- Fact sheets
- Facts in pictures

Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- Data collection tools
- Global Health Observatory
- Insights and visualizations
- COVID excess deaths
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Fact sheets /
- Globally in 2022, there were an estimated 249 million malaria cases and 608 000 malaria deaths in 85 countries.
- The WHO African Region carries a disproportionately high share of the global malaria burden.
- In 2022, the Region was home to 94% of malaria cases (233 million) and 95% (580 000) of malaria deaths.
- Children under 5 accounted for about 80% of all malaria deaths in the Region.
Malaria is a life-threatening disease spread to humans by some types of mosquitoes. It is mostly found in tropical countries. It is preventable and curable.
The infection is caused by a parasite and does not spread from person to person.
Symptoms can be mild or life-threatening. Mild symptoms are fever, chills and headache. Severe symptoms include fatigue, confusion, seizures, and difficulty breathing.
Infants, children under 5 years, pregnant women, travellers and people with HIV or AIDS are at higher risk of severe infection.
Malaria can be prevented by avoiding mosquito bites and with medicines. Treatments can stop mild cases from getting worse.
Malaria mostly spreads to people through the bites of some infected female Anopheles mosquitoes. Blood transfusion and contaminated needles may also transmit malaria. The first symptoms may be mild, similar to many febrile illnesses, and difficulty to recognize as malaria. Left untreated, P. falciparum malaria can progress to severe illness and death within 24 hours.
There are 5 Plasmodium parasite species that cause malaria in humans and 2 of these species – P. falciparum and P. vivax – pose the greatest threat. P. falciparum is the deadliest malaria parasite and the most prevalent on the African continent. P. vivax is the dominant malaria parasite in most countries outside of sub-Saharan Africa. The other malaria species which can infect humans are P. malariae, P. ovale and P. knowlesi .
The most common early symptoms of malaria are fever, headache and chills.
Symptoms usually start within 10–15 days of getting bitten by an infected mosquito.
Symptoms may be mild for some people, especially for those who have had a malaria infection before. Because some malaria symptoms are not specific, getting tested early is important.
Some types of malaria can cause severe illness and death. Infants, children under 5 years, pregnant women, travellers and people with HIV or AIDS are at higher risk. Severe symptoms include:
- extreme tiredness and fatigue
- impaired consciousness
- multiple convulsions
- difficulty breathing
- dark or bloody urine
- jaundice (yellowing of the eyes and skin)
- abnormal bleeding.
People with severe symptoms should get emergency care right away. Getting treatment early for mild malaria can stop the infection from becoming severe.
Malaria infection during pregnancy can also cause premature delivery or delivery of a baby with low birth weight.
Disease burden
According to the latest World malaria report , there were 249 million cases of malaria in 2022 compared to 244 million cases in 2021. The estimated number of malaria deaths stood at 608 000 in 2022 compared to 610 000 in 2021.
The WHO African Region continues to carry a disproportionately high share of the global malaria burden. In 2022 the Region was home to about 94% of all malaria cases and 95% of deaths. Children under 5 years of age accounted for about 78% of all malaria deaths in the Region.
Malaria can be prevented by avoiding mosquito bites and by taking medicines. Talk to a doctor about taking medicines such as chemoprophylaxis before travelling to areas where malaria is common.
Lower the risk of getting malaria by avoiding mosquito bites:
- Use mosquito nets when sleeping in places where malaria is present
- Use mosquito repellents (containing DEET, IR3535 or Icaridin) after dusk
- Use coils and vaporizers.
- Wear protective clothing.
- Use window screens.
Vector control
Vector control is a vital component of malaria control and elimination strategies as it is highly effective in preventing infection and reducing disease transmission. The 2 core interventions are insecticide-treated nets (ITNs) and indoor residual spraying (IRS).
Progress in global malaria control is threatened by emerging resistance to insecticides among Anopheles mosquitoes. As described in the latest World malaria report , other threats to ITNs include insufficient access, loss of nets due to the stresses of day-to-day life outpacing replacement, and changing behaviour of mosquitoes, which appear to be biting early before people go to bed and resting outdoors, thereby evading exposure to insecticides.
Chemoprophylaxis
Travellers to malaria endemic areas should consult their doctor several weeks before departure. The medical professional will determine which chemoprophylaxis drugs are appropriate for the country of destination. In some cases, chemoprophylaxis drugs must be started 2–3 weeks before departure. All prophylactic drugs should be taken on schedule for the duration of the stay in the malaria risk area and should be continued for 4 weeks after the last possible exposure to infection since parasites may still emerge from the liver during this period.
Preventive chemotherapies
Preventive chemotherapy is the use of medicines, either alone or in combination, to prevent malaria infections and their consequences. It requires giving a full treatment course of an antimalarial medicine to vulnerable populations at designated time points during the period of greatest malarial risk, regardless of whether the recipients are infected with malaria.
Preventive chemotherapy includes perennial malaria chemoprevention (PMC), seasonal malaria chemoprevention (SMC), intermittent preventive treatment of malaria in pregnancy (IPTp) and school-aged children (IPTsc), post-discharge malaria chemoprevention (PDMC) and mass drug administration (MDA). These safe and cost-effective strategies are intended to complement ongoing malaria control activities, including vector control measures, prompt diagnosis of suspected malaria, and treatment of confirmed cases with antimalarial medicines.
Since October 2021, WHO has recommended broad use of the RTS,S/AS01 malaria vaccine among children living in regions with moderate to high P. falciparum malaria transmission. The vaccine has been shown to significantly reduce malaria, and deadly severe malaria, among young children. In October 2023, WHO recommended a second safe and effective malaria vaccine, R21/Matrix-M. The availability of two malaria vaccines is expected to make broad-scale deployment across Africa possible.
Questions and answers on the RTS,S vaccine .
Early diagnosis and treatment of malaria reduces disease, prevents deaths and contributes to reducing transmission. WHO recommends that all suspected cases of malaria be confirmed using parasite-based diagnostic testing (through either microscopy or a rapid diagnostic test).
Malaria is a serious infection and always requires treatment with medicine.
Multiple medicines are used to prevent and treat malaria. Doctors will choose one or more based on:
- the type of malaria
- whether a malaria parasite is resistant to a medicine
- the weight or age of the person infected with malaria
- whether the person is pregnant.
These are the most common medicines for malaria:
- Artemisinin-based combination therapy medicines are the most effective treatment for P. falciparum malaria.
- Chloroquine is recommended for treatment of infection with the P. vivax parasite only in places where it is still sensitive to this medicine.
- Primaquine should be added to the main treatment to prevent relapses of infection with the P. vivax and P. ovale parasites.
Most medicines used are in pill form. Some people may need to go to a health centre or hospital for injectable medicines.
Antimalarial drug resistance
Over the last decade, partial artemisinin resistance has emerged as a threat to global malaria control efforts in the Greater Mekong subregion. WHO is very concerned about reports of partial artemisinin resistance in Africa, confirmed in Eritrea, Rwanda, Uganda and, most recently, Tanzania. Regular monitoring of antimalarial drug efficacy is needed to inform treatment policies in malaria-endemic countries, and to ensure early detection of, and response to, drug resistance.
For more on WHO’s work on antimalarial drug resistance in the Greater Mekong subregion, visit the Mekong Malaria Elimination Programme webpage. WHO has also developed a strategy to address drug resistance in Africa .
Elimination
Malaria elimination is defined as the interruption of local transmission of a specified malaria parasite species in a defined geographical area as a result of deliberate activities. Continued measures to prevent re-establishment of transmission are required.
In 2022, 34 countries reported fewer than 1000 indigenous cases of the disease, up from just 13 countries in 2000. Countries that have achieved at least 3 consecutive years of zero indigenous cases of malaria are eligible to apply for the WHO certification of malaria elimination . Since 2015, 12 countries have been certified by the WHO Director-General as malaria-free, including Maldives (2015), Sri Lanka (2016), Kyrgyzstan (2016), Paraguay (2018), Uzbekistan (2018), Argentina (2019), Algeria (2019), China (2021), El Salvador (2021), Azerbaijan (2023), Tajikistan (2023) and Belize (2023).
Countries and territories certified malaria-free by WHO .
Malaria surveillance is the continuous and systematic collection, analysis and interpretation of malaria-related data, and the use of that data in the planning, implementation and evaluation of public health practice. Improved surveillance of malaria cases and deaths helps ministries of health determine which areas or population groups are most affected and enables countries to monitor changing disease patterns. Strong malaria surveillance systems also help countries design effective health interventions and evaluate the impact of their malaria control programmes.
