
- Previous Article
- Next Article

Presentation
Clinical pearls, case study: complicated gestational diabetes results in emergency delivery.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Ginny Lewis; Case Study: Complicated Gestational Diabetes Results in Emergency Delivery. Clin Diabetes 1 January 2001; 19 (1): 25–26. https://doi.org/10.2337/diaclin.19.1.25
Download citation file:
- Ris (Zotero)
- Reference Manager
A.R. is a 33-year-old caucasian woman initially diagnosed with diabetes during a recent pregnancy. The routine glucose challenge test performed between 28 and 29 weeks gestation was elevated at 662 mg/dl. A random glucose completed 1–2 days later was also elevated at 500 mg/dl. A follow-up HbA 1c was elevated at 11.6%. Additional symptoms included a 23-lb weight loss over the past 3–4 weeks with ongoing “flu-like” symptoms, including fatigue, nausea, polyuria, and polydypsia.
A.R. had contacted her obstetrician’s office when her symptoms first appeared and was told to contact her primary care provider for the “flu” symptoms. She had called a nurse triage line several times over the previous 2–3 weeks with ongoing symptoms and was told to rest and take fluids.
She presented to her primary care provider 3 days after the HbA 1c was drawn for ongoing evaluation of hyperglycemia. At that time, she was symptomatic for diabetic ketoacidosis. She was hospitalized and started on an insulin drip.
A.R.’s hospitalization was further complicated with gram-negative sepsis, adult respiratory distress syndrome, and Crohn’s disease with a new rectovaginal fistula. She was intubated as her respiratory status continued to decline and was transferred to a tertiary medical center for ongoing management. She required an emergency Caesarian section at 30 1/7 weeks gestation due to increased fetal distress.
A.R. had no family history of diabetes with the exception of one sister who had been diagnosed with gestational diabetes. Her medical history was significant for Crohn’s disease diagnosed in 1998 with no reoccurrence until this hospitalization. Her pre-pregnancy weight was 114–120 lb. She had gained 25 lb during her pregnancy and lost 23 lb just before diagnosis.
A.R.’s blood glucose levels improved postpartum, and the insulin drip was gradually discontinued. She was discharged on no medications.
At her 2-week postpartum visit, home blood glucose monitoring indicated that values were ranging from 72 to 328 mg/dl, with the majority of values in the 200–300 mg/dl range. A repeat HbA 1c was 8.7%. She was restarted on insulin.
1. What is the differential diagnosis of gestational diabetes versus type 1 diabetes?
2. At what point during pregnancy should insulin therapy be instituted for blood glucose control?
3. How can communication systems be changed to provide for integration of information between multiple providers?
Gestational diabetes is defined as “any degree of carbohydrate intolerance with onset first recognized during pregnancy. This definition applies whether insulin ... is used for treatment and whether or not the condition persists after pregnancy.” 1 Risk assessment is done early in the pregnancy, with average-risk women being tested at 24–28 weeks’ gestation and low-risk women requiring no additional testing. 1 , 2 A.R. met the criteria for average risk based on age and a first-degree family member with a history of gestational diabetes.
Screening criteria for diagnosing diabetes include 1 ) symptoms of diabetes plus casual plasma glucose >200 mg/dl (11.1 mmol/l), or 2 ) fasting plasma glucose >126 mg/dl (7.0 mmol/l), or 3 ) 2-h plasma glucose >200 mg/dl (11.1 mmol/l) during an oral glucose tolerance test (OGTT). 3 For women who do not meet the first two criteria, a glucose challenge test (GCT) measuring a 1-h plasma glucose following a 50-g oral glucose load is acceptable. For those women who fail the initial screen, practitioners can then proceed with the OGTT. 1
In A.R.’s case, she most likely would have met the first criterion if a casual blood glucose had been measured. She had classic symptoms with weight loss, fatigue, polyuria, and polydypsia. Her 1-h plasma glucose following the glucose challenge was >600 mg/dl, which suggests that her casual glucose would also have been quite high.
Medical nutrition therapy (MNT) is certainly a major part of diabetes management. However, with this degree of hyperglycemia, MNT would not be adequate as monotherapy. Treatment for gestational diabetes includes the use of insulin if fasting blood glucose levels are >95 mg/dl (5.3 mmol/l) or 2-h postprandial values are >120 mg/dl (6.7 mmol/l). 1
Several days passed from the time of A.R.’s initial elevated blood glucose value and the initiation of insulin therapy. While HbA 1c values cannot be used for diagnostic purposes, in this case they further confirmed the significant degree of hyperglycemia.
Plasma blood glucose values initially improved in the immediate postpartum period. A.R. was sent home without medications but instructed to continue home glucose monitoring.
At her 2-week postpartum visit, whole blood glucose values were again indicating progressive hyperglycemia, and insulin was restarted. A.R.’s postpartum weight was 104 lb—well below her usual pre-pregnancy weight of 114–120 lb. Based on her ethnic background, weight loss, abrupt presentation with classic diabetes symptoms, and limited family history, she was reclassified as having type 1 diabetes.
In immune-mediated, or type 1, diabetes, b-cell destruction can be variable, with a slower destruction sometimes seen in adults. 3 Presentation of type 1 diabetes can also vary with modest fasting hyperglycemia that can quickly change to severe hyperglycemia and/or ketoacidosis in the presence of infection or other stress. 3 A.R. may have had mild hyperglycemia pre-pregnancy that increased in severity as the pregnancy progressed.
The final issue is communication among multiple health care providers. A.R. was part of a system that uses primary care providers, specialists, and triage nurses. She accessed all of these providers as instructed. However, the information did not seem to be clearly communicated among these different types of providers. A.R. called triage nurses several times with her concerns of increased fatigue, nausea, and weight loss. The specialist performed her glucose challenge with follow-up through the primary care office. It seems that if all of these providers had the full information about this case, the diagnosis could have been made more easily, and insulin could have been initiated more quickly.
1. Hyperglycemia diagnosed during pregnancy is considered to be gestational diabetes until it is reclassified in the postpartum period. Immune-mediated diabetes can cause mild hyperglycemia that is intensified with the increased counterregulatory hormone response during pregnancy.
2. Insulin therapy needs to be instituted quickly for cases in which MNT alone is inadequate.
3. The GCT is an appropriate screening test for an average-risk woman with no symptoms of diabetes. In the face of classic symptoms of diabetes, a casual plasma glucose test can eliminate the need for the glucose challenge.
4. As part of the health care industry, we need to continue to work on information systems to track patient data and share data among multiple providers. Patients can become lost in an ever-expanding system that relies on “protocols” and does not always allow for individual differences or for cases with unusual presentation.
Ginny Lewis, ARNP, FNP, CDE, is a nurse practitioner at the Diabetes Care Center of the University of Washington School of Medicine in Seattle.
Email alerts
- Online ISSN 1945-4953
- Print ISSN 0891-8929
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Research article
- Open access
- Published: 07 February 2020
Women’s experiences of a diagnosis of gestational diabetes mellitus: a systematic review
- Louise Craig 1 ,
- Rebecca Sims 1 ,
- Paul Glasziou 1 &
- Rae Thomas ORCID: orcid.org/0000-0002-2165-5917 1
BMC Pregnancy and Childbirth volume 20 , Article number: 76 ( 2020 ) Cite this article
29k Accesses
70 Citations
46 Altmetric
Metrics details
Gestational diabetes mellitus (GDM) - a transitory form of diabetes induced by pregnancy - has potentially important short and long-term health consequences for both the mother and her baby. There is no globally agreed definition of GDM, but definition changes have increased the incidence in some countries in recent years, with some research suggesting minimal clinical improvement in outcomes. The aim of this qualitative systematic review was to identify the psychosocial experiences a diagnosis of GDM has on women during pregnancy and the postpartum period.
We searched CINAHL, EMBASE, MEDLINE and PsycINFO databases for studies that provided qualitative data on the psychosocial experiences of a diagnosis of GDM on women across any stage of pregnancy and/or the postpartum period. We appraised the methodological quality of the included studies using the Critical Appraisal Skills Programme Checklist for Qualitative Studies and used thematic analysis to synthesis the data.
Of 840 studies identified, 41 studies of diverse populations met the selection criteria. The synthesis revealed eight key themes: initial psychological impact; communicating the diagnosis; knowledge of GDM; risk perception; management of GDM; burden of GDM; social support; and gaining control. The identified benefits of a GDM diagnosis were largely behavioural and included an opportunity to make healthy eating changes. The identified harms were emotional, financial and cultural. Women commented about the added responsibility (eating regimens, appointments), financial constraints (expensive food, medical bills) and conflicts with their cultural practices (alternative eating, lack of information about traditional food). Some women reported living in fear of risking the health of their baby and conducted extreme behaviours such as purging and starving themselves.
A diagnosis of GDM has wide reaching consequences that are common to a diverse group of women. Threshold cut-offs for blood glucose levels have been determined using the risk of physiological harms to mother and baby. It may also be advantageous to consider the harms and benefits from a psychosocial and a physiological perspective. This may avoid unnecessary burden to an already vulnerable population.
Peer Review reports
Gestational diabetes mellitus (GDM) is diagnosed by elevated blood glucose in pregnancy though the definition has changed repeatedly since its first description in the 1960’s [ 1 , 2 ]. The most frequently reported perinatal consequence of GDM is macrosomia (usually defined as a neonate weighing over 4 kg) which can increase the risk of caesarean section and shoulder dystocia. For the mother, there are also potential longer-term consequences including an increased risk of type 2 diabetes post-pregnancy and/or in later life [ 3 ]. The investigators of a large international Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study aimed to identify a cut-point in the continuum to decide the blood glucose level (BGL) thresholds that should be used to define GDM [ 4 ]. However, a definitive cut-point was not identified and using the HAPO data the International Association of the Diabetes and Pregnancy Study Groups (IADSPG) consensus panel recommended a BGL threshold associated with the risk of adverse infant outcomes (such as risk of macrosomia, excess infant adiposity and neonatal hyperinsulinemia) [ 5 ]. This change was controversial, and there is currently a lack of an agreed standard for diagnosing high blood glucose in pregnancy.
Pregnancy can be a vulnerable period when a woman is adapting and responding to changes in body perceptions, such as loss of strength or fitness, which can result in reduced self-esteem and depression [ 6 ]. Many women report depression and anxiety during pregnancy which often includes worry for the baby’s wellbeing [ 7 , 8 ]. A diagnosis of a health condition such as GDM could have a detrimental effect on a pregnant woman’s quality of life due to fears that the illness may affect her and/or her baby [ 9 ]. This has potential to convert pregnancy, a natural process, into one associated with risks, ill-health and increased surveillance [ 10 ]. Understanding a women’s response to the GDM diagnosis and its psychological impact has emerged as an important issue [ 11 ]. Some studies report women describing the initial response as one of ‘shock’ [ 12 , 13 ], ‘sadness’ and ‘guilt’ [ 13 ]. A women’s acceptance of risk and fear of complications is likely to influence the acceptability of various interventions. Therefore, it is imperative to amalgamate the findings of these studies to synthesise the array of potential psychosocial consequences of a diagnosis of GDM.
In many countries the prevalence of GDM is rising [ 14 , 15 , 16 ]. Some of this is due to the increasing age at which women are becoming pregnant, an increase in obesity amongst women, more testing during pregnancy, and better recording during pregnancy. However, much of the rise has occurred since 2013 when some countries adopted the new IADPSG criteria and testing regimen for gestational diabetes. This resulted in the anomalous position that two women in two countries with exactly the same glucose levels may or may not be diagnosed with GDM depending on the country’s definition. Caution had been previously raised that the new IADPSG definition would increase prevalence of women diagnosed with GDM by two-to-three-fold [ 17 ].
Despite a significant increase in prevalence of GDM after the introduction of the new IADPSG criteria [ 15 , 16 ], some pre-post studies suggest negligible clinical improvement in the adverse outcomes measured [ 17 , 18 ]. Findings from a qualitative study of 19 women of different cultural backgrounds investigating women’s experiences of a GDM diagnosis reported that the diagnostic criteria itself was viewed as ‘confusing’ by some women and treatment for their ‘borderline’ condition unnecessary [ 19 ].
Although multiple studies have considered the impact of a diagnosis of GDM, a systematic review to synthesise the evidence around the emotional impact of a diagnosis at different stages, i.e. time of diagnosis, after diagnosis, at the delivery of the baby, and post-delivery, is lacking. The findings could inform healthcare clinicians of women’s attitudes and the consequences of a diagnosis and illuminate potential opportunities to provide support and advise. Therefore, in this systematic review, we aim to synthesise the evidence of the psychosocial experiences a diagnosis of GDM has on women during pregnancy and the postpartum period.
We followed the Enhancing Transparency in Reporting the Synthesis of Qualitative Research Guidelines (ENTREQ; Additional file 1 : Table S1) [ 20 ]. We included primary studies published in peer-review journals that:
included pregnant women with a current diagnosis or women with a history of GDM;
provided qualitative data on the psychosocial experiences of a diagnosis of GDM on women across any stage of pregnancy and/or the postpartum period; and
where participants have provided an account of their experience or perspective of living with GDM
No restrictions were placed on country, written language, or year of publication.
Studies were excluded, if:
the primary aim was to identify barriers and/or facilitators to service as these focused on the management of GDM rather than the GDM diagnosis; or
participants were women diagnosed with diabetes before pregnancy
Abstracts, letters, editorials and commentaries were also excluded.
Search methods for identification of studies
The search strategy (MEDLINE version provided in the Additional file 1 ) was developed using a combination of Medical Subject Headings terms centred around three key areas: i) gestational diabetes mellitus ii) diagnostic testing for gestational diabetes mellitus and iii) patient experiences. The Systematic Review Accelerator software was used to translate the search strategy for each of the different databases and to remove duplicated articles [ 21 ]. We searched CINAHL, EMBASE, MEDLINE and PsycINFO databases from inception to April 2018. Forward and backward citation searching of included studies was conducted.
Selection process
A single reviewer (LC) screened the titles and abstracts of retrieved references using Endnote Version X7.7.1. Potentially eligible full-texts were independently reviewed by LC and RS with conflicts resolved via discussion. Two full-text studies published in Portuguese were first translated using Google Translate and then validated by a researcher with both spoken and written Portuguese language skills located within our research network.
Data extraction
All data labelled as results or findings including themes, categories, theories were extracted and imported into NVivo Version 12 by LC. Study characteristics were extracted by LC which included study location, reported research aims, study design, methodology and the analytical approach. Information about the diagnostic criteria used to determine GDM in women was also extracted.
Data synthesis and analysis
To synthesise the findings, we used a thematic synthesis described by Thomas and Harden [ 22 ]. Thematic synthesis has the potential for conclusions to be drawn based on common aspects across otherwise heterogeneous studies and produce findings that directly inform health practitioners [ 22 , 23 ]. Coding was inductive, with codes derived from the data. First, extracted text relevant to patient experiences and perspectives was coded line by line. A subset of studies ( n = 5) were coded independently by LC and RS to develop a coding framework. Disagreements were resolved by discussion. LC and RS coded a further subset ( n = 4) and established an inter-rater reliability of Kappa = 0.87. Following this, LC applied the coding framework to the remaining studies. New codes were iteratively developed as new concepts arose.
Second, relationships between the codes were identified by LC to form the basis of descriptive themes across the studies. Similar codes were grouped to generate themes and less frequently used codes were classified into sub-themes. In the final stage, analytical themes were developed to ‘go beyond’ the primary studies to amalgamate and interpret the findings. The relevant quotes to support each theme were tabulated.
Quality assessment
As recommended by the Cochrane Qualitative Research Methods Group, we assessed the quality of the included studies using the Critical Appraisal Skills Programme Qualitative Checklist (CASP). This tool uses a systematic approach to appraise three key areas: study validity, an evaluation of methodological quality, and an assessment of external validity [ 24 ]. Critical appraisal was conducted by one reviewer (LC) for all studies, with second reviewer appraisal (RS) for a sub-set of included papers. The findings from the two reviewers were compared and any contrasting items were discussed and re-reviewed.
