the PRISMA statement methodological article [ 37 ]. Although the SLR is a validated method for systematic reviews and meta-analyses, we believe that the workflow provided may benefit the replicability of the results [ 37 , 38 , 39 , 40 ]. Figure 1 summarises the researchers’ research steps, indicating that there are no results that can be referred to as a meta-analysis.
Source : Authors’ elaboration on Liberati et al. [ 37 ]
PRISMA workflow.
The third step is to specify the search strategy and search database. Our analysis is based on the search string “Artificial Intelligence” OR “AI” AND “Healthcare” with a focus on “Business, Management, and Accounting”, “Decision Sciences”, and “Health professions”. As suggested by [ 11 , 41 ] and motivated by [ 42 ], keywords can be selected through a top-down approach by identifying a large search field and then focusing on particular sub-topics. The paper uses data retrieved from the Scopus database, a multi-disciplinary database, which allowed the researchers to identify critical articles for scientific analysis [ 43 ]. Additionally, Scopus was selected based on Guo et al.’s [ 25 ] limitations, which suggest that “future studies will apply other databases, such as Scopus, to explore more potential papers” . The research focuses on articles and reviews published in peer-reviewed journals for their scientific relevance [ 11 , 16 , 17 , 29 ] and does not include the grey literature, conference proceedings or books/book chapters. Articles written in any language other than English were excluded [ 2 ]. For transparency and replicability, the analysis was conducted on 11 January 2021. Using this research strategy, the authors retrieved 288 articles. To strengthen the study's reliability, we publicly provide the full bibliometric extract on the Zenodo repository [ 44 , 45 ].
The fourth research phase is defining the code framework that initiates the analysis of the variables. The study will identify the following:
descriptive information of the research area;
source analysis [ 16 ];
author and citation analysis [ 28 ];
keywords and network analysis [ 14 ]; and
geographic distribution of the papers [ 14 ].
The final research phase is the article’s discussion and conclusion, where implications and future research trends will be identified.
At the research team level, the information is analysed with the statistical software R-Studio and the Bibliometrix package [ 15 ], which allows scientific analysis of the results obtained through the multi-disciplinary database.
The analysis of bibliometric results starts with a description of the main bibliometric statistics with the aim of answering RQ1, What are the most prominent authors, journal keywords and citations in the field of the research study?, and RQ2, How does artificial intelligence relate to healthcare, and what is the focus of the literature? Therefore, the following elements were thoroughly analysed: (1) type of document; (2) annual scientific production; (3) scientific sources; (4) source growth; (5) number of articles per author; (6) author’s dominance ranking; (7) author’s h-index, g-index, and m-index; (8) author’s productivity; (9) author’s keywords; (10) topic dendrogram; (11) a factorial map of the document with the highest contributions; (12) article citations; (13) country production; (14) country citations; (15) country collaboration map; and (16) country collaboration network.
Main information
Table 3 shows the information on 288 peer-reviewed articles published between 1992 and January 2021 extracted from the Scopus database. The number of keywords is 946 from 136 sources, and the number of keywords plus, referring to the number of keywords that frequently appear in an article’s title, was 2329. The analysis period covered 28 years and 1 month of scientific production and included an annual growth rate of 5.12%. However, the most significant increase in published articles occurred in the past three years (please see Fig. 2 ). On average, each article was written by three authors (3.56). Finally, the collaboration index (CI), which was calculated as the total number of authors of multi-authored articles/total number of multi-authored articles, was 3.97 [ 46 ].
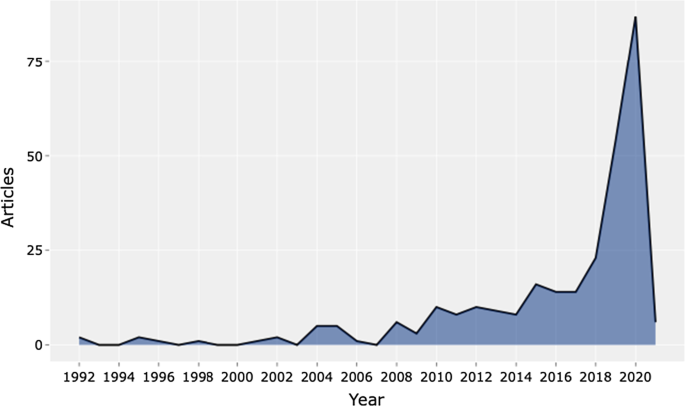
Source : Authors’ elaboration
Annual scientific production.
Table 4 shows the top 20 sources related to the topic. The Journal of Medical Systems is the most relevant source, with twenty-one of the published articles. This journal's main issues are the foundations, functionality, interfaces, implementation, impacts, and evaluation of medical technologies. Another relevant source is Studies in Health Technology and Informatics, with eleven articles. This journal aims to extend scientific knowledge related to biomedical technologies and medical informatics research. Both journals deal with cloud computing, machine learning, and AI as a disruptive healthcare paradigm based on recent publications. The IEEE Journal of Biomedical and Health Informatics investigates technologies in health care, life sciences, and biomedicine applications from a broad perspective. The next journal, Decision Support Systems, aims to analyse how these technologies support decision-making from a multi-disciplinary view, considering business and management. Therefore, the analysis of the journals revealed that we are dealing with an interdisciplinary research field. This conclusion is confirmed, for example, by the presence of purely medical journals, journals dedicated to the technological growth of healthcare, and journals with a long-term perspective such as futures.
The distribution frequency of the articles (Fig. 3 ) indicates the journals dealing with the topic and related issues. Between 2008 and 2012, a significant growth in the number of publications on the subject is noticeable. However, the graph shows the results of the Loess regression, which includes the quantity and publication time of the journal under analysis as variables. This method allows the function to assume an unlimited distribution; that is, feature can consider values below zero if the data are close to zero. It contributes to a better visual result and highlights the discontinuity in the publication periods [ 47 ].
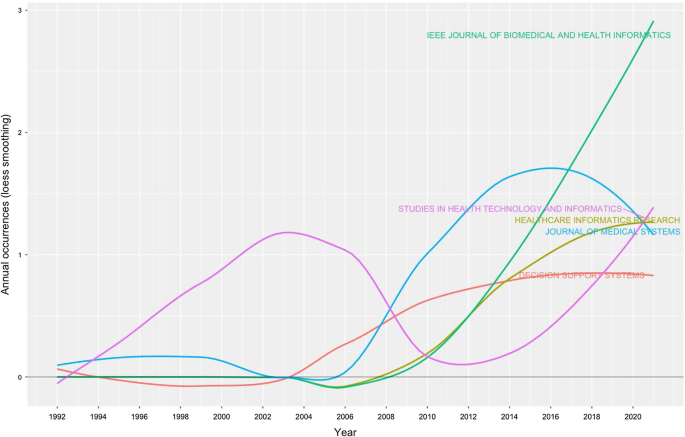
Source growth. Source : Authors’ elaboration
Finally, Fig. 4 provides an analytical perspective on factor analysis for the most cited papers. As indicated in the literature [ 48 , 49 ], using factor analysis to discover the most cited papers allows for a better understanding of the scientific world’s intellectual structure. For example, our research makes it possible to consider certain publications that effectively analyse subject specialisation. For instance, Santosh’s [ 50 ] article addresses the new paradigm of AI with ML algorithms for data analysis and decision support in the COVID-19 period, setting a benchmark in terms of citations by researchers. Moving on to the application, an article by Shickel et al. [ 51 ] begins with the belief that the healthcare world currently has much health and administrative data. In this context, AI and deep learning will support medical and administrative staff in extracting data, predicting outcomes, and learning medical representations. Finally, in the same line of research, Baig et al. [ 52 ], with a focus on wearable patient monitoring systems (WPMs), conclude that AI and deep learning may be landmarks for continuous patient monitoring and support for healthcare delivery.
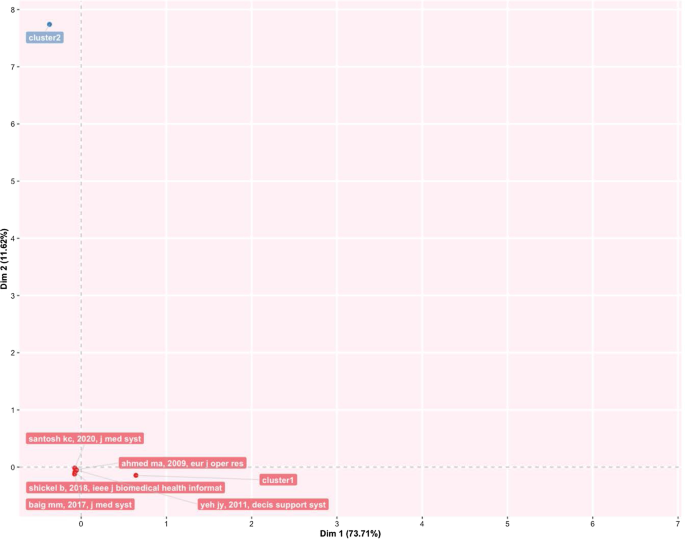
Factorial map of the most cited documents.
This section identifies the most cited authors of articles on AI in healthcare. It also identifies the authors’ keywords, dominance factor (DF) ranking, h-index, productivity, and total number of citations. Table 5 identifies the authors and their publications in the top 20 rankings. As the table shows, Bushko R.G. has the highest number of publications: four papers. He is the editor-in-chief of Future of Health Technology, a scientific journal that aims to develop a clear vision of the future of health technology. Then, several authors each wrote three papers. For instance, Liu C. is a researcher active in the topic of ML and computer vision, and Sharma A. from Emory University Atlanta in the USA is a researcher with a clear focus on imaging and translational informatics. Some other authors have two publications each. While some authors have published as primary authors, most have published as co-authors. Hence, in the next section, we measure the contributory power of each author by investigating the DF ranking through the number of elements.
Authors’ dominance ranking
The dominance factor (DF) is a ratio measuring the fraction of multi-authored articles in which an author acts as the first author [ 53 ]. Several bibliometric studies use the DF in their analyses [ 46 , 54 ]. The DF ranking calculates an author’s dominance in producing articles. The DF is calculated by dividing the number of an author’s multi-authored papers as the first author (Nmf) by the author's total number of multi-authored papers (Nmt). This is omitted in the single-author case due to the constant value of 1 for single-authored articles. This formulation could lead to some distortions in the results, especially in fields where the first author is entered by surname alphabetical order [ 55 ].
The mathematical equation for the DF is shown as:
Table 6 lists the top 20 DF rankings. The data in the table show a low level of articles per author, either for first-authored or multi-authored articles. The results demonstrate that we are dealing with an emerging topic in the literature. Additionally, as shown in the table, Fox J. and Longoni C. are the most dominant authors in the field.
Authors’ impact
Table 7 shows the impact of authors in terms of the h-index [ 56 ] (i.e., the productivity and impact of citations of a researcher), g-index [ 57 ] (i.e., the distribution of citations received by a researcher's publications), m-index [ 58 ] (i.e., the h-index value per year), total citations, total paper and years of scientific publication. The H-index was introduced in the literature as a metric for the objective comparison of scientific results and depended on the number of publications and their impact [ 59 ]. The results show that the 20 most relevant authors have an h-index between 2 and 1. For the practical interpretation of the data, the authors considered data published by the London School of Economics [ 60 ]. In the social sciences, the analysis shows values of 7.6 for economic publications by professors and researchers who had been active for several years. Therefore, the youthfulness of the research area has attracted young researchers and professors. At the same time, new indicators have emerged over the years to diversify the logic of the h-index. For example, the g-index indicates an author's impact on citations, considering that a single article can generate these. The m-index, on the other hand, shows the cumulative value over the years.
The analysis, also considering the total number of citations, the number of papers published and the year of starting to publish, thus confirms that we are facing an expanding research flow.
Authors’ productivity
Figure 5 shows Lotka’s law. This mathematical formulation originated in 1926 to describe the publication frequency by authors in a specific research field [ 61 ]. In practice, the law states that the number of authors contributing to research in a given period is a fraction of the number who make up a single contribution [ 14 , 61 ].
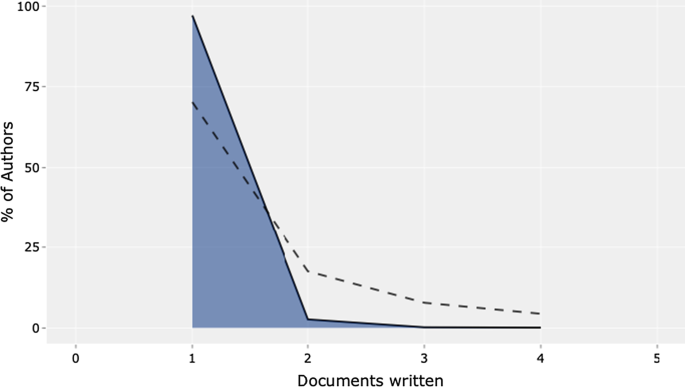
Lotka’s law.
The mathematical relationship is expressed in reverse in the following way:
where y x is equal to the number of authors producing x articles in each research field. Therefore, C and n are constants that can be estimated in the calculation.
The figure's results are in line with Lotka's results, with an average of two publications per author in a given research field. In addition, the figure shows the percentage of authors. Our results lead us to state that we are dealing with a young and growing research field, even with this analysis. Approximately 70% of the authors had published only their first research article. Only approximately 20% had published two scientific papers.
Authors’ keywords
This section provides information on the relationship between the keywords artificial intelligence and healthcare . This analysis is essential to determine the research trend, identify gaps in the discussion on AI in healthcare, and identify the fields that can be interesting as research areas [ 42 , 62 ].
Table 8 highlights the total number of keywords per author in the top 20 positions. The ranking is based on the following elements: healthcare, artificial intelligence, and clinical decision support system . Keyword analysis confirms the scientific area of reference. In particular, we deduce the definition as “Artificial intelligence is the theory and development of computer systems able to perform tasks normally requiring human intelligence, such as visual perception, speech recognition, decision-making, and translation between languages” [ 2 , 63 ]. Panch et al. [ 4 ] find that these technologies can be used in different business and management areas. After the first keyword, the analysis reveals AI applications and related research such as machine learning and deep learning.
Additionally, data mining and big data are a step forward in implementing exciting AI applications. According to our specific interest, if we applied AI in healthcare, we would achieve technological applications to help and support doctors and medical researchers in decision-making. The link between AI and decision-making is the reason why we find, in the seventh position, the keyword clinical decision support system . AI techniques can unlock clinically relevant information hidden in the massive amount of data that can assist clinical decision-making [ 64 ]. If we analyse the following keywords, we find other elements related to decision-making and support systems.
The TreeMap below (Fig. 6 ) highlights the combination of possible keywords representing AI and healthcare.
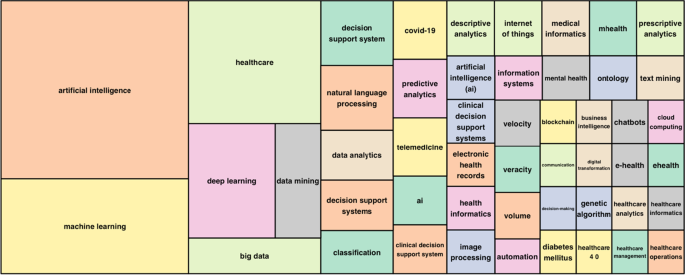
Keywords treemap.
The topic dendrogram in Fig. 7 represents the hierarchical order and the relationship between the keywords generated by hierarchical clustering [ 42 ]. The cut in the figure and the vertical lines facilitate an investigation and interpretation of the different clusters. As stated by Andrews [ 48 ], the figure is not intended to find the perfect level of associations between clusters. However, it aims to estimate the approximate number of clusters to facilitate further discussion.
Topic dendrogram.
The research stream of AI in healthcare is divided into two main strands. The blue strand focuses on medical information systems and the internet. Some papers are related to healthcare organisations, such as the Internet of Things, meaning that healthcare organisations use AI to support health services management and data analysis. AI applications are also used to improve diagnostic and therapeutic accuracy and the overall clinical treatment process [ 2 ]. If we consider the second block, the red one, three different clusters highlight separate aspects of the topic. The first could be explained as AI and ML predictive algorithms. Through AI applications, it is possible to obtain a predictive approach that can ensure that patients are better monitored. This also allows a better understanding of risk perception for doctors and medical researchers. In the second cluster, the most frequent words are decisions , information system , and support system . This means that AI applications can support doctors and medical researchers in decision-making. Information coming from AI technologies can be used to consider difficult problems and support a more straightforward and rapid decision-making process. In the third cluster, it is vital to highlight that the ML model can deal with vast amounts of data. From those inputs, it can return outcomes that can optimise the work of healthcare organisations and scheduling of medical activities.
Furthermore, the word cloud in Fig. 8 highlights aspects of AI in healthcare, such as decision support systems, decision-making, health services management, learning systems, ML techniques and diseases. The figure depicts how AI is linked to healthcare and how it is used in medicine.