WHO response
The WHO Global technical strategy for malaria 2016–2030 , updated in 2021, provides a technical framework for all malaria-endemic countries. It is intended to guide and support regional and country programmes as they work towards malaria control and elimination.
The strategy sets ambitious but achievable global targets, including:
- reducing malaria case incidence by at least 90% by 2030
- reducing malaria mortality rates by at least 90% by 2030
- eliminating malaria in at least 35 countries by 2030
- preventing a resurgence of malaria in all countries that are malaria-free.
Guided by this strategy, the Global Malaria Programme coordinates the WHO’s global efforts to control and eliminate malaria by:
- playing a leadership role in malaria, effectively supporting member states and rallying partners to reach Universal Health Coverage and achieve goals and targets of the Global Technical Strategy for Malaria;
- shaping the research agenda and promoting the generation of evidence to support global guidance for new tools and strategies to achieve impact;
- developing ethical and evidence based global guidance on malaria with effective dissemination to support adoption and implementation by national malaria programmes and other relevant stakeholders; and
- monitoring and responding to global malaria trends and threats.
- World malaria report 2023
- Global technical strategy for malaria 2016–2030, 2021 update
- A framework for malaria elimination
- WHO guidelines for malaria
- World Malaria Day 2024
- Malaria health topic page
- World Malaria Day (25 April)
- WHO Global Malaria Programme (GMP)
- Malaria Policy Advisory Group
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
- Open access
- Published: 16 July 2007
Vector control in a malaria epidemic occurring within a complex emergency situation in Burundi: a case study
- Natacha Protopopoff 1 , 2 ,
- Michel Van Herp 2 ,
- Peter Maes 2 ,
- Tony Reid 2 ,
- Dismas Baza 3 ,
- Umberto D'Alessandro 1 ,
- Wim Van Bortel 1 &
- Marc Coosemans 1 , 4
Malaria Journal volume 6 , Article number: 93 ( 2007 ) Cite this article
13k Accesses
28 Citations
Metrics details
African highlands often suffer of devastating malaria epidemics, sometimes in conjunction with complex emergencies, making their control even more difficult. In 2000, Burundian highlands experienced a large malaria outbreak at a time of civil unrest, constant insecurity and nutritional emergency. Because of suspected high resistance to the first and second line treatments, the provincial health authority and Médecins Sans Frontières (Belgium) decided to implement vector control activities in an attempt to curtail the epidemic. There are few reported interventions of this type to control malaria epidemics in complex emergency contexts. Here, decisions and actions taken to control this epidemic, their impact and the lessons learned from this experience are reported.
Case description
Twenty nine hills (administrative areas) were selected in collaboration with the provincial health authorities for the vector control interventions combining indoor residual spraying with deltamethrin and insecticide-treated nets. Impact was evaluated by entomological and parasitological surveys. Almost all houses (99%) were sprayed and nets use varied between 48% and 63%. Anopheles indoor resting density was significantly lower in treated as compared to untreated hills, the latter taken as controls. Despite this impact on the vector, malaria prevalence was not significantly lower in treated hills except for people sleeping under a net.
Indoor spraying was feasible and resulted in high coverage despite being a logistically complex intervention in the Burundian context (scattered houses and emergency situation). However, it had little impact on the prevalence of malaria infection, possibly because it was implemented after the epidemic's peak. Nevertheless, after this outbreak the Ministry of Health improved the surveillance system, changed its policy with introduction of effective drugs and implementation of vector control to prevent new malaria epidemics.
In the absence of effective drugs and sufficient preparedness, present study failed to demonstrate any impact of vector control activities upon the course of a short-duration malaria epidemic. However, the experience gained lead to increased preparedness and demonstrated the feasibility of vector control measures in this specific context.
Malaria epidemics are a growing problem in the African highlands with devastating effects on their immunologically naive population [ 1 , 2 ]. When occurring during complex emergency situations their control is even more difficult. According to WHO [ 3 ] "a complex emergency is a situation that affects large civilian populations with war or civil strife, food shortages and population displacement, resulting in excess mortality and morbidity". The approach to malaria control in the acute phases of emergencies, particularly in organized refugee camps, has been established and is based on surveillance, outbreak preparedness and case management [ 3 , 4 ]. However, there are a variety of situations that are much more complex where the control depends strongly on the local context.
Burundi has faced an ongoing conflict since 1993. Massive movements of the population have been recorded and according to the Office for the Coordination of Humanitarian Affairs (OCHA) more than 500,000 people were internally displaced in Burundi at the end of 2000. In addition to the civil war, Burundi faced, an increase in malaria cases in the whole country and small outbreaks were recorded in two highland provinces in the late nineties [ 5 ]. From October 2000 to March 2001, a large malaria epidemic occurred in the Burundian highlands [ 6 ], with 2.9 million registered cases over a population of 6.7 million. Between 1,000 to 8,900 probable malaria deaths were reported in three highland provinces, representing between 51% to 78% of the overall mortality [ 7 ]. This epidemic was the result of a combination of different factors including land use changes, population movements, climate variability, deteriorating health systems and malnutrition, further compounded by a high level of resistance against the main drugs chloroquine (CQ) and sulphadoxine/pyrimethamine (SP).
In Karuzi, one of the highland provinces, several actions were taken in progression to contain the increasing number of malaria cases (Figure 1 ). First, early November 2000, the health staff was increased, a simplified malaria treatment protocol was implemented, the hospital capacity was doubled and two mobile clinics were set up, the latter with the intention of decreasing the health facilities' workload and reaching more isolated populations. Secondly, mid-November, the Ministry of Health (MoH) declared the epidemic (Figure 1 ) and antimalarial drugs were provided free-of-charge. Médecins Sans Frontières Belgium (MSF-B) supplied all the public and private health facilities with CQ, SP and quinine. However, because of the suspected high CQ and SP resistance, the first and second line treatment at the time of the epidemic, the MoH in collaboration with MSF-B planned an evaluation of the resistance against these drugs. Using non efficacious drugs would not stop the epidemic and could even worsen it [ 8 , 9 ]. Hence, the need for an alternative strategy to control the transmission and reduce clinical malaria was required, before a new national drug policy based on the results of the resistance monitoring could be adopted.
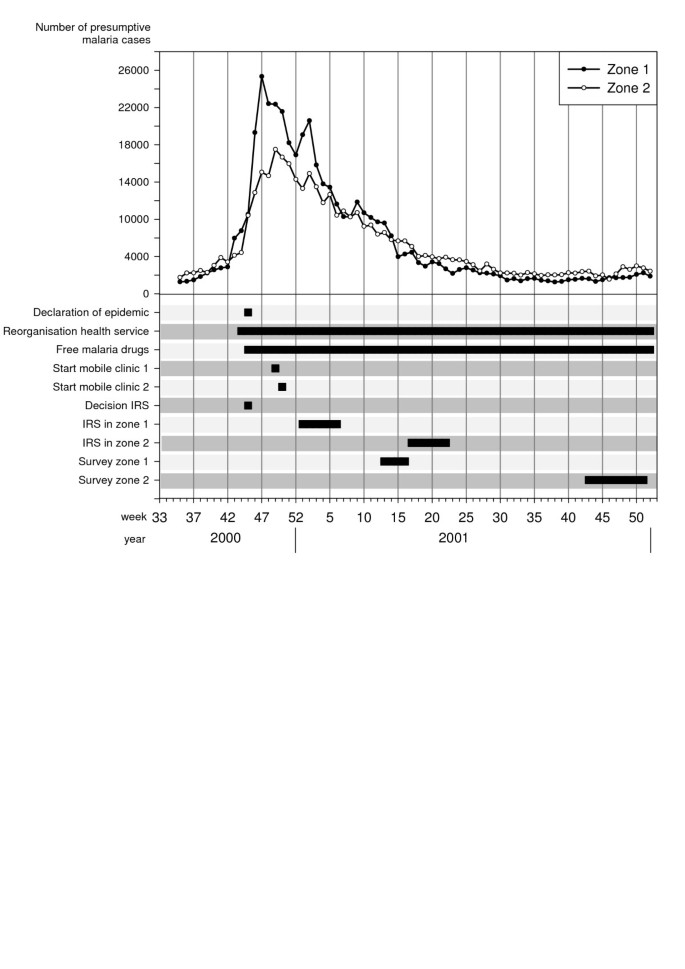
Overview of the malaria epidemic and control interventions in the highland of Karuzi province, Burundi . Number of presumptive malaria cases recorded separately in the health centre of Zone 1 and 2 by weeks. Decisions and actions are plot according the date of their implementation.