The search identified 840 studies. After deduplication and screening of titles and abstracts 88 full-text articles were assessed (Fig. 1 ). Seven further articles were identified through citation searching. Data were extracted from 41 studies meeting eligibility criteria and were included in the review [ 11 , 12 , 13 , 19 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 ].
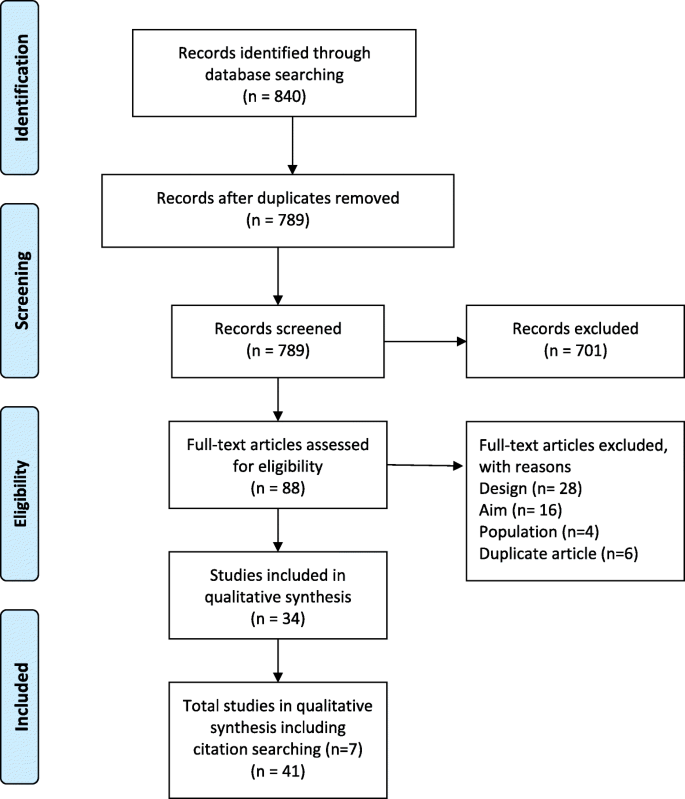
Prima flow diagram
Study characteristics
The studies reflected a variety of sampling methods and data collection methods. For example, interviews were conducted in 34 studies [ 10 , 12 , 13 , 25 , 27 , 28 , 30 , 31 , 32 , 34 , 35 , 36 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 60 , 61 ], focus group methods were used in three [ 19 , 32 , 37 ], and interviews and focus groups were used in two studies [ 29 , 51 ]. Two studies used a mixed method approach [ 26 , 59 ]. The sample sizes ranged from 6 to 57 women. Eighteen studies were conducted in Europe, 10 in Australia, 9 in North America, and 2 studies each in Asia and South America. Table 1 details the characteristics of the included studies.
Quality appraisal
Most studies were assessed as high quality (Additional file 1 : Table S2). Study aims were stated in all but one study [ 47 ]. As the purpose of all included studies was to explore or gain knowledge, opinions or attitudes about GDM, the qualitative methods employed in all the studies were appropriate. Different study designs were used and in some cases the lack of reporting details made judgments of the appropriateness of study methods difficult. Data were collected in a way that addressed the research issue, however, a few authors did not discuss or report details such as saturation of data [ 31 , 47 , 56 , 59 ]. The relationship between researcher and participants was considered in only two studies [ 51 , 61 ]. Appropriateness of data analysis was assessed as “unclear” when there was a lack of details about how themes were derived.
Thematic analyses
Eight themes were generated from the data synthesis: 1. initial psychological impact; 2. communicating the diagnosis; 3. knowledge of GDM; 4. risk perception; 5. management of GDM; 6. burden of GDM; 7. social support; and 8. gaining control. The relevant quotes to support each theme are presented in Table 2 .
Initial psychological impact
When initially diagnosed with GDM, most women reported reactions such as self-blame, failure, fear, sadness, concern and confusion. Women often focused on the uncertainty of diagnostic prognosis and some considered it to be a life-altering experience. Some women felt lost and unsure what to do next. Often women felt an overwhelming sense of vulnerability and guilt. In some cases, the diagnosis was received positively and was viewed as an opportunity for lifestyle improvements. For example, some women viewed the diagnosis as a ‘ wake up’ call and were grateful for the chance to intervene and potentially prevent adverse outcomes for their babies and themselves. Some women viewed gaining less weight than expected during their pregnancy as a benefit of having a GDM diagnosis.
Communicating the diagnosis
Communication with healthcare professionals (HCPs) and their families was a common theme throughout the findings of the included studies. Generally, the level and quality of communication with HCPs was mixed – with some women reporting positive and informative encounters, while others described brief encounters with overly technical language and unsupportive consultations. The main issues were limited time available to spend with the HCP, lack of continuity of care and lack of understanding about the role of the HCP at follow-up. In some instances, women felt that GDM was not a topic that HCPs were keen to discuss - ‘the nurses, they never talked to me about my gestational diabetes’. [ 23 ] The level and quality of information provided was often conflicting, confusing or insufficient. Areas of contention were appropriate foods and the dietary changes that should be made.
Some women formed a dependency on HCPs to know what to do and on the electronic reminders for follow-up appointments and monitoring. Often women reported having no choice in treatment resulting in them feeling threatened and frustrated. Often women were automatically booked in for a caesarean section without consultation or lived in fear of this occurring. One woman referred to GDM as being over medicalised. Receiving limited information also prompted women to independently seek information about the impact and management of GDM from other sources such as the internet. However, some women found the internet limited for specific information or confusing.
Knowledge of GDM
Women had varying levels of understanding of GDM which impacted on their initial reaction to the diagnosis. Those who were able to explain the cause of GDM were able to process and accept the diagnosis more readily than those who had little understanding of GDM, or were confused as to how GDM occurred. Lack of knowledge also extended to and impacted on relatives. Some women stated that they would have preferred to be more prepared to receive the diagnosis by having early knowledge about the testing for diabetes. Women reported being on a steep learning curve, especially the onerous approach of dietary trial and error whereby women learnt what foods would increase their blood glucose level (BGL) and what food to avoid. Women also reported challenges in adopting new habits to manage their GDM, including understanding food labels and nutritional values of food. Often this required a trial and error approach. There was also a lack of understanding about the impact of GDM on their baby with some women believing it would be transmitted to their baby via breastmilk.
Risk perception
Women’s perception of risk were reported before the diagnosis of GDM, after they were diagnosed in pregnancy, and after the delivery. Some women attempted to understand their level of risk in context of family history. Some were very surprised by the diagnosis, especially if they were asymptomatic; and some women found it difficult to come to terms with the diagnosis. There was uncertainty about the severity of the condition. Some women considered the condition to be mild, downplaying the disease and believing that too much ‘ fuss’ was being made about GDM and other women doubted the diagnosis and its seriousness. Women often discussed: the adverse effects that GDM would have on her baby; frustration that the focus was on risks to the baby and less so them; their worry about the consequences for the future; and questioned the impact of insulin on the baby. Women worried that their diet was too restrictive for their growing baby and would not provide the nutrients that the baby required. Some women held the view that GDM was a temporary condition and would disappear once the baby was born, and many women reverted to old eating habits after the baby’s birth. Often women referred to the birth as a ‘ moment of truth ’ or as an endpoint to their GDM. This was also reflected in the level of care that the women received after the birth of their baby.
Managing GDM
Dietary management-related stress was commonly reported amongst interviewed women and was experienced by both insulin and non-insulin users. Stress and frustrations often occurred as a consequence of an unexpected abnormal blood glucose reading following strict adherence to dietary advice. Maintaining stable BGL was an ongoing struggle and in some cases the burden proved too much, with a few women ceasing employment. Insulin users described the process as a ‘ roller coaster ’ as well as the emotional and physical discomfort of injecting, while non-insulin users often became obsessed with a well-controlled diet, with some women viewing this as a way to avoid the use of insulin. Conversely, some women felt relieved when they were transitioned onto insulin, as it reduced the need for dietary restriction.
Making lifestyle changes was considered stringent and restrictive by the majority of women, and for some required ‘ major restructuring’ to their diet and daily routines to incorporate exercise. Some women reported extreme behaviours, including falsifying blood glucose readings, self-starvation and hiding their condition, including from family members. Often the impact of non-adherence to lifestyle changes resulted in guilt and belief that the baby would know they have cheated. Other pregnancy related ailments and the need to care for other children interfered with the ability to make the required changes. Women who had a specific culture-related diet discussed the impact and difficulty of applying or tailoring the dietary recommendations to their diet.
The key motivator to making required lifestyle changes, despite the associated hardships, was to minimise the risks to their unborn baby. Women prioritised the health of the baby over their own health and were willing to do anything to ensure that the health of their baby was not compromised. Over time, management of the GDM became a part of their normal routine for many women. However, some women expressed a desire to have a ‘ normal’ pregnancy similar to their friends, discussing that a diagnosis of GDM made them feel as though their pregnancy was atypical, leading to defining their pregnancy as ‘ abnormal ’ or as an ‘ illness ’. For one woman, it made her feel like an ‘ illegal’ person.
Burden of GDM
Women reported that a diagnosis of GDM came with extra responsibility, which added pressure whilst trying to juggle life commitments such as work, childcare, and daily living responsibilities. Monitoring and treating GDM placed burden on women’s daily routines and most woman agreed that taking BGL measurements was time consuming and disruptive. There was a constant need to prudently plan meals and co-ordinate the attendance at additional hospital appointments, all of which were considered time intensive, especially with travel and wait times. Women expressed that GDM consumed a lot of their thinking time e.g., ‘ I think about diabetes everyday’ and felt that they had to acknowledge GDM all the time and became ‘ super-conscious’ . In some instances, women reported a GDM diagnosis took away some of the ‘ joy of pregnancy ’ . One woman described her pregnancy as a ‘ misfortune’ . Women mentioned the financial burden of buying healthier food – ‘it would take lots of money just because it is so expensive to eat healthy’. [ 25 ] Women also considered the physical burden of GDM such as fatigue and the side effects of treatment such as insulin. There was a longer-term impact on family planning, where in some cases women decided not to have another child because they were fearful of enduring a similar restrictive and stressful pregnancy due to GDM.
Social support
Social support, including family and HCP support, was an important aspect for women during their experience of a GDM diagnosis. Changes in lifestyle often had an overflow effect, with other family members adopting healthier lifestyles. Women not in their country of birth, and without family, often reported feeling isolated and lonely. Disappointment and isolation were also expressed by some women when they perceived a lack of healthcare system support. This often occurred postnatally when the expectations of postpartum care were high, however, in reality, support was absent. In some cases, women were stigmatised by their families and in a few cases received undesirable feedback that they were not doing enough to protect their unborn child.
Gaining control
Control was a frequently used word when women described living with and managing a GDM diagnosis. Initially women reported a lack of control especially over their emotions, however, over time women transitioned from feeling like a victim of diabetes, to being active agents in controlling their GDM. The terms ‘ balance’ and ‘ adjustment’ were used to describe how some women tried to offset the strict compliance and active self-management with reducing their risk to their unborn baby and their own future risk of developing diabetes after pregnancy. Some women reported feeling empowered as their pregnancies progressed, especially when they gained more knowledge about GDM and what action they could take to accept and make sense of the diagnosis. Taking control included realising the changes that were required to their lifestyle, self-initiated care, and self-education. Often investigating alternative options, such as natural remedies outside those recommended by HCPs, provided women with some autonomy in managing their condition and some believed that it was a safer option to medication.
Summary of main findings
This synthesis of the qualitative evidence of women’s experiences of being diagnosed with GDM highlighted the psychosocial consequences a diagnosis of GDM can have on women. The purported benefits of a GDM diagnosis identified from our review, were largely behavioural and included an opportunity to improve health, prevent excessive weight gain, control weight during pregnancy, and prompts to make healthy eating changes. However, the purported harms included the added responsibility (eating regimens, appointments), financial constraints (expensive food, medical bills), and conflicts with their cultural practices (alternative eating, lack of information about traditional food). The psychosocial consequences were wide reaching and often resulted in significant social isolation with women only sharing their diagnosis with partners. Furthermore, there were a few reports of over-medicalisation due to a GDM diagnosis, with the perception that HCPs were often authoritarian, focusing on physiological aspects, with little attempt to involve women in decision making. This is noteworthy considering a non-GDM pregnancy has already come under scrutiny as being over-medicalised with increasing levels of unnecessary intervention [ 62 ].
Women from studies included in our review frequently reported inconsistent information provision. Limited GDM information provision has been identified in another systematic review regarding healthcare seeking for GDM during the postpartum period [ 63 ]. In contrast, findings from another study which aimed to evaluate satisfaction with obtaining a diagnosis of GDM concluded that the majority of women were satisfied with their experience of being diagnosed [ 64 ]. Further, women in the latter study associated poor GDM control with perinatal complications and an increased risk of type 2 diabetes following pregnancy [ 64 ].
Another key finding from this review was low awareness of the potential risks of GDM, particularly in the long-term. Low health literacy levels could be one factor to explain knowledge deficits and understanding of GDM, especially given the sociodemographic diverse population included in this review. One study found that low literacy among disadvantaged women had a significant impact on their understanding of GDM information [ 65 ]. Other research found that women who live in an English-speaking country but primarily speak a non-English language, have lower rates of dietary awareness compared with their English speaking counterparts, and this may affect compliance to dietary interventions [ 66 ]. Therefore, it is important that new educational interventions are developed to target those with lower health literacy as well as cultural factors when diagnosing and managing multi-ethnic populations with GDM [ 66 ].
Interestingly, women with a borderline diagnosis of GDM did not seem as concerned as other women and in some cases were dismissive of the diagnosis and the potential consequences. Similarly, in a study which specifically included women with a borderline diagnosis of GDM, the majority of women reported that they were not worried by the diagnosis [ 67 ]. For some women, the potential transitory nature of GDM was emphasised and some reported that it didn’t seem like a real illness. The diagnostic criteria for GDM has previously been compared with the established criteria used to classify a condition as a disease. This comparison revealed disparity which Goer, in 1996, used to suggest that GDM did not pose a serious health risk, was neither easily nor accurately diagnosed, was not treated effectively and that treatment outweighed the risks of the condition [ 68 ]. Therefore, the levels of heightened psychological distress as reported by the women in our review, may actually be unnecessary and others have gone as far as saying that GDM is an example of ‘obstetric iatrogenesis’ [ 69 ].
The findings of this review did underline a few unmet service needs with recurring themes around the lack of individualised care and its continuity, lack of choice regarding important aspects of care such as birthing options, and the scarcity of comprehensive follow-up. There was a sense of abandonment amongst women after delivery in that they had experienced intensive intervention and then nothing. This could be viewed as a missed opportunity to capitalise on the motivation to make changes during pregnancy. Researchers have previously highlighted that adherence to postpartum screening and continued lifestyle modifications to prevent future diabetes seems to dissipate after birth, possibly because the driver to protect their unborn child is no longer there [ 70 ].
The studies included in our review had participants of varying cultures sampled from countries with different GDM definitions. However, there appeared no difference in the qualitative outcomes between studies/countries. In our review, the experiences of women diagnosed with GDM suggest psychosocial harms appear to outweigh the qualitative benefits. Quantitative studies [ 14 , 15 ] that report prevalence increases in GDM after the IADSPG [ 71 ] definition changed, also report minimal improvements to maternal and infant physical outcomes.
This synthesis of women’s experiences of a GDM diagnosis could be used to inform the content of communication materials both before and after a GDM diagnosis. For example, an awareness of GDM testing and basic information including cultural adaptations to dietary guidelines and addressing misconceptions around breastfeeding. There is also an opportunity for HCPs to use teachable moments with women who have been identified at risk of developing type 2 diabetes post-pregnancy and offer supportive, effective advice about lifestyle changes. This is particularly relevant considering a previous review highlighted a significant time is spent in sedentary behaviour during pregnancy [ 72 ]. A study which examined HCPs views of healthcare provision to women with GDM showed that HCPs themselves perceived that there was a shortfall in GDM education [ 73 ]. There are also signals for service improvement and potential for service redesign, such as increasing community-delivered care for women diagnosed with GDM. This would assist in alleviating the burden on women to attend hospital appointments and potentially offer flexible appointment times. Follow-up appointments post-pregnancy could be made with consideration of other appointments such as maternal and child health milestones and breastfeeding weaning classes, and could also focused on healthy eating for both mother and baby.
