- Advisory Board
- For Professionals
- For Caregivers
- Administrative Core
- Biomarker Core
- Clinical Core
- Data Management and Statistics Core
- Imaging Core
- Neuropathology Core
- Outreach, Recruitment and Engagement Core
- Research and Education Core
- Brain Donation
- Biospecimen and Data Requests
- Publications
- Events and Seminars
- Newsletters
- Clinical Trials
INFORMATION FOR
- Residents & Fellows
- Researchers

ADRC Funded Projects
Current research scholars, past research scholars, current pilot projects, past pilot projects, past research projects, development projects.
Award Year 05/01/2024 - 04/30/2025
- Dr. Schneeberger-Pané employs state-of-the-art technologies in neuroscience combining unbiased whole mount imaging of circuits, activity, and vasculature; molecular profiling single-cell gene expression technologies, neuromodulation (optogenetics, and chemogenetics) to understand the fundamental principles in the brain governing homeostasis. He received an ADRC Scholar award to facilitate entire new studies focused on the brain vasculature as a substrate of obesity-mediated cognitive dysfunction and neurodegeneration.
- The goal of Dr. Favuzzi's project is to advance the understanding of the role of the innate immune system in regulating homeostatic functions in the brain including brain activity patterns and to elucidate mechanisms of altered states in the brain as a result of aberrant interactions between neurons and innate immune microglial cells.
- Dr. Snell's research addresses a major gap in knowledge regarding the cellular and electrophysiological mechanisms controlling cognitive functions of the cerebellum. The ultimate goal of her lab is to use cutting-edge techniques to study mechanisms underlying rare cerebellar disorders to pioneer discoveries that contribute to the understanding of basic cerebellar function and increase the quality of life of patients worldwide. In addition to advancing the cellular understanding of cerebellar cognitive functions, Dr. Snell plans to utilize resources and samples from the ADRC to develop a comprehensive proteomic study using novel proximity labeling techniques in the cerebellum in collaboration with other ADRC members.
- Dr. Nandy's project investigates the entorhinal cortex (ERC) and its vulnerability to tau pathology in Alzheimer’s disease (AD). Focusing on the ERC’s role in memory and spatial navigation, the study explores the molecular mechanisms, including calcium dysregulation and NMDAR-GluN2B receptor involvement. Using marmosets as a primate model, it aims to compare ERC functions with rodents and macaques to better understand AD progression and identify potential therapeutic targets.
- The main objective of Dr. Vives-Rodriguez's project is to characterize the progression of positive psychiatric symptoms and their association with cognitive deficits across the spectrum of Lewy body disease, including patients with PD who are cognitively unimpaired, PD-MCI, PDD, DLB, and age-matched healthy controls. The neuropsychological deep phenotyping, DNA, and bio sample collections will set the foundation for future phenotype-genotype and biomarker investigations across the Lewy body spectrum.
- Dysregulated alternative splicing has been identified as a major factor in Alzheimer’s disease and related dementias (ADRD). Loss of proteins like TDP-43 and mutations in MAPT disrupt critical neuronal functions. Dr. Wei's project aims to develop a new method called region-specific Perturb-seq, which will identify cellular regulators of these splicing issues. By focusing on specific mRNA regions, this method will improve the detection of low-abundance splicing events and allow for high-throughput analysis. The ultimate goal is to uncover new therapeutic targets to treat ADRD effectively
Award Year 5/01/2023 - 4/30/2024
- This project integrates multimodal neuroimaging, genetics, and clinical data to identify preclinical biomarkers for Alzheimer’s disease (AD). By combining advanced imaging techniques with genetic analysis and clinical assessments, Dr. Xu aims to detect early signs of AD before clinical symptoms appear. The goal is to improve early diagnosis and intervention strategies, enhancing our understanding of AD progression and enabling more effective treatments.
- Dr. Tavares Da Silva Pereira's project focuses on frontotemporal dementia (FTD) and its genetic overlap with ALS. It aims to identify early FTD phenotypes using iPSC-derived neurons with TDP43 and C9ORF72 mutations and cell painting assays combined with AI. The study will compare FTD and ALS phenotypes, explore stressor responses, and conduct a small-scale drug screen to find potential early interventions, utilizing advanced imaging and FDA-approved drug libraries. This approach aims to uncover the mechanisms behind FTD and ALS and identify druggable targets for early intervention.
- This project investigates the molecular changes in the hippocampus and amygdala of Alzheimer’s disease (AD) patients with recent depression episodes. Dr. Pathak aims to identify differential gene expression in these brain regions to understand the comorbidity of depression and AD. By analyzing 70 samples from 35 donors, the study will identify key molecular pathways and potential therapeutic targets. Techniques include RNA sequencing, differential gene expression analysis, and cross-tissue correlation to uncover insights into the transcriptomic regulation in AD and depression.
- Persons living with dementia (PLWD) visit emergency departments (ED) more frequently than those without cognitive impairments. The ED is a crucial but underutilized setting for engaging PLWD and their caregivers. Dr. Gettel's project aims to examine the assistance provided by caregivers to PLWD and those with undiagnosed cognitive impairments, to inform ED-based best practices and improve early detection of cognitive issues.
- This project aims to uncover the mechanisms behind Amyloid Precursor Protein processing by γ-secretase and Aβ production in Alzheimer’s disease. Utilizing techniques like lipid photoaffinity crosslinking, click chemistry, and LC-MS/MS lipidomic analysis, Dr. Kim's study will explore lipid-protein interactions and membrane homeostasis. Supported by experts in mass spectrometry, γ-secretase functions, and neurodegenerative disease models, this research seeks to provide new insights into Alzheimer’s disease progression.
Award Year 5/01/2022 - 4/30/2023
- Yifei Cai, PhD : "Molecular mechansms of axonal pathology in Alzheimer's disease human neurons"
- Sathish Ramakrishnan, PhD : "Mechanism of exocytosis protein in Alzheimer's disease"
- Dibyadeep Datta : "Interrogating the molecular mechanisms mediating the emergence of biomarker pT217-tau in AD"
Award Year 5/01/2021 - 4/30/2022
- Eyiyemisi Damisah, MD : "Investigating statistical learning in the Dementias"
- Hongying Shen, PhD : "Expanding Neurometabolic Biochemistry Underlying Alzheimer's disease"
- Takuya Toyonaga, MD, PhD : "Whole brain in vivo characterization of synaptic density in Alzheimer's disease model rat with different rearing environments"
- Le Zhang, PhD : "Sex-Specific Single Cell Expression Profiles and Genetic Risk in Alzheimer's disease"
- Yize Zhao, PhD : "Connectome coupling and genetic underpinning in the Supernormal"
Award Year 6/15/2020 - 4/30/2021
- Adam Mecca, MD, PhD : "Novel Biomarkers of Synaptic Health in Alzheimer’s disease"
- Carolyn Fredericks, MD : "Tau PET & Network Integrity in Atypical Alzheimer’s disease"
- Evelyn Lake, PhD : "A multimodal PET, MR, and fluorescence CA2 imaging study of a murine model of CTE"
- Marcello DiStasio, MD, PhD : "Uncovering Proinflammatory Signatures in Alzheimer's Disease Using Spatial Transcriptomics"
- Juan Young, MD : "Investigating a CD8 + T cell associated aging gene signature in Alzheimer's disease"
- Daniel Jane-Wit, MD, PhD, RPVI : "ZFYVE21 and Cerebral Amyloid Angiopathy in Alzheimer's disease Related Dementias"
- Dibyadeep Datta : "Investigating the role of pT217-tau in the pathogenesis of AD and relevance for biomarker development"
Previously awarded pilot projects funded by a NIA ADRC grant.
Award Year 4/1/2015 - 3/31/2016
- Janghoo Lim, PhD : "The role of Neuroinflammation in the pathogenesis of Alzheimer's disease with focus on nemo-Like Kinase"
- Ming-Kai Chen, MD, PhD : "Brain PET Imaging of Synaptic Density in Alzheimer's disease"
Award Year 4/1/2016 - 3/31/2017
- Becky Carlyle, PhD: "Profiling 3D Amyloid Patterns"
Award Year 4/1/2017 - 3/31/2018
- Marc Hammarlund, PhD : "A new C. elegans model for AB42 toxicity"
Award Year 4/1/2018 - 3/31/2019
- Carla Rothlin, PhD : "Mechanisms of reactive astrogliosis - a critical feature in Alzheimer's disease"
- Sreeganga Chandra, PhD : "Testing Synapse Protection Strategies for Alzheimer's disease"
- Anita Huttner, MD : "Modeling Sporadic Alzheimer's disease with Human IPSC-Derived Cerebral Organoids"
Award Year 4/1/2019 - 3/31/2020
- Kurt Zilm, PhD : "Structural characterization by NMR of amyloid-beta oligomers bounds in hydrogel phase to prion protein"
- Le Zhang, PhD : "Single nucleus and single-cell profiling of human brain and CSF in Alzheimer's disease"
Award Year 5/1/23 - 4/30/24
- Insoo Kang : “Investigating comprehensive genomic profile and heterogeneity of viral antigen-specific T cells in patients with Alzheimer's disease using single cell RNA sequencing”
- Jianbing Zhou : “Ribonucleoprotein-mediated genome editing therapy for Alzheimer's Disease”
Award Year 5/1/2022 - 4/30/2023
- Caroline Fredericks, MD : "Predictive modeling of genetic Alzheimer's risk and memory performance in healthy older adults"
- Chao Zheng, PhD : "Longitudinal ROCK2-PET imaging study in the TgF344-AD transgenic rat model of AD"
- Pallavi Gopal, MD, PhD : "TDP-43 transport and spatiotemporal regulation of RNA in Alzheimer's disease"
- David Matuskey, MD and Arman Fesharaki, MD, PhD, BMath : "Novel in vivo synaptic imaging in Behavioral Variant Frontotemporal Dementia (bvFTD)"

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Research on Alzheimer’s Disease and Related Dementias

Alzheimer’s disease and related dementias are a series of complex brain disorders that affect millions of Americans and many more people worldwide. These disorders have an enormous impact on individuals and their families, long-term care facilities, health care providers, health care systems and infrastructure, and the communities in which we all live. As the economic, social, and personal costs of these diseases climb, the research community is working to discover solutions that will improve the lives of those with dementia, their caregivers, and their communities.
The federal government’s Alzheimer’s and related dementias research strategy focuses on engaging a cross-disciplinary team of geneticists, epidemiologists, gerontologists, behavioral scientists, disease and structural biologists, pharmacologists, clinical researchers, and others to bring the greatest and most diverse expertise to the field. This includes training new generations of researchers and clinician-scientists and engaging in innovative partnerships with private industry, nonprofit groups, and more to foster collaboration and broaden access to research resources and data.
Critically, the government’s research strategy includes the search to find treatment and prevention strategies, as well as interventions, services, and supports to improve quality of life for those already living with these diseases and their families.
Who Funds Alzheimer’s and Related Dementias Research?
The National Institutes of Health (NIH) is made up of Institutes, Centers, and Offices that conduct and fund research into all aspects of human health. The National Institute on Aging (NIA) leads NIH’s efforts in clinical, behavioral, and social research in Alzheimer’s and related dementias through efforts aimed at finding ways to treat and ultimately prevent the disorder. NIA collaborates closely with the National Institute of Neurological Disorders and Stroke (NINDS), which manages a research portfolio targeting Alzheimer’s-related dementias. While some of this research takes place in NIH laboratories, the vast majority of NIH support is provided through a competitive grants process to institutions and small businesses across the country. Other federal agencies support a range of activities focused on public health and community programs.
Advances in Alzheimer's and Related Dementias Research
As the nation’s biomedical research agency, NIH supports research ranging from basic biology to drug development and from clinical studies to evaluating public health outcomes. Within the past several decades, researchers have made great strides toward better understanding what causes Alzheimer’s and related dementias and discovering approaches that may prevent, diagnose, and treat them. Some highlights of these efforts include:
- Drug discovery and drug repurposing. Thanks to the substantial investment in Alzheimer’s and related dementias research over the past decade, NIH has increased drug discovery significantly. Of the many compounds in NIH-supported drug development programs for Alzheimer’s and related dementias, 18 new dementia drug candidates have now matured through the pipeline, from discovery in the lab all the way through preclinical development, to reach the stage of human testing. NIA currently supports more than 60 clinical trials testing drug candidates that target many different aspects of the disease. Several of these drug candidates are intended to stop or slow the disease process rather than only treat symptoms. For example, some target amyloid plaques and tau tangles in new ways. Researchers are also exploring multiple ways to repurpose drugs for the potential treatment of dementia, including FDA-approved drugs used to treat epilepsy and diabetes.
- Early detection and diagnosis. Researchers have made significant progress in developing, testing, and validating biomarkers that detect signs of the disease process. For example, in addition to PET scans that detect abnormal beta-amyloid plaques and tau tangles in the brain, NIH-supported scientists have developed the first commercial blood test for Alzheimer’s. This test and others in development can not only help support diagnosis but also be used to screen volunteers for research studies. Other discoveries are leading to the development of potential biomarkers for other dementias. These include the detection of abnormal TDP-43 protein, found in frontotemporal dementias, and a cerebrospinal fluid test to help diagnose Lewy body dementia and Parkinson’s disease. Researchers are also studying behavioral and social indicators, including problems with paying bills and a combined decline in memory and walking speed, that may be early signs of these diseases. Other early markers are also under study.
- Risks factors, genetics, and disease pathways. NIH’s research investments to identify the biological mechanisms that lead to Alzheimer’s and related dementias are fundamental for the discovery of potential drugs that target them. There are many biological pathways that scientists can target with investigational drugs. For example, several recent studies have further revealed how components of the immune system, brain inflammation, vascular disease, and possibly viruses and bacteria — including the many tiny organisms that live in the digestive system, known as the gut microbiome — contribute to the development of these diseases. Scientists are also exploring genetic variations that may contribute to or prevent disease. Recent research has revealed that the genetic risk for Alzheimer’s differs between ethnic and racial groups, highlighting the need for more diversity in genetic research studies. Scientists are also discovering genetic variants that may help protect against Alzheimer’s. Other studies are identifying the genetic underpinnings of related dementias, including new gene variants linked to the development of Lewy body dementia.
- Population studies and precision medicine. By studying large, diverse groups of people, researchers are identifying which genes, behaviors, and lifestyle choices are linked with dementia. Population studies have shown that sedentary behavior, low socioeconomic status, low level of education, and living in a poor neighborhood may increase the risk of developing dementia. These observational discoveries, along with knowledge of genetic and other factors, can be used to advance the development of methods for diagnosis, prevention, and treatment at an individualized level.
- Health disparities and dementia risk. NIH-funded researchers are examining the biological, social, and environmental factors that contribute to the higher prevalence of dementia in Hispanic Americans and Black Americans compared with other White Americans. Since dementia is also underdiagnosed in these populations, researchers are studying approaches to improve diagnoses in underserved communities. NIH is also investing in strategies to increase diversity in research study participants.
- Lifestyle interventions. Researchers are investigating interventions around exercise, healthy eating, cognitive training, preventive health care, and management of chronic conditions, such as high blood pressure, that — if made early in life — may be able to prevent or delay disease symptoms. Emerging areas of study include interventions to enhance cognitive reserve — the mind’s ability to cope with the effects of aging — and interventions to potentially compensate for premature cognitive decline and dementia linked to adverse exposures in early life, such as abuse and malnutrition. NIA currently supports more than 150 trials testing behavioral and lifestyle interventions.
- Dementia care and caregiver support. NIH has significantly expanded research on how to improve dementia care and support for care partners. Researchers are investigating new dementia care models and strategies to equip family caregivers with tools and knowledge to manage the challenges of caring for a loved one with dementia. Studies are also underway to examine ways to improve quality of life for people with dementia and their caregivers. Other studies aim to understand the costs and challenges of dementia, including lost wages and paying for long-term care. NIA currently supports more than 200 studies on dementia care and caregiving.
- Infrastructure development. NIH is continually investing in research infrastructure to advance Alzheimer’s and related dementias research. Efforts in this area include launching a consortium for Alzheimer’s clinical trials, a collaboratory to test interventions to improve care of people with dementia in real-world settings, research efforts to validate cognitive tests in a primary care setting, and centralized data-sharing platforms and other technologies.
Challenges for the Alzheimer’s Research Community
Even with the progress that we’ve made, there’s still a lot of work to do before we can find treatment and prevention strategies for the millions of people affected by Alzheimer’s and related dementias. These devastating diseases are highly complex conditions caused by an interplay of genetic, lifestyle, and environmental factors. They usually develop gradually — changes in the brain take place over years and even decades, long before the first symptoms appear. This complexity presents challenges to the discovery and development of new drugs and other prevention and treatment approaches.
Researchers believe Alzheimer’s and related dementias will likely require multiple treatments customized to individuals. We also know that as the older population continues to grow — aging remains the most important risk factor for dementia — we will see increased numbers of people living with these diseases. That’s why thousands of researchers around the country are working on this issue.
Setting the Federal Research Agenda
NIH takes a collaborative, methodical approach to reviewing progress, identifying gaps, and setting the future agenda for research into Alzheimer’s and related dementias. NIH funding in this area is guided by gaps and opportunities identified in research summits , which alternate yearly to focus on Alzheimer’s, Alzheimer’s-related dementias, or dementia care and services. Smaller, focused workshops are held more frequently on specific aspects of this research.
NIH outlines its Alzheimer’s research efforts in the NIH AD/ADRD Research Implementation Milestones , a research framework detailing specific steps and success criteria toward achieving the goals of the National Plan to Address Alzheimer’s Disease . The milestones also showcase funding initiatives, accomplishments, and highlights of progress toward accomplishing the National Plan goals.
NIH’s research progress is highlighted in the annual Alzheimer’s and related dementias professional judgment budget , which is submitted to Congress each year.
What Is a Professional Judgment Budget?
Each year NIH submits a professional judgment budget that estimates the additional funding needed to advance NIH-supported research into the treatment and prevention of Alzheimer’s and related dementias. The report also summarizes progress and promising research opportunities. Only two other areas of biomedical research — cancer and HIV/AIDS — follow a similar process designed to accelerate research discovery. This approach is often referred to as a “bypass budget” because of its direct transmission to the President and then to Congress without modification through the traditional federal budget process.
Clinical Research Into Alzheimer’s and Related Dementias
No major advance in Alzheimer’s and related dementias treatment, prevention, or care will be possible without robust clinical research. Clinical research includes studies that involve people so scientists can learn more about disease progression, how behavior and lifestyle factors may affect health, and the safety and effectiveness of an intervention. Advances made through clinical research rely on the volunteers who participate in these types of studies. NIA is working on multiple initiatives to enhance recruitment and retention of diverse populations in clinical research. View some of those resources below.
NIA-funded clinical research includes both observational studies through which researchers gather important information, and clinical trials in which researchers test interventions to treat or prevent disease, improve care and caregiver support, and enhance quality of life for people living with dementia. NIA is currently funding more than 400 active clinical trials .
NIA also funds more than 30 Alzheimer’s Disease Research Centers across the country. Scientists at these centers conduct clinical research to improve diagnosis and care for people with dementia and their families, and to find a treatment or increase prevention.

Volunteer for Research
You could help discover new ways to treat and prevent Alzheimer’s and related dementias.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Find More Resources on Alzheimer’s Research
Explore the resources on this website and linked below to find more information from federal government agencies.
View professional judgment budgets for Alzheimer’s and related dementias from NIH, including yearly updates on research progress.
Browse this database to learn more about research implementation plans and progress toward the goal of treating or preventing Alzheimer’s and related dementias.
Search this repository of resources to support the recruitment and retention of participants into clinical trials and studies on Alzheimer’s disease and related dementias.
Learn about the data sharing policies, considerations, resources, and guidance available to support researchers in safely and efficiently sharing data from their studies.
Visit IADRP to search a database of categorized research across public and private sources.
Learn about NIA's efforts toward the National Plan and NIH annual summits that shape research priorities.
View a list of all active NIA-funded clinical trials, including drug trials, intervention studies, and care and caregiver interventions.
Search for NIA-supported clinical research tools, datasets, samples, visualization tools, and more for Alzheimer’s and related dementias research.
Read the National Strategy for Recruitment and Participation in Alzheimer’s and Related Dementias Research and get resources to support study recruitment.
Read about the National Institute of Neurological Disorders and Stroke’s research into Alzheimer’s disease-related dementias.
Search NIH-funded research in Alzheimer’s and related dementias.
Other Articles in This Section
- Federal Response
- National Research Centers
Questions? Contact the ADEAR Center
The Alzheimer’s & related Dementias Education & Referral (ADEAR) Center is a service of the National Institute on Aging at the National Institutes of Health. Call 800-438-4380 or email [email protected] to talk with an information specialist.
Last updated: July 9, 2024
This content is provided by the National Institute on Aging (NIA), part of the National Institutes of Health. NIA scientists and other experts review this content to ensure it is accurate and up to date.
alzheimers.gov
An official website of the U.S. government, managed by the National Institute on Aging at the National Institutes of Health

Enter a Search Term
Alzheimer's disease research.
Driving innovative research around the world to end Alzheimer’s , we identify high-risk, high-reward projects that have the most promise to change the trajectory of the disease.
Our 360° Approach to Research
Tangling with tau, battling amyloid beta, blood and the brain in dementia, immunity and inflammation, biology of apoe and lipids, new approaches.
BrightFocus takes a 360 ° approach to fund innovative scientific research worldwide to defeat Alzheimer’s, exploring the full range of scientific paths toward better treatments and ultimately a cure. Watch this video and learn more.
Genes are the “master blueprint” that instructs our cells to make unique proteins which in turn build, operate, and repair human tissue. Humans have an estimated 24,000 genes along our 23 matched pairs of chromosomes (46 in all), and “genomics” refers to the field that studies all of them at once.

A biological marker (biomarker) is a measurable substance in an organism whose presence is indicative of some phenomenon such as disease or infection. Biomarkers can help doctors and scientists diagnose diseases and health conditions, find health risks in a person, monitor responses to treatment, and see how a person's disease or health condition changes over time.

Tau is a small protein with a short name but a large reputation because of its association with multiple brain diseases, including Alzheimer’s disease (AD). The tau protein is predominantly found in brain cells (neurons).

There are many versions of amyloid protein in the human body, and most serve a useful role. One of the hallmarks of Alzheimer’s disease (AD) is the accumulation of amyloid plaques (abnormally configured proteins) between nerve cells (neurons) in the brain.

Scientists are interested in developing a screening tool for Alzheimer’s disease (AD) in blood. A simple blood draw is much less invasive than a spinal tap and may prove more cost effective. Developing blood biomarkers that accurately depict brain changes has proven challenging, as levels of AD hallmark proteins in the blood are low, but there are some very recent promising results observing tau and the ratio of Aβ42 and Aβ40.

One theory about Alzheimer’s disease (AD) is that it may be triggered, in part, by a breakdown in the brain’s immune system.

Alzheimer's disease (AD). Its primary function is to regulate a class of proteins involved in the metabolism of fats (lipids) in the body. However, APOE has several common variants (or "alleles") whose effect vary.

The human brain has an estimated 100 billion neurons. Extending from each of them is a long fiber, known as an “axon,” which can run several feet. Each axon forms a connection, known as a “synapse” with another neuron, creating a circuit over which brain signals travel. In Alzheimer’s disease (AD), individual neurons die and do not regenerate; while others have brains that are more are resilient and respond to meeting changing demands.

Years of innovative and dedicated research have paid off with the discovery of numerous factors contributing to Alzheimer’s disease (AD) pathology. With a disease as complex as this one, it’s very helpful to find multiple points where it may be possible to slow or halt its progress.

Research We Fund
BrightFocus drives innovative research worldwide on Alzheimer’s, macular degeneration, and glaucoma. Search our grant awards to learn more.
Insights and Breakthroughs
Well-designed research pays off. With further research, each of these discoveries may contribute to the development of new treatments and preventions.

Driving Innovation in Diagnosis and Treatment: Alzheimer’s Disease Research Roundup

Searching the Eye for Signs of an Inherited Rare Form of Alzheimer’s

New Alzheimer’s Drug Leqembi Granted Full FDA Approval

What Can We Learn From 100-Year-Olds Without Alzheimer’s?

FDA Approves First Treatment for Alzheimer’s-Associated Agitation

Researchers Develop First-of-its-Kind Artificial Intelligence Model That Could Detect Alzheimer’s Through Retinal Photographs

Researchers Identify a Genetic Factor in People of African Ancestry That May Lower Alzheimer’s Risk

Moderate Alcohol Use May Accelerate Alzheimer’s Disease
Living with alzheimer's.
An Alzheimer’s diagnosis can be overwhelming. BrightFocus Foundation is a trusted resource to help you understand, manage and live with Alzheimer’s disease.
Learn from experts.
We're here to help.
Alzheimer's disease is the seventh leading cause of death in the United States. An irreversible degeneration of the brain that causes disruptions in memory, cognition, personality, and other functions, it eventually leads to death from complete brain failure.
Nearly 7 million Americans aged 65 and older are thought to have Alzheimer's disease. By 2050, that figure may increase to nearly 13 million.
Learn about Alzheimer's Disease
Alzheimer’s disease is the most common form of dementia, affecting more than six and a half million Americans aged 65 and older. In this section, you can find out more about Alzheimer’s and how you can manage care for yourself or a loved one.
- Alzheimer's Overview
- Brain Health
Donate to Alzheimer’s Disease Research
Your gift can help lead to treatments and a cure to end Alzheimer’s. Fund the latest, promising research and help provide valuable information to families living with this disease.
I would like to donate
The Eye, A Window on the Brain
It is often said that “the eyes are the window to the soul,” and while that may or may not be true, the eye is certainly a window into many health conditions.
In fact, sometimes an eye doctor will be the first physician to diagnose a medical condition because the first signs may appear in the eye. Thus, having your eyes thoroughly examined is a lot more than just getting a prescription for new glasses or contact lenses.
Useful Resources
BrightFocus Foundation offers vetted resources to help you and your loved ones better understand, manage and live with an Alzheimer’s diagnosis.
- Healthy Living
- Understanding Alzheimer's
- Managing Alzheimer's
- Living with Alzheimer's
Expert Advice
Useful articles to help you understand and manage symptoms, treatment, and the latest discoveries in Alzheimer's Disease Research.

When Alzheimer’s Disease Begins with Vision Problems

What’s Next for Alzheimer’s Disease Treatments: A 2024 Forecast

Alzheimer’s Treatment Coverage: 6 Facts to Know About Patient Registries

Facts About Leqembi, a New Alzheimer’s Drug

Exploring a Connection Between ADHD and Alzheimer’s

"Is It Something I'm Taking?" Medications That Can Mimic Dementia

Biohacking Brain Health: Research Exploring Fasting and Diet Changes Shows Promise in Delaying Alzheimer's Disease, Improving Cognition

Navigating Neurodegenerative Diseases: What Causes Neurodegeneration and Can It Be Stopped?

Can a Multivitamin Prevent Alzheimer’s Disease?

Alzheimer’s Blood Tests: How Do They Work and Should You Request One?

Alzheimer’s Disease and COVID-19—What’s the Connection?
Take action, be part of the cure.
The most valuable assets we have are the individuals dedicated to our cause. People like you are the reason we are able to boost our scientific agenda so that patients’ lives may be enhanced.
Find an Alzheimer's Clinical Trial
These studies are crucial to advancing medical approaches most effective for specific conditions or groups of people. Today’s clinical trials will lead to new standards of care in the future.
Connect with @BrightFocus
Let’s stay in touch. BrightFocus is a top source of accurate, helpful information on the latest scientific research and care for Alzheimer’s, glaucoma, and macular degeneration.

Current research projects
Our research aims to understand the underlying causes of the condition, improve diagnosis and care, identifying ways to prevent dementia and searching for a cure.
Below you can find out about the research projects that we are funding. Discover more about our researchers' work and how it will impact people affected by dementia.
In addition, learn about all our current:
- Fellowships
- PhD studentships
- Implementation and dissemination grants
- ADDF Drug Discovery grants
- International Research Partnerships
Latest research projects
Understanding the effects of the diabetes type 2 drug Metformin on models of Alzheimer’s disease
Lead Investigator: Dr Teresa Niccoli
Institution: University College London
Grant type: PhD Studentship
Awarded: 2020/21
Investigating how to clear toxic amyloid protein from the brain in Alzheimer’s disease
Lead Investigator: Professor K. Ravi Acharya
Institution: University of Bath
Mining a common food compound, Epicatechin, for a new Alzheimer’s disease treatment
Lead Investigator: Dr Robert Williams
Grant type: Project
Awarded: 2019/2020
Project grants
Developing new ways to study and test treatments for small vessel disease in vascular dementia
Lead Investigator: Professor Karen Horsburgh
Institution: University of Edinburgh
Grant type: Project
Awarded: 2016/2017
Understanding whether drugs for rheumatoid arthritis can reduce the risk of Alzheimer’s disease
Lead Investigator: Dr Bernadette McGuinness
Institution: Queen's Univeristy Belfast
Awarded: 2016/2017
Investigating a potential target for treatment of frontotemporal dementia
Lead Investigator: Dr Christopher Miller
Institution: Institute of Psychiatry, King's College London
Awarded: 2015/2016
Investigating heparins as a potential new drug for Alzheimer’s disease
Lead Investigator: Professor Jerry Turnbull
Institution: University of Liverpool
Testing the effect of the diabetes drug Liraglutide in Alzheimer's disease
Lead Investigator: Dr Paul Edison
Institution: Imperial College London
Awarded: 2014/2015
Fellowships
Taking an innovative approach to designing potential treatments for Alzheimer’s disease
Lead Investigator: Dr Francesco Antonio Aprile
Institution: University of Cambridge
Grant type: Senior Fellowship
Awarded: 2016/2017
PhD studentships
Can leptin, the anti-obesity hormone, protect brain cells?
Lead Investigator: Dr Jenni Harvey
Institution: University of Dundee Grant type: PhD studentship
Awarded: 2018/2019
Using an innovative approach to prevent the toxic build-up of amyloid in Alzheimer’s disease
Lead Investigator: Dr Jody Mason
Grant type: PhD studentship
Awarded: 2017/2018
Does targeting the immune system have the potential to treat Alzheimer’s disease?
Lead Investigator: Dr David Brough
Institution: University of Manchester
What factors affect the attitudes of young people to dementia?
Lead Investigator: Dr Nicolas Farina
Institution: Brighton and Sussex Medical School
Can we predict Alzheimer’s disease and its risk factors from the proteins found in the blood?
Lead Investigator: Dr Riccardo Marioni
Supporting people with dementia to continue their careers
Lead Investigator: Dr Louise Ritchie
Institution: University of West of Scotland
Supporting conversations about end-of-life care
Lead Investigator: Dr Nathan Davies
Redesigning care homes to improve the wellbeing of people with dementia
Lead Investigator: Professor Catherine Hennessy
Institution: University of Stirling
Improving exercise classes for people with dementia
Lead investigator: Dr Annabelle Long
Grant type: Fellowship
Awarded: 2020/21
Interacting with children: What are the benefits for people with dementia?
Lead Investigator: Dr Suzanne Beeke
Institution: University College London Grant type: PhD Studentship Awarded: 2019/20
Are smartphones a gamechanger for dementia research?
Lead Investigator: Dr Chris Hinds
Institution: University of Oxford Grant type: Project Awarded: 2019/20
Can blood tests be used to diagnose delirium and improve diagnosis?
Lead Investigator: Dr Valerie Page
Institution: Watford General Hospital Grant type: Project Awarded: 2019/20
Supporting person-centred care for people with dementia in hospitals
Lead Investigator: Dr Melanie Hanley
Institution: University of Hertfordshire Grant type: Project grant Awarded: 2019/20
Understanding the genetics of depression in Alzheimer’s disease
Lead Investigator: Dr Lindsey Sinclair
Institution: University of Bristol Grant type: Junior Fellowship Awarded: 2019/20
Supporting deaf carers of people with dementia
Lead Investigator: Dr Emma Ferguson-Coleman
Institution: University of Manchester Grant type: Junior Fellowship Awarded: 2019/20
Understanding the role of volunteering in the future of dementia care
Lead Investigator: Professor Heather Wilkinson
Institution: University of Edinburgh Grant type: PhD studentship Awarded: 2019/20 How can compassionate communities support end of life dementia care?
Lead Investigator: Dr Joseph Sawyer
Institution: University College London Grant type: Clinical Training Fellowship Awarded: 2019/20
Exploring dementia in the South Asian community Lead Investigator: Dr Naaheed Mukadam
Institution: University College London Grant type: Senior Fellowship Awarded: 2019/20
Care collaboration grants
Exchanging knowledge with independent care homes in Exeter
Lead Investigator: Dr Iain Lang
Institution: University of Exeter Grant type: Care Collaboration Grant Awarded: 2019/20
Can embedding a researcher in care homes help to improve care?
Lead Investigator: Professor Mo Ray
Institution: University of Lincoln Grant type: Care Collaboration Grant Awarded: 2019/20
A new approach to collaborative research with care homes in Worcester
Lead Investigator: Professor Tracey Williamson
Institution: University of Worcester Grant type: Care Collaboration Grant Awareded: 2019/20
Understanding the benefits of community singing groups for people with dementia
Lead investigator: Professor Justine Schneider
Awarded: 2018/2019
Improving night-time care and reducing hypnotic drug use in care homes
Lead Investigator: Dr Anne Corbett
Institution: University of Exeter Grant type: Project grant Awarded: 2019/2020 Optimising PET scanning to diagnose Alzheimer’s disease
Lead Investigator: Dr Paresh Malhotra
Awarded: 2018/2019
Understanding more about community based support for people affected by dementia
Lead investigator: Professor Dawn Brooker
Institution: University of Worcester
Awarded: 2017/2018
Residential respite care: Experiences, access and outcomes Lead Investigator: Dr Kritika Samsi
Institution: King's College London
Awarded: 2018/2019 Understanding and improving end of life care for people with dementia and their carers
Lead Investigator: Professor Martin Knapp Institution: London School of Economics and Political Science Grant awarded: 2017/2018
Detecting changes in the brain in frontotemporal dementias
Lead Investigator: Professor Jason Warren
Grant awarded: 2016/2017
Improving diagnosis and support services for people with younger onset dementia
Lead Investigator: Dr Janet Carter
Grant awarded: 2015/2016
Identifying factors that lead to problems with everyday tasks for people with dementia
Lead Investigator: Dr Eneida Mioshi
Institution: University of East Anglia
Grant awarded: 2014/2015
Implementation grants
Supporting end of life decision-making in care homes
Lead Investigator: Professor Kevin Brazil
Institution: Queen’s University Belfast Grant type: Implementation Awarded: 2018/2019
Beyond the Margins: Accessing support in the community Lead Investigator: Professor Charlotte Clarke Institution: University of Edinburgh
Grant type: Implementation
Awarded: 2018/2019
Knowledge Exchange Fellowships
A digital guide to support people with dementia to be part of the community
Lead Investigator: Dr Kieren Egan
Institution: University of Strathclyde Grant type: Knowledge exchange fellowship Awarded: 2019/20
How does your economic background affect the dementia care you access?
Lead Investigator: Clarissa Giebel
Grant type: Knowledge exchange fellowship
Awarded: 2019/20
Clinical Training Fellowships & Partnerships
Providing research training to increase support and improve care for people with dementia and their carers
Lead Investigator: Professor Linda Clare Institution: University of Exeter
Grant type: Clinical Training Partnership
A mouth care programme for people with dementia and swallowing problems
Lead investigator: Dr Julie Pollock
Nottingham University Hospitals NHS Trust
Grant type: Clinical Training Fellowship
Tackling critical issues in dementia diagnosis, care and treatment
Lead Investigator: Professor Dag Aarsland
Institution: King’s College London Grant type: Clinical Training Partnership Awarded: 2019/20
Using blood tests and psychological tests to predict familial Alzheimer’s disease Lead Investigator: Dr Antoinette O’Connor
Institution: Institute of Neurology, University College London
A mouth care programme for people with dementia and swallowing problems Lead Investigator: Julie Pollock Institution: Nottingham Healthcare NHS Trust
Helping people with dementia to better recover from delirium
Lead Investigator: Dr Daniel Davis (Clinical Training Partnership)
Grant awarded: 2017/2018
Can keeping up routine activities help people with dementia to remain independent during a hospital stay?
Lead Investigator: Ms Lisa Patrick (Clinical Training Fellowship)
Institution: Nottingham University Hospitals NHS Trust
Grant awarded: 2017/2018
Developing a technique to detect early signs of prion diseases
Lead Investigator: Dr Tze How Mok (Clinical Training Fellowship)
Institution: National Prion Clinic, University College London
Grant awarded: 2016/2017
Enabling people with dementia to access and understand assistive technology
Lead Investigator: Dr Lisa Newton (Clinical Training Fellowship)
Institution: Newcastle University
Understanding eating and drinking difficulties for people with dementia in care homes
Lead Investigator: Ms Lindsey Collins (Clinical Training Fellowship)
Grant awarded: 2014/2015
Adapting cognitive behavioural therapy for people with dementia
Lead Investigator: Dr Joshua Stott (Clinical Training Fellowship)
Institution: University College London
Grant awarded: 2014/2015
Understanding the role of delirium in dementia development
Lead Investigator: Dr Sarah Richardson (Clinical Training Fellowship)
Institution: Newcastle University
Junior Fellowships
Can new brains scans improve diagnosis of dementia with Lewy bodies ? Lead investigator: Dr Elijah Mak
Institution: University of Cambridge
Grant type: Junior Fellowship
Awarded: 2018/2019
Developing a tool to maintain compassion
Lead investigator: Dr Nuriye Kupeli
Improving coordination in Posterior Cortical Atrophy and Alzheimer’s disease Lead Investigator: Dr Kier Yong Institution: University College London
Improving diagnosis of Alzheimer’s disease by understanding changes seen in brain scans
Lead Investigator: Dr Kirsty Elizabeth McAleese (Junior Fellowship)
Reducing resistance to receiving personal care in advanced dementia
Lead Investigator: Dr Tamara Backhouse (Junior Fellowship)
Helping people with dementia to make decisions about continence products
Lead Investigator: Dr Catherine Murphy (Junior Fellowship)
Institution: University of Southampton
Creating a system to improve the treatment of additional medical conditions in people with dementia
Lead Investigator: Dr Joao Delgado (Junior Fellowship)
Institution: University of Exeter
What causes severe forgetting in Alzheimer’s disease?
Lead Investigator: Dr Michael Craig (Junior Fellowship)
Institution: Heriot-Watt University
Senior Fellowships
Creating a resource to help current carers to cope with feelings of grief
Lead Investigator: Dr Kirsten Moore (Senior Fellowship)
Understanding the needs and experiences of people affected by dementia in rural areas
Lead Investigator: Dr Fiona Jayne Marshall (Senior Fellowship)
Institution: University of Nottingham
PhD Studentships
Improving driving safety assessments for people with dementia
Lead Investigator: Dr Paul Donaghy
Institution: Newcastle University Grant type: PhD Studentship Awarded: 2019/20
Identifying brain features to diagnosis and monitor Alzheimer’s disease accurately
Lead Investigator: Dr Richard Killick Institution: Institute of Psychiatry, King’s College London Grant type: PhD studentship Awarded: 2019/2020
Brain scans to spot signs of Alzheimer’s disease in the choroid plexus Lead Investigator: Dr Jack Wells
Supporting family carers to carry out person-centred care
Lead Investigator: Dr Gerard Riley
Institution: University of Birmingham
Helping people in care homes with hearing problems to communicate
Lead Investigator: Dr Piers Dawes Institution: University of Manchester Grant awarded: 2017/2018
Understanding how dementia services can meet the needs of Black African and Caribbean people
Lead Investigator: Professor Paul Higgs Institution: University College London Grant awarded: 2017/2018
Understanding the impact of visual impairment on life with dementia
Lead Investigator: Dr Claire Hutchinson
Institution: University of Leicester
Understanding how people from minority ethnic backgrounds can access better support
Lead Investigator: Dr Jan Oyebode
Institution: University of Bradford
Keep talking for longer: speech therapy to help people living with dementia stay in the conversation
Lead Investigator: Dr Catherine Tattersall
Institution: University of Sheffield
Providing better care for care home residents with both dementia and cancer
Lead Investigator: Dr Laura Ashley
Institution: Leeds Beckett University
Finding drugs to manage bladder and bowel control in people with Alzheimer's disease
Lead Investigator: Dr Jerome Swinny
Institution: University of Portsmouth
Global Brain Health Institute grants
Investigating burdensome end of life care in people with advanced dementia
Lead investigator: Dr Elizabeth Dzeng
Institution: King’s College London, University of California and France. Grant round awarded: 2018/19
Identifying people with dementia who may benefit from palliative care
Lead Investigator: Corrina Grimes
Institution: Global Brain Health Institute, GP practices across Northern Ireland Grant round awarded: 2018/2019
Latest research projects
Understanding risk of dementia in professional rugby players
Lead Investigator: Professor Craig Ritchie
Does gum disease play a role in cognitive decline?
Lead Investigator: Dr Jing Kang
Institution: University of Leeds
Understanding the links between hearing problems and dementia Lead Investigator: Professor Jason Warren
Institution: University College London Grant type: Project Grant Awarded 2019/20
PREVENT Dementia: Understanding changes in the brain through mid-life
Lead Investigator: Professor Craig W Ritchie
Institution: University of Edinburgh Grant type: Project Awarded: 2019/20
Psychological interventions to tackle risk factors of dementia: Depression and anxiety
Lead Investigators: Professor Marcus Richards & Dr Joshua Stott
Institution: University College London Grant type: Project
Understanding the factors in mid-life that increase the risk of developing dementia
Institution: University of Edinburgh/Imperial College London
Grant type: Project
Awarded: 2015/2016 (phase 2)
Investigating the links between anticholinergic drugs and benzodiazepines and risk of dementia
Lead investigator: Dr George Savva
Awarded: 2013/2014
Understanding whether anticholinergic drugs to treat bladder problems increases risk of dementia
Lead Investigator: Dr Kathryn Richardson
Institution: University of East Anglia
Heart-brain link: how heart disease increases risk of dementia Lead Investigator: Dr Sana Suri Institution: University of Oxford
Grant type: Junior Fellowship
Understanding whether negative thinking influences dementia risk
Lead Investigator: Dr Natalie Marchant
Grant type: Senior Fellowship
PhD Studentships
Understanding the risk of developing dementia in UK military veterans
Lead Investigator: Professor Neil Greenberg
Institution: King’s College London Grant type: PhD studentship Awarded: 2019/20
‘Use it or lose it’: What’s the truth for dementia? Lead Investigator: Dr Dorina Cadar
Awarded: 2019/2010
Understanding links between diabetes, infections and dementia risk
Lead Investigator: Dr Charlotte Warren-Gash
Institution: London School of Hygiene and Tropical Medicine
Grant type: PhD studentship
Awarded: 2017/2018
Getting a ‘Clu’ about the causes of Alzheimer’s disease
Lead Investigator: Professor Paul Morgan
Institution: Cardiff University
Understanding the genetics of the Brains for Dementia Research participants
Lead Investigator: Dr Keeley Brookes
Institution: Nottingham Trent University
Understanding the prevalence of LATE, a new form of dementia
Lead Investigator: Professor Carol Brayne
Institution: Cambridge Institute of Public Health
Exploring the waste disposal system in brain cells and what it means for Alzheimer’s disease and Frontotemporal dementia
Lead Investigator: Dr Gemma Lace
Institution: University of Salford
Grant type: PhD
Understanding the relationship between the internal structure of the brain and Alzheimer’s disease
Lead Investigator: Dr Juan Varela
Institution: University of Cambridge Grant type: PhD Awarded: 2019/20
Exploring the relationship between blood pressure control and Alzheimer’s disease
Lead Investigator: Professor Mark Good
Institution: University of Cardiff Grant type: PhD Awarded: 2019/20
Using botox to investigate the spread of tau in Alzheimer’s disease Lead Investigator: Professor Giampietro Schiavo
Institution: University College London Grant type: PhD Studentship Awarded: 2019/20
Understanding the early events that lead to brain cell death in Alzheimer’s disease
Lead Investigator: Dr Katrin Deinhardt
Institution: University of Southampton Grant type: PhD studentship Awarded: 2019/20
Understanding neuroinflammation in Down syndrome dementia
Lead Investigator: Dr Frances Wiseman
Institution: University College London Grant type: PhD Studentship Awarded: 2019/20
Predicting when mild cognitive impairment progresses to dementia in the clinic
Lead investigator: Professor Karl Herholz
Institution: University of Manchester Grant type: Project grant Awarded: 2019/20
Exploring the role of the thalamus in early-stage Alzheimer’s disease
Lead Investigator: Dr Tim Viney
Institution: University of Oxford Grant type: PhD Studentship Awarded: 2019/20
Using the cell’s internal machinery to break down protein plaques in dementia
Lead Investigator: Dr Edward Avezov
Institution: University of Cambridge Grant type: PhD studentship Awarded: 2019/20
Investigating if peptide inhibitors can treat Alzheimer’s disease
Lead Investigator: Professor David Allsop
Institution: Lancaster University Grant type: Project Awarded: 2019/20
Spotting the early signs of inherited forms of frontotemporal dementia
Lead Investigator: Dr Martina Bocchetta
Institution: University College London Grant type: Junior Fellowship Awarded: 2019/20
Project grants
BioResource: 100,000 people to support dementia research
Lead Investigator: Professor Patrick Chinnery
Institution: University of Cambridge Grant type: Project Awarded: 2019/2020
Does failed waste removal explain tau’s role in dementia?
Lead Investigator: Professor Diane Hanger
Institution: King’s College London Grant type: Project Awarded: 2019/2020
How are changes to blood flow to the brain linked to memory and thinking problems? Lead Investigator: Dr Alastair Webb Institution: University of Oxford
Could misplaced ribonuclear proteins play a role in Alzheimer’s? Lead Investigator: Professor John Hardy
Awarded: 2018/2019
Does the tau protein stop brain cells from sending inhibitory signals?
Lead Investigator: Dr Francesco Tamagnini
Institution: University of Reading
Understanding how cells in the brain called astrocytes respond to damage in the brain
Lead Investigator: Professor Stephen Wharton Institution: University of Sheffield Grant awarded: 2017/2018
Investigating who develops memory and thinking problems following a stroke and why
Lead Investigator: Professor Joanna Wardlaw
Investigating how fluid is drained from the brain in vascular dementia
Lead Investigator: Professor Roxana Carare
Institution: University of Southampton
Can blocking the Dkk3 protein prevent the loss of connections between brain cells?
Lead Investigator: Professor Patricia Salinas
Understanding why dementia can occur after stroke
Lead Investigator: Professor Stephen Wharton
Investigating how changes to the immune system could be linked to development of Alzheimer’s disease
Understanding how brain cells called astrocytes prevent toxic tau from causing nerve cell death
Lead Investigator: Professor Maria Grazia Spillantini
Understanding whether chemical DNA tags affect the immune system in the development of Alzheimer’s disease
Lead Investigator: Dr Katie Lunnon
Understanding how small groups of proteins are toxic to brain cells
Lead Investigator: Professor Louise Serpell
Institution: University of Sussex
Investigating whether a particular type of amyloid protein is linked to Alzheimer’s disease development
Lead Investigator: Professor Johannes Attems
Improving how we study tau in the brain
Lead Investigator: Dr Diane Hanger
Institution: Institute of Psychiatry, King's College London
Understanding how the immune system contributes to Alzheimer's disease development
Lead Investigator: Professor Will Wood
Institution: University of Bristol
Grant awarded: 2015/2016
Investigating how connections between brain cells are lost in dementia
Lead Investigator: Dr Tara Spires-Jones
Building a resource to better understand the genetics of dementia
Lead Investigator: Professor Henry Houlden
Investigating the biological role of the Alzheimer's disease risk gene APOE
Lead Investigator: Professor Seth Love
Institution: University of Bristol
Investigating whether changes to chemical DNA tags contribute to Alzheimer's disease
Delving into how changes to blood circulation contributes to dementia
Lead Investigator: Dr William Whiteley
Grant type: Clinical training partnership
What is the role of microglia in frontotemporal dementia?
Lead Investigator: Dr Sarah Ryan
What can stem cells teach us about inflammation? Lead Investigator: Dr Charles Arber
Using artificial intelligence to understand the causes of Alzheimer’s disease Lead Investigator: Dr Ashwin Venkataraman Institution: Imperial College London
Awarded: 2018/2019
Using ultra-high resolutions MRI scans to understand more about Dementia with Lewy bodies
Lead investigator: Dr Elizabeth McKiernan
Understanding how Posterior Cortical Atrophy affects the brain
Lead Investigator: Dr Zeinab Abdi Institution: University College London Grant awarded: 2017/2018
Could ageing brain cells increase our risk of developing Alzheimer’s?
Lead investigator: Dr Nathaniel Woodling Institution: University College London Grant awarded: 2017/2018
Better understanding the role of a protein linked to frontotemporal dementia Lead Investigator: Dr Ryan West Institution: University of Manchester Grant awarded: 2016/2017
Understanding why certain parts of the brain are vulnerable to Alzheimer’s disease
Lead Investigator: Dr Lovesha Sivanantharajah Institution: Bangor University Grant awarded: 2016/2017
Understanding how Alzheimer’s disease affects different types of brain cells
Lead Investigator: Dr Lilach Soreq (Junior Fellowship)
Grant awarded: 2015/2016 Themes: Alzheimer's disease; cause; brain cells
Exploring the link between major depressive disorder and Alzheimer’s disease
Lead Investigator: Dr Magdalena Sastre
Institution: Imperial College London Grant type: PhD studentship Awarded: 2019/20
Understanding the role of mRNA and tau in recognition memory Lead Investigator: Professor Elizabeth Warburton
Institution: University of Bristol Grant type: PhD Studentship Awarded: 2019/20
Investigating cell waste removal genes in frontotemporal dementia
Lead Investigator: Professor John Hardy
Awarded: 2019/20
Understanding how to prevent the build-up of toxic tau in cell models Lead Investigator: Dr Will McEwan
Institution: University of Cambridge Grant type: PhD Studentship Awarded: 2019/20
Understanding the role of mitochondria in dementia with Lewy bodies Lead Investigator: Dr Ilse Pienaar
Institution: University of Sussex Grant type: PhD studentship
Saving synapses in Alzheimer's disease
Using virtual reality technology to detect the earliest stages of Alzheimer’s disease
Lead Investigator: Dr Dennis Chan Institution: University of Cambridge Grant awarded: 2017/2018
Investigating a potential pathway in the development of frontotemporal dementia
Lead Investigator: Dr Kurt De Vos
Understanding the role of the C9orf72 gene in frontotemporal dementia
Lead Investigator: Dr Kevin Talbot
Institution: University of Oxford
Understanding how tiny changes in several genes could contribute towards risk of Alzheimer’s disease
Lead Investigator: Dr Jonathan Mill
Further understanding of the role of the immune system in blood flow to the brain
Understanding what causes the increased risk of Alzheimer’s disease in Down’s syndrome
Think this page could be useful to someone? Share it:
- Email this page to a friend.
- Page last reviewed: 22 April 2021
Further reading
What kind of information would you like to read? Use the button below to choose between help, advice and real stories.
Choose one or more options
- Information
- Real stories
- Dementia directory
People affected by dementia interview Blood Biomarker Challenge researchers