Two additional interventions were considered. The first was indoor residual spraying (IRS), a treatment that can effectively control epidemics but usually only when implemented at an early stage of the outbreak [ 3 ]. Despite some reservations, regarding the timing of control activities, it was expected that IRS might work in this case. There is no literature describing field experiences of such an intervention to control an epidemic with conditions related to a complex emergency in the highlands. The second intervention was the use of insecticide-treated bed nets (ITN) that has been shown to reduce malaria morbidity and mortality where malaria is stable [ 10 – 12 ], though there is little documented evidence for the control or prevention of epidemics [ 13 ]. The malaria vectors in the Burundian highlands, Anopheles funestus and Anopheles gambiae s.l., are highly endophilic and endophagic [ 14 – 16 ] so that IRS or ITN or both combined had the potential of controlling the epidemic through their impact on the mosquito population.
The objective of this case study is to report on the decisions made and the actions taken to control the 2000/2001 epidemic in Karuzi province, by vector control and to present an evaluation of the programme and the lessons learned from this experience.
Karuzi is a poor highland province in North-East Burundi with a population of 302,000 people at the time of the epidemic. The area is hilly with altitudes ranging between 1,450 to 2,000 metres. The valleys are fertile and humid, offering breeding sites for An. gambiae and An. funestus . The annual rainfall ranges between 800 and 1,300 mm, generally between October and April. The highest mean temperatures occur between August and September (19°–20°C). The basic administrative unit is the "colline" (hill), 145 in the whole province distributed into seven communes.
Emergency context
In Burundi, there has been a civil war since 1993. Hundred thousands of people were internally displaced or crossed the Tanzanian border. An international economic embargo further impoverished the population. Since the beginning of the conflict, and until 2000, the complex emergency, on the background of general insecurity, was characterized by displaced people, a collapsing health system, environmental deterioration and poor housing conditions. In addition, the famine that occurred in Karuzi at the end of 2000, because of the drought and poor harvest, resulted in dramatic increase of malnourished cases. A nutritional survey in November 2000 reported that 24% of the population was acutely malnourished (MSF-B unpublished data). In Karuzi, a retrospective mortality survey from November 2000 to March 2001 reported a crude mortality rate of 1.1/10,000/day, an under-five mortality rate of 3.0/10,000/day which is far above the emergency threshold of 2.0/10,000/day [ 7 ].
MSF-B started to work in Karuzi in 1993 by opening a medical emergency programme providing assistance to the local population and supporting the public health services. By mid-October 2000, the number of malaria cases in the health centres doubled over one week, a clear sign that an epidemic was beginning. In just two weeks, malaria cases increased from 17,000 to 43,330. The epidemic peaked in December (Figure 1 ), with a 10-fold increase of cases reported by the health centres as compared to the previous three years. The weekly number of cases remained at around 30,000 throughout January and slowly decreased the following months to return to "normal" values in May 2001.
Vector control interventions
The vector control activities were carried out in collaboration with the Transmissible and Deficiency Disease Control Programme (LMTC) and the Provincial Health Office. Despite the decision to implement vector control measures, it was impossible to cover the whole province and intervention areas had to be chosen on the basis of the malaria burden. Unfortunately, the information available was not reliable; health services were so disorganized that the patients' origin was no longer recorded and, hence, a list of the most affected areas was unavailable. Therefore, 29 hills (4–5/communes) were selected (Figure 2 ), regardless of more specific criteria, based on anecdotal evidence given by provincial authorities and because of insecurity in other areas.
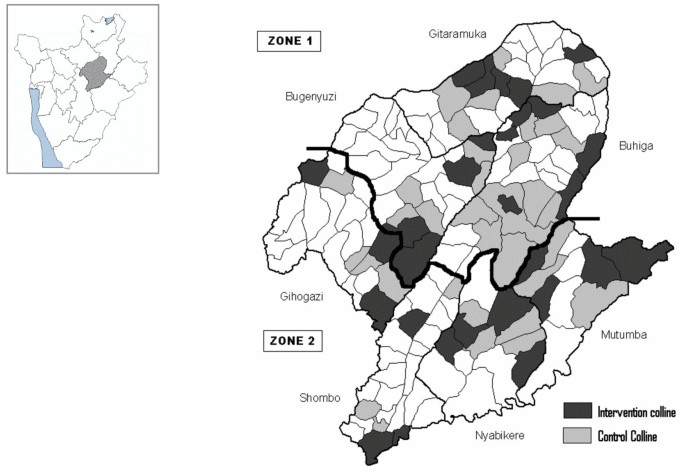
Map of Karuzi province showing the intervention (treated hills) and control hills . The Karuzi province is composed of "colline" (hills), represented by small polygons and regrouped in 7 communes (Buhiga, Bugenyuzi, Gitaramuka, Gihogazi, Nyabikere, Mutumba and Shombo). The dark grey polygons corresponded to the targeted hills for the vector control and light grey are the hills selected to be the control areas for the survey. The two zones (Zone 1: survey done in March-April 2001 two months after the intervention. Zone 2: survey done from October to December 2001, five months after the intervention) are separated by a thick black line.
In each commune, 14 teams (six people each) of local inhabitants were trained on IRS, following the recommended application procedure defined by Lacarin and Reed [ 17 ]. Deltamethrine 2.5 WP (K-Othrine) was applied at the target dose of 0.025 g a.i./m 2 . Each person would spray 10 houses by day. The team supervisor checked the quality of the spraying procedure and collected information on the insecticide used, the characteristics of the house and the corresponding number of people. Between December and January all health facilities, feeding centres and boarding schools were sprayed and provided with ITNs. The rest of the intervention started during the second week of January in the targeted hills of Buhiga, Bugenyuzi and Gitaramuka (Figures 1 and 2 ), called zone 1. The communes of Gihogazi, Mutumba, Nyabikere and Shombo were treated between April and June 2001 because of a delay in obtaining the insecticide. These communes were called zone 2 (Figures 1 and 2 ).
Each sprayman treated an average of 7.7 houses per day (Table 1 ), less than the planned target of 10 houses by day based on grouped camps or villages. Supervision was difficult due to the dispersion of the houses, the hilly environment and the absence of roads. At least once a week, some areas could not be reached because insecurity and this resulted in a delay of the supply of insecticide. Despite these problems and thanks to the good collaboration of the community, most houses (16494/16616; 99.3%) were covered by IRS (Table 1 ). On every intervention hill, an educational campaign for ITN was implemented before the distribution of one ITN (Permanet ® first generation) by household. A total of 16,781 ITNs were distributed (Table 1 ). In zone 2, most houses (91.8%; 95%CI: 83.8–96.6), had at least one ITN (installed or not) while this percentage was lower in zone 1 (61.2%; 95%CI: 50.0–71.6). However, the number of installed ITN was not significantly different in the two zones (zone 1: 78.8%; 95%CI: 65.3–88.9, zone 2: 69.2%; 95%CI: 57.8–79.2; P = 0.2).
Parasitological and entomological survey
Survey design.
Considering the emergency context no baseline survey before the vector control interventions was planned. In zone 1, a survey was carried out from 26 March to 21 April 2001 and in zone 2 from 22 October to 19 December 2001, or respectively two and five months after the end of the IRS (Figure 1 ). The survey includes all intervention hills. For each intervention hill, the nearest hill with the closest number of inhabitants was included as control hill (Figure 2 ). In each zone, the total number of houses to be selected was 85 in intervention hills and 85 in the thirty five selected control hills. The number of houses to be sampled by hill was calculated according the population density of every hill. Then from a list given by the local administration of the hill, houses were selected at random.
Daytime indoor resting mosquitoes were collected using the spray collection method [ 18 ]. After having spread white sheets on floor, the house was sprayed inside with pyrethrum, a non residual insecticide. The mosquitoes falling on the white sheets were collected and morphologically identified to species using M.T. Gillies's keys [ 19 ].
In each house, where the spray catches were done, one inhabitant was randomly selected and a rapid diagnostic test (RDT, Paracheck ® ) to detect Plasmodium falciparum specific antigens, was performed. People with a positive RDT were treated with oral quinine (10 mg/kg/day × 3 during seven days). Additional information on living conditions, past malaria history and treatment was also collected.
Participating individuals were informed of the objectives of the study and verbal consent was obtained. This study was a programme evaluation and was carried out with full cooperation and approval of the Burundi Ministry of Health and the Karuzi provincial authority. It was also reviewed and approved by the MSF Ethics Committee.