Strengths and limitations
This systematic review included studies with women of different demographic characteristics and multicultural samples. The themes identified were represented in the majority of studies which increased the internal validity. The relatively high participation rate in the included studies, and that most studies were conducted during pregnancy or shortly after delivery, contributes to the external validity of our study. Although some participants were interviewed antenatally and some postnatally, this distribution over different gestational stages assists the generalisability of the study findings.
The comparison of coding between authors, discussion of the results and reaching consensus was a robust approach to improve the credibility of the results. Overall, the quality of most studies was good, however, a third of the studies used convenience methods to recruit participants which could contribute to sampling bias and limit the external validity of our findings. Only two studies adequately described the facilitator’s prior experience and the relationship between the participants and the facilitator/researcher. Unfortunately, this review did not capture the perception of HCPs which might be used to explain some of the behaviours and attitudes of the women, particularly in relation to communication of the diagnosis and information provision. Finally, although the data were collected from diverse populations, the majority of the countries in which research were conducted in were high-income countries, which could be considered to have more established and evidence-based healthcare systems than low-income countries.
Further research
A previous study has suggested the need for more research on the benefits and harms of alternative treatment choices for women with GDM [ 33 ]. The findings from this review suggest a need for more investigation around the psychosocial benefits and harms of a diagnosis of GDM. Given some women viewed treatment of ‘borderline GDM’ as unimportant, a new model of care based on stratification or individual level of risk for pregnancy and birth complications could be further explored. This may reduce the need for all women to be labelled as having GDM and negate unnecessary anxiety and burden for those at the lower ‘borderline’ threshold. This would then potentially offer tailored treatment options, improve shared-decision making, and improve women’s knowledge about how a diagnosis of GDM might affect them.
Consequences of a GDM diagnosis are multidimensional and highly contextual. Despite the psychosocial challenges frequently experienced, many women (driven by the innate response to safeguard their unborn baby) were able to gradually adapt to the required lifestyle changes and monitoring regimens. Perhaps a question is whether some of them should have to. There is opportunity to improve lifestyle and to assist the prevention of diabetes after pregnancy, however, this needs to be managed alongside the potential harms of a GDM diagnosis such as the negative psychological impact and social isolation. In the context of rising prevalence [ 14 , 15 , 16 , 17 ], potential minimal clinical [ 14 , 15 , 16 ] improvements, and the wide range of psychosocial experiences identified in this study, the findings of this review highlight the need for HCPs to consider the implications that a GDM diagnosis may have on women. It is essential that women diagnosed with GDM receive consistent evidence-based information and ongoing psychological and social support.
Availability of data and materials
The datasets generated during the current systematic review are available from the lead author upon request.
Abbreviations
Blood glucose level
Critical Appraisal Skills Programme Checklist (Qualitative)
Enhancing Transparency in Reporting the Synthesis of Qualitative Research
- Gestational diabetes mellitus
Hyperglycemia and Adverse Pregnancy Outcomes
Health care professional
International Association of the Diabetes and Pregnancy Study Groups
O'Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278–85.
CAS PubMed Google Scholar
Mishra S, Rao CR, Shetty A. Trends in the diagnosis of gestational diabetes mellitus. Scientifica (Cairo). 2016;2016:5489015.
Google Scholar
Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes. Syst Rev. 2002;25(10):1862–8. https://doi.org/10.2337/diacare.25.10.1862 .
Article Google Scholar
HAPO Study Cooperative Research Group. Hyperglycaemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002.
McIntyre HD, Gibbons KS, Lowe J, Oats JJN. Development of a risk engine relating maternal glycemia and body mass index to pregnancy outcomes. Diabetes Res Clin Pract. 2018;139:331–8.
Article PubMed Google Scholar
Kamysheva E, Skouteris H, Wertheim EH, et al. Examination of a multi-factorial model of body-related experiences during pregnancy: The relationships among physical symptoms, sleep quality, depression, self-esteem, and negative body attitudes. Body Image. 2008;5(2):152–63 https://doi.org/10.1016/j.bodyim.2007.12.005 .
Carolan-Olah M, Barry M. Antenatal stress: an Irish case study. Midwifery. 2014;30(3):310–6. https://doi.org/10.1016/j.midw.2013.03.014 [published Online First: 2013/05/21].
Ferreira CR, Orsini MC, Vieira CR, et al. Prevalence of anxiety symptoms and depression in the third gestational trimester. Arch Gynecol Obstet. 2015;291(5):999–1003. https://doi.org/10.1007/s00404-014-3508-x [published Online First: 2014/10/15].
Dalfra MG, Nicolucci A, Bisson T, et al. Quality of life in pregnancy and post-partum: a study in diabetic patients. Qual Life Res. 2012;21(2):291–8. https://doi.org/10.1007/s11136-011-9940-5 .
Article CAS PubMed Google Scholar
Evans MK, O’Brien B. Gestational diabetes: the meaning of an at-risk pregnancy. Qual Health Res. 2005;15(1):66–81.
Devsam BU, Bogossian FE, Peacock AS. An interpretive review of women's experiences of gestational diabetes mellitus: proposing a framework to enhance midwifery assessment. Women Birth. 2013;26(2):E69–76.
Carolan-Olah M, Duarte-Gardea M, Lechuga J, et al. The experience of gestational diabetes mellitus (GDM) among Hispanic women in a U.S. border region. Sex Reprod Healthc. 2017;12:16–23. https://doi.org/10.1016/j.srhc.2016.11.003 .
Persson M, Winkvist A, Mogren I. ‘From stun to gradual balance’—women’s experiences of living with gestational diabetes mellitus. Scand J Caring Sci. 2010;24(3):454–62. https://doi.org/10.1111/j.1471-6712.2009.00735.x .
Cade TJ, Polyakov A, Brennecke SP. Implications of the introduction of new criteria for the diagnosis of gestational diabetes: a health outcome and cost of care analysis. BMJ Open. 2019;9(1):e023293–e93. https://doi.org/10.1136/bmjopen-2018-023293 .
Article PubMed PubMed Central Google Scholar
Sexton H, Heal C, Banks J, et al. Impact of new diagnostic criteria for gestational diabetes. J Obstet Gynaecol Res. 2018;44(3):425–31. https://doi.org/10.1111/jog.13544 [published Online First: 2018/01/13].
Erjavec K, Poljičanin T, Matijević R. Impact of the implementation of new WHO diagnostic criteria for gestational diabetes mellitus on prevalence and perinatal outcomes: a population-based study. J Pregnancy. 2016;2016:2670912. https://doi.org/10.1155/2016/2670912 .
Feldman RK, Tieu RS, Yasumura L. Gestational diabetes screening the international association of the diabetes and pregnancy study groups compared with carpenter-coustan screening. Obstet Gynecol. 2016;127(1):10–7. https://doi.org/10.1097/aog.0000000000001132 .
Pocobelli G, Yu O, Fuller S, et al. One-step approach to identifying gestational diabetes mellitus: association with perinatal outcomes. Obstet Gynecol. 2018;132(4):859–67. https://doi.org/10.1097/aog.0000000000002780 [published Online First: 2018/08/22].
Draffin CR, Alderdice FA, McCance DR, et al. Exploring the needs, concerns and knowledge of women diagnosed with gestational diabetes: a qualitative study. Midwifery. 2016;40:141–7. https://doi.org/10.1016/j.midw.2016.06.019 .
Tong A, Flemming K, McInnes E, et al. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol. 2012;12(1):181. https://doi.org/10.1186/1471-2288-12-181 .
Rathbone J, Carter M, Hoffmann T, et al. Better duplicate detection for systematic reviewers: evaluation of systematic review assistant-deduplication module. Syst Rev. 2015;4:6. https://doi.org/10.1186/2046-4053-4-6 [published Online First: 2015/01/16].
Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol. 2008;8(1):45. https://doi.org/10.1186/1471-2288-8-45 .
Lucas PJ, Baird J, Arai L, et al. Worked examples of alternative methods for the synthesis of qualitative and quantitative research in systematic reviews. BMC Med Res Methodol. 2007;7(1):4. https://doi.org/10.1186/1471-2288-7-4 .
Group CCQM. Chapter 4: critical appraisal of qualitative research. In: Noyes J, Booth A, Hannes K, Harden A, Harris J, Lewin S, Lockwood C, editors. Supplementary guidance for inclusion of qualitative research in cochrane systematic reviews of interventions. Version 1; 2011.
Abraham K, Wilk N. Living with gestational diabetes in a rural community. MCN Am J Matern Child Nurs. 2014;39(4):239–45.
Araujo MF, Pessoa SM, Damasceno MM, et al. Gestational diabetes from the perspective of hospitalized pregnant women. Rev Bras Enferm. 2013;66(2):222–7.
Bandyopadhyay M, Small R, Davey MA. Attendance for postpartum glucose tolerance testing following gestational diabetes among south Asian women in Australia: a qualitative study. Int J Gynecol Obstet. 2015;131:E149.
Bandyopadhyay M, Small R, Davey MA, et al. Lived experience of gestational diabetes mellitus among immigrant South Asian women in Australia. Aust N Z J Obstet Gynaecol. 2011;51(4):360–4. https://doi.org/10.1111/j.1479-828X.2011.01322.x .
Carolan M. Women’s experiences of gestational diabetes self-management: a qualitative study. Midwifery. 2013;29(6):637–45. https://doi.org/10.1016/j.midw.2012.05.013 .
Doran F. Gestational diabetes mellitus: perspectives on lifestyle changes during pregnancy and post-partum, physical activity and the prevention of future type 2 diabetes. Aust J Prim Health. 2008;14(3):85–92.
Doran F, Davis K. Gestational diabetes mellitus in Tonga: insights from healthcare professionals and women who experienced gestational diabetes mellitus. N Z Med J. 2010;123(1326):59–67.
PubMed Google Scholar
Eades CE, France EF, Evans JMM. Postnatal experiences, knowledge and perceptions of women with gestational diabetes. Diabet Med. 2018;35(4):519–29.
Figueroa Gray M, Hsu C, Kiel L, et al. “It's a very big burden on me”: Women’s experiences using insulin for gestational diabetes. Matern Child Health J. 2017;21(8):1678–85.
Ge L, Albin B, Hadziabdic E, et al. Beliefs about health and illness and health-related behavior among urban women with gestational diabetes mellitus in the south east of China. J Transcult Nurs. 2016;27(6):593–602.
Ge L, Wikby K, Rask M. ‘Is gestational diabetes a severe illness?’ Exploring beliefs and self-care behaviour among women with gestational diabetes living in a rural area of the south east of China. Aust J Rural Health. 2016;24(6):378–84. https://doi.org/10.1111/ajr.12292 .
Han S, Middleton PF, Bubner TK, et al. Women’s views on their diagnosis and management for borderline gestational diabetes mellitus. J Diabetes Res. 2015;2015:209215. https://doi.org/10.1155/2015/209215 .
Article CAS PubMed PubMed Central Google Scholar
Hirst JE, Tran TS, Do MAT, et al. Women with gestational diabetes in Vietnam: a qualitative study to determine attitudes and health behaviours. BMC Pregnancy Childbirth. 2012;12:81.
Hjelm K, Bard K, Apelqvist J. Gestational diabetes: prospective interview-study of the developing beliefs about health, illness and health care in migrant women. J Clin Nurs. 2012;21(21–22):3244–56.
Hjelm K, Bard K, Apelqvist J. A qualitative study of developing beliefs about health, illness and healthcare in migrant African women with gestational diabetes living in Sweden. BMC Womens Health. 2018;18(1):34.
Hjelm K, Bard K, Berntorp K, et al. Beliefs about health and illness postpartum in women born in Sweden and the Middle East. Midwifery. 2009;25(5):564–75.
Hjelm K, Bard K, Nyberg P, et al. Swedish and middle-eastern-born women’s beliefs about gestational diabetes. Midwifery. 2005;21(1):44–60.
Hjelm K, Berntorp K, Apelqvist J. Beliefs about health and illness in Swedish and African-born women with gestational diabetes living in Sweden. J Clin Nurs. 2012;21(9–10):1374–86. https://doi.org/10.1111/j.1365-2702.2011.03834.x .
Hjelm K, Berntorp K, Frid A, et al. Beliefs about health and illness in women managed for gestational diabetes in two organisations. Midwifery. 2008;24(2):168–82.
Hui AL, Sevenhuysen G, Harvey D, et al. Stress and anxiety in women with gestational diabetes during dietary management. Diabetes Educ. 2014;40(5):668–77.
Kaptein S, Evans M, McTavish S, et al. The subjective impact of a diagnosis of gestational diabetes among ethnically diverse pregnant women: a qualitative study. Can. 2015;39(2):117–22. https://doi.org/10.1016/j.jcjd.2014.09.005 .
Kilgour C, Bogossian FE, Callaway L, et al. Postnatal gestational diabetes mellitus follow-up: Australian women’s experiences. Women Birth. 2015;28(4):285–92. https://doi.org/10.1016/j.wombi.2015.06.004 .
Lawson EJ, Rajaram S. A transformed pregnacy – the psychological consequences of gestational diabetes. Sociol Health Illn. 1994;16(4):536–62.
Lie MLS, Hayes L, Lewis-Barned NJ, et al. Preventing type 2 diabetes after gestational diabetes: women’s experiences and implications for diabetes prevention interventions. Diabet Med. 2013;30(8):986–93.
Neufeld HT. Food perceptions and concerns of aboriginal women coping with gestational diabetes in Winnipeg, Manitoba. J Nutr Educ Behav. 2011;43(6):482–91. https://doi.org/10.1016/j.jneb.2011.05.017 .
Nielsen JH, Olesen CR, Kristiansen TM, et al. Reasons for women’s non-participation in follow-up screening after gestational diabetes. Women Birth. 2015;28(4):e157–63. https://doi.org/10.1016/j.wombi.2015.04.006 .
Parsons J, Sparrow K, Ismail K, et al. Experiences of gestational diabetes and gestational diabetes care: a focus group and interview study. BMC Pregnancy Childbirth. 2018;18:25.
Pennington AVR, O’Reilly SL, Young D, et al. Improving follow-up care for women with a history of gestational diabetes: perspectives of GPs and patients. Aust J Prim Health. 2017;23(1):66–74.
Rafii F, Vasegh Rahimparvar SF, Keramat A, et al. Procrastination as a key factor in postpartum screening for diabetes: A qualitative study of Iranian women with recent gestational diabetes. Iran Red Crescent Med J. 2017;19(5). https://doi.org/10.5812/ircmj.44833 .
Razee H, van der Ploeg HP, Blignault I, et al. Beliefs, barriers, social support, and environmental influences related to diabetes risk behaviours among women with a history of gestational diabetes. Health Promot J Austr. 2010;21(2):130–7.
Salomon IMM, Soares SM. Understanding the impact of gestational diabetes diagnosis. Revista Mineira de Enfermagem. 2004;8(3):349–57.
Svensson L, Nielsen KK, Maindal HT. What is the postpartum experience of Danish women following gestational diabetes? A qualitative exploration. Scand J Caring Sci. 2018;32(2):756–64.
Tang JW, Foster KE, Pumarino J, et al. Perspectives on prevention of type 2 diabetes after gestational diabetes: a qualitative study of Hispanic, African-American and White women. Matern Child Health J. 2015;19(7):1526–34. https://doi.org/10.1007/s10995-014-1657-y .
Tierney M, O'Dea A, Danyliv A, et al. Factors influencing lifestyle behaviours during and after a gestational diabetes mellitus pregnancy. Health Psychol Behav Med. 2015;3(1):204–16. https://doi.org/10.1080/21642850.2015.1073111 .
Trutnovsky G, Panzitt T, Magnet E, et al. Gestational diabetes: women's concerns, mood state, quality of life and treatment satisfaction. J Matern Fetal Neonatal Med. 2012;25(11):2464–6. https://doi.org/10.3109/14767058.2012.683900 .
Wah YYE, McGill M, Wong J, et al. Self-management of gestational diabetes among Chinese migrants: a qualitative study. Women Birth. 2019;32(1):e17–e23.
