Clinical trial search
From Health Canada
You may search by one or more of the criteria immediately below, or alternatively by either Protocol Number or Control Number. When typing inside fields, do not include punctuation marks such as hyphens, commas, colons, brackets and wildcard characters (%).

Application information
- Search tips
- CTA terminology
- Frequently asked questions
Related information
- Drug product database
- Content support
- Technical support
Language selection
- Français fr
WxT Search form
Clinical trials environment in canada.
Canada captures 4% of global clinical trials, fourth in number of clinical trials sites, and is the G7 leader in clinical trial productivity (number of trials/population). Canada is globally recognized for the quality and expertise of its research clinicians, many of whom are globally recognized for major medical discoveries and innovations, and its ability to conduct clinical research in complex therapeutic areas with diverse population bases.
Strong investments in Canadian clinical trials can be attributed to Canada's world-leading higher education system; its publicly-funded health care system known for quality; its internationally recognized networks and health charities dedicated to clinical trials in areas including cancer, cardiovascular, rheumatology.
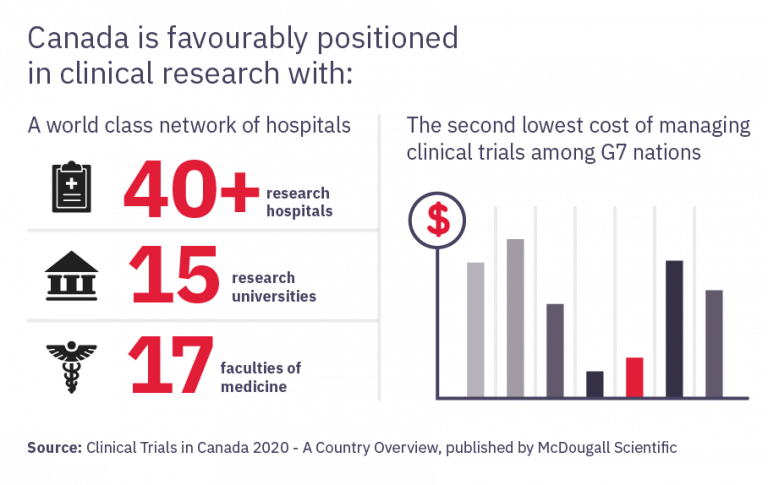
Canada's competitiveness in clinical trials is also supported by strong government support in public research infrastructure including over $1 billion investment by the Canadian Institutes of Health Research (CIHR) in health research funding; as well as a world-class contract research sector with extensive capabilities in phase I-IV clinical trials. Canada's extensive network of academic health institutions and research centres, which support clinical research includes 17 medical schools, approximately 40 groupings of academic healthcare organizations and about 15,000 researchers. CIHR has funded more than 400 COVID-19-related research projects totalling $250 million since March 2020 to develop diagnostics, treatments, public health measures and communication strategies
The Government of Canada has also committed funding through the Strategic Innovation Fund (SIF), to support research and development, clinical trials, and the manufacturing of vaccines and therapeutic drugs to fight COVID-19. SIF investments in clinical trials include
- In 2021, Canada’s Strategic Innovation Fund (SIF) invested $13.44 million in Immune Biosolutions to develop and biomanufacture its promising immunotherapy to treat covid-19 and its variants. This funding will allow the immunotherapy currently in pre-clinical studies to progress to Phase II clinical trials.
- In 2021, SIF invested $14 million in Edesa Biotech as they take their promising treatment for COVID-19 through clinical trials and subsequent approvals.
- In 2020, SIF invested $6.7 million in Arch Biopartners to support a treatment for the worst cases of COVID19. The funding is intended to advance the Phase II of their clinical trials.
- n 2020, SIF invested $173 million in Medicago Inc. to advance their virus-like particle vaccine to treat COVID-19, developed on the company’s unique plant-based production platform, through clinical trials.
- In 2020, SIF invested $56 million in Variation Biotechnologies Inc. (VBI) to support the development of the company’s coronavirus program, VBI-2900, through Phase 2 clinical trials.
All major global pharmaceutical companies conduct a large portion of their clinical trials in Canada. For some of these companies, Canada is the number two or three location globally for clinical trials. In fact, the pharmaceutical and biotechnology industry has the second largest Canadian business expenditures in R&D (BERD) expenditures intensity in 2020. As a result of Canada's world class clinical trials environment, leading multi-national pharmaceutical companies have made significant investments in Canadian clinical trial health research. Examples of recent investments at both the academic and industry level include:
- In 2011, Roche Canada invested $190 million to establish a North American clinical trials coordinating centre and development site (one of six world-wide) in Mississauga, Ontario.
- In 2014, Servier Canada invested $17 million to established a centre of excellence in clinical development in Laval, Quebec.
- AstraZeneca Canada was recently designated a global Clinical Hub for oncology and immuno-oncology clinical studies.
In order to improve the competitiveness of Canada's clinical trial environment, Canada continues to work to enhance the conditions for clinical trial in Canada. Major initiatives and supports for clinical trials include:
- $250 million over three years, starting in 2021-22, to increase clinical research capacity through a new CIHR Clinical Trials Fund.
- In January 20, 2021, Canada committed to invest $6 million in the Canadian Network of COVID-19 Clinical Trials Network, which will expand existing national and international clinical trial networks to coordinate research on tools that prevent, detect, manage and treat COVID-19.
- National and regional efforts to make Research Ethics Boards more efficient, including through the development of a national standard in this area.
- The June 4, 2015 launch of the Canadian Clinical Trials Asset Map which aims to market Canada as a leading destination for clinical trials in the global marketplace by providing a comprehensive picture of Canada’s clinical research assets.
Leading globally recognized academic research centres and institutes include:
- Vancouver Coastal Health Research Institute (Vancouver, British Columbia)
- Providence Health Care Research Institute (Vancouver, British Columbia)
- University of Alberta Hospital (Edmonton, Alberta)
- The Hospital for Sick Children (Toronto, Ontario)
- Population Health Research Institute (Hamilton, Ontario)
- McGill University Health Centre (Montreal, Quebec)
- BIOTIC (Biomedical Translational Imaging Centre (Halifax, Nova Scotia)
- Stem Cell and Cancer Research Institute (McMaster University–Hamilton, Ontario)
- Institute for Research in Immunology and Cancer (Université de Montréal–Montréal, Quebec)
- University Health Network (UHN) (Toronto, Ontario)
- Lawson Health Research Institute (London, Ontario)
- Ottawa Heart Institute (Ottawa, Ontario)
- Montreal Neurological Institute and Hospital (McGill University–Montréal, Quebec)
- Brain Repair Centre (Halifax, Nova Scotia)
- Sunnybrook Research Institute (Toronto, Ontario)
- BC Cancer Agency (Vancouver, British Colombia)

Clinical Trials and Research in Canada: An Overview

Clinical trials are crucial for the advancement of medical knowledge and the enhancement of patient care. They play a critical role in the drug development process by allowing researchers to assess the safety and effectiveness of new treatments prior to their release to the general public.
Canada has a rich history of conducting clinical trials and research. This article aims to present a variety of information and resources related to clinical trials and research in Canada, such as ethical guidelines, reasonable market value, contract samples, and additional resources.
Clinical Trials in Canada: Key Organizations
Clinical trials are research studies that involve human participants. They are conducted to evaluate the safety and effectiveness of new treatments, diagnostic tests, or medical devices. Clinical trials are a necessary step in the drug development process, as they provide researchers with critical data about the potential benefits and risks of new therapies.
Montreal InVivo
Montreal InVivo is a non-profit organization that works to promote the life sciences sector in Quebec. Founded in 1996, Montreal InVivo brings together industry, academia, and government stakeholders to support the development of innovative healthcare solutions and promote economic growth in the province.
One of Montreal InVivo’s key initiatives is the Quebec Clinical Research Organization (Q-CROC), a consortium of academic health centers and research institutes that collaborate to conduct high-quality clinical research studies in Quebec. Q-CROC aims to improve the efficiency and effectiveness of clinical research in Quebec, while also promoting the province as a hub for clinical research activities.
Montreal InVivo also supports the development of the life sciences sector in Quebec through its promotion of industry-academic partnerships, the provision of business development services, and its advocacy for policies and regulations that support the growth of the industry.
Canadian Cancer Trials Group
The Canadian Cancer Trials Group (CCTG) is a national, academic clinical trials organization that conducts research in the areas of cancer diagnosis, treatment, and prevention. Founded in 1980, the CCTG is headquartered at Queen’s University in Kingston, Ontario, and has over 80 member institutions across Canada and around the world.
The CCTG conducts a wide range of clinical trials, including Phase I-III studies of new cancer therapies, as well as studies focused on improving the quality of cancer care and patient outcomes. The organization’s research portfolio covers a broad range of cancer types, including breast cancer, lung cancer, and colorectal cancer, among others.
In addition to its clinical trial activities, the CCTG is also involved in research collaborations, education and training initiatives, and advocacy efforts to promote the interests of the cancer research community.
One of the CCTG’s key initiatives is the IND Program, a clinical trials program focused on the development of new cancer therapies. The IND Program provides support and guidance to researchers and industry partners throughout the drug development process, from preclinical research to clinical trials and regulatory approval.
Data and Statistics on Clinical Research in Canada
Here are some key data and statistics on clinical research in Canada:
- In 2022, there were over 3,500 clinical trials registered in Canada, according to the Canadian Clinical Trials Asset Map (CCTAM).
- Cancer clinical trials are a major area of focus in Canada, with over 1,300 cancer trials registered in 2021, according to CCTAM.
- According to a 2022 report by Innovative Medicines Canada, the pharmaceutical industry invested over $1.2 billion in clinical research in Canada in 2022.
- In a 2022 survey of Canadian clinical research professionals, over 90% of respondents agreed that ethical considerations were a top priority in clinical research.
- According to a 2022 report by the Canadian Academy of Health Sciences, the clinical research industry in Canada supports over 23,000 jobs and contributes over $1 billion to the Canadian economy each year.
- The national patient database in Canada, known as the Canadian Clinical Trials Network (CCTN), has over 6 million patient records as of 2021.
- In a 2022 survey of clinical research organizations (CROs) in Canada, over 75% of respondents reported that they were experiencing growth in their business, and over 60% reported that they were planning to hire additional staff in the near future.
These statistics highlight the significant impact that clinical research has on the Canadian healthcare system and economy. They also demonstrate the importance of ethical practices, patient recruitment, and job opportunities in the clinical research field. By continuing to prioritize these areas, Canada can maintain its leadership position in clinical research and continue to make meaningful contributions to the healthcare industry.
Ethical Practices in Clinical Research
Ethical considerations are of paramount importance in clinical research. The Tri-Council Policy Statement 2 (TCPS 2) is a set of guidelines developed by the Canadian government to ensure that research involving human participants is conducted in an ethical manner.
The TCPS 2 is based on four core principles: respect for persons, concern for welfare, justice, and integrity. These principles have important implications for research ethics, including issues related to informed consent, confidentiality, and privacy.
Innovative Medicines Canada is an industry association that represents Canada’s pharmaceutical companies. The organization has developed a Code of Ethical Practices to guide the conduct of clinical research in Canada.
The drug development process in Canada is highly regulated, with Health Canada playing a significant role in overseeing clinical trials. The agency is responsible for ensuring that clinical trials are conducted in a safe and ethical manner.
Fair Market Value in Clinical Research
Fair market value (FMV) refers to the price that a willing buyer would pay a willing seller for a particular asset or service. In the context of clinical research, FMV is an important consideration in determining the appropriate compensation for research-related services.
The calculation of FMV in Canada is based on a variety of factors, including the qualifications and experience of the service provider, the nature of the services provided, and the prevailing market rates for similar services.
Key considerations in FMV calculation include the need for transparency, consistency, and objectivity in the process. It is important to ensure that FMV rates are fair and reasonable while also providing sufficient compensation for research-related services.
Compared to other countries, FMV rates in Canada are generally considered to be reasonable and competitive. However, there are some challenges associated with FMV in clinical research, including the potential for conflicts of interest and the need to balance the interests of multiple stakeholders.
About Contract Templates and Agreements
Contract templates and agreements are an important part of the clinical research process. They help to define the roles and responsibilities of all parties involved in a clinical trial, as well as outline the terms and conditions of the study.
There are several types of contracts used in clinical research, including clinical trial agreements (CTAs), investigator-initiated trial agreements (IITs), and material transfer agreements (MTAs).
Examples of contract templates used in Canada include the Canadian Clinical Trials Asset Map (CCTAM) CTA template, the Clinical Trials Ontario (CTO) CTA template, and the Health Research Ethics Authority (HREA) IIT template.
Key clauses to include in clinical research contracts include those related to intellectual property, confidentiality, indemnification, and termination.
It is important to ensure that all contracts are reviewed by legal counsel before they are finalized to ensure that they are legally binding and enforceable.
Patient Recruitment and Oriented Research
Patient-oriented research is an important component of clinical research. It involves engaging patients as partners in the research process to ensure that their perspectives and experiences are taken into account in the design and conduct of clinical trials.
Patient recruitment is a critical aspect of clinical research, as it can be challenging to find eligible participants for clinical trials. Strategies for patient recruitment include advertising, physician referrals, social media outreach, and community outreach.
The national patient database is a valuable resource for patient recruitment, as it provides researchers with access to a large pool of potential participants.
Paid clinical trials are another option for patient recruitment, although there are some ethical considerations associated with this approach.
Lab trials are an innovative approach to patient recruitment, as they allow researchers to conduct clinical trials in a controlled laboratory environment. This approach may be particularly useful for studies involving healthy volunteers or those with rare conditions.
MS and Neurological Disorders Research
Multiple sclerosis (MS) and other neurological disorders are a major focus of clinical research in Canada. The National MS Society Canada is a leading organization that funds and conducts research in this field.
The MS Society Ottawa is another important player in MS research, providing funding and support for innovative research projects.
The MSN Art program is a unique initiative that uses art as a means of empowering MS patients and raising awareness about the disease.
Other key MS research initiatives in Canada include the Canadian MS Brain Bank and the MS Society of Canada’s EndMS research program.
UBC Cardiology is a prominent research institution that conducts research in a wide range of neurological disorders, including Alzheimer’s disease, Parkinson’s disease, and stroke.
Clinical Research Organizations and Jobs
Clinical research organizations (CROs) play an important role in the clinical research process. They provide a range of services to support the design, conduct, and execution of clinical trials, including study management, monitoring, and data analysis.
The clinical research job market in Canada is robust, with a wide range of opportunities available for individuals with the necessary qualifications and experience.
Job opportunities in clinical research include roles in clinical development, clinical trial management, and medical writing, among others.
The Alberta Innovates Health Solutions (AIHS) is a non-profit organization that funds and supports innovative health research in Alberta.
Pharma clinical research is another area of the job market that offers a wide range of opportunities for individuals with a background in science or healthcare.
TrialsMap is an online resource that provides information about clinical research jobs and opportunities in Canada, including job postings, training programs, and networking events.

Clinical Trials
A key component of JDRF’s research strategy is the support of clinical trials because they help advance work that has been tested extensively in the lab to testing in people. These real-life studies are when we truly put new therapies designed to prevent, treat or cure type 1 diabetes (T1D) to the test.
How Can I Contribute to Clinical Research?
One of the biggest challenges for clinical trials is finding volunteers to take part in studies. 80% of T1D trials are delayed, largely because of lack of participants. Choosing to participate in a clinical trial is a very personal decision. Early access to promising new treatment can be an enormous benefit. Others have found that by participating in a clinical trial, they learned more about their health or T1D management. By volunteering to participate in a clinical trial, you are helping all people living with T1D, by enabling research towards better health outcomes and cures.
Find a T1D Clinical Trial
To learn more about JDRF-funded trials and how to participate, click on the link below:
Participate in Research
Your questions about clinical trial participation answered
Despite what many believe, some clinical trials do not use ‘inactive’ placebos, like a sugar pill; some compare a potential new therapy against an already approved therapy. And participants who do receive a placebo still receive personalized, quality care from top doctors as part of the study and may be offered the intervention after the study ends if it’s found to be effective.
Diabetes Management
Concern that participation in a clinical trial could affect diabetes management can be a barrier to clinical trial recruitment.
Clinical trials may provide an opportunity to access brand new medical advances. In some cases, participants can continue the therapy after the end of the trial if it’s found to be effective. Most participants experience a benefit in diabetes management or understanding their condition just from being enrolled in a trial since they often receive frequent, high-quality care at top facilities throughout the duration of the trial.
Types of trials – Intervention vs observation
Interventional trials are actively ‘giving’ something to the participants (such as a drug, a surgery, an exercise program, access to a mental health app, etc.). These studies are looking for the effect of the intervention. Observational trials are just ‘recording’ something from the participant (such as taking blood for screening, asking for demographic information in a registry, etc.).
While an interventional trial is more ‘active’, versus a more ‘passive’ participation in observational trials, both provide critical information that helps to advance understanding of T1D and improve treatment outcomes.
Side effects
Side effects are possible for all clinical trials – this is one of the reasons that these trials are conducted. Any anticipated side effects are detailed prior to beginning the study in the informed consent document. Any side effects that occur are recorded and managed very closely, often by a dedicated safety committee.
Ethics and Approval processes
All clinical trials go through rigorous ethical assessment and, when required, Health Canada approval. Before joining a clinical trial, you will be asked to sign an informed consent form, which outlines the details of the trial, but this is not a binding contract—even after you sign it, you can leave the trial at any time, for any reason.
Equity of treatment
Concerns about equity can be a real barrier to clinical trial participation.
Clinical trials and the resulting interventions or observations are safer and more effective for everyone when diverse populations are included. All clinical research is HIGHLY scrutinized for ethical treatment of participants and participants will receive equal care regardless of age, sex, gender, sexual orientation, ethnicity, race, diabetes management practices, etc. Study teams keep the treatment of participants as EQUITABLE as possible to not influence the results.
Location & Travel
You may think that living in a rural or remote area prevents you from participating in clinical trials, but that is not the case. Many clinical trials have travel budgets to cover participant expenses such as driving/flying costs, accommodation, parking, sometimes even food and time compensation. There are also several clinical trials that can be done from the comfort of your home through virtual platforms.