Data analysis
Data were entered into MS Excel and analysed using Epi Info version 3.3.2 (Centers for Disease Control and Prevention, Atlanta). Descriptive statistics were used to summarize demography data. Chi squared analysis was used to compare the proportions. Bivariate analyses were performed to see the relative protective effect of IRS and ITN to the outcomes using a negative binomial regression for the Anopheles indoor resting density and a logistic regression for the malaria prevalence (Stata intercooled version Nine). Density ratios (DR = exponential of the regression coefficient) and odds ratios (OR) are reported.
Characteristics of the study population and selected houses are summarized in Table 2 and were similar for control and intervention hills in the same zone. In the intervention hills of zones 1 and 2 respectively, 34.1% and 44.7% of the selected persons declared having slept under a net the previous night, whereas in control areas only one person out of 170 did so. In each zone, the spray catches were done in the 170 selected households (85 in the intervention hills and 85 in the controls). In zone 1, the majority of Anopheles (95.2%) was An. gambiae s.l., the remaining being An. funestus while in zone 2 both species were present in almost equal proportions ( An. gambiae s.l.: 45.1%; An. funestus : 54.9%). In zone 1, the protective effect of IRS against Anopheles in treated houses was 95% (CI 95%: 80–99) compared to control houses and adjusted for net use, in zone 2, it reached 87% (CI 95%: 31–98) (Table 3 ). Using a net was not followed by a significant reduction of Anopheles indoor resting density (Table 3 ). No difference in malaria infection was found between sprayed and non-sprayed hills whereas in zone 1, prevalence was lower in people sleeping under a net (Table 4 ). The difference in prevalence detected between the two intervention zones (zone 1: 60%, zone 2: 30%) is probably due to the natural decline of the epidemic as survey in zone 2 was carried out several months after the survey in zone 1 (Figure 1 ). Moreover, the proportion of persons reporting a malaria attack during the past two months was similar between control and intervention hills but was lower in October December (zone 2: 37.1%) compared to the period of March-April (zone 1: 77.1%) (Table 2 ).
Despite the difficulties encountered, a vector control programme based on IRS and ITN was feasible in an open setting associated with a complex emergency situation. Excellent coverage was obtained for IRS and moderately good coverage for ITN.
Ideally un-treated sentinel houses should have been chosen to evaluate the mass effect of IRS on the vector population. In present study, vector density was estimated in treated houses providing an evaluation of the treatment status of the houses. However the endophillic behaviour of Anopheles is very pronounced in the highlands of Burundi [ 15 ] probably restricting the resting sites in houses or shelters where the average temperatures are 3 to 5°C above the outside temperatures [ 16 , 20 ]. Furthermore, more than 99% of the households were sprayed including the cattle sheds and separate kitchens. It can then be assumed that the used collection method provides also a representative picture of the vector density.
IRS reduced drastically the Anopheles indoor resting density, although the prevalence of malaria infection did not follow accordingly. However, sleeping under a net reduced the prevalence of 64% in zone 1 whereas no difference was seen in zone 2. The absence of impact of the ITN in zone 2 can be explained by the end of the transmission period and the natural decrease in prevalence in both intervention and control hills so that no potential protective effect of the net could be seen.
The malaria cases as reported by the health centres (Figure 1 ) started to decline during the vector control intervention in zone 1, which could hardly be explained by the intervention itself. In zone 2 the cases reached the pre-epidemic level before the intervention. Moreover, although observed in two different control zones, malaria attacks reported during the October-December survey was half of that observed during the March April survey. Both observations suggest that the decline of the malaria incidence was mainly natural and there is no evidence that vector control activities may have sped up the resolution of the epidemic. It was mentioned earlier that IRS is useful only if applied in a timely manner at the start of the epidemic and has little or no impact on malaria epidemics if implemented when peak is reached [ 3 ]. In Burundi, the malaria epidemic was recognized late because, after 10 years of civil war, the health services were unprepared for it. Surveillance, outbreak preparedness and responses were not well developed [ 6 ]. In addition, vector control activities were started only two months after the decision had been taken despite the availability of the expertise and equipment at the LMTC. This could be explained by an underestimation of the required time and equipment due to poor information on vector control strategies in open settings, the difficulties of establishing the areas most affected and the chronic insecurity in the province which delayed the beginning of the intervention. However, vector control activities were started because good case management could not be achieved due to presumptive poor efficacy of CQ and SP. The in vivo resistance tests carried out afterwards reported a failure by day 14 of 93% for CQ and 66% for SP (MSF-B, internal report). These results prompted the MoH to recommend an interim drug policy with SP as a first line drug and artemether-lumefantrine to be used during malaria epidemics. The final drug policy with amodiaquine-artesunate as first line treatment was implemented at the end of 2003 [ 21 ].
The lessons learned during the 2000 epidemic encouraged the MoH to undertake measures to improve the surveillance, the response and the prevention of future malaria outbreaks. Since 2001, a weekly collection of some infectious diseases, including malaria, has been set up in all health facilities. In January 2004, the MoH and WHO elaborated a national strategy [ 22 ] to prevent, to detect earlier and to control epidemics in Burundi. This plan included, increased epidemiological surveillance, improved case management with artemisinine-based combination treatment (ACT), the strengthening of human resources in the health facilities, the distribution of mosquito nets and focal IRS in areas most at risk. Since 2005, systematic distribution of long lasting mosquito nets to pregnant women and children under five years has been integrated within routine health services. Indeed, the target groups are provided with ITN through the first antenatal cares and measles vaccination. Furthermore, acquired experience at the provincial and national level on vector control will be useful for future activities and could, with improved epidemic preparedness, greatly reduce the risk of recurrent epidemics.
Since 2001, some highland provinces were affected by higher number of malaria cases, reaching emergency thresholds in 2002 and 2005 (MoH data). However these increases were limited in time and confined to smaller areas than the 2001 epidemic. The implementation of more systematic vector control activities could be one of the reasons for the absence of true epidemics. Furthermore the introduction of ACT in December 2003 could have reduced the malaria transmission as reported in low endemic areas [ 23 , 24 ]. The possible acquisition of a protective immunity as observed in the Kenyan highlands population [ 25 ] could even play a more important role to explain the absence of epidemics. In Karuzi, from 2002 to 2006 a change in endemicity was observed compared to the 1998 classification of the MoH with prevalence reaching 35 to 50% in age group of two to nine years old and with a high proportion of asymptomatic carriers recorded (unpublished data).
Vector control measures based on IRS and ITN may be more appropriate for the prevention of malaria epidemics in the highlands [ 26 , 27 ]. One round of IRS, before the transmission period and targeted to areas near the valley marshes, could reduce the vector population, the intensity of transmission levels and the human reservoir, hence, the risk of a devastating epidemic.
In the absence of effective drugs during an epidemic of malaria in the highlands of Burundi, vector control programme combining IRS and ITN was feasible despite a context of complex emergency. Vector populations were much reduced, but there is no evidence that the vector control intervention changed the natural evolution of the epidemic. This programme did, however, lead to better surveillance systems being established by the government so that future epidemics may be identified earlier. As well, the experience gained from the IRS and ITNs showed that these measures, known to be effective in preventing epidemics, could be feasibly introduced, even in the context of a complex emergency situation. The combination of improved prevention, earlier detection, and treatment with more effective drugs should help to make serious epidemics of malaria in the Burundi highlands a thing of the past.
Malakooti MA, Biomndo K, Shanks GD: Reemergence of epidemic malaria in the highlands of western Kenya. Emerg Infect Dis. 1998, 4: 671-676.
Article PubMed Central CAS PubMed Google Scholar
Mouchet J, Manguin S, Sircoulon J, Laventure S, Faye O, Onapa AW, Carnevale P, Julvez J, Fontenille D: Evolution of malaria in Africa for the past 40 years: impact of climatic and human factors. J Am Mosq Control Assoc. 1998, 14: 121-130.
CAS PubMed Google Scholar
World Health Organization: Malaria control in complex emergencies: An inter agency field handbook. 2005, WHO/HTM/MAL/2005.1107:
Google Scholar
World Health Organization: Guiding principles for malaria control in acute and chronic phase emergencies in Africa. 2004, DIP/MAL/05.08:
Webster J: 2001, WHO/CDS/RBM/: 1-23. RBM Complex Emergency Malaria Data Base Burundi RBM Complex Emergencies Technical Support Network,
Checchi F, Cox J, Balkan S, Tamrat A, Priotto G, Alberti KP, Zurovac D, Guthmann JP: Malaria epidemics and interventions, Kenya, Burundi, Southern Sudan, and Ethiopia, 1999-2004. Emerg Infect Dis. 2006, 12: 1477-1485.