The Blood Biomarker Challenge aims to revolutionise dementia diagnosis on the NHS. Anita Goundry and Tom Lawless interview two of its lead researchers.
Could finding changes common to different types of dementia lead to treatments?

Research into changes that are common to different types of dementia could point to similar ways of treating them.
How to spot dementia misinformation in the media

UK comes a step closer to blood tests for diagnosing dementia
Two world-class research teams will carry out countrywide trials to identify accurate and quick blood tests that can diagnose dementia, in a bid to improve the UK’s shocking diagnosis rate.
Researching why some people get dementia points to how we could reduce risk

Understanding more about what makes some people more likely to develop dementia can help us to reduce future risk.
Researching the links between oral health and dementia

Establishing how oral health and dementia are linked could point to better prevention and care.
Taking dementia research in new directions through South Asian music and dance

Meet Naaheed Mukadam, who connected South Asian community members with her dementia research through a ‘Music, Meaning and Memory’ event.
Find out more about Alzheimer’s Society research
Sign up to our monthly email update for all the latest news and developments in dementia research. You can change what you receive at any time and we will never sell your details to third parties. Here’s our Privacy Policy
Department of Neurology
Knight Alzheimer Disease Research Center
- Memory & Aging Project

What is the Memory & Aging Project?
Since 1979, the Memory & Aging Project (MAP) at Washington University has studied cognitive functioning in persons as they age. Our efforts are designed to provide information on the aging process in healthy older persons and in those diagnosed as having a dementia of the Alzheimer type or another related disorder. The Memory and Aging Project has enrolled hundreds of volunteers for our studies and is at the forefront of a worldwide effort to uncover key causal factors in the development of Alzheimer disease. Our goal is the development of more effective treatments and an eventual cure or prevention of Alzheimer disease.
Who can volunteer?
- Individuals age 40 and older with or without memory loss
- In stable, general health
- No problems with memory or thinking OR have mild dementia
- Have a study partner (spouse, family member or friend) who will be interviewed yearly
- Willing and able to complete all study procedures
- We are especially seeking volunteers from underrepresented populations (e.g., Hispanic and African American).
What study procedures are a part of MAP?
Yearly interviews in our office with the study participant and study partner are performed to assess the participant’s memory and thinking. These last between 2-3 hours.
Blood sample is taken to test DNA for genetic causes of AD.
A lumbar puncture is used to collect cerebrospinal fluid (CSF) and is performed every 2-3 years. CSF contains proteins and other chemicals that are important for brain health and provides a unique “window” into understanding how Alzheimer disease develops and progresses.
Yearly psychometric testing of the study participant’s memory and thinking is preformed in our office. This testing takes between 2-3 hours.
Brain scans, including magnetic resonance imaging (MRI) and positron emission tomography (PET) are conducted every 2-3 years. Scans can last up to 2 hours, and you are welcome to take a break if needed.
Additional testing, such as a sleep study, for selected volunteers is also possible.
What is brain donation?
We now know more about Alzheimer disease than ever before. This is in large part due to the dedication of research volunteers and the gift of brain donation for autopsy. A brain autopsy confirms a diagnosis of Alzheimer disease (AD) and identifies clues about other diseases that may be present in brain tissue. Information from the autopsy helps researchers better understand Alzheimer Disease and find a cure to help future generations. Learn more about this voluntary contribution from our Brain Donation Fact Sheet .
A Memory and Aging Project (MAP) team member can provide you with information about the study and answer any questions you may have. Use our online inquiry form or give us a call!
Call MAP at (314) 286-2683 for further information.
- Collaborations
- Importance of Research
- Adult Children Study
- Clinical Trials
- Dominantly Inherited Alzheimer Network
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 17 October 2022
Generalizable deep learning model for early Alzheimer’s disease detection from structural MRIs
- Sheng Liu 1 ,
- Arjun V. Masurkar 2 , 3 ,
- Henry Rusinek 4 , 5 ,
- Jingyun Chen 2 , 4 ,
- Ben Zhang 4 ,
- Weicheng Zhu 1 ,
- Carlos Fernandez-Granda 1 , 6 &
- Narges Razavian 1 , 2 , 4 , 7
Scientific Reports volume 12 , Article number: 17106 ( 2022 ) Cite this article
28k Accesses
46 Citations
93 Altmetric
Metrics details
- Alzheimer's disease
- Cognitive ageing
- Neuroscience
An Author Correction to this article was published on 02 October 2023
This article has been updated
Early diagnosis of Alzheimer’s disease plays a pivotal role in patient care and clinical trials. In this study, we have developed a new approach based on 3D deep convolutional neural networks to accurately differentiate mild Alzheimer’s disease dementia from mild cognitive impairment and cognitively normal individuals using structural MRIs. For comparison, we have built a reference model based on the volumes and thickness of previously reported brain regions that are known to be implicated in disease progression. We validate both models on an internal held-out cohort from The Alzheimer's Disease Neuroimaging Initiative (ADNI) and on an external independent cohort from The National Alzheimer's Coordinating Center (NACC). The deep-learning model is accurate, achieved an area-under-the-curve (AUC) of 85.12 when distinguishing between cognitive normal subjects and subjects with either MCI or mild Alzheimer’s dementia. In the more challenging task of detecting MCI, it achieves an AUC of 62.45. It is also significantly faster than the volume/thickness model in which the volumes and thickness need to be extracted beforehand. The model can also be used to forecast progression: subjects with mild cognitive impairment misclassified as having mild Alzheimer’s disease dementia by the model were faster to progress to dementia over time. An analysis of the features learned by the proposed model shows that it relies on a wide range of regions associated with Alzheimer's disease. These findings suggest that deep neural networks can automatically learn to identify imaging biomarkers that are predictive of Alzheimer's disease, and leverage them to achieve accurate early detection of the disease.
Similar content being viewed by others
Structure focused neurodegeneration convolutional neural network for modelling and classification of Alzheimer’s disease
Identification of Alzheimer's disease using a convolutional neural network model based on T1-weighted magnetic resonance imaging
Multimodal deep learning for Alzheimer’s disease dementia assessment
Introduction.
Alzheimer’s disease is the leading cause of dementia, and the sixth leading cause of death in the United States 1 . Improving early detection of Alzheimer’s disease is a critical need for optimal intervention success, as well as for counseling patients and families, clinical trial enrollment, and determining which patients would benefit from future disease-modifying therapy 2 . Alzheimer’s disease related brain degeneration begins years before the clinical onset of symptoms. In recent years, the development of PET imaging techniques using tracers for amyloid and tau have improved our ability to detect Alzheimer’s disease at preclinical and prodromal stages, but they have a significant disadvantage of being expensive and requiring specialized tracers and equipment. Many studies have shown that structural MRI-based volume measurements, particularly of the hippocampus and medial temporal lobe, are somewhat predictive of Alzheimer’s disease progression 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 . While the availability and cost of MRI is beneficial, these early attempts to discriminate healthy aging from Alzheimer’s disease based on volumetry had significant limitations, including small sample size and reliance on semi-automated segmentation methods. This motivated the emergence of more sophisticated methods to analyze MRI data based on machine learning.
In the last decade, machine learning and fully automatic segmentation methods have achieved impressive results in multiple computer vision and image processing tasks. Early applications of machine learning to Alzheimer’s disease diagnosis from MRIs were based on discriminative features selected a priori 14 , 15 , 16 , 17 . These features include regional volumes and cortical thickness segmented from brain regions known to be involved/implicated with memory loss and accelerated neurodegeneration that accompany Alzheimer’s disease 17 , 18 , 19 . Newer machine learning methods based on deep convolutional neural networks (CNNs) make it possible to extract features directly from image data in a data-driven fashion 20 , 21 , 22 , 23 , 24 , 25 , 26 . These methods have been shown to outperform traditional techniques based on predefined features in most image processing and computer vision tasks 27 , 28 . In the biomedical field, CNN-based methods also have the potential to reveal new imaging biomarkers 29 , 30 . Multiple studies have addressed mild Alzheimer’s disease dementia detection from MRI via deep learning, with notable examples of 3D convolutional neural networks based on 3D AlexNet, 3D Resnet, patch based models, Siamese networks, auto-encoder based models, among others 31 , 32 , 33 . Based on systematic reviews and survey studies 34 , 35 , many of previous approaches had major limitations in their design or validation: Most of these studies focus on distinguishing Alzheimer’s disease dementia patients from normal controls. However, in order to develop effective and clinically relevant early detection methods, it is crucial to also differentiate prodromal Alzheimer’s disease, otherwise known as mild cognitive impairment (MCI), from both normal controls and patients with manifest Alzheimer’s disease dementia. Some recent studies have made inroads to this end 36 , 37 , 38 , but do not evaluate their results on large independent cohorts where there can be more variability in image acquisition and clinical diagnosis, more representative of real world scenarios. The goal of this work is to address these significant challenges.
We propose a deep-learning model based on a novel CNN architecture that is capable of distinguishing between persons who have normal cognition, MCI, and mild Alzheimer’s disease dementia. The proposed model is trained using a publicly available dataset from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Although a multisite study, ADNI sites follow a rigorous standard protocol and stringent quality control to minimize site differences and improve our ability to reliably detect neuroanatomical changes. To assess the performance of the proposed methodology when applied in more realistic conditions, we evaluated our approach on an entirely independent cohort of 1522 subjects from the National Alzheimer’s Coordinating Center (NACC). Since (until very recently) each NIH/NIA funded center contributing to the NACC database is free to employ different acquisition parameters, this enables us to validate our approach on imaging data acquired with variable and non standardized protocols.
Our approach achieves an area-under-the-curve (AUC) of 85.12 (95% CI: 84.98–85.26) when distinguishing between cognitive normal subjects and subjects with either MCI or mild Alzheimer’s dementia in the independent NACC cohort. For comparison, we have built a reference model based on the volumes and thickness of previously reported brain regions that are known to be implicated early in disease progression. These measures were obtained by the automated segmentation tool Freesurfer 39 . We demonstrate that our proposed deep-learning model is more accurate and orders-of-magnitude faster than the ROI-volume/thickness model. Our results suggest that CNN-based models hold significant promise as a tool for automatic early diagnosis of Alzheimer’s disease across multiple stages.
Study participants
The study is based on data from ADNI and NACC. The cohorts are described in Table 1 . ADNI is a longitudinal multicenter study designed to develop clinical, imaging, genetic, and biochemical biomarkers for the early detection and tracking of Alzheimer’s disease 40 . NACC, established in 1999, is a large relational database of standardized clinical and neuropathological research data collected from Alzheimer’s disease centers across the USA 41 . Both datasets contain MRIs labeled with one of three possible diagnoses based on the cognitive status evaluated closest to the scanning time: cognitive normal (CN), mild cognitive impairment (MCI), or Alzheimer’s disease dementia. Labeling criteria are included/described in supplementary Table S1 .
We separated the ADNI subjects at random into three disjoint sets: a training set, with 1939 scans from 463 individuals, a validation set with 383 scans from 99 individuals, and a test cohort of 297 scans from 90 individuals. We built an additional independent test cohort based on NACC using the following inclusion criteria: individuals aged ≽ 55 years with MRIs within ± 6 months from the date of clinically-confirmed diagnosis of cognitively normal (CN), mild cognitive impairment (MCI), or mild Alzheimer’s disease dementia (AD). This resulted in a cohort of 1522 individuals (1281 CN, 322 MCI and 422 AD) and 2045 MRIs.
Table 1 reports the basic demographic and genetic characteristics of participants whose scans were used in this study. While cognitive groups in ADNI are well matched on age, in NACC cohort CN subjects were on the average ~ 5 years younger than the two impaired groups; they were 6–7 years younger than ADNI participants. There is a female predominance in NACC data, especially in CN and MCI, and a male predominance in ADNI, notable in the impaired stages. In the impaired population (MCI, AD), the prevalence of the AD genetic risk factor APOE4 is lower in NACC compared to ADNI. Considering these significant differences in cohort characteristics, using NACC as an external validation cohort allows us to assess the robustness of our method. In both ADNI and NACC, education seems to also be lower with progressive impairment stage, which may be indicative of lower structural reserve.
Identification of cognitive impairment status
Our deep-learning model is a 3D convolutional neural network (CNN) for multiclass classification, with an architecture that is specifically optimized for the task of distinguishing CN, MCI, and AD status based on MRIs (Fig. 1 b, see the “ Methods ” section for more details). We also designed a gradient-boosting model 42 based on 138 volumes and thickness of clinically-relevant brain ROIs (see supplementary Table S2 for the list) obtained by segmenting the MRIs using the Freesurfer software (v6.0, surfer.nmr.mgh.harvard.edu). Quality control was applied to the segmentations through sampling and visual inspection by a trained neuroimaging analyst (JC), in consultation with a clinical neurologist (AVM). Details of the quality control process are included in the “ Methods ” section.
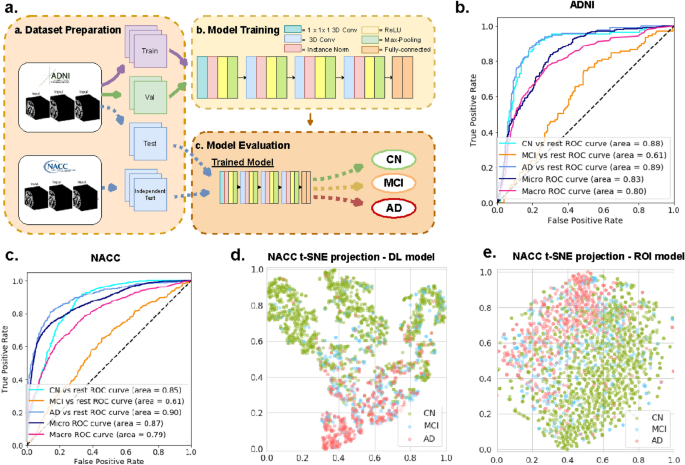
Overview of the deep learning framework and performance for Alzheimer’s automatic diagnosis. ( a ) Deep learning framework used for automatic diagnosis. ( b ) Receiver operating characteristic (ROC) curves for classification of cognitively normal (CN), mild cognitive impairment (MCI) and Alzheimer’s disease (AD), computed on the ADNI held-out test set. ( c ) ROC curves for classification of cognitively normal (CN), mild cognitive impairment (MCI) and Alzheimer’s disease (AD) on the NACC test set. ( d ) Visualization using t-SNE projections of the features computed by the proposed deep-learning model. Each point represents a scan. Green, blue, red colors indicate predicted cognitive groups. CN and AD scans are clearly clustered. ( e ) Visualization using t-SNE projections of the 138 volumes and thickness in the ROI-volume/thickness model. Compared to ( d ) the separation between CN and AD scans is less marked. The t-SNE approach is described in details in the methods section.
In order to evaluate the diagnostic performance of the machine-learning models, we computed ROC curves for the task of distinguishing each class (CN, MCI, or AD) from the rest. Table 2 includes our results. On the ADNI held-out test set, the proposed deep-learning model achieved the following AUCs: 87.59 (95% CI: 87.13–88.05) for CN vs the rest, 62.59 (95% CI: 62.01–63.17) for MCI vs the rest, and 89.21 (95% CI: 88.88–89.54) for AD vs the rest.
The AUCs of the ROI-volume/thickness model were statistically significantly lower than those of the deep-learning model: 84.45 (95% CI: 84.19–84.71) for CN vs the rest, 56.95 (95% CI: 56.27–57.63) for MCI vs the rest, and 85.57 (95% CI: 85.16–85.98) versus the rest.
The deep-learning model achieved similar performance on the NACC external validation data compared to ADNI held-out test set, achieving AUCs of 85.12 (95% CI: 85.26–84.98) for CN vs the rest, 62.45 (95% CI: 62.82–62.08) for MCI vs the rest, and 89.21 (95% CI: 88.99–89.43) for AD vs the rest. The ROI-volume/thickness model suffered a more marked decrease in performance, achieving AUCs of 80.77 (95% CI: 80.55–80.99) for CN vs the rest, 57.88 (95% CI: 57.53–58.23) for MCI vs the rest, and 81.03 (95% CI: 80.84–81.21) for AD vs the rest. Note that in Fig. 1 b and c, the Micro-AUC is worse in the ADNI dataset than in the external dataset (NACC). This is because of the imbalance between classes. Micro AUC tends to be driven by the classes with more examples, which are the MCI class in ADNI, and the CN class in NACC. The superior micro-AUC of NACC is therefore due to the fact that the model performs better on the CN class than on the MCI class, which is more common in ADNI. In Supplementary Figure F1 we also report the precision-recall curve for the deep learning model on both the ADNI held-out test set and NACC external validation set. In Supplementary Tables S5 and S6 we provide confusion matrices that show the misclassification rate between different classes.
We analyze the features extracted by the deep-learning model using t-distributed stochastic neighbour embedding (t-SNE), which is a projection method suitable for data with high-dimensional features, such as those learned via deep learning 43 . Figure 1 (d) shows the two dimensional t-SNE projections of the deep learning based features corresponding to all subjects in the NACC dataset. Points corresponding to CN and AD subjects are well separated. Figure 1 (e) shows the t-SNE projections of the ROI-volume/thickness features. In this case the separation between AD and CN scans is less clear. In both cases, points corresponding to MCI scans are not clustered together. This visualization is consistent with our results, which suggest that the features extracted by the deep-learning model are more discriminative than the ROI-based features, and also that distinguishing individuals diagnosed as MCI is more challenging.
Our deep learning model is significantly faster than classification based on regions of interest. On average, for each MRI, our deep learning model requires 0.07 s (plus 7 min for NMI normalization as preprocessing), compared to 11.2 h required for extracting the regions of interest with Freesurfer (we calculate the average running time of the Freesurfer software on each MRI scan, details on the computational settings can be found in the “ Methods ” section).
Progression analysis
We investigated whether the deep-learning model and ROI-volume/thickness model learn features that may be predictive of cognitive decline of MCI subjects to AD. In the held-out test set of ADNI, we divided the subjects into two groups based on the classification results of the deep learning model from the baseline date defined as the time of initial diagnosis as MCI: group A if the model classified the first scan as AD (n = 18), and group B if it did not (n = 26). Figure 2 shows the proportion of subjects in each group who progressed to AD at different months past the baseline. Based on the deep learning model, 23.02% (95% CI: 21.43%–24.62%) of subjects in group A (blue line) progress to AD, compared to 8.81% (95% CI: 8.09%–9.53%) of subjects in group B (red line). For the ROI-volume/thickness model, 20.22% (95% CI: 18.49%–21.95%) of subjects in group A (blue line) progress to AD, compared to 11.11% (95% CI: 10.32%–11.89%) of subjects in group B (red line). The forecasting ability of the deep learning model is therefore significantly higher than that of the ROI-volume/thickness based model. Our results suggest that deep-learning models could be effective in forecasting the progression of Alzheimer’s disease.
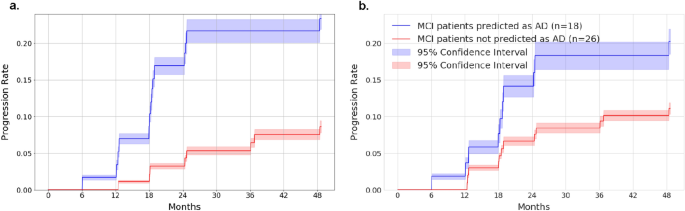
Progression analysis for MCI subjects. ( a ) Progression analysis based on the deep learning model. ( b ) Progression analysis based on the ROI-volume/thickness model. The subjects in the ADNI test set are divided into two groups based on the classification results of the deep learning model from their first scan diagnosed as MCI: group A if the prediction is AD, and group B if it is not. The graph shows the fraction of subjects that progressed to AD at different months following the first scan diagnosed as MCI for both groups. Subjects in group A progress to AD at a significantly faster rate, suggesting that the features extracted by the deep-learning model may be predictive of the transition.
Sensitivity to group differences
Figure 3 shows the performance of the deep learning and the ROI-volume/thickness model across a range of sub-cohorts based on age, sex, education and APOE4 status. Supplementary Table S3 includes the AUC values and 95% confidence intervals. The deep learning model achieves statistically significantly better performance on both ADNI and NACC cohorts. One exception is the ApoE4-positive group within NACC, for which classification of CN vs the rest, and MCI vs the rest by deep learning were worse than the ROI-volume/thickness model. Differences in sex and age representation in NACC versus ADNI, as discussed above, could influence this result. However, deep learning outperformed the ROI-volume/thickness model in both males and females, in both cohorts. The CN cohort in the NACC dataset is on average younger than that in the ADNI dataset (Table 1 ). In order to control for the influence of age, we stratified the NACC cohort into two groups (above and below the median age of 70 years old). However, the AUCs of the deep learning model for classification of CN vs the rest were very similar in both groups (85.2 for the younger cohort, to 86.1 for the older cohort). Another possible explanation for the ApoE4 difference is that NACC has a more clinically heterogeneous population, including participants with early stages of other diseases for which ApoE4 can be a risk factor, such as Lewy body and vascular dementia, and which can be clinically indistinguishable from AD at CN and MCI stages. In both ADNI and NACC, low education (< 15 years) also identified a subgroup in which deep learning was outperformed by ROI-volume/thickness model (on distinguishing MCI from others). This subgroup’s clinical presentation, especially at prodromal stages, may be more directly related to brain volume changes according to the cognitive reserve hypothesis 44 .
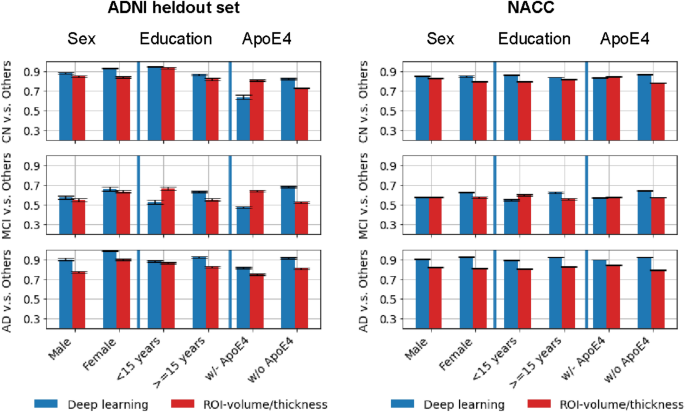
Performance across different subgroups. Performance of the deep learning model (in blue) and of the ROI-volume/thickness model (in red) for different subpopulations of individuals, separated according to sex, education, and ApoE4 status.
Impact of dataset size
In order to evaluate the impact of the training dataset size, we trained the proposed deep-learning model and the ROI-volume/thickness model on datasets of varying sizes, by randomly subsampling the training dataset. As shown in Fig. 4 , the performance of the ROI-volume/thickness model improves when the training data increases from 50 to 70%, but remains essentially stagnant after further increases. In contrast, the performance of the deep learning model consistently improves as the size of the training set increases. This is also observed in recent works 45 .
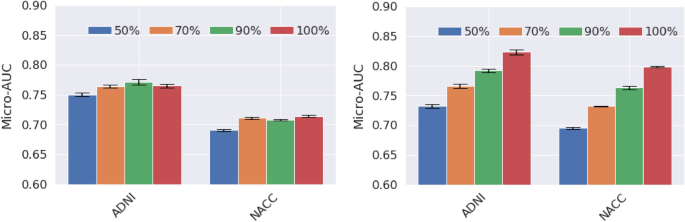
Datasize Impact. Performance of the baseline ROI-volume/thickness model (left) and the proposed deep learning model (right) when trained on datasets with different sizes (obtained by randomly subsampling the training set). The performance of the ROI-volume/thickness model improves when the training data increases from 50 to 70%, but remains essentially stagnant after further increases. In contrast, the performance of the deep learning model consistently improves as the size of the training set increases. Given that the deep learning model is trained on a very small dataset compared to standard computer-vision tasks for natural images, this suggests that building larger training sets is a promising avenue to further improving performance.
Model interpretation
In order to visualize the features learned by the deep learning model, we computed saliency maps highlighting the regions of the input MRI scans that most influence the probability assigned by the model to each of the three classes (CN, MCI, or AD), as described in the “ Methods ” section. Figure 5 shows the saliency maps corresponding to each class, aggregated over all scans in the ADNI held-out test set. The figure also reports the relative importance of the top 30 ROIs, quantified by a normalized count of voxels with high gradient magnitudes (see “ Methods ” section for more details). In Supplementary Table S4 we report the full list, as well as a quantification of the importance of the ROIs for the baseline volume/thickness model.
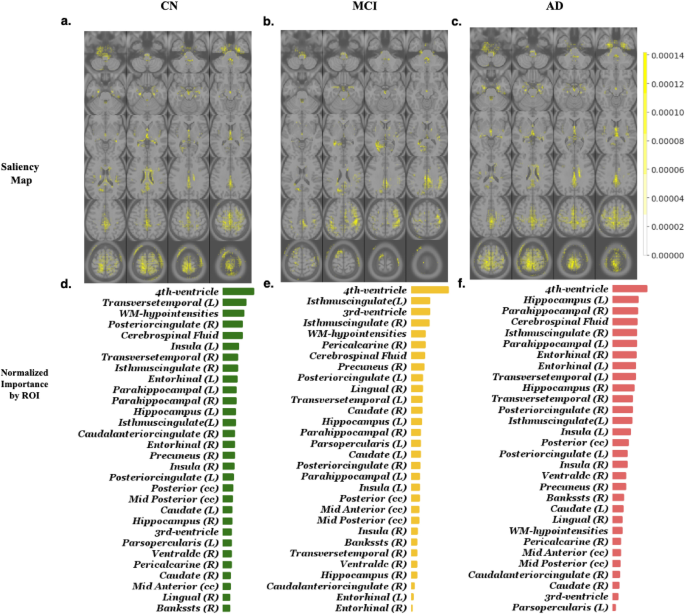
( a – c ) Visualization of the aggregated importance of each voxel (in yellow) in the deep learning model when classifying subjects into CN/MCI/AD. For each subject, the importance map was computed using the gradient of the deep-learning model with respect to its input (Details in “ Methods ” section). The computed gradients are visualized over the MNI T1-weighted template. ( d – f ) Top 30 regions of interest, sorted by their normalized gradient count, which quantifies their importance (see “ Methods ” section), for each of the classes.
Combining deep learning and the ROI-volume/thickness models
We combined the deep learning and ROI-volume/thickness model model by treating the predictions of the deep learning model as new features, and fusing them with the volume/thickness features to train a new gradient boosting model. This model achieved the following AUCs on ADNI test set: 89.25 (95% CI: 88.82–89.63) for CN versus the rest, 70.04 (95% CI: 69.40–70.68) for MCI versus the rest, and 90.12 (95% CI: 89.75–90.49) for AD vs the rest. It achieved similar performance on the NACC external validation data: AUCs of 85.49 (95% CI: 85.06–85.92) for CN versus the rest, 65.85 (95% CI: 65.37–66.33) for MCI versus the rest, and 90.12 (95% CI: 89.86–90.38) for AD versus the rest.
Our analysis supports the feasibility of automated MRI-based prioritization of elderly patients for further neurocognitive screening using deep learning models. Recent literature 46 has consistently shown high prevalence of missed and delayed diagnosis of dementia in primary care. Major contributory factors include insufficient training, lack of resources, and limited time to comprehensively perform early dementia detection. We show that deep learning is a promising tool to perform automatic early detection of Alzheimer’s disease from MRI data. The proposed model is able to effectively identify CN and AD subjects based on MRI data, clearly outperforming the model based on more traditional features such as ROI volumes and thicknesses. While identifying MCI is more challenging, our method still demonstrates improved performance compared to traditional methods. Moreover, as demonstrated in Fig. 3 , MCI misclassification as mild Alzheimer’s dementia may prove to be clinically useful in identifying a higher risk MCI subgroup that progresses faster. For example, in practice, if subsequent functional assessment confirms MCI rather than dementia, these patients may need to be monitored more closely, counselled differently, and more quickly introduced to disease-modifying therapy that may be available in the future.
Our results suggest that deep convolutional neural networks automatically extract features associated with Alzheimer's disease. The analysis of feature importance for the ROI-volume/thickness method shows that the importance of the left hippocampus is an order of magnitude larger than any of the other ROIs, suggesting that this region may dominate the output of the model (see Supplementary Table S4 ). This discovery is consistent with existing literature where a strong association between AD and the volume of the left hippocampus has been reported 47 , 48 , 49 . In contrast, the deep-learning model exploits a much wider range of regions. This highlights the potential of such models to exploit features in imaging data, which are not restricted to traditional measures such as volume and thickness. Many regions previously implicated in distinguishing stage severity of Alzheimer’s disease are recognized as salient by the deep-learning model (see Results). The left and right entorhinal cortex and hippocampus, which are considered the most relevant ROIs to the early stages of Alzheimer’s disease progression by the Braak staging method 50 , appear within the 11 most salient regions. Other regions identified as salient that are in agreement with previous literature include the inferior lateral ventricles (left and right), parahippocampal gyrus (left and right), and white matter hypo-intensities 51 . When comparing to another study that used segmented volumes to distinguish Alzheimer’s disease dementia from controls 52 , the 4th ventricle was uniquely and highly relevant to the deep-learning model whereas certain gyri (fusiform, temporal, angular, supramarginal) were not identified as particularly salient.
Our results suggest several avenues for improving deep learning models for early detection of Alzheimer’s disease. First, the available datasets to train these models is quite limited compared to standard benchmarks for computer vision tasks, which have millions of examples 53 . We show that the number of training data has a strong effect on performance, so gathering larger training sets is likely to produce a significant boost in accuracy. Second, we have shown that combining features learned using deep learning with more traditional ROI-based features such as volume and thickness improves the performance. However, using segmentation-based features is very costly computationally (segmentation takes 11.2 h on average, compared to the 7.8 min needed to apply the deep-learning model). Designing deep-learning models trained to extract volumetric information automatically may improve performance, without incurring such a heavy computational cost.
In this work, we limit our analysis to brain structural MRIs, in order to develop imaging biomarkers for early detection of Alzheimer’s disease. Integrating information such as age or education, genetic data (e.g. single nucleotide polymorphisms), clinical test data from electronic health records, and cognitive performance tests results could provide a more holistic view of Alzheimer’s disease staging analysis. Building deep learning models capable of effectively combining such information with imaging data is an important direction for future research.
Materials and methods
The data used in this study consists of imaging and diagnosis data from Alzheimer’s Disease Neuroimaging Initiative (ADNI) and National Alzheimer’s Coordinating Center (NACC). Since all the analyses were performed on de-identified data which is publically available, IRB Review was not required. In addition, all methods were carried out in accordance with the approved guidelines.
The structural MRI scans (T1 MRIs) were downloaded from the ADNI portal (n = 2619, https://adni.loni.usc.edu/ ). As the diagnoses are done for each screen visit, we directly used the current diagnosis (DXCURREN column), in the ADNI’s diagnosis summary table for each scan.
We used the NACC dataset for external evaluation (n = 2025 MRI scans). The NACC initiative was established in 1999, and maintains a large relational database of standardized clinical and neuropathological research data collected from Alzheimer’s disease centres across the USA 54 . The scan-level diagnostic labels were obtained based on diagnosis within 6 months of the scanning time (closest visit). Scans which did not have any diagnostic information within 6 month of the scan were excluded. Volumetric data for the same cohort were compiled from Freesurfer outputs (with built-in commands asegstats2table and aparcstats2table). Both of these two datasets were from large-scale multicenter studies, in which subject inclusion criteria and/or image acquisition protocols can vary by study center, leading to the potential differences in the scan and diagnosis rating (See Supplementary Table S0 for comparison on image acquisition protocols of two cohorts).
Our analysis was restricted to patients over the age of 55 in both cohorts, and we only considered T1 MRIs (without contrast) for the study.
MRI Data preprocessing
All scans in both cohorts were preprocessed by applying bias correction and spatial normalization to the Montreal Neurological Institute (MNI) template using the Unified Segmentation procedure 55 as implemented in step A of the T1-volume pipeline in the Clinica software 56 . The preprocessed images consist of 121 × 145 × 121 voxels, with a voxel size of 1.5 × 1.5 × 1.5 mm 3 .
Deep learning model
A 3D CNN, composed of convolutional layers, instance normalization 57 , ReLUs and max-pooling layers, was designed to perform classification of Alzheimer’s disease and mild cognitive impairment and normal cognition cases. The architecture is described in more detail in Fig. 1 a(b). In a preliminary work we showed that the proposed architecture is superior to state-of-the-art CNNs for image classification 54 . The proposed architecture contains several design choices that are different from the standard convolutional neural networks for classification of natural images: (1) instance normalization, an alternative to batch normalization 58 , which is suitable for small batch sizes and is empirically observed to achieve better performance (See Supplementary Table S8 ) (2) small kernel and stride in the initial layer for preventing losing information in small regions; (3) wider network architecture with more filters and less layers for the diversity of the features and ease of training. These techniques all independently contribute to boosting performance.
As is standard in deep learning for image classification 59 , we performed data augmentation via Gaussian blurring with mean zero and standard deviation randomly chosen between 0 and 1.5, and via random cropping (using patches of size 96 × 96 × 96).
Training and testing routines for the DL architectures were implemented on an NVIDIA CUDA parallel computing platform (accessing 2 Intel(R) Xeon(R) Gold 6230 CPU @ 2.10 GHz nodes on the IBM LSF cluster each with 2 NVIDIA Tesla V100 SXM2 32 GB GPUs) using GPU accelerated NVIDIA CUDA toolkit (cudatoolkit; https://developer.nvidia.com/cuda-toolkit ), CUDA Deep Neural Network (cudnn) and PyTorch 60 tensor libraries. The model was trained using stochastic gradient descent with momentum 0.9 (as implemented in the torch.optim package) to minimize a cross-entropy loss function. We used a batch size of 4 due to computational limitations. We used a learning rate of 0.01 with a total 60 epochs of training which were chosen by grid search based on validation set performance. During training, the model with the lowest validation loss was selected.
ROI-volume/thickness model
To build a model based on traditionally and commonly used ROI thickness and volumes, we first segmented each brain MRI using Freesurfer, and then computed volume and thickness from these ROIs (using the Freesurfer commands asegstats2table and aparcstats2table ). In order to get the volumetric data for each scan, we processed ADNI and NACC datasets with “recon-all” command at a high performance computer cluster. 16 parallel batch jobs were carried out together, each job was assigned with 320 RAM, 40 CPUs. The average processing time for each scan is about 12 h. 2730 scans of ADNI and 2999 of NACC were successfully processed.
For each brain MRI, a total of 138 MRI volume and thickness features (full list in Supplementary Table S2 ), were used as inputs to construct a Gradient Boosting (GB) classifier to predict Alzheimer's disease statuses. GB is a standard method to leverage pre-selected features as opposed to learning them from the data 61 . It constructs an ensemble of weak predictors by iteratively combining weaker base predictors in a greedy manner. We applied the implementation in the Python Sklearn package v0.24.1 sklearn.ensemble.GradientBoostingClassifier 62 . We set the learning rate to 0.1 (this value was selected based on validation performance). Other hyperparameters were set to their default values. After hyperparameter selection, we trained the model 5 times with different random seeds and reported average performances of these 5 models on the ADNI test set and the external NACC test dataset.
Quality control for freesurfer segmentation
Because of the large number of scans, we developed a two-stage approach for the quality control (QC) of a specific ROI. In the first stage, we located outlier cases within each cohort by fitting a Gaussian distribution to the volumes and centroids of all the segmented ROIs, using a cut-off of mean +/− 3 standard deviations. In the second stage, we conducted QC on the outlier and non-outlier cases separately. For the outliers, all cases were examined visually. For the non-outliers, 100 cases were randomly selected for visual examination. The visual examination was conducted by a trained neuroimaging researcher (JC), in consultation with a neurologist (AM) and a radiologist (HR). This two-stage approach was then repeated for two representative ROIs, namely hippocampus and entorhinal cortex, on each hemisphere, for each cohort. As a result, several segmentation errors were found in the outlier group of both ADNI and NACC cohorts (and excluded from follow-up machine-learning analyses), while no errors were found in the non-outlier group.
Performance metrics
We computed areas under the ROC curve (AUC), which are widely used for measuring the predictive accuracy of binary classification problems. This metric indicates the relationship between the true positive rate and false positive rate when the classification threshold varies. As AUC can only be computed for binary classification, we computed AUCs for all three binary problems of distinguishing between one of the categories and the rest. We also calculated two types of averages, micro- and macro-average denoted as Micro-AUC and Macro-AUC respectively. The micro average treats the entire set of data as an aggregated results, and consists of the sum of true positive rates divided by the sum of false positive rates. The macro average is computed by calculating the AUC for each of the binary cases, and then averaging the results.
t-SNE projection
t-SNE is a tool to visualize high-dimensional data. It converts similarities between data points to joint probabilities and minimizes the Kullback–Leibler divergence between the joint probabilities of the low-dimensional embedding and the high-dimensional data. We applied the implementation in the Python Sklearn package v0.24.1 sklearn.manifold.TSNE with default hyperparameters.
Interpretation of models in terms of ROIs
In order to analyze the features learned by the deep learning model, we computed saliency maps consisting of the magnitude of the gradient of the probability assigned by the model to each of the three classes (CN, MCI, or AD) with respect to its input 63 . Intuitively, changes in the voxel intensity of regions where this gradient is large have greater influence on the output of the model. As mentioned above, we segmented the MRI scans in our dataset to locate ROIs. To determine the relative importance of these regions for the deep-learning model, we calculated the total count of voxels where the gradient magnitude is above a certain threshold ( \({10}^{-3}\) , which is the magnitude observed in background regions where no brain tissue is present) within each ROI, and normalized it by the total number of voxels in the ROI. We excluded left-vessel, right-vessel, optic-chiasm, left-inf-lat-vent, and right-inf-lat-vent, due to their small size (less than 120 voxels).
For the ROI-volume/thickness models, we determined feature importance using a standard measure for gradient boosting methods 64 . This is obtained from the property feature_importances_ in the Python Sklearn package v0.24.1 sklearn.ensemble.GradientBoostingClassifier .
Statistical comparisons
To report statistical significance of descriptive statistics we employed 2-tailed, unpaired testing. We used python statsmodel v0.12.2 and scipy.stats v1.6.1. A p-value < 0.05 was reported as significant. To compute 95% confidence intervals, the bootstrapping method with 100 bootstrap iterations was used.
Reproducibility
The trained deep learning model, and corresponding code, notebooks, the IDs of subject-splits (training/validation/held-out) from publicly available ADNI, and the IDs of NACC participants included in our external validation study are all publicly available in our open-source repository: https://github.com/NYUMedML/CNN_design_for_AD .
The datasets used in this analysis are both de-identified and publicly available and therefore we did not need to get IRB approval for this study.
Data availability
Both cohorts used in our study are publicly available at no cost upon completion of the respective data-use agreements. We used all the T1 MRI scan and clinical data from ADNI (June 15th 2019 freeze), and NACC (Jan 5th 2019 freeze). The IDs of patients and scans used in our study and training, validation, test indicators are available in our github repository. Results of our Freesurfer segmentation (results of over 60,000 compute time on our HPC) for all of NACC and ADNI scans are also publicly available at no cost through NACC and ADNI websites, to anyone who has signed ADNI and NACC data-use agreements. Our trained model, training and validation scripts, and model predictions for each scan ID and patient ID, as well as Freesurfer segmentation volume and thickness features are also available as open-source on github.
Change history
02 october 2023.
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-43726-2
Deaths and Mortality. https://www.cdc.gov/nchs/fastats/deaths.htm (2021).
Rasmussen, J. & Langerman, H. Alzheimer’s disease—Why we need early diagnosis. Degener. Neurol. Neuromuscul. Dis. 9 , 123–130 (2019).
PubMed PubMed Central Google Scholar
Rusinek, H. et al. Alzheimer disease: Measuring loss of cerebral gray matter with MR imaging. Radiology 178 , 109–114 (1991).
Article CAS PubMed Google Scholar
Fox, N. C. et al. Presymptomatic hippocampal atrophy in Alzheimer’s disease. A longitudinal MRI study. Brain 119 (Pt 6), 2001–2007 (1996).
Article PubMed Google Scholar
Convit, A. et al. Specific hippocampal volume reductions in individuals at risk for Alzheimer’s disease. Neurobiol. Aging 18 , 131–138 (1997).
de Jong, L. W. et al. Strongly reduced volumes of putamen and thalamus in Alzheimer’s disease: An MRI study. Brain 131 , 3277–3285 (2008).
Article PubMed PubMed Central Google Scholar
Frisoni, G. B., Fox, N. C., Jack, C. R., Scheltens, P. & Thompson, P. M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 6 , 67–77 (2010).
de Leon, M. J. et al. The radiologic prediction of Alzheimer disease: The atrophic hippocampal formation. AJNR Am. J. Neuroradiol. 14 , 897–906 (1993).
Laakso, M. P. et al. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: An MRI study. Neurology 46 , 678–681 (1996).
Scahill, R. I., Schott, J. M., Stevens, J. M., Rossor, M. N. & Fox, N. C. Mapping the evolution of regional atrophy in Alzheimer’s disease: Unbiased analysis of fluid-registered serial MRI. Proc. Natl. Acad. Sci. USA 99 , 4703–4707 (2002).
Article ADS CAS PubMed PubMed Central Google Scholar
Ridha, B. H. et al. Tracking atrophy progression in familial Alzheimer’s disease: A serial MRI study. Lancet Neurol. 5 , 828–834 (2006).
Schuff, N. et al. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain 132 , 1067–1077 (2008).
Article Google Scholar
Bobinski, M. et al. MRI of entorhinal cortex in mild Alzheimer’s disease. Lancet 353 , 38–40 (1999).
Fan, Y., Batmanghelich, N., Clark, C. M. & Davatzikos, C. Alzheimer’s Disease Neuroimaging Initiative. Spatial patterns of brain atrophy in MCI patients, identified via high-dimensional pattern classification, predict subsequent cognitive decline. Neuroimage 39 , 1731–1743 (2008).
Cuingnet, R. et al. Automatic classification of patients with Alzheimer’s disease from structural MRI: A comparison of ten methods using the ADNI database. Neuroimage 56 , 766–781 (2011).
Liu, M., Zhang, D. & Shen, D. Ensemble sparse classification of Alzheimer’s disease. Neuroimage 60 , 1106–1116 (2012).
Tong, et al. Multiple instance learning for classification of dementia in brain MRI. Med. Image Anal. 18 , 808–818 (2014).
Chu, C., Hsu, A.-L., Chou, K.-H., Bandettini, P. & Lin, C. Does feature selection improve classification accuracy? Impact of sample size and feature selection on classification using anatomical magnetic resonance images. Neuroimage 60 , 59–70 (2012).
Lerch, J. P. et al. Automated cortical thickness measurements from MRI can accurately separate Alzheimer’s patients from normal elderly controls. Neurobiol. Aging 29 , 23–30 (2008).
Amoroso, N. et al. Deep learning reveals Alzheimer’s disease onset in MCI subjects: Results from an international challenge. J. Neurosci. Methods 302 , 3–9 (2018).
Suh, C. H. et al. Development and validation of a deep learning-based automatic brain segmentation and classification algorithm for Alzheimer disease using 3D T1-weighted volumetric images. AJNR Am. J. Neuroradiol. 41 , 2227–2234 (2020).
Article CAS PubMed PubMed Central Google Scholar
Kundaram, S. S. & Pathak, K. C. Deep learning-based Alzheimer disease detection.
Suresha, H. S. & Parthasarathy, S. S. Alzheimer disease detection based on deep neural network with rectified Adam optimization technique using MRI analysis. In 2020 Third International Conference on Advances in Electronics, Computers and Communications (ICAECC) (IEEE, 2020). https://doi.org/10.1109/icaecc50550.2020.9339504 .
Li, F. et al. Robust deep learning for improved classification of AD/MCI patients. In Machine Learning in Medical Imaging 240–247 (Springer International Publishing, 2014).
Rani, G. et al. Applying deep learning-based multi-modal for detection of coronavirus. Multimed. Syst . 1–12 (2021).
Dhaka, V. S., Rani, G., Oza, M. G., Sharma, T. & Misra, A. A deep learning model for mass screening of COVID-19. Int. J. Imaging Syst. Technol. https://doi.org/10.1002/ima.22544 (2021).
Voulodimos, A., Doulamis, N., Doulamis, A. & Protopapadakis, E. Deep learning for computer vision: A brief review. Comput. Intell. Neurosci. 2018 , 7068349 (2018).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521 , 436–444 (2015).
Article ADS CAS PubMed Google Scholar
Echle, A. et al. Deep learning in cancer pathology: A new generation of clinical biomarkers. Br. J. Cancer 124 , 686–696 (2021).
Fortino, V. et al. Machine-learning-driven biomarker discovery for the discrimination between allergic and irritant contact dermatitis. Proc. Natl. Acad. Sci. USA 117 , 33474–33485 (2020).
Qiu, S. et al. Development and validation of an interpretable deep learning framework for Alzheimer’s disease classification. Brain 143 , 1920–1933 (2020).
Cheng, D., Liu, M., Fu, J. & Wang, Y. Classification of MR brain images by combination of multi-CNNs for AD diagnosis. In Ninth International Conference on Digital Image Processing (ICDIP 2017) (2017). https://doi.org/10.1117/12.2281808 .
Lian, C., Liu, M., Zhang, J. & Shen, D. Hierarchical fully convolutional network for joint atrophy localization and Alzheimer’s disease diagnosis using structural MRI. IEEE Trans. Pattern Anal. Mach. Intell. 42 , 880–893 (2020).
Bäckström, K., Nazari, M., Gu, I. Y. & Jakola, A. S. An efficient 3D deep convolutional network for Alzheimer’s disease diagnosis using MR images. In 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018) 149–153 (2018).
Wen, J. et al. Convolutional neural networks for classification of Alzheimer’s disease: Overview and reproducible evaluation. Med. Image Anal. 63 , 101694 (2020).
Jo, T., Nho, K. & Saykin, A. J. Deep learning in Alzheimer’s disease: Diagnostic classification and prognostic prediction using neuroimaging data. Front. Aging Neurosci. 11 , 220 (2019).
Pan, D. et al. Early detection of Alzheimer’s disease using magnetic resonance imaging: A novel approach combining convolutional neural networks and ensemble learning. Front. Neurosci. 14 , 259 (2020).
Liu, S., Yadav, C., Fernandez-Granda, C. & Razavian, N. On the design of convolutional neural networks for automatic detection of Alzheimer’s disease. In Machine Learning for Health Workshop 184–201 (PMLR, 2020).
Fischl, B. et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33 , 341–355 (2002).
Petersen, R. C. et al. Alzheimer’s disease neuroimaging initiative (ADNI): Clinical characterization. Neurology 74 , 201–209 (2010).
Beekly, D. L. et al. The national Alzheimer’s coordinating center (NACC) database: The uniform data set. Alzheimer Dis. Assoc. Disord. 21 , 249–258 (2007).
Friedman, J. H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 29 , 1189–1232 (2001).
Article MathSciNet MATH Google Scholar
van der Maaten, L., van der Maaten, L. & Hinton, G. Visualizing non-metric similarities in multiple maps. Mach. Learn. 87 , 33–55 (2012).
Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8 , 448–460 (2002).
Sanaat, A., Shiri, I., Ferdowsi, S., Arabi, H. & Zaidi, H. Robust-deep: A method for increasing brain imaging datasets to improve deep learning models’ performance and robustness. J. Digit. Imaging https://doi.org/10.1007/s10278-021-00536-0 (2022).
Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimers. Dement. (2021) https://doi.org/10.1002/alz.12328 .
Goukasian, N. et al. Cognitive correlates of hippocampal atrophy and ventricular enlargement in adults with or without mild cognitive impairment. Dement. Geriatr. Cogn. Dis. Extra 9 , 281–293 (2019).
Cahn, D. A. et al. Structural MRI correlates of recognition memory in Alzheimer’s disease. J. Int. Neuropsychol. Soc. 4 , 106–114 (1998).
de Toledo-Morrell, L. et al. Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with Alzheimer’s disease. Hippocampus 10 , 136–142 (2000).
Braak, H., Alafuzoff, I., Arzberger, T., Kretzschmar, H. & Del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112 , 389–404 (2006).
Schwarz, C. G. et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. Neuroimage Clin. 11 , 802–812 (2016).
Ledig, C., Schuh, A., Guerrero, R., Heckemann, R. A. & Rueckert, D. Structural brain imaging in Alzheimer’s disease and mild cognitive impairment: biomarker analysis and shared morphometry database. Sci. Rep. 8 , 1–16 (2018).
Article CAS Google Scholar
Deng, J. et al. ImageNet: A large-scale hierarchical image database. in 2009 IEEE Conference on Computer Vision and Pattern Recognition 248–255 (2009).
Beekly, D. L. et al. The National Alzheimer’s Coordinating Center (NACC) database: An Alzheimer disease database. Alzheimer Dis. Assoc. Disord. 18 , 270–277 (2004).
PubMed Google Scholar
Ashburner, J. & Friston, K. J. Unified segmentation. Neuroimage 26 , 839–851 (2005).
Routier, A. et al. Clinica: An open source software platform for reproducible clinical neuroscience studies. (2019).
Ulyanov, D., Vedaldi, A. & Lempitsky, V. Instance normalization: The missing ingredient for fast stylization. arXiv [cs.CV] (2016).
Ioffe, S. & Szegedy, C. Batch normalization: Accelerating deep network training by reducing internal covariate shift. In Proceedings of the 32nd International Conferenceon Machine Learning, vol 37, 448–456 (eds Bach, F. & Blei, D.) (PMLR, 2015).
Google Scholar
Goodfellow, I., Bengio, Y., Courville, A. & Bengio, Y. Deep learning Vol. 1 (MIT press, 2016).
MATH Google Scholar
Paszke, A. et al. PyTorch: An imperative style, high-performance deep learning library. arXiv [cs.LG] (2019).
Prokhorenkova, L., Gusev, G., Vorobev, A., Dorogush, A. V. & Gulin, A. CatBoost: Unbiased boosting with categorical features. arXiv [cs.LG] (2017).
Garreta, R. & Moncecchi, G. Learning Scikit-Learn: Machine Learning in Python (Packt Publishing Ltd, 2013).
Simonyan, K., Vedaldi, A. & Zisserman, A. Deep Inside Convolutional Networks: Visualising Image Classification Models and Saliency Maps. arXiv [cs.CV] (2013).
Hastie, T., Tibshirani, R. & Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction (Springer, Berlin, 2013).
Download references
Acknowledgements
SL was supported by Alzheimer’s Association grant AARG-NTF-21-848627, and received partial support from NSF DMS-2009752, NSF NRT-HDR-1922658, and the Leon Lowenstein Foundation. NR was partially supported by Leon Lowenstein Foundation and NIH/NIA P30AG066512. AVM was partially supported by NIH/NIA P30AG008051 and P30AG066512. HR was partially supported by NIH/NIA P30AG066512 and NIA/NIBIB U24EB028980. JC was partially supported by NIH/NIA P30AG066512 and NIH-NINDS 1RF1NS110041-01. The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADRCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD). Data collection and sharing for the ADNI part of this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.;Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.;Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health ( www.fnih.org ). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database ( adni.loni.usc.edu ). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf .
Author information
Authors and affiliations.
Center for Data Science, NYU, 60 Fifth Avenue, 5th Floor, New York, NY, 10011, USA
Sheng Liu, Weicheng Zhu, Carlos Fernandez-Granda & Narges Razavian
Center for Cognitive Neurology, Department of Neurology, NYU Grossman School of Medicine, 60 Fifth Avenue, 5th Floor, New York, NY, 10011, USA
Arjun V. Masurkar, Jingyun Chen & Narges Razavian
Neuroscience Institute, NYU Grossman School of Medicine, 145 E 32nd St #2, New York, NY, 10016, USA
Arjun V. Masurkar
Department of Radiology, NYU Grossman School of Medicine, 660 First Avenue, New York, NY, 10016, USA
Henry Rusinek, Jingyun Chen, Ben Zhang & Narges Razavian
Department of Psychiatry, NYU Grossman School of Medicine, 227 East 30th St, 6th Floor, New York, NY, 10016, USA
Henry Rusinek
Courant Institute of Mathematical Sciences, NYU, 251 Mercer St # 801, New York, NY, 10012, USA
Carlos Fernandez-Granda
Department of Population Health, NYU Grossman School of Medicine, 227 East 30th street 639, New York, NY, 10016, USA
Narges Razavian
You can also search for this author in PubMed Google Scholar
Contributions
C.F.G. and N.R. co-led and supervised this study over all steps from design, development, and analysis. S.L. performed the majority of the data preprocessing, all of deep learning and baseline machine learning model development, training, validation and analysis. A.V.M. provided clinical supervision throughout the study from design to analysis. H.R. provided neuro-imaging supervision throughout the study from design to analysis. J.C. developed Freesurfer segmentation scripts, performed quality control analysis of the Freesurfer results, contributed to writing and analysis of the results overall, and provided neuro-imaging supervision during the study. B.Z. developed scalable Freesurfer segmentation scripts, performed all Freesurfer segmentation jobs in our institution’s high performance cluster. W.Z. performed pre-processing of all scans in the NACC dataset. All authors participated in the writing of the manuscript.
Corresponding authors
Correspondence to Carlos Fernandez-Granda or Narges Razavian .
Ethics declarations
Competing interests.
AVM is on the Council of the Alzheimer's Association International Research Grant Program and is a Steering Committee Member of the Alzheimer's Disease Cooperative Study. The other authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the Acknowledgements section: “SL was partially supported by NSF DMS-2009752, NSF NRT-HDR-1922658, and Leon Lowenstein Foundation.” now reads “SL was supported by Alzheimer’s Association grant AARG-NTF-21-848627, and received partial support from NSF DMS-2009752, NSF NRT-HDR-1922658, and the Leon Lowenstein Foundation.”
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Liu, S., Masurkar, A.V., Rusinek, H. et al. Generalizable deep learning model for early Alzheimer’s disease detection from structural MRIs. Sci Rep 12 , 17106 (2022). https://doi.org/10.1038/s41598-022-20674-x
Download citation
Received : 05 January 2022
Accepted : 16 September 2022
Published : 17 October 2022
DOI : https://doi.org/10.1038/s41598-022-20674-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
An explainable machine learning approach for alzheimer’s disease classification.
- Abbas Saad Alatrany
- Dhiya Al-Jumeily
Scientific Reports (2024)
Research of spatial context convolutional neural networks for early diagnosis of Alzheimer’s disease
- Yinsheng Tong
The Journal of Supercomputing (2024)
Revolutionizing Alzheimer’s detection: an advanced telemedicine system integrating Internet-of-Things and convolutional neural networks
- Mohamed A. Massoud
- Mohamed E. El-Bouridy
- Wael A. Ahmed
Neural Computing and Applications (2024)
A deep learning-based early alzheimer’s disease detection using magnetic resonance images
- S. Suchitra
- Lalitha Krishnasamy
- R. J. Poovaraghan
Multimedia Tools and Applications (2024)
Automated Quantification of Lateral and Medial Temporal Lobe Volumes for Improved Diagnosis of Early Alzheimer’s Disease
- Marufjon Salokhiddinov
- Dharmesh Singh
- Dilshod Tolibov
Applied Magnetic Resonance (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Center for Alzheimer's Disease Research
The Center for Alzheimer's Disease Research fosters collaborations across basic and clinical research groups that work toward uncovering when, where and how Alzheimer’s disease arises.
The Center for Alzheimer’s Disease Research is committed to catalyzing discovery into Alzheimer’s disease and related disorders and speeding the race to treatments.
More than six million people in the United States are living with Alzheimer’s disease. By 2050, this number is expected to rise to nearly 13 million. Alzheimer’s is the only cause of death in the top 10 nationally with no known cure. In order to advance prevention, treatments and cures, the Center for Alzheimer’s Disease Research bridges foundational research and clinical studies and supports collaborative thinking across multiple disciplines. The center brings together basic science researchers, clinicians and physician-scientists at Brown and its affiliated hospitals, including Butler Hospital , Rhode Island Hospital , Women and Infants Hospital and the Providence VA Medical Center .
- Brown has major strengths in brain science, biology and bioinformatics, with research programs in Alzheimer’s disease risk genes, neurodegeneration, aging and neuron-glial cell interactions.
- Clinical faculty members have an international reputation in diagnosis and treatment. They are leaders in clinical trials at the memory and aging clinics at Butler Hospital and Rhode Island Hospital.
- The center fosters research on questions essential to changing disease trajectory: how, where and when does Alzheimer’s disease begin? The center facilitates discovery research informed by genetic and biomarker data.
Alzheimer’s and other neurodegenerative diseases require collaborative thinking across disciplines in both basic and clinical research to generate the knowledge we need to advance prevention, treatments and cures.
Research Projects
Facilities and resources, biomarkers facility.
International Alzheimer's and Related Dementias Research Portfolio (IADRP)
Our database brings together funded research supported by public and private organizations both in the US and abroad all categorized using the Common Alzheimer’s and Related Dementias Research Ontology or CADRO .
Public and Private Funding Organizations in Over 10 Countries
Unique basic, translational, clinical and health services research projects and resources
Researchers across over 1000 academic institutions
Research Funding