Legacy of JDRF-Funded Trials in Canada
Canadian clinical researchers have made an immeasurable impact upon the lives of those with diabetes, ever since Dr. Banting and Best first discovered the insulin hormone and its role in diabetes. A highlight in our legacy is JDRF’s Canadian Clinical Trial Network (JDRF CCTN), which was established in 2009 to accelerate innovative solutions for the management, care, and cure of T1D.
Created in partnership with the Government of Canada, funding for JDRF CCTN came from a commitment of $20 million by the Federal Economic Development Agency for Southern Ontario (FedDev Ontario), with an additional $14 million contribution from JDRF. The $34 million investment is accelerating the testing of new technologies and treatments for Canadians and individuals around the world living with T1D and its complications. A generous $3 million investment from the WB Family Foundation later enabled JDRF CCTN to expand into western Canada, helping to fund new trials in Alberta and BC.
JDRF CCTN has supported over a dozen clinical trials, as well as multiple training awards and diabetes device projects. Clinical trials enabled by JDRF CCTN include CONCEPTT, which has led to use and coverage of continuous glucose monitors in pregnant women with T1D in many countries; ViaCyte’s first trial of encapsulated, stem cell-derived therapies; and trials testing whether a drug called ustekinumab can slow disease progression in young adults just diagnosed with T1D (in progress).

Canadian Cancer Trials - In partnership with cancer programs across Canada
Thank you for visiting the Canadian Cancer Trials website.
This website is being redesigned. We look forward to sharing news with you over the coming months.
Looking for cancer trials in Canada?
While the Canadian Cancer Trials website is redesigned, please visit ClinicalTrials.gov to find recruiting cancer trials in Canada.
Please speak with your healthcare team about participating in a clinical trial.
Questions about cancer?
For information about clinical trials, cancer, cancer treatment or programs and resources for people affected by cancer, please contact the Canadian Cancer Society at 1-888-939-3333, Live Chat with an Information Specialist on cancer.ca , or send an email to [email protected] .


1 866 262 7427
- Our studies
Become a volunteer

- arrow_drop_up

New name. Same mission: to contribute to the medicine of tomorrow!
Created through the merger of two industry leading companies – INC Research and inVentiv Health – we bring together approximately 27,000 clinical and commercial minds with the ability to support customers in more than 110 countries.
Learn more arrow_forward
Become a medical partner
Work at syneos health.

Discover Syneos Health

Latest news

Myths and truth about clinical research
Recommend a friend and you will be doubly rewarded! You and your friend will receive compensation up to $ 200
Newsletter subscription
Smoker or Non smoker
- ICH GCP (De)
- ICH GCP (En)
- ICH GCP (Es)
- ICH GCP (Fr)
- ICH GCP (It)
- ICH GCP (Pt)
- ICH GCP (Ru)
- AUSTRALIA (NHMRC)
- JAPAN (PMDA)
- US Clinical Trials Registry
- EU Clinical Trials Registry
- Pharmaceutical Companies
- Clinical Research Labs
- Service Companies
- Clinical Research Events
- Publications
- Researchers
List of Contract Research Organizations in Canada
Featured cros.

iNGENū is the FDA-centric Australian CRO championing disruptive, innovative biotech firms globally. Our core mission is to create access to high quality clinical research globally, for early to mid-stage biotechs by removing financial and other unnec...

Celero provides optimal returns to innovative biotechs & pharmaceutical companies by addressing unmet needs, accelerating clinical development and commercialising innovative healthcare products.Trial mismanagement is the main reason why 85%-90% o...
Local, small- and mid-size Contract Research Organizations in Canada
Allphase Clinical Research is a full-service, progressive, Contract Research Organization (CRO) providing high quality clinical development strategy and management services for early-stage to post-market programs. Allphase was founded with the mandat... View full profile
- United States
Welcome to the Biotrial website, where you will find extensive information on the services we offer, on our expertise and experience, and of course on how to contact us. At Biotrial, drug evaluation and pharmacology research is a tailor-made service.... View full profile
- United Kingdom
Canadian Centre for Clinical Trials is a contract research organization with more than a decade of experience, qualified experts, a personalized approach, and competitive prices. We will provide all tools to support your clinical trial all the way. I... View full profile
CMX Research Inc. collaborates with an elite portfolio of clients. They range from domestic and large global Pharmaceutical organizations, emerging Biotech firms and Device companies.CMX is a niche CRO that started as a Site Management Organization (... View full profile
Ethica CRO Inc. is a full-service Contract Research Organization (CRO) that conducts and manages ethical clinical research on drugs, biologics, medical devices and natural health products. Canadian life sciences companies pursuing market expansion t... View full profile
Innovaderm shares your mission of developing innovative treatments for patients living with skin diseases. With in-depth therapeutic expertise and our focus on dermatology, we are committed to the clinical development of the next generation of dermat... View full profile
JSS Medical Research is a full-service CRO built on a foundation of epidemiological and scientific expertise, with a strong network of academic affiliations and over 30 years of experience in multiple therapeutic areas. We are an international Canad... View full profile
Syreon is an expert contract research organization conducting international clinical trials, health economics and outcomes research, computational analysis and real-world evidence research across a broad range of chronic and complex diseases. Our par... View full profile
We are TRIO, a not-for-profit academic clinical research organization (CRO). We are academic leaders in our field and share your goal to find the shortest path to saving lives. With a worldwide network of over 700 cancer centres, we can deliver accur... View full profile
Vantage BioTrials is a Contract Research Organization (CRO) that provides Phase I-IV clinical trial management services to international pharmaceutical, biotechnology, generic pharmaceutical and medical device companies. We believe that success is... View full profile
Global Contract Research Organizations in Canada
Altasciences is a forward-thinking, mid-size contract research organization offering pharmaceutical and biotechnology companies a proven, flexible approach to preclinical and clinical pharmacology studies, including formulation, manufacturing, and an... View full profile
With 15 years of experience, we have defined a process that consistently achieves success for our clients.RESEARCH that uncovers the real needs and delivers actionable insights.At the heart of any healthcare challenge lies a compelling narrative. To... View full profile
- Switzerland
Cato Research is a contract research organization (CRO) that provides integrated services to pharmaceutical, biotechnology, and medical device companies. We specialize in complex development programs requiring innovative regulatory and clinical strat... View full profile
Celerion, a leader in early clinical research, delivers Applied Translational Medicine. In Applied Translational Medicine, Celerion applies our expertise and experience to translating information gained in research discoveries, to knowledge of drug a... View full profile
- South Korea
Celero provides optimal returns to innovative biotechs & pharmaceutical companies by addressing unmet needs, accelerating clinical development and commercialising innovative healthcare products.Trial mismanagement is the main reason why 85%-90% o... View full profile
- Netherlands
- Philippines
- South Africa
At Charles River, we are passionate about our role in improving the quality of people’s lives. Our mission, our excellent science and our strong sense of purpose guides us in all that we do, and we approach each day with the knowledge that our work h... View full profile
Address: 603-7 St Thomas Street Toronto, ON M5S 2B7, Canadadicentra is a full-service Contract Research Organization (CRO) and professional consulting firm that specializes in addressing all matters related to safety, quality, and compliance for all... View full profile
Emergo by UL is a leading regulatory consulting firm specializing in global medical device and IVD compliance. Our comprehensive solution is designed to help you achieve and maintain regulatory and commercial success. With a presence on six continent... View full profile
Since our foundation in Dublin, Ireland in 1990, our mission has been to help our clients to accelerate the development of drugs and devices that save lives and improve quality of life. We are a global provider of consulting, and outsourced developme... View full profile
- Czech Republic
- New Zealand
iNGENū is the FDA-centric Australian CRO championing disruptive, innovative biotech firms globally. Our core mission is to create access to high quality clinical research globally, for early to mid-stage biotechs by removing financial and other unnec... View full profile
IMS Health and Quintiles are now IQVIA, a world leader in using data, technology, advanced analytics and expertise to help customers drive healthcare - and human health - forward. Together with the companies we serve, we are enabling a more modern, m... View full profile
- Bosnia & Herzegovina
Keyrus Life Science is the C2RO launched by the Keyrus Group, the “Making Data Matter” company. Keyrus Life Science is a unique Connected Clinical Research & Development Organization. Keyrus Life Science helps connect industry expertise, Life Da... View full profile
PPD is a leading global contract research organization providing comprehensive, integrated drug development, laboratory and lifecycle management services. Our clients and partners include pharmaceutical, biotechnology, medical device, academic and go... View full profile
Our mission is to be the best CRO in the world as measured by our employees, clients, investigators, and vendors. Our teams work tirelessly to ensure that we deliver on time and on budget. You will always know what's going on with your study when you... View full profile
Life sciences services from SGS – optimize your development timelines to get medicines and medical devices to market quickly and safely. There is no other area of business that is more heavily regulated than the development, testing and distribution... View full profile
- Burkina Faso
- Congo, DR of
- Cote d'Ivoire
- Dominican Republic
- El Salvador
- Equatorial Guinea
- Papua New Guinea
- Saint Lucia
- Saudi Arabia
- Trinidad & Tobago
- Turkmenistan
Syneos Health (Nasdaq:SYNH) is the only fully integrated biopharmaceutical solutions organization purpose-built to accelerate customer success. We lead with a product development mindset, strategically blending clinical development, medical affairs a... View full profile
List of CROs by location
- United Arab Emirates
Your browser is ancient! Upgrade to a different browser or install Google Chrome Frame to experience this site.
Clinical Trials Network

The Clinical Trials Network (CTN) is able to conduct rapid clinical trials to address public health-relevant immunization questions in large and specialized groups with a focus on safety, immunogenicity, and mechanisms of immunity. CTN includes sites in Vancouver, Calgary, Winnipeg, Hamilton, Toronto, Ottawa, Sudbury, Montreal, Quebec City, and Halifax.
The primary goal of the Clinical Trials Network (CTN) is to increase the trial capacity for Canadian researchers and provide the institutional infrastructure where the lead for any one trial can be any member of the CTN, making it a truly pan-Canadian network. The CTN currently has systems and processes in place to support investigators to address diverse questions.
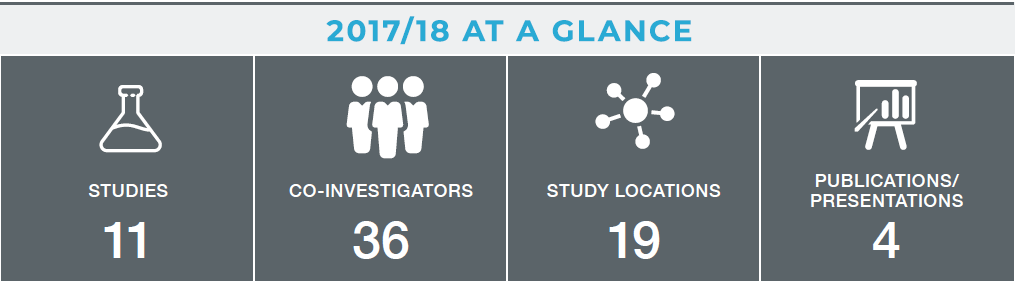
2017/18 was an important year for the CTN, with the culmination of years of work to start enrollment for the first African-Canadian collaboration for a phase II Ebola vaccine in HIV-affected populations study (ACHIV-Ebola). This collaboration between the International Development Research Centre (IDRC), CIRN and Merck began enrolling participants in 2018.
Launching the ACHIV-Ebola trial has required, and will continue to require, a tremendous amount of work behind the scenes. The CTN has coordinated with our partners at Merck across four different clinical sites, liaised with governments in three different countries to ensure ethics submissions and associated documentation were accurate, and planned the logistics of transporting the vaccine and patient samples to and from Africa. This would not have been possible without the tireless efforts of the project management team of Karen Inglis, May ElSherif, Jessica McCarthy and Donna MacKinnon-Cameron.
This year, the CTN welcomed new investigators, like Guillaume Poliquin, who is leading an Ebola vaccine study in Winnipeg for first responders and laboratory personnel who may come into contact with the virus.
In 2018/19, the CTN has four new trials beginning, two led by Manish Sadarangani in British Columbia, one led by Brenda Coleman in Toronto, and one led out of Halifax.
Co-Investigators:
- Joenel Alcantara , University of Calgary
- Houreratou Barry, Centre Muraz
- Julie Bettinger , BC Centre for Disease Control, University of British Columbia
- Kristin Burnett , Lakehead University
- Bill Cameron , Ottawa Hospital Research Institute and University of Ottawa
- Nicolas Chomont , Centre Hospitalier de L’Université de Montréal
- Brenda Coleman , Mount Sinai Hospital
- Jeannette Comeau , Dalhousie University
- Curtis Cooper , Ottawa Hospital Research Institute
- Mark Dionne, Université Laval
- Joanne Embree , University of Winnipeg
- Soren Gantt , University of British Columbia
- Scott Halperin , Dalhousie University
- Jia Hu, Alberta Health Services
- Jennifer Isenor , Dalhousie University
- Jim Kellner , University of Calgary
- Tobias Kollmann, BC Children’s Hospital
- Mark Loeb , McMaster University
- Judy MacDonald , University of Calgary
- Souleymane Mboup , Institut de Recherche en Santé, de Surveillance Epidémiologique et de Formations (IRESSEF)
- Allison McGeer , Mt. Sinai Hospital
- Shelly McNeil , Dalhousie University
- Monika Naus , BC Centre for Disease Control
- Jeff Pernica , McMaster University
- Birahim Pierre Ndiaye, Institut de Recherche en Santé, de Surveillance Epidémiologique et de Formations (IRESSEF)
- Guillaume Poliquin , Public Health Agency of Canada
- Caroline Quach , Université McGill
- Earl Rubin , McGill University
- Chris Sanders , Lakehead University
- David Scheifele , University of British Columbia
- Manish Sadarangani , University of British Columbia
- Mayank Singal, Alberta Health Services
- Kathryn Slayter , Dalhousie University
- Cecile Tremblay , Centre Hospitalier de l’Université de Montréal
- Innocent Valea , Centre Muraz
- Otto Vanderkooi , University of Calgary
- Brian Ward , McGill University
- Duncan Webster , Saint John Regional Hospital
Clinical Trials
Learn about clinical research.
A clinical trial is a scientific study that helps determine if and how a medicine works in people.
Why participate in a clinical trial? Finding new and improved medicines is possible only through the help of people like you. Every clinical trial participant provides valuable information that might help advance medical research and knowledge.
People take part in clinical trials for many reasons, including the chance to:
Help in the development of a new or existing medicine that may benefit people in the future
Receive study-related monitoring for their health
Help find new cures and treatments for the future
Clinical Trials in Canada
Health Canada must approve a clinical trial to validate its scientific value and ensure that it is being conducted according to good clinical practices and Health Canada standards.
In 2020, Lilly Canada managed research at 418 sites across Canada. For more information on Lilly’s clinical trials, visit www.ClinicalTrials.gov . Please note that this information is in English only. Or, you can contact our Customer Response Centre at 1-888-545-5972.
Diversity in Clinical Trials
Responses to medicines can vary depending on a number of factors, including someone’s genetic background, ethnicity, gender and lifestyle.
This is why it’s critical for Lilly to have diverse representation in clinical trials – to gain the insights necessary to make medicines that will be the most effective for all people who use them.
Regrettably, minority populations have been historically and consistently underrepresented in clinical trials. As a result, important information about how medicines work in minority populations is not always available. In response, Lilly has developed a strategy to increase diversity in our clinical trials and better understand the individual differences that may affect clinical outcomes. The ultimate goal of this strategy is to improve health outcomes for individual patients.
Transparency around Clinical Trials
Lilly has a history of commitment to transparency of our clinical studies. In 2004, Lilly became the first company to voluntarily disclose the initiation of our clinical studies and the results of our studies in a publicly available registry. Since the start of 2014, Lilly has enhanced our transparency initiatives in alignment with the PhRMA/EFPIA Principles for Responsible Clinical Trial Data Sharing. Lilly recognizes that the responsible sharing of data from clinical studies has the ability to enhance public health while safeguarding the privacy of patients, respecting the integrity of national regulatory systems and maintaining incentives for investments in biomedical research.
Independent Clinical Research
In addition to sponsoring our own clinical research and research collaborations, Lilly has programs for considering external requests for us to provide study drug and/or financial support for independent clinical research that is initiated, designed and sponsored by external researchers.
Lilly considers such requests for support based on the research projects’ scientific merit and strategic fit with Lilly’s areas of research interest. These reviews are carried out by global committees composed of members of Lilly’s medical and scientific staff from relevant therapeutic areas.
Health care and research professionals may submit a research concept online. Please note that this information is in English only.