Word cloud.
Figure 9 represents the search trends based on the keywords analysed. The research started in 2012. First, it identified research topics related to clinical decision support systems. This topic was recurrent during the following years. Interestingly, in 2018, studies investigated AI and natural language processes as possible tools to manage patients and administrative elements. Finally, a new research stream considers AI's role in fighting COVID-19 [ 65 , 66 ].
Keywords frequency.
Table 9 represents the number of citations from other articles within the top 20 rankings. The analysis allows the benchmark studies in the field to be identified [ 48 ]. For instance, Burke et al. [ 67 ] writes the most cited paper and analyses efficient nurse rostering methodologies. The paper critically evaluates tangible interdisciplinary solutions that also include AI. Immediately thereafter, Ahmed M.A.'s article proposes a data-driven optimisation methodology to determine the optimal number of healthcare staff to optimise patients' productivity [ 68 ]. Finally, the third most cited article lays the groundwork for developing deep learning by considering diverse health and administrative information [ 51 ].
This section analyses the diffusion of AI in healthcare around the world. It highlights countries to show the geographies of this research. It includes all published articles, the total number of citations, and the collaboration network. The following sub-sections start with an analysis of the total number of published articles.
Country total articles
Figure 9 and Table 10 display the countries where AI in healthcare has been considered. The USA tops the list of countries with the maximum number of articles on the topic (215). It is followed by China (83), the UK (54), India (51), Australia (54), and Canada (32). It is immediately evident that the theme has developed on different continents, highlighting a growing interest in AI in healthcare. The figure shows that many areas, such as Russia, Eastern Europe and Africa except for Algeria, Egypt, and Morocco, have still not engaged in this scientific debate.
Country publications and collaboration map
This section discusses articles on AI in healthcare in terms of single or multiple publications in each country. It also aims to observe collaboration and networking between countries. Table 11 and Fig. 10 highlight the average citations by state and show that the UK, the USA, and Kuwait have a higher average number of citations than other countries. Italy, Spain and New Zealand have the most significant number of citations.
Articles per country.
Figure 11 depicts global collaborations. The blue colour on the map represents research cooperation among nations. Additionally, the pink border linking states indicates the extent of collaboration between authors. The primary cooperation between nations is between the USA and China, with two collaborative articles. Other collaborations among nations are limited to a few papers.
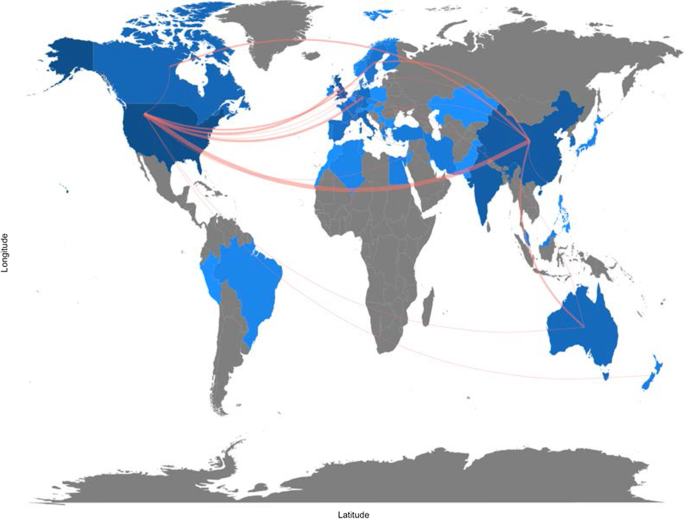
Collaboration map.
Artificial intelligence for healthcare: applications
This section aims to strengthen the research scope by answering RQ3: What are the research applications of artificial intelligence for healthcare?
Benefiting from the topical dendrogram, researchers will provide a development model based on four relevant variables [ 69 , 70 ]. AI has been a disruptive innovation in healthcare [ 4 ]. With its sophisticated algorithms and several applications, AI has assisted doctors and medical professionals in the domains of health information systems, geocoding health data, epidemic and syndromic surveillance, predictive modelling and decision support, and medical imaging [ 2 , 9 , 10 , 64 ]. Furthermore, the researchers considered the bibliometric analysis to identify four macro-variables dominant in the field and used them as authors' keywords. Therefore, the following sub-sections aim to explain the debate on applications in healthcare for AI techniques. These elements are shown in Fig. 12 .
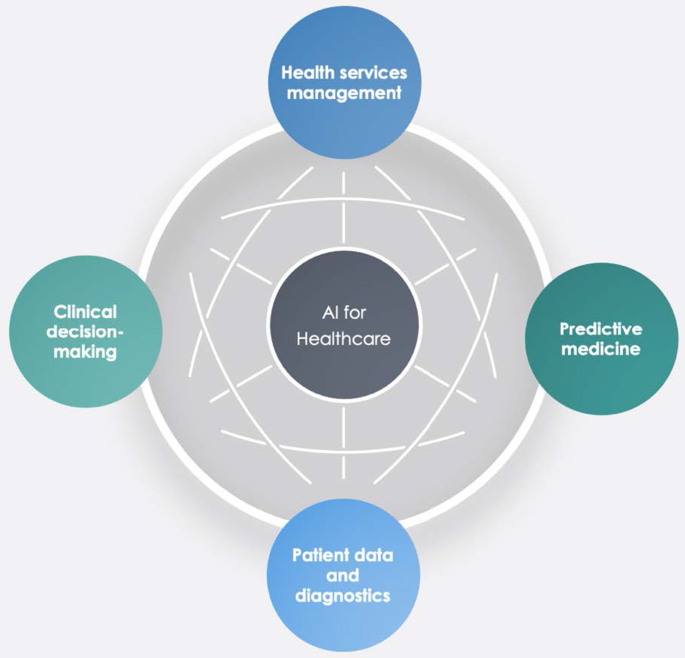
Dominant variables for AI in healthcare.
Health services management
One of the notable aspects of AI techniques is potential support for comprehensive health services management. These applications can support doctors, nurses and administrators in their work. For instance, an AI system can provide health professionals with constant, possibly real-time medical information updates from various sources, including journals, textbooks, and clinical practices [ 2 , 10 ]. These applications' strength is becoming even more critical in the COVID-19 period, during which information exchange is continually needed to properly manage the pandemic worldwide [ 71 ]. Other applications involve coordinating information tools for patients and enabling appropriate inferences for health risk alerts and health outcome prediction [ 72 ]. AI applications allow, for example, hospitals and all health services to work more efficiently for the following reasons:
Clinicians can access data immediately when they need it.
Nurses can ensure better patient safety while administering medication.
Patients can stay informed and engaged in their care by communicating with their medical teams during hospital stays.
Additionally, AI can contribute to optimising logistics processes, for instance, realising drugs and equipment in a just-in-time supply system based totally on predictive algorithms [ 73 , 74 ]. Interesting applications can also support the training of personnel working in health services. This evidence could be helpful in bridging the gap between urban and rural health services [ 75 ]. Finally, health services management could benefit from AI to leverage the multiplicity of data in electronic health records by predicting data heterogeneity across hospitals and outpatient clinics, checking for outliers, performing clinical tests on the data, unifying patient representation, improving future models that can predict diagnostic tests and analyses, and creating transparency with benchmark data for analysing services delivered [ 51 , 76 ].
Predictive medicine
Another relevant topic is AI applications for disease prediction and diagnosis treatment, outcome prediction and prognosis evaluation [ 72 , 77 ]. Because AI can identify meaningful relationships in raw data, it can support diagnostic, treatment and prediction outcomes in many medical situations [ 64 ]. It allows medical professionals to embrace the proactive management of disease onset. Additionally, predictions are possible for identifying risk factors and drivers for each patient to help target healthcare interventions for better outcomes [ 3 ]. AI techniques can also help design and develop new drugs, monitor patients and personalise patient treatment plans [ 78 ]. Doctors benefit from having more time and concise data to make better patient decisions. Automatic learning through AI could disrupt medicine, allowing prediction models to be created for drugs and exams that monitor patients over their whole lives [ 79 ].
- Clinical decision-making
One of the keyword analysis main topics is that AI applications could support doctors and medical researchers in the clinical decision-making process. According to Jiang et al. [ 64 ], AI can help physicians make better clinical decisions or even replace human judgement in healthcare-specific functional areas. According to Bennett and Hauser [ 80 ], algorithms can benefit clinical decisions by accelerating the process and the amount of care provided, positively impacting the cost of health services. Therefore, AI technologies can support medical professionals in their activities and simplify their jobs [ 4 ]. Finally, as Redondo and Sandoval [ 81 ] find, algorithmic platforms can provide virtual assistance to help doctors understand the semantics of language and learning to solve business process queries as a human being would.
Patient data and diagnostics
Another challenging topic related to AI applications is patient data and diagnostics. AI techniques can help medical researchers deal with the vast amount of data from patients (i.e., medical big data ). AI systems can manage data generated from clinical activities, such as screening, diagnosis, and treatment assignment. In this way, health personnel can learn similar subjects and associations between subject features and outcomes of interest [ 64 ].
These technologies can analyse raw data and provide helpful insights that can be used in patient treatments. They can help doctors in the diagnostic process; for example, to realise a high-speed body scan, it will be simpler to have an overall patient condition image. Then, AI technology can recreate a 3D mapping solution of a patient’s body.
In terms of data, interesting research perspectives are emerging. For instance, we observed the emergence of a stream of research on patient data management and protection related to AI applications [ 82 ].
For diagnostics, AI techniques can make a difference in rehabilitation therapy and surgery. Numerous robots have been designed to support and manage such tasks. Rehabilitation robots physically support and guide, for example, a patient’s limb during motor therapy [ 83 ]. For surgery, AI has a vast opportunity to transform surgical robotics through devices that can perform semi-automated surgical tasks with increasing efficiency. The final aim of this technology is to automate procedures to negate human error while maintaining a high level of accuracy and precision [ 84 ]. Finally, the -19 period has led to increased remote patient diagnostics through telemedicine that enables remote observation of patients and provides physicians and nurses with support tools [ 66 , 85 , 86 ].
This study aims to provide a bibliometric analysis of publications on AI in healthcare, focusing on accounting, business and management, decision sciences and health profession studies. Using the SLR method of Massaro et al. [ 11 ], we provide a reliable and replicable research protocol for future studies in this field. Additionally, we investigate the trend of scientific publications on the subject, unexplored information, future directions, and implications using the science mapping workflow. Our analysis provides interesting insights.
In terms of bibliometric variables, the four leading journals, Journal of Medical Systems , Studies in Health Technology and Informatics , IEEE Journal of Biomedical and Health Informatics , and Decision Support Systems , are optimal locations for the publication of scientific articles on this topic. These journals deal mainly with healthcare, medical information systems, and applications such as cloud computing, machine learning, and AI. Additionally, in terms of h-index, Bushko R.G. and Liu C. are the most productive and impactful authors in this research stream. Burke et al.’s [ 67 ] contribution is the most cited with an analysis of nurse rostering using new technologies such as AI. Finally, in terms of keywords, co-occurrence reveals some interesting insights. For instance, researchers have found that AI has a role in diagnostic accuracy and helps in the analysis of health data by comparing thousands of medical records, experiencing automatic learning with clinical alerts, efficient management of health services and places of care, and the possibility of reconstructing patient history using these data.
Second, this paper finds five cluster analyses in healthcare applications: health services management, predictive medicine, patient data, diagnostics, and finally, clinical decision-making. These technologies can also contribute to optimising logistics processes in health services and allowing a better allocation of resources.