Alzheimer's Clinical Trial Consortium
To provide an optimal infrastructure, utilizing centralized resources and shared expertise, to accelerate the development of effective interventions for Alzheimer’s disease and related disorders.
Latest Update
2023 Alzheimer’s Disease Bypass Budget
Alzheimer's Disease-Related Dementias Virtual Summit 2022
Agora: Discover Alzheimer's Disease Genes
Workshops, Conferences and Meetings
Alzheimer's Disease-Related Dementias Summit 2022
Funding Announcements
NIA Funding Opportunity Announcements
The IADRP Project Team is currently seeking participation from public, private and international biomedical and public health organizations who fund Alzheimer's disease (AD) research.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Neurol Genet
- v.3(5); 2017 Oct


The Alzheimer's Disease Sequencing Project: Study design and sample selection
Late-onset Alzheimer disease (LOAD) is the leading cause of dementia worldwide, with substantial economic and public health implications. 1 LOAD is a neurodegenerative disease characterized by progressive dementia typically manifesting in the seventh to ninth decades. Neuropathological changes precede clinical symptoms by 10–20 years, resulting in clinically asymptomatic individuals carrying neuropathologic features of LOAD. 2 Much of the heritability of LOAD remains unexplained, despite LOAD having a high heritability (60%–80%) and despite the identification of the APOE locus, a major genetic determinant for LOAD. 3 Genetic analyses have identified more than 25 other variants associated with smaller individual effects on disease risk. 4
To identify novel genetic variation influencing AD risk and protection, the Alzheimer's Disease Sequencing Project (ADSP) was implemented as a collaborative effort of the National Institutes on Aging, the National Human Genome Research Institute, and the Alzheimer disease research community. Individual contributors include the Alzheimer's Disease Genetics Consortium, the Neurology Phenotype Working Group of the Cohorts for Heart and Aging Research in Genomic Epidemiology consortium, and the Large Scale Sequencing and Analysis Centers at Baylor University, the Broad Institute, and Washington University.
Study design and sample selection were conducted to address issues of phenotypic heterogeneity and maximize statistical power. The study design includes 2 primary phases: a whole-genome sequencing (WGS) family-based study and a whole-exome sequencing (WES) case-control study. The WGS study was designed to target rarer variation through allelic segregation and linkage analyses in multiplex AD families. The WES case-control study was designed to target low-frequency coding variation in genes that contribute to AD risk or protection.
Approximately 1,400 multiplex LOAD families were reviewed for inclusion. Families were required to have multiple members with LOAD, genomic DNA, and available APOE genotypes. Families meeting initial criteria were assigned a priority rank based on number and age at onset of affected individuals, number of generations affected, and presence of APOE ε4 alleles. Priority was given to families heavily loaded for AD (≥4 affected members with DNA available) with minimal APOE ε 4 alleles. Cases met National Institute of Neurological Diseases–Alzheimer's NINCDS-ADRDA (National Institute of Neurological and Communicative Disorders and Stroke, and the Alzheimer's Disease and related Disorders Association; now, Alzheimer's Association) criteria for possible, probable, or definite AD. Controls were free of clinical AD on cognitive assessment. A detailed description of the family design is in Appendix 1 at Neurology.org/ng .
In total, we selected 582 individuals (498 affected and 84 unaffected) from 111 families for WGS to identify genomic regions associated with increased risk of LOAD. Selected individuals include 229 European ancestry and 353 Caribbean Hispanic (CH) individuals ( table ). The European ancestry families included 2 large Dutch families from the Erasmus Rucphen Family study. 5 Most of these families were recently analyzed for genetic linkage, an analysis that will be used in the analysis of the sequence data. 6 , 7 By design, no ε4/ε4 individuals were selected for sequencing, and we prioritized ε3/ε4 individuals with earlier disease onset. Twenty-seven percent of families had at least 1 case with autopsy confirmation.
Sample demographics for family and case-control studies

ADSP case-control design.
Over 30,000 samples were considered for inclusion in the case-control design. All cases met NINCDS-ADRDA criteria for possible, probable, or definite AD, had documented age at onset or age at death (for pathologically verified cases), and APOE genotyping. All controls were at least 60 years old and were free of dementia by direct, documented cognitive assessment. Three primary case-control selection strategies were evaluated, and ultimately, a design was chosen that targeted cases with minimal risk as predicted by known risk factors (age, sex, and APOE ) and targeted controls with the least probability of conversion to AD by age 85 years. The details and rationale of the case-control selection process and the evaluation of alternate study designs are described in detail in Appendix 2.
In total, we selected 5,096 cases and 4,965 controls under the chosen design ( table ). We selected 682 additional unrelated cases from additional multiplex families that had a strong family history for LOAD. Because some of these 682 cases arose from CH multiplex families, we included 171 cognitively normal CH control samples in the WES.
The sequencing of the nearly 600 whole genomes and 11,000 whole exomes has been completed; the data sets are currently available to the research community through qualified access (dbGaP study phs000572.v7.p4). This data set will be used to identify genetic factors influencing AD risk and protection and will be a critical resource for the LOAD research community.
This study has the approval of the institutional review boards of participating institutions, and informed consent was obtained from all patients.
Acknowledgments
Acknowledgment: The Alzheimer's Disease Sequencing Project (ADSP) comprises 2 Alzheimer's Disease (AD) genetics consortia and 3 National Human Genome Research Institute (NHGRI)-funded Large Scale Sequencing and Analysis Centers (LSAC). The 2 AD genetics consortia are the Alzheimer's Disease Genetics Consortium (ADGC) funded by the NIA (U01 AG032984), and the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) funded by the NIA (R01 AG033193), the National Heart, Lung, and Blood Institute (NHLBI), other NIH institutes, and other foreign governmental and nongovernmental organizations. The Discovery Phase analysis of sequence data is supported through UF1AG047133 (to G. Schellenberg, L.A. Farrer, M.A. Pericak-Vance, R. Mayeux, and J.L. Haines); U01AG049505 to S. Seshadri; U01AG049506 to E. Boerwinkle; U01AG049507 to E. Wijsman; and U01AG049508 to A. Goate. Data generation and harmonization in the Follow-up Phases is supported by U54AG052427 (to G. Schellenberg and Wang). The ADGC cohorts include Adult Changes in Thought (ACT), the Alzheimer's Disease Centers (ADC), the Chicago Health and Aging Project (CHAP), the Memory and Aging Project (MAP), Mayo Clinic (MAYO), Mayo Parkinson's Disease controls, the University of Miami, the Multi-Institutional Research in Alzheimer's Genetic Epidemiology Study (MIRAGE), the National Cell Repository for Alzheimer's Disease (NCRAD), the National Institute on Aging Late Onset Alzheimer's Disease Family Study (NIA-LOAD), the Religious Orders Study (ROS), the Texas Alzheimer's Research and Care Consortium (TARC), Vanderbilt University/Case Western Reserve University (VAN/CWRU), the Washington Heights-Inwood Columbia Aging Project (WHICAP) and the Washington University Sequencing Project (WUSP), the Columbia University Hispanic–Estudio Familiar de Influencia Genetica de Alzheimer (EFIGA), the University of Toronto (UT), and Genetic Differences (GD). The CHARGE cohorts with funding provided by 5RC2HL102419 and HL105756, include the following: the Atherosclerosis Risk in Communities (ARIC) Study which is conducted as a collaborative study supported by NHLBI contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C), the Austrian Stroke Prevention Study (ASPS), the Cardiovascular Health Study (CHS), the Erasmus Rucphen Family Study (ERF), the Framingham Heart Study (FHS), and the Rotterdam Study (RS). The 3 LSACs are the Human Genome Sequencing Center at the Baylor College of Medicine (U54 HG003273), the Broad Institute Genome Center (U54HG003067), and the Washington University Genome Institute (U54HG003079). Biological samples and associated phenotypic data used in primary data analyses were stored at Study Investigators institutions and at the National Cell Repository for Alzheimer's Disease (NCRAD, U24AG021886) at Indiana University funded by the NIA. Associated Phenotypic Data used in primary and secondary data analyses were provided by Study Investigators, the NIA-funded Alzheimer's Disease Centers (ADCs), and the National Alzheimer's Coordinating Center (NACC, U01AG016976) and the National Institute on Aging Genetics of Alzheimer's Disease Data Storage Site (NIAGADS, U24AG041689) at the University of Pennsylvania, funded by the NIA and at the Database for Genotypes and Phenotypes (dbGaP) funded by the NIH. This research was supported in part by the Intramural Research Program of the NIH and the National Library of Medicine. Contributors to the Genetic Analysis Data included Study Investigators on projects that were individually funded by the NIA and other NIH institutes, and by private U.S. organizations, or foreign governmental or nongovernmental organizations.
Supplemental data at Neurology.org/ng
Author contributions: All authors contributed to the work presented in this article. Drafting: the primary manuscript was prepared by G.W.B., with significant contributions from S.S., E.B., G.S., M.A.P.-V., and J.C.B. All authors participated in the revision and editing of the manuscript. Concept and design: primary study concept and design was by G.W.B., with significant contributions from E.R.M., J.C.B., M.A.P.-V., J.L.H., R.M., S.S., E.B., G.S., L.A.F., A.G., C.M.v.D., A.C.N., and A.D. Analysis and interpretation: review of family data was performed by M.A.P.-V., R.M., E.B., S.S., C.M.v.D., and T.M.F. Primary statistical analyses were performed by G.W.B., with additions from J.C.B., A.C.N., E.R.M., S.-H.C., A.D., and S.S. All authors participated in the interpretation and discussion of results. Acquisition of data: sample data were contributed by C.M.v.D., A.D., T.M.F., L.A.F., A.G., J.L.H., M.A.P.-V., E.B., R.M., S.S., and G.S. Statistical analyses: statistical analyses were primarily conducted by G.W.B.; additional analyses conducted by J.C.B., A.C.N., E.R.M., S.-H.C., A.D., and S.S. (affiliations noted above, all academic). Study supervision and coordination: primary study supervision and coordination was by G.S., R.M., E.B., M.A.P.-V., J.L.H., S.S., A.G., L.A.F., and E.W.
Study funding: Supported by the NIH, primarily the NIA, NHLBI, and NHGRI. Primary support includes the Alzheimer's Disease Genetics Consortium (ADGC) funded by the NIA (U01 {"type":"entrez-nucleotide","attrs":{"text":"AG032984","term_id":"16559857","term_text":"AG032984"}} AG032984 ), and the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) funded by the NIA (R01 AG033193), the Human Genome Sequencing Center at the Baylor College of Medicine (U54 HG003273), the Broad Institute Genome Center (U54HG003067), and the Washington University Genome Institute (U54HG003079). Additional funding of contributing sites is noted in the Acknowledgment section.
Disclosures: G.W. Beecham receives funding from the NIH and the Department of Defense. J.C. Bis reports no disclosures. E.R. Martin has served on the editorial board of Frontiers in Statistical Genetics and Methodology and holds US Patent No. 6697739 Test for Linkage and Association in General Pedigrees: The Pedigree Disequilibrium Test. S.-H. Choi reports no disclosures. A. DeStefano has received research support from the NIH. C.M. van Duijn and M. Fornage report no disclosures. S.B. Gabriel is an employee of a nonprofit entity and has been a consultant for WilmerHale Guidepoint Global. D.C. Koboldt receives a coinventor's share of license revenue for VarScan (a software tool for next-generation sequencing analysis), with licensing and disbursements handled by his former institution, Washington University in St. Louis. In the past 2 years, paying licensees included Bina Technologies, Janssen, Fera Science, Philips Electronics, and WuXi NextCODE. D.E. Larson has received research support from the NIH and St. Jude Children's Research Hospital. A.C. Naj has received speaker honoraria from Pfizer; has served on the editorial board of PLoS One ; and has received research support from the NIA, the BrightFocus Foundation, and Penn Institute on Aging. B.M. Psaty serves on the DSMB of a clinical trial for a device funded by the manufacturer (Zoll Lifecor) and on the Steering Committee for the Yale Open Data Access project funded by Johnson & Johnson; is a contributing writer for JAMA; and has received research support from an entity/entities listed in the Acknowledgment section. W. Salerno has been a consultant for Lasergen. W.S. Bush serves on the editorial boards of BMC BioData Mining and PLoS One ; and has received research support from the NIA. T.M. Foroud has served on the scientific advisory boards of the National Advisory Council on Alcohol Abuse and Alcoholism, the Washington University Alzheimer's Disease Research Center, and the NIA Genetics of Alzheimer's Disease Data Storage Site; has received travel funding from the Michael J. Fox Foundation for Parkinson's Research, the NIH, the University of Pittsburgh, and the University of Chicago; has received travel funding and speaker honoraria from the University of Texas at Austin; and has received research support from the NIH, the US Department of Defense, Columbia University, San Diego State University, the University of California, San Diego, the University of Massachusetts, the University of Pennsylvania, the State University of New York, and the Michael J. Fox Foundation for Parkinson's Research. E. Wijsman has served on the scientific advisory board of NIH NHLBI National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions; has served on the editorial boards of BMC Proceedings and Bioinformatics ; and has received research support from the NIH and the Metropolitan Life Foundation Award for Medical Research. L.A. Farrer has served on the editorial boards of the American Journal of Alzheimer's Disease & Other Dementias and Clinical Genetics ; has 1 patent pending for the use of PLXNA4 as a drug target and biomarker for Alzheimer disease; has been a consultant for Novartis Pharmaceuticals, Gerson Lehrman, Guidepoint Global, and Finnegan & Associates, LLP; and has received research support from the NIH, the Fidelity Foundation, and the Thome Memorial Foundation. A. Goate has served on the scientific advisory board of Denali Therapeutics; has received travel funding from the Rainwater Foundation; has served on the editorial board of eLife ; holds patents for PSEN mutations in AD, Tau mutations in FTD, and TDP43 mutations in ALS\FTD; has been a consultant for Cognition Therapeutics and AbbVie; has received research support from F-Prime, the NIA, the Rainwater Charitable Foundation, and the JPB Foundation; and receives royalty payments from Taconic Industries for tau mutation patent. J.L. Haines has served on the editorial boards of Neurogenetics , Current Protocols in Human Genetics , and Human Molecular Genetics ; receives publishing royalties from John Wiley & Sons; and has received research support from the NIH. M.A. Pericak-Vance serves on the editorial boards of Genetic Epidemiology , Molecular Autism , and Advances in Genomics and Gene Expression ; her immediate family member Dr. Jeffery Vance has served on the editorial boards of Neurology Genetics and American Journal of Neurodegenerative Disease ; and she has received research support from the NIH and the JJ Vance Foundation. E. Boerwinkle has received a speaker honorarium from the American Society for Bone and Mineral Research; is a Scientific Officer at Codified Genomics, LLC; and has received research support from the NIH. R. Mayeux has received research support from the NIH. S. Seshadri serves on the editorial boards of Journal of Alzheimer's Disease , Stroke , and Neurology and has received research support from the NIA. G. Schellenberg has served on the scientific advisory boards of Alzheimer's Association, the Society of Progressive Supranuclear Palsy, the Alzheimer Research Consortium, the Peebler PSP Research Foundation, the United Kingdom Parkinson Disease Center, University College London, the Alzheimer's Disease Sequence Project, the Structural Variant Work Group, Mayo Clinic, Rochester, Udall Center, the University of Miami, and the Oxford Parkinson's Disease Centre; has received travel funding/speaker honoraria from the Alzheimer's Disease Center, CurePSP, the University of California, San Diego, Keystone Symposia, the University of California, the Institute for Memory Impairment and Neurological Disorders, Biomarkers in Neuropsychiatric Disorders (Toronto, Canada), the NIH, Novartis, the McKnight Brain Institute, the University of Florida, the NIA, the Keep Memory Alive Center (Cleveland Clinic), the Lou Ruvo Center for Brain Health, PSP/Lewy Body Disease Think-Tank, the American Association of Neuropathologists, the Fusion Conference, “What does the future hold?” (Tucson, AZ), “Progressive supranuclear palsy genetics—update” (La Jolla, CA), the Center for Public Health Genomics, Genome Sciences Seminar, the University of Virginia, Neurology Grand Rounds, and Columbia University; has served on the editorial boards of the Journal of Neural Transmission , Alzheimer's Research , the American Journal of Alzheimer's Disease and other Dementias , Neurodegenerative Diseases , Current Alzheimer Research , and Pathology and Laboratory Medicine International ; is a professor at the University of Pennsylvania; and has received research support from the NIA/NIH, CurePSP, and CBD Solutions. Go to Neurology.org/ng for full disclosure forms. The Article Processing Charge was funded by the authors.
- Start Your Own Fundraiser
- Find a Community Fundraiser
- Shop at AFA
- Other Ways To Donate

Alzheimer's Foundation of America
Our research projects.