Research and Scientific Discovery
Principles of Medical Research
We use cookies to deliver the best possible experience. By using our website, you're agreeing to our use of cookies.
Certificate in Clinical Research
Drive the next generation of treatments and therapies
On this page
- Register to a specific offering
- Program information
Next Enrolment
September 23rd, January 20th
Delivery Format
Blended (Online + Live Online Classes)
$6,594 (Domestic)
Program Length
Select an Offering to Register
Sep 23, 24 - Jul 20, 25
Program Type
Course Descriptions and Schedule
Cscr1000 principles of clinical trials, research & drug development.
This introductory course will orient you to the drug development process and the clinical research function. Pharmaceutical drug development will be used as a model, with some exposure to the development of devices and biologics. Basic concepts in clinical research, such as trial designs, trial phases, randomization, and blinding, will be discussed. By the end of the course, you will have developed a high-level overview of all phases of drug development and be able to explain the key components and principles governing clinical trial execution.
September 23 to November 03, 2024
Classes Sat,Sun 9:00 AM-12:00 PM (12 Oct 2024 to 13 Oct 2024); Sat,Sun 9:00 AM-12:00 PM (02 Nov 2024 to 03 Nov 2024)
CSCR1010 Regulatory & Ethical Issues in Clinical Trials
In this course, you will be familiarized with the regulations and ethical principles that govern the conduct of research, as well as their practical application in clinical trials. Following the presentation of the historical rationale for regulatory oversight, you will gain familiarity with the definitions and terminology used in laws directing clinical trials, as well as the national and international guidelines that apply to clinical research. By the end of the course, you will possess a strong understanding of the key ethical principles underlying ICH-GCP and their application in clinical research.
November 04 to December 15, 2024
Classes Sat,Sun 9:00 AM-12:00 PM (23 Nov 2024 to 24 Nov 2024); Sat,Sun 9:00 AM-12:00 PM (07 Dec 2024 to 08 Dec 2024)
CSCR1020 Clinical Trial Design & Planning
This course will provide you with sound knowledge of key clinical trial design principles, including how to design a protocol and other important aspects of conducting a clinical trial. Today’s healthcare industry is focused on following science, and on designing studies that answer important questions that advance the practice of medicine. This course has been developed to help you effectively navigate important decisions typically faced by clinical researchers when designing and planning clinical studies.
January 06 to February 16, 2025
Classes Sat,Sun 9:00 AM-12:00 PM (25 Jan 2025 to 26 Jan 2025); Sat,Sun 9:00 AM-12:00 PM (15 Feb 2025 to 16 Feb 2025)
CSCR1030 Clinical Research Operations
We will focus on the day-to-day operations of leading a clinical trial. You will gain applied knowledge in financial management, essential documents, recruitment, data management and strategies for safety reporting. Upon completion, you will possess the necessarily skills needed to effectively and efficiently execute clinical research trials.
February 24 to April 20, 2025
Classes Sat,Sun 9:00 AM-12:00 PM (08 Mar 2025 to 09 Mar 2025); Sat,Sun 9:00 AM-12:00 PM (29 Mar 2025 to 30 Mar 2025)
CSCR1040 Clinical Trial Monitoring
We will examine oversight mechanisms in clinical research operations, including monitoring, audits, and inspections, as well as safety, medical, and data oversight. The primary aim of this course is to empower you with the knowledge and practical skill sets required for end-to-end monitoring activities. You will gain valuable knowledge of monitoring practices which are essential to clinical trial conduct and management.
April 28 to June 08, 2025
Classes Sat,Sun 9:00 AM-12:00 PM (24 May 2025 to 25 May 2025); Sat,Sun 9:00 AM-12:00 PM (07 Jun 2025 to 08 Jun 2025)

CSCR1050 Clinical Research Capstone
After the completion of the first five courses and their applied learning assignments, this course is intended to further simulate real-world experience by combining all previous learnings to an applied clinical research management simulation. You will leverage all your knowledge, assignments and experiences to date to further develop your competencies in critical thinking and problem solving, teamwork and collaboration, as well as agility and adaptability, to ensure the successful execution of clinical trials. By taking your hands-on experience to the next level, you will be ready to begin work in the field and add immediate value to any clinical research team.
June 09 to July 20, 2025
Classes Sat,Sun 9:00 AM-12:00 PM (05 Jul 2025 to 06 Jul 2025); Sat,Sun 9:00 AM-12:00 PM (19 Jul 2025 to 20 Jul 2025)
Winter 2025
Jan 20 - Nov 02, 25
Blended (On Campus and Online)
January 20 to March 02, 2025
Classes Sat,Sun 9:00 AM-4:00 PM (08 Feb 2025 to 09 Feb 2025)
March 10 to April 20, 2025
Classes Sat,Sun 9:00 AM-4:00 PM (05 Apr 2025 to 06 Apr 2025)
April 21 to June 01, 2025
Classes Sat,Sun 9:00 AM-4:00 PM (10 May 2025 to 11 May 2025)
June 09 to August 03, 2025
Classes Sat,Sun 9:00 AM-4:00 PM (12 Jul 2025 to 13 Jul 2025)
August 11 to September 21, 2025
Classes Sat,Sun 9:00 AM-4:00 PM (20 Sep 2025 to 21 Sep 2025)
September 22 to November 02, 2025
Classes Sat,Sun 9:00 AM-4:00 PM (18 Oct 2025 to 19 Oct 2025)
Become an essential member of the clinical research process by learning how to protect patient safety, ensure trial integrity, and manage adherence to research ethics, best practices, and regulations.
What you will learn.
In our part-time Certificate in Clinical Research, you’ll prepare for a career on the cutting edge of this field with instruction from leaders in clinical research who bring a practical perspective to the curriculum. This program will help you:
- Understand the stages in setting up clinical trials
- Plan, manage, and monitor clinical research and trials
- Adhere to good clinical practice including patient consent, privacy, and data integrity protocols
- Abide by regulations and legislation to ensure that trials are conducted ethically while upholding scientific research principles
- Demonstrate accuracy and reliability in data collection, management, and analysis
Program Benefits
- Practice applying clinical trial procedures, regulations, and best practices through experiential assignments, projects, and case studies
- Advance through the program with the same cohort of peers, allowing you to develop a strong professional network
- Apply your learnings in an applied clinical research management simulation
- Balance your commitments with our blended study option which combines live classes with asynchronous online learning
- Complete the program faster by earning your certificate in only nine months
- Careers in Clinical Research with Instructor Taymour Bibi [01:02:37] [ Watch Now ] Find out more about this dynamic program and the emerging careers in the Clinical Research field. *From 00:23:10 – Instructor Taymour Bibi speaks about the careers in Clinical Research
- Opportunities and Challenges for Clinical Research Post COVID-19 [00:55:08] [ Watch Now ] * From 00:19:25 – Instructor Miran Kenk speaks about COVID-19 fundamentally changing medicine in Canada and around the world, drastically altering how we conduct clinical research.
Career Potential
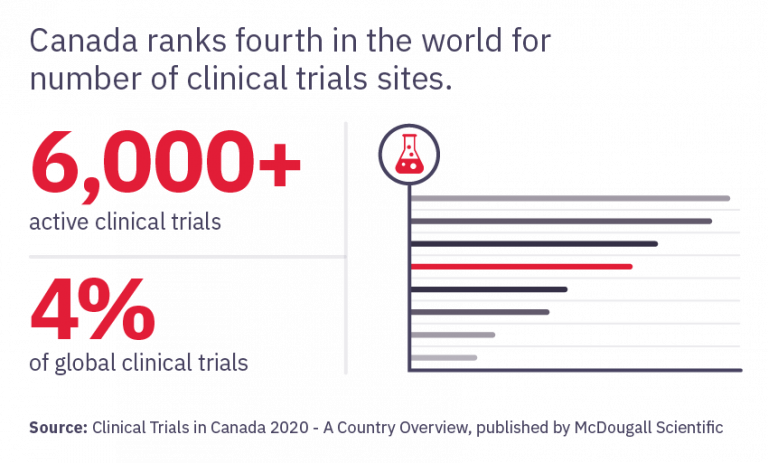
Canada is a World Leader in Clinical Research Canada currently ranks fourth in the world for number of clinical trial sites. The volume and growth of clinical trials taking place nationally signals promising job and career advancement opportunities.
The Need for Qualified, Confident Clinical Research Professionals is on the Rise The clinical research and trials industry has quickly entered a new phase, initiated and expedited by the COVID-19 pandemic. As the only university-level clinical research certificate program in the GTA, you will learn about the technological and operational changes this industry is undergoing firsthand.
Get Hired for Jobs Like:
- Clinical Research Assistant
- Clinical Research Associate
- Clinical Research Coordinator
- Clinical Trials Coordinator
- Clinical Study Specialist
- Clinical Project Associate
- Clinical Project Manager
Gain These Cross-Functional Skills:
- Relationship and stakeholder management
- Critical thinking and problem solving
- Teamwork and collaboration
- Agility and adaptability to a changing environment
- Patient interaction and interpersonal skills

Prospective students for this program include:
- Internationally Educated Medical Doctors (IMDs) and Internationally Educated Health Practitioners (IEHPs) who wish to meaningfully apply their existing skills and experience
- New graduates and early career professionals with a related degree who want to specialize their skills and experience in this growing industry
- Trained Nurses (RNs, RPNs) seeking opportunities for professional growth and advancement
Enrolment Requirements:
The Certificate in Clinical Research is a direct registration program. No application process is required; simply enrol in the session of your choice to get started.
Prerequisites:
Carefully review the prerequisites below to determine if the Certificate in Clinical Research is the right program for you. Individuals who wish to register for this program should have the following:
- University Degree in Health Sciences or a related degree in fields that can include—but not limited to—life sciences, biology, medicine, nursing, nutrition, physiology, anatomy, pharmacy, pharmacology, kinesiology, biochemistry, epidemiology, or health informatics
English Language Proficiency The metrics outlined below are recommended levels of competency:
| IELTS (Academic Only) | 6.5 (with no score less than 6.5) |
| TOEFL Paper | 550 |
| TOEFL Computer | 213 |
| TOEFL Internet | 79-80 |
| TOEIC | 736 |
| Cambridge ESOL | 176 (CI Advanced) |
| PTE Academic | 58 |
| YUELI AP Level | 9 |
| GSPP (Graduate Studies Program) | Pass |
| Duolingo | 115 |
If you have any questions about your eligibility for this program, please contact [email protected] and we would be happy to assist you.
Technology Requirements for Remote/Online Courses Please review the technology and software requirements you will need to access our courses remotely.
School Policies
Funding and payments.
Ask us anything about this program and we’ll get back to you within 2 business days.
[email protected] | +1 416 736 5616 | +1 416 650 8042 (Fax)
Please tell us how we can help you.
[email protected] | +1 416 736 5353 | +1 416 736 5908 (Fax)
[email protected] | +1 416.736.5616
- Name * First Last
- Previous York University Student Number
- I WOULD LIKE TO receive information about York University School of Continuing Studies, which may include, but is not limited to my program(s) of choice, admission requirements and event alerts via email. I can withdraw my consent to receive communications from York University School of Continuing Studies at any time.
We will respond to your inquiry within two business days.
Take the Next Step
Your journey to success begins with us. Learn how to apply today. If you have any questions, we're here to help guide you every step of the way.


Clinical Trials
Clinical trials are research studies that are done to investigate new treatments through the observation of patients. by observing how many patients respond on the tested therapy, researchers can confirm whether new treatments are beneficial for patients., many clinical trials are currently in progress and taking part in a clinical trial may provide valuable information to help researchers make advancements in treatment possible. in some cases, patients who participate in a trial may benefit from receiving cutting – edge treatments resulting in an improvement in a patient’s quality of life that cannot be achieved by standard therapy. .

Clinical trials can be used for different types and stages of pancreatic cancer. Every clinical trial will have enrollment guidelines and requirements for participants to meet which may vary based on the trial. Clinical trials can be considered at time of diagnosis and when making decisions about treatment. If you are considering a clinical trial, it is important to establish that the proposed treatment is a better choice for you compared to your current treatment. It is essential to discuss the trial thoroughly with your physician. Since pancreatic cancer is a rare disease, many trials have difficulty recruiting enough patients and are constantly looking for new entrants.
Below is a list of links to clinical trial registry websites:

316-4211 Yonge Street Toronto, ON M2P 2A9 Toll Free: 1-888-726-2269 [email protected] Charitable Registration Number 84870 1967 RR0001