Third, the authors analysing the research findings and the issues under discussion strongly support AI's role in decision support. These applications, however, are demonstrated by creating a direct link to data quality management and the technology awareness of health personnel [ 87 ].
The importance of data quality for the decision-making process
Several authors have analysed AI in the healthcare research stream, but in this case, the authors focus on other literature that includes business and decision-making processes. In this regard, the analysis of the search flow reveals a double view of the literature. On the one hand, some contributions belong to the positivist literature and embrace future applications and implications of technology for health service management, data analysis and diagnostics [ 6 , 80 , 88 ]. On the other hand, some investigations also aim to understand the darker sides of technology and its impact. For example, as Carter [ 89 ] states, the impact of AI is multi-sectoral; its development, however, calls for action to protect personal data. Similarly, Davenport and Kalakota [ 77 ] focus on the ethical implications of using AI in healthcare. According to the authors, intelligent machines raise issues of accountability, transparency, and permission, especially in automated communication with patients. Our analysis does not indicate a marked strand of the literature; therefore, we argue that the discussion of elements such as the transparency of technology for patients is essential for the development of AI applications.
A large part of our results shows that, at the application level, AI can be used to improve medical support for patients (Fig. 11 ) [ 64 , 82 ]. However, we believe that, as indicated by Kalis et al. [ 90 ] on the pages of Harvard Business Review, the management of costly back-office problems should also be addressed.
The potential of algorithms includes data analysis. There is an immense quantity of data accessible now, which carries the possibility of providing information about a wide variety of medical and healthcare activities [ 91 ]. With the advent of modern computational methods, computer learning and AI techniques, there are numerous possibilities [ 79 , 83 , 84 ]. For example, AI makes it easier to turn data into concrete and actionable observations to improve decision-making, deliver high-quality patient treatment, adapt to real-time emergencies, and save more lives on the clinical front. In addition, AI makes it easier to leverage capital to develop systems and facilities and reduce expenses at the organisational level [ 78 ]. Studying contributions to the topic, we noticed that data accuracy was included in the debate, indicating that a high standard of data will benefit decision-making practitioners [ 38 , 77 ]. AI techniques are an essential instrument for studying data and the extraction of medical insight, and they may assist medical researchers in their practices. Using computational tools, healthcare stakeholders may leverage the power of data not only to evaluate past data ( descriptive analytics ) but also to forecast potential outcomes ( predictive analytics ) and to define the best actions for the present scenario ( prescriptive analytics ) [ 78 ]. The current abundance of evidence makes it easier to provide a broad view of patient health; doctors should have access to the correct details at the right time and location to provide the proper treatment [ 92 ].
Will medical technology de-skill doctors?
Further reflection concerns the skills of doctors. Studies have shown that healthcare personnel are progressively being exposed to technology for different purposes, such as collecting patient records or diagnosis [ 71 ]. This is demonstrated by the keywords (Fig. 6 ) that focus on technology and the role of decision-making with new innovative tools. In addition, the discussion expands with Lu [ 93 ], which indicates that the excessive use of technology could hinder doctors’ skills and clinical procedures' expansion. Among the main issues arising from the literature is the possible de-skilling of healthcare staff due to reduced autonomy in decision-making concerning patients [ 94 ]. Therefore, the challenges and discussion we uncovered in Fig. 11 are expanded by also considering the ethical implications of technology and the role of skills.
Implications
Our analysis also has multiple theoretical and practical implications.
In terms of theoretical contribution, this paper extends the previous results of Connelly et al., dos Santos et al, Hao et al., Huang et al., Liao et al. and Tran et al. [ 2 , 19 , 20 , 21 , 22 , 24 ] in considering AI in terms of clinical decision-making and data management quality.
In terms of practical implications, this paper aims to create a fruitful discussion with healthcare professionals and administrative staff on how AI can be at their service to increase work quality. Furthermore, this investigation offers a broad comprehension of bibliometric variables of AI techniques in healthcare. It can contribute to advancing scientific research in this field.
Limitations
Like any other, our study has some limitations that could be addressed by more in-depth future studies. For example, using only one research database, such as Scopus, could be limiting. Further analysis could also investigate the PubMed, IEEE, and Web of Science databases individually and holistically, especially the health parts. Then, the use of search terms such as "Artificial Intelligence" OR "AI" AND "Healthcare" could be too general and exclude interesting studies. Moreover, although we analysed 288 peer-reviewed scientific papers, because the new research topic is new, the analysis of conference papers could return interesting results for future researchers. Additionally, as this is a young research area, the analysis will be subject to recurrent obsolescence as multiple new research investigations are published. Finally, although bibliometric analysis has limited the subjectivity of the analysis [ 15 ], the verification of recurring themes could lead to different results by indicating areas of significant interest not listed here.
Future research avenues
Concerning future research perspectives, researchers believe that an analysis of the overall amount that a healthcare organisation should pay for AI technologies could be helpful. If these technologies are essential for health services management and patient treatment, governments should invest and contribute to healthcare organisations' modernisation. New investment funds could be made available in the healthcare world, as in the European case with the Next Generation EU programme or national investment programmes [ 95 ]. Additionally, this should happen especially in the poorest countries around the world, where there is a lack of infrastructure and services related to health and medicine [ 96 ]. On the other hand, it might be interesting to evaluate additional profits generated by healthcare organisations with AI technologies compared to those that do not use such technologies.
Further analysis could also identify why some parts of the world have not conducted studies in this area. It would be helpful to carry out a comparative analysis between countries active in this research field and countries that are not currently involved. It would make it possible to identify variables affecting AI technologies' presence or absence in healthcare organisations. The results of collaboration between countries also present future researchers with the challenge of greater exchanges between researchers and professionals. Therefore, further research could investigate the difference in vision between professionals and academics.
In the accounting, business, and management research area, there is currently a lack of quantitative analysis of the costs and profits generated by healthcare organisations that use AI technologies. Therefore, research in this direction could further increase our understanding of the topic and the number of healthcare organisations that can access technologies based on AI. Finally, as suggested in the discussion section, more interdisciplinary studies are needed to strengthen AI links with data quality management and AI and ethics considerations in healthcare.
In pursuing the philosophy of Massaro et al.’s [ 11 ] methodological article, we have climbed on the shoulders of giants, hoping to provide a bird's-eye view of the AI literature in healthcare. We performed this study with a bibliometric analysis aimed at discovering authors, countries of publication and collaboration, and keywords and themes. We found a fast-growing, multi-disciplinary stream of research that is attracting an increasing number of authors.
The research, therefore, adopts a quantitative approach to the analysis of bibliometric variables and a qualitative approach to the study of recurring keywords, which has allowed us to demonstrate strands of literature that are not purely positive. There are currently some limitations that will affect future research potential, especially in ethics, data governance and the competencies of the health workforce.
Availability of data and materials
All the data are retrieved from public scientific platforms.
Tagliaferri SD, Angelova M, Zhao X, Owen PJ, Miller CT, Wilkin T, et al. Artificial intelligence to improve back pain outcomes and lessons learnt from clinical classification approaches: three systematic reviews. NPJ Digit Med. 2020;3(1):1–16.
Article Google Scholar
Tran BX, Vu GT, Ha GH, Vuong Q-H, Ho M-T, Vuong T-T, et al. Global evolution of research in artificial intelligence in health and medicine: a bibliometric study. J Clin Med. 2019;8(3):360.
Article PubMed Central Google Scholar
Hamid S. The opportunities and risks of artificial intelligence in medicine and healthcare [Internet]. 2016 [cited 2020 May 29]. http://www.cuspe.org/wp-content/uploads/2016/09/Hamid_2016.pdf
Panch T, Szolovits P, Atun R. Artificial intelligence, machine learning and health systems. J Glob Health. 2018;8(2):020303.
Article PubMed PubMed Central Google Scholar
Yang X, Wang Y, Byrne R, Schneider G, Yang S. Concepts of artificial intelligence for computer-assisted drug discovery | chemical reviews. Chem Rev. 2019;119(18):10520–94.
Article CAS PubMed Google Scholar
Burton RJ, Albur M, Eberl M, Cuff SM. Using artificial intelligence to reduce diagnostic workload without compromising detection of urinary tract infections. BMC Med Inform Decis Mak. 2019;19(1):171.
Meskò B, Drobni Z, Bényei E, Gergely B, Gyorffy Z. Digital health is a cultural transformation of traditional healthcare. Mhealth. 2017;3:38.
Cho B-J, Choi YJ, Lee M-J, Kim JH, Son G-H, Park S-H, et al. Classification of cervical neoplasms on colposcopic photography using deep learning. Sci Rep. 2020;10(1):13652.
Article CAS PubMed PubMed Central Google Scholar
Doyle OM, Leavitt N, Rigg JA. Finding undiagnosed patients with hepatitis C infection: an application of artificial intelligence to patient claims data. Sci Rep. 2020;10(1):10521.
Shortliffe EH, Sepúlveda MJ. Clinical decision support in the era of artificial intelligence. JAMA. 2018;320(21):2199–200.
Article PubMed Google Scholar
Massaro M, Dumay J, Guthrie J. On the shoulders of giants: undertaking a structured literature review in accounting. Account Auditing Account J. 2016;29(5):767–801.
Junquera B, Mitre M. Value of bibliometric analysis for research policy: a case study of Spanish research into innovation and technology management. Scientometrics. 2007;71(3):443–54.
Casadesus-Masanell R, Ricart JE. How to design a winning business model. Harvard Business Review [Internet]. 2011 Jan 1 [cited 2020 Jan 8]. https://hbr.org/2011/01/how-to-design-a-winning-business-model
Aria M, Cuccurullo C. bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetr. 2017;11(4):959–75.
Zupic I, Čater T. Bibliometric methods in management and organization. Organ Res Methods. 2015;1(18):429–72.