Article PubMed Central PubMed Google Scholar
Guthmann JP, Bonnet M, Ahoua L, Dantoine F, Balkan S, Van Herp M, Tamrat A, Legros D, Brown V, Checchi F: Death rates from malaria epidemics, Burundi and Ethiopia. Emerging Infectious Diseases. 2007, 13: 140-143.
Bousema JT, Gouagna LC, Meutstege AM, Okech BE, Akim NI, Githure JI, Beier JC, Sauerwein RW: Treatment failure of pyrimethamine-sulphadoxine and induction of Plasmodium falciparum gametocytaemia in children in western Kenya. Trop Med Int Health. 2003, 8: 427-430. 10.1046/j.1365-3156.2003.01047.x.
Article CAS PubMed Google Scholar
Sowunmi A, Fateye BA: Plasmodium falciparum gametocytaemia in Nigerian children: before, during and after treatment with antimalarial drugs. Trop Med Int Health. 2003, 8: 783-792. 10.1046/j.1365-3156.2003.01093.x.
Nevill CG, Some ES, Mung'ala VO, Mutemi W, New L, Marsh K, Lengeler C, Snow RW: Insecticide-treated bednets reduce mortality and severe morbidity from malaria among children on the Kenyan coast. Trop Med Int Health. 1996, 1: 139-146.
Snow RW, Lindsay SW, Hayes RJ, Greenwood BM: Permethrin-treated bed nets (mosquito nets) prevent malaria in Gambian children. Trans R Soc Trop Med Hyg. 1988, 82: 838-842. 10.1016/0035-9203(88)90011-9.
Van Bortel W, Delacollette C, Barutwanayo M, Coosemans M: Deltamethrin-impregnated bednets as an operational tool for malaria control in a hyper-endemic region of Burundi: impact on vector population and malaria morbidity. Trop Med Int Health. 1996, 1: 824-835. 10.1046/j.1365-3156.1996.d01-14.x.
World Health Organization: Malaria epidemics: forecasting, prevention, early detection and control. From policy to practice : Report of an Informal Consultation.8-10 December 2003. 2003, Leysin.Switzerland., WHO/HTM/MAL/2004.1098: [ http://www.who.int/malaria/docs/Leysinreport.pdf ]
Jadin J, Fain A: Contribution à l'étude du paludisme en pays d'altitude. Ann Soc Belg Med Trop. 1951, 31: 353-363.
CAS Google Scholar
Meyus H, Lips M, Caubergh H: L'état actuel du problème du paludisme d'altitude au Ruanda-Urundi. Ann Soc Belg Med Trop. 1962, 42: 771-782.
Vincke IH, Jadin JB: Contribution à l'étude de l'anophélisme en pays d'altitude. Ann Soc Belg Med Trop. 1946, 26: 483-500.
Lacarin CJ, Reed RA: Emergency, vector control using chemicals. 1999, Loughborough, Water, Engineering and development centre (WEDC)
World Health Organization: Manual on practical entomology in malaria. PartII: Methods and Techniques. 1975, Division of Malaria and other Parasitic Diseases
Gillies M.T., Coetzee M.: A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical region). 1987, 55
Garnham PCC: Malaria epidemics at exceptionally high altitudes in Kenya. British Medical Journal. 1945, 2: 45-47.
Ndayiragije A, Niyungeko D, Karenzo J, Niyungeko E, Barutwanayo M, Ciza A, Bosman A, Moyou-Somo R, Nahimana A, Nyarushatsi JP, Barihuta T, Mizero L, Ndaruhutse J, Delacollette C, Ringwald P, Kamana J: Efficacité de combinaisons thérapeutiques avec des dérivés de l'artémisinine dans le traitement de l'accès palustre non-compliqué au Burundi. Trop Med Int Health. 2004, 9: 673-679. 10.1111/j.1365-3156.2004.01255.x.
Article PubMed Google Scholar
Ministère de la Santé Publique du Burundi: Plan de lutte contre les épidémies de paludisme au Burundi: Année 2004-2005. 2004
Barnes KI, Durrheim DN, Little F, Jackson A, Mehta U, Allen E, Dlamini SS, Tsoka J, Bredenkamp B, Mthembu DJ, White NJ, Sharp BL: Effect of artemether-lumefantrine policy and improved vector control on malaria burden in KwaZulu-Natal, South Africa. PLoS Med. 2005, 2: e330-10.1371/journal.pmed.0020330.
Nosten F, van Vugt M, Price R, Luxemburger C, Thway KL, Brockman A, McGready R, ter Kuile F, Looareesuwan S, White NJ: Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet. 2000, 356: 297-302. 10.1016/S0140-6736(00)02505-8.
Hay SI, Noor AM, Simba M, Busolo M, Guyatt HL, Ochola SA, Snow RW: Clinical epidemiology of malaria in the highlands of western Kenya. Emerg Infect Dis. 2002, 8: 543-548.
Guyatt HL, Corlett SK, Robinson TP, Ochola SA, Snow RW: Malaria prevention in highland Kenya: indoor residual house-spraying vs. insecticide-treated bednets. Trop Med Int Health. 2002, 7: 298-303. 10.1046/j.1365-3156.2002.00874.x.
Romi R, Razaiarimanga MC, Raharimanga R, Rakotondraibe EM, Ranaivo LH, Pietra V, Raveloson A, Majori G: Impact of the malaria control campaign (1993-1998) in the highlands of Madagascar: parasitological and entomological data. Am J Trop Med Hyg. 2002, 66: 2-6.
Download references
Acknowledgements
The authors express their sincere thanks to the authorities of the Ministry of Health in Burundi, the LMTC and the provincial administration of Karuzi for their collaboration and their support during the field work. This work was partly funded by MSF-Belgium and the Belgian co-operation (DGDC).
Author information
Authors and affiliations.
Department of Parasitology, Prince Leopold Institute of Tropical Medicine, Nationalestraat 155, B-2000, Antwerp, Belgium
Natacha Protopopoff, Umberto D'Alessandro, Wim Van Bortel & Marc Coosemans
Medical Department, Médecins Sans Frontières Belgium, 94 rue Dupré, Brussels, Belgium
Natacha Protopopoff, Michel Van Herp, Peter Maes & Tony Reid
Programme de Lutte contre les Maladies Transmissibles et Carentielles, Ministry of Health, Bujumbura, Burundi
Dismas Baza
Department of Biomedical Sciences, Faculty of Pharmaceutical, Veterinary and Biomedical Sciences, University of Antwerp, Universiteitplein 1, B-2610, Belgium
Marc Coosemans
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Natacha Protopopoff .
Additional information
Authors' contributions.
NP collected and analysed the data and drafted the manuscript. MC and WVB provided crucial inputs in the data analysis and the writing up of the manuscript. UD and TR improved the manuscript. MVH designed the survey and with, PM and DB facilitated the data collection and gave technical advice on the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Protopopoff, N., Van Herp, M., Maes, P. et al. Vector control in a malaria epidemic occurring within a complex emergency situation in Burundi: a case study. Malar J 6 , 93 (2007). https://doi.org/10.1186/1475-2875-6-93
Download citation
Received : 19 March 2007
Accepted : 16 July 2007
Published : 16 July 2007
DOI : https://doi.org/10.1186/1475-2875-6-93
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Malaria Case
- Indoor Residual Spray
- Vector Control Intervention
- Malaria Epidemic
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 07 August 2020
The potential public health consequences of COVID-19 on malaria in Africa
- Ellie Sherrard-Smith 1 na1 ,
- Alexandra B. Hogan ORCID: orcid.org/0000-0002-6271-9921 1 na1 ,
- Arran Hamlet 1 na1 ,
- Oliver J. Watson ORCID: orcid.org/0000-0003-2374-0741 1 na1 ,
- Charlie Whittaker ORCID: orcid.org/0000-0002-5003-2575 1 na1 ,
- Peter Winskill 1 ,
- Fatima Ali 2 ,
- Audu B. Mohammad 2 ,
- Perpetua Uhomoibhi 2 ,
- Ibrahim Maikore 2 ,
- Nnenna Ogbulafor 2 ,
- Jamilu Nikau 2 ,
- Mara D. Kont 1 ,
- Joseph D. Challenger 1 ,
- Robert Verity 1 ,
- Ben Lambert 1 ,
- Matthew Cairns ORCID: orcid.org/0000-0003-1068-9713 3 ,
- Bhargavi Rao 4 ,
- Marc Baguelin 1 , 5 ,
- Lilith K. Whittles 1 ,
- John A. Lees 1 ,
- Sangeeta Bhatia 1 ,
- Edward S. Knock 1 ,
- Lucy Okell ORCID: orcid.org/0000-0001-7202-6873 1 ,
- Hannah C. Slater 1 , 6 ,
- Azra C. Ghani 1 ,
- Patrick G. T. Walker 1 ,
- Okefu Oyale Okoko 2 &
- Thomas S. Churcher ORCID: orcid.org/0000-0002-8442-0525 1
Nature Medicine volume 26 , pages 1411–1416 ( 2020 ) Cite this article
26k Accesses
110 Citations
514 Altmetric
Metrics details
- Computational models
The burden of malaria is heavily concentrated in sub-Saharan Africa (SSA) where cases and deaths associated with COVID-19 are rising 1 . In response, countries are implementing societal measures aimed at curtailing transmission of SARS-CoV-2 2 , 3 . Despite these measures, the COVID-19 epidemic could still result in millions of deaths as local health facilities become overwhelmed 4 . Advances in malaria control this century have been largely due to distribution of long-lasting insecticidal nets (LLINs) 5 , with many SSA countries having planned campaigns for 2020. In the present study, we use COVID-19 and malaria transmission models to estimate the impact of disruption of malaria prevention activities and other core health services under four different COVID-19 epidemic scenarios. If activities are halted, the malaria burden in 2020 could be more than double that of 2019. In Nigeria alone, reducing case management for 6 months and delaying LLIN campaigns could result in 81,000 (44,000–119,000) additional deaths. Mitigating these negative impacts is achievable, and LLIN distributions in particular should be prioritized alongside access to antimalarial treatments to prevent substantial malaria epidemics.