Canada Profile Updated
The Canada profile in ClinRegs has been reviewed and updated with the following information:
- Health Canada (HC) guidance on filing submissions electronically, including electronic Common Technical Document (eCTD) submission, non-eCTD electronic submission, and validation rules (See Submission Process )
- HC implementation of International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidance on a selective approach to safety data collection in specific late-stage pre-approval or post-approval clinical trials as of 2023 (See Risk & Quality Management )
- Additional information from HC guidance on end-of-study reporting and requirements (See Progress Reporting )
- Additional information from HC guidance on sponsor notification to HC when a study has been suspended (See Risk & Quality Management )
- Additional information regarding Canada's ICH membership and implementation of ICH guidelines (See Regulatory Authority )
Sources Added During this Update :
(Not Available Online) Common Electronic Submissions Gateway (CESG) Health Canada Reference Guide (Last Updated April 2022) (*Note: Only available upon request. See Submission Process section for details) (G-CESG)
(Guidance) Guidance Document: Preparation of Regulatory Activities in the Electronic Common Technical Document (eCTD) Format ( G-eCTD ) (Effective March 13, 2020) Health Canada (*Note: Latest version available upon request. See Submission Process section for details)
(Guidance) Guidance Document: Preparation of Regulatory Activities in Non-eCTD Format ( G-Non-eCTD ) (Effective May 15, 2024) Health Canada (*Note: Latest version available upon request. See Submission Process section for details)
(Guidance) Preparation of Clinical Trial Applications for Use of Cell Therapy Products in Humans ( G-CTACell ) (August 21, 2015) Health Canada
(Guidance) Quality Requirements for Investigational Biologic Drugs Used in Clinical Trials: Notice to Clinical Trial Sponsors ( G-QltyBioCTs ) (Last Updated July 23, 2024) Health Canada
(Guidance) Validation Rules for Regulatory Transactions Provided to Health Canada in the Electronic Common Technical Document (eCTD) Format ( Rules-eCTD ) (January 30, 2024) Health Canada
(Notice) Implementation of ICH E19: A Selective Approach to Safety Data Collection in Specific Late-stage Pre-approval or Post-approval Clinical Trials ( HCNotice-ICH-E19 ) (Last Updated February 27, 2024) Health Canada
(Not Available Online) Health Canada – Clinical Trial Applications in eCTD format (September 2022) (*Note: Only available upon request. See Submission Process section for details) (CAN-36)
(Form) Dossier ID Request Form for Biologic Clinical Trial Dossiers ( CAN-20 ) (Last Updated November 2, 2023) Health Canada
(Form) Dossier ID Request Form for Pharmaceutical Clinical Trial Dossiers ( CAN-21 ) (Last Updated November 2, 2023) Health Canada
(International Guidance) A Selective Approach to Safety Data Collection in Specific Late-Stage in Specific Pre-Approval or Post-Approval Clinical Trials E19 ( CAN-15 ) (Step 4 Version) (September 27, 2022) International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
(Webpage) Electronic Submissions Gateway ( CAN-51 ) (Current as of July 11, 2024) U.S. Food and Drug Administration
(Webpage) Frequently Asked Questions - Common Electronic Submissions Gateway ( CAN-28 ) (Last Updated January 17, 2020) Health Canada
(Webpage) How to Use the Common Electronic Submissions Gateway to Send Regulatory Transactions to Health Canada ( CAN-34 ) (Last Updated May 23, 2024) Health Canada
(Webpage) International Council for Harmonisation (ICH) – Health Canada Role in ICH ( CAN-10 ) (Last Updated February 15, 2022) Health Canada
(Webpage) Resources ( CAN-11 ) (Current as of July 25, 2024) Network of Networks (N2)
(Webpage) User Guide - Electronic Submissions Gateway ( CAN-47 ) (March 1, 2022) U.S. Food and Drug Administration
Sources Revised During this Update :
(Guidance) Filing Submissions Electronically ( ElecSubms ) (Last Updated June 17, 2024) Health Products and Food Branch, Health Canada
(Not Available Online) NIAID Communication with Health Canada (May 2024) (CAN-44)
Language selection
- Français fr
Government of Canada strengthens Canada’s clinical trials environment
From: Canadian Institutes of Health Research
News release
Clinical trials are essential scientific studies that evaluate the safety, effectiveness, and outcomes of health interventions such as vaccines and treatments used across Canada to protect Canadians from diseases. The Government of Canada is working to reinforce the clinical trials environment so that we can improve health outcomes for Canadians while ensuring Canada is well-positioned to respond to future pandemics and other health priorities.
Clinical Trials Fund will lead to improved treatments for all Canadians
June 22, 2022 — Ottawa, Ontario — Canadian Institutes of Health Research
Today, the Honourable Jean-Yves Duclos, Minister of Health, officially launched the Clinical Trials Fund (CTF), supported by a Budget 2021 investment of $250 million over three years for the Canadian Institutes of Health Research (CIHR). As an integral component of Canada’s Biomanufacturing and Life Sciences Strategy , the CTF will strengthen the clinical trials infrastructure in Canada and support the training of new clinical researchers.
Through the CTF, CIHR will reinforce the clinical trials ecosystem by investing in three funding streams:
- The Pan-Canadian Clinical Trials Consortium will create a new platform to strengthen coordination between domestic and international clinical trial networks. This will serve to advance equitable access to clinical trials, build capacity, and improve their impact.
- The Clinical Trials Training Platforms will improve recruitment, training, and mentoring strategies to better position the next generation of clinical trial researchers within the biomanufacturing sector.
- Clinical Trials Projects will support the clinical trials pipeline, from discovery to implementation, through operating grants targeting priority research areas.
By investing in these streams, researchers across the country will conduct all stages of clinical trials to develop new vaccines, therapeutics, and other interventions, addressing a broad range of health conditions. This will ensure that Canada can solve current and future health challenges as well as remain globally recognized for the quality and expertise of its research clinicians.
“We are committed to protecting the health and safety of all Canadians by ensuring we have the best available treatments. The Clinical Trials Fund will strengthen Canada’s expertise in clinical research and enable researchers across the country to move their discoveries into clinical phases of development. This will ensure that Canada remains well positioned to respond to future pandemics or other health emergencies.” The Honourable Jean-Yves Duclos Minister of Health
“The health and safety of Canadians have always been our top priority. For Canadian researchers to bring their ideas from discovery to market, they need the right tools and support to undertake their innovative work. The Clinical Trials Fund will be critical to making more life-saving vaccines, therapeutics and other medicines, right here in Canada. This fund will also support the continued development and growth of Canada’s vibrant life sciences industry and help us be better prepare for future health crises.” The Honourable François-Philippe Champagne Minister of Innovation, Science and Industry
“Canada is globally recognized for its expertise in clinical research. The CTF will strengthen Canada’s existing scientific excellence and support equitable access to clinical trials. This investment will make an important contribution to fulfilling CIHR’s vision for a healthier future and the best health for all.” Dr. Michael J. Strong President Canadian Institutes of Health Research
Quick facts
Clinical trials are studies that evaluate the safety and effectiveness of vaccines, therapeutics, and other health interventions. Data from clinical trials can be used to support the approval of drugs for Canadians or to compare different medicines or treatments. The data can also help us determine which treatments are best for specific populations.
The Government of Canada has invested $250 million through CIHR to create the Clinical Trials Fund as part of a $2.2 billion investment in Canada’s Biomanufacturing and Life Sciences Strategy, which aims to boost the biomanufacturing and life science sector and protect Canadians against future pandemics and health emergencies.
The CTF has three funding streams:
- Pan-Canadian Clinical Trials Consortium to better coordinate clinical trials in Canada and strengthen Canadian participation in international clinical trials.
- Clinical Trials Training Platforms to attract and develop high-caliber trainees, researchers, and healthcare professionals and better position the next generation of clinical trials researchers within the biomanufacturing sector.
- Clinical Trials Projects to support an array of clinical trials phases, designs, and objectives across a broad range of health research areas.
CIHR has launched the first funding opportunities in each these streams. The results of these funding opportunities will be released in Fall 2022.
Associated links
- Clinical Trials Fund
- Canada’s Biomanufacturing and Life Sciences Strategy
Marie-France Proulx Press Secretary Office of the Honourable Jean-Yves Duclos Minister of Health 613-957-0200
Media Relations Canadian Institutes of Health Research [email protected]
Page details
- Skip to main content
- Skip to "About this site"
- Departments
Language selection
- Search and menus
What are clinical trials?
- Canada’s Biomanufacturing and Life Sciences Strategy
- Learn the requirements to register your clinical trial and disclose the results
A clinical trial is a research study involving human participants that evaluates the safety and/or effects of one or more interventions on health outcomes.
Interventions include, but are not limited to, drugs, vaccines, radiopharmaceuticals, cells and other biological products, surgical procedures, radiologic procedures, devices, genetic therapies, natural health products, process-of-care changes, preventive care, manual therapies, and psychotherapies.
Data from clinical trials can be used to support the approval of drugs for Canadians or to compare different medicines or treatments. The data can also help us determine which treatments are best for specific populations.
Clinical Trials at CIHR
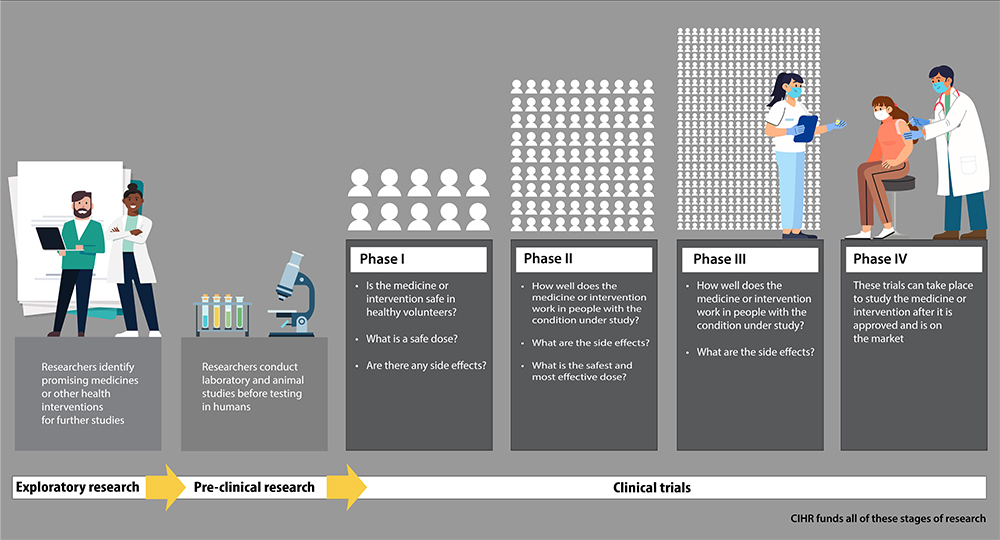
Exploratory research
Researchers identify promising medicines or other health interventions for future studies.
Pre-clinical research
Researchers conduct laboratory and animal studies before testing in humans.
Clinical trials: Phase 1
Is the medicine or intervention safe in healthy volunteers? What is a safe dose? Are there any side effects?
Clinical trials: Phase 2
How well does the medicine or intervention work in people with the condition under study? What are the side effects? What is the safest and most effective dose?
Clinical trials: Phase 3
How well does the medicine or intervention work in people with the condition under study? What are the side effects?
Clinical trials: Phase 4
These trials can take place to study the medicine or intervention after it is approved and is on the market.
CIHR funds all these stages of research.

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Health Topics
- Brochures and Fact Sheets
- Help for Mental Illnesses
- Clinical Trials
Clinical Trials – Information for Participants
Esta página también está disponible en español .
What are clinical trials?
Clinical trials are research studies that look at ways to prevent, detect, or treat diseases and conditions. They are critical to understanding and treating mental illnesses. Clinical trials are the primary way researchers determine if a new treatment is safe and effective in people.
Clinical trials can study:
- New drugs or combinations of drugs.
- New medical procedures (such as a new blood test or scan).
- New medical devices (such as a brain stimulation device ).
- New therapies or behavioral interventions, which help people change their behaviors, thoughts, and feelings to improve their mental health
- New ways to prevent health conditions or find a disease early, sometimes even before symptoms occur.
Watch these videos to learn more about clinical trials
Why are clinical trials important.
Clinical trials are the foundation of most medical advances. Without clinical trials, many of the medical treatments and cures we have today wouldn’t exist.
By testing new treatments and interventions in a carefully designed and controlled way, researchers learn more about the underlying mechanisms of disease and develop new ways to diagnose, treat, and prevent illness.
The results of clinical trials help inform medical decision-making and provide evidence-based information about the benefits and risks of different treatments or interventions. Researchers and doctors use this information to decide which treatments should be recommended and which require more study.
Why should I participate in a clinical trial?
People volunteer for clinical trials for many reasons. Some people join clinical trials to help doctors and researchers learn more about a disease and improve health care. Other people, such as those with health conditions, join to try treatments that aren’t widely available.
Researchers usually study people who have a specific health condition. Researchers sometimes need to compare data from volunteers with no health conditions to data from people with specific health conditions so they can use that information to learn more about the disease.
Participating in a clinical trial is entirely up to you. If you volunteer for a clinical trial and later decide it’s not right for you, you can withdraw anytime.
Clinical Research Trials and You: Questions and Answers
Find more information about the risks and benefits of joining a clinical trial, how your safety is protected, and what happens when a clinical trial ends.
Download this free fact sheet about clinical trials