Secinaro S, Calandra D. Halal food: structured literature review and research agenda. Br Food J. 2020. https://doi.org/10.1108/BFJ-03-2020-0234 .
Rialp A, Merigó JM, Cancino CA, Urbano D. Twenty-five years (1992–2016) of the international business review: a bibliometric overview. Int Bus Rev. 2019;28(6):101587.
Zhao L, Dai T, Qiao Z, Sun P, Hao J, Yang Y. Application of artificial intelligence to wastewater treatment: a bibliometric analysis and systematic review of technology, economy, management, and wastewater reuse. Process Saf Environ Prot. 2020;1(133):169–82.
Article CAS Google Scholar
Huang Y, Huang Q, Ali S, Zhai X, Bi X, Liu R. Rehabilitation using virtual reality technology: a bibliometric analysis, 1996–2015. Scientometrics. 2016;109(3):1547–59.
Hao T, Chen X, Li G, Yan J. A bibliometric analysis of text mining in medical research. Soft Comput. 2018;22(23):7875–92.
dos Santos BS, Steiner MTA, Fenerich AT, Lima RHP. Data mining and machine learning techniques applied to public health problems: a bibliometric analysis from 2009 to 2018. Comput Ind Eng. 2019;1(138):106120.
Liao H, Tang M, Luo L, Li C, Chiclana F, Zeng X-J. A bibliometric analysis and visualization of medical big data research. Sustainability. 2018;10(1):166.
Choudhury A, Renjilian E, Asan O. Use of machine learning in geriatric clinical care for chronic diseases: a systematic literature review. JAMIA Open. 2020;3(3):459–71.
Connelly TM, Malik Z, Sehgal R, Byrnes G, Coffey JC, Peirce C. The 100 most influential manuscripts in robotic surgery: a bibliometric analysis. J Robot Surg. 2020;14(1):155–65.
Guo Y, Hao Z, Zhao S, Gong J, Yang F. Artificial intelligence in health care: bibliometric analysis. J Med Internet Res. 2020;22(7):e18228.
Choudhury A, Asan O. Role of artificial intelligence in patient safety outcomes: systematic literature review. JMIR Med Inform. 2020;8(7):e18599.
Forliano C, De Bernardi P, Yahiaoui D. Entrepreneurial universities: a bibliometric analysis within the business and management domains. Technol Forecast Soc Change. 2021;1(165):120522.
Secundo G, Del Vecchio P, Mele G. Social media for entrepreneurship: myth or reality? A structured literature review and a future research agenda. Int J Entrep Behav Res. 2020;27(1):149–77.
Dal Mas F, Massaro M, Lombardi R, Garlatti A. From output to outcome measures in the public sector: a structured literature review. Int J Organ Anal. 2019;27(5):1631–56.
Google Scholar
Baima G, Forliano C, Santoro G, Vrontis D. Intellectual capital and business model: a systematic literature review to explore their linkages. J Intellect Cap. 2020. https://doi.org/10.1108/JIC-02-2020-0055 .
Dumay J, Guthrie J, Puntillo P. IC and public sector: a structured literature review. J Intellect Cap. 2015;16(2):267–84.
Dal Mas F, Garcia-Perez A, Sousa MJ, Lopes da Costa R, Cobianchi L. Knowledge translation in the healthcare sector. A structured literature review. Electron J Knowl Manag. 2020;18(3):198–211.
Mas FD, Massaro M, Lombardi R, Biancuzzi H. La performance nel settore pubblico tra misure di out-put e di outcome. Una revisione strutturata della letteratura ejvcbp. 2020;1(3):16–29.
Dumay J, Cai L. A review and critique of content analysis as a methodology for inquiring into IC disclosure. J Intellect Cap. 2014;15(2):264–90.
Haleem A, Javaid M, Khan IH. Current status and applications of Artificial Intelligence (AI) in medical field: an overview. Curr Med Res Pract. 2019;9(6):231–7.
Paul J, Criado AR. The art of writing literature review: what do we know and what do we need to know? Int Bus Rev. 2020;29(4):101717.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Biancone PP, Secinaro S, Brescia V, Calandra D. Data quality methods and applications in health care system: a systematic literature review. Int J Bus Manag. 2019;14(4):p35.
Secinaro S, Brescia V, Calandra D, Verardi GP, Bert F. The use of micafungin in neonates and children: a systematic review. ejvcbp. 2020;1(1):100–14.
Bert F, Gualano MR, Biancone P, Brescia V, Camussi E, Martorana M, et al. HIV screening in pregnant women: a systematic review of cost-effectiveness studies. Int J Health Plann Manag. 2018;33(1):31–50.
Levy Y, Ellis TJ. A systems approach to conduct an effective literature review in support of information systems research. Inf Sci Int J Emerg Transdiscipl. 2006;9:181–212.
Chen G, Xiao L. Selecting publication keywords for domain analysis in bibliometrics: a comparison of three methods. J Informet. 2016;10(1):212–23.
Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB J. 2007;22(2):338–42.
Article PubMed CAS Google Scholar
Sicilia M-A, Garcìa-Barriocanal E, Sànchez-Alonso S. Community curation in open dataset repositories: insights from zenodo. Procedia Comput Sci. 2017;1(106):54–60.
Secinaro S, Calandra D, Secinaro A, Muthurangu V, Biancone P. Artificial Intelligence for healthcare with a business, management and accounting, decision sciences, and health professions focus [Internet]. Zenodo; 2021 [cited 2021 Mar 7]. https://zenodo.org/record/4587618#.YEScpl1KiWh .
Elango B, Rajendran D. Authorship trends and collaboration pattern in the marine sciences literature: a scientometric Study. Int J Inf Dissem Technol. 2012;1(2):166–9.
Jacoby WG. Electoral inquiry section Loess: a nonparametric, graphical tool for depicting relationships between variables q. In 2000.
Andrews JE. An author co-citation analysis of medical informatics. J Med Libr Assoc. 2003;91(1):47–56.
PubMed PubMed Central Google Scholar
White HD, Griffith BC. Author cocitation: a literature measure of intellectual structure. J Am Soc Inf Sci. 1981;32(3):163–71.
Santosh KC. AI-driven tools for coronavirus outbreak: need of active learning and cross-population train/test models on multitudinal/multimodal data. J Med Syst. 2020;44(5):93.
Shickel B, Tighe PJ, Bihorac A, Rashidi P. Deep EHR: a survey of recent advances in deep learning techniques for electronic health record (EHR) analysis. IEEE J Biomed Health Inform. 2018;22(5):1589–604.
Baig MM, GholamHosseini H, Moqeem AA, Mirza F, Lindén M. A systematic review of wearable patient monitoring systems—current challenges and opportunities for clinical adoption. J Med Syst. 2017;41(7):115.
Kumar S, Kumar S. Collaboration in research productivity in oil seed research institutes of India. In: Proceedings of fourth international conference on webometrics, informetrics and scientometrics. p. 28–1; 2008.
Gatto A, Drago C. A taxonomy of energy resilience. Energy Policy. 2020;136:111007.
Levitt JM, Thelwall M. Alphabetization and the skewing of first authorship towards last names early in the alphabet. J Informet. 2013;7(3):575–82.
Saad G. Exploring the h-index at the author and journal levels using bibliometric data of productive consumer scholars and business-related journals respectively. Scientometrics. 2006;69(1):117–20.
Egghe L. Theory and practise of the g-index. Scientometrics. 2006;69(1):131–52.
Schreiber M. A modification of the h-index: the hm-index accounts for multi-authored manuscripts. J Informet. 2008;2(3):211–6.
Engqvist L, Frommen JG. The h-index and self-citations. Trends Ecol Evol. 2008;23(5):250–2.
London School of Economics. 3: key measures of academic influence [Internet]. Impact of social sciences. 2010 [cited 2021 Jan 13]. https://blogs.lse.ac.uk/impactofsocialsciences/the-handbook/chapter-3-key-measures-of-academic-influence/ .
Lotka A. The frequency distribution of scientific productivity. J Wash Acad Sci. 1926;16(12):317–24.
Khan G, Wood J. Information technology management domain: emerging themes and keyword analysis. Scientometrics. 2015;9:105.
Oxford University Press. Oxford English Dictionary [Internet]. 2020. https://www.oed.com/ .
Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230–43.
Calandra D, Favareto M. Artificial Intelligence to fight COVID-19 outbreak impact: an overview. Eur J Soc Impact Circ Econ. 2020;1(3):84–104.
Bokolo Anthony Jnr. Use of telemedicine and virtual care for remote treatment in response to COVID-19 pandemic. J Med Syst. 2020;44(7):132.
Burke EK, De Causmaecker P, Berghe GV, Van Landeghem H. The state of the art of nurse rostering. J Sched. 2004;7(6):441–99.
Ahmed MA, Alkhamis TM. Simulation optimization for an emergency department healthcare unit in Kuwait. Eur J Oper Res. 2009;198(3):936–42.
Forina M, Armanino C, Raggio V. Clustering with dendrograms on interpretation variables. Anal Chim Acta. 2002;454(1):13–9.
Wartena C, Brussee R. Topic detection by clustering keywords. In: 2008 19th international workshop on database and expert systems applications. 2008. p. 54–8.
Hussain AA, Bouachir O, Al-Turjman F, Aloqaily M. AI Techniques for COVID-19. IEEE Access. 2020;8:128776–95.
Agrawal A, Gans JS, Goldfarb A. Exploring the impact of artificial intelligence: prediction versus judgment. Inf Econ Policy. 2019;1(47):1–6.
Chakradhar S. Predictable response: finding optimal drugs and doses using artificial intelligence. Nat Med. 2017;23(11):1244–7.
Fleming N. How artificial intelligence is changing drug discovery. Nature. 2018;557(7707):S55–7.
Guo J, Li B. The application of medical artificial intelligence technology in rural areas of developing countries. Health Equity. 2018;2(1):174–81.
Aisyah M, Cockcroft S. A snapshot of data quality issues in Indonesian community health. Int J Netw Virtual Organ. 2014;14(3):280–97.
Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6(2):94–8.
Mehta N, Pandit A, Shukla S. Transforming healthcare with big data analytics and artificial intelligence: a systematic mapping study. J Biomed Inform. 2019;1(100):103311.
Collins GS, Moons KGM. Reporting of artificial intelligence prediction models. Lancet. 2019;393(10181):1577–9.
Bennett CC, Hauser K. Artificial intelligence framework for simulating clinical decision-making: a Markov decision process approach. Artif Intell Med. 2013;57(1):9–19.
Redondo T, Sandoval AM. Text Analytics: the convergence of big data and artificial intelligence. Int J Interact Multimed Artif Intell. 2016;3. https://www.ijimai.org/journal/bibcite/reference/2540 .
Winter JS, Davidson E. Big data governance of personal health information and challenges to contextual integrity. Inf Soc. 2019;35(1):36–51.
Novak D, Riener R. Control strategies and artificial intelligence in rehabilitation robotics. AI Mag. 2015;36(4):23–33.
Tarassoli SP. Artificial intelligence, regenerative surgery, robotics? What is realistic for the future of surgery? Ann Med Surg (Lond). 2019;17(41):53–5.
Saha SK, Fernando B, Cuadros J, Xiao D, Kanagasingam Y. Automated quality assessment of colour fundus images for diabetic retinopathy screening in telemedicine. J Digit Imaging. 2018;31(6):869–78.
Gu D, Li T, Wang X, Yang X, Yu Z. Visualizing the intellectual structure and evolution of electronic health and telemedicine research. Int J Med Inform. 2019;130:103947.
Madnick S, Wang R, Lee Y, Zhu H. Overview and framework for data and information quality research. J Data Inf Qual. 2009;1:1.
Chen X, Liu Z, Wei L, Yan J, Hao T, Ding R. A comparative quantitative study of utilizing artificial intelligence on electronic health records in the USA and China during 2008–2017. BMC Med Inform Decis Mak. 2018;18(5):117.
Carter D. How real is the impact of artificial intelligence? Bus Inf Surv. 2018;35(3):99–115.
Kalis B, Collier M, Fu R. 10 Promising AI Applications in Health Care. 2018;5.
Biancone P, Secinaro S, Brescia V, Calandra D. Management of open innovation in healthcare for cost accounting using EHR. J Open Innov Technol Market Complex. 2019;5(4):99.
Kayyali B, Knott D, Van Kuiken S. The ‘big data’ revolution in US healthcare [Internet]. McKinsey & Company. 2013 [cited 2020 Aug 14]. https://healthcare.mckinsey.com/big-data-revolution-us-healthcare/ .
Lu J. Will medical technology deskill doctors? Int Educ Stud. 2016;9(7):130–4.