Whitty-Rogers J, Caine V, Cameron B. Aboriginal women’s experiences with gestational diabetes mellitus: a participatory study with mi'kmaq women in Canada. ANS Adv Nurs Sci. 2016;39(2):181–98. https://doi.org/10.1097/ANS.0000000000000115 .
Johanson R, Newburn M, Macfarlane A. Has the medicalisation of childbirth gone too far? BMJ. 2002;324(7342):892–5.
Van Ryswyk E, Middleton P, Shute E, et al. Women's views and knowledge regarding healthcare seeking for gestational diabetes in the postpartum period: a systematic review of qualitative/survey studies. Diabetes Res Clin Pract. 2015;110(2):109–22.
Goldstein RF, Gibson-Helm ME, Boyle JA, et al. Satisfaction with diagnosis process for gestational diabetes mellitus and risk perception among Australian women. Int J Gynaecol Obstet. 2015;129(1):46–9.
Carolan M. Diabetes nurse educators’ experiences of providing care for women, with gestational diabetes mellitus, from disadvantaged backgrounds. J Clin Nurs. 2014;23(9–10):1374–84. https://doi.org/10.1111/jocn.12421 .
Yuen L, Wong VW. Gestational diabetes mellitus: challenges for different ethnic groups. World J Diabetes. 2015;6(8):1024–32. https://doi.org/10.4239/wjd.v6.i8.1024 [published Online First: 2015/07/25].
Carolan M, Steele C, Margetts H. Knowledge of gestational diabetes among a multi-ethnic cohort in Australia. Midwifery. 2010;26(6):579–88. https://doi.org/10.1016/j.midw.2009.01.006 .
Goer H. Gestational diabetes: the emperor has no clothes. The Birth Gazette Summertown: Second Foundation; 1996. p. 32–5.
Han S, Bubner T, Middleton PF, et al. A qualitative study of women's views on diagnosis and management for borderline gestational diabetes. J Paediatr Child Health. 2013;49:128. https://doi.org/10.1111/jpc.12133 .
Nielsen KK, Kapur A, Damm P, et al. From screening to postpartum follow-up - the determinants and barriers for gestational diabetes mellitus (GDM) services, a systematic review. BMC Pregnancy Childbirth. 2014;14:41. https://doi.org/10.1186/1471-2393-14-41 .
International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups Recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82.
Article PubMed Central Google Scholar
Fazzi C, Saunders DH, Linton K, et al. Sedentary behaviours during pregnancy: a systematic review. Int J Behav Nutr Phys Act. 2017;14:32. https://doi.org/10.1186/s12966-017-0485-z .
Van Ryswyk E, Middleton P, Hague W, et al. Clinician views and knowledge regarding healthcare provision in the postpartum period for women with recent gestational diabetes: A systematic review of qualitative/survey studies. Diabetes Res Clin Pract. 2014;106(3):401–11 https://doi.org/10.1016/j.diabres.2014.09.001 .
Download references
Acknowledgements
Not applicable.
LC is supported by a National Health and Medical Research Council Partnership Centre for Health System Sustainability grant (#9100002). RS and RT are supported by a National Health and Medical Research Council Program grant (#1106452) and PG is supported by a NHMRC Research Fellowship (#1080042). The funders had no role in design, data collection, analysis, interpretation or writing of the manuscript.
Author information
Authors and affiliations.
Institute for Evidence-Based Healthcare, Bond University, Gold Coast, Australia
Louise Craig, Rebecca Sims, Paul Glasziou & Rae Thomas
You can also search for this author in PubMed Google Scholar
Contributions
PG, LC and RT conceived the project design, LC conducted the search, extracted, analysed and interpreted the data assisted by RS. RT assisted in data interpretation. All authors contributed to the drafting of the manuscript and approve the final version.
Corresponding author
Correspondence to Rae Thomas .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:.
Table S1. Enhancing Transparency in Reporting the Synthesis of Qualitative Research Guidelines Checklist. Table S2. Assessment of quality of included studies using the CASP tool.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Craig, L., Sims, R., Glasziou, P. et al. Women’s experiences of a diagnosis of gestational diabetes mellitus: a systematic review. BMC Pregnancy Childbirth 20 , 76 (2020). https://doi.org/10.1186/s12884-020-2745-1
Download citation
Received : 24 September 2019
Accepted : 15 January 2020
Published : 07 February 2020
DOI : https://doi.org/10.1186/s12884-020-2745-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Systematic review
- Qualitative
- Diagnostic impacts
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Advertisement
Determinants of gestational diabetes mellitus: a hospital-based case–control study in coastal South India
- Original Article
- Published: 23 July 2020
- Volume 41 , pages 108–113, ( 2021 )
Cite this article

- Archana Ganapathy 1 , 2 ,
- Ramesh Holla ORCID: orcid.org/0000-0002-2296-3719 3 , 4 ,
- B. B. Darshan 3 , 4 ,
- Nithin Kumar 3 , 4 ,
- Vaman Kulkarni 3 , 4 ,
- Bhaskaran Unnikrishnan 3 , 4 ,
- Rekha Thapar 3 , 4 ,
- Prasanna Mithra 3 , 4 &
- Avinash Kumar 3 , 4
344 Accesses
6 Citations
Explore all metrics
A public health problem that has been on the rise in the twenty-first century is gestational diabetes mellitus (GDM). There are serious adverse effects on both maternal and fetal health following GDM. Potential complications can be reduced by early detection of risk factors, which predispose women to GDM.
This study aims to identify the risk factors associated with GDM.
A case–control study was carried out among antenatal women admitted to hospitals affiliated to Kasturba Medical College, Mangalore. The study population consisted of cases, who were GDM patients, and controls, who were age-matched, non-GDM patients. Statistical Package for Social Sciences (SPSS) version 25.0 was used for entering and analysing data. Both univariate and multivariate analysis was done for determining the factors responsible for GDM.
The mean age of cases was 29.54 (± 4.3) years and of controls was also 29.54 (± 4.2). There was no significant difference while comparing the socioeconomic status across the study groups. Irregular menstrual cycle (OR = 2.78, CI = 0.94–08.4, P = 0.06) and history of type 2 diabetes mellitus in first-degree relatives (OR = 5.26, CI = 2.13–12.99, P ≤ 0.001) were found to be significant risk factors.
It was found in our study that irregular menstrual history, history of GDM in previous pregnancy, history of type 2 diabetes mellitus in first-degree relative and history of GDM in first-degree relative are all independent risk factors of GDM.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Prevalence and risk factors of gestational diabetes in the health region of Lleida: a retrospective observational cohort study
Maternal age at pregnancy and risk for gestational diabetes mellitus among Chinese women with singleton pregnancies

Gestational diabetes mellitus, its associated factors, and the pregnancy outcomes among pregnant women attending tertiary care hospitals of Bhubaneswar, India
Bhat M, Ramesha KN, Sarma SP, Menon S, Sowmini CV, Ganesh Kumar S. Determinants of gestational diabetes mellitus: a case control study in a district tertiary care hospital in South India. Int J Diabetes Dev Ctries. 2010;30(2):91–6.
Article Google Scholar
Lakshmi D, Felix AJW, Devi R, Manobharathi M. Study on knowledge about gestational diabetes mellitus and its risk factors among antenatal mothers attending care, urban Chidambaram. Int J Commun Med Public Health. 2018;5(10):4388–92.
Larrabure Torrealva GT, Martinez S, Luque Fernandez MA, Sanchez SE, Mascaro PA, Ingar HA, et al. Prevalence and risk factors of gestational diabetes mellitus: findings from a universal screening feasibility program in Lima, Peru. BMC Pregnancy Childbirth. 2018;18(1):303.
Carey Rubin R 2016 Gestational diabetes mellitus—risk factors and screening. today’s dietitian, [online] available at: < https://www.todaysdietitian.com/pdf/courses/CareyGDM.pdf > [Accessed 19 Mar 2020].
Agrawal S, Das V, Agarwal A, Pandet A, Namrata. Prevalence of gestational glucose intolerance and gestational diabetes in a tertiary care centre in Northern India. J Clin Diagn Res. 2018;12(8):QC04–6.
Google Scholar
Sivakumar V, Rajasekeran AM, Vijayakumar A. Assessment of risk factors for the early detection of gestational diabetes mellitus. Int J Pharm Sci Res. 2014;5:114–7.
CAS Google Scholar
Karagiannis T, Bekiari E, Manolopoulos K, Paletas K, Tsapas A. Gestational diabetes mellitus: why screen and how to diagnose. Hippokratia. 2010;14(3):151–4.
CAS PubMed PubMed Central Google Scholar
Mangal A, Kumar V, Panesar S, Talwar R, Raut D, Singh S. Updated BG Prasad socioeconomic classification, 2014: a commentary. Indian J Public Health. 2015;59:42–2.
Haver MC, Locksmith GJ, Emmet E. Irregular menses: an independent risk factor for gestational diabetes mellitus. Am J Obstet Gynecol. 2003;188:1189–91.
Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–97.
Article CAS Google Scholar
Waldman IN, Legro RS. Chapter 26—polycystic ovary syndrome. The Ovary (Third Edition); 2019. p. 415–35.
Khomami MB, Joham AE, Boyle JA, Piltonen T, Silagy M, Arora C, et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity—a systematic review, meta-analysis, and meta-regression. Obes Rev. 2019;20(5):659–74.
Oviya C, Mohanraj U, Sathya N, Deeksha N. The study of gestational diabetes mellitus among pregnant women with and without polycystic ovary syndrome—cohort study. Paripex - Indian J Res. 2019;8(4).
Boomsma CM, Fauser BCJM, Macklon NS. Pregnancy complications in women with polycystic ovary syndrome. Semin Reprod Med. 2008;26(1):072–84.
Barnes RA, Wong T, Ross GP, Griffiths MM, Smart CE, Collins CE, et al. Excessive weight gain before and during gestational diabetes mellitus management: what is the impact? Diabetes Care. 2019;43(1):74–81.
Wang YY, Liu Y, Li C, Lin J, Liu XM, Sheng JZ, et al. Frequency and risk factors for recurrent gestational diabetes mellitus in primiparous women: a case control study. BMC Endocr Disord. 2019;19:22.
Gangadhara Goud T, Pavan Kumar K, Ramesh K. Risk factors of gestational diabetes in Karnataka. Int J Curr Res Acad Rev. 2014;2(9):286–91.
Alexander B. After-meals walks may help control diabetes, study suggests. NBC news [Internet]. 2013 12 [cited Oct 13 2016]. Available from: http://www.nbcnews.com/health/after-meal-walks-may-help-control-diabetes-study-suggests-6C10287692 . Accessed 27 Mar 2018.
Feleke BE. Determinants of gestational diabetes mellitus: a case–control study. J Matern Fetal Neonatal Med. 2018;31(19):2584–9.
Sreekanthan K, Belicita A, Rajendran K, Vijayakumar A. Prevalence of gestational diabetes mellitus in a medical college in South India: a pilot study. Indian J Clin Pract. 2014;25(4).
Ming WK, Ding W, CJP Z, Zhong L, Long Y, Li Z, et al. The effect of exercise during pregnancy on gestational diabetes mellitus in normal-weight women: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18(440).
Tobias DK, Zhang C, van Dam RM, Bowers K, Hu FB. Physical activity before and during pregnancy and risk of gestational diabetes mellitus. Diabetes Care. 2011;34(1):223–9.
Muller PS, Nirmala M. Effects of pre-pregnancy maternal body mass index on gestational diabetes mellitus. Int J Eng Technol. 2018;7(1.9):279–82.
Faurholt-Jepsen D, Range N, PrayGod G, Jeremiah K, Faurholt-Jepsen M, Aabye MG, et al. The association between conventional risk factors and diabetes is weak among urban Tanzanians. Diabetes Care. 2014;37(1):e5–6.
Arora GP, Thaman RG, Prasad RB, Almgren P, Brøns C, Groop LC, et al. Prevalence and risk factors of gestational diabetes in Punjab, North India: results from a population screening program. Eur J Endocrinol. 2015;173(2):257–67.
Kongubol A, Phupong V. Prepregnancy obesity and the risk of gestational diabetes mellitus. BMC Pregnancy Childbirth. 2011;11:59.
Download references
Author information
Authors and affiliations.
Kasturba Medical College, Mangalore, India
Archana Ganapathy
Manipal Academy of Higher Education, Manipal, India
Department of Community Medicine, Kasturba Medical College, Mangalore, India
Ramesh Holla, B. B. Darshan, Nithin Kumar, Vaman Kulkarni, Bhaskaran Unnikrishnan, Rekha Thapar, Prasanna Mithra & Avinash Kumar
Faculty of Health Sciences, Manipal Academy of Higher Education, Manipal, India
You can also search for this author in PubMed Google Scholar

Contributions
1.2. conceptualized the study design and carried out the experiment, including data acquisition. 1.2.3. Carried out the data analysis and contributed to interpretation of the results. 6. Conceived the original idea and supervised the project. 1.2.3 wrote the manuscript and 4.5.6.7.8.9. Reviewed and edited the manuscript. 1.2.3.4.5.6.7.8.9 have seen and approved the final version of the manuscript and all the subsequent versions.
Corresponding author
Correspondence to Ramesh Holla .
Ethics declarations
Approval was sought from the Institutional Ethics Committee of Kasturba Medical College before commencement of the study. During the study, informed written consent was taken from all participants after clearly explaining the study objectives in a language known to them. If the participant was unable to read/write, the informed consent was taken from a Legally Authorized representative or next of kin.
Ethical approval
Prior to the commencement of the study, the study protocol was submitted for approval to the Institutional Ethics Committee (IEC) of Kasturba Medical College. Thereafter, permission was obtained from the Medical Superintendents of the concerned hospitals for conduction of the study. Study objectives were clearly explained to the participants in a language familiar to them. Anonymity and discretion of the information given by the patients were maintained with utmost care and a written informed consent was obtained from the participants.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Ganapathy, A., Holla, R., Darshan, B.B. et al. Determinants of gestational diabetes mellitus: a hospital-based case–control study in coastal South India. Int J Diabetes Dev Ctries 41 , 108–113 (2021). https://doi.org/10.1007/s13410-020-00844-1
Download citation
Received : 13 April 2019
Accepted : 13 June 2020
Published : 23 July 2020
Issue Date : January 2021
DOI : https://doi.org/10.1007/s13410-020-00844-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gestational diabetes mellitus
- Case–control study
- Risk factors
- Multivariate analysis
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 19 August 2024
The impact of PM 2.5 and its constituents on gestational diabetes mellitus: a retrospective cohort study
- Weiqi Liu 1 na1 ,
- Haidong Zou 2 na1 ,
- Weiling Liu 3 na1 &
- Jiangxia Qin 2
BMC Public Health volume 24 , Article number: 2249 ( 2024 ) Cite this article
Metrics details
There is increasing evidence that exposure to PM 2.5 and its constituents is associated with an increased risk of gestational diabetes mellitus (GDM), but studies on the relationship between exposure to PM 2.5 constituents and the risk of GDM are still limited.
A total of 17,855 pregnant women in Guangzhou were recruited for this retrospective cohort study, and the time-varying average concentration method was used to estimate individual exposure to PM 2.5 and its constituents during pregnancy. Logistic regression was used to assess the relationship between exposure to PM 2.5 and its constituents and the risk of GDM, and the expected inflection point between exposure to PM 2.5 and its constituents and the risk of GDM was estimated using logistic regression combined with restricted cubic spline curves. Stratified analyses and interaction tests were performed.
After adjustment for confounders, exposure to PM 2.5 and its constituents (NO 3 − , NH 4 + , and OM) was positively associated with the risk of GDM during pregnancy, especially when exposure to NO 3 − and NH 4 + occurred in the first to second trimester, with each interquartile range increase the risk of GDM by 20.2% (95% CI: 1.118–1.293) and 18.2% (95% CI. 1.107–1.263), respectively. The lowest inflection points between PM 2.5 , SO 4 2− , NO 3 − , NH 4 + , OM, and BC concentrations and GDM risk throughout the gestation period were 18.96, 5.80, 3.22, 2.67, 4.77 and 0.97 µg/m 3 , respectively. In the first trimester, an age interaction effect between exposure to SO 4 2− , OM, and BC and the risk of GDM was observed.