What is it like to participate in a clinical trial?
During a clinical trial, you will see a team of researchers, sometimes called a study team, clinical trial team, or clinical research team, who will monitor your health closely.
You may have more tests and medical exams than you would if you were getting mental health care but not participating in a clinical trial. The study team may also ask you to do other tasks, such as keeping a log about your health or filling out forms about how you feel.
Clinical trials occur in medical centers, doctors’ offices, and community-based organizations nationwide. You may need to travel or stay in a hospital to participate in a clinical trial.
Are clinical trials safe?
Clinical trials are generally safe. Though there are risks to participating in clinical research, clinical trials are designed to minimize risks and keep you safe.
Before a clinical trial can start, it must be reviewed and approved by an institutional review board (IRB) for U.S.-based studies or an independent ethics committee outside the U.S. This review ensures that it is safe and that the potential benefits of the trial are worth the potential risks. The study team will also make sure you meet certain requirements and that it is safe for you to participate.
Clinical studies might make you feel a little uncomfortable for a short time, but how much risk you face depends on the type of study you join. For instance, if you are participating in a study testing a new drug, the medication might make you feel sick or tired when you first start taking it. In some studies, instead of trying a new medicine, you might take computer-based tests or have a non-invasive magnetic resonance imaging (MRI) done, which carries different risks. The research team and the IRB continuously monitor studies to ensure ongoing safety.
Speak with the study team to understand the risks involved in a particular study. Potential risks are included in the informed consent process, and the research team will be able to explain anything you don’t understand.
Are clinical trials paid?
Some clinical trials pay participants, including some trials that take place at the National Institutes of Health (NIH) Clinical Center in Bethesda, MD.
The amount of money you get paid depends on things like how long the trial takes, how much time you need to give, and what kind of trial it is. Sometimes, the trial may also cover your travel, lodging, and food costs. Not all clinical trials are paid, and you should consider all aspects of the study, including risks and benefits, before making a final decision.
How do I find a clinical trial?
The National Institute of Mental Health (NIMH) is the lead federal agency for research on mental disorders. NIMH supports clinical trials at the NIH campus in Bethesda, MD and across the United States.
Find a study at the NIH campus
NIMH researchers conduct many clinical trials at the NIH Clinical Center . Located on the NIH campus in Bethesda, Maryland, the Clinical Center is the largest research hospital in the world.
Learn more about how to join an NIMH clinical trial at the NIH Clinical Center. These studies enroll volunteers from the local area and across the nation.
Find NIMH clinical trials for adults and children that are currently accepting volunteers:
- Join a Research Study: Adults
- Join a Research Study: Children
- Frequently Asked Questions About Participating in NIMH Research Studies for Adults & Children
You can also subscribe to receive email updates about clinical trials conducted at NIH.
Find other studies around the United States
NIMH also funds many studies that are currently recruiting people around the country on different mental health disorders, including:
- Anxiety Disorders
- Attention-Deficit/Hyperactivity Disorder (ADHD)
- Autism Spectrum Disorder (ASD)
- Bipolar Disorder
- Borderline Personality Disorder
- Eating Disorders
- Generalized Anxiety Disorder
- Obsessive-Compulsive Disorder (OCD)
- Panic Disorder
- Post-Traumatic Stress Disorder (PTSD)
- Schizophrenia
- Social Anxiety Disorder
- Studies Recruiting Only Men
- Studies Recruiting Only Women
- Conditions Related to Mental Disorders
Other ways to find a clinical trial
- Search clinicaltrials.gov , a database of privately and publicly funded clinical studies conducted worldwide.
- Talk to your health care provider about studies that may be right for you. You can also learn about studies in newspapers, TV, or online.
- Join a national registry of research volunteers , such as ResearchMatch . ResearchMatch is a nonprofit program funded by NIH that helps connect people interested in research studies with researchers from medical centers across the United States.
- Join the NIH All of Us Research Program , which is enrolling a large group of people that reflects the diversity of the United States. The program aims to build a diverse database that can inform thousands of studies on various health conditions.
How do I sign up to participate in a clinical trial?
After you find a clinical trial you're interested in, contact the study team to learn more about it. You can usually find the study teams’ contact information in the trial’s description. The staff can give you information that will help you decide whether to participate.
Check out this resource from the U.S. Department of Health and Human Services (HHS) for a list of specific questions to ask about volunteering for a research study .
Let your health care provider know if you decide to join a clinical trial. They may want to talk to the study team to help coordinate your care and ensure the trial is safe for you.
How can I learn more about participating in a clinical trial?
Federal resources
- Clinical Trials : The National Institute on Aging offers articles about how clinical trials work and how to participate about clinical trials.
- NIH Clinical Research Trials and You : Answers from the NIH to many common questions about participating in a clinical trial
- Clinical Trials (MedlinePlus - also en español) : Information about clinical trial protocols and institutional review boards
- Federal Government Health Insurance Programs : Information about federal programs that help pay the costs of care in clinical trials
- NIH Clinical Research Trials and You: Personal Stories : Stories about volunteers and researchers
- Videos sobre la investigación clínica : Spanish-language videos about participating in research
- What is a clinical trial?
- Should I participate in a clinical trial? What’s in it for me?
- What should I know to participate in a clinical trial?
- HHS: Human Research Volunteer Informational videos : Basic information about research, including questions to ask and what to think about when deciding whether to participate in a study
Last reviewed : April 2024
Unless otherwise specified, the information on our website and in our publications is in the public domain and may be reused or copied without permission. However, you may not reuse or copy images. Please cite the National Institute of Mental Health as the source. Read our copyright policy to learn more about our guidelines for reusing NIMH content.
You need to enable JavaScript to run this app.
- Introduction
- Conclusions
- Article Information
a Inclusion criteria for tau pathology: low/medium or high tau indicated by standardized uptake value ratio >1.10 or positive visual read assessed by 18 F-flortaucipir positron emission tomography (PET) imaging.
b Inclusion criteria for amyloid pathology (≥37 Centiloids) assessed with 18 F-florbetapir or 18 F-florbetaben PET.
c Inclusion criteria for Mini-Mental State Examination: score of 20 to 28.
d Phosphorylated tau 181 (P-tau181) screening criterion was not implemented for the entire trial duration (eMethods in Supplement 3 ).
e Exclusion criteria for MRI include presence of amyloid-related imaging abnormalities of edema/effusion, >4 cerebral microhemorrhages, >1 area of superficial siderosis, and any intracerebral hemorrhage >1 cm or severe white matter disease.
f Summary of other screen failure can be found in eTable 3 in Supplement 3 (lists reason if ≥20 participants).
g Stratified by baseline tau categorization and enrolling sites.
h One additional death occurred after treatment completion and in the follow-up period.
i Alzheimer disease progression to a degree prompting study discontinuation, per investigator judgment.
j Treatment completion criteria: amyloid plaque level of 11 Centiloids on any single scan or 11 to <25 Centiloids on 2 consecutive scans.
k Participants who met treatment completion criteria are included in discontinuation and completion numbers.
l Percentage calculated as No./total No. of participants with a PET scan at visit: n = 761 at 24 wk, n = 672 at 52 wk, and n = 620 at 76 wk. Corresponding number of participants and percentages for the low/medium tau population were 20.3% (n = 106) at 24 wk, 51.9% (n = 241) at 52 wk, and 73.5% (n = 321) at 76 wk.
A, 35.1% slowing (95% CI, 19.90%-50.23%) of clinical progression. B, 22.3% slowing (95% CI, 11.38%-33.15%) of clinical progression. C, 36.0% slowing (95% CI, 20.76%-51.15%) of clinical progression. D, 28.9% slowing (95% CI, 18.41%-39.44%) of clinical progression. iADRS data were analyzed using the natural cubic spline model with 2 degrees of freedom (NCS2) and CDR-SB data were analyzed with mixed models for repeated measures (MMRM). For MMRM analyses, 95% CIs for least-squares mean changes were calculated with the normal approximation method. For the Alzheimer Disease Cooperative Study—Instrumental Activities of Daily Living, 13-item cognitive subscale of the Alzheimer Disease Assessment Scale, and CDR-SB clinical assessments analyzed with NCS2, see eFigure 1 (low/medium tau population) and eFigure 2 (combined population) in Supplement 3 and Table 2. For all clinical assessments analyzed with MMRM, see eFigure 3 (low/medium tau population) and 4 (combined population) in Supplement 3 and Table 2. P < .001 for all 76 week time points.
Biomarker data shown were analyzed using mixed models for repeated measures (MMRM). For MMRM analyses, 95% CIs for the least-squares mean changes were calculated with the normal approximation method. P < .001 for all time points in panels A-D. B, P value is from Fisher exact test comparing the percent amyloid negative by treatment groups at each visit. E and F, The analysis was conducted using a Cox proportional hazards model. There were 163 events among 573 participants in the placebo group and 100 events among 555 participants in the donanemab group in the low/medium tau population and 288 events among 844 participants in the placebo group and 186 events among 805 participants in the donanemab group in the combined population. CDR-G indicates Clinical Dementia Rating Global Score.
Trial protocol
Statistical analysis plan
Nonauthor collaborators
Data sharing statement
- Donanemab for Alzheimer Disease—Who Benefits and Who Is Harmed? JAMA Editorial August 8, 2023 Jennifer J. Manly, PhD; Kacie D. Deters, PhD
- Amyloid-Targeting Monoclonal Antibodies for Alzheimer Disease JAMA Editorial August 8, 2023 Gil D. Rabinovici, MD; Renaud La Joie, PhD
- Novel Alzheimer Disease Treatments and Reconsideration of US Pharmaceutical Reimbursement Policy JAMA Editorial August 8, 2023 Meredith B. Rosenthal, PhD
- Ushering in a New Era of Alzheimer Disease Therapy JAMA Editorial August 8, 2023 Eric W. Widera, MD; Sharon A. Brangman, MD; Nathaniel A. Chin, MD
- Role of Registries in Medicare Coverage of New Alzheimer Disease Drugs JAMA Viewpoint October 10, 2023 This Viewpoint discusses how the design of the Centers for Medicare & Medicaid Services (CMS) registry could impact Medicare’s ability to evaluate whether monoclonal antibodies are reasonable and necessary for patients with Alzheimer disease and help physicians understand when the drug is most beneficial. Ilina C. Odouard, MPH; Mariana P. Socal, MD, PhD; Gerard F. Anderson, PhD
- Who Should Get the New Alzheimer Disease Drug? JAMA Medical News & Perspectives October 17, 2023 This Medical News story examines the complexity of determining who to treat with lecanemab, the new Alzheimer disease drug. Rita Rubin, MA
- Use of Donanemab in Early Symptomatic Alzheimer Disease—Reply JAMA Comment & Response December 19, 2023 Cynthia D. Evans, PhD; John R. Sims, MD
- Use of Donanemab in Early Symptomatic Alzheimer Disease JAMA Comment & Response December 19, 2023 Nunzio Pomara, MD; Bruno Pietro Imbimbo, PhD
- Risks of Harm in Alzheimer Disease by Amyloid Lowering JAMA Viewpoint June 18, 2024 This Viewpoint discusses how data gaps in published research impede clinicians’ ability to clearly discuss the risks and benefits of amyloid-lowering drugs for treating Alzheimer disease. Madhav Thambisetty, MD, PhD; Robert Howard, MD
- FDA Greenlights Second Alzheimer Drug, Donanemab JAMA Medical News in Brief July 26, 2024 Emily Harris
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Sims JR , Zimmer JA , Evans CD, et al. Donanemab in Early Symptomatic Alzheimer Disease : The TRAILBLAZER-ALZ 2 Randomized Clinical Trial . JAMA. 2023;330(6):512–527. doi:10.1001/jama.2023.13239
Manage citations:
© 2024
- Permissions
Donanemab in Early Symptomatic Alzheimer Disease : The TRAILBLAZER-ALZ 2 Randomized Clinical Trial
- 1 Eli Lilly and Company, Indianapolis, Indiana
- 2 Boston Center for Memory and Boston University Alzheimer’s Disease Center, Boston, Massachusetts
- 3 Department of Neurology and Department of Psychiatry, Alpert Medical School of Brown University, Providence, Rhode Island
- 4 Butler Hospital, Providence, Rhode Island
- 5 Department of Neurology, Indiana University School of Medicine, Indianapolis
- 6 Clinical Memory Research Unit, Department of Clinical Sciences Malmö, Lund University, Lund, Sweden; Memory Clinic, Skåne University Hospital, Lund, Sweden
- 7 Scottish Brain Sciences, Edinburgh, United Kingdom
- Editorial Donanemab for Alzheimer Disease—Who Benefits and Who Is Harmed? Jennifer J. Manly, PhD; Kacie D. Deters, PhD JAMA
- Editorial Amyloid-Targeting Monoclonal Antibodies for Alzheimer Disease Gil D. Rabinovici, MD; Renaud La Joie, PhD JAMA
- Editorial Novel Alzheimer Disease Treatments and Reconsideration of US Pharmaceutical Reimbursement Policy Meredith B. Rosenthal, PhD JAMA
- Editorial Ushering in a New Era of Alzheimer Disease Therapy Eric W. Widera, MD; Sharon A. Brangman, MD; Nathaniel A. Chin, MD JAMA
- Viewpoint Role of Registries in Medicare Coverage of New Alzheimer Disease Drugs Ilina C. Odouard, MPH; Mariana P. Socal, MD, PhD; Gerard F. Anderson, PhD JAMA
- Medical News & Perspectives Who Should Get the New Alzheimer Disease Drug? Rita Rubin, MA JAMA
- Comment & Response Use of Donanemab in Early Symptomatic Alzheimer Disease—Reply Cynthia D. Evans, PhD; John R. Sims, MD JAMA
- Comment & Response Use of Donanemab in Early Symptomatic Alzheimer Disease Nunzio Pomara, MD; Bruno Pietro Imbimbo, PhD JAMA
- Viewpoint Risks of Harm in Alzheimer Disease by Amyloid Lowering Madhav Thambisetty, MD, PhD; Robert Howard, MD JAMA
- Medical News in Brief FDA Greenlights Second Alzheimer Drug, Donanemab Emily Harris JAMA
Question Does donanemab, a monoclonal antibody designed to clear brain amyloid plaque, provide clinical benefit in early symptomatic Alzheimer disease?
Findings In this randomized clinical trial that included 1736 participants with early symptomatic Alzheimer disease and amyloid and tau pathology, the least-squares mean change in the integrated Alzheimer Disease Rating Scale score (range, 0-144; lower score indicates greater impairment) at 76 weeks was −6.02 in the donanemab group and −9.27 in the placebo group for the low/medium tau population and −10.19 in the donanemab group and −13.11 in the placebo group in the combined study population, both of which were significant differences.
Meaning Among participants with early symptomatic Alzheimer disease and amyloid and tau pathology, donanemab treatment significantly slowed clinical progression at 76 weeks.
Importance There are limited efficacious treatments for Alzheimer disease.
Objective To assess efficacy and adverse events of donanemab, an antibody designed to clear brain amyloid plaque.
Design, Setting, and Participants Multicenter (277 medical research centers/hospitals in 8 countries), randomized, double-blind, placebo-controlled, 18-month phase 3 trial that enrolled 1736 participants with early symptomatic Alzheimer disease (mild cognitive impairment/mild dementia) with amyloid and low/medium or high tau pathology based on positron emission tomography imaging from June 2020 to November 2021 (last patient visit for primary outcome in April 2023).
Interventions Participants were randomized in a 1:1 ratio to receive donanemab (n = 860) or placebo (n = 876) intravenously every 4 weeks for 72 weeks. Participants in the donanemab group were switched to receive placebo in a blinded manner if dose completion criteria were met.
Main Outcomes and Measures The primary outcome was change in integrated Alzheimer Disease Rating Scale (iADRS) score from baseline to 76 weeks (range, 0-144; lower scores indicate greater impairment). There were 24 gated outcomes (primary, secondary, and exploratory), including the secondary outcome of change in the sum of boxes of the Clinical Dementia Rating Scale (CDR-SB) score (range, 0-18; higher scores indicate greater impairment). Statistical testing allocated α of .04 to testing low/medium tau population outcomes, with the remainder (.01) for combined population outcomes.
Results Among 1736 randomized participants (mean age, 73.0 years; 996 [57.4%] women; 1182 [68.1%] with low/medium tau pathology and 552 [31.8%] with high tau pathology), 1320 (76%) completed the trial. Of the 24 gated outcomes, 23 were statistically significant. The least-squares mean (LSM) change in iADRS score at 76 weeks was −6.02 (95% CI, −7.01 to −5.03) in the donanemab group and −9.27 (95% CI, −10.23 to −8.31) in the placebo group (difference, 3.25 [95% CI, 1.88-4.62]; P < .001) in the low/medium tau population and −10.2 (95% CI, −11.22 to −9.16) with donanemab and −13.1 (95% CI, −14.10 to −12.13) with placebo (difference, 2.92 [95% CI, 1.51-4.33]; P < .001) in the combined population. LSM change in CDR-SB score at 76 weeks was 1.20 (95% CI, 1.00-1.41) with donanemab and 1.88 (95% CI, 1.68-2.08) with placebo (difference, −0.67 [95% CI, −0.95 to −0.40]; P < .001) in the low/medium tau population and 1.72 (95% CI, 1.53-1.91) with donanemab and 2.42 (95% CI, 2.24-2.60) with placebo (difference, −0.7 [95% CI, −0.95 to −0.45]; P < .001) in the combined population. Amyloid-related imaging abnormalities of edema or effusion occurred in 205 participants (24.0%; 52 symptomatic) in the donanemab group and 18 (2.1%; 0 symptomatic during study) in the placebo group and infusion-related reactions occurred in 74 participants (8.7%) with donanemab and 4 (0.5%) with placebo. Three deaths in the donanemab group and 1 in the placebo group were considered treatment related.
Conclusions and Relevance Among participants with early symptomatic Alzheimer disease and amyloid and tau pathology, donanemab significantly slowed clinical progression at 76 weeks in those with low/medium tau and in the combined low/medium and high tau pathology population.
Trial Registration ClinicalTrials.gov Identifier: NCT04437511
Deposition of β-amyloid in the brain is an early event in Alzheimer disease that leads to neurofibrillary tangles composed of tau protein and other characteristic brain changes referred to as the amyloid cascade . 1 , 2 Abnormal β-amyloid is a key pathological hallmark of Alzheimer disease defined by the 2018 National Institute on Aging and the Alzheimer’s Association Research Framework 3 and is one of the major targets in Alzheimer disease research and drug development.
Over the past decade, considerable advances occurred in testing the amyloid cascade hypothesis in Alzheimer disease clinical trials. Numerous amyloid-targeting therapy trials failed to show appreciable slowing of clinical disease progression 4 - 7 ; however, aducanumab, lecanemab, and donanemab recently showed promising amyloid plaque clearance, potentially benefitting patients. 8 - 10
Donanemab is an immunoglobulin G1 monoclonal antibody directed against insoluble, modified, N-terminal truncated form of β-amyloid present only in brain amyloid plaques. Donanemab binds to N-terminal truncated form of β-amyloid and aids plaque removal through microglial-mediated phagocytosis. 11 In the phase 2 TRAILBLAZER-ALZ trial of donanemab vs placebo, the primary outcome was met, as measured by the integrated Alzheimer Disease Rating Scale (iADRS), an integrated assessment of cognition and daily function. 9 Adverse events of interest included amyloid-related imaging abnormalities and infusion-related reactions. 9 To confirm and expand results from TRAILBLAZER-ALZ, we report results from TRAILBLAZER-ALZ 2, a global phase 3 randomized clinical trial that assessed donanemab efficacy and adverse events in a larger group of participants with low/medium tau pathology (the population studied in the phase 2 trial) and in a combined population including those with high tau pathology, a population hypothesized to be more difficult to treat due to more advanced disease.
TRAILBLAZER-ALZ 2 was a 76-week, phase 3, randomized, double-blind, parallel, multicenter, placebo-controlled trial with participants screened at 277 sites in 8 countries (eTable 1 in Supplement 3 ). Enrollment began June 19, 2020, and ended November 5, 2021, and database lock/unblinding (double-blind phase) occurred on April 28, 2023. The trial was originally designed as a phase 2 trial but was subsequently amended to a larger phase 3 trial in February 2021 in an effort to confirm and expand the results of the previous TRAILBLAZER-ALZ trial. The trial was conducted according to the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice Guideline, and local regulatory requirements. An independent ethics committee/institutional review board at each site approved the study protocol ( Supplement 1 ), which is provided alongside the statistical analysis plan ( Supplement 2 ). Participants and study partners provided written consent. An independent data and safety monitoring board provided trial oversight.
The trial included participants aged 60 to 85 years with early symptomatic Alzheimer disease (mild cognitive impairment [MCI] 12 or Alzheimer disease with mild dementia). 3 P-tau181 screening was removed in an early protocol amendment (eMethods in Supplement 3 ). Eligible participants had screening Mini-Mental State Examination (MMSE) scores of 20 to 28, amyloid pathology (≥37 Centiloids) assessed with 18 F-florbetapir 13 or 18 F-florbetaben 14 positron emission tomography (PET), and presence of tau pathology assessed by 18 F-flortaucipir PET imaging with central image evaluation. 13 , 15 Tau PET scans were categorized as low/medium or high tau by visual and quantitative reads as previously described 16 - 20 ( Supplements 1 and 2 ). Screening procedures also included magnetic resonance imaging (MRI), and key exclusion criteria included presence of amyloid-related imaging abnormalities of edema/effusion, more than 4 cerebral microhemorrhages, more than 1 area of superficial siderosis, and any intracerebral hemorrhage greater than 1 cm or severe white matter disease on MRI. For all eligibility criteria, see Supplement 1 . Demographic information, including race and ethnicity, was collected to potentially understand any differences in disease course, treatment effects, or adverse events. The participants self-reported race and ethnicity based on fixed categories.
Eligible participants were randomly assigned in a 1:1 ratio ( Figure 1 ) by a computer-generated sequence using interactive web response systems, with stratification by baseline tau categorization and enrolling sites; the randomization block size was 4. Randomized participants received either donanemab (700 mg for the first 3 doses and 1400 mg thereafter) or placebo, administered intravenously every 4 weeks for up to 72 weeks. If amyloid plaque level (assessed at 24 weeks and 52 weeks) was less than 11 Centiloids on any single PET scan or less than 25 but greater than or equal to 11 Centiloids on 2 consecutive PET scans (TRAILBLAZER-ALZ cutoffs 9 ), donanemab was switched to placebo in a blinded procedure. Final adverse events and efficacy assessments were performed at 76 weeks. Amyloid-related imaging abnormality monitoring occurred with scheduled MRIs at 4, 12, 24, 52, and 76 weeks and unscheduled MRIs at investigator discretion. Any participant with detected amyloid-related imaging abnormalities had imaging every 4 to 6 weeks until resolution or stabilization. Amyloid-related imaging abnormality management and treatment interruption guidelines (eTable 2 in Supplement 3 ) depended on severity and symptoms. If infusions were held, investigators were advised to await resolution of amyloid-related imaging abnormalities of edema/effusion on radiographic imaging and stabilization of amyloid-related imaging abnormalities of microhemorrhages and hemosiderin deposits before resuming infusions. Permanent discontinuation was advised for macrohemorrhages. Investigators made final amyloid-related imaging abnormality management decisions.
The primary outcome was change in the iADRS score from baseline to 76 weeks in either the low/medium tau population or combined (low/medium and high tau) population. The iADRS is an integrated assessment of cognition and daily function from the 13-item cognitive subscale of the Alzheimer Disease Assessment Scale (ADAS-Cog 13 ) and Alzheimer Disease Cooperative Study—Instrumental Activities of Daily Living (ADCS-iADL), measuring global disease severity across the Alzheimer disease continuum as a single summary score. The iADRS is validated and captures clinical progression from MCI due to Alzheimer disease through moderate dementia due to Alzheimer disease, and treatment effects have been demonstrated across MCI and Alzheimer disease with mild dementia. 6 , 9 , 21 - 27 The possible scores on the iADRS range from 0 to 144 (lower scores indicate greater impairment), and the meaningful within-patient change (MWPC) is a change of 5 points for those with Alzheimer disease with MCI and 9 points for those with Alzheimer disease with mild dementia. The MWPC, or minimal clinically important difference (MCID) as in Supplement 1 and 2 , is a threshold for outcome scores (either patient-reported or physician-measured) above which a patient or physician would consider the change meaningful. 28
Prespecified secondary outcomes included changes from baseline to 76 weeks by sum of boxes of the Clinical Dementia Rating Scale (CDR-SB), the ADAS-Cog 13 , the ADCS-iADL, and MMSE in the low/medium tau or combined population. Amyloid plaque reduction at 76 weeks, percentage of participants reaching amyloid clearance (<24.1 Centiloids measured by amyloid PET 9 , 29 ) at 24 weeks and 76 weeks, tau PET 1 (frontal cortical regions) change, volumetric MRI (vMRI; whole brain, hippocampus, and ventricles) change, and adverse events were additional secondary outcomes. Supplement 1 provides a complete listing and methodology of adverse events assessments. Amyloid-related imaging abnormalities of edema/effusion, amyloid-related imaging abnormalities of microhemorrhages and hemosiderin deposits, and infusion-related reactions were adverse events of special interest because they were considered class effects or observed in previous trials. 9 , 30 - 32 Secondary outcomes related to pharmacokinetics and antidrug antibodies were also prespecified and are planned for subsequent studies. Exploratory outcomes included change in plasma P-tau217 (C 2 N Diagnostics) at 76 weeks and time-based analyses: progression risk using the CDR Global score (CDR-G; progression defined as any increase from baseline in CDR-G at consecutive visits), participants with no progression at 1 year on the CDR-SB, and clinical progression delay (ie, months saved with treatment) on the iADRS and CDR-SB. Additional information about outcome measures, including score ranges and MWPCs, is provided in eMethods in Supplement 3 .
Prespecified primary and secondary outcomes were controlled for multiplicity (gated) at 76 weeks ( Supplement 2 and eMethods in Supplement 3 ) except for MMSE, changes in vMRI measurements, and adverse event assessments. Additional time points were gated for amyloid clearance and P-tau217. Nominal P values are reported for gated and nongated outcomes.
The trial was originally designed as a phase 2 trial with a plan to enroll 500 participants and assess CDR-SB as the primary outcome, but was subsequently amended to a phase 3 trial assessing the iADRS score as the primary outcome in February 2021 in an effort to confirm and expand the results of the TRAILBLAZER-ALZ trial. No unblinded data analysis of TRAILBLAZER-ALZ 2 was performed or used to inform design or analyses. Further details regarding major protocol or study adjustments are in eMethods in Supplement 3 and the trial protocol in Supplement 1 .
Revised study sample size and power calculations were based on the primary results from the TRAILBLAZER-ALZ trial, 9 where mean progression in the placebo and donanemab groups on iADRS was −10.06 and −6.86 (approximately 32% slowing of disease progression) over 76 weeks, respectively. Multiple longitudinal data sets were simulated and the natural cubic spline model with 2 degrees of freedom (NCS2) was fit to each sample to determine the power. The powering and sample size determination of the trial was based on the low/medium tau population. With a sample size of approximately 1000 randomized participants in the low/medium tau population and an assumed 30% discontinuation rate, the NCS model provided greater than 95% power to achieve statistical significance at a 2-sided α level of .05. The total planned enrollment (including both the low/medium and high tau populations) was 1800.
Most statistical analyses were done with SAS version 9.4 (SAS Institute). Some time-based progression analyses were analyzed with R Project version 4.3.0 (R Foundation).
The efficacy analyses were conducted by using the evaluable efficacy population (participants with a baseline and at least 1 postbaseline efficacy measurement based on randomized treatment). A prespecified gated testing scheme 33 , 34 was used to control for study-wise type I error rate at 2-sided α level of .05, with 80% of initial α spend (.04) for multiplicity control allocated to the low/medium tau population and 20% of initial α spend (.01) for multiplicity control allocated to the combined population (testing scheme in Supplement 2 ; eMethods in Supplement 3 also describes time-based analyses not described below).
Clinical outcomes (except for CDR-SB) were primarily analyzed using an NCS2 model. The protocol-specified week value for each participant was used as a continuous variable to create NCS basis functions with knot locations at 0 weeks, the median observation time, and 76 weeks. The model restricted baseline estimates to be the same for treatment and placebo groups. The baseline score and each scheduled postbaseline score were dependent variables in the model. The model’s independent variables included NCS basis expansion terms (2 terms), NCS basis expansion term × treatment interaction (2 terms), baseline age, concomitant acetylcholinesterase inhibitor and/or memantine use at baseline (yes/no), and randomization stratifying factors (pooled site and baseline tau category [baseline tau category in combined population only]). An unstructured variance covariance matrix was used to model the within-participant errors using restricted maximum likelihood. The Kenward-Roger approximation was used to estimate the denominator degrees of freedom.
The MMRM was used to primarily assess CDR-SB, plasma P-tau217, amyloid PET, and vMRI. The analysis model used change from baseline as the dependent variable. The model was adjusted for age, baseline value, visit as a categorical variable, treatment, baseline × visit interactions, treatment × visit interactions, concomitant acetylcholinesterase inhibitor/memantine use at baseline (CDR-SB only), and randomization stratifying factors of pooled site and, for combined population only, baseline tau category. For vMRI, only age and baseline brain volumes were covariates. The covariance matrix structure used was the same as NCS. Plasma P-tau 217 value was log 10 -transformed to meet the normality assumption.
Both the NCS2 and MMRM use the same protocol-specified time values for each participant in the analysis; the NCS2 model makes additional parametric assumptions for the shape of the longitudinal mean structure that can lead to increased efficiency.
The percent slowing relative to placebo was calculated by dividing the least-squares mean (LSM) change from baseline treatment differences at 76 weeks by the LSM change from baseline with placebo at 76 weeks and multiplying by 100.
ANCOVA analysis was conducted for tau PET standardized uptake value ratio (SUVR), with change from baseline to 76 weeks as the dependent variable and covariates of baseline tau SUVR, age, and, for the combined population, tau burden.
MMRM, NCS with 3 degrees of freedom model (NCS3), and bayesian disease progression model (DPM) were applied as sensitivity analyses for the primary outcome. DPM was applied to measure the proportion of disease progression in donanemab-treated participants relative to placebo-treated participants using a disease progression ratio, as previously described. 35 Details on sensitivity analyses for censoring after amyloid-related imaging abnormalities or infusion-related reactions, per-protocol analysis, and analysis of study completers are in eMethods in Supplement 3 . Details of subgroup analyses and time-based analyses are also described in eMethods in Supplement 3 .
Cox proportional hazard models were applied to CDR-G (gated), iADRS (nongated), and CDR-SB (nongated). Progression to next clinical stage was defined as any increase in CDR-G at 2 consecutive visits from baseline. MWPC was established as an iADRS change of greater than or equal to 5 for those with Alzheimer disease with MCI and greater than or equal to 9 points for those with Alzheimer disease with mild dementia and a CDR-SB change of greater than or equal to 1 point for those with Alzheimer disease with MCI and greater than or equal to 2 points for Alzheimer disease with mild dementia at 2 consecutive visits from baseline.
Analyses of the high tau population alone (ie, not combined with the low/medium tau population) for primary and secondary outcomes was performed post hoc.
Adverse events were evaluated in all participants exposed to study drug and were summarized according to event frequency by treatment assignment.
If less than 30% of the ADCS-iADL, 3 or fewer items of the ADAS-Cog 13, or 1 box of the CDR were missing, the total score for these assessments was imputed. If more items were missing than defined, the total score at that visit was considered missing ( Supplement 2 ). If either the ADCS-iADL or ADAS-Cog 13 scores were missing, the iADRS score was considered as missing. The missing data for NCS and MMRM analyses were handled by the likelihood-based mixed-effect model and the model parameters were estimated using restricted likelihood estimation incorporating all the observed data.
All presented primary, secondary, and exploratory outcomes were controlled for multiplicity (gated) in at least 1 population except for MMSE, vMRI measurements, and safety assessments. Of the 24 gated outcomes (eMethods in Supplement 3 ), 23 were statistically significant.
Of 8240 participants screened, 1736 were enrolled (mean age, 73.0 years; 996 [57.4%] women) and 76% completed the trial: 860 were assigned to receive donanemab and 876 were assigned to receive placebo ( Figure 1 ). Baseline characteristics are summarized by treatment groups in both low/medium tau (n = 1182) and combined populations (n = 1736) ( Table 1 ). As expected, the combined population had higher tau biomarkers at baseline due to the inclusion of participants with high tau pathology and showed greater impairment across baseline clinical assessments.
In the low/medium tau population, LSM change from baseline in the iADRS score at 76 weeks was −6.02 (95% CI, −7.01 to −5.03) in the donanemab group and −9.27 (95% CI, −10.23 to −8.31) in the placebo group (difference, 3.25 [95% CI, 1.88-4.62]; P < .001), representing a 35.1% (95% CI, 19.90%-50.23%) slowing of disease progression ( Figure 2 , Table 2 ).
In the combined population, LSM change from baseline in the iADRS score at 76 weeks was −10.19 (95% CI, −11.22 to −9.16) in the donanemab group and −13.11 (95% CI, −14.10 to −12.13) in the placebo group (difference, 2.92 [95% CI, 1.51-4.33]; P < .001), representing a 22.3% (95% CI, 11.38%-33.15%) slowing of disease progression ( Figure 2 , Table 2 ).
In the low/medium tau population, the differences between treatment groups in the LSM change from baseline at 76 weeks was −0.67 (95% CI, −0.95 to −0.40) (36.0% [95% CI, 20.76%-51.15%] slowing of clinical progression) for CDR-SB, 1.83 (95% CI, 0.91-2.75) (39.9% [95% CI, 19.15%-60.58%] slowing of clinical progression) for ADCS-iADL, and −1.52 (95% CI, −2.25 to −0.79) (32.4% [95% CI, 16.55%-48.35%] slowing of clinical progression) for ADAS-Cog 13 ( Figure 2 , Table 2 ; eFigure 1 and 3 in Supplement 3 ).
In the combined population, the differences in the LSM change from baseline to 76 weeks between the donanemab and placebo groups were −0.70 (95% CI, −0.95 to −0.45) (28.9% [95% CI, 18.26%-39.53%] slowing of clinical progression) for CDR-SB, 1.70 (95% CI, 0.84-2.57) (27.8% [95% CI, 13.48%-42.13%] slowing of clinical progression) for ADCS-iADL, and −1.33 (95% CI, −2.09 to −0.57) (19.5% [95% CI, 8.23%-30.83%] slowing of clinical progression) for ADAS-Cog13 ( Figure 2 , Table 2 ; eFigures 2 and 4 in Supplement 3 ).
At 76 weeks, brain amyloid plaque level decreased by 88.0 Centiloids (95% CI, −90.20 to −85.87) with donanemab treatment and increased by 0.2 Centiloids (95% CI, −1.91 to 2.26) in the placebo group in the low/medium tau population; in the combined population, amyloid plaque level decreased by 87.0 Centiloids (95% CI, −88.90 to −85.17) with donanemab treatment and decreased by 0.67 Centiloids (95% CI, −2.45 to 1.11) in the placebo group ( Figure 3 A). The percentages of donanemab-treated participants in the low/medium tau population who reached amyloid clearance 29 , 38 were 34.2% (95% CI, 30.22%-38.34%) at 24 weeks and 80.1% (95% CI, 76.12%-83.62%) at 76 weeks compared with 0.2% (95% CI, 0.03%-1.02%) at 24 weeks and 0% (95% CI, 0.00%-0.81%) at 76 weeks of placebo-treated participants. In the combined population, amyloid clearance was reached in 29.7% (95% CI, 26.56%-33.04%) of participants at 24 weeks and 76.4% (95% CI, 72.87%-79.57%) at 76 weeks of donanemab-treated participants compared with 0.2% (95% CI, 0.07%-0.90%) at 24 weeks and 0.3% (95% CI, 0.08%-1.05%) at 76 weeks of placebo-treated participants ( Figure 3 B).
Evaluation of the LSM change from baseline to 76 weeks in frontal tau SUVR (cerebellar gray reference) did not show a significant difference in the low/medium tau or in the combined population (eFigure 5 in Supplement 3 ). The difference in LSM change in tau SUVR from placebo in the frontal lobe at 76 weeks was −0.0002 (95% CI, −0.01 to 0.01; P = .97) in the low/medium tau population and −0.0041 (95% CI, −0.01 to 0.01; P = .45) in the combined population.
For both the low/medium tau and combined populations, at 76 weeks, vMRI (a non-gated secondary outcome) showed a greater decrease in whole brain volume, a lesser decrease in the hippocampal volume, and a greater increase in ventricular volume in the donanemab group than in the placebo group (eFigure 6 in Supplement 3 ).
P-tau217 was significantly reduced from baseline with donanemab treatment compared with placebo in the low/medium tau and combined population. The difference in LSM change in tau SUVR (log 10 -based) vs placebo was −0.25 (95% CI, −0.28 to −0.22; P < .001) in the low/medium tau population and −0.22 (95% CI −0.24 to −0.20; P < .001) in the combined population at 76 weeks ( Figure 3 C and D).
There was a 38.6% (CDR-G hazard ratio, 0.61 [95% CI, 0.47-0.80]; P < .001) lower risk of disease progression in the low/medium tau population and a 37.4% (CDR-G hazard ratio, 0.63 [95% CI, 0.51-0.77; P < .001) lower risk of disease progression in the combined population with donanemab treatment compared with placebo over the 18-month trial ( Figure 3 E and F; see eFigure 7 in Supplement 3 for nongated disease progression analyses of iADRS and CDR-SB). Substantial decline in the low/medium tau population occurred in 100 (18%) donanemab-treated participants and 163 (28%) placebo-treated participants and, in the combined population, occurred in 186 (23%) donanemab-treated and 288 (34%) placebo-treated participants. In addition, in the low/medium tau population, an estimated 47% of participants were stable (showed no decline in CDR-SB from baseline) with donanemab at 1 year compared with 29% of participants receiving placebo ( P < .001) (eTable 6 in Supplement 3 ). At 76 weeks, disease progression with donanemab treatment in the low/medium tau population was delayed by 4.36 months (95% CI, 1.87-6.85) on the iADRS and 7.53 months (95% CI, 5.69-9.36) on the CDR-SB.
Sensitivity analyses of the iADRS score (eFigure 8 in Supplement 3 ) using NCS3, MMRM, and DPM analyses, NCS2 in the completers and per protocol populations, and censoring change scores after amyloid-related imaging abnormalities edema/effusion and/or infusion-related reaction observations were consistent with the primary analysis (33.4%-39.6% slowing of clinical progression).
The findings as measured by iADRS and CDR-SB were generally consistent across baseline characteristic subgroups where the subgroup was sufficiently large (eFigure 9 in Supplement 3 ).
Analysis of the smaller (n = 552) high tau population alone (ie, not combined with the low/medium tau population) for all primary and secondary outcomes was completed post hoc. The difference between the donanemab and placebo groups in the LSM change from baseline at 76 weeks was 1.26 (95% CI, −1.77 to 4.28; P = .42) for the iADRS score and −0.69 (95% CI −1.19 to −0.20; P = .006) for the CDR-SB score. For additional assessments in the high tau population, see eTables 4, 5, and 10 and eFigures 10-13 in Supplement 3 .
The incidence of death was 1.9% in the donanemab group and 1.1% in the placebo group, while the incidence of serious adverse events was 17.4% in the donanemab group and 15.8% in the placebo group ( Table 3 ). In the donanemab group, 3 participants with serious amyloid-related imaging abnormalities subsequently died (2 APOE ε4 heterozygous carriers and one noncarrier; none were prescribed anticoagulant or anti-platelet medications; one resumed treatment after resolution of severe amyloid-related imaging abnormalities edema/effusion that was accompanied by severe amyloid-related imaging abnormalities microhemorrhages and hemosiderin deposits and one had superficial siderosis at baseline) (eTable 9 in Supplement 3 ). Treatment-emergent adverse events were reported by 759 of 853 participants (89.0%) receiving donanemab and 718 of 874 participants (82.2%) receiving placebo. Treatment discontinuation due to adverse events was reported in 112 participants receiving donanemab and 38 participants receiving placebo. The most common adverse events that led to treatment discontinuation were infusion-related reactions, either amyloid-related imaging abnormalities edema/effusion or microhemorrhages and hemosiderin deposits, and hypersensitivity (eTable 7 in Supplement 3 ).
Either amyloid-related imaging abnormalities of edema/effusion or microhemorrhages and hemosiderin deposits occurred in 314 participants (36.8%) receiving donanemab and 130 (14.9%) receiving placebo. Amyloid-related imaging abnormalities of edema/effusion, determined via MRI, occurred in 205 participants (24.0%) in the donanemab group and in 18 (2.1%) in the placebo group. Most amyloid-related imaging abnormalities of edema/effusion events were mild to moderate (see eTable 2 in Supplement 3 ) (n = 188 [93.1%] in the donanemab group; n = 17 [100%] in the placebo group). Symptomatic amyloid-related imaging abnormalities of edema/effusion were reported by 52 participants (6.1%) in the donanemab group (25.4% of those with amyloid-related imaging abnormalities of edema/effusion), with 45 participants (86.5%) having symptom resolution. Most cases (57.9%) of first amyloid-related imaging abnormalities of edema/effusion occurred after receiving up to 3 donanemab infusions. Serious amyloid-related imaging abnormalities of edema/effusion (see Table 3 ) occurred in 13 participants (1.5%) receiving donanemab. First events of amyloid-related imaging abnormalities of edema/effusion radiographically resolved in 198 (98.0%) donanemab-treated participants and 11 (64.7%) placebo-treated participants, with a mean amyloid-related imaging abnormalities of edema/effusion resolution time of 72.4 days for those receiving donanemab and 63.5 days for those receiving placebo. Edema/effusion were numerically less common among APOE ε4 noncarriers than carriers, with higher frequency among homozygotes than heterozygotes ( Table 3 ; further details in eTable 8 in Supplement 3 ).
The incidence of amyloid-related imaging abnormalities of microhemorrhages and hemosiderin deposits, determined via MRI, was higher in the donanemab group than the placebo group (268 participants [31.4%] vs 119 participants [13.6%]). Incidence of amyloid-related imaging abnormalities of microhemorrhages and hemosiderin deposits in the absence of amyloid-related imaging abnormalities of edema/effusion was not different between treatments (12.7% in the donanemab group vs 12.4% in the placebo group). The incidence of microhemorrhage and superficial siderosis was greater in the donanemab group than in the placebo group (microhemorrhage: 26.8% vs 12.5%; superficial siderosis: 15.7% vs 3.0%). Three intracerebral hemorrhages greater than 1 cm were recorded in the donanemab group and 2 were recorded in the placebo group ( Table 3 ).
Infusion-related reactions were reported by 74 participants (8.7%) in the donanemab group and 4 (0.5%) in the placebo group. Serious infusion-related reactions or hypersensitivity occurred in 3 participants (0.4%) in the donanemab group. Most infusion-related reactions were mild to moderate and occurred during or within 30 minutes of the end of the infusion and between the second and fifth infusion (73.6%). Anaphylactic reaction occurred in 3 participants (0.4%) in the donanemab group and were considered to be mild to moderate.
In this phase 3 trial, donanemab significantly slowed Alzheimer disease progression, based on the iADRS score, compared with placebo in the low/medium tau and combined tau populations and across secondary clinical outcomes of CDR-SB, ADAS-Cog 13 , and ADCS-iADL scores.
Donanemab treatment resulted in clinically meaningful benefit (considered to be >20% slowing of clinical progression 39 - 41 ) on the iADRS and CDR-SB scales for both the low/medium tau and combined populations, regardless of statistical model. Additional support for clinical relevance is the 38.6% risk reduction of disease progression as measured on the CDR-G score and the 4.4 to 7.5 months saved over the 18-month study (low/medium tau population). Furthermore, an estimated 47% of participants receiving donanemab had no change in the CDR-SB at 1 year (no disease progression), compared with 29% of participants receiving placebo.
This trial used a definition of a MWPC 28 based on any incremental change on the CDR-G scale (Alzheimer disease with MCI to mild Alzheimer disease or mild Alzheimer disease to moderate Alzheimer disease) or point changes of −5 on the iADRS and 1 on the CDR-SB for those with Alzheimer disease with MCI or −9 on the iADRS and 2 on the CDR-SB for those with Alzheimer disease with mild dementia at consecutive visits from baseline. In analyses assessing whether individual participants reached thresholds of clinically important progression over the course of the trial, donanemab resulted in significantly lower risk of meaningful change on the CDR-G as well as the prespecified nongated analyses of the iADRS and CDR-SB outcomes.
These clinical outcomes were achieved in 52% of low/medium tau participants completing donanemab treatment by 1 year, based on when a participant met amyloid clearance criteria. Limited-duration dosing was a distinct trial design feature reflecting donanemab binding specificity for amyloid plaque and implemented to decrease burden, cost, and potentially unnecessary treatments. 11 Early significant changes on both brain amyloid PET scans and P-tau217 blood test results suggest opportunities for clinical monitoring of therapy. Donanemab treatment resulted in significantly reduced brain amyloid plaque in participants at all time points assessed, with 80% (low/medium tau population) and 76% (combined population) of participants achieving amyloid clearance at 76 weeks. Clearance beyond 76 weeks, and associated Alzheimer disease biomarkers levels, are currently being studied in the ongoing extension phase. The lack of response in frontal tau-PET is inconsistent with the TRAILBLAZER-ALZ phase 2 results. 9 , 38 Additional regions have yet to be analyzed and reported. Factors resulting in this inconsistency will be examined. Changes in vMRI (including a greater decrease in whole brain volume in the donanemab group) were consistent with previous reports 9 , 42 and would benefit from further exploration.
The general belief is that treating Alzheimer disease at the earliest disease stage is likely to result in more clinically meaningful effects. 43 , 44 Post hoc evaluation in only high tau participants demonstrated no differences ( P < .05) on the primary outcome or on most secondary clinical outcomes in donanemab-treated compared with placebo-treated participants within the 18-month trial, with the exception of CDR-SB. Compared with significant differences in the low/medium tau population, this supports the hypothesis that a greater benefit from amyloid-lowering therapies may occur when initiated at an earlier disease stage.
Similar to other amyloid-lowering drugs, and the phase 2 TRAILBLAZER-ALZ trial, amyloid-related imaging abnormalities are an associated adverse event. When amyloid-related imaging abnormalities occur, they are mostly asymptomatic and resolve in approximately 10 weeks. When symptoms occur, they are usually mild, consisting of a headache or increase in confusion, but can have more severe symptoms such as seizures. In some instances, these events can be life-threatening and result in, or lead to, death. For 1.6% of participants in the donanemab treatment group, amyloid-related imaging abnormalities led to serious outcomes, such as hospitalization, and required supportive care and/or corticosteroid use. It is also important to note that 3 deaths in TRAILBLAZER-ALZ 2 occurred after serious amyloid-related imaging abnormalities. Further evaluation of the risks associated with serious and life-threatening amyloid-related imaging abnormalities will be important to identify the best approaches for managing risks and maximizing benefit, in addition to earlier treatment of the disease when less amyloid pathology is present and, theoretically, when amyloid-related imaging abnormalities risk is lower.
This study has several limitations. First, an inherent limitation to limited-duration dosing was variability in total donanemab doses received and/or duration of donanemab dosing. Second, data collection was for 76 weeks, limiting long-term understanding of donanemab; however, a study extension is ongoing. Third, the studied populations were primarily White (91.5%), which may limit generalizability to other populations due to a lack of racial and ethnic diversity. Fourth, although no related protocol amendments were necessary, this trial was conducted during the COVID-19 pandemic, and COVID-19 was the most commonly reported adverse event across treatment groups (see eMethods in Supplement 3 ). Fifth, direct comparison of results to other amyloid-targeting trials is not possible due to trial design differences such as stratification by baseline tau PET category. Sixth, amyloid-related imaging abnormality and infusion-related reaction occurrences may have caused participants and investigators to infer treatment assignment; attempts to minimize bias included blinding CDR raters to adverse event information and, based on sensitivity analyses, censoring change scores after the first observation of amyloid-related imaging abnormalities of edema/effusion and/or infusion-related reactions did not impact the results.
Among participants with early symptomatic Alzheimer disease and amyloid and tau pathology, donanemab significantly slowed clinical progression at 76 weeks in those with low/medium tau and in the combined low/medium and high tau pathology population.
Accepted for Publication: June 28, 2023.
Published Online: July 17, 2023. doi:10.1001/jama.2023.13239
Corresponding Author: John R. Sims, MD, Eli Lilly and Company, Lilly Corporate Center DC 1532, Indianapolis, IN 46285 ( [email protected] ).
Author Contributions: Dr Solomon had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Sims, Zimmer, Ardayfio, Sparks, Wessels, Wang, Collins, Salloway, Mintun, Skovronsky.
Acquisition, analysis, or interpretation of data: Sims, Zimmer, Evans, Lu, Ardayfio, Sparks, Wessels, Shcherbinin, Wang, Nery, Collins, Solomon, Apostolova, Hansson, Ritchie, Brooks, Mintun, Skovronsky.
Drafting of the manuscript: Sims, Evans, Ardayfio, Wang, Nery, Collins, Ritchie, Skovronsky.
Critical review of the manuscript for important intellectual content: Sims, Zimmer, Ardayfio, Wessels, Shcherbinin, Salloway, Apostolova, Ritchie, Mintun, Skovronsky.
Statistical analysis: Lu, Ardayfio, Sparks, Wang, Salloway, Skovronsky.
Obtained funding: Sims, Brooks, Mintun, Skovronsky.
Administrative, technical, or material support: Zimmer, Evans, Wessels, Shcherbinin, Collins, Salloway, Brooks, Mintun, Skovronsky.
Supervision: Sims, Wessels, Nery, Collins, Solomon, Brooks, Mintun, Skovronsky.
Other - imaging and biomarker analysis: Collins.
Other - suggested additional analyses: Apostolova.
Conflict of Interest Disclosures: Dr Sims reported being an employee of Eli Lilly and Company during the conduct of the study. Dr Zimmer reported receiving personal fees from and being a shareholder in Eli Lilly and Company during the conduct of the study. Dr Evans reported being an employee of and minority shareholder in Eli Lilly and Company during the conduct of the study. Dr Lu reported being an employee of and stockholder in Eli Lilly. Dr Ardayfio reported being an employee of and stockholder in Eli Lilly during the conduct of the study. Dr Wessels reported being a minor shareholder in Eli Lilly and Company outside the submitted work. Dr Shcherbinin reported being an employee of and stockholder in Eli Lilly and Company during the conduct of the study and Eli Lilly and Company having patents pending relevant to this research. Dr Nery reported being an employee of and shareholder in Eli Lilly and Company during the conduct of the study. Dr Collins reported being an employee of and stockholder in from Eli Lilly and Company during the conduct of the study. Dr Salloway reported receiving personal fees and grants from Biogen, Eli Lilly, Genentech, Avid, Roche, Eisai, Novartis, Acumen, NovoNordisk, and Prothena during the conduct of the study. Dr Apostolova reported receiving grants from NIA, Alzheimer Association, AVID Radiopharmaceuticals, Life Molecular Imaging, and Roche Diagnostics and personal fees from Eli Lilly, Biogen, Two Labs, IQVIA, Genentech, Siemens, Corium, GE Healthcare, Eisa, Roche Diagnostics, Alnylam, Alzheimer Association, and from the US Food And Drug Administration outside the submitted work. Dr Hansson reported personal fees from AC Immune, Amylyx, Alzpath, BioArtic, Biogen, Cerveau, Eisai, Eli Lilly, Fujirebio, Merk, Novartis, Novo Nordisk, Roche, Sanofi, and Siemens outside the submitted work. Dr Ritchie reported receiving personal fees from Actinogen, Biogen, Cogstate, Eisai, Eli Lilly, Janssen Cilag, Merck, Novo Nordisk, Roche Diagnostics, and Signant and being founder of and majority shareholder in Scottish Brain Sciences outside the submitted work. Dr Brooks reported being an employee of and shareholder in Eli Lilly and Company. Dr Mintun reported being an employee of and shareholder in Eli Lilly and Company and having a patent pending with Eli Lilly and Company. Dr Skovronsky reported being an employee of and shareholder in Eli Lilly and Company. No other disclosures were reported.
Funding/Support: This work was funded by Eli Lilly and Company.
Role of the Funder/Sponsor: Eli Lilly and Company was responsible for design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Group Information: The TRAILBLAZER-ALZ 2 Investigators appear listed in Supplement 4 .
Data Sharing Statement: See Supplement 5 .
Additional Contributions: We thank all the trial participants and their families and caregivers who participated in the TRAILBLAZER-ALZ 2 trial as well as the site staff, raters, and site investigators (see list in Supplement 4 ); members of the data and safety monitoring board; vendor partners including BioAgilitix, Clario, Clinical Trial Media, Cogstate, C 2 N Diagnostics, Invicro, IQVIA, Labcorp and Quanterix. The authors would like to thank the following salaried employees of Eli Lilly and Company for their contributions to TRAILBLAZER-ALZ 2, for which they received no additional compensation: Andrea Abram, MBA; Hrideep Antony, BS; Anupa Arora, MD; Theresa Bauer, BS; Jude Burger, MS; Yang Dai, MS; Russell A Delgiacco, MS; Marybeth Devine, BS; Dawn East, BS; Tim Edison, PharmD; Naohisa Hatekeyama, MS; Jeremy T Hemiup, MS, MBA; Stacy A Huckins, BS; Blaire Iris Kaufman, BS; Rashna Khanna, MD; Min Jung Kim, MS; Albert Lo, MD, PhD; Dedeepya Masarapu, B Pharm; Shoichiro Sato, MD, PhD; Adam Schaum, MAS; Linda Shurzinske, MS; Andrea L Speas, RNN, BSN; LisaAnn Trembath, MS; Giulia Tronchin, PhD; Melissa Veenhuizen, DVM, MS; Wen Xu, PhD; and Wei Zhou, MS. The authors would like to acknowledge Paula Hauck, PhD; Deirdre Hoban, PhD; and Carmen Deveau, PhD, salaried employees of Eli Lilly and Company, for project management support, and strategic scientific communication expertise, for which they received no additional compensation.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