Hoff T. Deskilling and adaptation among primary care physicians using two work innovations. Health Care Manag Rev. 2011;36(4):338–48.
Picek O. Spillover effects from next generation EU. Intereconomics. 2020;55(5):325–31.
Sousa MJ, Dal Mas F, Pesqueira A, Lemos C, Verde JM, Cobianchi L. The potential of AI in health higher education to increase the students’ learning outcomes. TEM J. 2021. ( In press ).
Download references
Acknowledgements
The authors are grateful to the Editor-in-Chief for the suggestions and all the reviewers who spend a part of their time ensuring constructive feedback to our research article.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Department of Management, University of Turin, Turin, Italy
Silvana Secinaro, Davide Calandra & Paolo Biancone
Ospedale Pediatrico Bambino Gesù, Rome, Italy
Aurelio Secinaro
Institute of Child Health, University College London, London, UK
Vivek Muthurangu
You can also search for this author in PubMed Google Scholar
Contributions
SS and PB, Supervision; Validation, writing, AS and VM; Formal analysis, DC and AS; Methodology, DC; Writing; DC, SS and AS; conceptualization, VM, PB; validation, VM, PB. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Davide Calandra .
Ethics declarations
Ethical approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Secinaro, S., Calandra, D., Secinaro, A. et al. The role of artificial intelligence in healthcare: a structured literature review. BMC Med Inform Decis Mak 21 , 125 (2021). https://doi.org/10.1186/s12911-021-01488-9
Download citation
Received : 24 December 2020
Accepted : 01 April 2021
Published : 10 April 2021
DOI : https://doi.org/10.1186/s12911-021-01488-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Artificial intelligence
- Patient data
BMC Medical Informatics and Decision Making
ISSN: 1472-6947
- General enquiries: [email protected]
More From Forbes
Medical education needs radical reform: ai, alone, isn’t the answer.
- Share to Facebook
- Share to Twitter
- Share to Linkedin
Medical school needs radical reform. AI coursework, alone, won't prep students for the medical ... [+] challenges that await.
America’s medical schools, with their state-of-the-art facilities and history of groundbreaking clinical advancements, have built a reputation as global leaders in scientific and technological innovation. But does reputation match reality?
In an episode of the Fixing Healthcare podcast recorded in 2023, Deep Medicine author Eric Topol highlighted a significant oversight in medical education.
“It’s pretty embarrassing,” he said. “If you go across 150 medical schools, not one has AI as a core curriculum.”
A year later, most U.S. medical schools have responded by weaving AI into their programs. But look closer and you’ll find that most of the coursework—a mix of theoretical application, ethical consideration and the use of AI to streamline routine tasks: billing, coding, charting—fails to arm future physicians with the training they’ll need to improve medical care and save lives.
Medical Education Is Failing Patients, Doctors
In 1910, education reformer Abraham Flexner published a groundbreaking condemnation of American medical schools. His controversial findings revealed significant inadequacies in the training of future doctors.
The Flexner Report led to the closure of about half the nation’s medical schools and the restructuring of medical education, resulting in more scientifically rigorous and clinically relevant curricula. Flexner’s goal was not just to standardize clinical training but to stop thousands of needless deaths caused by substandard medical practices.
Best Short-Term Health Insurance Companies Of 2022
Best health insurance companies of 2022.
More than a century later, American medicine faces a similar opportunity. Hundreds of thousands of people die each year from mostly preventable chronic diseases, misdiagnoses, medical errors and gaps in research.
Having extensively studied and written about the future of generative AI in medicine , I believe this technology can revolutionize medical care. However, this will only happen if medical schools fundamentally redesign their curricula to teach students how to use GenAI to radically improve medical practices and processes.
The time is now to reform medical education. Here are three GenAI-powered opportunities students must master to improve clinical outcomes and save patient lives:
1. Chronic Disease Management: From Episodic To Continuous
Traditional medical education focuses on students memorizing tens of thousands of facts and committing to memory diagnostic and treatment “algorithms.”
By the time they graduate, doctors are expected to apply these memorized facts and algorithms toward helping patients manage chronic diseases and prevent their complications. Clinicians are taught the drugs to prescribe, the lifestyle modifications to recommend and the protocols to follow, including scheduling a routine follow-up visit three to four months later.
The problem with this “standard” episodic approach is that it leaves physicians with no actionable data between visits. The lack of continuous monitoring leads to:
- Delayed adjustments to medication.
- Inconsistent adherence to treatment plans.
- Poor disease control that remains unnoticed until the next office appointment.
Today, chronic diseases like diabetes and hypertension afflict 6 in 10 Americans, and are responsible for 1.7 million American deaths each year from heart attacks, strokes, cancer and other complications.
These deaths are directly tied to a lack of prevention and effective disease management. Today, hypertension is the leading cause of stroke and is adequately controlled only 55% of the time. Diabetes, the leading cause of kidney failure and major contributor to cardiovascular disease, is controlled even less often. We know that control rates of 90% or more are possible with best practices, but not with today’s approach.
According to the CDC, 30% to 50% of the life-threatening complications from chronic disease could be avoided with effective management. Teaching medical students how to use generative AI for continuous—not episodic—monitoring would radically improve the health of patients and our nation as a whole.
Today’s doctors have access to wearable monitors capable of measuring blood pressure and blood sugar. When linked with GenAI, these tools can reliably analyze patient health data and provide medical advice based on the expectations set by a clinician.
With this combination, patients don’t have to guess whether they need a physician’s medical attention. They know. And that expertise allows physicians to intervene sooner when there’s a problem while reducing unnecessary office visits when chronic diseases are well-controlled.
Based on CDC data, successfully training the next generation of doctors to effectively monitor and manage chronic illnesses will save an estimated 510,000 to 850,000 lives each year with an annual reduction in healthcare spending of $163 billion to $272 billion.
2. Diagnosis: From Confirmation Bias To Constant Second Opinion
In classrooms and on clinical rotations, medical students are still being taught to rely on their memory to establish a diagnosis and recommend optimal treatment.
Often, in the rush of clinical practice, they fall prey to cognitive human biases , leading to unintended but frequent errors. Each year, misdiagnoses kill 400,00 Americans.
American doctors are smart, skilled and committed to their patients. And yet, errors occur. GenAI provides doctors the opportunity to double check their assumptions and reduce the risk of error—all at no added cost.
AI can analyze vast amounts of patient data, including symptoms, medical histories and diagnostic test results. And cognitive errors like confirmation, over-confidence and proximity bias don’t happen with computer applications. GenAI isn’t perfect, but the technology can serve as a valuable complement to human analysis. And because it can compare a patient’s data against a massive, comprehensive database of known diseases and medical conditions, it can identify possible diagnoses that a doctor might overlook.
Already, it has shown great potential to reduce misdiagnoses in emergency room settings . One recent study evaluated AI’s ability to triage patients, finding that the AI performed on par with doctors and nurses, accurately identifying patients at higher risk. A second study assessed AI’s diagnostic accuracy based on patient symptoms and lab results, and the AI consistently proved more accurate than physicians in correctly identifying the likely diagnosis.
And the technology is only getting better. Experts predict that by the time today’s matriculating medical students finish their fellowship programs in 10 years, generative AI will be 1,000 times more powerful .
3. Research: From Human Hypotheses To Data Mining
Clinical research is the backbone of medical advancement, providing doctors with the information they need to improve health outcomes and save lives. In school, physicians are taught how to design research studies, analyze data and write journal articles.
When crafting studies, researchers today pose a clinical question, extract information from medical records and perform a statistical analysis. With the availability of GenAI, we have the opportunity to reverse-engineer this traditional approach.
By using GenAI, doctors now have the ability to analyze vast amounts of data generated by bedside monitors, operative robots and other digital sources. At present, U.S. hospitals create 50 petabytes of data every year , with 97% of it going unused. That’s the data equivalent to the entire published works of mankind through all of recorded history in every language. All this data is currently omitted from clinical research because the sheer volume exceeds researchers’ ability to analyze the patterns embedded within it, making it impossible to separate the signal from the noise.
By using GenAI models to dissect this data, doctors will be able to advance medical knowledge much faster than today. The technology will help clinicians accurately predict which hospitalized patients will deteriorate over the next 24 hours and take preventive action. It will allow oncologists to determine optimal chemotherapy doses with fewer complications. It will enable surgeons to identify the best operative techniques for cancer resection. By leveraging GenAI to analyze data, questions that would take years to answer can be resolved quickly.
Imagine if researchers from dozens of academic facilities agreed to pool and share their monitoring and patient data. With this wealth of information, dozens of researchers could access and learn from this data simultaneously. Rather than asking a narrow, specific question and having to find clinical information to answer it, these scientists could take on larger questions and find themselves capable of advancing clinical practice in a fraction of the time.
This approach of pre-loading vast amounts of data and asking the technology to organize and analyze it mirrors how GenAI tools are designed and would be a radical departure from traditional research endeavors.
But before this can happen, physician researchers will need to be trained in new data analysis methods and equipped with interpersonal tools to enhance collaboration and cooperation. These skills, often taught in business schools, can be easily adapted for medical students.
The next era of medicine is upon us, and the call to action is clear. Academic medical centers must not only weave generative AI into their core curricula. They also must teach the next generation of clinicians how to use this technology to radically improve chronic disease management, diagnostic accuracy, clinical research and dozens of other outdated medical processes. With classes scheduled to start this fall, the time to act is now.