Conclusions
This study demonstrates a positive association between exposure to PM 2.5 and its constituents and the risk of GDM. Specifically, exposure to NO 3 − , NH 4 + , and OM was particularly associated with an increased risk of GDM. The present study contributes to a better understanding of the effects of exposure to PM 2.5 and its constituents on the risk of GDM.
Peer Review reports
Gestational diabetes mellitus (GDM) is a common metabolic disorder of pregnancy, and its incidence has increased in recent years. It is estimated that GDM affects approximately 16.7% of pregnancies worldwide, affecting approximately 21 million live births, and in China, the prevalence of GDM has reached 8.6% [ 1 ]. GDM affects not only the health of pregnant women, [ 2 , 3 , 4 ] but also the potential occurrence of adverse pregnancy outcomes, including macrosomia and neonatal hypoglycaemia, and increases the long-term risk of diabetes in both mothers and children [ 5 , 6 , 7 ]. Therefore, to reduce the risk of GDM and its associated complications, it is particularly important to study the pathogenic factors of GDM.
The mechanisms through which fine particulate matter (PM 2.5 ) exposure leads to GDM are not fully understood and may involve multiple pathways that increase the risk of GDM. Animal experiments by Xu J et al. have shown that PM 2.5 exposure in mice induces oxidative stress mediated by nuclear factor erythroid 2 related factor 2 and activates inhibitory signaling pathways mediated by c-Jun N-terminal kinase, leading to hepatic insulin resistance (IR). [ 8 ] PM 2.5 contains thousands of chemical constituents, with polycyclic aromatic hydrocarbons (PAHs) being the most prominent organic constituents. Research suggests that lipophilic PAHs may contribute to IR through methylation-mediated suppression of the insulin receptor substrate 2 gene. [ 9 ] Additionally, PM 2.5 also interferes with the inflammatory response in visceral adipose tissue, lipid metabolism in hepatocytes and glucose metabolism in skeletal muscle by altering the CC-chemokine receptor 2 signalling pathway, further exacerbating insulin resistance. [ 10 ] An increasing number of studies suggest that exposure to PM 2.5 is associated with an increased risk of developing diabetes [ 11 , 12 , 13 , 14 ]. According to a study of 395,927 pregnant women in southern California, exposure to ambient PM 2.5 increases the likelihood of developing gestational diabetes mellitus (GDM) [ 15 ]. A case‒control study by Shen HN et al. [ 16 ] revealed that exposure to PM 2.5 in early and mid-pregnancy increased the risk of GDM by 9% (95% CI 1.02‒1.17) and 7% (95% CI 1.01‒1.14), respectively. A positive association between PM 2.5 exposure in the second trimester and GDM risk was found in a study of 2,078,669 people in Florida between 2005 and 2015 [ 17 ]. However, there is also evidence that exposure to PM 2.5 is not associated with an increased risk of GDM. [ 18 , 19 ] Therefore, the relationship between PM 2.5 exposure and the risk of gestational diabetes is controversial and needs to be clarified by further large-scale studies.
PM 2.5 is composed of a variety of substances, including sulfate (SO 4 2− ), nitrate (NO 3 − ), ammonium (NH 4 + ), organic matter (OM), and black carbon (BC). The toxicity of PM 2.5 constituents to people is variable. Wang X et al. [ 20 ] conducted a study on PM 2.5 constituents and asthma in six low- and middle-income countries and found that ammonia may be the main cause of asthma. Li S et al. [ 21 ] conducted a large-scale epidemiological survey in Southwest China and showed that OM may be the main cause of the association between PM 2.5 exposure and diabetes mellitus risk. BC and OM were found to be the PM 2.5 constituents that are most strongly and consistently associated with cardiovascular mortality and morbidity. [ 22 ] However, evidence on the relationship between exposure to PM 2.5 constituents and GDM risk is limited. Previous studies have focused on the relationship between PM 2.5 exposure and GDM risk, and a further understanding of the relationship between exposure to different PM 2.5 constituents and the risk of GDM could rationally explain which component is responsible for the relationship between PM 2.5 exposure and GDM risk and provide new opportunities to reduce the burden of GDM associated with PM 2.5 exposure.
To address the research needs in this area, this retrospective cohort study evaluated the association of exposure to PM 2.5 and its constituents with the risk of GDM in a population from Guangzhou city, Guangdong Province, China, to provide a basis for the targeted prevention and control of PM 2.5 constituents.
Study cohort
This retrospective study focused on pregnant women who visited the Maternal and Children Health Care Hospital of Huadu in Guangzhou between 2020 and 2022. This specialized hospital primarily serves pregnant women and children, and its services cover the entire Guangzhou territory. The data of the study participants were obtained from the electronic case management system of the hospital, and GDM diagnoses were made according to the ICD-10 classification criteria for participants with diagnosis code O24. Participants who met the following criteria were included in the study: lived in Guangzhou during pregnancy, had complete relevant data, were not pregnant with twins, had no history of diabetes or hypertension before pregnancy, and conceived naturally. Notably, as this study used deidentified information, it was not necessary to obtain informed consent. This study was approved by the Ethics Committee of t the Maternal and Child Health Hospital of Huadu District (No. 2024-001).
Exposure to PM 2.5 and its constituents
To obtain daily concentrations of PM 2.5 and its constituents, including SO 4 2− , NO 3 − , NH 4 + , OM, and BC, at a spatial resolution of 10 km × 10 km, we used data from the Tracking Air Pollution in China (TAP) project. This dataset, accessible via the web portal ( http://tapdata.org.cn ), consolidates ground-level measurements from various publications and supplements them with satellite-derived estimates. The estimation process used aerosol optical depth (AOD) data in conjunction with the GEOS-Chem atmospheric chemistry transport model, as described by Liu et al. [ 23 ] The temperature and relative humidity data used in this study were obtained from a website ( https://rp5.ru/ ), and the monitoring site used was Guangzhou Airport.
To assess the exposure concentrations of PM 2.5 and its constituents for each study participant, we used the time-varying average concentration method. Specifically, since all participants lived in Guangzhou, we first collected daily average concentrations of PM 2.5 and its constituents for the city. Using the average for the entire region and each participant’s gestational week and delivery date, we estimated their average exposure concentrations during the first trimester (1–13 gestational weeks, T1), the second trimester (14–28 gestational weeks, T2), and first to second trimester (T1 + T2).
Based on earlier studies [ 24 , 25 ] and information obtained from electronic medical records, we selected potential confounders, including age, ethnicity, occupation type, marital status, blood type, nonprimiparous status, anaemia status, infant weight, preeclampsia status, vaginitis status, gestational hypertension status, thyroid disease status, temperature, and relative humidity. Participants self-reported their ethnicity (Han, Hui, Miao, Tujia, etc.), occupation type (employee, civil servant, professional, self-employed, farmer, unemployed, etc.), marital status (married, divorced), blood type (A, B, O, AB), and whether they were first-time mothers or had given birth to at least one child. Ethnicity was reclassified as Han or other; occupation type was reclassified as employed, self-employed, or other; and infant weight was classified as low birth weight (< 2500 g), normal birth weight (2500–4000 g), or macrosomia (> 4000 g) based on the recorded birth weight. Assessing exposure to temperature and relative humidity using the same methodology as for PM 2.5 and its constituents.
Diagnosis of GDM
According to the diagnostic criteria for GDM, [ 26 , 27 ] all pregnant women underwent oral glucose tolerance tests after fasting for at least 8 h between the 24th and 28th weeks of pregnancy. During the test, the pregnant woman had to drink 300 ml of a solution containing 75 g of glucose within 5 min. Blood glucose levels were measured before, 1 h after, and 2 h after glucose ingestion. According to medical guidelines, the blood glucose levels of pregnant women should be kept below 5.1 mmol/L, 10.0 mmol/L and 8.5 mmol/L at these three times. If a pregnant woman’s blood glucose level meets or exceeds any of the above criteria, she will be diagnosed with GDM by a health care professional.
Statistical analyses
We used chi-squared or nonparametric tests for baseline characteristics. and Spearman’s rank correlation test was used to assess the correlations between exposure to PM 2.5 and its constituents. Logistic regression analyses were used to estimate the odds ratios (ORs) and 95% confidence intervals (95% CIs) associated with the development of GDM, adjusting for potential confounders, including age, ethnicity, occupation type, marital status, blood type, nonprimary status, anaemia status, infant weight, preeclampsia status, vaginitis status, gestational hypertension status, thyroid disease status, temperature, and relative humidity. We used a logistic regression combined with restricted cubic spline curves to assess the relationship between exposure to PM 2.5 and its constituents and the risk of GDM, with the reference value (OR = 1) set at the 10th percentile and the nodes set at the 5th, 35th, 65th, and 95th percentiles of the concentrations of PM 2.5 and its constituents. Furthermore, we conducted stratified analyses to evaluate the impact of exposure to PM 2.5 and its constituents on GDM risk.
Statistical analyses were performed with STATA 16.0 (StataCorp, USA) and R 4.3.2 (Lucent Technologies, USA) using the “rcssci” and “autoReg” packages. A two-tailed p < 0.05 was considered to indicate statistical significance.
Baseline characteristics
In total, 17,855 pregnant women were included in our study, and 22.14% of the participants had GDM. The median (P25, P75) age of the participants was 29 years (26 years, 33 years), and 14.86% of the pregnant women were of an advanced maternal age. The median exposure concentrations for PM 2.5 , SO 4 2− , and OM in the GDM group were greater than those in the non-GDM group, and the temperature and relative humidity in the GDM group were greater than those in the non-GDM group. Further details are shown in Table 1 .
Correlation analysis of PM 2.5 , SO 4 2− , NO 3 − , NH 4 + , OM, and BC concentrations
Table 2 shows the concentrations of PM 2.5 , SO 4 2− , NO 3 − , NH 4 + , OM, and BC during the study period. There was a strong correlation among PM 2.5 , SO 4 2− , NO 3 − , NH 4 + , OM, and BC concentrations (Spearman’s correlation coefficient > 0.8). To ensure that the results of the correlation analysis were not affected by outliers, we performed a sensitivity analysis. Specifically, we chose the 95th percentile of PM 2.5 concentration as a threshold to exclude extreme values from the dataset and recalculated the correlation coefficients. We found that the correlation coefficients between PM 2.5 and its components did not significantly change after removing the extreme values (Table S1 ).
Relationship between PM 2.5 , SO 4 2− , NO 3 − , NH 4 + , OM, and BC exposure and GDM risk
Table 3 shows the associations between exposure to PM 2.5 , SO 4 2− , NO 3 − , NH 4 + , OM, and BC and the risk of GDM. After adjusting for confounding factors, in the first trimester, the ORs per Interquartile range (IQR) increase in PM 2.5 , SO 4 2− , NO 3 − , NH 4 + , OM, and BC concentrations were associated with an increase in the risk of GDM by 9.2% (95% CI: 1.034–1.154), 8. 6% (95% CI: 1.035–1.140), 11.6% (95% CI: 1.034–1.023), 11.1% (95% CI: 1.037–1.190), 9.7% (95% CI: 1.040–1.158), and 8.5% (95% CI: 1.039–1.134), respectively. Exposure to PM 2.5 , NO 3 − , NH 4 + , and OM in the second trimester and exposure to PM 2.5 , SO 4 2− , NO 3 − , NH 4 + , OM, and BC from the first to second trimester also increased the risk of GDM.
After adjusting for confounders, we found that the inflection points between PM 2.5 , OM, and BC concentrations and GDM risk were lowest in the second trimester, at 18.96, 4.77 and 0.97 µg/m 3 , respectively. The inflection points between SO 4 2− , NO 3 − and NH 4 + concentrations and GDM risk were lowest in the first to second trimester, at 5.80, 3.22 and 2.67 µg/m 3 , respectively. In addition, a nonlinear relationship between PM 2.5 , NO 3 − , NH 4 + , and OM exposure and GDM risk was observed only in the first trimester (p values for nonlinearity of 0.002, 0.008, 0.001 and 0.022, respectively) (Figs. 1 , 2 Figure S1 - S2 ).

Flowchart of participant screening

Association between predicted exposure to PM 2.5 and its constituents during the first trimester and GDM risk. The solid line indicates the OR, and the dashed area indicates the 95% CI. The reference point is the lowest value for PM 2.5 and its constituents, and the nodes are at the 5th, 35th, 65th, and 95th percentiles for PM 2.5 and its constituents
Subgroup analysis
To evaluate the association between exposure to PM 2.5 and its constituents and GDM risk, stratified and interaction analyses of the study participants’ age, ethnicity, occupation type, marital status, blood type, nonprimiparous status, anaemia status and infant sex were performed. In the first trimester, significant associations between PM 2.5 , SO 4 2− , NO 3 − , NH 4 + , OM and BC exposure and GDM risk were observed in the nonprimiparous, anaemic and infant sex subgroups ( p < 0.05) (Table 4 ; Fig. 3 , Table S2 - S5 ). A similar pattern of increased GDM risk was found in the second trimester and the first to second trimester subgroups. Details of the exposure effect sizes for the second trimester subgroup are given in Tables S6-S11. The exposure effect sizes for the first to second trimester subgroup are presented in Tables S12 - S17 . In addition, an interaction by age subgroup was observed only between exposure to SO 4 2− , OM and BC in the first trimester and GDM risk (p values for the interaction were 0.046, 0.046 and 0.044, respectively).

Forest plot of subgroup analysis of the relationship between SO 4 2− exposure in the first trimester and GDM risk
In this study, we found that exposure to the air pollutant PM 2.5 and its constituents (SO 4 2− , NO 3 − , NH 4 + , OM and BC) is positively associated with an increased risk of GDM. In addition, nonlinear associations were found between PM 2.5 , NO 3 − , NH 4 + , OM exposure during the first trimester and GDM risk, while subgroup analyses revealed age interactions between exposure to SO 4 2− , OM and BC during the first trimester and GDM risk.
Numerous epidemiological studies have consistently revealed a correlation between exposure to PM 2.5 and the risk of GDM, [ 28 , 29 , 30 ] which is consistent with the findings of this study. Tang et al. [ 31 ] analysed 13 studies (including 9 retrospective studies, 3 prospective studies and 1 case‒control study) and found that PM 2.5 exposure in the second trimester was associated with an increased risk of GDM (OR 1.07, 95% CI 1.00 to 1.13), while PM 2.5 exposure in the first trimester did not increase the risk of GDM (OR 1.01; 95% CI 0.96 to 1.07). A retrospective cohort study conducted in Shanghai, China, from 2014 to 2016 revealed that a 10 µg/m 3 increase in PM 2.5 exposure during the first trimester, second trimester, and first to second trimester increased the risk of GDM by 9% (95% CI: 1.02, 1.16), 9% (95% CI: 1.03, 1.16), and 15% (95% CI: 1.04, 1.28), respectively. [ 32 ] However, a study from Hebei, China, showed that PM 2.5 exposure in the first trimester, second trimester, or first to second trimester did not increase the risk of GDM. [ 33 ] The results of this study showed that exposure to PM 2.5 increased the risk of GDM by 9.2% (95% CI: 1.034–1.154), 8.2% (95% CI: 1.014–1.154), and 10.5% (95% CI: 1.046–1.167) in the first, second, and first to second trimester, respectively. This finding is consistent with a previous study conducted in Foshan city, Guangdong Province, from 2015 to 2019, which was a birth cohort study. The results showed that exposure to PM 2.5 during the first, second, and first to second trimester increased the risk of GDM [ 34 ]. This may be due to the proximity of Foshan to Guangzhou and their similar geographical and climatic conditions. Such similarities could result in comparable sources, concentrations and compositions of PM 2.5 pollution in both areas, leading to consistent research results between the two locations. In addition, similarities in residents’ lifestyles, dietary habits and other factors may contribute to similar sensitivities to PM 2.5 exposure and susceptibility to GDM, further explaining the consistency of the research findings.