AFA provides funding for research projects aimed at improving treatment and quality of life for the millions of people living with Alzheimer’s disease.
Click here to donate and support AFA
The following are some of the research projects AFA has supported:
Identifying At-Risk Individuals
Researchers at NYU Langone Hospital-Long Island are conducting an innovative study called “Platelet-Rich Plasma in the Study of Alzheimer’s Pathophysiology.” The team is studying platelet-rich plasma of individuals with and without Alzheimer’s disease, as well as interactions of the blood with neural progenitor cells. The study focuses on amyloid, an abnormal protein in the brains of people with Alzheimer’s, which some scientists believe to be part of what kills healthy brain cells. The research has potential in both biomarker development-diagnosing who is at risk early on- and developing drug therapies to treat Alzheimer’s disease.
Exploring Role of Neuroimmune Interactions and Alzheimer’s Disease
A study by The Broad Institute of Harvard & MIT and One Mind is examining the role of the brain’s immune cells in the onset and progression of Alzheimer’s disease. Research is showing how, with increasing age and specific genetic influences, microglia (resident immune cells in the brain) respond to amyloid-beta peptides in a particular way that causes inflammation. In turn, researchers hypothesize that this triggers microglia to inappropriately remove synapses in the brain, resulting in dementia. The Broad Institute will leverage new single-cell RNA sequencing tools that allow deep characterization of individual microglia and immune cells. This could lead to new biological insight and inform the identification of biomarkers used for early detection and monitoring of progression and therapies.
Treating Hallucination and Aggressive Behaviors
Conducted by the Litwin-Zucker Research Center for the Study of Alzheimer’s Disease at the Feinstein Institute for Medical Research in New York, this study is exploring the causes of hallucination, agitation and aggression in relation to Alzheimer’s disease and how they can be better treated. These types of behaviors are among the most troubling behaviors associated with Alzheimer’s disease and are often one of the main causes that lead to families moving their loved one living with the disease from their homes to a residential healthcare setting.
Improving Early Detection
The Haddasah Medical Organization in Israel is creating ways to detect Alzheimer’s disease earlier so that it can be treated more quickly and effectively. The research team, led by Dr. Shahar Arzy, is focusing on the brain’s orientation system to design new types of Alzheimer’s testing and a revolutionary diagnostic App which will enable doctors to diagnose and begin treating Alzheimer’s disease earlier, when brain tissue is healthier. Treatment at this stage can help slow the progression of Alzheimer’s disease and enable individuals living with it to have a higher and more meaningful quality of life.
AFA also awarded grant funding for Hadassah to purchase a semi-automated Quanterix system to use at the new Hadassah Center for Healthy Brain Aging. The system will be used to screen the aging population in order to identify “at risk” patients and assemble a clinical cohort, with the ultimate goal of improving early detection at the pre-symptomatic phase and developing personalized treatment plans.
Uncovering APP’s Role in Alzheimer’s
The amyloid precursor protein (APP) gene family is essential for viability in mammals, but its function is unclear. Mutations in the genes for APP and in the enzymes that interact with APP have been found in familial Alzheimer’s disease (a form of Alzheimer’s disease which is linked to genes and affects at least two generations of a family), suggesting that disruption of APP can lead to Alzheimer’s disease. Researchers at the City College of New York (CCNY) are aiming to identify the role that APP plays in brain health and Alzheimer’s disease using the C. elegans model system. This research can then be translated into discoveries in mammals that could potentially lead to the development of new medications to treat Alzheimer’s that do not interfere with APP function.
Minority Outreach Program
Emory University’s Alzheimer’s Disease Research Center (ADRC) is undertaking a comprehensive, grassroots outreach program to help African-Americans in the Atlanta-metropolitan area. According to Emory ADRC, African-American seniors are two to three times more likely to develop Alzheimer’s disease as compared to Caucasians; part of the reason stems from a higher reluctance among African-Americans to see a physician about memory loss and other symptoms of Alzheimer’s, often stemming from experienced and perceived discrimination by medical providers. Emory’s grassroots outreach program is successfully working with leaders in the African-American community to connect African-Americans with free memory screenings and information about warning signs, ways to reduce their risk of Alzheimer’s and how to participate in research.
Developing More Effective Treatments for Memory Loss
Researchers at Stony Brook University are undertaking innovative research project that utilizes Positron Emission Technology (PET) imaging in an effort to further drug development. The most commonly used drugs to improve memory in neurodegenerative diseases such as Alzheimer’s target a set of neurons critical for memory called cholinergic neurons. Loss of cholinergic function is a hallmark of cognitive decline. But these medications, which target the cholinergic system and are known as cholinesterase inhibitors, have only a modest effect. By gaining a better understanding of exactly how these neurons are damaged by Alzheimer’s, Stony Brook’s research team hopes to improve therapeutic strategies that can more effectively target and treat the damage and return these neurons to a normal state to help improve memory.
Read more about some of these and other AFA-funded research projects by clicking below (links to a pdf).
Click to enlarge text

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
NIA-Funded Active Alzheimer’s and Related Dementias Clinical Trials and Studies
The National Institute on Aging (NIA) is currently supporting 507 active clinical trials on Alzheimer’s disease and related dementias (AD/ADRD). These trials reflect diverse drug and mechanistic targets, as well as diversity in the stages of AD/ADRD they address. NIA’s active trials include: Clinical Drug Development – Phase I and II (57), Clinical Drug Development – Phase II/III and Phase III (15), Nondrug (159), Dementia Care and Caregiving (226), Understanding Disease Processes (24), Diagnostic Tools, Assessments, & Imaging Studies (19), and Treatments for Neuropsychiatric Symptoms (7). Please see the tables below for more details about these trials.*