Immune Modulation in Solid Tumors: A Phase 1b Study of RO6870810 (BET Inhibitor) and Atezolizumab (PD-L1 Inhibitor)
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Daniel Marbach
- For correspondence: [email protected]
- ORCID record for Iakov I Davydov
- Info/History
- Supplementary material
- Preview PDF
Purpose: Bromodomain and extra-terminal domain (BET) inhibitors (BETi) have demonstrated epigenetic modulation capabilities, specifically in transcriptional repression of oncogenic pathways. Preclinical assays suggest that BETi potentially attenuates the PD1/PD-L1 immune checkpoint axis, supporting its combination with immunomodulatory agents. Patients and Methods: A Phase 1b clinical trial was conducted to elucidate the pharmacokinetic and pharmacodynamic profiles of the BET inhibitor RO6870810, as monotherapy and in combination with the PD-L1 antagonist atezolizumab, in patients with advanced ovarian carcinomas and triple-negative breast cancer (TNBC). Endpoints included maximum tolerated dosages, adverse event profiling, pharmacokinetic evaluations, and antitumor activity. Pharmacodynamic and immunomodulatory effects were assessed in tumor tissue (by immunohistochemistry and RNA-seq) and in peripheral blood (by flow cytometry and cytokine analysis). Results: The study was terminated prematurely due to a pronounced incidence of immune-related adverse effects in patients receiving combination of RO6870810 and atezolizumab. Anti-tumor activity was limited to 2 patients (5.6%) showing partial response. Although target engagement was confirmed by established BETi pharmacodynamic markers in both blood and tumor samples, BETi failed to markedly decrease tumor PD-L1 expression and had a suppressive effect on anti-tumor immunity. Immune effector activation in tumor tissue was solely observed with the atezolizumab combination, aligning with this checkpoint inhibitor's recognized biological effects. Conclusions: The combination of BET inhibitor RO6870810 with the checkpoint inhibitor atezolizumab presents an unfavorable risk-benefit profile for ovarian cancer and TNBC (triple-negative breast cancer) patients due to the increased risk of augmented or exaggerated immune reactions, without evidence for synergistic anti-tumor effects.
Competing Interest Statement
Authors1-5 are employees and/or shareholders of F Hoffmann-La Roche. All authors have received grants and non-financial or other support from F. Hoffmann-La Roche, during the conduct of the study. Editorial support, funded by the sponsor, was provided by an independent medical writer under the guidance of the authors.
Clinical Trial
NCT03292172
Funding Statement
This study was funded by F. Hoffmann-La Roche Ltd.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
This study was approved by each center's ethics committee or institutional review board, and the study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. All participants provided written informed consent. List of independent Ethics Committees/Institutional Review Boards with dates of approval: (1) University Health Network Research Ethics Board, 700 Bay Street, 17th Floor, Suite 1700, M5G 1Z6, Toronto, Ontario, CANADA (Approval: 12-Oct-2017); (2) Dana Farber Cancer Institute/Dana-Farber/Harvard Cancer center, 450 Brookline Ave, OS-200, Boston, MA, 02215, UNITED STATES (Approval: 07-Nov-2017); (3) Western Institutional Review Board, 1019 39th Avenue SE, Ste 120, Puyallup, WA, 98374, UNITED STATES (Approval: 18-Oct-2017); (4) Peter MacCallum Cancer Centre Ethics Committee, 305 Grattan Street, 3000, Melbourne, Victoria, AUSTRALIA (Approval: 01-Aug-2018); (5) IntegReview Ethical Review Board, 3001 S. Lamar Blvd., Suite 210, Austin, TX, 78704, UNITED STATES (Approval: 03-Sept-2018).
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Data Availability
Qualified researchers may request access to individual participant-level data through the clinical study data request platform (https://vivli.org/ourmember/roche/). Further details on F. Hoffmann-La Roche Ltd's criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on F. Hoffmann-La Roche Ltd's Global Policy on the Sharing of Clinical Information and the procedure to request access to related clinical study documents, see https://www.roche.com/innovation/process/clinical-trials/data-sharing
View the discussion thread.
Supplementary Material
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Addiction Medicine (334)
- Allergy and Immunology (657)
- Anesthesia (177)
- Cardiovascular Medicine (2561)
- Dentistry and Oral Medicine (309)
- Dermatology (217)
- Emergency Medicine (389)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (905)
- Epidemiology (12064)
- Forensic Medicine (10)
- Gastroenterology (741)
- Genetic and Genomic Medicine (3978)
- Geriatric Medicine (375)
- Health Economics (666)
- Health Informatics (2567)
- Health Policy (991)
- Health Systems and Quality Improvement (951)
- Hematology (357)
- HIV/AIDS (823)
- Infectious Diseases (except HIV/AIDS) (13563)
- Intensive Care and Critical Care Medicine (783)
- Medical Education (394)
- Medical Ethics (106)
- Nephrology (421)
- Neurology (3743)
- Nursing (206)
- Nutrition (558)
- Obstetrics and Gynecology (717)
- Occupational and Environmental Health (684)
- Oncology (1946)
- Ophthalmology (565)
- Orthopedics (233)
- Otolaryngology (299)
- Pain Medicine (246)
- Palliative Medicine (72)
- Pathology (469)
- Pediatrics (1087)
- Pharmacology and Therapeutics (452)
- Primary Care Research (442)
- Psychiatry and Clinical Psychology (3342)
- Public and Global Health (6406)
- Radiology and Imaging (1354)
- Rehabilitation Medicine and Physical Therapy (790)
- Respiratory Medicine (857)
- Rheumatology (393)
- Sexual and Reproductive Health (395)
- Sports Medicine (336)
- Surgery (430)
- Toxicology (51)
- Transplantation (184)
- Urology (161)
- OIG Reports that Clinical Trials Lack Diverse Subjects: What Role Can Artificial Intelligence Play?