Strong seasonal and regional variations in PM 2.5 constituents were suggested by Bell et al. [ 35 ] However, it is still unclear which PM 2.5 constituents have the greatest effect on GDM risk, and research on the association between exposure to PM 2.5 constituents and the risk of GDM remains limited. A cross-sectional survey conducted in 55 hospitals across 24 provinces in China from 2015 to 2016, with a total of 54,517 participants, revealed that organic compounds, black carbon, and nitrate may be the main causes behind the occurrence of GDM. [ 36 ] A retrospective cohort study conducted in the United States between 2002 and 2008 involving 201,015 participants revealed that each IQR increase in nitrate exposure during the first trimester was associated with a 5% (95% CI: 1.02–1.09) increased risk of GDM. However, exposure to elemental carbon, organic compounds, ammonium ions and sulfate did not increase the risk of GDM. [ 37 ] A recent meta-analysis of 31 eligible cohort studies revealed that second-trimester BC exposure and first-trimester NO 3 − exposure increased the risk of GDM, with RRs of 1.128 (1.032–1.231) and 1.128 (1.032–1.231), respectively. A recent meta-analysis of 31 eligible cohort studies revealed that NO 3 − exposure in the first trimester and BC exposure in the first to second trimester increased the risk of GDM by 5.6% (95% CI: 1.008–1.107) and 18.5% (95% CI: 1.026–1.368), respectively [ 38 ]. This finding is not entirely consistent with our findings in this retrospective cohort study, which revealed that although SO 4 2− and BC exposure in the second trimester was negatively associated with GDM risk, SO 4 2− , NO 3 − , NH 4 + , OM, and BC exposure in other exposure windows were positively associated with GDM risk. The reason for this inconsistency may be due to significant variations in the levels of exposure to PM 2.5 and its constituents in different countries and regions, as well as significant differences in the methods used to assess the exposure levels of the study participants.
Previous studies on the relationship between exposure to PM 2.5 and its constituents and the risk of GDM have focused on risk assessment and exposure windows, [25, 39, 40]while the critical concentrations defining the association between these variables have been less explored. This study provides clearer evidence for the prevention of GDM in individuals with exposure to PM 2.5 and its constituents by analysing the cut-off values of PM 2.5 and its constituents associated with the occurrence of GDM. This study also provides a more precise basis for targeted interventions and policy development. In addition, we investigated the potential impacts of age, ethnicity, occupation type, marital status, blood type, nonprimiparous status, anaemia status, and infant sex. Our findings revealed a statistically significant age interaction between exposure to SO 4 2− , OM, and BC during the first trimester and the risk of GDM. Our results revealed a statistically significant age interaction effect between SO 4 2− , OM and BC exposure in the first trimester and GDM risk. This may be due to several factors. First, pregnant women of different ages have marked physiological differences, such as variations in metabolic rate, hormone levels and organ function, which may lead to different sensitivities to PM 2.5 constituents. Second, with increasing age, prolonged exposure to environmental pollutants and the adoption of unhealthy lifestyles may increase the susceptibility of pregnant women to air pollutants in early pregnancy, thereby increasing the risk of GDM. Third, differences in prenatal nutrition, health care, work and family stress among age groups may differentially affect pregnant women’s susceptibility to air pollution. Finally, age-related changes in the immune system may lead to different immune responses to air pollutants in pregnant women. Such differences could increase the susceptibility of certain age groups to the effects of air pollutants, thereby increasing the likelihood of GDM.
There are a number of advantages to this study. First, the study population consists of pregnant women from Guangzhou, a large city in China, with a large sample size covering all 11 administrative districts of the city, which enhances the generalisability and applicability of the results. Second, we used logistic regression combined with restricted cubic splines, a method that allows us to accurately capture the exposure-response relationship and its non-linear effects. Finally, we adjusted the analysis for various confounding factors, such as age, ethnicity, occupation, marital status and blood group, and conducted subgroup analyses to explore heterogeneity among different subgroups. These measures increase the credibility of the results and provide new directions for future research. However, several limitations of this study need to be considered. First, there is a potential risk of exposure misclassification, as individual mobility was not taken into account during the exposure assessment, which may have affected the accuracy of the exposure estimates. Second, the cut-off for defining the onset of GDM in our study population was set at 28 weeks gestation rather than the clinically meaningful threshold of 24 weeks. This extended timeframe may have introduced ambiguity, potentially weakening the directness and clarity of the association between exposures and outcomes. Additionally, this study used a spatial resolution of 10 km x 10 km to estimate exposure to PM2.5 and its components, and the low spatial resolution of the exposure assessment may not be fine enough in some areas, especially localised urban pollution hotspots, which may affect the precision of the exposure estimates for the study population.
Our results suggest that exposure to SO 4 2− and BC during mid-pregnancy is negatively associated with GDM risk, whereas exposure to PM 2.5 and its constituents during other windows is positively associated with an increased risk of GDM, adding to the evidence on the effects of exposure to PM 2.5 and its constituents on the development of GDM. Furthermore, we identified thresholds for the effects of exposure to PM 2.5 and its constituents on the risk of GDM during different exposure periods. These results have important implications for the prevention of GDM and call for further research to confirm our findings and elucidate the underlying mechanisms involved.
Data availability
No datasets were generated or analysed during the current study.
International Diabetes Federation: IDF Diabetes Atlas 10th edition. 2021, https://diabetesatlas.org/
Morimitsu LK, Fusaro AS, Sanchez VH, Hagemann CC, Bertini AM, Dib SA. Fibrinolytic dysfunction after gestation is associated to components of insulin resistance and early type 2 diabetes in latino women with previous gestational diabetes. Diabetes Res Clin Pract. 2007;78(3):340–8.
Article CAS PubMed Google Scholar
Lin CH, Wen SF, Wu YH, Huang YY, Huang MJ. The postpartum metabolic outcome of women with previous gestational diabetes mellitus. Chang Gung Med J. 2005;28(11):794–800.
PubMed Google Scholar
Greco E, Calanducci M, Nicolaides KH, Barry EVH, Huda MSB, Iliodromiti S. Gestational diabetes mellitus and adverse maternal and perinatal outcomes in twin and singleton pregnancies: a systematic review and meta-analysis. Am J Obstet Gynecol. 2024;230(2):213–25.
Article PubMed Google Scholar
Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;377:e067946.
Article PubMed PubMed Central Google Scholar
Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(Suppl 2):14–20.
Flores-le Roux JA, Sagarra E, Benaiges D, Hernandez-Rivas E, Chillaron JJ, Puig de Dou J, Mur A, Lopez-Vilchez MA, Pedro-Botet J. A prospective evaluation of neonatal hypoglycaemia in infants of women with gestational diabetes mellitus. Diabetes Res Clin Pract. 2012;97(2):217–22.
Xu J, Zhang W, Lu Z, Zhang F, Ding W. Airborne PM(2.5)-Induced hepatic insulin resistance by Nrf2/JNK-Mediated signaling pathway. Int J Environ Res Public Health 2017, 14(7).
Kim YH, Lee YS, Lee DH, Kim DS. Polycyclic aromatic hydrocarbons are associated with insulin receptor substrate 2 methylation in adipose tissues of Korean women. Environ Res. 2016;150:47–51.
Liu C, Xu X, Bai Y, Wang TY, Rao X, Wang A, Sun L, Ying Z, Gushchina L, Maiseyeu A, et al. Air pollution-mediated susceptibility to inflammation and insulin resistance: influence of CCR2 pathways in mice. Environ Health Perspect. 2014;122(1):17–26.
Yang Y, Ma X, Pang W, Jiang C. Causal associations of PM2.5 and GDM: a two-sample mendelian randomization study. Toxics 2023, 11(2).
Ren Z, Yuan J, Luo Y, Wang J, Li Y. Association of air pollution and fine particulate matter (PM2.5) exposure with gestational diabetes: a systematic review and meta-analysis. Ann Transl Med. 2023;11(1):23.
Article CAS PubMed PubMed Central Google Scholar
He D, Wu S, Zhao H, Qiu H, Fu Y, Li X, He Y. Association between particulate matter 2.5 and diabetes mellitus: a meta-analysis of cohort studies. J Diabetes Invest. 2017;8(5):687–96.
Article Google Scholar
Zeng X, Zhan Y, Zhou W, Qiu Z, Wang T, Chen Q, Qu D, Huang Q, Cao J, Zhou N. The influence of Airborne Particulate Matter on the risk of gestational diabetes Mellitus: a large retrospective study in Chongqing, China. Toxics 2023, 12(1).
Sun Y, Li X, Benmarhnia T, Chen JC, Avila C, Sacks DA, Chiu V, Slezak J, Molitor J, Getahun D, et al. Exposure to air pollutant mixture and gestational diabetes mellitus in Southern California: results from electronic health record data of a large pregnancy cohort. Environ Int. 2022;158:106888.
Shen HN, Hua SY, Chiu CT, Li CY. Maternal exposure to Air pollutants and Risk of Gestational Diabetes Mellitus in Taiwan. Int J Environ Res Public Health 2017, 14(12).
Zheng Y, Wen X, Bian J, Lipkind H, Hu H. Associations between the chemical composition of PM(2.5) and gestational diabetes mellitus. Environ Res. 2021;198:110470.
Zhang H, Wang Q, He S, Wu K, Ren M, Dong H, Di J, Yu Z, Huang C. Ambient air pollution and gestational diabetes mellitus: a review of evidence from biological mechanisms to population epidemiology. Sci Total Environ. 2020;719:137349.
Zhang H, Dong H, Ren M, Liang Q, Shen X, Wang Q, Yu L, Lin H, Luo Q, Chen W, et al. Ambient air pollution exposure and gestational diabetes mellitus in Guangzhou, China: a prospective cohort study. Sci Total Environ. 2020;699:134390.
Wang X, Guo Y, Cai M, Qian ZM, Zhang S, Zhang Z, Yang Y, Vaughn MG, Aaron HE, Wu F, et al. Constituents of fine particulate matter and asthma in 6 low- and middle-income countries. J Allergy Clin Immunol. 2022;150(1):214–e222215.
Li S, Guo B, Jiang Y, Wang X, Chen L, Wang X, Chen T, Yang L, Silang Y, Hong F, et al. Long-term exposure to ambient PM2.5 and its components Associated with Diabetes: evidence from a large Population-based Cohort from China. Diabetes Care. 2023;46(1):111–9.
Yang Y, Ruan Z, Wang X, Yang Y, Mason TG, Lin H, Tian L. Short-term and long-term exposures to fine particulate matter constituents and health: a systematic review and meta-analysis. Environ Pollut. 2019;247:874–82.
Liu S, Geng G, Xiao Q, Zheng Y, Liu X, Cheng J, Zhang Q. Tracking daily concentrations of PM(2.5) Chemical composition in China since 2000. Environ Sci Technol. 2022;56(22):16517–27.
Yang C, Wang W, Wang F, Wang Y, Zhang F, Liang Z, Liang C, Wang J, Ma L, Li P, et al. Ambient PM2.5 components and prevalence of chronic kidney disease: a nationwide cross-sectional survey in China. Environ Geochem Health. 2024;46(2):70.
Zheng Y, Bian J, Hart J, Laden F, Soo-Tung Wen T, Zhao J, Qin H, Hu H. PM(2.5) Constituents and Onset of Gestational Diabetes Mellitus: Identifying Susceptible Exposure Windows. Atmos Environ (1994) 2022, 291.
American Diabetes Association Professional Practice C: 2. Classification and diagnosis of diabetes: standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–38.
Huang J, Li Y, Liu M, Wu W. Guide Interpretation/Contrast the Guideline of Gestational Hyperglycemia from CMA and the Guideline of Gestational Diabetes Mellitus from ADA in 2022. J Int Obstet Gynecol. 2022;49:691–9.
Google Scholar
Miron-Celis M, Talarico R, Villeneuve PJ, Crighton E, Stieb DM, Stanescu C, Lavigne E. Critical windows of exposure to air pollution and gestational diabetes: assessing effect modification by maternal pre-existing conditions and environmental factors. Environ Health. 2023;22(1):26.
Liu W, Zhang Q, Liu W, Qiu C. Association between air pollution exposure and gestational diabetes mellitus in pregnant women: a retrospective cohort study. Environ Sci Pollut Res Int. 2023;30(2):2891–903.
Elshahidi MH. Outdoor Air Pollution and Gestational Diabetes Mellitus: a systematic review and Meta-analysis. Iran J Public Health. 2019;48(1):9–19.
PubMed PubMed Central Google Scholar
Tang X, Zhou JB, Luo F, Han Y, Heianza Y, Cardoso MA, Qi L. Air pollution and gestational diabetes mellitus: evidence from cohort studies. BMJ Open Diabetes Res Care 2020, 8(1).
Cheng X, Ji X, Yang D, Zhang C, Chen L, Liu C, Meng X, Wang W, Li H, Kan H, et al. Associations of PM(2.5) exposure with blood glucose impairment in early pregnancy and gestational diabetes mellitus. Ecotoxicol Environ Saf. 2022;232:113278.
Cao L, Diao R, Shi X, Cao L, Gong Z, Zhang X, Yan X, Wang T, Mao H. Effects of Air Pollution Exposure during Preconception and Pregnancy on Gestational Diabetes Mellitus. Toxics 2023, 11(9).
Lin Q, Zhang S, Liang Y, Wang C, Wang C, Wu X, Luo C, Ruan Z, Acharya BK, Lin H, et al. Ambient air pollution exposure associated with glucose homeostasis during pregnancy and gestational diabetes mellitus. Environ Res. 2020;190:109990.
Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM. Spatial and temporal variation in PM(2.5) chemical composition in the United States for health effects studies. Environ Health Perspect. 2007;115(7):989–95.
Yu G, Ao J, Cai J, Luo Z, Martin R, Donkelaar AV, Kan H, Zhang J. Fine particular matter and its constituents in air pollution and gestational diabetes mellitus. Environ Int. 2020;142:105880.
Robledo CA, Mendola P, Yeung E, Mannisto T, Sundaram R, Liu D, Ying Q, Sherman S, Grantz KL. Preconception and early pregnancy air pollution exposures and risk of gestational diabetes mellitus. Environ Res. 2015;137:316–22.
Liang W, Zhu H, Xu J, Zhao Z, Zhou L, Zhu Q, Cai J, Ji L. Ambient air pollution and gestational diabetes mellitus: an updated systematic review and meta-analysis. Ecotoxicol Environ Saf. 2023;255:114802.
Download references
Acknowledgements
Not applicable.
This research was not funded.
Author information
Weiqi Liu, Haidong Zou and Weiling Liu contributed equally to this work.
Authors and Affiliations
Department of Clinical Laboratory, The Maternal and Children Health Care Hospital (Huzhong Hospital) of Huadu, Guangzhou, 510800, Guangdong, People’s Republic of China
Department of Obstetrics, The Maternal and Children Health Care Hospital (Huzhong Hospital) of Huadu, Guangzhou, 510800, Guangdong, People’s Republic of China
Haidong Zou & Jiangxia Qin
Department of Clinical Laboratory, Foshan Fosun Chancheng Hospital, Foshan, 528000, Guangdong, People’s Republic of China
Weiling Liu
You can also search for this author in PubMed Google Scholar
Contributions
Weiqi Liu conceived the study. Weiqi Liu and Weiling Liu drafted the manuscript. Weiqi Liu performed formal analyses, investigation, methodology, software and verification. Weiqi Liu and Haidong Zou revised the manuscript. Jiangxia Qin and Haidong Zou supported data collection. All authors participated in the interpretation of the results and approved the final version of the manuscript.
Corresponding author
Correspondence to Weiqi Liu .
Ethics declarations
Ethics approval and consent to participate.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Maternal and Children Health Care Hospital of Huadu (approval no. 2024-001). Informed consent for this study was not obtained (and was exempted by the Ethics Committee of the Maternal and Children Health Care Hospital of Huadu) because de-identified data were analyzed.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Liu, W., Zou, H., Liu, W. et al. The impact of PM 2.5 and its constituents on gestational diabetes mellitus: a retrospective cohort study. BMC Public Health 24 , 2249 (2024). https://doi.org/10.1186/s12889-024-19767-1
Download citation
Received : 01 June 2024
Accepted : 12 August 2024
Published : 19 August 2024
DOI : https://doi.org/10.1186/s12889-024-19767-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Black carbon
- Fine particulate matter
- Gestational diabetes mellitus
- Odds ratios
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Case 6–2020: A 34-Year-Old Woman with Hyperglycemia
Presentation of case.