Please note: The data in the graphics are from March 2024.
On this page:
- Section 1: Clinical Drug Development – Phase I and II (57 trials)
- Section 2: Clinical Drug Development – Phase II/III and Phase III (15 trials)
- Section 3: Nondrug (159 trials)
- Section 4: Dementia Care and Caregiver (226 trials)
- Section 5: Understanding Disease Processes (24 trials)
- Section 6: Diagnostic Tools, Assessments, & Imaging Studies (19 trials)
- Section 7: Treatments for Neuropsychiatric Symptoms (7 trials)
*The data in the tables below are from March 2024
Section 1: Clinical Drug Development – Phase I and II
| Michael S. Rafii, University of Southern California | Anti-Aβ immunotherapy to remove amyloid plaques | Non-demented adults with down syndrome (Ages 35 - 55) | 2025 | ||
| TBD | Douglas Galasko, University of California San Diego | Gamma secretase modulator (GSM)776890, an Allosteric modulator of amyloid precursor protein (APP) processing, to attenuate Alzheimer's disease pathology | Healthy adult participants | 2025 | |
| Howard Feldman, University of California San Diego | PQ 912, a small molecule glutaminyl cyclase (QC) inhibitor to treat both amyloidopathy and neuroinflammation | Adults with mild cognitive impairment or mild probable Alzheimer's disease (Ages 50 - 89) | 2024 | ||
| Anthony Caggiano, Cognition Therapeutics Inc | CT1812, a small molecule sigma2 receptor antagonist that displaces Aß oligomers bound to neuronal receptors at synapses | Individuals with mild to moderate Lewy Body Dementia | 2024 | ||
| Anthony Caggiano, Cognition Therapeutics, Inc. | CT1812, a small molecule sigma2 receptor antagonist that displaces Aß oligomers bound to neuronal receptors at synapses | Adults with Mild to Moderate Alzheimer's Disease (Ages 50 - 85) | 2024 | ||
| Michael Agadjanyan, Institute For Molecular Medicine | AV-1959D, a DNA based vaccine targeting amyloid beta to reduce amyloid plaque | Early Alzheimer's disease participants (Ages 60 - 85 | 2027 | ||
| Anthony Caggiano, Cognition Therapeutics, Inc. | CT1812, a small molecule sigma2 receptor antagonist that displaces Aß oligomers bound to neuronal receptors at synapses | Older adults with early Alzheimer's disease, late mild cognitive impairment, and mild Alzheimer's disease dementia | 2027 |
| Natalie Denburg, University of Iowa | Melatonin, a hormone that regulates sleep, to improve cognition in older adults | Older adults (Ages 60 - 75) | 2025 | ||
| TBD | Victoria Pak, Emory University | Examine whether Citicoline (a dietary choline supplement) can significantly increase choline levels and decrease inflammatory and potentially Alzheimer's disease (AD)-associated biomarkers during a prodromal stage of AD (mild cognitive impairment due to AD) | Mild cognitive impairment (Ages 60+) | 2025 | |
| Brendan Lucey, Washington University in St. Louis | Suvorexant, a dual orexin receptor antagonist (DORA), to decrease cerebrospinal fluid tau and phosphorylated tau and to prevent Alzheimer's disease | Cognitively healthy, amyloid-positive people with symptomatic insomnia (Ages 65+) | 2028 | ||
| Barry Greenberg, Johns Hopkins Bayview Medical Center | Trazadone, an antidepressant to treat sleep disturbance and improve cognitive outcomes | Individuals with prodromal Alzheimer's disease or amnestic Mild Cognitive Impairment and sleep complaints | 2026 |
| Mark Tuszynski, University of California San Diego | Brain Derived Neurotrophic Factor (BDNF) Gene Therapy to potentially slow or even reverse cognitive decline in Alzheimer’s disease | Adults with early Alzheimer's disease and/or mild cognitive impairment | 2026 | ||
| Tracy Butler, Joan and Sanford I Weill Medical College of Cornell University | Lupron, a small molecule gonadotropin-releasing hormone (GnRH) receptor agonist to slow cognitive decline in women with mild to moderate Alzheimer's disease | Women with mild-moderate Alzheimer's disease who are also taking acetylcholinesterase (AChE) inhibitors (Ages 65 - 90) | 2025 | ||
| TBD | John Rinehart, Neutherapeutics, Llc | PhytoSERM, a selective estrogen receptor beta (ERß) modulator; a rationally designed formulation of 3 phytoestrogens, genistein, daidzein, and S-equol | Peri- and post-menopausal women (Ages 45 - 60) | 2024 | |
| TBD | Roberta Brinton, University of Arizona | PhytoSERM, a selective estrogen receptor beta (ERß) modulator to improve brain metabolism and cognitive function | Peri- and post-menopausal women (Ages 45 - 60) | 2027 | |
| Project Name | |||||
|---|---|---|---|---|---|
| Eti Yoles, Immunobrain Checkpoint Inc. | IBC-Ab002, an antibody therapy targeting the peripheral immune system | Individuals with early Alzheimer's disease (Ages 50 - 75) | 2024 | ||
| Jeffrey Pelletier, Neurokine Therapeutics | MW150, a small molecule inhibitor of p38alphaMAPK serine/threonine protein kinase to reduce neuroinflammation and improve synaptic function. | Mild to Moderate Alzheimer's Disease (Ages 50 - 85) | 2024 | ||
| Huntington Potter, University of Colorado Anschutz Medical Campus | Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Sargrammostim, an immune system modulator to improve cognition | Young adults with Down Syndrome | 2024 | ||
| Marwan Sabbagh, Dignity Health's St. Joseph's Hospital and Medical Center | Lenalidomide, a small molecule Anti-inflammatory immunomodulator to reduce neuroinflammation and slow or prevent Alzheimer's disease | Adults with amnestic Mild Cognitive Impairment (Ages 50+) | 2024 | ||
| Huntington Potter, University Of Colorado Denver | Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Sargrammostim, an immune system modulator to improve cognition | Older adults with mild-to-moderate Alzheimer's disease | 2025 | ||
| Daniel Hanley, Johns Hopkins Bayview Medical Center | MW150, a small molecule inhibitor of p38alphaMAPK serine/threonine protein kinase to reduce neuroinflammation and improve synaptic function | Participants with spontaneous, non-traumatic, Intracranial Hemorrhage | 2025 | ||
| TBD | Alireza Faridar, Houston Methodist Hospital Research Institute | Low-dose Interleukin-2 (IL-2) immunotherapy to reduce neuroinflammation and ameliorate Alzheimer's disease pathology | Participants with Alzheimer's disease (Ages 55 - 86) | 2025 | |
| TBD | Ilya Ilin, General Biophysics, LLC | Evaluation of Xenon gas to reduce inflammation as a treatment for Alzheimer's disease | Healthy older adults (Ages 55 - 75) | 2025 |
| Fei Du, McLean Hospital | Nicotinamide riboside (NR), an orally bioavailable precursor of NAD+, to enhance mitochondrial function, and improve cognition | Adults with mild cognitive impairment and mild Alzheimer’s disease (Ages 55 - 89) | 2025 | ||
| TBD | Gary Gibson, Winifred Masterson Burke Medical Research Institute | Benfotiamine, a small molecule thiamine derivative to improve cognition and overall global function | Individuals with early Alzheimer's disease, including mild cognitive impairment and mild dementia with plasma evidence of amyloid positivity | 2027 | |
| Andriy Yabluchanskiy, University of Oklahoma | Nicotinamide Adenine Dinucleotide (NAD) supplementation to improve brain health and memory | Older adults (Ages 60 - 85) | 2027 | ||
| Christopher Martens, University of Delaware | Nicotinamide Riboside, a precursor molecule of Nicotinamide adenine dinucleotide (NAD+) to improve cerebrovascular function and improve memory in patients with mild cognitive impairment | People with amnestic mild cognitive impairment (Ages 60 - 90) | 2024 | ||
| Carleara Weiss, State University of New York at Buffalo | Nicotinamide riboside (NR) supplementation to enhance cognition by improving objective sleep duration and sleep quality in older persons | Older adults with poor sleep quality (Ages 65 - 85) | 2024 | ||
| Rajesh Kumar, University of California Los Angeles | Thiamine (vitamin B1), an essential micronutrient known to reduce anaerobic metabolism, intervention to improve quality of life and daily activities and reduce cognitive deficits | Adults with coronary heart disease undergoing coronary artery bypass grafting (Ages 60 – 80) | 2025 |
| Lewis Lipsitz, Hebrew SeniorLife / Hebrew Rehabilitation Center | Quercetin and Dasatinib, a senolytic combination, to improve brain blood flow | Older adults with slow gate speed and Mild Cognitive Impairment (Ages 70 - 90) | 2024 | ||
| Shalender Bhasin, Brigham and Women's Hospital | ß nicotinamide mononucleotide (ßNMN): An NAD+ precursor to slow Alzheimer's disease progression and improve cognition | Mild Alzheimer's disease dementia participants | 2025 | ||
| TBD | Marwan Sabbagh, Dignity Health's St. Joseph's Hospital and Medical Center | Siponimod, an immunomodulatory small molecule used to treat multiple sclerosis and serves as a neuroprotective | Individuals with early Alzheimer's disease (Ages 60 - 85) | 2026 | |
| Vikas Kotagal, University of Michigan | Citalopram, a selective serotonin-reuptake inhibitor to test effect on amyloid-beta levels in the visuospatial cortex and visuospatial cognitive abilities in patients with Parkinson’s disease | Older adults with Parkinson's disease diagnosis and no active depression (Ages 65+) | 2025 | ||
| Roberta Brinton, University of Arizona | Allopregnanolone, a small molecule that stimulates anti-inflammatory mechanisms and mitochondrial function to regenerate neurons and restore cognition in Alzheimer's disease | Adults who are APOE e4 positive and diagnosed with mild Alzheimer's disease (Ages 55+) | 2025 | ||
| William Raschke, Virogenics, Inc. | CMS121, a small molecule therapy to reduce neuroinflammation | Healthy adult volunteers | 2024 | ||
| TBD | Kent Hutchison, University of Colorado Anschutz Medical Campus | An intervention with full spectrum hemp-derived cannabidiol (CBD) and CBD to determine the influence on Alzheimer's disease biomarkers | Older adults with mild cognitive impairment (Ages 55 - 85) | 2028 |
| Rajagopal Sekhar, Baylor College of Medicine | Glutathione supplementation to improve cognition in Alzheimer's disease | Adults with mild cognitive impairment (Ages 65 - 80) | 2024 | ||
| Rajagopal Sekhar, Baylor College of Medicine | Glutathione supplementation to improve cognition in Alzheimer's disease | Adults with mild cognitive impairment and mild Alzheimer’s disease (Ages 55 - 85) | 2025 | ||
| Peter Ljubenkov, University of California San Francisco | Verdiperistat, a microglial myeloperoxidase inhibitor that modulates proinflammatory microglia | Participants with Semantic variant primary progressive aphasia (Ages 18 - 80) | 2026 |
| Hussein Yassine, University of Southern California | Docosahexaenoic acid (DHA) supplementation, a dietary lipid to improve cognition in Alzheimer's disease | Healthy adult carriers and non-carriers of APOE e4 (Ages 60 - 80) | 2024 | ||
| Paul Newhouse, Vanderbilt University Medical Center | Nicotine to improve cognition in Alzheimer's disease | People with mild cognitive impairment (Ages 55+) | 2025 | ||
| Adam Mecca, Yale University | BMS-984923, blocks aberrant Amyloid beta activation of mGluR5 | Adults with early Alzheimer's disease (Ages 50 - 80) | 2024 | ||
| TBD | Sharon Rosenzweig-Lipson, AgeneBio, Inc. | BPN-27473, a potent, selective and orally active GABA-A a5 Positive Allosteric Modulator (PAM) for the treatment of mild cognitive impairment due to Alzheimer's disease | Healthy Older Adults | 2026 | |
| Jan Johansson, Artery Therapeutics, Inc. | CS6253, an ABCA1 transporter agonist to treat APOE4 associated dementia | Healthy young adults | 2024 |
| TBD | Lon S. Schneider, University of Southern California | (-)-PHENSERINE, a small molecule that inhibits neuronal pre-programmed cell death, to prevent neuronal dysfunction and death | Adults with probable Alzheimer's disease (Ages 70 - 82) | 2025 | |
| Mouhsin Shafi, Beth Israel Deaconess Medical Center | Levetiracetam, a small molecule synaptic vesicle protein (SV2A) modulator, to improve cognition in Alzheimer's disease | Adults with probable Alzheimer's disease (Ages 50 - 90) | 2024 | ||
| Charbel Moussa, Georgetown University | Nilotinib, a small molecule inhibitor of Abelson kinase to facilitate degradation of neurotoxic proteins and promote survival of neurons | Adults with clinical diagnosis of Lewy body dementia with both dementia and Parkinsonism (Ages 25 - 90) | 2024 | ||
| TBD | Chien-Liang Glenn Lin, Ohio State University | A single ascending dose (SAD) study to determine safety and tolerability of a novel compound that has the potential for modifying Alzheimer's disease progression/onset | Healthy men and women (Ages 18 - 60) | 2024 |
| TBD | William Erhardt, Oligomerix, Inc | OLX-07010 is a tau self-association inhibitor small molecule that targets tau, a protein involved in disease progression | Healthy adults (ages 18 - 50) and healthy older adults (ages 51 - 75) | 2024 | |
| TBD | Adam Boxer, University of California San Francisco | Tau-directed therapies, alone and in combination with an anti-amyloid therapy, to slow Alzheimer's disease progression | Older adults with elevated brain amyloid and tau (Ages 60 - 75) | 2028 |
| Rong Zhang, University of Texas Southwestern Medical Center | Intensive lowering of systolic blood pressure (SBP) using antihypertensive medications to reduce Alzheimer's disease pathology (i.e., excessive brain amyloid and tau protein deposition) in older adults at high risk for memory decline or dementia | Older adults who have hypertension (SBP=130 mmHg), family history of dementia, and/or subjective memory complaints (Ages 60 - 80) | 2027 | ||
| Gregory Jicha, University of Kentucky | Nicorandil, a small molecule agonist for sulfonyl urea receptor 2 protein (SUR2), to improve cognition and hippocampal pathology | Adults with probable Hippocampal sclerosis (HS)-aging (Ages 75+) | 2025 | ||
| Akira Sekikawa, University of Pittsburgh | Equol supplementation as an intervention to improve cognitive decline by targeting arterial stiffness and white matter lesions in the brain | Older adults (Ages 65+) | 2027 | ||
| Oh Sung Kwon, University Of Connecticut Storrs | Determine if MitoQ supplementation will affect peripheral vasodilation, peripheral and cerebral nitric oxide (NO) bioavailability and mitochondrial reactive oxygen species (mtROS), cognitive function and gait speed | Older adults who are healthy, frail with slow walking speed (0.4m/s based on a 4m walk), and those who meet criteria for mild cognitive impairment (Ages 65 - 80) | 2028 |
| John Olichney, University of California Davis | Vitamin D supplements, to improve cognitive outcomes in elderly groups at risk for dementia | People with mild cognitive impairment or mild Alzheimer’s disease (Ages 65 - 90) | 2024 | ||
| Davangere Devanand, Columbia University | Valacyclovir, an Antiviral (Herpes Simplex Virus drug) to examine if HSV contributes to the pathology of Alzheimer's disease and whether antiviral treatment can improve cognition | People with mild Alzheimer's disease | 2024 | ||
| David Hasan, Duke University | Deferiprone to improve cognitive outcomes | Adults with aneurysmal subarachnoid hemorrhage at risk for cognitive decline (Ages 18 - 70) | 2025 |
Back to top
Section 2: Clinical Drug Development – Phase II/III and Phase III
| Reisa Sperling, University of Southern California | BAN2401 , anti-amyloidß antibody to prevent cognitive decline in Alzheimer's disease by preventing amyloid accumulation | Cognitively healthy older adults with "intermediate" amyloid levels on screening PET (Ages 55 - 80; adults 55 - 64 must also carry at least one APOE e4 allele) | 2024 | ||
| Reisa Sperling, Brigham And Women's Hospital | Solanezumab, an anti-amyloidß antibody to prevent cognitive decline associated with early Alzheimer's disease pathology | Cognitively healthy older adults who are amyloid positive on brain imaging (Ages 65+) | 2024 | ||
| Paul Aisen, University of Southern California | BAN2401, anti-amyloidß antibody to slow or prevent cognitive decline in cognitively normal preclinical individuals with Alzheimer's disease | Cognitively healthy older adults with "elevated" amyloid levels on screening PET (Ages 55 - 80; adults ages 55 - 64 must have an additional risk factor) | 2025 | ||
| Susan Abushakra, Alzheon Inc. | ALZ-801 (tramiprosate pro-drug), an inhibitor of Aß oligomer formation | Individuals with APOE e4 and early Alzheimer's disease diagnosis | 2025 | ||
| Eric Mcdade, Washington University in St. Louis | Anti-amyloid therapy to prevent Alzheimer's disease | Cognitively healthy adults who are Alzheimer's disease genetic mutation carriers | 2026 |
| JJohn Alam, Eip Pharma, Inc. | Neflamapimod, inhibitor of p38 mitogen activated protein kinase alpha (p38a), to reduce neuronal degeneration | Mild-to-moderate dementia with Lewy bodies (Ages 55+) | 2026 | ||
| TBD | E. Wesley Ely, Vanderbilt University Medical Center | Barcitinib, an immunomodulator for acute COVID-19 (FDA approved), administered orally in patients with Long COVID to determine if Barcitinib improves brain function and physical impairment | Adults with Long COVID and cognitive impairment at high risk for long-term Alzheimer's disease and related dementias (ages 18+) | 2028 |
| Jose Alejandro Luchsinger, Columbia University | Metformin, a medication with proven efficacy in decreasing hyperinsulinemia and preventing diabetes, repurposed for the prevention of Alzheimer’s dementia | Adults with amnestic mild cognitive impairment (Ages 55 - 90) | 2025 |
| Ariel Gildengers, University of Pittsburgh | Lithium, a GSK3a and GSK3ß inhibitor to prevent or slow cognitive decline in older adults with mild cognitive impairment | People with mild cognitive impairment (Ages 60+) | 2024 | ||
| Nunzio Pomara, New York University School of Medicine | Escitalopram Oxalate, a SSRI antidepressant to examine effects of Alzheimer's disease biomarkers | Older cognitively unimpaired adults with Major Depressive Disorder (Ages 60+) | 2026 |
| Project Name | Trial Name | Principal Investigator/Institution | Trial Description | Population | Anticipated Completion Date |
|---|---|---|---|---|---|
| Alison Huang, University of California San Francisco | Tolterodine and Mirabegron, anticholinergic bladder medications | Older women with urinary incontinence and without pre-existing dementia | 2027 |
| Project Name | Trial Name | Principal Investigator/Institution | Trial Description | Population | Anticipated Completion Date |
|---|---|---|---|---|---|
| and | Randall Bateman, Washington University in St. Louis | Anti-tau therapy to prevent AD | Cognitively healthy or mildly impaired adults who are Alzheimer's disease genetic mutation carriers | 2026 |
| Karen Alexander, Duke University | Atorvastatin, a statin to reduce death, dementia, and persistent disability | Community-dwelling adults without clinically evident cardiovascular disease, significant disability, or dementia (Ages 75+) | 2027 | ||
| Katherine Mills, Tulane University | Multifaceted intensive blood pressure intervention to reduce blood pressure-related cognitive decline in underserved populations | Adults with hypertension who receive primary care from clinics that predominately manage underserved populations with health disparities | 2025 |
| Katie Schenning, Oregon Health and Science University | IV vs. inhalational anesthesia on post-operative delirium, post-operative cognitive dysfunction, functional status, and patient-reported outcomes | Older adults undergoing elective inpatient non-cardiac surgery (Ages 75+) | 2027 |
Section 3: Nondrug
| Hanzhang Xu, Duke University | A mobile app-based cognitive training (aka mHealth) intervention that is culturally and linguistically relevant to older Chinese Americans to, ultimately, enhance cognitive function in the population. Includes dementia knowledge education, culturally-appropriate app content, cognitive training activities, and training reminders. | Older Chinese Americans without cognitive impairment and adult children of older Chinese Americans | 2025 | ||
| Jerri Edwards, University of South Florida | Combination of cognitive training exercises to modify the functional trajectories of adults with mild cognitive impairment | Older adults with mild cognitive impairment (Ages 65 - 89) | 2027 | ||
| Davangere Devanand, Columbia University | Computerized cognitive training to improve cognitive performance | People with Mild Cognitive Impairment (Ages 55 - 95) | 2029 | ||
| Ivan Lee, University of Massachusetts Boston | Serious games (Neuro-World), to stimulate working memory and improve cognition | Older adults with Mild Cognitive Impairment | 2024 | ||
| Allison Magnuson, University of Rochester | Memory and Attention Adaptation Training-Geriatrics (MAAT-G), a cognitive behavioral therapy-based intervention comprised of a series of videoconferencing workshops | Older cancer survivors with Mild Cognitive Impairment (Ages 65+) | 2025 | ||
| Walter Boot, Florida State University | Mobile platform-based support system intervention to improve adherence to home-based cognitive assessment | Non-cognitively impaired adults (Ages 65+) | 2025 | ||
| Erin Foster, Washington University in St. Louis | Memory encoding strategies to improve prospective memory | Adults who meet criteria for typical idiopathic Parkinson's disease (Ages 50+) | 2025 | ||
| Jin Han, Vanderbilt University Medical Center | Cognitive training rehabilitation program for individuals with delirium to improve working memory | Hospitalized older delirious patients with and without Alzheimer's disease and related dementias (Ages 65+) | 2025 | ||
| Gitendra Uswatte, University of Alabama at Birmingham | Web-based computer game that trains cognitive processing speed to improve everyday activities | Stroke Patients | 2025 | ||
| Jennifer O'Brien, University of South Florida | Speed of processing training intervention to reduce incidence of mild cognitive impairment or dementia | Older adults who are cognitively normal | 2026 | ||
| Nancy Chiaravalloti, Kessler Foundation, Inc. | Kessler Foundation modified Story Memory Technique (KF-mSMT), a 10-session structured cognitive rehabilitation treatment composed of the application of imagery and context to facilitate learning | Older adults with mild cognitive impairment and no evidence of dementia (Ages 60+) | 2027 | ||
| Sharon Sanz Simon, Columbia University | The intervention includes a web-based cognitive training of Executive Control (EC) using an ecological Emphasis Change (EmCh) approach with a task named the Breakfast Game (B-Game) | Cognitively healthy older adult participants (Ages 60 - 75) | 2024 | ||
| Tanvi Bhatt, University of Illinois at Chicago | A novel four-week perturbation-based cognitive-motor intervention for improving reactive balance control and cognition | Older adults with mild cognitive impairment or cognitively intact older adults (Ages 55+) | 2026 | ||
| Joseph Gullett, University of Florida | Use of artificial intelligence in cognitive training for memory deficit-based disorders, which may increase the risk of Alzheimer’s disease | Adults with amnestic mild cognitive impairment (Ages 55 - 100) | 2028 | ||
| Feng Lin, Stanford University | Personalized cognitive training program to slow cognitive decline; intervention is tailored using a participant's biofeedback and cognitive performance | People with mild cognitive impairment due to Alzheimer's disease (Ages 60 - 89) | 2026 | ||
| Miyeon Jung, Indiana University-Purdue University Indianapolis | Preliminary efficacy study of a combined intervention involving virtual reality-based cognitive restoration (Vita) coupled with computerized cognitive training (Com), compared to each intervention alone, and standard of care in a 4-group randomized controlled trial using a 2X2 factorial design | Dyads composed of older (65+ years) patients with HF (chronic HF Stage C validated from echocardiography or comparable measure) and MCI (MoCA score 23 and lower) and their informants | 2027 | ||
| Hyun Kyu Lee, Posit Science Corporation | Cognitive training intervention to improve cognitive and functional performance, while reducing pathology associated with Alzheimer's disease | Cognitively normal older adults (Ages 75+) | 2026 | ||
| TBD | Michelle Voss, University of Iowa | Computerized physical activity behavior change (PABC) programs to improve physical activity behavior change in inactive middle-age adults | Inactive middle-aged adults (Ages 40 - 65) | 2027 | |
| Feng Lin, Stanford University | The ventromedial prefrontal cortex (vmPFC) function will be strengthened, via resonance frequency breathing training to achieve greater adherence to VSOP, a preventative strategy for cognitive aging | Persons with amnestic mild cognitive impairment and healthy controls (Ages 60-89) | 2025 |
| Fang Yu, Arizona State University | Aerobic exercise (cycling) and a specific type of cognitive training (speed of processing training) intervention to improve reasoning and memory, as well as to improve brain structure and function | People with Mild Cognitive Impairment (Ages 65 - 74) | 2024 | ||
| Daniel Clark, Indiana University-Purdue University Indianapolis | Nutrition intervention (with foods high in polyphenols) combined with cognitive training exercises to test impact on cognitive performance | Older adults (60+) | 2024 | ||
| Juleen Rodakowski, University of Pittsburgh | Strategy Training to slow the emergence of disability and decline in cognition | People with Mild Cognitive Impairment (Ages 60+) | 2024 | ||
| Jordan Glenn, Neurotrack Technologies | MindMate + Health Coaching, a digital intervention consisting of the MindMate cognitive health app (digital multi-domain lifestyle intervention designed to address modifiable risk factors for dementia) and Neurotrack's personalized health coaching platform | At-risk adults (Ages 45 - 64) | 2024 | ||
| Cay Anderson-Hanley, iPACES LLC | iPACES v3, a physical and cognitive exercise system that can be used at home | People with Mild Cognitive Impairment or subjective cognitive complaints (Ages 50+) | 2024 | ||
| Judy Pa, University of California San Diego | Physical and cognitive activity program in a virtual reality environment to improve cognition | People with early Mild Cognitive Impairment (Ages 55 - 80) | 2026 | ||
| Hyun Kim, New York State Psychiatric Institute Research Foundation For Mental Hygiene, Inc. | Assess the neurocognitive effects of two sleep interventions (cognitive behavioral therapy for insomnia [CBTI] and acoustic slow-wave activity enhancement [SWAE]) and their underlying mechanisms in individuals with amnestic mild cognitive impairment | Older adults with sleep disturbance who meet criteria for amnestic mild cognitive impairment (Ages 60 - 85) | 2028 | ||
| Daniel Clark, Indiana University-Purdue University Indianapolis | Mediterranean-Dash Intervention for Neurodgenerative Delay (MIND) and aerobic training (MAT) | Non-Hispanic black adults with hypertension (Ages 40 - 64) | 2027 | ||
| Dustin Hammers, Indiana University-Purdue University Indianapolis | To conduct behavioral interventions for the management of patients with early-onset Alzheimer’s disease | Adults with a Clinical Dementia Rating scale of 0.5 to 1.0 at the time of enrollment (Ages 40 - 64) | 2028 | ||
| TBD | David Clark, University of Florida | Brain electrical stimulation intervention to improve walking performance and executive function in older adults with cognitive decline | Older adults with age-related cognitive decline (Ages 65 – 89) | 2027 | |
| TBD | Maya Elias, University of Washington | Daily 30-minute cognitive training sessions and/or sleep promotion intervention to improve cognitive function and sleep | Older adults with no diagnosis of dementia and an intensive care unit stay of 24+ hours (Ages 60+) | 2027 | |
| Mansha Mirza, University of Illinois at Chicago | IPROACTIF is an occupational therapist-delivered primary care intervention for aging and chronic disease management | Community-dwelling adults (Ages 55+) | 2024 | ||
| Christopher Hughes, Vanderbilt University Medical Center | A comprehensive cognitive and physical training program compared to active control to improve outcomes after surgery in the elderly | Patients ≥60 years old undergoing elective major non-cardiac surgery with expected hospitalization ≥3 days and high likelihood of ICU admission | 2025 | ||
| TBD | Tom Plocher, Moai Technologies LLC | Combination of virtual reality cognitive training combined with aerobic cycling to improve cognition and aerobic fitness to prevent Alzheimer's disease | Older adults with subjective cognitive decline (Ages 65+) | 2026 | |
| TBD | Jun Ma, University of Illinois at Chicago | Digital behavioral intervention for racial and ethnic minorities that combines a virtual coaching concept for depression management and a video-based weight loss program to target comorbid depression and obesity | Adults with depression and obesity who self-identify as racial or ethnic minorities (Ages 50-74) | 2029 | |
| Thomas Van Vleet, Posit Science Corporation | An innovative multimodal brain health program to improve cognitive function and functional abilities | Individuals at risk for Alzheimer's disease and related dementias | 2024 |
| Susan Roberts, Tufts University Medical School | Nutrition-based intervention to improve cognitive and brain functioning | Older adults who are overweight/ obese | 2024 | ||
| Suzanne Craft, Wake Forest University | Ketogenic diet to improve cognitive symptoms and pathology in mild cognitive impairment | People with amnestic Mild Cognitive Impairment (Ages 55 - 85) | 2024 | ||
| Yasmin Mossavar-Rahmani, Albert Einstein College of Medicine | An anti-inflammatory, multicultural health diet (MHD) to improve cognitive function in a culturally diverse population of adults | Adults (Ages 40 - 65) | 2024 | ||
| Debra Sullivan, University of Kansas Medical Center | To investigate the effects of a Mediterranean diet (MedD) that is enhanced with extra virgin olive oil (EVOO), almonds, and omega-3 supplements (Med+O) on cognition, brain volume, cerebral antioxidant status, and cardiometabolic biomarkers in adults without cognitive impairment | Adults (Ages 65+) | 2024 | ||
| Russell Swerdlow, University of Kansas Medical Center | Ketogenic diet (KD) to test whether manipulating brain energy metabolism through the KD will benefit individuals with Alzheimer's disease | People with Alzheimer's disease (Ages 50 - 90) | 2024 | ||
| Ashley Shaw, University of Kansas Medical Center | Culturally-adapted, brain-healthy diet to assess body composition, cardiovascular risk, nutritional health status, and changes in cognition among older African American adults | Older African American adults (Ages 55+) | 2025 | ||
| Neelum Aggarwal, Rush University Medical Center | Mediterranean-DASH Diet Intervention for Neurodegenerative Delay (MIND), to test impact on cognition and brain biomarkers related to dementia in patients who are recovering from acute ischemic stroke | Individuals who have suffered acute stroke (Ages 60 - 80) | 2026 | ||
| Lauren Ptomey, University of Kansas Medical Center | Dietary intervention to determine if weight loss or changes can help prevent of delay adults with Down syndrome from developing Alzheimer's disease | Adults with Down syndrome and a body mass index (BMI) of 25 to 45 (Ages 18 - 64) | 2024 |
| Kirk Erickson, University of Pittsburgh | Exercise program to decrease cognitive decline associated with aging | Sedentary adults (Ages 65 - 80) | 2024 | ||
| Jonathan Hakun, Penn State Health Milton S. Hershey Medical Center | Adaptive Step Goals with Interim Goal Setting: participants receive daily step goals, self-monitoring, and interim goal setting on study smart phone to promote increased goal maintenance | Middle-aged adults with increased risk for Alzheimer's disease and related dementia due to obesity | 2027 | ||
| Jerome Smith, University of Maryland | Exercise and flexibility training programs to improve quality of life and physical function | Physically inactive adults (Ages 60 - 80) | 2024 | ||
| Jennifer Etnier, University of North Carolina at Greensboro | Physical Activity program intervention to delay the onset of Alzheimer's disease | Adults with a family history of Alzheimer's disease (Ages 40 - 65) | 2025 | ||
| Kirk Erickson, University of Pittsburgh | To investigate whether African Dance improves cognitive, neurological, physical, psychosocial, and mood outcomes in 60- to 80-year-old African Americans | African Americans (Ages 60 - 80) | 2024 | ||
| Robert Newton, Louisiana State University Pennington Biomedical Research Center | Aerobic exercise & strength training programs to promote cognitive function in older African American adults | African American adults (Ages 65 - 85) | 2025 | ||
| Daniel Zondervan, Flint Rehabilitation Devices (FlintRehab) | FitMi AD system, a wireless, sensorized at-home exercise device | Individuals with mild cognitive impairment or mild dementia | 2024 | ||
| Molly Maxfield, Arizona State University | The intervention is a Physical Activity (PA) Goal Setting Technique | Adults with BMI of 30 kg/m2 or greater (Ages 45 - 65) | 2024 | ||
| TBD | Cynthia Benjamin, Together Senior Health, Inc. | Brain Health Together, a 12-week, group-based, live streaming, digital program focused on addressing physical activity and social isolation to improve cognitive function | People living with mild cognitive impairment | 2024 | |
| Fuzhong Li, Oregon Research Institute | Tai Ji Quan (martial arts practice) intervention to slow cognitive decline in older adults with mild cognitive impairment | People with Amnestic Mild Cognitive Impairment (Ages 65+) | 2025 | ||
| Madeleine Hackney, Emory University | Partnered Rhythmic Rehabilitation, an intervention that simultaneously targets cardiovascular, social and motor-cognitive domains, to improve motor-cognitive function, and neuronal, vascular and inflammatory intermediaries | Adults with prodromal Alzheimer's disease (Ages 50 - 80) | 2025 | ||
| Lauren Ptomey, University of Kansas Medical Center | Moderate-to-vigorous physical activity in adults with down syndrome to prevent Alzheimer's disease | Adults with down syndrome (Ages 18+) | 2024 | ||
| Jian Kong, Massachusetts General Hospital | Baduanjin (BDJ), a mind-body exercise to reduce cognitive decline | Adults (Ages 50 - 80) | 2024 | ||
| G. Adriana Perez, University of Pennsylvania | "Tiempo Juntos para la Salud," a multilevel intervention for Latino adults with mild cognitive impairment that includes empowerment education for behavior change and individual motivation, building support network, and promoting community resources for safe walking to reduce inactivity | Spanish language dominant Latino adults with mild cognitive impairment (Ages 55+) | 2024 | ||
| Jill Morris, University of Kansas Medical Center | Exercise intervention to characterize the brain metabolic response to different exercise intensity levels and measure changes in cognition | People with Alzheimer's Disease (Ages 60+) | 2025 | ||
| Neha Gothe, Northeastern University | Yoga training and aerobic exercise programs to test effects on cognitive functions, neural activity and structure, and physiological parameters | Adults with normal cognition (Ages 55 - 79) | 2025 | ||
| Stephen Rao, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University | Indoor Cycling (high intensity; 60-90% of heart rate reserve) to slow or delay disease onset | Healthy elders at genetic risk for Alzheimer's disease; APOE e4 carriers (Ages 65 - 80) | 2025 | ||
| Mark Gluck, Rutgers University Newark | Cardio Dance Fitness (CDF) intervention, and a Strength, Flexibility, & Balance intervention | Sedentary older African Americans (Ages 60+) | 2027 | ||
| Robert Newton, Louisiana State University Pennington Biomedical Research Center | Exercise intervention to reduce risk in African Americans with Mild Cognitive Impairment | African Americans with mild cognitive impairment (Ages 65 - 85) | 2026 | ||
| Eric Vidoni, University of Kansas Medical Center | Aerobic and resistance exercise programs, to test combined and independent effects on cognitive, neural, and physical function | Healthy individuals (Ages 65 - 80) | 2026 | ||
| Christina Hugenschmidt, Wake Forest University | Dance movement and music appreciation classes to improve cardiorespiratory fitness and cognition | Adults at risk for Alzheimer's disease due to subjective cognitive decline (Ages 65+) | 2027 | ||
| Jenna-Lee Taylor, Mayo Clinic Rochester | Exercise intervention, comparing effects of High Intensity Interval Training (HIIT) vs. Medium Intensity Continuous Training (MICT) on cognitive function | Mid-life adults with cardiovascular disease | 2024 | ||
| Laura Korthauer, Rhode Island Hospital | A 24-session healthy living education program, with enhanced content about health beliefs and mechanisms of behavior change | Cognitively healthy/normal adults with at least two Alzheimer's disease risk factors (Ages 45 - 69) | 2024 | ||
| Ulf Bronas, Columbia University | Home-based walking program to improve cognitive function in patients with co-morbid chronic kidney disease and mild cognitive impairment | Older adults with mild cognitive impairment and chronic kidney disease | 2027 | ||
| Fuzhong Li, Oregon Research Institute | A three-arm, single-blind, randomized controlled trial comparing efficacy of the two tai ji quan fall prevention interventions (Dual-TJQMBB, standard TJQMBB) relative to a stretching control for reducing the incidence of falls among older adults with mild cognitive impairment | Adults with mild cognitive impairment (Ages 65+) | 2028 | ||
| Bijan Najafi, BioSensics | A tele-exercise system that allows a qualified therapist to remotely supervise and interact with the patient during goal-oriented game-like and low risk exercise tasks that have been designed to improve balance and cognition | Individuals with Mild Cognitive Impairment and/or mild dementia | 2025 | ||
| Joseph Kaholokula, Washington State University | A tailored Hula dance program to prevent or slow cognitive decline | Older Native Hawaiians Pacific Islanders with uncontrolled hypertension (Ages 55+) | 2026 | ||
| Jill Morris, University of Kansas Medical Center | Aerobic exercise and lactate infusions to understand changes in brain glucose metabolism and promote cognition | Cognitively normal people and people with Alzheimer's disease (Ages 60 - 90) | 2025 | ||
| Fang Yu, Arizona State University | Aerobic exercise program to improve cognition and memory | People with mild Alzheimer’s disease (Ages 65+) | 2028 | ||
| Peixuan Zheng, University of Illinois at Chicago | Remotely-delivered aerobic and resistance exercise training to improving cognitive and physical function | Adults diagnosed with multiple sclerosis who have cognitive and walking impairment (Ages 50+) | 2024 | ||
| Maiya Geddes, Douglas Mental Health University Institute | The Intergenerational Social Motivation Behavioral Intervention is a technology-based platform to enhance physical activity in older adults at risk for Alzheimer’s disease | Cognitively normal older adults with a first-degree family history of Alzheimer's disease (Ages 60+) | 2025 | ||
| Junxin Li, Johns Hopkins Bayview Medical Center | Personalized physical activity intervention to improve cognition and sleep | Sedentary low-income older adults with sleep difficulties (Ages 65+) | 2028 | ||
| Kueifang Hsieh, University of Illinois at Chicago | We Walk Plus intervention to promote physical activity and improve cognition for older adults with intellectual disabilities | Older adults with intellectual disabilities | 2024 | ||
| TBD | Brianne Tomaszewski, University of North Carolina at Chapel Hill | Fitness classes and coaching for persons with intellectual disabilities to increase physical activity | People with intellectual disabilities and no clinically elevated symptoms of Alzheimer's disease or related dementias (Ages 18+) | 2028 | |
| TBD | Jian Kong, Brain Thrive Technology LLC | Digital self-directed multimodal mind and body approach intervention that integrates Baduanjin, acupressure, and relaxation to improve the cognitive function for mild cognitive impairment | Individuals with mild cognitive impairment (Ages 55+) | 2025 | |
| Pariya Wheeler, University of Alabama at Birmingham | Physical activity (High-intensity interval training versus continuous moderate exercise) and coaching interventions (psychotherapy, lifestyle counseling) to test the effects on cognitive function. | People living with HIV who are stable on HIV antiretroviral therapy (Ages 50+) | 2028 | ||
| TBD | Angela Bryan, University of Colorado Anschutz Medical Campus | Moderate to vigorous physical activity intervention with goal setting that aims to target biomarkers associated with cognitive decline | Middle aged and older adults (Ages 45+) | 2025 | |
| Laura Martin, University of Kansas Medical Center | The application will develop and refine guided imagery exercise and test whether it increases adherence to exercise among mid-life adults | Older adults able to participate in high-intensity interval training (HIIT) exercise (Ages 65 - 85) | 2027 | ||
| TBD | Trisha Kesar, Emory University | Walking intervention to improve cognition and mobility | Adults with or without mild cognitive impairment or Alzheimer's disease diagnosis (Ages 50 - 90) | 2025 | |
| TBD | Jennifer Etnier, University of North Carolina at Greensboro | Rhythmic Auditory Stimulation that promotes physical activity behavior change in low-active older adults | Older adults (Ages 65+) | 2025 | |
| TBD | Dori Rosenberg, Kaiser Foundation Hospitals - Washington | Culturally adapted intervention using goal setting to reduce sedentary time and gradually increase moderate to vigorous physical activity (MVPA) in older Latinx adults | Middle-aged and older Latinx adults (Ages 55 - 89) | 2027 | |
| TBD | Raymond Jones, University of Alabama at Birmingham | High Impact Interval Training (HIIT) interventions for improvement of arterial stiffness and cognition in older adults with HIV | Sixty older adults living with HIV and neurocognitive disorder (Ages 50+) | 2028 |
| TBD | Julene Johnson, University of California San Francisco | A music improvisation intervention on self-regulation, psychosocial function, and cognitive engagement to promote cognitive health and well-being | Older adults with and without mild cognitive impairment (Ages 60+) | 2026 |
| Simon Davis, Duke University | Closed-loop transcranial magnetic stimulation (TMS) to establish parameters that can reliably control brain states during normal memory functioning in healthy aging and mild cognitive impairment | Healthy older adults and individuals with mild cognitive impairment (Ages 60 - 75) | 2027 | ||
| Psyche Loui, Northeastern University | Non-invasive gamma-frequency (40 Hz) light-flickering and auditory tone-stimulation | Participants with mild Alzheimer’s disease (Ages 55 - 90) | 2027 | ||
| Joan Camprodon, Massachusetts General Hospital | Closed-loop transcranial Alternating Current Stimulation (tACS) at 40 Hz to modulate brain oscillations and cognition, as an individualized and potential disease-modifying precision therapy for Alzheimer's disease | Participants with Alzheimer's disease (Ages 50 - 80) | 2024 | ||
| Benjamin Hampstead, University of Michigan | Transcranial direct current stimulation (tDCS) to treat mild cognitive impairment | People with mild cognitive impairment (Ages 65+) | 2024 | ||
| Benjamin Hampstead, University of Michigan | Transcranial direct current stimulation | Older adults with mild cognitive impairment | 2024 | ||
| Emiliano Santarnecchi, Massachusetts General Hospital | Transcranial Alternating Current Stimulation (tACS) to induce increase in gamma oscillations, and assess acute and long-lasting changes in Aß and p-tau levels via PET imaging to investigate the clinical, neurophysiological and cognitive impact | Adults with mild to moderate Alzheimer's disease (Ages 45+) | 2024 | ||
| Roy Hamilton, University of Pennsylvania | High definition transcranial direct stimulation (tDCS) of the left frontal lobe with the goal of developing a potential treatment for primary progressive aphasia (PPA) | Adults with either nonfluent/ | 2025 | ||
| Robert Wilson, University of Arizona | Transcranial Magnetic Stimulation (TMS) to vertex, right inferior frontal gyrus, and right frontal pole to explore effects on decision-making | Healthy younger adults (Ages 18 - 30) and older adults (Ages 65 - 74), with no subjective memory complaints. | 2024 | ||
| Dawn Bowers, University of Florida | Infrared stimulation to assess improvements in cognition function and network connectivity | Older adults with subjective cognitive complains and family history of Alzheimer's disease (Ages 65 - 89) | 2024 | ||
| Ying-Hui Chou, University of Arizona | Repetitive Transcranial Magnetic Stimulation (rTMS) in hippocampus to determine its therapeutic effect on memory function and brain plasticity | People with Amnestic Mild Cognitive Impairment (Ages 50 - 80) | 2025 | ||
| Joe Verghese, Albert Einstein College of Medicine | Transcranial direct current neurostimulation (tDCS) at home intervention to improve cognitive performance and symptoms in patients with mild to moderate Alzheimer’s Disease | People with Alzheimer's disease (Ages 60+) | 2025 | ||
| Dan Iosifescu, New York University School of Medicine | Transcranial Photobiomodulation (t-PBM), which penetrates robustly into the cerebral cortex, stimulating the mitochondrial respiratory chain, and significantly increasing cerebral blood flow, to improve cognitive deficits | People with Amnestic Mild Cognitive Impairment (Ages 65 - 85) | 2025 | ||
| Nanthia Suthana, University of California Los Angeles | Theta burst transcranial magnetic stimulation, a non-invasive neuromodulation method applied to brain areas that are functionally connected to the hippocampus in order to improve/restore memory function | People with Amnestic Mild Cognitive Impairment (Ages 60 - 90) | 2025 | ||
| Kyrana Tsapkini, Johns Hopkins Bayview Medical Center | Transcranial direct current neurostimulation (tDCS) to improve clinical symptoms of mild cognitive impairment | People with probable Alzheimer's disease (Ages 50+) | 2025 | ||
| Heidi Jacobs, Massachusetts General Hospital | Transcutaneous vagus nerve stimulation (tVNS) to improve cognitive function | At-risk older adults (Ages 60 - 85) | 2026 | ||
| Kyrana Tsapkini, Johns Hopkins Bayview Medical Center | Transcranial direct current stimulation (tDCS) applied to brain networks that underlie several language-specific vs. executive cognitive functions to maximize the benefits of tDCS in the treatment of neurodegenerative disease | Individuals with primary progressive aphasia (Ages 50 - 80) | 2027 | ||
| Junxin Li, Johns Hopkins Bayview Medical Center | Feasibility and preliminary efficacy of 40 Hz music (Condition A), 40 Hz sound (Condition B) and preferred music (Condition C) on neural gamma activity and cognitive function in community-dwelling older adults with amnestic mild cognitive impairment | Older adults with amnestic MCI (65+ years of age) | 2025 | ||
| Ying-Hui Chou, University of Arizona | Spaced Theta burst stimulation (TBS) to improve memory performance and hippocampal function in mild cognitive impairment | Individuals with amnestic mild cognitive impairment | 2024 | ||
| Alvaro Pascual-Leone, Hebrew SeniorLife and Hebrew Rehabilitation Center | Transcranial alternating current stimulation targeting the left angular gyrus (tACS) at gamma frequency and transcranial direct current stimulation (tDCS) targeting the left dorsolateral prefrontal cortex | Older adults with mild dementia (Ages 55+) | 2027 | ||
| Kyrana Tsapkini, University of Pennsylvania | Transcranial direct current stimulation (tDCS) therapy in primary progressive aphasia (PPA) | Older adults with primary progressive aphasia (Ages 50 - 80) | 2027 | ||
| TBD | David Ziegler, University of California San Francisco | Closed-loop digital meditation (MediTrain) and transcranial alternating current stimulation (tACS) to improve cognition and wellbeing in adults with mild cognitive impairment | Mild cognitive impairment (Ages 60 - 85) | 2025 | |
| Susan Bookheimer, University of California Los Angeles | Low intensity focused ultrasound pulsation (LIFUP) to improve learning and memory | Adults (Ages 75+) | 2026 | ||
| TBD | Psyche Loui, Northeastern University | Gamma frequency stimulation is administered via lights, and tuned to user-defined music; participants receive the intervention by listening to music while watching lights | Neurologically healthy older and younger adults | 2025 | |
| Andreana Benitez, Medical University of South Carolina | Intermittent theta-burst repetitive transcranial magnetic stimulation (iTBS-rTMS) to improve cognitive function and depression | People with Mild Cognitive Impairment and Major Depressive Disorder (Ages 60 – 85) | 2028 | ||
| TBD | Alexandra Touroutoglou, Massachusetts General Hospital | Repetitive transcranial magnetic stimulation (rTMS) to improve brain function and improve symptoms in Alzheimer's disease | Amyloid-positive people with and without amnestic mild cognitive impairment (Ages 65 – 85) | 2025 | |
| Robert Reinhart, Boston University | Personalized non-invasive brain stimulation to improve memory | Cognitively healthy older adults, persons with mild cognitive impairment, or persons with Alzheimer's disease (Ages 50 - 100) | 2028 | ||
| Nicolaas Bohnen, University of Michigan | Transcranial direct current neurostimulation (tDCS) to test whether tDCS to relevant brain regions may help manage future fluctuations in alertness in people with Lewy body dementias | Adults with Lewy Body Dementia | 2024 |
| Mariana Figueiro, Icahn School of Medicine at Mount Sinai | 8-week Tailored Lighting intervention (TLI). The active TLI will provide high circadian stimulation during the day produced by light sources that provide moderate light levels of spectra that are tuned to the sensitivity of the circadian system | Older adults with mild to moderate Alzheimer's Disease diagnosis and Type 2 diabetes (Ages 55+) | 2024 | ||
| Catherine Siengsukon, University of Kansas Medical Center | CBT-I, cognitive behavioral therapy for insomnia | People with difficulty falling asleep, maintaining sleep, or waking up too early at least 3 nights per week for the past 3 months (Ages 60 - 85) | 2024 | ||
| TBD | Girardin Jean-Louis, University of Miami School of Medicine | Personalized OSA Treatment Adherence Model (PRAISE), a personalized obstructive sleep apnea (OSA) treatment adherence model; participants will be exposed to either personalized OSA videos (intervention arm) or standard online videos (control arm) | Older black adults newly diagnosed with obstructive sleep apnea (Ages 60 – 85) | 2027 | |
| Mariana Figueiro, Icahn School of Medicine at Mount Sinai | Light intervention to improve sleep and mild cognitive impairment | People with amnestic mild cognitive impairment or mild Alzheimer's disease (Ages 65+) | 2024 | ||
| Atul Malhotra, University of California San Diego | Supplemental oxygen and Continuous Positive Airway Pressure (CPAP) machine to determine if oxygen is a viable therapeutic strategy to improve memory | Cognitively healthy older adults (Ages 65-85) | 2025 | ||
| Kristine Wilckens, University of Pittsburgh | Habitual slow-wave sleep enhancement to improve cognition | Older adults (Ages 65 - 85) | 2026 | ||
| Mariana Figueiro, Icahn School of Medicine at Mount Sinai | Rhythmic Light Therapy to improve sleep and cognition | Older adults with mild cognitive impairment | 2026 | ||
| TBD | Don Tucker, Brain Electrophysiology Laboratory Company, Llc | Neurosom® Electric Sleep Therapy (NEST) system to allow researchers to conduct transcranial electrical stimulation (TES) studies to improve sleep in seniors with mild cognitive impairment | Adults with amnestic mild cognitive impairment (Ages 55 - 85) | 2023 | |
| Meghan Mattos, University of Virginia | Internet-delivered cognitive behavioral therapy for insomnia (CBT-I) intervention | Older adults with mild cognitive impairment and insomnia (Ages 65+) | 2027 | ||
| Mariana Figueiro, Icahn School of Medicine at Mount Sinai | Tailored Lighting Intervention to improve sleep disturbances, inflammation, insulin sensitivity (Si) and glucose disposal (Sg) and cognition in patients with mild cognitive impairment or mild Alzheimer's disease and related dementias (AD/ADRD) and sleep disturbances | Adults with Alzheimer's disease and related dementias (AD/ADRD) who live in controlled environments (i.e., assisted living facilities and nursing homes) | 2024 | ||
| Mariana Figueiro, Icahn School of Medicine at Mount Sinai | Light exposure treatment to investigate the effect on sleep, mood, and agitation in persons with Alzheimer's disease or related dementias living in assisted living and nursing home settings | Individuals with Alzheimer's disease or related dementias and sleep problems (Ages 65+) | 2025 | ||
| TBD | Katie Stone, California Pacific Medical Center (Sutter Health) | Obstructive sleep apnea therapies including positive airway pressure, oral appliance therapy, and positional therapy, to promote cognitive function in older adults | Cognitively normal older adults with moderate to severe obstructive sleep apnea (Ages 55 - 75) | 2028 |
| TBD | Dillon Myers, Potluck, LLC | OneClick, an online social engagement platform, to benefit health outcomes affected by social engagement (survival, onset of dementia) | Adults with mild cognitive impairment | 2024 | |
| Sara Czaja, Joan and Sanford I Weill Medical College of Cornell University | Virtual reality program for social activity and cognitive engagement | Community dwelling older adults with mild cognitive impairment | 2027 | ||
| Sara Czaja, Joan and Sanford I Weill Medical College of Cornell University | Intelligent Adaptive System, an innovative intelligent adaptive software package aimed at providing social and cognitive support to older adults with mild cognitive impairment | Cognitively diverse older adults | 2027 |
| Ambar Kulshreshtha, Emory University | A web-based multidisciplinary stress reduction program to examine the association of stress and cognition, role of cardiovascular disease risk factors, and related health disparities | African Americans and White adults with mild cognitive impairment | 2025 | ||
| TBD | David Ziegler, University of California San Francisco | MediTrain, a digital meditation intervention to reduce cognitive decline and emotional stress | Older adults | 2027 | |
| Dimitris Kiosses, Joan and Sanford I Weill Medical College of Cornell University | Problem Adaptation Therapy for Pain (PATH-Pain) primary-care based psychosocial intervention designed to reduce stress | Adults with mild cognitive impairment or early stage Alzheimer's disease (Ages 60+) | 2026 | ||
| Elena Salmoirago-Blotcher, Miriam Hospital | A mindfulness training intervention to improve cognitive function in patients with co-morbid heart failure and cognitive impairment | Individuals with mild cognitive impairment and heart failure | 2027 | ||
| TBD | David Ziegler, University of California San Francisco | Closed-loop digital meditation to improve cognitive function, sleep, and stress in older adults | Older adults with mild cognitive impairment and cognitively normal older adults (Ages 60 - 85) | 2025 |
| Ana-Maria Vranceanu, Massachusetts General Hospital | Two symptom management programs, Active Brains-Digital (AB-D) and Health Enhancement Program (HEP), to assess how each program may help in improving multimodal physical, cognitive, and emotional function | Older adults with nonmalignant chronic pain for more than 3 months and early cognitive decline (Ages 60+) | 2027 | ||
| Michal Schnaider Beeri, Icahn School of Medicine at Mount Sinai | Hyperbaric oxygen therapy intervention to improve cognitive function and increase cerebral blood flow and glucose utilization in people with mild cognitive impairment | People with mild cognitive impairment and diabetes (Ages 65+) | 2024 | ||
| Frank Lin, Johns Hopkins Bayview Medical Center | Follow up of ACHIEVE cohort for an additional three years to determine the long- term effects of hearing intervention (i.e., participants randomized to hearing intervention at Year 0) versus successful aging/delayed hearing intervention control (i.e., participants randomized to successful aging intervention at Year 0 and offered the hearing intervention after their Year 3 visit) on cognitive and brain outcomes | Older adults with mild-to-moderate hearing loss and a MMSE score greater than or equal to 23 (Ages 73 - 89) | 2027 | ||
| Sumeet Seth, EvON Medics | Computerized Olfactory Training Program (COT), a portable, home-based product to prevent progressive cognitive decline and progressive dementia in early Alzheimer's Disease | Adults with diagnosis of probable mild Alzheimer's disease (Ages 65 - 85) | 2024 | ||
| Richard Holden, Indiana University | Digital Brain Safe mobile app to reduce exposure to anticholinergic drugs | Adults who have taken at least 1 anticholinergic medication within the past 90 days (Ages 65+) | 2025 | ||
| Noll Campbell, Purdue University | Pharmacist-driven de-prescribing protocol focused only on targeted anticholinergic medications | Older adults with subjective cognitive dysfunction and at least one order for a strong anticholinergic medication in the previous 12 months (Ages 65+) | 2025 | ||
| Ruth Ottman, Columbia University | Information about risk of Alzheimer's disease given to participants based on their APOE genotypes, in addition to Latino ethnicity and family history, to assess the psychosocial and behavioral impacts | Latino or Hispanic adults (Ages 40 - 64) | 2024 | ||
| TBD | Mark McInnis, RETAIN Health, Inc. | Digital therapeutic designed to reduced risk of Alzheimer's disease | Normal healthy volunteers with a family history of Alzheimer's disease | 2025 | |
| Sarah Hartz, Washington University in St. Louis | Returning research results to participants to measure impact on cognitive and psychosocial outcomes | Cognitively normal individuals who have had imaging studies within 18 months, APOE genotyping, and are scheduled for annual visits | 2025 | ||
| David Hasan, Duke University | Endovascular techniques (or hybrid of carotid endarterectomy and endovascular techniques) to improve cognitive outcomes in individuals with complete occlusion of the internal carotid artery (COICA) | Adults with complete occlusion of the Internal carotid artery (ICA) due to atherosclerotic disease, a recent history of a transient ischemic attack or stroke, and a MoCA < 26 (Ages 21 - 70) | 2024 | ||
| Fiza Singh, University of California San Diego | EEG-neurofeedback (EEG-NFB) computer-based brain training program to improve working memory in individuals with amnestic mild cognitive impairment | People with Amnestic Mild Cognitive Impairment (Ages 60 - 85) | 2025 | ||
| Barry Rovner, Thomas Jefferson University | Diabetes Mellitus-Specific Behavioral Activation (DM-BA) to prevent decline in verbal memory in African American adults with amnestic multiple-domain mild cognitive impairment | African-American adults with amnestic mild cognitive impairment and poorly controlled diabetes mellitus (Ages 65+) | 2025 | ||
| Sarah Tomasweski-Farias, University of California Davis | Digital Memory Notebook intervention, an interactive application used to facilitate behavioral change and enhanced motivation | People with Alzheimer's disease or mild cognitive impairment (Ages 65 - 90) | 2025 | ||
| Adrienne Johnson, University of Wisconsin - Madison | Motivation to Quit Intervention to increase motivation to quit, quit attempts, and use of evidence-based smoking treatments (EBSTs) for older adult smokers | Older adult smokers (Ages 50 - 80) | 2026 | ||
| Yuri Agrawal, University of Colorado Anschutz Medical Campus | Vestibular therapy to reduce falls and improve balance in older adults with mild to moderate Alzheimer's disease and vestibular loss | Adults with mild-moderate Alzheimer's disease & vestibular loss (Ages 60+) | 2026 | ||
| Xiangrong Shi, University of North Texas Health Science Center | An intermittent-hypoxia training (IHT) program to prevent or reverse cognitive decline and neurodegeneration in mild cognitive impairment | Individuals with amnestic mild cognitive impairment | 2025 | ||
| TBD | Aaron Seitz, Northeastern University | An auditory training intervention to mitigate hearing issues that promote dementia risk | Older adults (ages 60 - 85) with no more than typical hearing loss for their age, no evidence of dementia and comparison younger adults (ages 18 - 30) with normal hearing | 2027 | |
| Sara Czaja, Joan and Sanford I Weill Medical College of Cornell University | Intelligent Decision Support Tool, an innovative intelligent decision tool for health decisions aims at providing health management support for older adults with and without mild cognitive impairment | Cognitively diverse older adults | 2027 | ||
| Ryan Mace, Massachusetts General Hospital | My Healthy Brain (MHB) teaches education, mindfulness skills, and behavior change principles. All MHB skills were adapted to account for commonly reported Subjective Cognitive Decline symptoms (e.g., forgetfulness) and co-morbid psychological barriers (e.g., low motivation) that can interfere with lifestyle behavior change. | Medical providers and older adults at risk for Alzheimer's disease and related dementia (Ages 60+) | 2027 | ||
| Justin Golub, Columbia University | Hearing aids to prevent cognitive decline in those at risk for Alzheimer’s Disease and Alzheimer’s Disease Related Dementias (AD/ADRD) | Older adults with early-stage age-related hearing loss (ARHL) and amnestic mild cognitive impairment (55 - 75 years of age) | 2027 | ||
| TBD | Mara Mather, University of Southern California | Ten weeks of daily paced breathing sessions to determine whether resonance breathing improves plasma Alzheimer's disease (AD) biomarkers, identify how resonance-frequency breathing affects plasma AD biomarkers, assess whether daily resonance-frequency breathing accelerates consolidation of new learning, and compare effects in African Americans vs. European Americans | Cognitively normal African-American and European-American adults (Ages 50 - 70) | 2028 | |
| Patrick Smith, University of North Carolina at Chapel Hill | Time restricted fasting intervention to improve metabolic and cognitive health | Amnestic mild cognitive impairment (Ages 65 - 80) | 2025 | ||
| Jeffrey Burns, University of Kansas Medical Center | Remote blood pressure monitoring and management through a virtual Collaborative Care Clinic (vCCC) | Older adults with hypertension | 2025 | ||
| Mariana Figueiro, Icahn School of Medicine at Mount Sinai | Gamma oscillation entrainment for persons with amnestic mild cognitive impairment or mild Alzheimer's disease to improve sleep and cognition | People with amnestic mild cognitive impairment or mild Alzheimer's disease (Ages 55+) | 2026 | ||
| TBD | Jill Morris, University of Kansas Medical Center | Heat therapy to improve systemic blood glucose metabolism to reduce risk for Alzheimer's disease | Cognitively healthy people (Ages 65+) | 2028 | |
| TBD | Emily Rogalski, University of Chicago | Speech-language intervention combined with psychosocial education and counselling to improve communication participation and caregiver burden | People with primary progressive aphasia and their care partners (Ages 18+) | 2028 |
Section 4: Dementia Care and Caregiver
| Mi-Kyung Song Emory University | SPIRIT (Sharing Patient's Illness Representation to Increase Trust), a patient- and family-centered advanced care planning (ACP) intervention based on the Representational Approach to Patient Education to promote cognitive and emotional preparation for end-of-life decision making | Patient (mild Alzheimer's disease)-surrogate dyads (Ages 18+) | 2024 | ||
| Jennifer Wolff, Johns Hopkins Bayview Medical Center | SHARING Choices (Sharing access to Health records, Agenda setting and RespectING Choices to Engage Families), a multicomponent communication intervention that seeks to proactively engage family members and support advance care planning (ACP) in primary care | Adults with Alzheimer's disease and related dementias (AD/ADRD) (Ages 65+) | 2024 | ||
| Alexia Torke, Indiana University-Purdue University Indianapolis | Physician Orders for Scope of Treatment (POST), a virtual at-home advanced care planning (ACP) intervention to make decisions about care goals and to record preferences in writing | Frail, older community dwelling adults (Ages 65+) and their surrogate decision maker | 2024 | ||
| Jennifer Wolff, Johns Hopkins Bayview Medical Center | Sharing Healthcare Wishes in Primary Care (SHARE), a multicomponent communication intervention to proactively engage family members or friends to support advance care planning in primary care | Persons with cognitive impairment (Ages 80+) and family dyads | 2024 | ||
| Lee Lindquist, Northwestern University | Plan Your Lifespan, a web-based decision-making and planning tool to examine the mediating/moderating factors involved in decision-making, timing of decision implementation, and goal concordance for older adults aging-in-place/long term care | Community-dwelling older adults (Ages 55+) | 2025 | ||
| Hillary Lum, University of Colorado Anschutz Medical Campus | ENgaging in Advance Care planning Talks (ENACT) Group Visits intervention to increase advance care planning (ACP) documentation among older adults in primary care settings, including older adults with cognitive impairment | Older adults that have at least one primary care clinic visit in the past 12 months (Ages 70+) | 2026 | ||
| TBD | Jennifer Gabbard, Wake Forest University | ACPdSmart is a dementia-specific portal-based tool for advance care planning engagement | Adults with recognized or probable mild cognitive impairment or mild dementia, yet retain decisional capacity to complete advance care planning (Ages 65+) | 2027 | |
| Erin Kross, University of Washington | "Jumpstart", a one-page guide using patient-specific information to prompt and guide clinicians in goals-of-care discussions | Adults with an ICD10 code for Alzheimer's disease and related dementias (AD/ADRD) in the electronic health record (EHR) in the prior two years (Ages 55+) | 2027 | ||
| Michael Paasche-Orlow, Tufts Medical Center | COVID-19 advance care planning (ACP) Educator-led, video-assisted palliative care intervention to improve patient-clinician communication, increase ACP documentation, and lead to more patient-centered care at the end of life | Hospitalized patients and any patient with Alzheimer's disease and related dementias, as well as proxy decision-makers (Ages 65+) | 2024 | ||
| TBD | Christine Kistler, University of Pittsburgh | Toolkit with modules that include information and training on Alzheimer’s disease and related dementias education, advance care planning communication skills, and clinical implementation guidance | Physicians/ | 2028 |
| Joseph Gaugler, University of Minnesota Twin Cities | The Porchlight Project is multicomponent training delivered to volunteers and includes three established online training modules on person-centered dementia care; a four-session online training program that demonstrates to volunteers how to apply person-centered dementia care knowledge to their interactions with persons with dementia and their caregivers; and ongoing monthly coaching sessions | Family caregivers, persons with dementia, and senior volunteers | 2027 | ||
| Catherine Riffin, Joan and Sanford I Weill Medical College of Cornell University | Caregiver screening-referral protocol for family caregivers who accompany older adults to their primary care visits | Patient-caregiver dyads and healthcare providers | 2024 | ||
| TBD | Francesca Falzarano, University of Southern California | Online self-assessment and tailored referral platform to support caregivers of individuals with Alzheimer's disease and related dementia | Caregivers of individuals with Alzheimer's disease and related dementia | 2026 | |
| Veronica Yank, University of California, San Francisco | Building Better Caregivers, an online, self-management and skills building intervention to support isolated dementia caregivers in rural areas | Rural dementia caregivers (Ages 18+) | 2024 | ||
| Alan Stevens, Baylor Research Institute | GamePlan4Care (GP4C), an internet-based system designed to provide immediate, tailored education and skills training to caregivers | Caregivers of veterans with Alzheimer's disease and related dementias (AD/ADRD) admitted to the inpatient general medicine service at MEDVAMC | 2024 | ||
| TBD | Alan Stevens, Baylor Research Institute | Hospital GamePlan4Care (GP4C), an internet-based system designed to provide immediate, tailored education and skills training to caregivers in a hospital setting | Dementia caregivers in a hospital setting (Ages 18+) | 2024 | |
| Darby Morhardt, Northwestern University | Psychoeducational program for caregivers of persons with primary progressive aphasia | Caregivers of persons living with a documented diagnosis of primary progressive aphasia (PPA) | 2024 | ||
| Laura Gitlin, Drexel University | WeCareAdvisor, an online tool to help caregivers manage behavioral and psychological symptoms in persons with dementia | Caregivers (Ages 21+) | 2024 | ||
| Jacqueline Eaton, University of Utah | Enhancing Active Caregiver Training intervention to manage common behavioral symptoms associated with Alzheimer's disease and related dementias | Caregivers of persons living with Alzheimer's disease and related dementias | 2025 | ||
| TBD | Lauren Parker, Johns Hopkins Bayview Medical Center | Mechanism study to identify how adult day services influences caregiving related stressors and needs for African American caregivers of individuals with Alzheimer's disease and related dementias | African American Caregivers | 2025 | |
| Allison Lindauer, Oregon Health and Science University | Telehealth intervention using education and peer support to reduce stress and behavioral symptoms for family care partners for those with Alzheimer's disease and related dementias | Rural, African American, and White care partners for those living with Alzheimer's disease and related dementias | 2026 | ||
| Rita Jablonski, University of Alabama at Birmingham | Care-Resistant Behavior Internet Training (CuRB-IT) to enhance coping skills of family caregivers and reduce elder abuse and neglect (EAN) | Caregivers for persons with Alzheimer's disease and related dementias (AD/ADRD) | 2026 | ||
| Matthew Golden, MapHabit, Inc. | MapHabit System and Caregiver Training Program (CTP) to improve caregiver quality of life and overall patient care | Dyads, caregivers and individuals living with dementia | 2025 | ||
| Sato Ashida, University of Iowa | Disaster PrepWise (DPW), an intervention program to enhance emergency preparedness and support networks to increase caregiver resilience and minimize distress | Caregivers of individuals with Alzheimer's disease and related dementias (AD/ADRD) | 2027 | ||
| Kylie Meyer, Case Western Reserve University | Learning Skills Together (LST), a complex care skills intervention to improve caregiver self-efficacy | Caregivers to persons living with mid-stage Alzheimer's disease and related dementia (AD/ADRD) | 2027 | ||
| Shih-Yin Lin, New York University | Aliviado DSD Caregiving Mastery Program includes Family-centered training on delirium superimposed dementia (DSD) plus an mHealth app-based care plan of DSD prevention, detection, and management tasks and tailored mobile push notification task reminders and encouragements | Family caregivers for individuals living with Alzheimer's disease and related dementias (AD/ADRD) | 2024 | ||
| Kenneth Hepburn, Emory University | Couple in Control (CiC), a toolkit of communication skills and decisional strategies to help couples experiencing mild cognitive impairment cope with various challenges | Dyads of individuals diagnosed with mild cognitive impairment and their care partners | 2024 | ||
| Catherine Riffin, Joan and Sanford I Weill Medical College of Cornell University | Pain Identification and Communication Toolkit (PICT), a multicomponent intervention for caregivers of people with Alzheimer's disease and related dementias to provide training in observational pain assessment and coaching in effective pain communication techniques | Patients with moderate/severe Alzheimer's disease and related dementia and their caregivers | 2028 | ||
| Alicia Hong, George Mason University | Wellness Enhance for Caregivers (WECARE) is a 7-week social media-based behavioral intervention designed for Chinese American family caregivers of persons with Alzheimer’s disease and related dementias | Chinese American family caregivers of persons with dementia | 2024 | ||
| Kylie Meyer, Case Western Reserve University | An AI-Embedded Intervention that increases caregiver resourcefulness and displaces out-of-pocket caregiving costs with community and other resources to help mitigate financial strain | Adult Latino/Hispanic family caregivers to persons living with dementia (Ages 18+) | 2025 | ||
| Julian Montoro-Rodriguez, University of North Carolina at Charlotte | Latino Caregiver Thrive, Learn & Connect (TLC) is a Spanish telehealth caregiver educational and skill-building program to improve caregiver experience | Latino caregivers (Ages 45+) | 2025 | ||
| Candace Kemp, Georgia State University | The intervention, “Improving Care though Improv,” involves four highly interactive weekly two-hour sessions delivered over a one-month designed to build care partner skills and improve mastery and care experiences and outcomes | Care partners of persons living with dementia | 2024 | ||
| TBD | Carolyn Clevenger, Emory University | Psychoeducation program that seeks to enhance caregivers’ knowledge, skills and mastery of the many tasks they perform and responsibilities they assume in assisting the person living with dementia | Individuals serving as caregivers for family members and friends living with Alzheimer’s disease and related dementias | 2028 | |
| Jori Fleisher, Rush University Medical Center | A caregiver education and peer mentor program comprised of discussion and coaching through specific Lewy Body Dementia knowledge and social support topics | Lewy Body Dementia family caregivers | 2028 | ||
| TBD | Liron Sinvani, Feinstein Institutes for Medical Research | A comprehensive repository for Oropharyngeal Dysphagia related information for care partners of persons with Alzheimer’s disease and AD-related dementias and Oropharyngeal Dysphagia | Care partners of hospitalized patients with Alzheimer’s disease and related dementias and Oropharyngeal Dysphagia (Ages 65+) | 2025 | |
| Joseph Chung, KINTO | Kinto Care Coaching, a multilingual care coaching platform utilizing financial literacy content | Caregivers of persons living with Alzheimer's disease and related dementias | 2024 | ||
| TBD | Victoria Panzer, Brookside Research and Development Company | FallScape for Dementia (FS-D), an innovative caregiver-provided daily treatment utilizing proprietary multimedia and behavioral intervention methods to reduce falls for people living with dementia | Dyads of caregivers and persons living with dementia | 2024 |
| Fayron Epps, Emory University | Faith-based home activity toolkit (Faith-HAT), meaningful religious and spiritual activities for persons living with dementia to engage in at home with their family caregiver | Dyads of persons living with dementia and caregiver (Ages 18+) | 2024 | ||
| Robert Penfold, Kaiser Foundation Hospitals - Washington | STAR Virtual Training and Follow-up (STAR- VTF), a training using web-based Electronic Health Record portal to help in expand access to and delivery of empirically supported behavioral health services for caregivers and people with dementia | Dyads of people with dementia (Ages 55+) and their caregiver | 2024 | ||
| Nancy Hodgson, University of Pennsylvania | Care of Persons in the Environments (COPE), an online training program for caregivers and care staff of persons with dementia to improve care for persons with dementia | Dyads from PACE sites (Ages 21 - 100) | 2024 | ||
| TBD | Sarah Bannon, Icahn School of Medicine at Mount Sinai | Resilient Together for Dementia (RT-D), a live video dyadic resiliency intervention | Persons Living With Dementia, and Spousal Partner (SP) dyads, along with staff employed at MGH Department of Neurology | 2027 | |
| TBD | Hayley Belli, New York University School of Medicine | Behavioral economics mobile health (BE-mHealth) digital tool that will incorporate BE nudges into 2-3 evidence-based non-pharmacologic strategies for managing behavioral and psychological symptoms of dementia (BPSD) | Persons living with dementia and their primary care partners | 2027 |
| Kathi Heffner, University of Rochester | Mindfulness Based Stress Reduction (MBSR) and the Living Well program to see if the programs might be associated with better immune function (response to current influenza vaccine), physical and emotional health, and well-being for family caregivers | Adults who currently live with and are the primary caregivers for a community dwelling dementia patient (Ages 60+) | 2023 | ||
| Nicole Fowler, Indiana University-Purdue University Indianapolis | An early screening intervention involving sending letters to dyads and the primary care physician informing them of the results of the screening of Alzheimer's disease for earlier diagnosis | Dyads of older adults (Ages 65+) and family members (Ages 21+) | 2024 | ||
| Eun-Ok Im, Emory University | The intervention is BC-Care (BrainCheck), a cognitive care planning tool that integrates patient, provider, and caregiver input to generate personalized Alzheimer's disease and related dementias (AD/ADRD) recommendations and follow-up care to be implemented during an AD/ADRD's patient's medical visit | Asian American women who are family caregivers of persons living with Alzheimer’s disease, and experts in gerontology/ | 2024 | ||
| Judith Moskowitz, Northwestern University | LEAF, Life Enhancing Activities technology-based intervention to reduce burden and increase well-being for family caregivers of persons living with Alzheimer's disease or related dementias | Caregivers (Ages 34 - 87) | 2024 | ||
| Brent Mausbach, University of California San Diego | Mobile Pleasant Events Program (mPEP), a Behavioral Activation Therapy program to increase caregiver's engagement in pleasant activities | Dementia caregivers (Ages 40 - 90) | 2024 | ||
| Rebecca Utz, University of Utah | Time for Living & Caring (TLC), an online, self-administered intervention to provide informal family caregivers with resources, support, and education to improve their respite time-use (respite is defined as planned time away from caregiving | Caregivers (Ages 18+) | 2024 | ||
| Bruno Kajiyama, Photozig, Inc. | Caring Mind App, a mindfulness-based Cognitive Coping intervention to reduce caregiver stress and depressive symptoms | Caregivers of individuals living with Alzheimer's disease and related dementia (AD/ADRD) | 2023 | ||
| Susan Aguinaga, University of Illinois | A Latin dance program (BAILAMOS™) and Mediterranean diet (MIND) to improve cognitive function | Older Latino adults (Ages 50+) | 2024 | ||
| Benzi Kluger, University of Rochester | Social LEAF (Life Enhancing Activities for Family Caregivers), a group video-conference intervention to teach caregivers coping skills to improve social relationships and social connection | Caregivers of community-dwelling family member with dementia with Lewy Bodies (DLB) | 2025 | ||
| Lee Kehoe, University of Rochester | Life Review therapy, a behavioral intervention to address social connection in caregivers by focusing on the caregiving relationship | Caregivers and care receiver with mild cognitive impairment and advanced cancer (Ages 50+) | 2025 | ||
| Debra Parker Oliver, Washington University in St. Louis | Caregiver Speakers, a technologically-mediated storytelling intervention using photos to illustrate various feelings and meanings | Adult hospice family caregivers of person's living with dementia (Ages 18 - 120) | 2024 | ||
| Sunmoo Yoon, Columbia University Health Sciences | X-based intervention to enhance the social support (informational, instrumental, emotional, appraisal) for Hispanic and Black dementia caregivers | Black or Hispanic dementia caregivers (Ages 18+) | 2024 | ||
| Walter Hinton, University of California Davis | Multidomain intervention program that includes physical and cognitive exercises led by physiotherapists and management of metabolic and vascular risk factors by a cardiologist to address cognitive decline, quality of life, and functional ability | Individuals living in the community who have a diagnosis of mild-moderate vascular dementia (Ages 60+) | 2025 | ||
| Walter Hinton, University of California Davis | Adapted (i.e., Distant REACH VN) intervention training protocol for distance delivery to provide support for family caregivers of people living with dementia | Caregiver-care recipient dyads and other staff | 2025 | ||
| Walter Hinton, University of California Davis | Vietnam Resources for Enhancing Alzheimer’s Caregiver’s Health (REACH VN), a culturally adapted behavioral intervention to support family caregivers of persons with dementia in Vietnam | Family caregivers (Ages 18+) | 2025 | ||
| Walter Hinton, University of California Davis | Paper-based cognitive training | Vietnamese patients with early Alzheimer's disease and their caregivers | 2025 | ||
| Walter Hinton, University of California Davis | Information including knowledge and skills related to dementia patient care administered to the participants via a smartphone app (Zalo app) | Primary caregivers of patients with dementia | 2025 | ||
| Matthew Knutson, Minnesota HealthSolutions Corporation | Algorithm to connect caregivers of people with dementia to each other for emotional support | Caregivers of persons living with dementia, such as Alzheimer’s disease, Lewy Body dementia and Frontotemporal degeneration | 2025 | ||
| Sheria Robinson-Lane, University of Michigan | Culturally tailored support intervention for African American caregivers of persons living with dementia to reduce health disparities | African American caregivers of a person living with dementia (Ages 55+) | 2025 | ||
| Felipe Jain, Massachusetts General Hospital | Guided imagery and mindfulness therapy delivered via mobile app to augment dementia caregiver skills training | Primary caregivers (ages 60+) for a relative with Alzheimer's disease and related dementias (AD/ADRD) that uses a smartphone at least 5 days/week | 2025 | ||
| Jung-Ah Lee, University of California Irvine | Stress reduction and dementia education intervention with wearable technology to measure behavioral and physiological measures | Adult family caregivers of persons living with dementia | 2025 | ||
| Christina Mccrae, University of South Florida | NiteCAPP, a web cognitive behavioral treatment for insomnia (CBT-I) for rural dementia caregivers to improve caregiver health, mood, burden and cognition | Rural dementia caregivers and persons living with dementia | 2026 | ||
| George Demiris, University of Pennsylvania | ENCODE (Empowering Caregivers of Patients with Dementia) is a behavioral intervention to support family caregivers in pain management for their hospice patients based on the transcriptional theory of stress and coping | Caregivers of hospice patients with Alzheimer's disease and related dementias (AD/ADRD) | 2026 | ||
| Kathy Wright, Ohio State University | Mindfulness in Motion (MIM) plus the Dietary Approaches to Stop Hypertension (DASH) interventions to improve caregiver stress and quality of life | African American female caregivers with hypertension (Ages 40+) | 2025 | ||
| TBD | Bryan Denny, Rice University | A smartphone-based cognitive emotion regulation training to improve caregiver health and well-being | Caregivers of individuals with Alzheimer's disease and related dementia (AD/ADRD) | 2027 | |
| TBD | Kerry Evers, Pro-Change Behavior Systems, Inc. | Caring4Caregivers (C4C), a theory-driven mobile solution to promote self-care and well-being among caregivers | Caregivers for individuals living with Alzheimer's disease and related dementia (AD/ADRD) | 2025 | |
| Allison Marziliano, Feinstein Institutes for Medical Research | RELOAD-C, an intervention leveraging concepts from Meaning-Centered Psychotherapy to reduce loneliness | Individuals with Alzheimer's disease and related dementias (AD/ADRD) and their care partners | 2027 | ||
| Kelsey Gabel, University of Illinois at Chicago | An intermittent fasting intervention (time restricted eating, TRE), which involves confining the eating window to 8-10 hours and fasting for the remaining hours of the day, in combination with either resistance training (TRE-RT) or aerobic training (TRE-AT) to examine the effects on body weight, body composition, metabolic disease risk, and cognition in overweight and pre-diabetic adults | Adults who are overweight/obese and pre-diabetic (Ages 50 - 85) | 2024 | ||
| Naoko Muramatsu, University of Illinois Chicago | Pro-Home MeC, a 12-week in-home physical activity (PA) program, delivered using an app on a tablet hat has built-in motivational enhancement (e.g., music), that consists of: motivational enhancement components, including initial PA discussion between the patient and the memory care clinician, and goal setting with the interventionist; 5 moves for aerobics, strength, flexibility, and balance exercises that target daily functions | Adults diagnosed with mild cognitive impairment or mild dementia (Ages 50+) | 2024 | ||
| Kathi Heffner, University of Rochester | Social Engage Coaching (S-ENG), a behavioral intervention that involves one-on-one coaching with a care manager to increase social engagement and reduce loneliness | Caregivers for individuals living with Alzheimer's disease and related dementia (AD/ADRD) | 2025 | ||
| TBD | Angie Leroy, Baylor University | An internet-based cognitive-behavioral writing intervention to improve caregiver health and well-being | Spousal caregivers for individuals living with Alzheimer's disease and related dementia (AD/ADRD) | 2027 | |
| TBD | Manka Nkimbeng, University of Minnesota Twin Cities | The proposed intervention is The Active Caregiving Empowering Skills (ACES) intervention which is a psychoeducational program that is based on Cognitive Behavioral Therapy (CBT) to decrease caregiver stress and decrease behavioral distress in persons living with dementia | Black older adult immigrants (ages 50+) living with dementia and their informal caregivers | 2027 | |
| Richard Holden, Indiana University | Brain CareNotes app, a mobile telehealth app used by informal caregivers for behavioral and psychological symptoms of dementia (BPSD) management | Informal caregivers of people living with Alzheimer's disease and related dementias (AD/ADRD), and the care-recipients | 2027 | ||