As the largest public funder of biomedical research in the world, the National Institutes of Health (“NIH”) annually funds over $38 billion in extramural research, including about $6 billion for clinical trials. On May 28, 2024, OIG published a report (“OIG Report”) summarizing its finding that, of the NIH‑funded clinical trials randomly selected by OIG for review, a majority failed to meet NIH’s requirements for inclusion of diverse trial participants.
As reiterated in the OIG Report, the 1993 NIH Revitalization Act recognizes that diversity in clinical trial enrollment is crucial for closing health disparities and for producing research that is scientifically generalizable. Accordingly, NIH requires biomedical researchers seeking NIH funding for clinical trials to submit, as part of their grant applications, enrollment plans that justify enrollment targets broken down by sex, race, and ethnicity, unless they can present some clear and compelling rationale (generally excluding cost considerations) as to why inclusion of certain groups (children, minority groups, and women, including pregnant women) is inappropriate. More recently, pursuant to the 21st Century Cures Act , NIH also monitors compliance with inclusion enrollment plans through periodic reports submitted by researchers to Clinicaltrials.gov , which must include valid analyses of outcomes by sex or gender and race and/or ethnicity.
Nonetheless, as the OIG Report reflects, NIH has had limited success in improving enrollment of diverse subjects. One of OIG’s recommendations to NIH is to “develop additional ways of supporting researchers in meeting inclusion enrollment targets.” Per the OIG Report, “[c]ommon hurdles for diverse enrollment include increased costs (e.g., providing translated informed consent for patients with limited English proficiency or pregnancy tests for females), lack of outreach to diverse communities about local clinical trials, and implicit biases, among other barriers.”
Use and Regulation of AI in Clinical Trial Enrollment
Artificial intelligence (“AI”) is already being explored for its potential to improve clinical trial design and management and shows promise for patient recruitment, such as by optimizing site selection and creating more targeted engagement initiatives. For example, during a recent Food & Drug Administration (“FDA”) podcast , the Director of the Office of Medical Policy within the FDA’s Center for Drug Evaluation and Research noted that, “AI [is] being used to assist in recruitment, and is being really developed and used … to more effectively connect individuals as part of the trials. This can … involve mining vast amounts of data from diverse sources … including social media, medical literature, registries, and structured and unstructured data in electronic health records.” However, as numerous studies have shown , bias in AI—including underrepresentation of certain populations in the data sets used to train the AI—can further exacerbate inequities.
In a development that could arguably help ameliorate problems of bias in clinical research, on May 6, 2024, the United States Department of Health and Human Services (“HHS”) Office for Civil Rights (“OCR”) published a final rule, “Nondiscrimination in Health Programs and Activities,” 89 Fed. Reg. 37522 (“Final Rule”). The Final Rule, codified at 45 C.F.R. § 92.210 and effective May 1, 2025, prohibits specified covered entities from discriminating on the basis of race, color, national origin, sex, age, or disability in certain “health programs or activities” through the use of “patient care decision support tools,” and expressly addresses certain clinical research activities.
Pursuant to the Final Rule, covered entities include (1) recipients of Federal financial assistance, including any grant, loan, credit, subsidy, contract (other than a procurement contract but including a contract of insurance), or any other arrangement by which the federal government, directly or indirectly, provides assistance or otherwise makes assistance available; (2) HHS; and (3) any health benefit exchange created under Title I of the Affordable Care Act (“ACA”). As described in more detail, below, a “health program or activity” is defined to span clinical care, health insurance coverage and health education activities, expressly includes clinical research, and quite broadly extends to “all of the operations of any entity principally engaged” in one of the defined health programs or activities.
The Final Rule also defines “patient care decision support tool” as “any automated or non‑automated tool, mechanism, method, technology, or combination thereof used by a covered entity to support clinical decision‑making in its health programs or activities,” such as clinical guidance produced from an algorithm or risk adjustment modeling. This definition is very broad and encompasses any tools in a provider or payer’s health programs and activities that assist with screening, predicting risk, diagnosing, treating, planning, allocating resources, determining eligibility, conducting utilization review, or health care operations that “affect the patient care that individuals receive.”
Potential Implications of the Final Rule for Clinical Research
Importantly, the Final Rule explicitly includes clinical research within the definition of “health program or activity.” This indicates that even if, for example, a drug study is experimental in nature and offers no direct medical or curative benefit to its research subjects, if the treatment of research subjects under the study involves patient care decision support tools, the Final Rule’s anti‑bias standards would appear to apply. Commentary published in the Final Rule indicates that the potential for clinical research to impact “the most promising advances in patient care” and the involvement of human subjects (unlike in laboratory research) is a sufficient nexus to patient care for clinical research to be included. Notably, the Final Rule is not intended to alter the requirement that NIH‑funded clinical trials enroll diverse subjects unless doing so would be inappropriate.
With respect to the obligations imposed on covered entities using patient care decision support tools in its health program or activities, the Final Rule takes an approach consistent with the National Institutes of Standards and Technology’s AI Risk Management Framework , and imposes on covered entities:
- An ongoing duty to “make reasonable efforts to identify uses of patient care decision support tools in its health programs or activities that employ input variables or factors that measure race, color, national origin, sex, age, or disability,” and
- A duty to “mitigate the risk of discrimination resulting from the tool’s use in its health programs or activities.”
This means that hospitals, health systems, provider entities, and payers now have an affirmative obligation to identify bias in any patient care decision support tools and to take reasonable steps to mitigate discrimination once the covered entity becomes aware, or if the covered entity should be aware, of the potential for discrimination resulting from use of such tools.
In assessing the sufficiency of a covered entity’s efforts, OCR may consider, among other factors:
- The size and resources of the covered entity;
- Whether the covered entity used the tool as intended by the developer and, as applicable, as approved by regulators;
- Whether the developer informed the covered entity about relevant risks of bias; and
- “[W]hether the covered entity has a methodology or process in place for evaluating the patient care decision support tools it adopts or uses, which may include seeking information from the developer, reviewing relevant medical journals and literature, obtaining information from membership in relevant medical associations, or analyzing comments or complaints received about patient care decision support tools.”
In addition, under the definition of “health program or activity,” where a covered entity is principally engaged in clinical research or another health care program or activity enumerated by the Final Rule, all uses of patient care decision support tools by the covered entity, across the entity, must comply with the Final Rule.
Notably, although the Final Rule would certainly capture academic medical centers and other recipients of NIH clinical trial funding, it would not necessarily capture clinical trials funded solely by industry sponsors and conducted, for example, by clinical research organizations, outside of an academic setting, unless they receive federal funding. Nonetheless, the Final Rule has the potential indirectly to shape the entire field of clinical research, as developers of AI for use in clinical trials may wish to create a product that is marketable to academic medical centers and other kinds of covered entities, regardless of funding source. Moreover, on May 29, the FDA announced that, in accordance with President Biden’s Executive Order 14110 , it is planning to release AI guidance later this year, which would extend to all phases of drug development regulated by the FDA and can be expected to align with the Final Rule.
OCR is requesting additional comments, including whether to expand the scope of the Final Rule to include other decision support tools that are being used in covered entities’ health programs and activities that do not directly impact patient care and clinical decision‑making, but may violate Section 1557 of the ACA. For example, automated billing and coding decision support tools.
[ View source .]
Related Posts
- OIG Reaffirms Its Concern About “Carving Out” Federal Health Care Program Business
- OIG Issues Final Information Blocking Enforcement Rule and Highlights the Potential for Referrals to the FTC and FCA Liability
- Another Unique Integrity Agreement Signals a Trend towards HHS-OIG’s Comfort with a Belt and Suspenders
Latest Posts
- No Surprises Here! Divergent Court Rulings Spotlight Ongoing Challenges in No Surprises Act Implementation; Tee Up Split in Authority on Award Enforcement Mechanisms
See more »
DISCLAIMER: Because of the generality of this update, the information provided herein may not be applicable in all situations and should not be acted upon without specific legal advice based on particular situations. Attorney Advertising.
Refine your interests »
Written by:

PUBLISH YOUR CONTENT ON JD SUPRA NOW
- Increased visibility
- Actionable analytics
- Ongoing guidance
Published In:
Proskauer - health care law brief on:.

"My best business intelligence, in one easy email…"

IMAGES
VIDEO
COMMENTS
The database is managed by Health Canada and provides a source of information about Canadian clinical trials involving human pharmaceutical and biological drugs. Access the Clinical Trials Database. Patients can access the database to determine if a clinical trial has met the regulatory requirements. The database may also assist Canadians in ...
Health Canada does not sponsor or conduct drug research. Sponsors of clinical trials are usually: drug companies; researchers from a hospital, university or research organization; Clinical trial application. Before conducting a trial, the sponsor submits a clinical trial application to Health Canada. Our scientists review the application to ...
A clinical trial is a research study involving human participants that evaluates the safety and/or effects of one or more interventions on health outcomes. The CTF earmarked $250M to reinforce Canada's clinical trials infrastructure, training, and research.
From Health Canada. You may search by one or more of the criteria immediately below, or alternatively by either Protocol Number or Control Number. When typing inside fields, do not include punctuation marks such as hyphens, commas, colons, brackets and wildcard characters (%). Search criteria. Medical condition:
CIHR is strengthening the clinical trials ecosystem to improve health care and health outcomes for all Canadians. By investing in key initiatives such as the Clinical Trials Fund and the SPOR Innovative Clinical Trials Initiative, CIHR is accelerating the development and implementation of the best methods in clinical trials to ensure Canada is prepared to face health challenges.
Canada's competitiveness in clinical trials is also supported by strong government support in public research infrastructure including over $1 billion investment by the Canadian Institutes of Health Research (CIHR) in health research funding; as well as a world-class contract research sector with extensive capabilities in phase I-IV clinical ...
s. (Chart 1 and Chart 5)The majority of clinical trial activities in Canada took pl. ce in Ont. io and Quebec. (Chart 6)Oncology was the main therapeutic area accounting for 38% of ongoing trials, followed by trials for central nervous system drugs (15%), cardiovascular drugs (8%), and infe. tious dis.
The Canadian Institutes of Health Research (CIHR) is committed to strengthening the clinical trials ecosystem to improve health care and health outcomes for all Canadians. CIHR is now looking to build a long-term strategy to support Canada's clinical trials ecosystem. In the fall of 2022, CIHR launched Building the future of clinical trials ...
Message from the President: Proposed Clinical Trials Strategy for Canada. In the winter of 2023, CIHR published the summary of a public consultation outlining the state of clinical trials in Canada. Over 140 individuals representing researchers, patient and citizen groups, research and health networks, government departments, and several ...
Health Canada. As per the CanadaFDA, the CanadaFDR, and the G-CanadaCTApps, Health Canada (HC) is the competent authority responsible for clinical trial approvals, oversight, and inspections in Canada. The G-CanadaCTApps states that the HC grants permission for clinical trials to be conducted in the country, and regulates the sale and importation of drugs for use in clinical trials in ...
The Canadian Cancer Trials Group (CCTG) is a national, academic clinical trials organization that conducts research in the areas of cancer diagnosis, treatment, and prevention. Founded in 1980, the CCTG is headquartered at Queen's University in Kingston, Ontario, and has over 80 member institutions across Canada and around the world.
Clinical trials are research studies that look at new ways to prevent, find and treat cancer. They can help us understand cancer better or discover what works best for particular types of cancer or groups of people. ... We can give information about cancer care and support services in Canada only.
A highlight in our legacy is JDRF's Canadian Clinical Trial Network (JDRF CCTN), which was established in 2009 to accelerate innovative solutions for the management, care, and cure of T1D. Created in partnership with the Government of Canada, funding for JDRF CCTN came from a commitment of $20 million by the Federal Economic Development ...
For information about clinical trials, cancer, cancer treatment or programs and resources for people affected by cancer, please contact the Canadian Cancer Society at 1-888-939-3333, Live Chat with an Information Specialist on cancer.ca , or send an email to [email protected] . Canadian Cancer Trials is a national, bilingual, searchable database ...
Syneos Health | Clinical Trials Montreal and Quebec. New name. Same mission: to contribute to the medicine of tomorrow! Created through the merger of two industry leading companies - INC Research and inVentiv Health - we bring together approximately 27,000 clinical and commercial minds with the ability to support customers in more than 110 ...
List of Contract Research Organizations and representative ofices of global СROs in Canada. ... Canadian Centre for Clinical Trials is a contract research organization with more than a decade of experience, qualified experts, a personalized approach, and competitive prices. We will provide all tools to support your clinical trial all the way. I...
The Clinical Trials Network (CTN) is able to conduct rapid clinical trials to address public health-relevant immunization questions in large and specialized groups with a focus on safety, immunogenicity, and mechanisms of immunity. CTN includes sites in Vancouver, Calgary, Winnipeg, Hamilton, Toronto, Ottawa, Sudbury, Montreal, Quebec City, and Halifax.
In 2020, Lilly Canada managed research at 418 sites across Canada. For more information on Lilly's clinical trials, visit www.ClinicalTrials.gov. Please note that this information is in English only. Or, you can contact our Customer Response Centre at 1-888-545-5972.
Health Canada. As per the CanadaFDA, the CanadaFDR, the G-CanadaCTApps, and CAN-29, Health Canada (HC) is the competent authority responsible for clinical trial approvals, oversight, and inspections in Canada. The G-CanadaCTApps states that the HC grants permission for clinical trials to be conducted in the country, and regulates the sale and importation of drugs for use in clinical trials in ...
Canada is a World Leader in Clinical Research Canada currently ranks fourth in the world for number of clinical trial sites. The volume and growth of clinical trials taking place nationally signals promising job and career advancement opportunities. ... The clinical research and trials industry has quickly entered a new phase, initiated and ...
Clinical trials are research studies that are done to investigate new treatments through the observation of patients. By observing how many patients respond on the tested therapy, researchers can confirm whether new treatments are beneficial for patients. Many clinical trials are currently in progress and taking part in a clinical trial may provide valuable information to help researchers make ...
Health Canada (Form) Dossier ID Request Form for Pharmaceutical Clinical Trial Dossiers (Last Updated November 2, 2023) Health Canada (International Guidance) A Selective Approach to Safety Data Collection in Specific Late-Stage in Specific Pre-Approval or Post-Approval Clinical Trials E19 (Step 4 Version) (September 27, 2022)
The Government of Canada has invested $250 million through CIHR to create the Clinical Trials Fund as part of a $2.2 billion investment in Canada's Biomanufacturing and Life Sciences Strategy, which aims to boost the biomanufacturing and life science sector and protect Canadians against future pandemics and health emergencies.
Interventional study (clinical trial) — studies new tests, treatments, drugs, surgical procedures or devices. Medical records research — uses historical information collected from medical records of large groups of people to study how diseases progress and which treatments and surgeries work best.
What are clinical trials? A clinical trial is a research study involving human participants that evaluates the safety and/or effects of one or more interventions on health outcomes. Interventions include, but are not limited to, drugs, vaccines, radiopharmaceuticals, cells and other biological products, surgical procedures, radiologic ...
Clinical trials are research studies that look at ways to prevent, detect, or treat diseases and conditions. They are critical to understanding and treating mental illnesses. Clinical trials are the primary way researchers determine if a new treatment is safe and effective in people. Clinical trials can study: New drugs or combinations of drugs.
Research; Participate in Cancer Research; Find NCI-Supported Clinical Trials; ... Share this clinical trial with your doctor: Print this trial Email this trial. Have a question? We're here to help. Chat with us: LiveHelp Call us: 1-800-4-CANCER (1-800-422-6237) Which trials are right for you?
Findings In this randomized clinical trial that included 1736 participants with early symptomatic Alzheimer disease and amyloid and tau pathology, the least-squares mean change in the integrated Alzheimer Disease Rating Scale score (range, 0-144; lower score indicates greater impairment) at 76 weeks was −6.02 in the donanemab group and −9. ...
Purpose: Bromodomain and extra-terminal domain (BET) inhibitors (BETi) have demonstrated epigenetic modulation capabilities, specifically in transcriptional repression of oncogenic pathways. Preclinical assays suggest that BETi potentially attenuates the PD1/PD-L1 immune checkpoint axis, supporting its combination with immunomodulatory agents. Patients and Methods: A Phase 1b clinical trial ...
As the largest public funder of biomedical research in the world, the National Institutes of Health ("NIH") annually funds over $38 billion in extramural research, including about $6 billion ...