Dr. Max C. Petersen (Medicine): A 34-year-old woman was evaluated in the diabetes clinic of this hospital for hyperglycemia.
Eleven years before this presentation, the blood glucose level was 126 mg per deciliter (7.0 mmol per liter) on routine laboratory evaluation, which was performed as part of an annual well visit. The patient could not recall whether she had been fasting at the time the test had been performed. One year later, the fasting blood glucose level was 112 mg per deciliter (6.2 mmol per liter; reference range, <100 mg per deciliter [<5.6 mmol per liter]).
Nine years before this presentation, a randomly obtained blood glucose level was 217 mg per deciliter (12.0 mmol per liter), and the patient reported polyuria. At that time, the glycated hemoglobin level was 5.8% (reference range, 4.3 to 5.6); the hemoglobin level was normal. One year later, the glycated hemoglobin level was 5.9%. The height was 165.1 cm, the weight 72.6 kg, and the body-mass index (BMI; the weight in kilograms divided by the square of the height in meters) 26.6. The patient received a diagnosis of prediabetes and was referred to a nutritionist. She made changes to her diet and lost 4.5 kg of body weight over a 6-month period; the glycated hemoglobin level was 5.5%.
Six years before this presentation, the patient became pregnant with her first child. Her prepregnancy BMI was 24.5. At 26 weeks of gestation, the result of a 1-hour oral glucose challenge test (i.e., the blood glucose level obtained 1 hour after the oral administration of a 50-g glucose load in the nonfasting state) was 186 mg per deciliter (10.3 mmol per liter; reference range, <140 mg per deciliter [<7.8 mmol per liter]). She declined a 3-hour oral glucose tolerance test; a presumptive diagnosis of gestational diabetes was made. She was asked to follow a meal plan for gestational diabetes and was treated with insulin during the pregnancy. Serial ultrasound examinations for fetal growth and monitoring were performed. At 34 weeks of gestation, the fetal abdominal circumference was in the 76th percentile for gestational age. Polyhydramnios developed at 37 weeks of gestation. The child was born at 39 weeks 3 days of gestation, weighed 3.9 kg at birth, and had hypoglycemia after birth, which subsequently resolved. Six weeks post partum, the patient’s fasting blood glucose level was 120 mg per deciliter (6.7 mmol per liter), and the result of a 2-hour oral glucose tolerance test (i.e., the blood glucose level obtained 2 hours after the oral administration of a 75-g glucose load in the fasting state) was 131 mg per deciliter (7.3 mmol per liter; reference range, <140 mg per deciliter). Three months post partum, the glycated hemoglobin level was 6.1%. Lifestyle modification for diabetes prevention was recommended.
Four and a half years before this presentation, the patient became pregnant with her second child. Her prepregnancy BMI was 25.1. At 5 weeks of gestation, she had an elevated blood glucose level. Insulin therapy was started at 6 weeks of gestation, and episodes of hypoglycemia occurred during the pregnancy. Serial ultrasound examinations for fetal growth and monitoring were performed. At 28 weeks of gestation, the fetal abdominal circumference was in the 35th percentile for gestational age, and the amniotic fluid level was normal. Labor was induced at 38 weeks of gestation; the child weighed 2.6 kg at birth. Neonatal blood glucose levels were reported as stable after birth. Six weeks post partum, the patient’s fasting blood glucose level was 133 mg per deciliter (7.4 mmol per liter), and the result of a 2-hour oral glucose tolerance test was 236 mg per deciliter (13.1 mmol per liter). The patient received a diagnosis of type 2 diabetes mellitus; lifestyle modification was recommended. Three months post partum, the glycated hemoglobin level was 5.9% and the BMI was 30.0. Over the next 2 years, she followed a low-carbohydrate diet and regular exercise plan and self-monitored the blood glucose level.
Two years before this presentation, the patient became pregnant with her third child. Blood glucose levels were again elevated, and insulin therapy was started early in gestation. She had episodes of hypoglycemia that led to adjustment of her insulin regimen. The child was born at 38 weeks 5 days of gestation, weighed 3.0 kg at birth, and had hypoglycemia that resolved 48 hours after birth. After the birth of her third child, the patient started to receive metformin, which had no effect on the glycated hemoglobin level, despite adjustment of the therapy to the maximal dose.
One year before this presentation, the patient became pregnant with her fourth child. Insulin therapy was again started early in gestation. The patient reported that episodes of hypoglycemia occurred. Polyhydramnios developed. The child was born at 38 weeks 6 days of gestation and weighed 3.5 kg. The patient sought care at the diabetes clinic of this hospital for clarification of her diagnosis.
The patient reported following a low-carbohydrate diet and exercising 5 days per week. There was no fatigue, change in appetite, change in vision, chest pain, shortness of breath, polydipsia, or polyuria. There was no history of anemia, pancreatitis, hirsutism, proximal muscle weakness, easy bruising, headache, sweating, tachycardia, gallstones, or diarrhea. Her menstrual periods were normal. She had not noticed any changes in her facial features or the size of her hands or feet.
The patient had a history of acne and low-back pain. Her only medication was metformin. She had no known medication allergies. She lived with her husband and four children in a suburban community in New England and worked as an administrator. She did not smoke tobacco or use illicit drugs, and she rarely drank alcohol. She identified as non-Hispanic white. Both of her grandmothers had type 2 diabetes mellitus. Her father had hypertension, was overweight, and had received a diagnosis of type 2 diabetes at 50 years of age. Her mother was not overweight and had received a diagnosis of type 2 diabetes at 48 years of age. The patient had two sisters, neither of whom had a history of diabetes or gestational diabetes. There was no family history of hemochromatosis.
On examination, the patient appeared well. The blood pressure was 126/76 mm Hg, and the heart rate 76 beats per minute. The BMI was 25.4. The physical examination was normal. The glycated hemoglobin level was 6.2%.
A diagnostic test was performed.
DIFFERENTIAL DIAGNOSIS
Dr. Miriam S. Udler: I am aware of the diagnosis in this case and participated in the care of this patient. This healthy 34-year-old woman, who had a BMI just above the upper limit of the normal range, presented with a history of hyperglycemia of varying degrees since 24 years of age. When she was not pregnant, she was treated with lifestyle measures as well as metformin therapy for a short period, and she maintained a well-controlled blood glucose level. In thinking about this case, it is helpful to characterize the extent of the hyperglycemia and then to consider its possible causes.
CHARACTERIZING HYPERGLYCEMIA
This patient’s hyperglycemia reached a threshold that was diagnostic of diabetes 1 on two occasions: when she was 25 years of age, she had a randomly obtained blood glucose level of 217 mg per deciliter with polyuria (with diabetes defined as a level of ≥200 mg per deciliter [≥11.1 mmol per liter] with symptoms), and when she was 30 years of age, she had on the same encounter a fasting blood glucose level of 133 mg per deciliter (with diabetes defined as a level of ≥126 mg per deciliter) and a result on a 2-hour oral glucose tolerance test of 236 mg per deciliter (with diabetes defined as a level of ≥200 mg per deciliter). On both of these occasions, her glycated hemoglobin level was in the prediabetes range (defined as 5.7 to 6.4%). In establishing the diagnosis of diabetes, the various blood glucose studies and glycated hemoglobin testing may provide discordant information because the tests have different sensitivities for this diagnosis, with glycated hemoglobin testing being the least sensitive. 2 Also, there are situations in which the glycated hemoglobin level can be inaccurate; for example, the patient may have recently received a blood transfusion or may have a condition that alters the life span of red cells, such as anemia, hemoglobinopathy, or pregnancy. 3 These conditions were not present in this patient at the time that the glycated hemoglobin measurements were obtained. In addition, since the glycated hemoglobin level reflects the average glucose level typically over a 3-month period, discordance with timed blood glucose measurements can occur if there has been a recent change in glycemic control. This patient had long-standing mild hyperglycemia but met criteria for diabetes on the basis of the blood glucose levels noted.
Type 1 and Type 2 Diabetes
Now that we have characterized the patient’s hyperglycemia as meeting criteria for diabetes, it is important to consider the possible types. More than 90% of adults with diabetes have type 2 diabetes, which is due to progressive loss of insulin secretion by beta cells that frequently occurs in the context of insulin resistance. This patient had received a diagnosis of type 2 diabetes; however, some patients with diabetes may be given a diagnosis of type 2 diabetes on the basis of not having features of type 1 diabetes, which is characterized by autoimmune destruction of the pancreatic beta cells that leads to rapid development of insulin dependence, with ketoacidosis often present at diagnosis.
Type 1 diabetes accounts for approximately 6% of all cases of diabetes in adults (≥18 years of age) in the United States, 4 and 80% of these cases are diagnosed before the patient is 20 years of age. 5 Since this patient’s diabetes was essentially nonprogressive over a period of at least 9 years, she most likely does not have type 1 diabetes. It is therefore not surprising that she had received a diagnosis of type 2 diabetes, but there are several other types of diabetes to consider, particularly since some features of her case do not fit with a typical case of type 2 diabetes, such as her age at diagnosis, the presence of hyperglycemia despite a nearly normal BMI, and the mild and nonprogressive nature of her disease over the course of many years.
Less Common Types of Diabetes
Latent autoimmune diabetes in adults (LADA) is a mild form of autoimmune diabetes that should be considered in this patient. However, there is controversy as to whether LADA truly represents an entity that is distinct from type 1 diabetes. 6 Both patients with type 1 diabetes and patients with LADA commonly have elevated levels of diabetes-associated autoantibodies; however, LADA has been defined by an older age at onset (typically >25 years) and slower progression to insulin dependence (over a period of >6 months). 7 This patient had not been tested for diabetes-associated autoantibodies. I ordered these tests to help evaluate for LADA, but this was not my leading diagnosis because of her young age at diagnosis and nonprogressive clinical course over a period of at least 9 years.
If the patient’s diabetes had been confined to pregnancy, we might consider gestational diabetes, but she had hyperglycemia outside of pregnancy. Several medications can cause hyperglycemia, including glucocorticoids, atypical antipsychotic agents, cancer immunotherapies, and some antiretroviral therapies and immunosuppressive agents used in transplantation. 8 However, this patient was not receiving any of these medications. Another cause of diabetes to consider is destruction of the pancreas due to, for example, cystic fibrosis, a tumor, or pancreatitis, but none of these were present. Secondary endocrine disorders — including excess cortisol production, excess growth hormone production, and pheochromocytoma — were considered to be unlikely in this patient on the basis of the history, review of symptoms, and physical examination.
Monogenic Diabetes
A final category to consider is monogenic diabetes, which is caused by alteration of a single gene. Types of monogenic diabetes include maturity-onset diabetes of the young (MODY), neonatal diabetes, and syndromic forms of diabetes. Monogenic diabetes accounts for 1 to 6% of cases of diabetes in children 9 and approximately 0.4% of cases in adults. 10 Neonatal diabetes is diagnosed typically within the first 6 months of life; syndromic forms of monogenic diabetes have other abnormal features, including particular organ dysfunction. Neither condition is applicable to this patient.
MODY is an autosomal dominant condition characterized by primary pancreatic beta-cell dysfunction that causes mild diabetes that is diagnosed during adolescence or early adulthood. As early as 1964, the nomenclature “maturity-onset diabetes of the young” was used to describe cases that resembled adult-onset type 2 diabetes in terms of the slow progression to insulin use (as compared with the rapid progression in type 1 diabetes) but occurred in relatively young patients. 11 Several genes cause distinct forms of MODY that have specific disease features that inform treatment, and thus MODY is a clinically important diagnosis. Most forms of MODY cause isolated abnormal glucose levels (in contrast to syndromic monogenic diabetes), a manifestation that has contributed to its frequent misdiagnosis as type 1 or type 2 diabetes. 12
Genetic Basis of MODY
Although at least 13 genes have been associated with MODY, 3 genes — GCK , which encodes glucokinase, and HNF1A and HNF4A , which encode hepatocyte nuclear factors 1A and 4A, respectively — account for most cases. MODY associated with GCK (known as GCK-MODY) is characterized by mild, nonprogressive hyperglycemia that is present since birth, whereas the forms of MODY associated with HNF1A and HNF4A (known as HNF1A-MODY and HNF4A-MODY, respectively) are characterized by the development of diabetes, typically in the early teen years or young adulthood, that is initially mild and then progresses such that affected patients may receive insulin before diagnosis.
In patients with GCK-MODY, genetic variants reduce the function of glucokinase, the enzyme in pancreatic beta cells that functions as a glucose sensor and controls the rate of entry of glucose into the glycolytic pathway. As a result, reduced sensitivity to glucose-induced insulin secretion causes asymptomatic mild fasting hyperglycemia, with an upward shift in the normal range of the fasting blood glucose level to 100 to 145 mg per deciliter (5.6 to 8.0 mmol per liter), and also causes an upward shift in postprandial blood glucose levels, but with tight regulation maintained ( Fig. 1 ). 13 This mild hyperglycemia is not thought to confer a predisposition to complications of diabetes, 14 is largely unaltered by treatment, 15 and does not necessitate treatment outside of pregnancy.

Key features suggesting maturity-onset diabetes of the young (MODY) in this patient were an age of less than 35 years at the diagnosis of diabetes, a strong family history of diabetes with an autosomal dominant pattern of inheritance, and hyperglycemia despite a close-to-normal body-mass index. None of these features is an absolute criterion. MODY is caused by single gene–mediated disruption of pancreatic beta-cell function. In MODY associated with the GCK gene (known as GCK-MODY), disrupted glucokinase function causes a mild upward shift in glucose levels through-out the day and does not necessitate treatment. 13 In the pedigree, circles represent female family members, squares male family members, blue family members affected by diabetes, and green unaffected family members. The arrow indicates the patient.
In contrast to GCK-MODY, the disorders HNF1A-MODY and HNF4A-MODY result in progressive hyperglycemia that eventually leads to treatment. 16 Initially, there may be a normal fasting glucose level and large spikes in postprandial glucose levels (to >80 mg per deciliter [>4.4 mmol per liter]). 17 Patients can often be treated with oral agents and discontinue insulin therapy started before the diagnosis of MODY. 18 Of note, patients with HNF1A-MODY or HNF4A-MODY are typically sensitive to treatment with sulfonylureas 19 but may also respond to glucagon-like peptide-1 receptor agonists. 20
This patient had received a diagnosis of diabetes before 35 years of age, had a family history of diabetes involving multiple generations, and was not obese. These features are suggestive of MODY but do not represent absolute criteria for the condition ( Fig. 1 ). 1 Negative testing for diabetes-associated autoantibodies would further increase the likelihood of MODY. There are methods to calculate a patient’s risk of having MODY associated with GCK , HNF1A , or HNF4A . 21 , 22 Using an online calculator ( www.diabetesgenes.org/mody-probability-calculator ), we estimate that the probability of this patient having MODY is at least 75.5%. Genetic testing would be needed to confirm this diagnosis, and in patients at an increased risk for MODY, multigene panel testing has been shown to be cost-effective. 23 , 24
DR. MIRIAM S. UDLER’S DIAGNOSIS
Maturity-onset diabetes of the young, most likely due to a GCK variant.
DIAGNOSTIC TESTING
Dr. Christina A. Austin-Tse: A diagnostic sequencing test of five genes associated with MODY was performed. One clinically significant variant was identified in the GCK gene ( {"type":"entrez-nucleotide","attrs":{"text":"NM_000162.3","term_id":"167621407","term_text":"NM_000162.3"}} NM_000162.3 ): a c.787T→C transition resulting in the p.Ser263Pro missense change. Review of the literature and variant databases revealed that this variant had been previously identified in at least three patients with early-onset diabetes and had segregated with disease in at least three affected members of two families (GeneDx: personal communication). 25 , 26 Furthermore, the variant was rare in large population databases (occurring in 1 out of 128,844 European chromosomes in gnomAD 27 ), a feature consistent with a disease-causing role. Although the serine residue at position 263 was not highly conserved, multiple in vitro functional studies have shown that the p.Ser263Pro variant negatively affects the stability of the glucokinase enzyme. 26 , 28 – 30 As a result, this variant met criteria to be classified as “likely pathogenic.” 31 As mentioned previously, a diagnosis of GCK-MODY is consistent with this patient’s clinical features. On subsequent testing of additional family members, the same “likely pathogenic” variant was identified in the patient’s father and second child, both of whom had documented hyperglycemia.