| Ana-Maria Vranceanu, Massachusetts General Hospital | "CALM ", a smartphone-based meditation app that teaches users the basics of mindfulness and how to integrate it into daily life to promote sustained practice and provides 10- to 12-minute guided meditations with embedded behavioral principles [e.g., reminders] to facilitate adherence | Older adults with chronic pain comorbid with early cognitive decline (Ages 60+) | 2025 | ||
| TBD | Christine Ritchie, Massachusetts General Hospital | MASC combines evidence-based skills from mindfulness programs, self-compassion programs, and behavioral management programs. An open pilot trial was delivered to 10 informal caregivers of ADRD individuals | Caregivers of persons living with dementia | 2025 | |
| Jonathan Singer, Texas Tech University | Dialectical behavior therapy skills training groups tailored to the unique experiences and needs of informal caregivers of persons with Alzheimer's Disease and related dementias to reduce suicide-related outcomes | Informal caregivers (Ages 50+) of persons with Alzheimer's disease and related dementias | 2026 | ||
| TBD | Julia Burgdorf, Visiting Nurse Service of New York | DECLARE, a self-assessment/web-based survey platform that asks caregivers about their needs and concerns, to improve home health care team communication with and in support of caregivers | Home health staff and Alzheimer’s disease and related dementias (AD/ADRD) caregivers | 2028 | |
| Holly Prigerson, Joan and Sanford I Weill Medical College of Cornell University | LMH-4-Dementia Care Pairs (LMH-4-DCP), a reminiscence-based intervention for family caregiver and person with dementia to address family caregivers' pre-loss grief and enhance relationship quality | Caregivers and persons with dementia | 2025 | ||
| TBD | Fayron Epps, Emory University | Asynchronous/ | Adult family caregivers (Ages 40-75) to persons with Alzheimer’s disease and related dementia | 2025 | |
| TBD | Sokha Koeuth, Plans4care Inc. | Software system that provides caregivers of persons with Alzheimer's disease or Alzheimer's disease related dementia with personalized, evidence-based strategies on-demand to address self-identified care challenges | Caregivers of persons living with Alzheimer's disease or Alzheimer's disease related dementia from a care setting | 2024 | |
| TBD | Bruno Kajiyama, Photozig, Inc. | Recharge behavioral therapy delivered through the recharge mobile app to help reduce depressive symptoms and alleviate stress of caregivers of individuals with Alzheimer’s disease | Adult family caregivers (Ages 18+) to individuals with Alzheimer’s disease | 2024 | |
| TBD | Jordan Lewis, University of Minnesota Twin Cities | Psychoeducational program informed by stress mediation and social cognitive theories aimed at alleviating caregiving strains and to strengthen knowledge, skills, and outlook of caregiving | American Indian family caregivers (Ages 18+) of persons living with Alzheimer’s disease and related dementias | 2028 | |
| TBD | Andrew Pickett, Indiana University | A four-week feasibility test of the developed physical activity intervention | Sexual and gender minority caregivers of people living with Alzheimer’s disease and related dementias | 2028 |
| Nicole Fowler, Indiana University-Purdue University Indianapolis | A mammography decision aid that will help caregivers of patients with Alzheimer's make decisions about stopping or continuing breast cancer screening | Dyads consisting of an older woman with Alzheimer's disease (Ages 75+) and a family caregiver (Ages undefined) | 2024 | ||
| Bradford Dickerson, Massachusetts General Hospital | Video-based decision aids (user manuals) to improve decision making skills for caregivers of persons with early-onset dementia | Caregivers (Ages 18+) of those with young-onset Alzheimer's disease and related dementias (onset age <65 years old) | 2024 | ||
| Sara Czaja, Joan and Sanford I Weill Medical College of Cornell University | Communication-based intervention to improve preparedness for advance care planning (ACP) | Adults with Alzheimer's disease and related dementias and their caregivers | 2024 | ||
| Marian Betz, University of Colorado Anschutz Medical Campus | RCT of the “Safety in Dementia” (SiD) online tool to address firearm access, thereby reducing injury risk, among informal caregivers of community-dwelling adults with dementia | Informal caregivers of community-dwelling adults with dementia | 2024 | ||
| Ellen Brown, Florida International University | Assistive alternative communication device for persons living with dementia to communicate care preferences to caregiver and provider | Dyads of caregivers and persons living with dementia | 2025 | ||
| Kalisha Johnson, Emory University | Tailored strategies to support formal care decision-making processes | African-American parent-adult daughter dyad | 2026 | ||
| TBD | Emily Largent, University of Pennsylvania | A toolkit to improve decision-making | Individuals living with Alzheimer's disease and related dementia (AD/ADRD), their care partners, and providers | 2027 | |
| TBD | Allison Magnuson, University of Rochester | COACH-Cog, an adapted tool to facilitate conversations about aging-related conditions, such as cognition, with older patients and their care partners | Dyads of people living with dementia and their care partners | 2027 | |
| TBD | Paul Barr, Dartmouth College | HealthPAL, a visiting recording platform to improve tridiatic interpersonal communications | IIndividuals with Alzheimer's disease and related dementia (AD/ADRD), care partners, and providers | 2027 | |
| Beth Fields, University of Wisconsin - Madison | Care Partner Hospital Assessment Tool (CHAT): a novel approach designed to increase care partner preparedness for caregiving tasks during their family member or friends’ hospitalization | Patients living with Alzheimer's disease and related dementia (AD/ADRD) and their care partners, healthcare system administrators, and clinicians | 2027 | ||
| Lee Lindquist, Northwestern University | Negotiation and dispute resolution training program for caregivers of persons living with dementia | Adult family caregivers of persons living with dementia that provide at least 10 hours weekly support | 2025 |
| Susan Hickman, Indiana University-Purdue University Indianapolis | APPROACHES, an Advance Care Planning (ACP) Specialist Program to improve care and reduce unwanted, burdensome hospitalizations through improved ACP procedures, standardized staff education on ACP, and systematic ACP facilitation delivered by nursing home staff | Adults with Alzheimer's disease and related dementias, their family caregivers, and nursing home providers | 2024 | ||
| Vincent Mor, Brown University | MUSIC & MEMORY (M&M) Music Program, a personalized music program in which caregivers (nursing home staff, family, or others) provide people with dementia with music playlists tailored to their personal history of music preferences | Nursing home residents with moderate to severe dementia | 2024 | ||
| Abraham Brody, New York University | Dementia Symptom Management at Home Program Hospice Edition (Aliviado Dementia Care-Hospice Edition), a multi-modal quality assurance performance improvement (QAPI) program for improving the quality of care provided to persons with dementia and support to their informal caregivers through hospice | Hospice agencies | 2024 | ||
| Wen Liu, University of Iowa | Person-centered mealtime care intervention for nursing home residents with Alzheimer's disease and related dementias | Interventionalists and nursing home residents living with Alzheimer's disease and related dementias (Ages 55+) | 2024 | ||
| Ruth Engelberg, University of Washington | Electronic health record (EHR)-based Clinician Jumpstart intervention to promote goals-of-care communication for older patients with serious illness | Older adults (Ages 65+; adults ages 65 - 79 must have 1+ chronic condition) | 2025 | ||
| Barbara Resnick, University of Maryland, Baltimore | Function Focused Care for Acute Care (FFC-AC-EIT), a care interaction intervention to increase physical activity and prevent functional decline during hospitalization | Older adults with Alzheimer's disease and related dementias (AD/ADRD) admitted to the hospital (Ages 55+) | 2024 | ||
| Lorella Palazzo, Kaiser Permanente Washington Research Institute | Intervention led by clinical value champions embedded within primary care settings to decrease the use of potentially inappropriate medications in patients living with dementia | Persons living with dementia | 2024 | ||
| l | Michael Lepore, University of Maryland School of Nursing | Cognitive Stimulation Therapy (CST), an online group intervention to actively engage and stimulate cognitive processes of persons living with dementia to reduce depressive symptoms | Persons living with dementia | 2024 | |
| Xiaojuan Li, Harvard Pilgrim Health Care, Inc. | Dementia Care Consultation (DCC) program, a collaborative care coordination program delivered by a care consultant to reduce patient healthcare utilization and improve caregiver knowledge and well-being | Dyads of persons living with dementia and their caregiver | 2024 | ||
| Medha Munshi, Harvard Medical School | An educational intervention directed at Long Term Care Facility (LTCF) staff to deprescribe and reduce the number of high risk medications (HRMs) in long term care facility (LTCF) residents with diabetes and ADRD | Long-term care facility (LTCF) providers and LTCF residents with diabetes and Alzheimer's disease and related dementias (AD/ADRD) | 2024 | ||
| Joan Carpenter, University of Maryland School of Nursing | Telehealth intervention called Palliative Care Consultation in Post-Acute Care (PCC-PAC) to meet the needs of persons living with dementia newly admitted to nursing homes | Persons living with dementia | 2024 | ||
| Aanand Naik, University of Texas Health Science Center at Houston | Patient Priorities Care (PCC), an EHR-based intervention to improve care decisional strategies and align with patient priorities, for older adults with multiple chronic conditions | Hispanic people living with dementia and chronic conditions | 2024 | ||
| Richard Fortinsky, University of Connecticut Health Center | Tele-Savvy, an online program to improve caregiver mastery | Family members/informal caregivers of people living with dementia | 2024 | ||
| Annette Totten, Oregon Health and Science University | ADVANCE-PC delivered using remote technology (ECHO), a communications and implementation support intervention to provide training and technical assistance tailored to the needs of primary care clinicians and clinics | Persons living with dementia, clinicians, and staff | 2024 | ||
| Magaly Ramirez, University of Washington | STAR-VTF is a program that teaches caregivers skills to reduce behavioral and psychological symptoms of dementia | Caregivers of persons living with dementia | 2024 | ||
| Elizabeth Ciemins, American Medical Group Association | MIND at Home is a dementia care coordination model that includes coordination between persons living with dementia, their care partner, a memory care coordinator and their provider | Dyads of caregivers and individuals with dementia | 2024 | ||
| Leah Hanson, HealthPartners Institute | The Mindfulness-Based Dementia Care (MBDC) is a program for care partners of persons living with dementia to incorporate mindfulness practices into day-to-day life to help cope with the challenges and stresses of dementia care | Care partners of persons living with dementia | 2024 | ||
| TBD | Halima Amjad, Johns Hopkins Bayview Medical Center | Pragmatic intervention to facilitate high quality, comprehensive post-diagnosis care and improve quality of care and patient outcomes | Persons living with dementia, in traditional primary care settings | 2025 | |
| I | Nilanjan Sarkar, Vanderbilt University | Socially assistive robot to interact with long-term care setting residents with either dementia or mild cognitive impairment | Person living with dementia or mild cognitive impairment in assisted living or nursing home facilities (Ages 70+) | 2025 | |
| Miguel Vazquez, University of Texas, Southwestern Medical Center | Pragmatic trial of intensive blood pressure management | Older adults (Ages 70+) | 2025 | ||
| TBD | Jessica Palakshappa, Wake Forest University | Cognitive screening program for incident mild cognitive impairment or Alzheimer's disease and related dementias | Older adults with critical illness | 2026 | |
| April Savoy, Indiana University-Purdue University Indianapolis | Shared Decision-Making Tool: new internet based interactive information visualizations designed to facilitate shared decision-making and recognize certain needs; Continuous Glucose Monitoring: a wearable device that continuously monitors glucose levels in near real-time | Individuals with Dementia and Diabetes and their caregivers | 2027 | ||
| Donovan Maust, University of Michigan | The intervention, EMPOWER, is an educational brochure focused on the risks associated with psychotropic and opioid polypharmacy that will be sent to participant via mail to "nudge”/encourage them to have discussions with the prescribing clinicians about the inappropriateness of the regimen to lower polypharmacy exposure for persons living with dementia | Persons living with dementia | 2024 | ||
| Jennifer Carnahan, Indiana University Health | Patient Priorities Care (PPC), is an evidence-based program to identify the goals and values of patients and care partners by 1) the clinician contacting the patient and care partner for a discussion of their priorities and 2) integration of priorities into the patient’s care plan | Patients with mild cognitive impairment or dementia | 2024 | ||
| Lisa Kern, Joan and Sanford I Weill Medical College of Cornell University | Intervention to identify and assign care coordinators to persons living with dementia based on their care partners’ self-reported difficulty with care coordination as compared to the usual approach of assigning care coordinators after hospital discharge | Persons living with dementia | 2024 | ||
| Ira Hofer, Icahn School of Medicine at Mount Sinai | CDS prompts, a series of prompts following the notification that a patient is at increased risk of postoperative delirium (POD) to promote best clinical practices for the prevention of POD | Attending physicians and their adult patients with a history of cognitive impairment who will be undergoing surgery | 2024 | ||
| Helen Kales, University of California Davis | Describe, Investigate, Create, Evaluate (DICE) is an intervention to manage behavioral and psychological symptoms of dementia (BPSD). DICE includes a 6-hour online modular training, manual, and additional training as needed coaching from a DICE trainer that will be completed by clinic social workers (called Onsite DICE Coordinators or ODCs). | Persons living with dementia and their care partner dyads | 2024 | ||
| TBD | Katherine Possin, University of California San Francisco | The Care Ecosystem, a team-based care model that can lead to quality improvements for family caregivers and formal care providers | Family caregivers and formal care providers | 2025 | |
| Michelle Keller, Cedars-Sinai Medical Center | Pharmacist-led deprescribing intervention aimed at reducing inappropriate medications | Patients with 5 medications prescribed and a face-to-face primary care encounter in the last year (Ages 65+) | 2027 | ||
| TBD | Monique Pappadis, University of Texas Medical Branch | Caregiver and Patient Abuse assessment For Older and Vulnerable populations (CAPA-OV), a screening and intervention tool for use in primary care settings to screen for elder abuse among older and vulnerable adults with mild cognitive impairment or Alzheimer's disease and related dementias (AD/ADRD), as well as the inclusion of a risk assessment for accompanying caregivers | Persons living with dementia (Ages 65+), their caregivers, and healthcare providers | 2024 | |
| Ying-Ling Jao, Pennsylvania State University | The intervention is a smart ambient bright light (SABL) intervention which includes tunable LED lights, photosensors, and controllers | Nursing home residents with Alzheimer's disease and related dementia (AD/ADRD) (Ages 55+) | 2025 | ||
| TBD | Gregg Gorzelle, Hopeful Aging LLC | Making Connections Thru Music (MCTM) sessions, an evidence-based music and discussion intervention to improve engagement, enhance quality of life, and reduce behavioral expressions in persons with dementia | Caregiving volunteers and resident living staff for persons living with dementia | 2025 | |
| Katherine Abbott, Brown University | A Individualized Positive Psychosocial Intervention (IPPI) program that tailors activities to resident preferences and provides people living with dementia with preference-based, person-centered care to improve their mood and reduce communication of distress. | Persons Living With Dementia residing in nursing homes | 2024 | ||
| TBD | E. Wesley Ely, Voicelove LLC | VoiceLove is a HIPAA-compliant mobile application that enables expanded family engagement for patients in the Intensive Care Unit (ICU) | Clinicians, patients, and family members within medical, surgical, and coronary Intensive Care Units (ICU) | 2025 | |
| Denise Dillard, Washington State University | Positive airway pressure therapy, a behavioral intervention for obstructive sleep apnea, to improve sleep quality, cognitive function, and vascular risk factors for Alzheimer's disease and related dementias | American Indians from Strong Heart Study communities (Ages 55+) | 2026 | ||
| Sheryl Zimmerman, University of North Carolina at Chapel Hill | A lighting intervention to reduce nighttime falls | Individuals with Alzheimer's disease and related dementia | 2027 | ||
| A. Lynn Snow, University of Alabama | Improve clinical outcomes for nursing home residents with Alzheimer's disease or related dementias by testing an evidence-based intervention to improve these residents' sleep | Individuals with Alzheimer's disease or related dementia (Ages 50+) | 2027 | ||
| Kamakshi Lakshminarayan, University of Minnesota Twin Cities | An intervention (mGlide-Care) to address uncontrolled hypertension in people with mild cognitive impairment and early stage Alzheimer's disease and related dementia (AD/ADRD) | Participants with uncontrolled hypertension and early stage Alzheimer's disease and related dementia (AD/ADRD) or mild cognitive impairment (Ages 60 - 85) | 2028 | ||
| Vincent Mor, Brown University | MUSIC & MEMORY (M&M) Music Program, music is preloaded on personalized music devices to increase nursing use of the music with residents | Nursing home residents with moderate to severe dementia | 2024 | ||
| Holly Holmes and Aanand Naik, University of Texas Health Science Center at Houston | Patient Priorities Care (PPC), a three-step care plan regarding the implementation of priorities identification and alignment of deprescribing practices as part of the routine care of adults living with dementia | Adults with dementia (ages 65+) and their caregivers | 2024 | ||
| Joshua Chodosh, New York University School of Medicine | Emergency and post-emergency care through patient-care partner focused interventions: 1) Emergency Care Redesign; 2) Nurse-led Telephonic Care; and 3) Community Paramedic-led Transitions Intervention | Persons living with dementia who visit the emergency department (Ages 66+) | 2028 | ||
| Barbara Resnick, University of Maryland, Baltimore | The study will evaluate the efficacy and treatment fidelity of an intervention to improve assessment, diagnosis and management of pain (Pain Management Clinical Practice Guideline, PAIN-CPG-EIT) among nursing home residents living with dementia | Nursing home residents living with dementia (Ages 65+) | 2028 | ||
| TBD | Jamie Labuzetta, University of California San Diego | Determining if the frequency of checking neurological health affects cognitive outcomes of Intensive Care Unit patients | Adult patients (ages 55+) after uncomplicated elective coiling of unruptured cerebral aneurysm | 2028 | |
| Jamie Labuzetta, University of California San Diego | Determining if the frequency of checking neurological health affects cognitive outcomes of Intensive Care Unit patients after an acute brain injury | Older adult patients with spontaneous acute intracerebral hemorrhage with radiographic and clinical stability greater than 6 hours following admission to the Intensive Care Unit (Ages 55+) | 2028 | ||
| TBD | Teresita Hogan, University of Chicago | A Clinical Decision Support (CDS) to propel the PAIN-Advanced Dementia (PAINAD) scale into the routine emergency care | Medically stable emergency department patients with co-existent hip pain and history of dementia | 2024 | |
| TBD | Veronica Yank, Brown University | Online, peer-led small group workshop intervention for family caregivers to increase self-management behaviors, dementia caregiving skills, and peer social support | Family caregivers (Ages 18+) of persons living with dementia | 2024 | |
| TBD | Yuval Malinsky, Vigorous Mind Inc. | The Vigorous Mind Robot (VMR), an autonomous robot with an infra-red camera that will be employed in nursing home dementia units to alert staff and identify falls for early intervention | Subjects living on Dementia Memory Unit in three different nursing homes | 2024 |
| Natalie Regier, Johns Hopkins Bayview Medical Center | Service user-led meaningful activity intervention to treat mild dementia | People that are mild or early stages of dementia (Ages 60+) | 2024 | ||
| Quincy Samus, Johns Hopkins Bayview Medical Center | Activity-based home healthcare to treat people with dementia | People with dementia (Ages 55+) and caregivers (Ages 18+) | 2024 | ||
| Tania Giovannetti, Temple University | Virtual reality training intervention for persons with Alzheimer's Disease to improve performance of everyday tasks | Older adults with mild to moderate Alzheimer's disease (Ages 65+); Informants with knowledge of person with Alzheimer's disease's level of function | 2024 | ||
| Ashkan Vaziri, BioSensics | Care4AD, a comprehensive care platform to improve care for people with Alzheimer's disease | Adults with mild to moderate Alzheimer's disease (Ages 56+) | 2024 | ||
| Nicole Rogus-Pulia, University of Wisconsin - Madison | Dysphagia rehabilitative interventions (lingual strengthening and saliva substitute use) to improve swallowing-related outcomes | Older adults with diagnosis of dementia, cognitive impairment, or memory loss (Ages 55 - 90) | 2024 | ||
| Marian Betz, University of Colorado Anschutz Medical Campus | Web-based driving decision aid (DDA), an online tool that guides an individual to optimize decision-making | Older drivers without severe cognitive impairment (Ages 70+) and family members | 2025 | ||
| Lauren Ptomey, University of Kansas Medical Center | Moderate physical activity intervention for adults with Alzheimer’s Disease and their Caregivers to improve mobility | Adults with mild moderate Alzheimer's disease and their caregivers (Ages 55+) | 2025 | ||
| Jerry Gurwitz, University of Massachusetts Medical School | Patient/caregiver-centered, multifaceted educational intervention on inappropriate prescribing | Older adults with Alzheimer's disease and related dementias (AD/ADRD) | 2025 | ||
| TBD | Glenna Brewster Glasglow, Emory University | T-Dyadic Sleep, a cognitive behavioral therapy for insomnia (CBTI) intervention to examine acceptability and feasibility as well as preliminary efficacy on objective and subjective sleep outcomes | Persons living with mild cognitive impairment or early-stage dementia and their caregivers | 2026 | |
| TBD | Darina V. Petrovsky, Rutgers Biomedical And Health Sciences | Tailored music-based mobile application aimed at improving insomnia symptoms in persons living with dementia | Underrepresented community-dwelling persons living with dementia and their caregivers | 2026 | |
| Yeonsu Song, University of California Los Angeles | Dyadic sleep intervention to improve subjective and objective sleep quality | Dyads of people living with dementia and their care partners | 2027 | ||
| TBD | Esther Oh, Johns Hopkins Bayview Medical Center | A hearing care intervention to treating neuropsychiatric symptoms | Individuals living with Alzheimer's disease and related dementia (AD/ADRD) | 2027 | |
| TBD | Elizabeth Rhodus, University of Kentucky | Harmony at HOME will provide caregiver training for assessment of the environment as an antecedent to behaviors of the person with Alzheimer's disease and related dementias & implementation of environmental cueing as a tool to create a supportive environment for functional behaviors and activity participation | Persons living with dementia and their care partners | 2027 | |
| Norman Schmidt, Florida State University | Cognitive anxiety sensitivity treatment (CAST): a brief, fully computerized CBT-based intervention in people living with mild cognitive impairment | Dyads consisting of persons with mild cognitive impairment or mild Alzheimer's disease and related dementias (AD/ADRD) and their care partners | 2027 | ||
| Joseph Gaugler, University of Minnesota Twin Cities | Home Alone combines behavioral activation (BA) with other evidence-based intervention approaches (i.e., Tailored Activity Program/TAP; Skills2Care®) that target the environment in order to tailor activity that is fulfilling and meaningful to persons with cognitive impairment | Persons with cognitive impairment who live alone | 2024 | ||
| Lisbeth Sanders, LifeBio Inc | LifeBio Memory, an online platform that stores stories, videos, and photos and uses information to make personalized reminiscence therapy exercises | Adult staff (Ages 18+) in assisted living facility with dementia care residents in assisted living care setting (Ages 55+) | 2024 | ||
| TBD | Ariel Green, Johns Hopkins Bayview Medical Center | eALIGN, a patient portal intervention to align medication decisions with persons living with Alzheimer's disease and related dementias (AD/ADRD) and care partners' goals, provide care partner training in the use of nonpharmacologic methods to address behavioral and psychological symptoms of dementia, and reduce polypharmacy and potentially inappropriate medication use | Older adults with diagnosis of Alzheimer's disease and related dementia who receive 5 or more chronic daily medications (Ages 65+) | 2027 | |
| Alyssa Lanzi, University of Delaware | A behavioral treatment, Structured External Memory Aid Treatment (SEMAT), to promote independent living skills | Adults with mild cognitive impairment (Ages 65 - 85) | 2026 | ||
| Jennifer Kim, Vanderbilt University | A patient/ surrogate-centered deprescribing intervention adapted for dementia (Shed-MEDS) to reduce exposure to unnecessary or potentially harmful medications among residents with dementia living in an assisted living facility | Residents of an assisted living facility with dementia who are taking 5+ medications or 1 potentially inappropriate medication | 2024 | ||
| TBD | Alyssa Weakley, University of California Davis | Interactive-Care (I-Care), remote caregiver platform to improve everyday functioning and independence of care receivers, remote caregiver involvement, and the emotional bond between the dyad | Mild Alzheimer's disease and related dementia / cognitively impaired care receiver and remote caregivers | 2028 | |
| Annalisa Na, Drexel University | A program where participants will start each session with pain, vital signs, and environmental assessments; then receive an individualized home-based exercise program | People living with mild to very mild dementia with osteoarthritis (Ages 65+) and their caregiver | 2027 | ||
| TBD | Saleh Kalantari, Cornell University | A VR-based intervention to improve the wellbeing and quality of life of older adults with mild cognitive impairment | Older adults with mild cognitive impairment and non-impaired older adult volunteers | 2028 | |
| TBD | Chantal Kerssens, care.coach | A digital technology avatar-based fall prevention program to reduce the rate and risk of falling | Community dwelling mild cognitively impaired and cognitively normal participants (Ages 65+) | 2024 | |
| Mary Janevic, University of Michigan | STEPS-CI, a community health worker-led chronic pain self-management program designed to improve pain-related outcomes for older adults with chronic pain and mild to moderate cognitive impairment | Older adults who report chronic musculoskeletal pain and mild to moderate cognitive impairment (Ages 50+) | 2026 | ||
| Michael Skrajner, Hopeful Aging LLC | Social Activities For Engagement at Home or SAFE at Home (SaH), a social engagement intervention | Persons living with dementia | 2024 |
| Laura Gitlin, Johns Hopkins Bayview Medical Center | Adult day service (ADS) Plus, including care management, referral/linkage, education about dementia, situational counseling, emotional support, stress reduction techniques, and skills to manage behavioral symptoms | Caregivers initially enrolling their relative in one of the Adult Day Service sites (Ages 21+) | 2024 | ||
| B.R. Simon Rosser, University of Minnesota Twin Cities | Long-term services and support staff (Ages 18+), Sexual and Gender Minority (SGM) residents, and SGM resident caregivers | 2026 | |||
| TBD | Chanee Fabius, Johns Hopkins Bayview Medical Center | A feasibility test of a home care role and preference guide designed to improve information sharing between family caregivers and home care aides of persons living with Alzheimer's disease and related dementias (AD/ADRD) receiving Medicaid Home and Community-Based Services in Maryland | Family caregivers and home care aides of older adults living with Alzheimer's disease and related dementia (AD/ADRD) | 2027 | |
| TBD | Patricia Prusaczyk, Washington University in St. Louis | A communication and care coordination tool for providers of patients with Alzheimer's disease and related dementias | Providers of care for individuals living with Alzheimer's disease and related dementias | 2026 | |
| TBD | Peter Serina, Brown University | A care coordination intervention to improve communication between primary care and emergency department physicians to reduce unnecessary hospitalizations | Assisted living center residents with dementia | 2024 | |
| Alexia Torke, Indiana University | Virtual collaborative care program to reduce emergency department utilization and the secondary outcomes of medication use | Persons living with dementia (Ages 65+) and their care partners (Ages 18+) | 2024 |
| Greg Sachs, Indiana University-Purdue University Indianapolis | Palliative care to improve the care of community dwelling patients with dementia and their family caregivers through an innovative model of supportive care that combines an existing, evidence-based intervention for dementia care with an innovative intervention | People with moderate to severe stage dementia (Ages 65+) | 2024 | ||
| Donald Sullivan, Oregon Health and Science University | Education information sheet for the promotion of advanced care planning (ACP) among family members of persons with advanced dementia in long-term services and support facilities | Clinicians and family members of persons with advanced dementia in long-term services and support facilities | 2024 | ||
| TBD | Laura Hanson, University of North Carolina at Chapel Hill | A machine-learning mortality prediction model to identify nursing residents with late-stage Alzheimer's disease and related dementias appropriate for hospice informational referrals to reduce goal-discordant hospital transfers | Nursing home residents with late-stage Alzheimer's disease and related dementia | 2024 | |
| Elizabeth Luth, Rutgers Biomedical And Health Sciences | Training program and tool to improve clinicians' knowledge of dementia-related challenges in home hospice care, reduce family caregiver burden, and reduce hospice disenrollment | Nurses or social workers who provides home hospice to persons living with dementia and family caregivers | 2024 | ||
| Christopher Cox, Duke University | PCplanner-augmented care and PCplanner mobile, augmented collaborative palliative care that will allow patients and their family members to report their needs in a platform viewable by ICU physicians to 1) reduce family caregivers' unmet needs and psychological distress, 2) increase the frequency of goal concordant treatment among older adult patients, and 3) reduce hospital length of stay | Older adults (Ages 65+) managed in an ICU and their family members (Ages 18+), and physicians and nurses (Ages 18+) from academic and community settings | 2025 | ||
| Vyjeyanthi S. Periyakoil, Stanford University | Nurse-led early palliative care intervention (EPC) involving conducting systematic assessment and providing coaching to patients to improve care and well-being | Community-dwelling Stanford ADRC participants (Ages 60 - 100) | 2024 | ||
| Laura Hanson, University of North Carolina at Chapel Hill | Dementia-specific palliative care intervention involving caregiver education and transitional care | Dyads of hospitalized patients with late stage Alzheimer's disease and related dementias (AD/ADRD) and their family caregivers | 2025 | ||
| Kathleen Unroe, Indiana University-Purdue University Indianapolis | Palliative care intervention utilizing palliative care champions to consult and help surrogate decision makers | Surrogate decision makers for persons living with dementia in nursing homes | 2025 | ||
| Kara Dassel, University of Utah | Tool to help persons with preclinical awareness of Alzheimer's disease and related dementia risk and those with early-stage cognitive impairment to begin advance care planning conversations with a care partner | Community-dwelling pairs consisting of persons in the preclinical or early stage of Alzheimer's disease and related dementia (AD/ADRD) and their care partner | 2027 | ||
| Nathan Goldstein, Icahn School of Medicine at Mount Sinai | A new novel home-based palliative care intervention for patients with advanced AD/ADRD and their caregivers. Dyads will be cared for by a pyramid of palliative care and dementia care trained providers who will continue to work with the patient / caregiver through face-to-face visits, video visits, and (at least) weekly phone calls for 12 months | Older adults (ages 65+) with advanced dementia, based on Global Deterioration Score (GDS) >6 who are impairment in at least one activity of daily living (ADL) and their family caregiver | 2027 | ||
| TBD | Carey Candrian, University of Colorado Anschutz Medical Campus | 1) Stakeholder-based behavioral intervention: trains hospice interdisciplinary staff in person-centered communication to promote authentic end-of-life care for sexual and gender minority patients (SGM) patients; and 2) Communication training intervention: teaching hospice interdisciplinary staff to sensitively collect and incorporate sexual orientation and gender identity (SOGI) data to more effectively communicate to accomplish assessment and person-centered care when providing end of life | Hospice interdisciplinary team (IDT) staff, sexual and gender minority (SGM) former/current patients, and SGM older adults current/former caregivers for people living with Alzheimer's disease and related dementia (AD/ADRD) | 2027 | |
| Elizabeth Luth, Rutgers Biomedical And Health Sciences | Enhanced palliative care communication strategies and clinician workflow intervention to alter patients’ and family care partners’ engagement with palliative care | Persons living with dementia (Ages 18+) | 2024 |
| Sheryl Zimmerman, University of North Carolina at Chapel Hill | A program to provide daily mouth care to reduce bacteria in the mouth that can lead to aspiration pneumonia for assisted living residents | Residents with dementia, care staff, and dental hygienists (Ages 18+) | 2024 | ||
| Kristine N. Williams, University of Kansas Medical Center | Elderspeak and communication training to reduce behavioral symptoms in long term care settings | Nursing home staff | 2026 | ||
| TBD | Sheryl Zimmerman, University of North Carolina at Chapel Hill | Two training arms (essentiALZ alone and essentiALZ + ECHO) in terms of adoption, reach, and reaction. The trial will determine effectiveness of two training arms in relation to each other and to a control arm | Staff at Assisted Living facilities | 2027 | |
| TBD | E-Shien Chang, Joan and Sanford I Weill Medical College of Cornell University | A new staff education module aimed to prevent and mitigate race/ethnicity-related resident-to-resident aggression in long-term care facilities | Nursing home healthcare providers (Ages 18+) | 2028 | |
| Sheryl Zimmerman, University of North Carolina at Chapel Hill | A program to provide daily mouth care to reduce bacteria in the mouth that can lead to aspiration pneumonia for assisted living residents | Residents with dementia, care staff, and dental hygienists (Ages 18+) | 2024 |
| Gene Wang, People Power Company | People Power Caregiver is a newly developed hardware and software system that is designed to create a safer and more supportive home environment for caregivers and persons with dementia and mild cognitive impairment; People Power Caregiver combines modern Internet-of-Things and artificial intelligence technologies | Dyads of caregivers and individuals with dementia or mild cognitive impairment who live in a Spanish language home. Caregivers must have an iPhone or Android phone. | 2024 | ||
| Gene Wang, People Power Company | People Power Caregiver is a newly developed hardware and software system that is designed to create a safer and more supportive home environment for caregivers and persons with dementia and mild cognitive impairment; People Power Caregiver combines modern Internet-of-Things and artificial intelligence technologies | Dyads of caregivers and individuals with dementia or mild cognitive impairment who live in a rural home. Caregivers must have an iPhone or Android phone. | 2024 | ||
| Gene Wang, People Power Company | People Power Caregiver is a newly developed hardware and software system that is designed to create a safer and more supportive home environment for caregivers and persons with dementia and mild cognitive impairment; People Power Caregiver combines modern Internet-of-Things and artificial intelligence technologies | Dyads of caregivers and individuals with dementia or mild cognitive impairment in homes with Apple Watch. Caregivers must have an iPhone or Android phone. | 2024 | ||
| Jenay Beer, Applied Universal Dynamics, Corp. | Socially-assistive robot to deliver cognitive training in the form of a music (piano) learning intervention | Older adults with mild cognitive impairment (Ages 65+) | 2024 | ||
| Gary Havey, Advanced Medical Electronics Corp. | Smartwatch Reminder (SR) system, to automatically transmit pictures and relevant information, such as name or relationship, to a smartwatch worn by the person with memory concerns when family or friends visit | Dyads of persons with memory concerns (Ages 30 - 70) and their care partners (Ages 21+) | 2024 | ||
| Matthew Golden, MapHabit, Inc. | MapHabitTM system, an assistive technology app that helps accomplish activities of daily living, maintain independence, and improves overall quality of life for users | Older adults with Alzheimer's disease and related dementias (AD/ADRD) | 2024 | ||
| Sarah Stahl, University of Pittsburgh | iCARE is a multicomponent intervention that uses mobile technology and motivational health coaching to optimize behaviors that keep the body’s biological clock in sync: sleep, exercise, and social activities | Caregivers of persons living with dementia | 2025 | ||
| Chao-Yi Wu, Oregon Health and Science University | Staying Sharp, an online multi-component health intervention to promote a healthier lifestyle and improved skills for coping with the stress involved in dementia caregiving | Caregivers of persons with Alzheimer's disease and related dementias (AD/ADRD) | 2025 | ||
| Feng Yang, Georgia State University | Perturbation training, a motor learning intervention to prevent falls | Older adults with mild Alzheimer's disease | 2024 | ||
| Raina Croff, Oregon Health and Science University | The SHARP Android application preloaded with 72 GPS-linked routes, each route with three “Memory Markers” (historical localized images with two associated questions) designed to prompt conversational reminiscence about local Black history, culture, and life experiences while walking | Triads of 1) primary caregiver, 2) caregiver support person, and 3) African American person with early-stage dementia | 2025 | ||
| TBD | Nilanjan Sarkar, Vanderbilt University | HMD-AR, a collaborative head mounted display augmented reality to maximize social connection and engagement | Older adults residing in LTC facilities, family members of the older adults, and formal staff caregivers employed in the Department of Recreational Activities | 2025 | |
| TBD | Sajay Arthanat, University of New Hampshire | Mobile Assistive Robot with Smart Sensing (MARSS) is a smart home-based social assistive robot with multi modal care protocols which combines best practices and existing research on home automation, human-robot interaction, and Alzheimer's disease care giving | Individuals with Alzheimer’s diseases and caregiver dyads | 2027 | |
| Kyle Rand, Rendever | Rendever virtual reality (VR) platform designed to improve quality of life of residents with Alzheimer's disease and related dementias in senior living communities and their adult children who live at a distance | Dyads of residents with Alzheimer's disease and related dementias in senior living communities and their adult children | 2024 | ||
| Leanne Boehm, Vanderbilt University | Collaborative telemedicine-delivered ICU-Recovery Clinic intervention to identify and improve long-term cognitive impairment, physical and mental health dysfunction, social integration, and self-management behaviors for older critically ill adults | Older adults treated in a medical or surgical ICU for acute respiratory failure and/or septic shock and at high risk for developing Post-Intensive Care Syndrome (Ages 45+) | 2028 | ||
| TBD | Erik Page, Blue Iris Labs, Inc. | Speck Sr., an active lighting intervention designed to add morning light and reduce evening light to optimize light exposure and support circadian entrainment | Healthy adults and adults living at home with mild cognitive impairment or early stage Alzheimer's disease and related dementias | 2024 | |
| TBD | Erik Page, Blue Iris Labs, Inc. | Speck Sr., an active lighting intervention designed to add morning light and reduce evening light to optimize light exposure and support circadian entrainment | Healthy older adults | 2024 | |
| TBD | Pamela Souza, Northwestern University | Hearing or communication intervention to improve cognition and overall communication ability | Adults with mild hearing loss and early-stage Alzheimer's disease, related dementia, or mild cognitive impairment (Ages 60+) | 2025 | |
| TBD | Michael Scullin, Baylor University | Prospective memory training for persons with mild cognitive impairment or mild dementia using smart-phone based strategies | People with mild cognitive impairment or mild dementia from underserved or digitally disadvantaged populations and their study partners | 2028 | |
| TBD | Michael Skrajner, Hopeful Aging LLC | BRAIN APP is a non-pharmacological intervention that provides person-centered activities for persons with dementia through the use of Artificial Intelligence | Persons living with dementia (Ages 65+), professional care partners and family members (Ages 18+) | 2025 |
| Carrie Nieman, Johns Hopkins Bayview Medical Center | Communication intervention delivered at home (counseling program combined with a low-cost, over-the-counter amplification device) to optimize hearing and communication for older adults with Alzheimer's disease and related dementia | Older adults with dementia and their caregivers (Ages 18+) | 2024 | ||
| Naoko Muramatsu, University of Illinois at Chicago | Healthy Moves for Aging Well (Healthy Moves), a safe physical activity program delivered by home care aides, which consists of a brief motivational enhancement and three movements to be performed in a seated position | Pairs of eligible patients and their home care aides | 2024 | ||
| David Reuben, University of California Los Angeles | Pragmatic intervention to compare clinical effectiveness and cost-effectiveness of community-based dementia care and health system-based care | Dyads of persons with dementia and their primary caregivers (Ages 18+) | 2024 | ||
| Hae-Ra Han, Johns Hopkins Bayview Medical Center | PLAN, a dementia literacy training used to train community health workers to improve caregiver's dementia literacy, self-efficacy in dementia care and service use, social support, depression, and quality of life | Community health workers and dyads of individuals with probable dementia (Ages 65+) and their caregiver (Ages 18+) | 2025 | ||
| Annalise Rahman-Filipiak, University of Michigan | Diagnostic Disclosure Protocol, where participants receive information about current cognitive performance to assess long-term behavior, safety, and efficacy in patients with amnestic Mild Cognitive Impairment (aMCI) following biomarker disclosure | Adults diagnosed with amnestic mild cognitive impairment within the past 12 months (Ages 50+) | 2026 | ||
| TBD | Stephanie Nothelle, Johns Hopkins Bayview Medical Center | Develop and implement a practical primary care based intervention to facilitate collaboration across care management programs | Older adults with Alzheimer's disease and related dementia (AD/ADRD) | 2026 | |
| TBD | Patrik Johansson, Washington State University | INTACT, a clinic-level intervention to improve Alzheimer's disease and related dementias diagnosis and quality of care for American Indian and Alaska Natives | Rural clinics serving American Indian and Alaska Natives | 2026 | |
| Bhavana Patel, University of Florida | Telemedicine model of Lewy Body Dementia interdisciplinary care | Individuals living with Lewy Body Dementia and their caregivers | 2026 | ||
| TBD | Bin Huang, Braincheck, Inc. | BC-Care (BrainCheck), a cognitive care planning tool that integrates patient, provider, and caregiver input to generate personalized Alzheimer's disease and related dementias (AD/ADRD) recommendations and follow-up care to be implemented during an AD/ADRD's patient's medical visit | Adults with an existing diagnosis of mild to moderate dementia (Ages 65+), their caregiver, and their health care provider | 2024 | |
| Richard Lee, Duke University | Donepezil, an acetylcholinesterase inhibitor, compared to placebo to measure the effect on fracture risk factors including bone mineral density (BMD), bone turnover markers, and bone quality | Older adults with biomarker-diagnosed Alzheimer’s disease and related dementias at the Duke Memory Disorders Clinic | 2025 | ||
| TBD | Clara Li, Icahn School of Medicine at Mount Sinai | Chinese adapted memory support system that uses a day planner and note-taking system as a support tool to train individuals with mild cognitive impairment to perform their instrumental of activities of daily living independently | Chinese American older adults (Ages 65+) and their care partners who are primarily Mandarin and Cantonese-speaking | 2025 | |
| TBD | Fuad Abujarad, Yale University | VOICES elder mistreatment program, a tablet-based intervention that utilizes digital coaching, interactive multimedia libraries, electronic screening, and brief motivational interviewing to enhance self-reporting of elder mistreatment | Persons living with dementia or mild cognitive impairment (Ages 60+) | 2024 | |
| TBD | Scott Dresden, Northwestern University | Deprescribing recommendations and monitoring of medication supply data for the reduction of potentially inappropriate medications use | Emergency department patients with diagnosed Alzheimer’s disease and related dementias or mild cognitive impairment | 2024 | |
| TBD | Julie Lauffenburger, Brigham and Women's Hospital | Electronic health record deprescribing to facilitate deprescribing of high-risk medications | Primary care providers, patients living with dementia or mild cognitive impairment and their care partners | 2024 | |
| TBD | Ariel Green, Kaiser Permanente Colorado | Pharmacist-led deprescribing intervention in primary care to optimize medication usage by reducing or stopping medications that are harmful or unlikely to be beneficial | People living with dementia (Ages 65+) | 2024 | |
| Gregg Gorzelle, Hopeful Aging LLC | All About Me (AAM), an innovative cross-platform software app to improve relationships and increase sense of community | Persons living with dementia, their families, and residential care staff | 2024 |
Section 5: Understanding Disease Processes
| Julie Dumas, University of Vermont and State Agricultural College | Testing the effect of dietary fat on working memory | Cognitively normal men and women with a healthy body mass index of 20 to 40 kg/m2 (Ages 65 - 75) | 2028 | ||
| Andriy Yabluchanskiy, University of Oklahoma | Time restricted eating intervention to improve neurovascular function and cognitive performance | Older adults (Ages 55 - 80) | 2025 | ||
| TBD | Michael Howell, University of Minnesota Twin Cities | Compare serotonergic rapid eye movement (REM) sleep Behavior Disorder (5-HT RBD) participants and to adults without RBD who are taking a serotonergic antidepressants in order to: (1) detect systemic alpha-synuclein pathology; (2) examine for evidence of brainstem neurodegeneration using 7T magnetic resonance imaging; (3) quantify evolving speech deficits | Adults with serotonergic rapid eye movement (REM) sleep Behavior Disorder (5-HT RBD) and adults without RBD who take serotonergic antidepressants (Ages 18 - 75) | 2028 | |
| Kimberly Albert, Vanderbilt University Medical Center | Mecamylamine, a centrally and peripherally active non-competitive antagonist of acetylcholine at C6 (ganglionic) type nicotinic receptors, to better identify older adults at risk for Alzheimer’s Disease | Community-dwelling Chinese American older adults (Ages 55+) | 2028 | ||
| TBD | Dominika Pindus, University of Illinois | Feasibility trial of brief high intensity interval training (HIIT) exercise breaks to interrupt sitting and improve cognitive function | Healthy, sedentary older adults without dementia (Ages 60 - 75) | 2025 | |
| Mara Mather, University of Southern California | Measure the plasma beta-amyloid response to an acute laboratory stressor using the socially evaluated cold pressor test (SECPT) to aid in understanding how stress contributes to Alzheimer's disease development and progression in people | Healthy adults (Ages 18 - 65) | 2024 | ||
| Stephanie Grasso, University of Texas at Austin | Naming intervention for individuals with logopenic or semantic variant primary progressive aphasia (PPA) or script training intervention for individuals with nonfluent/agrammatic PPA | Bilingual individuals with primary progressive aphasia | 2027 | ||
| Priti Balchandani, Icahn School of Medicine at Mount Sinai | High-resolution multi-modal 7T imaging to investigate the impact of lighting intervention therapy (LIT) on sleep physiology and brain structure, function, and connectivity in people with mild cognitive impairment and mild Alzheimer's disease | Amnestic mild cognitive impairment or mild Alzheimer's disease patients with a confirmed Aß+ PET scan | 2024 | ||
| Christian Lobue, University of Texas Southwestern Medical Center | Mechanistic study using high definition transcranial direct current stimulation (HD-tDCS) and blood-derived biomarker tools | Individuals with amnestic mild cognitive impairment with and without a history of mild traumatic brain injury (TBI) | 2027 | ||
| Phillip Vlisides, University of Michigan | Test the effects of caffeine on neurocognitive and clinical recovery after major surgery | Adult surgical patients presenting for major non-cardiac, non-intracranial, non-major vascular surgery (Ages 70+) | 2027 | ||
| Tae-Ho Lee, Virginia Polytechnic Institute and State University | Two interventions to facilitate neural plasticity in the brain, either multi-modal adaptive attention training or practice on a training version of the criterion attention task, to examine how this may modulate locus coeruleus-salience network connectivity and mitigate distractibility | Healthy participants from the Roanoke and Blacksburg metropolitan area in Virginia (Ages 55 – 75) | 2027 | ||
| Cynthia Munro, Johns Hopkins Bayview Medical Center | Response to an acute stressor (trier social stress test) in mild cognitive impairment | Adults with mild cognitive impairment | 2027 | ||
| Natalie Ebner, University of Florida | Neurofeedback training using real time fMRI to increase activity in the anterior cingulate gyrus to improve learning | Healthy older adults (Ages 60 - 85) | 2027 | ||
| Eric Lenze, Washington University in St. Louis | Exercise and mindfulness interventions to measure the effects of COVID-induced stress on Alzheimer's disease-related cognitive decline | Healthy, sedentary, community living individuals (Ages 65 - 84) | 2026 | ||
| Ricardo Osorio, New York University School of Medicine | Locus coeruleus (LC) targeted imaging coupled with other biomarker testing to determine whether LC dysfunction can be measured in preclinical Alzheimer's disease stages | Cognitively normal older adults (Ages 60 - 75) | 2024 | ||
| Angela Hanson, University of Washington | Ingestion of a high fat drink (heavy dairy cream) to gain a more in-depth understanding of how high fat feeding (HFF) exerts influence on brain function and metabolism for APOE e4 carriers and non-carriers, and identify brain regions which are particularly vulnerable to the acute effects of HFF | Older adults with and without APOE E4 (Ages 55+) | 2025 | ||
| Mariana Figueiro, Icahn School of Medicine at Mount Sinai | Lighting interventions to suppress melatonin and promote better sleep and cognition | Older adults with mild cognitive impairment | 2024 | ||
| Manus Donahue, Vanderbilt University Medical Center | MRI and PET imaging to evaluate levels of beta-amyloid burden and assess lymphatic drainage function | Older adults with Parkinson's disease (Ages 55 - 80) | 2025 | ||
| Julie Dumas, University of Vermont and State Agricultural College | Mecamylamine, a cholinergic antagonist, to study the relations between the cholinergic system's functional integrity, cognition, and known Alzheimer's disease biomarkers | Postmenopausal women (Ages 50 - 70) | 2025 | ||
| Robert Reinhart, Boston University | High-definition transcranial alternating-current stimulation of the frontal and temporal cortex, to improve working memory and cognition | Younger adults (Ages 18 - 30) and older adults (Ages 68 - 80) | 2025 | ||
| Mouna Attarha, Posit Science Corporation | INHANCE, a computerized cognitive training program based on neuroplasticity principles to reduce long-term risk of dementia | Healthy older adults (Ages 65+) | 2024 | ||
| Richard Sloan, Columbia University | Cocoa-flavanol supplement to reduce systemic inflammation, improve cerebral blood volume to the hippocampus, and improve cognition | Adults (Ages 50 - 69) | 2024 | ||
| Rong Zhang, University of Texas Southwestern Medical Center | Amlodipine (calcium channel blocker), Losartan (angiotensin II receptor blocker), and other antihypertensive drugs to reduce systolic blood pressure and alter brain function | Older adults with hypertension (Ages 55 - 79) | 2024 | ||
| Michelle Voss, University of Iowa | Exercise program to improve learning and increase functional hippocampal-cortical communication that otherwise declines with aging | Sedentary Adults (Ages 55 - 80) | 2024 |
Section 6: Diagnostic Tools, Assessments, & Imaging Studies
| Malaz Boustani, Indiana University-Purdue University Indianapolis | The Passive Digital Marker (PDM), a Machine Learning (ML) algorithm, and the Quick Dementia Rating Scale (QDRS), a patient reported outcome (PRO) tool, to detect cognitive impairment and improve the annual rate of new documented Alzheimer's disease and related dementias (AD/ADRD) diagnosis in primary care practices | Eligible primary care clinics within the UMiami Health system and adults with at least one visit to a primary care physician within the past year (Ages 65+) | 2025 | ||
| Malaz Boustani, Indiana University-Purdue University Indianapolis | The Passive Digital Marker (PDM), a Machine Learning (ML) algorithm, and the Quick Dementia Rating Scale (QDRS), a patient reported outcome (PRO) tool, to detect cognitive impairment and improve the annual rate of new documented Alzheimer's disease and related dementias (AD/ADRD) diagnosis in primary care practices | Eligible primary care clinics within the Eskenazi Health system and adults with at least one visit to a primary care physician within the past year (Ages 65+) | 2025 | ||
| Neal Swerdlow, University of California San Diego | Identify sensitivity levels of memantine, an NMDA receptor antagonist approved for treatment of moderate-to-severe Alzheimer's disease | Older adults with Alzheimer's disease | 2025 | ||
| TBD | Stella Sarraf, Amydis Diagnostics Inc. | Ophthalmic diagnostic probe to detect cerebral amyloid angiopathy (vascular disease) | Healthy adults and Cerebral Amyloid Angiopathy adult patients | 2024 | |
| Ben Zarzaur, University of Wisconsin - Madison | Emergency General Surgery Delirium Recovery Program, a care collaborative program to cognitive, functional and psychological recovery after episode(s) of delirium | Surgery patients who suffer at least one episode of delirium in the post-operative period (Ages 65+) | 2027 | ||
| Kristan Leech, University of Southern California | Biomechanical analyses, neuropsychological assessments, and brain imaging techniques to examine the structural integrity of the dorsolateral prefrontal cortex in explicit locomotor learning and inform the development of a gait rehabilitation intervention for older adults post-stroke | Older adults with post-stroke cognitive impairment | 2026 | ||
| Sascha Dublin, Kaiser Foundation Hospitals - Washington | EHR dementia screening tool to follow up with high risk patients with potential undiagnosed dementia | Older adults enrolled in Kaiser Washington or UCSF care within the past 2 years with no prior diagnosis of dementia (Ages 65+) | 2024 | ||
| Brianne Bettcher, University of Colorado Anschutz Medical Campus | Home-based video Tele-neuropsychological assessment (TeleNP) versus a face-to-face (FF) neuropsychological assessment in older adults with suspected Alzheimer's disease or related dementias for the delivery of neuropsychological evaluations | Care recipient (Ages 60 - 89) who are undergoing evaluation for possible Alzheimer's disease and whose severity ranges from mild cognitive impairment to mild dementia and their care partner | 2025 | ||
| Sascha Dublin, Kaiser Foundation Hospitals - Washington | Electronic health record (EHR) dementia screening tool to detect cognitive impairment | Older adults enrolled in Kaiser Washington or UCSF care within the past two years with no prior diagnosis of dementia (Ages 65+) | 2026 | ||
| Cindy Nowinski, Northwestern University | Cognitive assessment toolbox to detect cognitive decline in Medicare Annual Wellness visits. | Older Adults/Patients (Ages 65+) | 2025 | ||
| TBD | Sanjay Mohanty, Indiana University-Purdue University Indianapolis | Test a clinical decision support tool incorporating a Passive Digital Marker (PDM) that predicts postoperative delirium based on EHR data and machine learning techniques for feasibility, acceptability, and effect size | Patients scheduled for elective major abdominal surgery requiring inpatient stay of more than 24 hours (Ages 50+) | 2027 | |
|
| Maria Edelen, RAND Corporation | Electronic health record (EHR) dementia screening tool, implemented in clinical practice to increase early detection of cognitive impairment | Providers of patients (Ages 65+) | 2024 | |
| TBD | Darlene Floden, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University | Cognitive screening tools (EHR-based Cognitive Risk Calculator and iPad-based Brief Assessment of Cognitive Health) used in primary care settings to detect dementia | Providers caring for patients (Ages 60+) | 2025 | |
|
| Leah Hanson, HealthPartners Institute | Electronic health record (EHR) cognitive impairment clinical decision support tool to improve early detection and management of cognitive impairment | Patients with elevated dementia risk | 2025 | |
| David Wilson, University of California San Francisco | A novel PET imaging tracer, RP-115, to evaluate changes in astrocytes in healthy versus cognitively impaired Alzheimer's disease patients by quantitative PET imaging of the excitatory amino acid transporter 2 (EAAT2; an astrocytic glutamate transporter) | Individuals with Alzheimer's disease and healthy older adults | 2026 | ||
| Donna Fick, Pennsylvania State University | Daily nurse-led delirium screening using the Ultra-brief Confusion Assessment Method in routine care among all hospitalized older adults to inform best practices for delirium identification, including in vulnerable individuals such as those with Alzheimer’s disease and related dementias | Adults, including a subgroup of participants with Alzheimer’s disease and Alzheimer’s related dementia, with an expected hospital length of stay of 3 or more days (Ages 70+) | 2028 | ||
| Robin Hilsabeck, University of Texas at Austin | Cognitive screening tool (RACS app) for primary care providers to conduct cognitive screenings on a regular basis | Cognitively normal and cognitively impaired adults (Ages 60+) | 2026 | ||
| TBD | Donald Royall, University of Texas Health Science Center at San Antonio | Donepezil, a cholinesterase inhibitor, to measure the impact on the latent dementia severity metric, and Adipokines, inflammatory proteins, to test the effects of donepezil on the latent dementia severity metric | People with Alzheimer's disease or mild cognitive impairment (Ages 65 – 100) | 2028 | |
| TBD | Narges Razavian, New York University School of Medicine | Electronic health records/artificial intelligent predictive model used to improve Alzheimer’s disease and related dementias screening rates and early diagnosis and healthcare metrics related to cognitive health | Primary care providers and patients at high risk for mild cognitive impairment or Alzheimer’s disease and related dementias | 2028 |
Section 7: Treatments for Neuropsychiatric Symptoms
| Brent Forester, McLean Hospital | Electroconvulsive Therapy to treat severe agitation in moderate to severe stage Alzheimer's disease | People with moderate to severe stage Alzheimer's disease (Ages 55+) | 2024 | ||
| Mariana Figueiro, Icahn School of Medicine at Mount Sinai | Light treatment designed to promote better sleep, cognition, mood and behavior in people living with Alzheimer's disease or related dementias | Individuals with Alzheimer's disease or related dementias and sleep problems (Ages 65+) | 2025 | ||
| Dimitris Kiosses, Joan and Sanford I Weill Medical College of Cornell University | Problem Adaptation Therapy (PATH), a novel psychosocial intervention designed to reduce depression and disability in older adults with major depression | People with Mild Cognitive Impairment and depression (Ages 60+) | 2024 | ||
| Mark Eldaief, Massachusetts General Hospital | Repetitive Transcranial Magnetic Stimulation (rTMS) to modulate cortico-striatal circuits in neurodegenerative patients with apathy | Alzheimer's disease participants with apathy (Ages 50 - 80) | 2024 |
| Constantine Lyketsos, Johns Hopkins Bayview Medical Center | Escitalopram, a small molecule selective serotonin reuptake inhibitor (SSRIs) antidepressant to treat agitation in Alzheimer's disease | People with Alzheimer's disease and agitation | 2024 | ||
| Jacobo Mintzer, Medical University of South Carolina | Cannabidol and Tetrahydrocannabinol, Cannabis chemotypes, to improve quality of life in adults with Alzheimer's disease | Adults with Alzheimer's disease | 2024 | ||
| Paul Rosenberg; Brent Forester, Johns Hopkins University; McLean Hospital | Dronabinol, a synthetic form of THC and partial agonist of CB1/2 endocannabinoid receptors to treat severe agitation in Alzheimer's disease | People with Alzheimer's disease and agitation (Ages 60-90) | 2024 |
Subscribe to the NIA blog for weekly updates on funding policies and research priorities
nia.nih.gov
An official website of the National Institutes of Health
Increasing investment and innovation in dementia research is a key aim of ADI. Find out about the projects we are involved in.
One of the key strands of ADI’s mission is to increase investment and innovation in dementia research.
Facilitating research into treatments and hopefully a cure, plus research into improving care, underpins much of our advocacy work.
For over 10 years, ADI has tackled important dementia research issues through our World Alzheimer Reports , that provide the most comprehensive data on dementia worldwide, including prevalence and economic impact.
ADI is actively involved in a number of research projects:
- WW-FINGERS : ADI is a partner in this project which builds on the successful experience of the FINnish GERiatric Intervention Study to prevent Cognitive Impairment and Disability. The FINGER model was the first randomized controlled trial that demonstrated how to benefit cognition using a multi-domain lifestyle intervention among older at-risk individuals. The results highlighted the value of addressing multiple dementia risk factors as a strategy to protect brain health, and promote overall health and functioning. WW-FINGERS aims to test, adapt, and optimize the FINGER model in different settings, across populations from a variety of geographical and cultural backgrounds.
- STRiDE project (Strengthening responses to dementia in developing countries): In partnership with the London School of Economics and Political Science (LSE), ADI joined forces on the STRiDE project, whose aim is to build research capacity, develop research evidence into what interventions work most effectively, and to better understand the impact and cost of dementia. The project works across seven countries – Brazil, India, Indonesia, Jamaica, Kenya, Mexico and South Africa – and 12 work packages. The four-year project aims to develop evidence to support development of national dementia plans and improve quality of life for people affected by dementia.
- COGNISANCE Project : An EU Joint Programme for Neurodegenerative Disease Research (JPND). A 3-year project working in 5 countries, led by Henry Brodaty in Australia, with partners in Australia, Canada, Netherlands, UK, and Poland. ADI is an external collaborator. The project’s focus is to co-design dementia diagnosis and post diagnostic care; develop a tool-kit to be disseminated internationally; develop a set of standards to guide the diagnostic and post-diagnostic process.
- CST International : ADI sits on the Advisory Board of this UCL (University College London) project focusing on work in Brazil, India, and Tanzania. The project will take place over three-years in four phases. The project will develop, test, refine and disseminate implementation strategies for people living with dementia – looking to increase quality of life and cognition, and to increase awareness and skills in the detection and management of dementia.
- DISTINCT project : ADI is an external collaborator on this project of nine key academic partners. The aim is to develop a multi-disciplinary, multi-professional education and training research framework for Europe aimed at improving technology and care for people with dementia and their carers. ADI offers expertise in areas of community-based practice and national policies through participating in training and education of the early stage researchers (ESRs).
- INDUCT project : Interdisciplinary network for dementia using current technology). A partnership across University of Nottingham, UCL, Maastricht University, University of Amsterdam, Karolinska Institutet, Vrije Universiteit Brussel, Charles University, and IDES in Spain, INDUCT aims to develop a multi-disciplinary, inter-sectorial educational research framework for Europe to improve technology and care for people with dementia, and to provide the evidence to show how technology can improve the lives of people with dementia. ADI sits on the project’s supervisory board an provides training a yearly summer school focusing on turning research into policy. ADI also provided internship opportunities for two early career researchers.
- The 10/66 Dementia Research Group are researchers who are redressing the fact that, when the group was created, less than 10% of all population-based research into dementia had been directed towards the 66% of people with dementia who live in developing countries. The group looks at the numbers of people with dementia, care arrangements and support services in developing countries.
In addition to our project-specific work, ADI continues to support and promote research into treatments and ultimately a cure for dementia. Through our Medical and Scientific Advisory Panel (MSAP), we maintain our close connection to cutting-edge research on drug development and often represent the patient and carer voice on advisory panels for clinical trials.
ADI proposes that, nationally, 1% of the societal cost of dementia should be devoted to funding research in basic science, care improvements, prevention and risk reduction, drug development and public health. Without significant investments in these areas of dementia research we will be unable to venture into new frontiers.
More information about research can be found on the web sites of Alzheimer’s Association (USA) , Alzheimer’s Society (UK) , Alzheimer Society of Canada , or the association in your country .
Related content
Stride project website.
Project website for STRiDE (Strengthening responses to dementia in developing countries)
World Alzheimer Reports
The World Alzheimer Reports are a comprehensive source of global socioeconomic information on dementia. Each World Alzheimer Report is on a different topic, so the previous reports remain important sources of information on a range of topics of worldwide relevance.
- Investigates
- Houston Life
- Newsletters
WEATHER ALERT
10 warnings in effect for 11 counties in the area
Blood tests for alzheimer's may be coming to your doctor's office. here's what to know.
Lauran Neergaard
Associated Press
WASHINGTON – New blood tests could help doctors diagnose Alzheimer’s disease faster and more accurately, researchers reported Sunday – but some appear to work far better than others.
It’s tricky to tell if memory problems are caused by Alzheimer’s. That requires confirming one of the disease’s hallmark signs — buildup of a sticky protein called beta-amyloid — with a hard-to-get brain scan or uncomfortable spinal tap. Many patients instead are diagnosed based on symptoms and cognitive exams.
Recommended Videos
Labs have begun offering a variety of tests that can detect certain signs of Alzheimer's in blood. Scientists are excited by their potential but the tests aren't widely used yet because there's little data to guide doctors about which kind to order and when. The U.S. Food and Drug Administration hasn't formally approved any of them and there's little insurance coverage.
“What tests can we trust?” asked Dr. Suzanne Schindler, a neurologist at Washington University in St. Louis who’s part of a research project examining that. While some are very accurate, “other tests are not much better than a flip of a coin.”
Demand for earlier Alzheimer's diagnosis is increasing
More than 6 million people in the United States and millions more around the world have Alzheimer’s, the most common form of dementia. Its telltale “biomarkers” are brain-clogging amyloid plaques and abnormal tau protein that leads to neuron-killing tangles.
New drugs, Leqembi and Kisunla, can modestly slow worsening symptoms by removing gunky amyloid from the brain. But they only work in the earliest stages of Alzheimer’s and proving patients qualify in time can be difficult. Measuring amyloid in spinal fluid is invasive. A special PET scan to spot plaques is costly and getting an appointment can take months.
Even specialists can struggle to tell if Alzheimer’s or something else is to blame for a patient’s symptoms.
“I have patients not infrequently who I am convinced have Alzheimer’s disease and I do testing and it’s negative,” Schindler said.
New study suggests blood tests for Alzheimer’s can be simpler and faster
Blood tests so far have been used mostly in carefully controlled research settings. But a new study of about 1,200 patients in Sweden shows they also can work in the real-world bustle of doctors' offices — especially primary care doctors who see far more people with memory problems than specialists but have fewer tools to evaluate them.
In the study, patients who visited either a primary care doctor or a specialist for memory complaints got an initial diagnosis using traditional exams, gave blood for testing and were sent for a confirmatory spinal tap or brain scan.
Blood testing was far more accurate, Lund University researchers reported Sunday at the Alzheimer's Association International Conference in Philadelphia. The primary care doctors' initial diagnosis was 61% accurate and the specialists' 73% — but the blood test was 91% accurate, according to the findings, which also were published in the Journal of the American Medical Association.
Which blood tests for Alzheimer’s work best?
There’s almost “a wild West” in the variety being offered, said Dr. John Hsiao of the National Institute on Aging. They measure different biomarkers, in different ways.
Doctors and researchers should only use blood tests proven to have a greater than 90% accuracy rate, said Alzheimer’s Association chief science officer Maria Carrillo.
Today's tests most likely to meet that benchmark measure what’s called p-tau217, Carrillo and Hsiao agreed. Schindler helped lead an unusual direct comparison of several kinds of blood tests, funded by the Foundation for the National Institutes of Health, that came to the same conclusion.
That type of test measures a form of tau that correlates with how much plaque buildup someone has, Schindler explained. A high level signals a strong likelihood the person has Alzheimer’s while a low level indicates that’s probably not the cause of memory loss.
Several companies are developing p-tau217 tests including ALZpath Inc., Roche, Eli Lilly and C2N Diagnostics, which supplied the version used in the Swedish study.
Who should use blood tests for Alzheimer’s?
Only doctors can order them from labs. The Alzheimer’s Association is working on guidelines and several companies plan to seek FDA approval, which would clarify proper use.
For now, Carrillo said doctors should use blood testing only in people with memory problems, after checking the accuracy of the type they order.
Especially for primary care physicians, “it really has great potential to help them in sorting out who to give a reassuring message and who to send on to memory specialists,” said Dr. Sebastian Palmqvist of Lund University, who led the Swedish study with Lund’s Dr. Oskar Hansson.
The tests aren't yet for people who don't have symptoms but worry about Alzheimer's in the family — unless it's part of enrollment in research studies, Schindler stressed.
That's partly because amyloid buildup can begin two decades before the first sign of memory problems, and so far there are no preventive steps other than basic advice to eat healthy, exercise and get enough sleep. But there are studies underway testing possible therapies for people at high risk of Alzheimer's, and some include blood testing.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.
Copyright 2024 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed without permission.
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Sustainability
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Study across multiple brain regions discerns Alzheimer’s vulnerability and resilience factors
Press contact :.
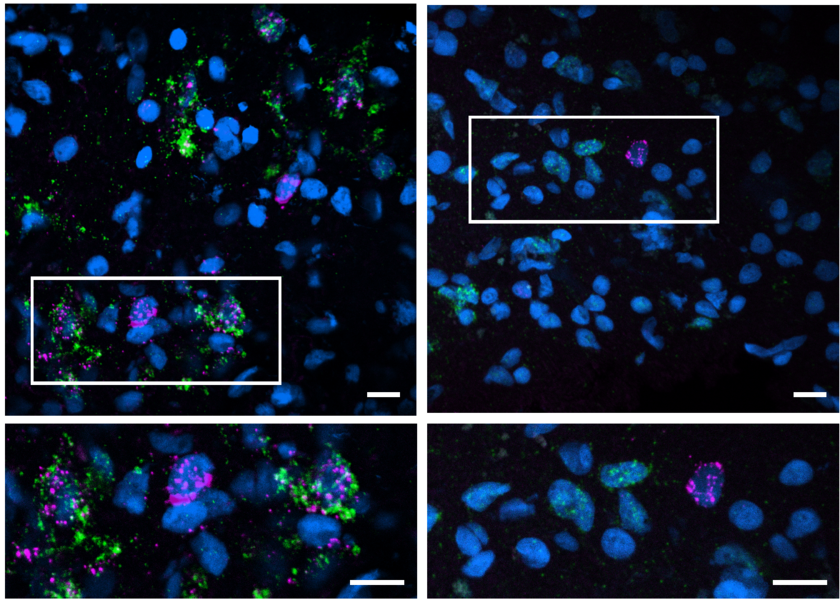
Previous image Next image
An open-access MIT study published today in Nature provides new evidence for how specific cells and circuits become vulnerable in Alzheimer’s disease, and hones in on other factors that may help some people show resilience to cognitive decline, even amid clear signs of disease pathology.
To highlight potential targets for interventions to sustain cognition and memory, the authors engaged in a novel comparison of gene expression across multiple brain regions in people with or without Alzheimer’s disease, and conducted lab experiments to test and validate their major findings.
Brain cells all have the same DNA but what makes them differ, both in their identity and their activity, are their patterns of how they express those genes. The new analysis measured gene expression differences in more than 1.3 million cells of more than 70 cell types in six brain regions from 48 tissue donors, 26 of whom died with an Alzheimer’s diagnosis and 22 of whom without. As such, the study provides a uniquely large, far-ranging, and yet detailed accounting of how brain cell activity differs amid Alzheimer’s disease by cell type, by brain region, by disease pathology, and by each person’s cognitive assessment while still alive.
“Specific brain regions are vulnerable in Alzheimer’s and there is an important need to understand how these regions or particular cell types are vulnerable,” says co-senior author Li-Huei Tsai , Picower Professor of Neuroscience and director of The Picower Institute for Learning and Memory and the Aging Brain Initiative at MIT. “And the brain is not just neurons. It’s many other cell types. How these cell types may respond differently, depending on where they are, is something fascinating we are only at the beginning of looking at.”
Co-senior author Manolis Kellis , professor of computer science and head of MIT’s Computational Biology Group, likens the technique used to measure gene expression comparisons, single-cell RNA profiling, to being a much more advanced “microscope” than the ones that first allowed Alois Alzheimer to characterize the disease’s pathology more than a century ago.
“Where Alzheimer saw amyloid protein plaques and phosphorylated tau tangles in his microscope, our single-cell ‘microscope’ tells us, cell by cell and gene by gene, about thousands of subtle yet important biological changes in response to pathology,” says Kellis. “Connecting this information with the cognitive state of patients reveals how cellular responses relate with cognitive loss or resilience, and can help propose new ways to treat cognitive loss. Pathology can precede cognitive symptoms by a decade or two before cognitive decline becomes diagnosed. If there’s not much we can do about the pathology at that stage, we can at least try to safeguard the cellular pathways that maintain cognitive function.”
Hansruedi Mathys, a former MIT postdoc in the Tsai Lab who is now an assistant professor at the University of Pittsburgh; Carles Boix PhD '22, a former graduate student in Kellis’s lab who is now a postdoc at Harvard Medical School; and Leyla Akay, a graduate student in Tsai’s lab, led the study analyzing the prefrontal cortex, entorhinal cortex, hippocampus, anterior thalamus, angular gyrus, and the midtemporal cortex. The brain samples came from the Religious Order Study and the Rush Memory and Aging Project at Rush University.
Neural vulnerability and Reelin
Some of the earliest signs of amyloid pathology and neuron loss in Alzheimer’s occur in memory-focused regions called the hippocampus and the entorhinal cortex. In those regions, and in other parts of the cerebral cortex, the researchers were able to pinpoint a potential reason why. One type of excitatory neuron in the hippocampus and four in the entorhinal cortex were significantly less abundant in people with Alzheimer’s than in people without. Individuals with depletion of those cells performed significantly worse on cognitive assessments. Moreover, many vulnerable neurons were interconnected in a common neuronal circuit. And just as importantly, several either directly expressed a protein called Reelin, or were directly affected by Reelin signaling. In all, therefore, the findings distinctly highlight especially vulnerable neurons, whose loss is associated with reduced cognition, that share a neuronal circuit and a molecular pathway.
Tsai notes that Reelin has become prominent in Alzheimer’s research because of a recent study of a man in Colombia. He had a rare mutation in the Reelin gene that caused the protein to be more active, and was able to stay cognitively healthy at an advanced age despite having a strong family predisposition to early-onset Alzheimer’s. The new study shows that loss of Reelin-producing neurons is associated with cognitive decline. Taken together, it might mean that the brain benefits from Reelin, but that neurons that produce it may be lost in at least some Alzheimer’s patients.
“We can think of Reelin as having maybe some kind of protective or beneficial effect,” Akay says. “But we don’t yet know what it does or how it could confer resilience.”
In further analysis the researchers also found that specifically vulnerable inhibitory neuron subtypes identified in a previously study from this group in the prefrontal cortex also were involved in Reelin signaling, further reinforcing the significance of the molecule and its signaling pathway.
To further check their results, the team directly examined the human brain tissue samples and the brains of two kinds of Alzheimer’s model mice. Sure enough, those experiments also showed a reduction in Reelin-positive neurons in the human and mouse entorhinal cortex.
Resilience associated with choline metabolism in astrocytes
To find factors that might preserve cognition, even amid pathology, the team examined which genes, in which cells, and in which regions, were most closely associated with cognitive resilience, which they defined as residual cognitive function, above the typical cognitive loss expected given the observed pathology.
Their analysis yielded a surprising and specific answer: across several brain regions, astrocytes that expressed genes associated with antioxidant activity and with choline metabolism and polyamine biosynthesis were significantly associated with sustained cognition, even amid high levels of tau and amyloid. The results reinforced previous research findings led by Tsai and Susan Lundqvist in which they showed that dietary supplement of choline helped astrocytes cope with the dysregulation of lipids caused by the most significant Alzheimer’s risk gene, the APOE4 variant. The antioxidant findings also pointed to a molecule that can be found as a dietary supplement, spermidine, which may have anti-inflammatory properties, although such an association would need further work to be established causally.
As before, the team went beyond the predictions from the single-cell RNA expression analysis to make direct observations in the brain tissue of samples. Those that came from cognitively resilient individuals indeed showed increased expression of several of the astrocyte-expressed genes predicted to be associated with cognitive resilience.
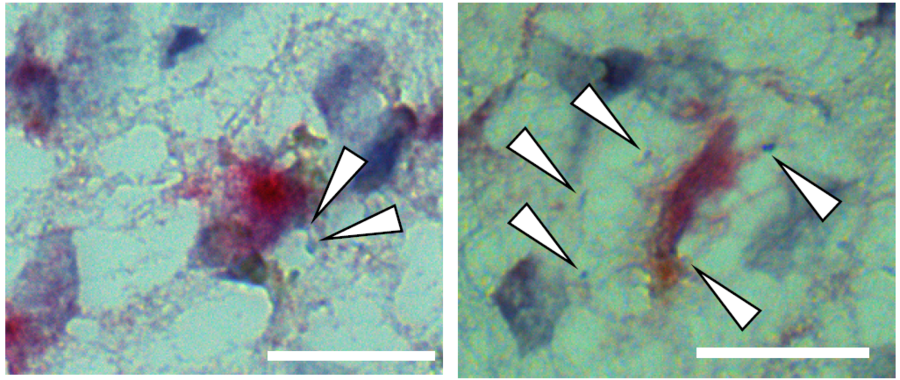
Previous item Next item
New analysis method, open dataset
To analyze the mountains of single-cell data, the researchers developed a new robust methodology based on groups of coordinately-expressed genes (known as “gene modules”), thus exploiting the expression correlation patterns between functionally-related genes in the same module.
“In principle, the 1.3 million cells we surveyed could use their 20,000 genes in an astronomical number of different combinations,” explains Kellis. “In practice, however, we observe a much smaller subset of coordinated changes. Recognizing these coordinated patterns allow us to infer much more robust changes, because they are based on multiple genes in the same functionally-connected module.”
He offered this analogy: With many joints in their bodies, people could move in all kinds of crazy ways, but in practice they engage in many fewer coordinated movements like walking, running, or dancing. The new method enables scientists to identify such coordinated gene expression programs as a group.
While Kellis and Tsai’s labs already reported several noteworthy findings from the dataset, the researchers expect that many more possibly significant discoveries still wait to be found in the trove of data. To facilitate such discovery the team posted handy analytical and visualization tools along with the data on Kellis’s website .
“The dataset is so immensely rich. We focused on only a few aspects that are salient that we believe are very, very interesting, but by no means have we exhausted what can be learned with this dataset,” Kellis says. “We expect many more discoveries ahead, and we hope that young researchers (of all ages) will dive right in and surprise us with many more insights.”
Going forward, Kellis says, the researchers are studying the control circuitry associated with the differentially expressed genes, to understand the genetic variants, the regulators, and other driver factors that can be modulated to reverse disease circuitry across brain regions, cell types, and different stages of the disease.
Additional authors of the study include Ziting Xia, Jose Davila Velderrain, Ayesha P. Ng, Xueqiao Jiang, Ghada Abdelhady, Kyriaki Galani, Julio Mantero, Neil Band, Benjamin T. James, Sudhagar Babu, Fabiola Galiana-Melendez, Kate Louderback, Dmitry Prokopenko, Rudolph E. Tanzi, and David A. Bennett.
Support for the research came from the National Institutes of Health, The Picower Institute for Learning and Memory, The JPB Foundation, the Cure Alzheimer’s Fund, The Robert A. and Renee E. Belfer Family Foundation, Eduardo Eurnekian, and Joseph DiSabato.
Share this news article on:
Press mentions.
Prof. Li-Huei Tsai, director of the Picower Institute, speaks with NPR host Jon Hamilton about her work identifying a protein called reelin that appears to protect brain cells from Alzheimer's. “Tsai says she and her team are now using artificial intelligence to help find a drug that can replicate what reelin does naturally,” says Hamilton.
Related Links
- Manolis Kellis
- Li-Huei Tsai
- Aging Brain Initiative
- The Picower Institute for Learning and Memory
- Department of Brain and Cognitive Sciences
- Computer Science and Artificial Intelligence Laboratory (CSAIL)
Related Topics
- Alzheimer's
- Brain and cognitive sciences
- Neuroscience
- Computational biology
- Electrical Engineering & Computer Science (eecs)
- Picower Institute
- National Institutes of Health (NIH)
Related Articles
Decoding the complexity of Alzheimer’s disease
Atlas of human brain blood vessels highlights changes in Alzheimer’s disease
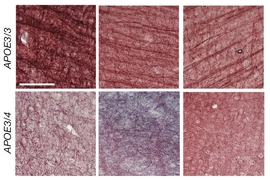
Alzheimer’s risk gene undermines insulation of brain’s “wiring”
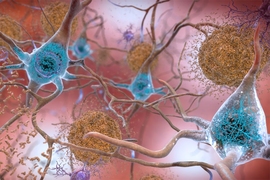
A comprehensive map of how Alzheimer’s affects the brain