- Editorial Standards
- Reprints & Permissions
Join The Conversation
One Community. Many Voices. Create a free account to share your thoughts.
Forbes Community Guidelines
Our community is about connecting people through open and thoughtful conversations. We want our readers to share their views and exchange ideas and facts in a safe space.
In order to do so, please follow the posting rules in our site's Terms of Service. We've summarized some of those key rules below. Simply put, keep it civil.
Your post will be rejected if we notice that it seems to contain:
- False or intentionally out-of-context or misleading information
- Insults, profanity, incoherent, obscene or inflammatory language or threats of any kind
- Attacks on the identity of other commenters or the article's author
- Content that otherwise violates our site's terms.
User accounts will be blocked if we notice or believe that users are engaged in:
- Continuous attempts to re-post comments that have been previously moderated/rejected
- Racist, sexist, homophobic or other discriminatory comments
- Attempts or tactics that put the site security at risk
- Actions that otherwise violate our site's terms.
So, how can you be a power user?
- Stay on topic and share your insights
- Feel free to be clear and thoughtful to get your point across
- ‘Like’ or ‘Dislike’ to show your point of view.
- Protect your community.
- Use the report tool to alert us when someone breaks the rules.
Thanks for reading our community guidelines. Please read the full list of posting rules found in our site's Terms of Service.
10 recent scientific breakthroughs
From cell reparation to reef restoration
- Newsletter sign up Newsletter
1. Finding the root cause of lupus
2. restoring brain cells, 3. menstrual blood as a diagnostic tool, 4. cell therapy for melanoma, 5. rhino ivf, 6. pristine configuration, 7. restoring reefs, 8. ai to find aliens, 9. inverse vaccines, 10. sequencing the y-chromosome.
Scientists in many fields received little recognition for the last couple of years, as the world focused on the emergency push to develop vaccines and treatments for Covid-19. But that doesn't mean they weren't still busy researching a dizzying series of developments that are now being reported as major discoveries and achievements.
Scientists have discovered a cause of lupus and a possible way to reverse it. A study published in the journal Nature points to abnormalities in the immune system of lupus patients that is caused by a molecular abnormality. "What we found was this fundamental imbalance in the types of T cells that patients with lupus make," Deepak Rao, one of the study authors, said to NBC News . Specifically, "people with lupus have too much of a particular T cell associated with damage in healthy cells and too little of another T cell associated with repair," NBC News said.
The good news is that this could be reversed. A protein called interferon is mainly to blame for the T-cell imbalance. Too much interferon blocks another protein called the aryl hydrocarbon receptor, which helps regulate how the body responds to bacteria or environmental pollutants. In turn, too many T-cells are produced that attack the body itself. "The study found that giving people with lupus anifrolumab, a drug that blocks interferon, prevented the T-cell imbalance that likely leads to the disease," said NBC News.
Subscribe to The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Scientists have found a way to repair brain cells impaired by a rare genetic disorder. A study published in the journal Nature found that a drug called antisense oligonucleotide allowed human neurons to develop normally despite carrying a mutation due to a genetic disorder called Timothy syndrome. "It's the beginning of a new era for many of these diseases that we first thought were untreatable," Dr. Huda Zoghbi, a professor at Baylor College of Medicine, said to NPR .
Timothy syndrome is caused by a mutation of a single gene in a person's DNA. The new drug develops an "antisense nucleotide, a small piece of synthetic genetic material that alters the proteins made by a cell," said NPR. The antisense nucleotide for Timothy syndrome was designed to replace a defective protein with a healthy version — "in effect counteracting the mutation responsible for the disorder." This same approach could potentially be used to treat other genetic disorders, "including some that cause schizophrenia, epilepsy, ADHD and autism spectrum disorder."
Menstrual blood can potentially be used to measure blood sugar. In early 2024, the U.S. Food and Drug Administration (FDA) approved a new diagnostic menstrual pad called the Q-Pad and A1C Test by the biotechnology research company Qvin. The Q-Pad is an organic cotton period pad that "collects the blood, which a laboratory then uses to analyze the individual's average blood sugar over three weeks through the A1C biomarker," said Forbes .
"There is a lot of clinically relevant information in this bodily fluid that comes every month," Sara Naseri, the CEO and co-founder of Qvin, said to Axios . "We've built a way for women to get insights about their health regularly. Non-invasively, using blood that comes every month, the menstrual blood." Diagnostic capabilities can potentially be extended to diagnose HPV or endometriosis.
The U.S. Food and Drug Administration (FDA) approved the first cellular therapy for aggressive forms of melanoma. The treatment, called Amtagvi, is "designed to fight off advanced forms of melanoma by extracting and replicating T cells derived from a patient's tumor," said NPR . These cells are also called tumor-infiltrating lymphocytes (TIL). T cells are integral in the immune system but can become "dysfunctional inside tumors."
"The approval of Amtagvi represents the culmination of scientific and clinical research efforts leading to a novel T cell immunotherapy for patients with limited treatment options," Dr. Peter Marks, the director of the FDA's Center for Biologics Evaluation and Research, said in a statement . The treatment won't work for everyone, but research by the National Institutes of Health showed a "56% response rate among patients with melanoma, and 24% of patients had a complete disappearance of their melanoma, regardless of where it was," Axios said. "This is the tip of the iceberg of what TIL can bring to the future of medicine," Patrick Hwu, CEO of Moffitt Cancer Center, said to Axios .
Scientists were able to impregnate a southern white rhino using in-vitro fertilization (IVF). Researchers in Kenya implanted a southern white rhino embryo into another of the same species using the technique in September 2023, resulting in a successful pregnancy. The technique could be used to save the northern white rhino from total extinction. "We achieved together something which was not believed to be possible," Thomas Hildebrandt , head of the reproduction management department at the Leibniz Institute for Zoo and Wildlife Research, said in a press conference.
There are two species of white rhinos: northern and southern. The northern white rhino is on the verge of extinction due to poaching, with only two females remaining. Luckily, scientists have sperm preserved from the last male rhino, which could be combined with an egg from the female and implanted into a southern white rhino female to act as a surrogate. Using a white rhino embryo to test the procedure was a "proof of concept" which is a "milestone to allow us to produce northern white rhino calves in the next two, two and a half years," Hildebrandt said.
Scientists discovered six exoplanets that revolve around a star in a rare pattern called orbital resonance, said a study published in the journal Nature . This means that "for every six orbits completed by planet b, the closest planet to the star, the outermost planet g completes one," CNN said, adding that "as planet c makes three revolutions around the star, planet d does two, and when planet e completes four orbits, planet f does three."
The system was deemed a "rare fossil" by Rafael Luque, a postdoctoral scholar in the University of Chicago's Department of Astronomy and Astrophysics. "We think only about one percent of all systems stay in resonance," Luque said in a statement . "It shows us the pristine configuration of a planetary system that has survived untouched." The discovery could help further the study of sub-Neptunes, which are planets larger than Earth but smaller than Neptune. They are not present in our solar system. "There is little agreement among astronomers about how these planets form and what they're made of — so an entire system consisting of sub-Neptunes could help scientists determine more about their origin," Luque said.
Coral bleaching has been a rapidly growing problem as climate change worsens. Without intervention, the reefs will continue to deteriorate. To counter this, scientists have explored the idea of a "coral gym," essentially a "laboratory to make corals stronger," NPR said. The goal is to "train" coral to survive more extreme conditions.
Warming oceans and rising temperatures are the largest contributors to coral degradation. "One of the things that we do in this lab is subject them to different environmental conditions and evaluate who's a little bit stronger," Ian Enochs, lead of the Coral Program at the Atlantic Oceanographic and Meteorological Laboratory at the National Oceanic and Atmospheric Administration, said to NPR. Researchers created a "complex matrix of aquariums" where they can "subject different types of corals to different environments and not only understand how they might survive, but perhaps help them to do so."
Scientists have created an artificial intelligence model that can detect alien life , said a study published in the journal PNAS . The algorithm can "distinguish between samples of biological and nonbiological origin 90% of the time," after being "trained using living cells, fossils, meteorites and lab-made chemicals," Live Science said. "Put another way, the method should be able to detect alien biochemistries, as well as Earth life," Robert Hazen, co-author of the study, said in a statement .
The AI "does not involve a machine having to look for specific things," but rather "looks for differences between samples," BBC said. "These results mean that we may be able to find a lifeform from another planet, another biosphere, even if it is very different from the life we know on Earth," Hazen said. "And, if we do find signs of life elsewhere, we can tell if life on Earth and other planets derived from a common or different origin."
Scientists may have found a way to calm immune responses for those with autoimmune disorders using an " inverse vaccine ," said a study published in the journal Nature Biomedical Engineering . The immune system responds to specific identifying markers on invaders like viruses and bacteria called antigens, "but some immune cells react to self-antigens," which are "molecules from our own cells," said Science . "In autoimmune diseases, these misguided immune cells turn against patients' own tissues."
The new research worked by "directing potential self-antigens to the liver," where "immune cells there pick up self-antigens and then stifle T cells that could target these molecules." The experiment was performed on mice. "The method they use is promising and potentially can induce better tolerance," neurologist and neuroimmunologist A.M. Rostami said to Science, adding that "we don't know" whether this approach is "applicable to human disease in which we don't know the antigen."
Scientists have finally sequenced the entire Y chromosome, one of the human sex chromosomes present in those assigned male at birth. The feat has been "notoriously difficult" because of the Y chromosome's "complex repeat structure," said a research paper published in the journal Nature .
"Just a few years ago, half of the human Y chromosome was missing" from knowledge of the human genome, Monika Cechova, co-lead author on the paper, said to CNN . "I would credit new sequencing technologies and computational methods for this," Arang Rhie, who also worked on the paper, said to Reuters . The X chromosome was fully sequenced back in 2020.
Understanding the Y chromosome can help with a number of health issues, including fertility. Genes have also "been shown to be required for the prevention of cancer and cardiovascular disease," Kenneth Walsh, a professor of biochemistry and molecular genetics at the University of Virginia School of Medicine, said to CNN.
Sign up for Today's Best Articles in your inbox
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Devika Rao has worked as a staff writer at The Week since 2022, covering science, the environment, climate and business. She previously worked as a policy associate for a nonprofit organization advocating for environmental action from a business perspective.
- Harold Maass, The Week US