DISCUSSION OF MANAGEMENT
Dr. Udler: In this patient, the diagnosis of GCK-MODY means that it is normal for her blood glucose level to be mildly elevated. She can stop taking metformin because discontinuation is not expected to substantially alter her glycated hemoglobin level 15 , 32 and because she is not at risk for complications of diabetes. 14 However, she should continue to maintain a healthy lifestyle. Although patients with GCK-MODY are not typically treated for hyperglycemia outside of pregnancy, they may need to be treated during pregnancy.
It is possible for a patient to have type 1 or type 2 diabetes in addition to MODY, so this patient should be screened for diabetes according to recommendations for the general population (e.g., in the event that she has a risk factor for diabetes, such as obesity). 1 Since the mild hyperglycemia associated with GCK-MODY is asymptomatic (and probably unrelated to the polyuria that this patient had described in the past), the development of symptoms of hyperglycemia, such as polyuria, polydipsia, or blurry vision, should prompt additional evaluation. In patients with GCK-MODY, the glycated hemoglobin level is typically below 7.5%, 33 so a value rising above that threshold or a sudden large increase in the glycated hemoglobin level could indicate concomitant diabetes from another cause, which would need to be evaluated and treated.
This patient’s family members are at risk for having the same GCK variant, with a 50% chance of offspring inheriting a variant from an affected parent. Since the hyperglycemia associated with GCK-MODY is present from birth, it is necessary to perform genetic testing only in family members with demonstrated hyperglycemia. I offered site-specific genetic testing to the patient’s parents and second child.
Dr. Meridale V. Baggett (Medicine): Dr. Powe, would you tell us how you would treat this patient during pregnancy?
Dr. Camille E. Powe: During the patient’s first pregnancy, routine screening led to a presumptive diagnosis of gestational diabetes, the most common cause of hyperglycemia in pregnancy. Hyperglycemia in pregnancy is associated with adverse pregnancy outcomes, 34 and treatment lowers the risk of such outcomes. 35 , 36 Two of the most common complications — fetal overgrowth (which can lead to birth injuries, shoulder dystocia, and an increased risk of cesarean delivery) and neonatal hypoglycemia — are thought to be the result of fetal hyperinsulinemia. 37 Maternal glucose is freely transported across the placenta, and excess glucose augments insulin secretion from the fetal pancreas. In fetal life, insulin is a potent growth factor, and neonates who have hyperinsulinemia in utero often continue to secrete excess insulin in the first few days of life. In the treatment of pregnant women with diabetes, we strive for strict blood sugar control (fasting blood glucose level, <95 mg per deciliter [<5.3 mmol per liter]; 2-hour postprandial blood glucose level, <120 mg per deciliter) to decrease the risk of these and other hyperglycemia-associated adverse pregnancy outcomes. 38 – 40
In the third trimester of the patient’s first pregnancy, obstetrical ultrasound examination revealed a fetal abdominal circumference in the 76th percentile for gestational age and polyhydramnios, signs of fetal exposure to maternal hyperglycemia. 40 – 42 Case series involving families with GCK-MODY have shown that the effect of maternal hyperglycemia on the fetus depends on whether the fetus inherits the pathogenic GCK variant. 43 – 48 Fetuses that do not inherit the maternal variant have overgrowth, presumably due to fetal hyperinsulinemia ( Fig. 2A ). In contrast, fetuses that inherit the variant do not have overgrowth and are born at a weight that is near the average for gestational age, despite maternal hyperglycemia, presumably because the variant results in decreased insulin secretion ( Fig. 2B ). Fetuses that inherit GCK-MODY from their fathers and have euglycemic mothers appear to be undergrown, most likely because their insulin secretion is lower than normal when they and their mothers are euglycemic ( Fig. 2D ). Because fetal overgrowth and polyhydramnios occurred during this patient’s first pregnancy and neonatal hypoglycemia developed after the birth, the patient’s first child is probably not affected by GCK-MODY.

Pathogenic variants that lead to GCK-MODY, when carried by a fetus, change the usual relationship of maternal hyperglycemia to fetal hyperinsulinemia and fetal overgrowth. GCK-MODY–affected fetuses have lower insulin secretion than unaffected fetuses in response to the same maternal blood glucose level. In a hyperglycemic mother carrying a fetus who is unaffected by GCK-MODY, excessive fetal growth is usually apparent (Panel A). Studies involving GCK-MODY–affected hyperglycemic mothers have shown that fetal growth is normal despite maternal hyperglycemia when a fetus has the maternal GCK variant (Panel B). The goal of treatment of maternal hyperglycemia when a fetus is unaffected by GCK-MODY is to establish euglycemia to normalize fetal insulin levels and growth (Panel C); whether this can be accomplished in the case of maternal GCK-MODY is controversial, given the genetically determined elevated maternal glycemic set point. In the context of maternal euglycemia, GCK-MODY–affected fetuses may be at risk for fetal growth restriction (Panel D).
In accordance with standard care for pregnant women with diabetes who do not meet glycemic targets after dietary modification, 38 , 39 the patient was treated with insulin during her pregnancies. In her second pregnancy, treatment was begun early, after hyperglycemia was detected in the first trimester. Because she had not yet received the diagnosis of GCK-MODY during any of her pregnancies, no consideration of this condition was given during her obstetrical treatment. Whether treatment affects the risk of hyperglycemia-associated adverse pregnancy outcomes in pregnant women with known GCK-MODY is controversial, with several case series showing that the birth weight percentile in unaffected neonates remains consistent regardless of whether the mother is treated with insulin. 44 , 45 Evidence suggests that it may be difficult to overcome a genetically determined glycemic set point in patients with GCK-MODY with the use of pharmacotherapy, 15 , 32 and affected patients may have symptoms of hypoglycemia when the blood glucose level is normal because of an enhanced counterregulatory response. 49 , 50 Still, to the extent that it is possible, it would be desirable to safely lower the blood glucose level in a woman with GCK-MODY who is pregnant with an unaffected fetus in order to decrease the risk of fetal overgrowth and other consequences of mildly elevated glucose levels ( Fig. 2C ). 46 , 47 , 51 In contrast, there is evidence that lowering the blood glucose level in a pregnant woman with GCK-MODY could lead to fetal growth restriction if the fetus is affected ( Fig. 2D ). 45 , 52 During this patient’s second pregnancy, she was treated with insulin beginning in the first trimester, and her daughter’s birth weight was near the 16th percentile for gestational age; this outcome is consistent with the daughter’s ultimate diagnosis of GCK-MODY.
Expert opinion suggests that, in pregnant women with GCK-MODY, insulin therapy should be deferred until fetal growth is assessed by means of ultrasound examination beginning in the late second trimester. If there is evidence of fetal overgrowth, the fetus is presumed to be unaffected by GCK-MODY and insulin therapy is initiated. 53 After I have counseled women with GCK-MODY on the potential risks and benefits of insulin treatment during pregnancy, I have sometimes used a strategy of treating hyperglycemia from early in pregnancy using modified glycemic targets that are less stringent than the targets typically used during pregnancy. This strategy attempts to balance the risk of growth restriction in an affected fetus (as well as maternal hypoglycemia) with the potential benefit of glucose-lowering therapy for an unaffected fetus.
Dr. Udler: The patient stopped taking metformin, and subsequent glycated hemoglobin levels remained unchanged, at 6.2%. Her father and 5-year-old daughter (second child) both tested positive for the same GCK variant. Her father had a BMI of 36 and a glycated hemoglobin level of 7.8%, so I counseled him that he most likely had type 2 diabetes in addition to GCK-MODY. He is currently being treated with metformin and lifestyle measures. The patient’s daughter now has a clear diagnosis to explain her hyperglycemia, which will help in preventing misdiagnosis of type 1 diabetes, given her young age, and will be important for the management of any future pregnancies. She will not need any medical follow-up for GCK-MODY until she is considering pregnancy.
FINAL DIAGNOSIS
Maturity-onset diabetes of the young due to a GCK variant.
Acknowledgments
We thank Dr. Andrew Hattersley and Dr. Sarah Bernstein for helpful comments on an earlier draft of the manuscript.
This case was presented at the Medical Case Conference.
No potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org .

IMAGES
COMMENTS
Gestational Diabetes Mellitus Case Study AUG.17, 2021 copy - Free download as PDF File (.pdf), Text File (.txt) or read online for free. This document provides an overview of gestational diabetes mellitus (GDM), including its definition, causes, risk factors, signs and symptoms, complications, incidence, and prognosis. It discusses the three major types of diabetes and two classifications of GDM.
This document presents a case study of a 40-year-old pregnant woman diagnosed with gestational diabetes mellitus. It defines gestational diabetes as a condition caused by pregnancy hormones that make insulin less effective. The clinical pathway for screening and diagnosing gestational diabetes outlined by Diabetes Canada is presented. Statistics provided show the global and national prevalence ...
Gestational diabetes mellitus (GDM) is a condition where high blood sugar levels develop during pregnancy due to the placenta preventing the body from properly using insulin. It affects around 2-10% of pregnancies in the United States. If poorly managed, GDM can increase risks for both mother and baby during pregnancy and childbirth. It is diagnosed through blood tests measuring glucose levels ...
Gestational diabetes is defined as "any degree of carbohydrate intolerance with onset first recognized during pregnancy. This definition applies whether insulin ... is used for treatment and whether or not the condition persists after pregnancy."1 Risk assessment is done early in the pregnancy, with average-risk women being tested at 24-28 weeks' gestation and low-risk women requiring ...
Chapter 20 Case Study: Gestational Diabetes (Margaret) Use p. 705-715 in your Ricci text to answer the following questions. Margaret is admitted to the high-risk perinatal unit for adjustment to her insulin. She is a gestational diabetic for the first time. Her obstetrical history includes two previous infants both over 9 pounds.
Diabetes The name diabetes mellitus has its roots in both the Greek and Latin languages. Diabetes, a Greek word, means to siphon in a continual flow. Mellitus has Latin origins and refers to the honey or sweet urine of diabetics. High blood sugar or hyperglycemia (generally, blood glucose levels >180 ml/dL) causes the urine of diabetic individuals to be high in glucose.6
Abstract. Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy. Screening for GDM is usually done at 24-28 weeks of gestation. In this case, we report a 31-year-old woman who developed gestational diabetes at 6 weeks in two successive pregnancies.
The Case Study of Gestational Diabetes Mellitus (GDM) Underwent Elective Lower Segment Caesarean Section. Glob J Reprod Med. 2017; 1(3): 555563. DOI: 10.19080/GJORM.2017.01.555563 0062 Global Journal of Reproductive Medicine delivery and 1 miscarriage. During pregnancy, she was diagnosed as GDM. She was reluctant taking Glucophage tablet and non-
25 weeks of gestational age, when she weighed 67 Kg of BW and had a BMI of 27.9 Kg.m-2. At the OGTT: The fasting serum glycemia was Abstract Background: How best to define Gestational Diabetes Mellitus (GDM) is the object of debate, with International Association of Diabetes in Pregnancy Study Groups criteria (IADPSGc) differing
Page: 47. This series of interactive case studies is aimed at all healthcare professionals in primary and community care who would like to broaden their understanding of diabetes. These two cases provide an overview of gestational diabetes (GDM). The scenarios cover the screening, identification and management of GDM, as well as the steps that ...
1.1. Need for developing case definitions and guidelines for data collection, analysis, and presentation for gestational diabetes mellitus as an adverse event following immunization. Gestational diabetes mellitus (GDM) is a common condition in pregnancy that can result in significant morbidity and mortality to both mother and fetus.
This document presents a case study of a 40-year-old pregnant woman diagnosed with gestational diabetes mellitus (GDM) at 30 weeks gestation. GDM occurs when pregnancy hormones prevent the body from properly using insulin, resulting in high blood sugar. Left untreated, GDM can increase risks for the mother and baby. The case study aims to demonstrate the student's knowledge of assessing and ...
Gestational diabetes mellitus (GDM) is diagnosed by elevated blood glucose in pregnancy though the definition has changed repeatedly since its first description in the 1960's [1, 2].The most frequently reported perinatal consequence of GDM is macrosomia (usually defined as a neonate weighing over 4 kg) which can increase the risk of caesarean section and shoulder dystocia.
Background A public health problem that has been on the rise in the twenty-first century is gestational diabetes mellitus (GDM). There are serious adverse effects on both maternal and fetal health following GDM. Potential complications can be reduced by early detection of risk factors, which predispose women to GDM. Objectives This study aims to identify the risk factors associated with GDM ...
Gestational diabetes mellitus (GDM) is a state of hyperglycemia (fasting plasma glucose ≥ 5.1 mmol/L, 1 h ≥ 10 mmol/L, 2 h ≥ 8.5 mmol/L during a 75 g oral glucose tolerance test according to IADPSG/WHO criteria) that is first diagnosed during pregnancy [ 1 ]. GDM is one of the most common medical complications of pregnancy, and its ...
Gestational diabetes. k here to access a new interactive case studyHolly is a 31-year-old lady. who is now 26 weeks into her first pregnancy. She sees you with a 3-day hi. tory of dysuria and frequency of micturition. T. ere is no history of abdominal pain or fever.A urine dipstick reveals a positive test.
Whether treatment of gestational diabetes before 20 weeks' gestation improves maternal and infant health is unclear. We randomly assigned, in a 1:1 ratio, women between 4 weeks' and 19 weeks 6 ...
This document presents a case study of a 28-year-old pregnant woman with gestational diabetes mellitus (GDM). It includes sections on the patient's profile, physical assessment findings across multiple systems, and laboratory test results. Key details provided include the patient's medical and pregnancy history, vital signs and physical exam findings over three days, and results of urinalysis ...
Gestational Diabetes Management case studies by diabetesasia.org - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Our aim is to alleviate human suffering related to diabetes and its complications among those least able to withstand the burden of the disease. From 2002 to March 2017, the World Diabetes Foundation provided USD 130 million in funding to 511 projects ...
To investigate the association between gestational diabetes mellitus (GDM) and blood levels of gelsolin (an inflammation-related protein thought to be reduced in type 2 diabetes mellitus) and to determine its role in potential diagnosis and neonatal outcomes. ... This prospective case-control study was conducted at Ankara Etlik City Hospital ...
The increased morbidity and mortality from NRD associated with diabetes in pregnancy has been known for over 60 years. 4-6 A recent meta-analysis reported an odds ratio (OR) of the risk of RDS of 1.57 (95% CI 1.28-1.93) for GDM and 2.66 (95% CI 2.06-3.44) for pre-gestational diabetes mellitus (PGD: largely type 1 and type 2 diabetes in ...
Scribd adalah situs bacaan dan penerbitan sosial terbesar di dunia.
Gestational diabetes - Case study 2 - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or view presentation slides online.
This case study describes a 34-year-old pregnant woman seen for prenatal care at 24 weeks gestation who presented several risk factors for gestational diabetes mellitus (GDM), including obesity, family history of diabetes, and glycosuria on a urine dipstick. Tests showed elevated blood glucose levels, leading to a diagnosis of GDM. The patient was treated with dietary and insulin therapy ...
There is increasing evidence that exposure to PM2.5 and its constituents is associated with an increased risk of gestational diabetes mellitus (GDM), but studies on the relationship between exposure to PM2.5 constituents and the risk of GDM are still limited. A total of 17,855 pregnant women in Guangzhou were recruited for this retrospective cohort study, and the time-varying average ...
PRESENTATION OF CASE. Dr. Max C. Petersen (Medicine): A 34-year-old woman was evaluated in the diabetes clinic of this hospital for hyperglycemia.. Eleven years before this presentation, the blood glucose level was 126 mg per deciliter (7.0 mmol per liter) on routine laboratory evaluation, which was performed as part of an annual well visit.