Epigenomics of Alzheimer’s disease progression
More mit news.

Edgerton Center hosts workshop for deaf high school students in STEM
Read full story →

MIT Global SCALE Network expands by adding center at Loughborough University
New transistor’s superlative properties could have broad electronics applications

When learning at MIT means studying thousands of miles away

Flying high to enable sustainable delivery, remote care

Professor Emeritus Ralph Gakenheimer, mobility planner and champion of international development, dies at 89
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
July 24, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New analysis offers most comprehensive roadmap to date for more targeted Alzheimer's research, drug discovery
by Jackson Laboratory
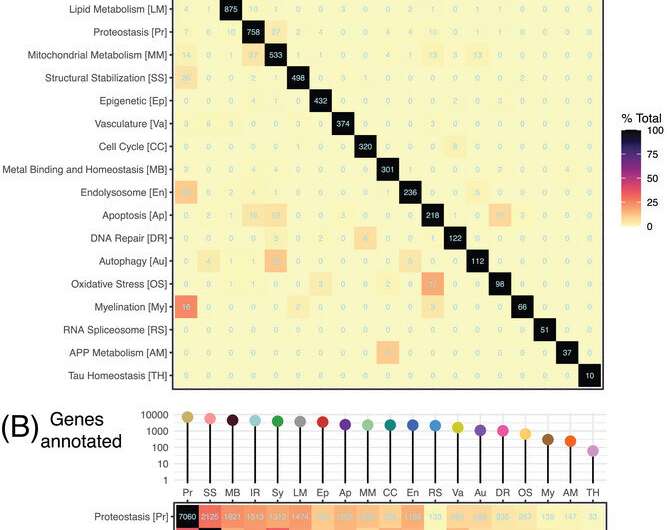
From studying the human genome to analyzing the way proteins are encoded, or monitoring RNA expression, researchers are rapidly gaining a far richer understanding of the complex genetic and cellular mechanisms that underpin dementia. But there's a catch: While new technologies are revealing myriad avenues for Alzheimer's research, it's impossible to know in advance which research pathways will lead to effective treatments.
"We have countless potential targets, but we don't know which ones to aim at," said Greg Carter, the Bernard and Lusia Milch Endowed Chair at the Jackson Laboratory (JAX), who led the study. "Drug development is slow and costly, so to make use of these new insights, we need a way to prioritize them effectively."
Now, Carter and his colleagues at JAX—in collaboration with partners from Stanford University School of Medicine, Emory University, and Sage Bionetworks—are doing just that, offering the first comprehensive ranking of the relative role and significance of every gene and protein in the disease's development.
The research is reported in Alzheimer's & Dementia , in advance of the Alzheimer's Association International Conference on July 28, where the work will be presented.
"This is the most comprehensive study to date of Alzheimer's patients' brains," Carter said. "We're integrating research from multiple fields, including genetics and -omics, across the patient's lifespan, and at a much larger scale than has previously been possible."
The team used machine learning to draw together and overlay findings from more than two dozen large-scale genetic studies , along with multi-omic analyses of almost 2,900 brains, to identify thousands of potential targets for therapeutic interventions. The targets were then sorted into 19 separate "biodomains" reflecting biological mechanisms believed to contribute to Alzheimer's disease.
Carter and his colleagues didn't just want a long list of undifferentiated gene and protein targets. Instead, each target is associated with a specific therapeutic hypothesis—making it easier to understand how it works, and to identify candidates for experimental validation.
The team was also able to flag targets likely to play a role in the early stages of Alzheimer's, supporting the development of new diagnostic and therapeutic tools for pre-symptomatic interventions.
"This is incredibly important, but also very challenging: most of our data come from post-mortem brains, so our job was like trying to deduce where a forest fire began after everything has been incinerated," said Carter. "Our computer modeling effectively rewinds the progression of the disease to identify early markers that correspond to late-stage pathology."
That approach is already generating important insights, including new evidence that mitochondria—the powerhouse of the cell—could play a significant role in the early stages of Alzheimer's disease. The team found a number of promising targets in this biodomain, suggesting that mitochondrial function could be a very strong early indicator of Alzheimer's—and a key driver of the disease's progression.
The findings and their full dataset are being made publicly available through the Emory-Sage-Structural Genomics Consortium-JAX TREAT-AD Center, part of a consortium dedicated to de-risking Alzheimer's research, giving researchers and biotech innovators a foundational tool to support smarter and more targeted future research.
"We're taking an aggressively open approach," said Carter. "If any biotech or pharmaceutical company wants to pick this up and run with it, they can—and we hope they will."
Explore further
Feedback to editors

Short-term vegan diet associated with reductions in biological age estimates
4 hours ago

Study finds big disparities in stroke services across the US
Jul 27, 2024

Study identifies biomarker that could predict whether colon cancer patients benefit from chemotherapy

New study shows 'dancing molecules' can regenerate cartilage in 3 days
Jul 26, 2024

Study uncovers key immune cells for combating aggressive Merkel cell carcinoma

Study finds unhealthy air quality from wildfires may impact fertility treatments

Higher CEO pay in large health care systems linked to hospital consolidations, study suggests

Researchers discover potential therapeutic target for degenerative eye disease

Researchers move a step closer to developing at-home test to detect dementia

Double mastectomy may offer no survival benefit to women with breast cancer
Related stories.