Cartoons Saturday's cartoons - Secret Service insomnia, fact-checking, and more
By The Week US Published 20 July 24

The Week Recommends There is an amazing variety of things to see and experience in this diverse region of Canada
By Yasemen Kaner-White Published 20 July 24

Cartoons Artists take on a fear of loud noises, Trump's luck, and more

Speed Read Two astronauts are stuck on the International Space Station due to problems with Boeing’s Starliner
By Peter Weber, The Week US Published 11 July 24

Today's Big Question People could be living in lunar 'houses' by 2040, says Nasa
By Chas Newkey-Burden, The Week UK Published 11 July 24

The Explainer Though taxonomy is hundreds of years old, scientists are still striving to create a universal and easily understood system
By Abby Wilson Published 8 July 24

The Explainer Meteor showers, eclipses and more are coming to the skies
By Devika Rao, The Week US Published 1 July 24

In Depth A running list of the space agency's most exciting developments

In Depth These events are becoming more common thanks to climate change, and are "affecting every corner of the world"
By Devika Rao, The Week US Published 18 June 24

The explainer Getting to the Red Planet requires planning and a whole lot of knowledge
By Devika Rao, The Week US Published 28 May 24

In Depth The number of records set in the past year is a stark reminder of the destructiveness of climate change
By Devika Rao, The Week US Published 17 May 24
- Contact Future's experts
- Terms and Conditions
- Privacy Policy
- Cookie Policy
- Advertise With Us
The Week is part of Future plc, an international media group and leading digital publisher. Visit our corporate site . © Future US, Inc. Full 7th Floor, 130 West 42nd Street, New York, NY 10036.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Family Med Prim Care
- v.8(7); 2019 Jul
Overview of artificial intelligence in medicine
1 Department of Medicine, All India Institute of Medical Sciences (AIIMS), Rishikesh, Uttarakhand, India
Paras Malik
Monika pathania, vyas kumar rathaur.
2 Department of Paediatrics, Government Doon Medical College, Dehradun, Uttarakhand, India
Background:
Artificial intelligence (AI) is the term used to describe the use of computers and technology to simulate intelligent behavior and critical thinking comparable to a human being. John McCarthy first described the term AI in 1956 as the science and engineering of making intelligent machines.
This descriptive article gives a broad overview of AI in medicine, dealing with the terms and concepts as well as the current and future applications of AI. It aims to develop knowledge and familiarity of AI among primary care physicians.
Materials and Methods:
PubMed and Google searches were performed using the key words ‘artificial intelligence’. Further references were obtained by cross-referencing the key articles.
Recent advances in AI technology and its current applications in the field of medicine have been discussed in detail.
Conclusions:
AI promises to change the practice of medicine in hitherto unknown ways, but many of its practical applications are still in their infancy and need to be explored and developed better. Medical professionals also need to understand and acclimatize themselves with these advances for better healthcare delivery to the masses.
Introduction
Alan Turing (1950) was one of the founders of modern computers and AI. The “Turing test” was based on the fact that the intelligent behavior of a computer is the ability to achieve human level performance in cognition related tasks.[ 1 ] The 1980s and 1990s saw a surge in interest in AI. Artificial intelligent techniques such as fuzzy expert systems, Bayesian networks, artificial neural networks, and hybrid intelligent systems were used in different clinical settings in health care. In 2016, the biggest chunk of investments in AI research were in healthcare applications compared with other sectors.[ 2 ]
AI in medicine can be dichotomized into two subtypes: Virtual and physical.[ 3 ] The virtual part ranges from applications such as electronic health record systems to neural network-based guidance in treatment decisions. The physical part deals with robots assisting in performing surgeries, intelligent prostheses for handicapped people, and elderly care.
The basis of evidence-based medicine is to establish clinical correlations and insights via developing associations and patterns from the existing database of information. Traditionally, we used to employ statistical methods to establish these patterns and associations. Computers learn the art of diagnosing a patient via two broad techniques - flowcharts and database approach.
The flowchart-based approach involves translating the process of history-taking, i.e. a physician asking a series of questions and then arriving at a probable diagnosis by combining the symptom complex presented. This requires feeding a large amount of data into machine-based cloud networks considering the wide range of symptoms and disease processes encountered in routine medical practice. The outcomes of this approach are limited because the machines are not able to observe and gather cues which can only be observed by a doctor during the patient encounter.
On the contrary, the database approach utilizes the principle of deep learning or pattern recognition that involves teaching a computer via repetitive algorithms in recognizing what certain groups of symptoms or certain clinical/radiological images look like. An example of this approach is the Google's artificial brain project launched in 2012. This system trained itself to recognize cats based on 10 million YouTube videos with efficiency improving by reviewing more and more images. After 3 days of learning, it could predict an image of a cat with 75% accuracy.[ 4 , 5 ]
Materials and Methods
PubMed and Google searches were performed using the key words “artificial intelligence.” Further references were obtained by cross-referencing the key articles. An overview of different applications utilizing AI technologies currently in use or in development is described.
A lot of AI is already being utilized in the medical field, ranging from online scheduling of appointments, online check-ins in medical centers, digitization of medical records, reminder calls for follow-up appointments and immunization dates for children and pregnant females to drug dosage algorithms and adverse effect warnings while prescribing multidrug combinations. Summarized in the pie chart [ Figure 1 ] are the broad applications of AI in medicine.

Applications of artificial intelligence in health care
Radiology is the branch that has been the most upfront and welcoming to the use of new technology.[ 6 ] Computers being initially used in clinical imaging for administrative work like image acquisition and storage to now becoming an indispensable component of the work environment with the origin of picture archiving and communication system. The use of CAD (computer-assisted diagnosis) in a screening mammography is well known. Recent studies have indicated that CAD is not of a lot of diagnostic aid, based on positive predictive values, sensitivity, and specificity. In addition, the false-positive diagnoses may distract the radiologist resulting in unnecessary work-ups.[ 7 , 8 ] As suggested by a study,[ 6 ] AI could provide substantial aid in radiology by not only labeling abnormal exams but also by identifying quick negative exams in computed tomographies, X-rays, magnetic resonance images especially in high volume settings, and in hospitals with less available human resources.
A decision support system known as DXplain was developed by the university of Massachusetts in 1986, which gives a list of probable differentials based on the symptom complex and it is also used as an educational tool for medical students filling the gaps not explained in standard textbooks.[ 9 ] Germwatcher is a system developed by the University of Washington to detect and investigate hospital acquired infections.[ 10 ] An online application in UK known as Babylon can be used by the patients to consult the doctor online, check for symptoms, get advice, monitor their health, and order test kits. Apart from that, the spectrum of AI has expanded to provide therapeutic facilities as well. AI-therapy is an online course that helps patients treat their social anxiety using therapeutic approach of cognitive behavior therapy. It was developed from a program CBTpsych.com at University of Sydney.[ 11 ]
The Da Vinci robotic surgical system developed by Intuitive surgicals has revolutionized the field of surgery especially urological and gynecological surgeries. The robotic arms of the system mimics a surgeon's hand movements with better precision and has a 3D view and magnification options which allow the surgeon to perform minute incisions.[ 3 ] Since 2018, Buoy Health and the Boston children's hospital are collaboratively working on a web interface-based AI system that provides advice to parents for their ill child by answering questions about medications and whether symptoms require a doctor visit.[ 12 ] The National Institute of Health (NIH) has created an AiCure App, which monitors the use of medications by the patient via smartphone webcam access and hence reduce nonadherence rates.[ 13 ]
Fitbit, Apple, and other health trackers can monitor heart rate, activity levels, sleep levels, and some have even launched ECG tracings as a new feature. All these new advances can alert the user regarding any variation and let the doctor have a better idea of the patient's condition. The Netherlands uses AI for their healthcare system analysis - detecting mistakes in treatment, workflow inefficiencies to avoid unnecessary hospitalizations.
Apart from the inventions which already exist, there are certain advances in various phases of development, which will help physicians be better doctors. IBM's Watson Health being a prime example of the same, which will be equipped to efficiently identify symptoms of heart disease and cancer. Stanford University is making a program AI-assisted care (PAC). PAC has intelligent senior wellbeing support system and smart ICUs, which will sense any behavioral changes in elderly people living alone[ 14 ] and ICU patients,[ 15 ] respectively, via the use of multiple sensors. PAC is also extending its projects over Intelligent Hand Hygiene support and Healthcare conversational agents. Hand hygiene support is using depth sensors refining computer vison technology to achieve perfect hand hygiene for clinicians and nursing staff reducing hospital acquired infections.[ 16 ] Healthcare conversational projects analyzes how Siri, Google Now, S voice, and Cortana respond to mental health, interpersonal violence, and physical health questions from mobile phone users allowing patients to seek care earlier. Molly is a virtual nurse that is being developed to provide follow-up care to discharged patients allowing doctors to focus on more pressing cases.
AI is growing into the public health sector and is going to have a major impact on every aspect of primary care. AI-enabled computer applications will help primary care physicians to better identify patients who require extra attention and provide personalized protocols for each individual. Primary care physicians can use AI to take their notes, analyze their discussions with patients, and enter required information directly into EHR systems. These applications will collect and analyze patient data and present it to primary care physicians alongside insight into patient's medical needs.
A study conducted in 2016[ 17 ] found that physicians spent 27% of their office day on direct clinical face time with their patients and spent 49.2% of their office day on electronic hospital records and desk work. When in the examination room with patients, physicians spent 52.9% of their time on EHR and other work. In conclusion, the physicians who used documentation support such as dictation assistance or medical scribe services engaged in more direct face time with patients than those who did not use these services. In addition, increased AI usage in medicine not only reduces manual labor and frees up the primary care physician's time but also increases productivity, precision, and efficacy.
Searching and developing pharmaceutical agents against a specific disease via clinical trials take years and cost a gazillion dollars. To quote a recent example, AI was used to screen existing medications, which could be used to fight against the emerging Ebola virus menace which would have taken years to process otherwise. With the help of AI, we would be able to embrace the new concept of “precision medicine.”
Some studies have been documented where AI systems were able to outperform dermatologists in correctly classifying suspicious skin lesions.[ 18 ] This because AI systems can learn more from successive cases and can be exposed to multiple cases within minutes, which far outnumber the cases a clinician could evaluate in one mortal lifetime. AI-based decision-making approaches bring used in situations where experts often disagree, such as identifying pulmonary tuberculosis on chest radiographs.[ 19 ]
This new era of AI-augmented practice has an equal number of skeptics as proponents [ Figure 2 ]. The increased utilization of technology has reduced the number of job opportunities, which many doctors in the making and practicing doctors are concerned about. Analytically and logically machines may be able to translate human behavior, but certain human traits such as critical thinking, interpersonal and communication skills, emotional intelligence, and creativity cannot be honed by the machines.

Advantages and disadvantages of artificial intelligence in medicine
In 2016, the Digital Mammography DREAM Challenge was done where several networks of computers were connected, and the goal was to establish an AI-based algorithm by reviewing 640,000 digital mammograms. The best which was achieved was a specificity of 0.81, sensitivity of 0.80, area under receiver operator curve was 0.87, which is roughly approximated to bottom 10% radiologists.[ 20 ] In conclusion, AI has potential, but it is unlikely that AI will replace doctors out rightly.
AI would be an integral part of medicine in the future. Hence, it is important to train the new generation of medical trainees regarding the concepts and applicability of AI and how to function efficiently in a workspace alongside machines for better productivity along with cultivating soft skills like empathy in them.
In conclusion, it is important that primary care physicians get well versed with the future AI advances and the new unknown territory the world of medicine is heading toward. The goal should be to strike a delicate mutually beneficial balance between effective use of automation and AI and the human strengths and judgment of trained primary care physicians. This is essential because AI completely replacing humans in the field of medicine is a concern which might otherwise hamper the benefits which can be derived from it.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
- HIPAA compliance and regulation

Getty Images
Examining Health Data Privacy, HIPAA Compliance Risks of AI Chatbots
Healthcare organizations seeking to reap the benefits of ai chatbots must consider the hipaa compliance and data privacy risks that come along with them..