Similar content being viewed by others
Factors associated with the decline of malaria in Myanmar’s Ayeyarwady Region between 2013 and 2017
The effectiveness of malaria camps as part of the malaria control program in Odisha, India
Malaria elimination on Hainan Island despite climate change
Globally, COVID-19 has the potential to overburden health systems. Interventions aimed at curbing transmission of SARS-CoV-2, such as restrictions to movement, absenteeism, behavioral changes, closure of institutions and interruption of supply chains, are also expected to result in malaria prevention activities being scaled back 6 , 7 . These antimalarial activities include mass distribution of LLINs, which are the most effective current tool for reducing malaria 5 . LLINs are typically distributed centrally within a community at gatherings that could be canceled or poorly attended as COVID-19 spreads. Other important focal preventive measures, such as seasonal malaria chemoprevention (SMC) and indoor residual spraying of insecticide (IRS), which are conducted house to house, could also be reduced. The World Health Organization (WHO) has emphasized that all routine prevention and case management activities should be continued to the fullest extent possible 8 ; however, early statistical modeling suggests that disrupting LLIN distribution and malaria treatment could have a substantial impact on the malaria burden in Africa 6 .
In the present study, we attempt to quantify the potential impact of the spread of COVID-19 on Plasmodium falciparum malaria morbidity and mortality in Nigeria and across SSA using mathematical models of COVID-19 4 and malaria 9 . We assume that one disease does not directly influence the transmission or severity of the other, but that COVID-19 impacts malaria via the response to the epidemic and its repercussions on health systems. Predictions of the timing and magnitude of COVID-19 epidemics across African countries are highly uncertain and will vary according to how individual countries respond to COVID-19. We use illustrative examples to show how different COVID-19 mitigation and suppression strategies could influence malaria burden. A summary of the main findings, limitations and policy implications of our study is shown in Table 1 . The pervasive and potentially large consequences of COVID-19 on African communities, such as increased poverty, malnutrition and social instability, which themselves can influence malaria burden, are not captured.
We consider four scenarios for the COVID-19 epidemic that will determine the period of malaria service interruption (Fig. 1 ): (1) unmitigated COVID-19 epidemic—although unlikely to occur, this scenario illustrates how a rapid epidemic would be highly disruptive to malaria services, but for a limited period; (2) mitigation—social contact is reduced but the effective reproduction number ( R t ) remains >1, causing a longer-lasting COVID-19 epidemic; (3) suppression—social distancing reducing R t < 1 remains in place until alternative strategies to contain COVID-19 are available, with malaria activities potentially disrupted for a year; and (4) suppression lift—suppression is sustained but then subsequently lifted resulting in a resurgence of the COVID-19 epidemic.
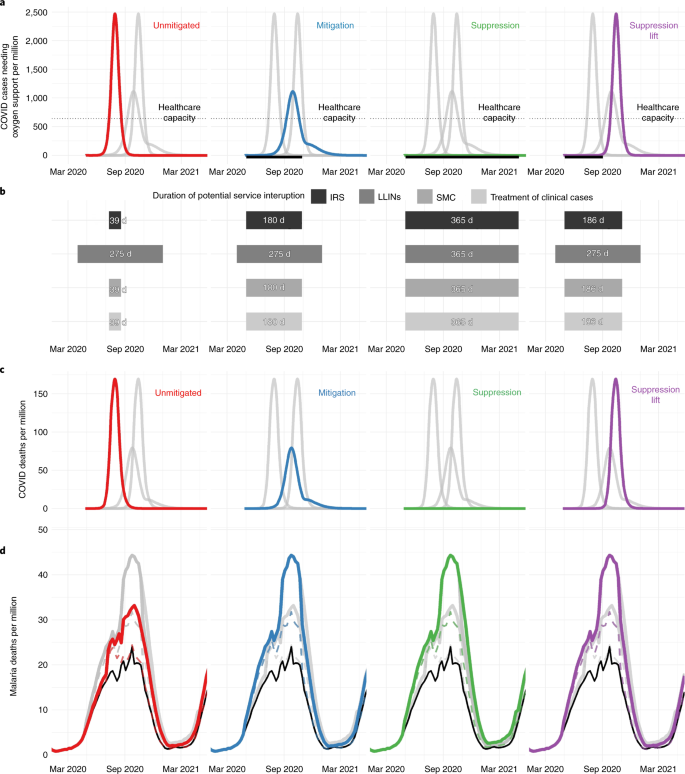
a , The COVID-19 epidemic and the number of people needing oxygen support per week for four different COVID-19 scenarios: an unmitigated epidemic (red), mitigation (blue), continued suppression (green) and suppression lift (purple). The thin dotted horizontal gray line indicates estimated health-care capacity for a typical African country. The thick black horizontal line beneath each figure shows the period when COVID-19 mitigation or suppression activities are assumed to be in operation. b , The assumed duration of interruption where COVID-19 interventions affect different malaria prevention activities (IRS, LLINs and SMC) or case management of clinical cases, with the level of this disruption presented in Table 2 . c , The predicted deaths due to COVID-19 per week in each scenario. d , Predicted malaria deaths per week for each scenario (colored lines) and for the counter-factual where there was no COVID-19-induced disruption (black lines). The top colored lines indicate a scenario in which nets and SMC are halted and case management reduced by half (see Supplementary Table 3 , row 1), whereas the bottom dashed colored lines show the most well-managed scenario (see Supplementary Table 3 , row 3).
We assume that malaria services could be interrupted if COVID-19 mitigation or suppression activities are ongoing or if health-care capacity is exceeded due to COVID-19. The impact of different levels of malaria service interruption is investigated. LLIN campaigns can either continue as normal or be delayed for a year, and clinical case treatments and SMC remain as planned, are reduced or are halted.
Currently, it is unclear how COVID-19 will spread in Africa, although all four COVID-19 scenarios are projected to result in substantial additional deaths from malaria. Implementing COVID-19 mitigation strategies substantially reduces COVID-19 mortality but the prolonged period of health system disruption risks considerably increased malaria deaths (Table 2 ). This is especially evident in Nigeria, where the longer malaria service disruption due to a mitigated (for 6 months) or suppressed (for 1 year) COVID-19 epidemic overlaps with the malaria transmission season, which peaks around September (Fig. 1 ). Considering the effect of the COVID-19 mitigation scenario across SSA over the coming year, if SMC and IRS were halted, the treatment of clinical cases was reduced by half for the next 6 months from 1 May 2020, and if LLIN campaigns due in 2020 were canceled, malaria cases are estimated to increase by 206 million (95% uncertainty interval (UI) = 157–254 million) (see Supplementary Table 1 ), and malaria deaths by 379,000 (95% UI = 221,000–537,000) (Table 2 ), with a corresponding additional 19 (95% UI = 11–26) million life-years lost (see Supplementary Table 2 ).