Scientists are closing in on a mouse model for late-onset Alzheimer's disease
Jul 24, 2024

Alzheimer's Association publishes final version of its new diagnostic criteria for the disease
Jul 3, 2024

Multi-omics analysis identifies molecularly defined Alzheimer's disease subtypes
Jun 12, 2024

Consistent gene changes in Alzheimer's disease across studies
Nov 25, 2019

Team presents new approach to discover targets for Alzheimer's, other diseases with protein phase separation
Sep 25, 2023

Researchers discover key role of DNA methylation in Alzheimer's disease
Feb 22, 2023
Recommended for you

European medicines watchdog rejects new Alzheimer's drug

New shingles vaccine could reduce risk of dementia
Jul 25, 2024

Cell aging discovery could help detect early warning signs for neurodegenerative diseases

Large study shows early-onset dementia more common than previously reported
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Targeted Alzheimer's research and drug discovery
Researchers at The Jackson Laboratory offer the first comprehensive ranking of the relative role and significance of every known gene and protein in the development of Alzheimer's Disease in advance of the Alzheimer's Association International Conference, July 28.
From studying the human genome, to analyzing the way proteins are encoded, or monitoring RNA expression, researchers are rapidly gaining a far richer understanding of the complex genetic and cellular mechanisms that underpin dementia. But there's a catch: While new technologies are revealing myriad avenues for Alzheimer's research, it's impossible to know in advance which research pathways will lead to effective treatments.
"We have countless potential targets, but we don't know which ones to aim at," said Greg Carter, the Bernard and Lusia Milch Endowed Chair at the Jackson Laboratory (JAX), who led the study. "Drug development is slow and costly, so to make use of these new insights, we need a way to prioritize them effectively."
Now, Carter and his colleagues at JAX -- in collaboration with partners from Stanford University School of Medicine, Emory University, and Sage Bionetworks -- are doing just that, offering the first comprehensive ranking of the relative role and significance of every gene and protein in the disease's development. The work is reported in the July issue of Alzheimer's & Dementia in advance of the Alzheimer's Association International Conference on July 28, where the work will be presented.
"This is the most comprehensive study to date of Alzheimer's patients' brains," Carter said. "We're integrating research from multiple fields, including genetics and -omics, across the patient's lifespan, and at a much larger scale than has previously been possible."
The team used machine learning to draw together and overlay findings from more than two dozen large-scale genetic studies, along with multi-omic analyses of almost 2,900 brains, to identify thousands of potential targets for therapeutic interventions. The targets were then sorted into 19 separate "biodomains" reflecting biological mechanisms believed to contribute to Alzheimer's disease.
Carter and his colleagues didn't just want a long list of undifferentiated gene and protein targets. Instead, each target is associated with a specific therapeutic hypothesis -- making it easier to understand how it works, and to identify candidates for experimental validation.
The team was also able to flag targets likely to play a role in the early stages of Alzheimer's, supporting the development of new diagnostic and therapeutic tools for pre-symptomatic interventions. "This is incredibly important, but also very challenging: most of our data come from post-mortem brains, so our job was like trying to deduce where a forest fire began after everything has been incinerated," said Carter. "Our computer modeling effectively rewinds the progression of the disease to identify early markers that correspond to late-stage pathology."
That approach is already generating important insights, including new evidence that mitochondria -- the powerhouse of the cell -- could play a significant role in the early stages of Alzheimer's disease. The team found a number of promising targets in this biodomain, suggesting that mitochondrial function could be a very strong early indicator of Alzheimer's -- and a key driver of the disease's progression.
The findings and their full dataset are being made publicly available through the Emory-Sage-Structural Genomics Consortium-JAX TREAT-AD Center, part of an NIH-funded consortium dedicated to de-risking Alzheimer's research, giving researchers and biotech innovators a foundational tool to support smarter and more targeted future research. "We're taking an aggressively open approach," said Carter. "If any biotech or pharmaceutical company wants to pick this up and run with it, they can -- and we hope they will."
- Alzheimer's Research
- Healthy Aging
- Diseases and Conditions
- Alzheimer's
- Huntington's Disease
- Alzheimer's disease
- Dementia with Lewy bodies
- Bioinformatics
- Gene therapy
- Huntington's disease
- Vector (biology)
Story Source:
Materials provided by Jackson Laboratory . Note: Content may be edited for style and length.
Journal Reference :
- Gregory A. Cary, Jesse C. Wiley, Jake Gockley, Stephen Keegan, Sai Sruthi Amirtha Ganesh, Laura Heath, Robert R. Butler, Lara M. Mangravite, Benjamin A. Logsdon, Frank M. Longo, Allan Levey, Anna K. Greenwood, Gregory W. Carter. Genetic and multi‐omic risk assessment of Alzheimer's disease implicates core associated biological domains . Alzheimer's & Dementia: Translational Research & Clinical Interventions , 2024; 10 (2) DOI: 10.1002/trc2.12461
Cite This Page :
Explore More
- 'Dancing Molecules' Heal Cartilage Damage
- Robotics and How Flies Assess Winds
- Lampreys: 'Jaw-Dropping' Evolutionary Origin
- Making AI 1,000 Times More Energy Efficient
- Controlling Mosquitoes Through Genetic Breeding
- Hidden Elements from Ancient Alchemy Lab
- Drug Shows Promise in Clearing HIV from Brain
- Nitrogen Emissions Have a Net Cooling Effect
- New Zealand's Flightless Birds Retreating
- Ice Sheets Melt Fast, Regrow Slowly: Sponginess
Trending Topics
Strange & offbeat.
- Meet the Acting Dean
- Dean's Executive Team
- Sustainability Council
- Vision, Mission, and Goals
- College Org. Chart
- Facts and Figures
- Faculty Honors and Awards
- Science Rankings
- Undergraduate Enrollment
- Climate and Diversity
- Diversity in STEM
- Office of Diversity and Inclusion
- College Guide program
- Rainbow Science Network
- Black in STEM
- Location and Maps
- Instruction and Curricula
- Staff Resources
- Frontiers of Science
- Science Journal
- Program Directory
- Department of Astronomy and Astrophysics
- Department of Biochemistry and Molecular Biology
- Department of Biology
- Department of Chemistry
- Department of Mathematics
- Department of Physics
- Department of Statistics
- Integrative Science major
- Science BS/MBA
- Premedical/Medical (BS/MD)
- Premedicine Major
- Office for Undergraduate Students
- First Year Living Options
- Scholarships
- Admitted Undergraduates
- Schedule a Visit
- Graduate Students
- Advising and Student Services
- Career Development
- Collaborative Learning Center
- Education Abroad
- Financial Aid and Scholarships
- Graduation and Commencement
- Information for Parents
- Student Organizations in Science
- Transfer or Change of Campus Students
- Undergraduate Research
- Commencement
- Interdisciplinary Research Centers
- Office for Innovation
- Funding for Research
- Information for Researchers
- Broader Impacts Resource Center
- Corporate Partners Program
- Empower community growth
- Undergraduate educational experience

Potential new target for early treatment of Alzheimer's disease
A class of proteins that regulates cell repair and enhances cell growth-signaling systems could be a promising new target for the treatment of Alzheimer's and other neurodegenerative diseases, according to a new study led by researchers at Penn State. They found that disrupting necessary sugar modifications of these proteins promotes cell repair and reverses cellular abnormalities that occur in neurodegenerative diseases.
The study appeared today (July 2) in the journal iScience , and the researchers have a patent related to this work .
“Strategies to treat Alzheimer's disease to date have largely focused on pathological changes prominent in the late stages of the disease,” said Scott Selleck, professor of biochemistry and molecular biology in the Penn State Eberly College of Science and leader of the research team. “Although recently [U.S. Food and Drug Administration]-approved drugs have shown the ability to modestly slow the disease by targeting one of these changes, amyloid accumulation, drugs that affect the earliest cellular deficits might provide important tools to stop or reverse the disease process. We are interested in understanding the earliest cellular changes that are found not only in Alzheimer's, but shared across other neurodegenerative diseases, including Parkinson's and amyotrophic lateral sclerosis (ALS).”
Roughly 6.9 million Americans over the age of 65 are estimated to be living with Alzheimer’s disease, according to the Alzheimer’s Association . Despite its widespread impact, there is no agreed upon biological cause or mechanism for the disease. Cell-signaling molecules called heparan sulfate–modified proteins have been implicated in the development of Alzheimer’s, but their specific role has remained unclear, Selleck said. In this study, the research team first performed a series of analyses in human cell lines and mouse brain cells that express aspects of Alzheimer’s, showing that these proteins regulate cellular processes known to be affected in several neurodegenerative diseases.
Heparan sulfate–modified proteins are found both on the surface of animal cells and in the matrix between cells. This class of proteins are named for a sugar polymer that bears many sulfate groups, called heparan sulfate. Heparan sulfate chains are attached to specific proteins, and this modification allows these proteins to assemble signaling complexes that affect cell growth and influence how the cell interacts with its environment. These signaling pathways also regulate autophagy, a process of cell repair that clears out damaged or dysfunctional components in the cell.

“In the early stages of several neurodegenerative diseases, autophagy is compromised, which means cells have a reduced repair capacity,” Selleck said. “In this study, we determined that heparan sulfate-modified proteins suppress autophagy-dependent cell repair. What’s more, we show that by compromising the structure and function of the sugar modifications of these proteins, the levels of autophagy increase so cells can take care of damage.”
The researchers found that, in human and mouse cells, reducing the function of heparan sulfate-modified proteins also rescued other pathologies that arise early in neurodegenerative diseases, improving the function of mitochondria — which are responsible for energy production in the cell — and reducing build-up of lipids, or fatty compounds, inside cells.
The researchers then evaluated the role of heparan sulfate-modified proteins in an animal model of Alzheimer's, a fruit fly with deficits in a presenilin protein. Presenilin mutations cause early onset disease in humans and likewise in fruit flies; defective presenilin causes cell death and brain degeneration. In flies with deficits in presenilin, reducing the function of heparan sulfate chains suppressed the death of neurons and corrected other cell defects as well. These results are directly relevant to recent human genetics research, the researchers said.
“Individuals with mutations in a presenilin gene, PSEN1, develop Alzheimer's in their mid-40s. But if they also inherit a rare genetic change in a specific protein called APOE , the disease is delayed, sometimes by decades ,” Selleck said, explaining that APOE plays an important role in lipid transport and binds to heparan sulfate. “This change in APOE — which has been in the news lately — greatly reduces APOE binding to heparan sulfate. Our work builds on and extends these findings, directly implicating heparan sulfate in Alzheimer’s pathology involving both PSEN1 and APOE. Targeting the enzymes that make heparan sulfate could provide a means of blocking neurodegeneration in humans.”
Collectively, these results show that disrupting the structure of heparan sulfate modifications, blocks or reverses early cellular problems in these models of Alzheimer's.
“We save the animal from neuron cell loss, mitochondrial defects and rescue behavior deficits that serve as a measure of nervous system function,” Selleck said. “These findings suggest a promising target for future treatments that could rescue the earliest abnormalities that occur in many neurodegenerative diseases.”

The researchers also explored how gene expression changed when they eliminated the capacity of human cells to make heparan sulfate chains. They found that expression levels of more than 50% of approximately 70 genes known to be associated with late-onset Alzheimer's disease were modulated, including APOE, suggesting a link between heparan sulfate-modified proteins and the more common and late onset forms of Alzheimer’s disease.
“There is a critical need to focus on cellular changes that occur at the earliest times in disease progression and develop treatments that block or reverse them,” Selleck said. “We demonstrate that reduced autophagy, mitochondrial defects and lipid build-up — all common changes in neurodegenerative disease — can be blocked by altering one class of proteins, those with heparan sulfate modifications. We think these molecules are promising targets for drug development.”
The researchers suspect that disrupting this pathway to promote cell repair systems could be important for a wide variety of other diseases where autophagy defects occur.
“The applications of manipulating this pathway may be broadly useful across a number of human medical conditions,” Selleck said.

The research team at Penn State also includes co-authors Nicholas Schultheis, doctoral student in the Biochemistry, Microbiology and Molecular Biology program; Alyssa Connell, research assistant; and Richard Mueller, Alexander Kapral, Robert Becker, Shalini Shah, Mackenzie O’Donnell, Matthew Roseman, Lindsey Swanson and Sophia DeGuara, all undergraduate students. Contributions were also made by researcher Weihua Wang and Associate Professor of Pharmacology Fei Yin at the University of Arizona and graduate student Tripti Saini and Assistant Professor of Biochemistry and Molecular Biology Ryan Weiss at the University of Georgia.
Funding from the National Institutes of Health and the Penn State Eberly College of Science supported this research.
Call our 24 hours, seven days a week helpline at 800.272.3900

- Professionals

- Younger/Early-Onset Alzheimer's
- Is Alzheimer's Genetic?
- Women and Alzheimer's
- Creutzfeldt-Jakob Disease
- Dementia with Lewy Bodies
- Down Syndrome & Alzheimer's
- Frontotemporal Dementia
- Huntington's Disease
- Mixed Dementia
- Normal Pressure Hydrocephalus
- Posterior Cortical Atrophy
- Parkinson's Disease Dementia
- Vascular Dementia
- Korsakoff Syndrome
- Traumatic Brain Injury (TBI)
- Know the 10 Signs
- Difference Between Alzheimer's & Dementia
- 10 Steps to Approach Memory Concerns in Others
- Medical Tests for Diagnosing Alzheimer's
- Why Get Checked?
- Visiting Your Doctor
- Life After Diagnosis
- Stages of Alzheimer's
- Earlier Diagnosis
- Part the Cloud
- Research Momentum
- Our Commitment to Research
- TrialMatch: Find a Clinical Trial
- What Are Clinical Trials?
- How Clinical Trials Work
- When Clinical Trials End
- Why Participate?
- Talk to Your Doctor
- Clinical Trials: Myths vs. Facts
- Can Alzheimer's Disease Be Prevented?
- Brain Donation
- Navigating Treatment Options
- Aducanumab Discontinued as Alzheimer's Treatment
- Donanemab Approved for Treatment of Early Alzheimer's Disease
- Lecanemab Approved for Treatment of Early Alzheimer's Disease
- Medicare Treatment Coverage
- Questions for Your Doctor
- Medications for Memory, Cognition and Dementia-Related Behaviors
- Treatments for Behavior
- Treatments for Sleep Changes
- Alternative Treatments
- Facts and Figures
- Assessing Symptoms and Seeking Help
- Now is the Best Time to Talk about Alzheimer's Together
- Get Educated
- Just Diagnosed
- Sharing Your Diagnosis
- Changes in Relationships
- If You Live Alone
- Treatments and Research
- Legal Planning
- Financial Planning
- Building a Care Team
- End-of-Life Planning
- Programs and Support
- Overcoming Stigma
- Younger-Onset Alzheimer's
- Taking Care of Yourself
- Reducing Stress
- Tips for Daily Life
- Helping Family and Friends
- Leaving Your Legacy
- Live Well Online Resources
- Make a Difference
- Daily Care Plan
- Communication and Alzheimer's
- Food and Eating
- Art and Music
- Incontinence
- Dressing and Grooming
- Dental Care
- Working With the Doctor
- Medication Safety
- Accepting the Diagnosis
- Early-Stage Caregiving
- Middle-Stage Caregiving
- Late-Stage Caregiving
- Aggression and Anger
- Anxiety and Agitation
- Hallucinations
- Memory Loss and Confusion
- Sleep Issues and Sundowning
- Suspicions and Delusions
- In-Home Care
- Adult Day Centers
- Long-Term Care
- Respite Care
- Hospice Care
- Choosing Care Providers
- Finding a Memory Care-Certified Nursing Home or Assisted Living Community
- Changing Care Providers
- Working with Care Providers
- Creating Your Care Team
- Long-Distance Caregiving
- Community Resource Finder
- Be a Healthy Caregiver
- Caregiver Stress
- Caregiver Stress Check
- Caregiver Depression
- Changes to Your Relationship
- Grief and Loss as Alzheimer's Progresses
- Home Safety
- Dementia and Driving
- Technology 101
- Preparing for Emergencies
- Managing Money Online Program
- Planning for Care Costs
- Paying for Care
- Health Care Appeals for People with Alzheimer's and Other Dementias
- Social Security Disability
- Medicare Part D Benefits
- Tax Deductions and Credits
- Planning Ahead for Legal Matters
- Legal Documents
- ALZ Talks Virtual Events
- ALZNavigator™
- Veterans and Dementia
- The Knight Family Dementia Care Coordination Initiative
- Online Tools
- Asian Americans and Pacific Islanders and Alzheimer's
- Native Americans and Alzheimer's
- Black Americans and Alzheimer's
- Hispanic Americans and Alzheimer's
- LGBTQ+ Community Resources for Dementia
- Educational Programs and Dementia Care Resources
- Brain Facts
- 50 Activities
- For Parents and Teachers
- Resolving Family Conflicts
- Holiday Gift Guide for Caregivers and People Living with Dementia
- Trajectory Report
- Resource Lists
- Search Databases
- Publications
- Favorite Links
- 10 Healthy Habits for Your Brain
- Stay Physically Active
- Adopt a Healthy Diet
- Stay Mentally and Socially Active
- Online Community
- Support Groups
- Find Your Local Chapter
- Any Given Moment
- New IDEAS Study
- Bruce T. Lamb, Ph.D., Chair
- Christopher van Dyck, M.D.
- Cynthia Lemere, Ph.D.
- David Knopman, M.D.
- Lee A. Jennings, M.D. MSHS
- Karen Bell, M.D.
- Lea Grinberg, M.D., Ph.D.
- Malú Tansey, Ph.D.
- Mary Sano, Ph.D.
- Oscar Lopez, M.D.
- Suzanne Craft, Ph.D.
- RFI Amyloid PET Depletion Following Treatment
- Criteria for Diagnosis and Staging
- About Our Grants
- Andrew Kiselica, Ph.D., ABPP-CN
- Arjun Masurkar, M.D., Ph.D.
- Benjamin Combs, Ph.D.
- Charles DeCarli, M.D.
- Damian Holsinger, Ph.D.
- David Soleimani-Meigooni, Ph.D.
- Donna M. Wilcock, Ph.D.
- Elizabeth Head, M.A, Ph.D.
- Fan Fan, M.D.
- Fayron Epps, Ph.D., R.N.
- Ganesh Babulal, Ph.D., OTD
- Hui Zheng, Ph.D.
- Jason D. Flatt, Ph.D., MPH
- Jennifer Manly, Ph.D.
- Joanna Jankowsky, Ph.D.
- Luis Medina, Ph.D.
- Marcello D’Amelio, Ph.D.
- Marcia N. Gordon, Ph.D.
- Margaret Pericak-Vance, Ph.D.
- María Llorens-Martín, Ph.D.
- Nancy Hodgson, Ph.D.
- Shana D. Stites, Psy.D., M.A., M.S.
- Walter Swardfager, Ph.D.
- ALZ WW-FNFP Grant
- Capacity Building in International Dementia Research Program
- ISTAART IGPCC
- Alzheimer’s Disease Strategic Fund: Endolysosomal Activity in Alzheimer’s (E2A) Grant Program
- Imaging Research in Alzheimer’s and Other Neurodegenerative Diseases
- Zenith Fellow Awards
- 2024 Part the Cloud Translational (PTC) Gene Targeting Challenge
- National Academy of Neuropsychology & Alzheimer’s Association Funding Opportunity
- Part the Cloud-Gates Partnership Grant Program: Bioenergetics and Inflammation
- Pilot Awards for Global Brain Health Leaders (Invitation Only)
- Robert W. Katzman, M.D., Clinical Research Training Scholarship
- Funded Studies
- How to Apply
- Portfolio Summaries
- Supporting Research in Health Disparities, Policy and Ethics in Alzheimer’s Disease and Dementia Research (HPE-ADRD)
- Annual Conference: AAIC
- Alzheimer's & Dementia
- Alzheimer's & Dementia: DADM
- Alzheimer's & Dementia: TRCI
- Professional Society: ISTAART
- International Network to Study SARS-CoV-2 Impact on Behavior and Cognition
- Alzheimer’s Association Business Consortium (AABC)
- Global Biomarker Standardization Consortium (GBSC)
- Global Alzheimer’s Association Interactive Network
- International Alzheimer's Disease Research Portfolio
- Alzheimer’s Disease Neuroimaging Initiative Private Partner Scientific Board (ADNI-PPSB)
- Research Roundtable
- About WW-ADNI
- North American ADNI
- European ADNI
- Australia ADNI
- Taiwan ADNI
- Argentina ADNI
- WW-ADNI Meetings
- Submit Study
- RFI Inclusive Language Guide
- Scientific Conferences
- AUC for Amyloid and Tau PET Imaging
- Make a Donation
- Walk to End Alzheimer's
- The Longest Day
- RivALZ to End ALZ
- Ride to End ALZ
- Donate Gold & Sterling Silver
- Tribute Pages
- Gift Options to Meet Your Goals
- Founders Society
- Fred Bernhardt
- Anjanette Kichline
- Lori A. Jacobson
- Pam and Bill Russell
- Gina Adelman
- Franz and Christa Boetsch
- Adrienne Edelstein
- For Professional Advisors
- Free Planning Guides
- Contact the Planned Giving Staff
- Workplace Giving
- Do Good to End ALZ
- Donate a Vehicle
- Donate Stock
- Donate Cryptocurrency
- Donor-Advised Funds
- Use of Funds
- Giving Societies
- Why We Advocate
- Ambassador Program
- About the Alzheimer’s Impact Movement
- Research Funding
- Improving Care
- Support for People Living With Dementia
- Public Policy Victories
- Planned Giving
- Community Educator
- Community Representative
- Support Group Facilitator or Mentor
- Faith Outreach Representative
- Early Stage Social Engagement Leaders
- Data Entry Volunteer
- Tech Support Volunteer
- Other Local Opportunities
- Visit the Program Volunteer Community to Learn More
- Become a Corporate Partner
- A Family Affair
- A Message from Elizabeth
- The Belin Family
- The Eliashar Family
- The Fremont Family
- The Freund Family
- Jeff and Randi Gillman
- Harold Matzner
- The Mendelson Family
- Patty and Arthur Newman
- The Ozer Family
- Salon Series
- No Shave November
- Other Philanthropic Activities
- Still Alice
- The Judy Fund E-blast Archive
- The Judy Fund in the News
- The Judy Fund Newsletter Archives
- Sigma Kappa Foundation
- Alpha Delta Kappa
- Parrot Heads in Paradise
- Tau Kappa Epsilon (TKE)
- Sigma Alpha Mu
- Alois Society Member Levels and Benefits
- Alois Society Member Resources
- Zenith Society
- Founder's Society
- Joel Berman
- JR and Emily Paterakis
- Legal Industry Leadership Council
- Accounting Industry Leadership Council

Find Local Resources
Let us connect you to professionals and support options near you. Please select an option below:
Use Current Location Use Map Selector
Search Alzheimer’s Association

There's No Better Time to Support the Fight Against Alzheimer’s
Alzheimer's disease was first described in 1906. Since then, scientists have made remarkable strides in understanding how Alzheimer's affects the brain and learning how to make life better for affected individuals and families. Below are some important milestones in our progress, including the founding of the Alzheimer's Association in 1980, which has played a key role in advancing research and raising awareness of the disease.
1906-1960: First discovery
1970-1979: modern research, 1980-1989: awareness and momentum, 1990-1999: treatments emerge, 2000-2009: progress and hope, 2010-2019: setting a national agenda, 2020-present: a new era of treatment, dr. alois alzheimer first describes "a peculiar disease".
German physician Alois Alzheimer, a pioneer in linking symptoms to microscopic brain changes, describes the haunting case of Auguste D., a patient who had profound memory loss, unfounded suspicions about her family, and other worsening psychological changes. In her brain at autopsy, he saw dramatic shrinkage and abnormal deposits in and around nerve cells. Dr. Alzheimer died in 1915, never suspecting that his encounter with Auguste D. would one day touch the lives of millions and ignite a massive international research effort. Scientists recognize Dr. Alzheimer not only for his groundbreaking characterization of a major disease but also as a role model. He set a new standard for understanding neurodegenerative disorders by establishing a close clinical relationship with his patients and using new scientific tools to determine how symptoms related to physical brain changes.
Alzheimer's disease named
Emil Kraepelin, a German psychiatrist who worked with Dr. Alzheimer, first names "Alzheimer's Disease" in the eighth edition of his book "Psychiatrie."
Invention of electron microscope allows further study of brain
In 1931, Germans Max Knoll and Ernst Ruska co-invent the electron microscope, which can magnify up to 1 million times. It is not until after WWII that the electron microscope becomes common in major research settings, enabling scientists to study brain cells in more detail.
Development of cognitive measurement scales
Researchers develop the first validated measurement scale for assessing cognitive and functional decline in older adults, paving the way to correlate the level of measured impairment with estimates of the number of brain lesions and the volume of damaged tissue.
Founding of National Institute on Aging
An act of Congress establishes the National Institute on Aging (NIA) as one of our National Institutes of Health (NIH). The NIA is our primary federal agency supporting Alzheimer's research.
Alzheimer's recognized as most common cause of dementia
Neurologist Robert Katzman identifies Alzheimer's disease as the most common cause of dementia and a major public health challenge in his editorial published in Archives of Neurology.
Alzheimer's Association founded

In 1979, Jerome H. Stone and representatives from several family support groups met with the National Institute on Aging to explore the value of a national, independent, nonprofit organization to complement and stimulate federal efforts on Alzheimer's disease. That meeting resulted in the 1980 formation of the Alzheimer's Association with Mr. Stone as founding president. Today, the Alzheimer’s Association is the leading voluntary health organization in Alzheimer’s care, support and research.
Declaration of National Alzheimer's Disease Month
Awareness of Alzheimer's disease increases, leading Congress to designate November 1983 as the first National Alzheimer's Disease Month.
Beta-amyloid identified
Researchers George Glenner and Cai'ne Wong report identification of "a novel cerebrovascular amyloid protein," known as beta-amyloid — the chief component of Alzheimer's brain plaques and a prime suspect in triggering nerve cell damage.
Nationwide infrastructure for Alzheimer's research established
The NIA begins funding its network of Alzheimer's Disease Centers at flagship medical institutions, establishing a nationwide infrastructure for research, diagnosis and treatment.
Tau protein identified
Researchers discover that tau protein is a key component of tangles — the second pathological hallmark of Alzheimer's disease and another prime suspect in nerve cell degeneration.
First Alzheimer's drug trial
The Alzheimer's Association assists the NIA and Warner-Lambert Pharmaceutical Company (now Pfizer) in launching and recruiting participants for clinical trials of tacrine, the first drug specifically targeting symptoms of Alzheimer's disease.
First deterministic Alzheimer's gene identified
Researchers identify the first gene associated with rare, inherited forms of Alzheimer's disease. This gene on chromosome 21 codes amyloid precursor protein (APP), the parent molecule from which beta-amyloid is formed. Chromosome 21 is also the chromosome of which those with Down syndrome have three copies rather than two. Many individuals with Down syndrome develop Alzheimer's disease, often as young as their 30s and 40s.
Federal clinical study consortium launched
The NIA established the Alzheimer's Disease Cooperative Study (ADCS), a nationwide medical network to facilitate clinical research and conduct federally funded clinical trials.
First Alzheimer's risk factor gene identified
Researchers identify APOE-e4, a form of the apolipoprotein-E (APOE) gene on chromosome 19, as the first gene that raises risk for Alzheimer's but does not determine that a person who has it will develop the disease.
First Alzheimer's drug approved by FDA
The Food and Drug Administration (FDA) approves tacrine (Cognex) as the first drug specifically targeting Alzheimer's memory and thinking symptoms. Four additional drugs are approved over the next 10 years.
President Reagan's diagnosis announced

Former U.S. President Ronald Reagan shares with the American people that he has been diagnosed with Alzheimer's disease. In an open letter to the American people about his decision to share his diagnosis, President Reagan wrote, "In opening our hearts, we hope this might promote greater awareness of this condition. Perhaps it will encourage a clearer understanding of the individuals and families who are affected by it."
First World Alzheimer's Day
The first World Alzheimer's Day (WAD) launches on September 21 by Alzheimer's Disease International, the umbrella organization of Alzheimer's associations.
First transgenic mouse model announced
Researchers announce the first transgenic mouse model that developed Alzheimer-like brain pathology. The mouse was developed by inserting one of the human APP genes linked to a rare, inherited form of Alzheimer's disease. The Alzheimer's Association first awarded a grant to develop a mouse model of a rare neurodegenerative disorder called Gerstmann-Sträussler-Scheinker syndrome in 1989, laying the technical foundation for Alzheimer's mouse models.
"Alzheimer's vaccine" successful in mice
The first in a series of reports is published showing that injecting transgenic "Alzheimer" mice with beta-amyloid prevents the animals from developing plaques and other Alzheimer-like brain changes.
National Alzheimer's Disease Genetics Study begins
The Alzheimer's Association partners with the National Institute on Aging to recruit participants for the National Alzheimer's Disease Genetics Study, a federal initiative to collect and bank blood samples from families with several members who developed Alzheimer's disease late in life in order to identify additional Alzheimer's risk genes.
First report on Pittsburgh Compound B (PIB)
Researchers at the Alzheimer's Association International Conference on Alzheimer's Disease (AAICAD) share their first report on an imaging agent called Pittsburgh Compound B (PIB), a major potential breakthrough in disease monitoring and early detection. PIB enters the brain through the bloodstream and attaches itself to beta-amyloid deposits, where it can be detected by positron emission tomography (PET). The Alzheimer's Association provided significant support to initiatives to develop PIB and conduct preclinical testing it in animal studies.
Neuroimaging Initiative (ADNI)

The Alzheimer's Association joins public and private donors as a major sponsor of the Alzheimer's Disease Neuroimaging Initiative (ADNI), a nationwide study to establish standards for obtaining and interpreting brain images. The ultimate goal of ADNI is to determine whether standardized images, possibly combined with laboratory and psychological tests, can identify high-risk individuals; provide early detection; and track and monitor treatment effects, especially in clinical trials of disease-modifying drugs. In 2006, the Association launched the European Alzheimer's Disease Neuroimaging Initiative (E-ADNI), to expand ADNI's scope by combining data from several European brain imaging initiatives with ADNI data. This effort has now grown into World Wide ADNI (WW-ADNI), a global network of flagship research sites united in a common effort to improve diagnosis and speed treatment development with standardized protocols and data shared internationally.
Alzheimer's & Dementia® journal launched
The Alzheimer's Association launches Alzheimer's & Dementia®: The Journal of the Alzheimer's Association to further support a global, interdisciplinary exchange within the Alzheimer's research community.
Healthy Brain Initiative launched
The Alzheimer’s Association and the U.S. Centers for Disease Control and Prevention launch the Healthy Brain Initiative with the publication of A National Public Health Road Map to Maintaining Cognitive Health. The Road Map advances 44 science-based actions emphasizing primary prevention of cognitive impairment. The goal of this initiative is to maintain or improve the cognitive performance of all adults.
International Society to Advance Alzheimer's Research and Treatment formed
To further the work of the global Alzheimer's research community, the Alzheimer's Association creates the International Society to Advance Alzheimer's Research and Treatment (ISTAART), the first and only professional society dedicated to Alzheimer's and dementia.
International Conference on Alzheimer's Disease becomes an annual event
With accelerating progress intensifying the need for global information exchange, the Alzheimer's Association International Conference on Alzheimer's Disease® (AAICAD®) becomes an annual event.
Effort to standardize biomarkers begins
The Alzheimer's Association announces funding of the Alzheimer's Association QC Program for CSF Biomarkers to help overcome variation among institutions in measuring potential biomarkers in cerebrospinal fluid (CSF).
Alzheimer's researchers unite to raise awareness and concern
Dozens of Alzheimer's researchers unite with the Alzheimer's Association for an "Alzheimer's Breakthrough Ride®," a 66-day bike relay across America to raise public and congressional awareness of the urgent need for more federal funding to support the search for effective Alzheimer's treatments.
Alzheimer's clinical trial database established
The Alzheimer's Association and its partners in the Coalition Against Major Diseases (CAMD) released a first-of-its kind database of 4,000 patients who participated in 11 pharmaceutical industry-sponsored clinical trials of Alzheimer's treatments. The combined data, accessible to any qualified researcher, will offer unprecedented power to understand the course of Alzheimer's.
Alzheimer's Association TrialMatch® launched

The Association launches TrialMatch®, a free, easy-to-use clinical studies matching service that connects individuals living with Alzheimer's disease, caregivers, and healthy volunteers with current research studies. Stakeholders unanimously identified building awareness of research studies and increasing enrollment as key strategies to accelerate treatment development.
An influential model of biomarker changes during Alzheimer’s disease progression is first published
A group of researchers publish a working model relating changes in Alzheimer’s biomarkers to disease stage and symptom severity. The model has become a focal point of research into Alzheimer’s biomarkers, and is revised periodically to account for new research.
President Obama signs National Alzheimer's Project Act (NAPA) into law
Groundbreaking legislation establishes our first-ever framework for a national strategic plan to address the Alzheimer's crisis and to coordinate our response on multiple fronts, including research, care and support.
New criteria and guidelines for Alzheimer's disease diagnosis
Three workgroups convened by the Alzheimer's Association and the National Institute on Aging issue updated criteria and guidelines for diagnosing Alzheimer's disease and propose a research agenda to define a new preclinical stage.
Annual assessment for cognitive impairment for all Medicare Beneficiaries implemented as part of Annual Wellness visits
The Centers for Medicare and Medicaid Services implement Annual Wellness Visits for all Medicare Beneficiaries under the Patient Protection and Affordable Care Act. A mandatory part of the Annual Wellness Visit is an assessment for detection of cognitive impairment.
First major clinical trial for prevention of Alzheimer’s disease is initiated
A multinational research consortium, the Dominantly Inherited Alzheimer Network, launches the first major clinical trial testing drug therapy to prevent the onset of Alzheimer’s disease symptoms in people who inherited an autosomal dominant mutation putting them at high risk for the disease.
International Genomics of Alzheimer’s Project (IGAP) researchers identify new genetic risk factors for Alzheimer’s disease
Hundreds of researchers from around the world collaborate to perform a meta-analysis of genome-wide association studies intended to identify genetic variations linked with an increased risk for Alzheimer’s disease. The collaboration revealed 20 genetic variations associated with increased risk, 11 of which had not been linked with Alzheimer’s before. Some of the newly identified genetic variations are thought to be specific to the immune system, adding to mounting evidence of a role for the immune system in Alzheimer’s disease.
Rates of death caused by Alzheimer’s disease found to be much higher than reported on death certificates
Researchers at Rush University find that the annual number of deaths attributable to Alzheimer’s disease in the U.S. among people at least 75 years old is about 500,000, much higher than the number reported on death certificates (>84,000).
Alzheimer's Accountability Act signed into law
The Alzheimer's Association led the fight for this revolutionary law that allows scientists at the NIH to submit an annual research budget directly to Congress.
Historic funding increase
Historic $400 million increase for federal Alzheimer’s disease research funding signed into law, bringing annual funding to $1.4 billion.
Dementia Care Practice Recommendations published
Alzheimer’s Association released Dementia Care Practice Recommendations aimed at helping professional care providers deliver optimal quality, person-centered care for those living with Alzheimer’s and other dementias.
International consortium established to improve care and psychosocial outcomes
The National Institutes of Health (NIH) awarded the Alzheimer’s Association $1.34 million over five years for an international research network, Leveraging an Interdisciplinary Consortium to Improve Care and Outcomes for Persons Living with Alzheimer’s and Dementia (LINC-AD), to improve care and psychosocial outcomes for individuals living with dementia.
Bill Gates helps fund Part the Cloud Research Grant Program
Bill Gates joined the Alzheimer’s Association’s Part the Cloud global research grant program with a $10 million award that will stimulate an additional $20 million in funding to the Alzheimer’s Association.
Alzheimer’s funding reaches all-time high

A $350 million increase for Alzheimer’s and dementia research funding at the National Institutes of Health (NIH) was signed into law, bringing the annual funding to $2.8 billion. An additional $10 million was also approved for the BOLD Infrastructure for Alzheimer’s Act.
Aducanumab approved for treatment of Alzheimer’s disease
Aducanumab (Aduhelm®) received accelerated approval as a treatment for Alzheimer's disease by the U.S. Food and Drug Administration (FDA). This is the first FDA-approved therapy to address the underlying biology of Alzheimer's disease. (Aducanumab will be discontinued on Nov. 1, 2024. Please connect with your provider on treatment options.)
Lecanemab receives traditional approval for treatment of Alzheimer's disease
Lecanemab (Leqembi®) received traditional approval as a treatment for early Alzheimer's from the FDA. It is the first traditionally approved treatment that addresses the underlying biology of Alzheimer's and changes the course of the disease in a meaningful way for people in the early stages.
CMS decides to cover PET imaging for Alzheimer's disease diagnosis
A policy change by the Centers for Medicare & Medicaid Services (CMS) expanded coverage of brain amyloid positron emission tomography (PET) imaging for the diagnosis of Alzheimer's disease, making a valuable tool more accessible across the country.
Alzheimer's Association announces milestone $100 million research investment
The Alzheimer's Association, the world's largest nonprofit funder of Alzheimer's and dementia research, invested a milestone $100 million in research initiatives in 2023, the largest single-year total since the organization's founding in 1980.
Donanemab receives traditional approval for treatment of Alzheimer's disease
Donanemab (Kisunla™) received traditional approval as a treatment for early Alzheimer's from the FDA. It is the second traditionally approved treatment that addresses the underlying biology of Alzheimer's and changes the course of the disease in a meaningful way for people in the early stages.
Keep Up With Alzheimer’s News and Events

IMAGES
VIDEO
COMMENTS
Alzheimer's and dementia research - find the latest information on research funding, grants, clinical trials and global research news. Get information and resources for Alzheimer's and other dementias from the Alzheimer's Association.
Current Pilot Projects. These awards are aimed at investigators at the associate research scientist/instructor level, although exceptions are possible for early-stage assistant professors. Award Year 5/01/2022 - 4/30/2023. Marcello DiStasio, MD, PhD: "Uncovering Proinflammatory Signatures in Alzheimer's Disease Using Spatial Transcriptomics".
The federal government's Alzheimer's and related dementias research strategy focuses on engaging a cross-disciplinary team of geneticists, epidemiologists, gerontologists, behavioral scientists, disease and structural biologists, pharmacologists, clinical researchers, and others to bring the greatest and most diverse expertise to the field.
An irreversible degeneration of the brain that causes disruptions in memory, cognition, personality, and other functions, it eventually leads to death from complete brain failure. Nearly 7 million Americans aged 65 and older are thought to have Alzheimer's disease. By 2050, that figure may increase to nearly 13 million.
1. Introduction. Alzheimer's disease (AD) (named after the German psychiatric Alois Alzheimer) is the most common type of dementia and can be defined as a slowly progressive neurodegenerative disease characterized by neuritic plaques and neurofibrillary tangles (Figure 1) as a result of amyloid-beta peptide's (Aβ) accumulation in the most affected area of the brain, the medial temporal ...
Grant type: Project. Awarded: 2015/2016. Investigating heparins as a potential new drug for Alzheimer's disease. Lead Investigator: Professor Jerry Turnbull. Institution: University of Liverpool. Grant type: Project. Awarded: 2015/2016. Testing the effect of the diabetes drug Liraglutide in Alzheimer's disease. Lead Investigator: Dr Paul Edison.
The Memory and Aging Project has enrolled hundreds of volunteers for our studies and is at the forefront of a worldwide effort to uncover key causal factors in the development of Alzheimer disease. ... Knight Alzheimer Disease Research Center. Department of Neurology. 4488 Forest Park Ave. Suite 200. St. Louis, MO 63108. Research: (314) 273 ...
In 2023, the Alzheimer's Association invested a record-breaking $100 million in research initiatives. This unparalleled commitment to dementia science is our biggest single-year investment since the Alzheimer's Association was founded in 1980. And we have the momentum to keep going. As the world's largest nonprofit funder of Alzheimer's ...
The Alzheimer's Disease Research Center, which is jointly based at the Mayo Clinic campuses in Jacksonville, Florida, and Rochester, Minnesota, also provides care and services for patients with dementia disorders and their families. Ultimately, researchers in the Alzheimer's Disease Research Center hope to prevent, delay and possibly cure ...
Early diagnosis of Alzheimer's disease plays a pivotal role in patient care and clinical trials. In this study, we have developed a new approach based on 3D deep convolutional neural networks to ...
The Center for Alzheimer's Disease Research fosters collaborations across basic and clinical research groups that work toward uncovering when, where and how Alzheimer's disease arises. ... The center supports research projects that integrate knowledge across human biological systems. Visit Page. Open details for Research Projects.
The Alzheimer's disease sequencing project is a study of human genetic variation and its relationship to health and disease. ... The Alzheimer's Disease Research Center Autopsy (ADRC) Cohort includes 1,500 cases with autopsy and 1,372 controls from the National Institute on Aging's ADRC's. These individuals are well phenotyped and ...
Unique basic, translational, clinical and health services research projects and resources. SVG. 8000+ ... to accelerate the development of effective interventions for Alzheimer's disease and related disorders. ... private and international biomedical and public health organizations who fund Alzheimer's disease (AD) research. Join Now ...
Late-onset Alzheimer disease (LOAD) is the leading cause of dementia worldwide, with substantial economic and public health implications. 1 LOAD is a neurodegenerative disease characterized by progressive dementia typically manifesting in the seventh to ninth decades. Neuropathological changes precede clinical symptoms by 10-20 years, resulting in clinically asymptomatic individuals carrying ...
Our Research Projects. AFA provides funding for research projects aimed at improving treatment and quality of life for the millions of people living with Alzheimer's disease. Click here to donate and support AFA. The following are some of the research projects AFA has supported: Identifying At-Risk Individuals.
Women's Alzheimer's Research Initiative (WARI) Step Up the Pace is a special initiative to increase philanthropic investment in four key dementia research outcomes areas: Discovery Science, Early Detection, Treatment and Prevention. Learn more about the Alzheimer's Association's history of identifying high-potential research opportunities to ...
NIA is currently supporting over 500 active clinical trials on Alzheimer's disease and dementia in many areas of research. See the comprehensive list. ... Natives Engaged in Alzheimer's Research: IKE Kupuna (Elder Wisdom) Project: Natives Engaged in Alzheimer's Research - 'Ike Kupuna (NEAR) Joseph Kaholokula, Washington State University ...
One of the key strands of ADI's mission is to increase investment and innovation in dementia research. Facilitating research into treatments and hopefully a cure, plus research into improving care, underpins much of our advocacy work. For over 10 years, ADI has tackled important dementia research issues through our World Alzheimer Reports ...
The Mayo Clinic Alzheimer's Disease Research Center has established a developmental projects program. Awards for developmental projects are in the amount of $50,000 to $100,000 of direct funds a year for project terms of 1 to 3 years. The funding mechanism is intended to give junior investigators opportunities to develop projects and generate ...
Another factor in NIA's decision, according to the report, is that the institute had seen "an increase in high-quality applications for Alzheimer's disease research grants." Even though the project was mentioned as part of its budget request for the 2025 fiscal year, NIA never announced RWDP's cancellation. Silverberg referred a ...
WASHINGTON - New blood tests could help doctors diagnose Alzheimer's disease faster and more accurately, researchers reported Sunday - but some appear to work far better than others.. It's ...
An open-access MIT study published today in Nature provides new evidence for how specific cells and circuits become vulnerable in Alzheimer's disease, and hones in on other factors that may help some people show resilience to cognitive decline, even amid clear signs of disease pathology.. To highlight potential targets for interventions to sustain cognition and memory, the authors engaged in ...
The research is reported in Alzheimer's & Dementia, in advance of the Alzheimer's Association International Conference on July 28, where the work will be presented.
The Alzheimer's Association International Research Grant Program (IRGP) funds investigations to advance our understanding of Alzheimer's disease, identify new treatment strategies, improve care for people with dementia and further our knowledge of brain health and disease prevention. Our 2024 Grants Application Process Is Open.
Targeted Alzheimer's research and drug discovery Date: July 24, 2024 Source: Jackson Laboratory Summary: Researchers offer the first comprehensive ranking of the relative role and significance of ...
Search for Alzheimer's and dementia research studies funded by the Alzheimer's Association International Research Grant Program. ... Resilience in Andean American Indigenous with Alzheimer's disease. Nilton Custodio, M.D., Ph.D. Lima, Peru 2024 Genetic and environmental contributions to longitudinal tau trajectories.
Mutations in the presenilin gene, PSEN1, causes early onset of Alzheimer's disease in humans and in fruit flies modified to have this gene. A new study led by researchers at Penn State revels that disrupting heparan sulfate-modified proteins in fruit flies suppressed neuronal death and corrected other cell deficits common in early stages of Alzheimer's and other neurodegenerative diseases.
Research and Progress This is a time of unprecedented promise in the quest to end Alzheimer's. Today, we are growing philanthropic support for Alzheimer's research, fostering a dynamic community of Alzheimer's scientists and securing increased federal funding for research - all of which are instrumental to finding new treatments to stop, slow and prevent Alzheimer's disease.
Alzheimer's Association announces milestone $100 million research investment. The Alzheimer's Association, the world's largest nonprofit funder of Alzheimer's and dementia research, invested a milestone $100 million in research initiatives in 2023, the largest single-year total since the organization's founding in 1980.