- Jill McKeon, Assistant Editor
AI chatbots, such as Google’s Bard and OpenAI’s ChatGPT, have sparked continuous conversation and controversy since they became available to the public. In the healthcare arena, patients may be tempted to tell their symptoms to a chatbot rather than a physician, and clinicians may be able to leverage these tools to easily craft medical notes and respond to portal messages.
However, AI chatbots present unique and varied challenges when it comes to protecting patient privacy and complying with HIPAA. Two recent viewpoints published in JAMA explored the health privacy and compliance risks of AI chatbots, each offering thoughts on how providers can navigate HIPAA compliance and honor their duty to protect patient data as these tools gain prominence.
Navigating HIPAA Compliance While Using AI Chatbots
In the first of two viewpoint articles published in JAMA on the topic, researchers noted that the use of AI to improve workflows in healthcare is not a new development. Healthcare organizations have been known to contract with data analytics firms to analyze electronic health records with a business associate agreement (BAA) in place that allows them to do so.
“The innovation—and risk—with an AI chatbot therefore does not lie with its AI engine but with its chat functionality,” the article suggested. For example, physicians may enter a transcript of a patient-physician encounter into a chatbot, which can then produce medical notes in seconds.
This task may be tempting to complete with the help of an AI chatbot, but doing so without a BAA in place may expose patient data.
“Clinicians may not realize that by using ChatGPT, they are submitting information to another organization, OpenAI, the company that owns and supports the technology,” the article stated.
“In other words, the clinical details, once submitted through the chat window, have now left the confines of the covered entity and reside on servers owned and operated by the company. Given that OpenAI has likely not signed a business associate agreement with any health care provider, the input of PHI into the chatbot is an unauthorized disclosure under HIPAA.”
The easiest way to avoid this compliance roadblock, the authors suggested, is to avoid entering any protected health information (PHI) into a chatbot. For example, if a physician wanted to enter a transcript into a chatbot, they would first have to manually deidentify the transcript according to HIPAA’s deidentification standards.
“Although some of the burden of purging PHI from chat inputs falls on the querying clinician, covered entities can take measures to create environments that prevent inadvertent PHI disclosure. At a minimum, covered entities should provide training specifically on chatbot risks, beginning now and continuing in the context of annual HIPAA training,” the article continued.
“Other more restrictive approaches include limiting chatbot access to only workforce members who have had training or blocking network access to chatbots.”
As chatbots continue to develop, healthcare organizations will be faced with the decision to embrace or reject these technologies. In the future, the authors predicted that AI chatbot developers will work directly with healthcare providers to develop HIPAA-compliant chat functionalities.
In the meantime, HIPAA-covered entities will have to take care to prevent the unauthorized disclosure of PHI themselves, should they choose to use chatbots.
Is HIPAA Strong Enough?
In the second viewpoint article published in JAMA surrounding AI chatbots and health data privacy, the authors posited that AI chatbots simply cannot comply with HIPAA in any meaningful way, even with industry assurances.
“Even if they could, it would not matter because HIPAA is outdated and inadequate to address AI-related privacy concerns,” the authors wrote. “Consequently, novel legal and ethical approaches are warranted, and patients and clinicians should use these products cautiously.”
The authors suggested that when HIPAA was enacted in 1996, lawmakers could not have predicted how healthcare would digitally transform. HIPAA was enacted when paper records were still used, and when stealing physical records was the primary security risk.
The authors argued that even deidentified data can pose privacy risks via reidentification and that asking whether chatbots “could be made HIPAA compliant is to pose the wrong question. Even if compliance were possible, it would not ensure privacy or address larger concerns regarding power and inequality.”
The true extent of the privacy risks that these chatbots pose is not yet known, but the authors urged clinicians to remember their duty to protect patients from the unauthorized use of their personal information.
“As alluring as offloading repetitive tasks or obtaining quick information might be, patients and clinicians should resist chatbots’ temptation. They must remember that even if they do not input personal health information, AI can often infer it from the data they provide,” the authors suggested.
“Because HIPAA is antiquated, clinicians should not rely on HIPAA compliance as a proxy for fulfilling their duty to maintain patient confidentiality.”
- HHS Settles HIPAA Investigation With Healthcare Business Associate
- 24 Attorneys General Express Support For Bolstering Reproductive Care HIPAA Protections
- Medical Record Snooping Case Leads to $240K HIPAA Settlement
Dig Deeper on HIPAA compliance and regulation

Industry groups seek clarity from HHS on Change Healthcare breach reporting

AI Chatbots Provide Inconsistent Musculoskeletal Health Information

Lawmakers Ask HHS to Expand Proposed HIPAA Rule, Require Warrant For PHI

How to Properly Dispose of Paper Medical Records, Physical PHI Under HIPAA
While awareness of TEFCA is rising nationwide, gaps exist across smaller, critical access and independent hospitals, according to...
United States Core Data for Interoperability Version 5 (USCDI v5) introduces new data classes and data elements to enhance health...
Healthcare stakeholders have successfully advocated for updated race and ethnicity data standards, but more work is needed to ...
A machine learning tool could bolster early detection of vision-threatening inflammation associated with drugs used to treat ...
Race is often included as a biological construct in clinical guidance, but experts assert that its use must be reexamined to ...
Learn how an existing AI chatbot for antidepressant recommendation will be validated for use in Black patients with funding from ...

IMAGES
VIDEO
COMMENTS
Artificial intelligence in healthcare: transforming the practice of medicine is a review article that explores the current and potential applications of AI in various domains of medicine, such as diagnosis, treatment, research, and education. The article also discusses the challenges and ethical issues of implementing AI in healthcare, and provides some recommendations for future directions ...
This paper also covers AI's impact on patient care and monitoring, with a look at AI-powered wearables and virtual nursing assistants, and the expansion of telemedicine. ... This review provides insights into the current state of multimodal medical data fusion in healthcare research. ... Zand R., Zhang Y. Health information management ...
The remainder of this paper is oriented toward the main AI applications. ... Proceedings of International Conference on Advancements of Medicine and Health Care through Technology; 2009 Sep 23-26; Cluj-Napoca, Romania (2009) ... Global evolution of research in artificial intelligence in health and medicine: a bibliometric study. J Clin Med, 8 ...
Abstract. Artificial intelligence (AI) is poised to broadly reshape medicine, potentially improving the experiences of both clinicians and patients. We discuss key findings from a 2-year weekly ...
Research topics. Artificial Intelligence (AI) is a novel topic based on already known knowledge, which has several healthcare implications. Due to its continuous growth, there is the potential for a structured literature review (SLR) investigating how AI can contribute to healthcare implementation. 5069 results.
The advancements in artificial intelligence (AI) have provided a wealth of opportunities for clinical practice and healthcare. Large language models (LLMs), such as BERT, GPT, and LaMDA have experienced exponential growth, with some now containing over a trillion parameters. 1 This growth in AI capabilities allows for seamless integration between different types of data and has led to ...
The editors announce both a series of articles focusing on AI and machine learning in health care and the 2024 launch of a new journal, NEJM AI, a forum for evidence, resource sharing, and discussi...
Abstract. Artificial Intelligence (AI) is evolving rapidly in healthcare, and various AI applications have been developed to solve some of the most pressing problems that health organizations currently face. It is crucial for health leaders to understand the state of AI technologies and the ways that such technologies can be used to improve the ...
1. Introduction1.1. Background. The emergence of artificial intelligence (AI) within healthcare holds much promise. AI in healthcare helps to enhance patient diagnoses, improve prevention and treatment, increase cost-efficiency, and serve as a way to provide equitable access and treatment for all [1, 2].The term AI innovation refers to the creation of new knowledge, tools, and ideas [3] using ...
Artificial intelligence (AI) has the potential to transform care delivery by improving health outcomes, patient safety, and the affordability and accessibility of high-quality care. AI will be ...
Medical schools of South Asian countries need to incorporate artificial intelligence-powered chatbots in existing undergraduate medical curricula, Journal of Integrative Medicine and Public Health ...
LLMs have already been used to pass the United States Medical Licensing Examination 4, write research articles 5, and interpret electronic medical record data 6. This last use case is perhaps ...
Introduction. Artificial intelligence (AI) rapidly dominates the health service system. It removes the manual health system into automatic, in which humans conduct the routine works/tasks in medical practice to the management of patients and medical resources. The technical challenges of digitizing health services pose new problems when ...
The modern healthcare landscape involves collection, processing and analysis of large volumes of data, related to medicines, patient records, treatment paradigms, medical reports, immunization, and various other attributes. These data points need to be handled precisely and efficiently, as they lead to critical health decisions. The role of emerging technologies like AI, ML, Deep Learning, IoT ...
In recent years, artificial intelligence (AI) technologies have greatly advanced and become a reality in many areas of our daily lives. In the health care field, numerous efforts are being made to implement the AI technology for practical medical treatments. With the rapid developments in machine learning algorithms and improvements in hardware ...
The transformative role of artificial intelligence (AI) in health care has been forecast for decades, 1 but only recently have technological advances appeared to capture some of the complexity of health and disease and how health care is delivered. 2 Recent emergence of large language models (LLMs) in highly visible and interactive applications 3 has ignited interest in how new AI technologies ...
The integration of artificial intelligence (AI) into healthcare practice has improved patient management and care. ... Feature papers represent the most advanced research with significant potential for high impact in the field. ... Challenges, and Applications of Artificial Intelligence in Medical Education Curriculum. JMIR Med. Educ. 2022, 8 ...
Artificial Intelligence (AI) is becoming increasingly common in healthcare, with applications ranging from screening and triage to clinical risk prediction and diagnosis. 1,2 As a clinical tool, AI has the potential to improve diagnostic accuracy and the efficiency of health services. 3 While AI can incorporate a spectrum of diverse tasks, broadly, it refers to computer-based technology ...
Artificial intelligence (AI) research within medicine is growing rapidly. In 2016, healthcare AI projects attracted more investment than AI projects within any other sector of the global economy. 1 However, among the excitement, there is equal scepticism, with some urging caution at inflated expectations. 2 This article takes a close look at current trends in medical AI and the future ...
The MITRE Corporation 7515 Colshire Drive McLean, VA 22102-7508 (703) 983-6997. This study centers on how computer-based decision procedures, under the broad umbrella of artificial intelligence (AI), can assist in improving health and health care.
The paper, titled "The Potential for Artificial Intelligence in Healthcare," also questioned whether health care providers will be able to adequately explain to a patient how deep learning algorithms led to, say, a cancer diagnosis. In turn, that could impact the doctor-patient relationship.
AI/ML technologies have the potential to transform health care by deriving new and important insights from the vast amount of data generated during the delivery of health care every day. Medical ...
Artificial intelligence systems are increasingly being applied to healthcare. In surgery, AI applications hold promise as tools to predict surgical outcomes, assess technical skills, or guide ...
Artificial intelligence (AI) has the potential to transform healthcare delivery by enhancing diagnostic accuracy, streamlining administrative operations, and increasing patient engagement. From 2017-2021, the healthcare sector received more private AI investment globally than any other, attracting $28.9 billion.
The potential for using AI to continuously assess vital sign data is vast, and would represent a fundamental shift in patient care and medical research. Yet there are significant challenges to ...
This paper evaluated AI in healthcare research streams using the SLR method [].As suggested by Massaro et al. [], an SLR enables the study of the scientific corpus of a research field, including the scientific rigour, reliability and replicability of operations carried out by researchers.As suggested by many scholars, the methodology allows qualitative and quantitative variables to highlight ...
Open AI & Thrive's AI Health Coach Is A Bold Step Towards Hyper-personalized Healthcare Jul 19, 2024, 08:06am EDT Ozempic Shortages Raise Questions About Prioritizing Access For Diabetics
The treatment won't work for everyone, but research by the National Institutes of Health showed a "56% response rate among patients with melanoma, and 24% of patients had a complete disappearance ...
In 2016, the biggest chunk of investments in AI research were in healthcare applications compared with other sectors. AI in medicine can be dichotomized into two subtypes: Virtual and physical. The virtual part ranges from applications such as electronic health record systems to neural network-based guidance in treatment decisions.
However, AI chatbots present unique and varied challenges when it comes to protecting patient privacy and complying with HIPAA. Two recent viewpoints published in JAMA explored the health privacy and compliance risks of AI chatbots, each offering thoughts on how providers can navigate HIPAA compliance and honor their duty to protect patient data as these tools gain prominence.