Many countries are pursuing strategies to suppress COVID-19 to minimize deaths 1 . Our results illustrate that, even if COVID-19 suppression is well managed and LLIN campaigns remain unaffected, with SMC coverage and case management reduced by 50% relative to the norm, prolonged service interruption could increase malaria deaths in Nigeria by approximately 42,000 (95% UI = 22,000–62,000) (see Supplementary Table 3 ) and across SSA by 200,000 (95% UI = 115,000–285,000) (Table 2 ). The impact of disruption to malaria services lasting ≥6 months from 1 May 2020 will be greatest in countries where the malaria transmission is high at the end of the year (see Extended Data Fig. 1 ). Failure to maintain a COVID-19 suppression strategy is likely to lead to a large resurgence, potentially resulting in worse outcomes for both COVID-19 and malaria.
Our findings demonstrate that provision of LLINs is critical. Of the 47 malaria-endemic countries in SSA, 27 were due LLIN campaigns in 2020, with delivery of 228 million LLINs expected ( https://netmappingproject.allianceformalariaprevention.com ). Across SSA, maintaining routine LLIN distribution in a COVID-19 mitigation scenario is predicted to halve deaths attributable to malaria (Table 2 ). This year, many LLINs in SSA will be 3 years old and have diminished efficacy due to insecticide loss and physical degradation 10 . The increased spread of mosquitoes resistant to LLIN insecticides may exacerbate this problem 11 . Effects can vary substantially within countries according to existing LLIN protection, and whether the COVID-19 epidemic will delay scheduled LLIN campaigns (Fig. 2a and see also Extended Data Fig. 2 ).
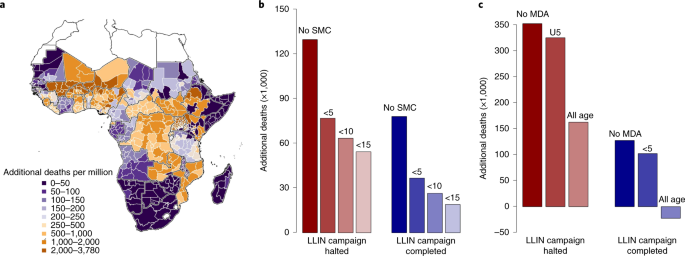
a , Estimated additional deaths per million people when all malaria interventions (LLIN campaigns, SMC and clinical treatment of cases) are halted for 6 months relative to normal service in the absence of COVID-19 for each administrative region (maps for other COVID-19 scenarios are presented in Extended Data Fig. 2 ). b , Reduction in additional malaria deaths by expanding the age of those eligible for SMC in regions within the Sahel where it was conducted in 2019 relative to all malaria interventions canceled (Table 2 , row 11: red bars) or LLIN distributions continue while clinical treatment ceases (Table 2 , row 8: blue bars). Absolute values are shown in Supplementary Table 7 . c , Reduction in additional malaria deaths by introducing a single round of MDA (using the prophylactic with a similar profile to amodiaquine + sulfadoxine–pyrimethamine) for regions where SMC is not currently conducted (see Supplementary Table 9 ). MDA is assumed to be implemented at the optimal time, before the transmission peak for each administration unit. In both SMC and MDA scenarios, we assume that 70% of the respective populations receive the intervention. Negative values indicate that there are fewer malaria deaths than would have been predicted if routine antimalarial interventions had been maintained without a COVID-19 epidemic. The map was prepared using GADM v.3.6 ( https://gadm.org/ ).
Disruption to case management increases the case fatality ratio (see Supplementary Table 4 ) and is predicted to have a similar effect on morbidity to canceling LLIN campaigns if services are stopped for equivalent time periods (illustrated in the COVID-19 suppression scenario when both LLINs and clinical treatment are interrupted for 1 year; Table 2 ). Maintaining 50% of the normal level of treatment over a 6-month period could still prevent up to 100,000 deaths if prevention activities ceased. In Nigeria, case management was estimated to be particularly important due to mass LLIN campaigns scheduled in just 7 of the 37 states in 2020. SMC is currently implemented in the Sahel region of West Africa, which reduces the continental effects of this antimalarial activity. However, the consequences of canceling SMC in operational regions are predicted to be large. A successful 2020 SMC campaign (in regions covered in 2019) is predicted to reduce deaths by 40% in a COVID-19 mitigation scenario if LLIN distributions and case management are also halted (see Supplementary Table 5 ).
There is considerable uncertainty about how COVID-19 will spread in Africa and how countries will respond 2 , 12 . A lower basic reproduction number, R 0 , would slow the epidemic and reduce COVID-19 deaths, yet potentially increase malaria mortality as a result of prolonged antimalarial service interruption. Social-distancing measures may reduce the spread of COVID-19 in Africa, but it is unclear for how long these measures will be maintained and what their effects on health-care capacity will be (see Extended Data Fig. 3 ). This uncertainty substantially influences not only estimates of COVID-19 mortality but also the interruption of malaria services. For example, in Nigeria, if COVID-19 spreads with an R 0 of 2.5 compared with 3, service interruption in the COVID-19 mitigation scenario would be extended from 6 months to 9 months to prevent a resurgence of COVID-19 (see Extended Data Fig. 3 ), which would increase malaria deaths by ~17%, even if LLINs were distributed and some case management was maintained (see Supplementary Table 6 ). Overall, the effects of COVID-19 on malaria are predicted to be greater than early estimates by the World Health Organization (WHO) 6 . This is probably due to the inclusion of SMC and IRS in our analysis, which have a substantial public health impact. The model also mechanistically captures differences in population immunity (determined by the history of malaria infection) and the impact of insecticide-resistant mosquitoes, both of which could increase malaria resurgence. Nevertheless, the numbers of deaths presented here should be considered illustrative because there are large uncertainties in how COVID-19 will spread and communities respond.
After the 2014 West African Ebola crisis, the WHO now recommends the use of mass drug administration (MDA) to prevent excess mortality during complex emergencies 13 . We explored the extent to which introducing or extending chemoprevention could mitigate excess malaria deaths during the COVID-19 epidemic. If LLIN campaigns in 2020 are delayed during a mitigated COVID-19 scenario, increasing the target age of SMC across the Sahel region from children aged <5 years to children aged <10 and 15 years could save 13,500 and 22,500 lives, respectively (Fig. 2b , and see also Extended Data Fig. 4a and Supplementary Table 7 ). Almost half the lives saved would be in Nigeria SMC regions (see Supplementary Table 8 ). Outside current SMC areas, a single round of MDA to 70% of the population is predicted to avert up to 266 deaths per million people over the next year (see Extended Data Fig. 4b and Supplementary Table 9 ) depending on the region in which it is implemented (see Extended Data Figs. 4b and 5 and Supplementary Table 10 ). Such emergency measures will depend on the feasibility of increasing the supply of appropriate drugs in areas where SMC interventions are not currently planned.
Symptoms of both COVID-19 and malaria include fever, which can confuse diagnosis in settings with limited testing for both diseases. In COVID-19 cases, the likelihood of developing fever increases with age (Fig. 3a ), whereas malaria fever declines with age. The percentage of fevers attributable to malaria compared with COVID-19 is predicted to vary temporally according to the synchrony of the two epidemics (Fig. 3b,c ). Furthermore, the proportion of febrile children in whom fever is attributable to malaria is likely to be higher than shown in our results, due to the data on COVID-19 fever in children primarily being sourced from hospital settings (see Supplementary Data 1 ). Many countries are advising that suspected COVID-19 cases should self-isolate ( https://www.acaps.org/covid19-government-measures-dataset ), which might further reduce malaria diagnosis. Providing simple age-based guidelines could substantially reduce malaria burden if malaria tests are unavailable. For example, presumptively treating 70% of febrile children aged <5, 10 and 15 years with antimalarials could save 122,000, 159,000 and 178,000 lives over the next year, respectively. Further work is needed to consider the implications of this strategy on the supply of drugs and burden of nonmalarial fevers 14 . Adhering to social-distancing guidelines will also remain critical because many people who are infected with COVID-19 could also harbor malaria parasites. For example, our modeled results indicate that, at the malaria transmission season peak in Mali (an example of a country with seasonal transmission; Fig. 3b ), in individuals aged >15 years, 30% of those infected with COVID-19 would also have malaria parasites, and therefore may not self-isolate if diagnosed with malaria as the cause of their fever.
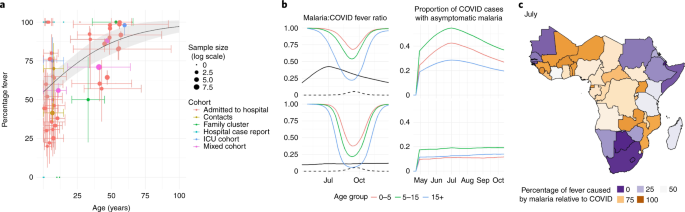
a , A systematic review of the literature showing how the percentage of COVID-19 cases with fever varies with respect to age. Points show published estimates colored according to the cohort in which they were observed: patients admitted to hospital (red), patients admitted to ICUs (green), contacts of known cases (blue) or a mixed cohort (purple). A summary of all data including precise estimates and sample sizes for each study are provided in Supplementary Table 13 . The solid line shows best-fit logistic regression line fit to all groups, and the shaded region indicates 95% confidence interval estimates in the mean. Vertical colored lines show the interquartile range for the proportion of fevers (when available) whereas the horizontal colored lines show the range of ages reported in each cohort. b , Left column figures show estimates of how the proportion of malaria fevers relative to COVID-19 fevers (that is, proportion of fevers due to malaria divided by malaria + COVID-19 fevers) varies over time; the right column shows the proportion of COVID-19 cases co-infected with asymptomatic malaria. The top row shows predictions for seasonal Mali; the bottom row shows the more perennial Uganda. In all panels in b , black lines indicate prevalence of malaria (as detected by microscopy) and dashed lines show COVID-19 prevalence; colored lines indicate age of group in years, 0–5 (red), 5–15 (green) or >15 (blue) years of age (scaling COVID-19 fevers by age using the regression line presented in a ). c , Country-level mean estimates of the fraction of fevers due to malaria compared with those due to malaria and/or COVID-19 in children aged <5 years in July 2020. Maps were prepared using GADM v.3.6 ( https://gadm.org ).
The rapid global spread of the SARS-CoV-2 virus has demonstrated the global vulnerability to new infectious diseases. Continued malaria prevention and treatment programs will be essential to reduce pressure on health systems during the COVID-19 pandemic.
COVID-19 transmission model
Potential COVID-19 trajectories were produced through a modeling framework from Walker et al. 4 . We used an age-structured, susceptible, exposed, infectious, then susceptible again model of transmission with age-specific patterns of disease severity captured according to age-dependent probabilities that infection leads to disease requiring hospitalization (and the need for treatment with high-pressure oxygen), to more severe disease requiring intensive care and subsequently to mortality. Model parameters are based on an analysis of age-specific severity and infection:mortality ratios observed in China and the United Kingdom 4 , 15 , 16 because comparable data from SSA are currently not available. To produce simulations representative of a malaria-endemic setting, the model was calibrated to typical social contact patterns observed within surveys in SSA, which show less substantial declines in contact rates by age 17 , and the demography of Nigeria, our case study and the country with the highest burden of malaria globally 18 . Our projections therefore incorporate a lower per-infection demand for health care such as oxygen and mechanical ventilation driven by the younger populations within malaria-endemic settings. Life-years lost were calculated under this demography using the corresponding life tables.
To capture the probable constraints within a health system, we contrasted this demand for health care with a representative level of supply using the median estimated provision of hospital beds and intensive care units (ICUs) for a low-income country 4 . This threshold was chosen on the basis that, although many countries in SSA are lower–middle income and therefore likely to have a lower total number of hospital beds and ICUs, access to high-pressure oxygen and mechanical ventilation within hospitals is lower than within equivalent high-income settings 19 . During the course of a projected scenario, as health-care capacity is exceeded, individuals requiring either mechanical ventilation or high-pressure oxygen who are unable to receive these interventions are then subject to a substantially higher degree of mortality, leading to excess mortality during time periods in which health systems are overwhelmed (for full details, code and parameterization, see https://github.com/mrc-ide/squire ).
Representative scenarios were simulated using a basic reproduction number, R 0 , of 3 representing a 3.5-day doubling time in cases and deaths reflecting many trajectories currently observed globally 20 . A full list of the parameter values is provided in Supplementary Table 11 . Once a threshold of 0.1 deaths per million (approximately reflecting the COVID-19 mortality observed in many countries in Africa to date) has been exceeded, the pandemic trajectory follows four potential scenarios:
‘Unmitigated’: no direct action is taken but contact rates are reduced by 20% relative to baseline, according to assumed behavior change given the pandemic even in the absence of specific, coordinated public health interventions.
‘ Mitigation’: through combinations of isolation and social distancing, contact rates are reduced by 45% for 6 months, after which infections fall to low levels and contact rates return to pre-pandemic levels. This scenario approximates the maximum reduction in the final size of the epidemic that can be achieved while generating sufficient levels of immunity capable of preventing a second wave once measures have been lifted (assuming infection leads to high levels of immunity from reinfection). It thus produces the lowest final numbers of COVID-19 infections of the three strategies that do not involve indefinite suppression.
‘Indefinite suppression’: stringent suppression-targeting interventions are implemented to reduce contact rates by 75%, and these are maintained indefinitely in the hope that a pharmaceutical intervention (for example, effective vaccine) is developed and deployed. We run this scenario for 12 months. (After this period, lifting suppression without such a pharmaceutical intervention would lead to a second wave of equivalent size as in the ‘Suppression lift’ scenario.)
‘Suppression lift’: the stringent ‘lockdown’-type interventions implemented by many countries are assumed to reduce contact rates by 75%. This reduction is maintained for 2 months, then lifted, and contact rates return to 80% of their pre-pandemic levels for the remainder of the epidemic.
These scenarios represent four possible projections of what could happen to the epidemic, not what policy strategy was adopted by the different countries. The number of deaths associated with COVID-19 between 1 May 2020 and 30 April 2021 is estimated, for African populations at risk of malaria, to provide a direct comparison with the predictions of malaria mortality.
It is assumed that malaria control is impeded by either the health system being overwhelmed or because mitigation or suppression social-distancing measures are in place. The health system is classified as being overwhelmed when the model estimates that the number of people currently requiring noncritical care in hospitals for COVID-19 is 50% more than current hospital capacity (here defined for Africa as 1,281 per million people 4 ). The timing and duration of service interruption for the different COVID-19 scenarios are shown in the second row of Extended Data Fig. 1 .
The trajectory of the COVID-19 pandemic in Africa is highly uncertain. To illustrate this uncertainty two different sensitivity analyses are conducted: (1) a univariate sensitivity analysis that shows how R 0 influences the severity of the epidemic and (2) a multivariate sensitivity analysis that varies all parameters to indicate the wider uncertainty.
In the univariate sensitivity analysis we vary R 0 between 2.0 and 3.5 to cover the range of estimates currently predicted for the region 2 , 12 . This is repeated for the four different COVID-19 scenarios described above. Estimates of the number of people requiring supplementary oxygen over time are presented in Extended Data Fig. 3a . Note that, in the COVID-19 mitigation scenario when R 0 < 3, the epidemic is not predicted to have peaked after 6 months when the social-distancing measures are assumed to be lifted (and many people have not been infected). In this scenario, if social-distancing measures are relaxed, then there is predicted to be a large rebound epidemic with a high death rate as hospitals are overwhelmed (similar to the suppression lift scenario). This means that lower R 0 simulations may counterintuitively have higher deaths due to COVID-19. An alternative assumption could be that social-distancing measures in the mitigation scenario are extended for 9 or 12 months. These simulations indicate a lower peaked epidemic with fewer deaths. Both possible mitigation scenarios with different periods of social distancing are presented in Extended Data Fig. 3a .
In the multivariate sensitivity analysis, we vary all the main parameters within the model for the four different COVID-19 scenarios. These include R 0 , the effectiveness of social distancing at reducing the contact rate, parameters determining the duration of hospitalization and the different severity parameters of the disease (the probability of death if critical care is required but not received; probability of death if hospitalized and oxygen is available; probability of death if hospitalized, but oxygen is not available; and probability of death if hospitalization is required but no hospital bed is available). A total of 500 parameter draws were independently sampled using a log-scaled triangular distribution centered around 1, which spanned the range of values presented in Supplementary Table 11 . To capture uncertainty in the infection fatality ratio and how this varies by age, the probabilities of death reported in Supplementary Table 11 were applied to 500 posteriors sampled from the fitted joint posterior distribution of Verity et al. 16 . This provides 500 different estimates of the magnitude of the infection fatality rate and how it increases with age. These values were then used to parameterize 500 different simulations of the COVID-19 transmission model. For each run, the period of potential malaria service interruption was calculated from the introduction of mitigation measures to the time when health care is no longer over capacity (see Extended Data Fig. 3b–e ). Results show how varying the parameters of the COVID-19 mitigation scenario can produce COVID-19 trajectories similar to the other three COVID-19 scenarios considered. For example, a high R 0 generates short periods of service interruption similar to the unmitigated scenario, whereas a low R 0 may recreate the period of interruption of either the suppression lift scenario (if social-distancing measures are released after 6 months) or a suppression scenario (if social distancing is maintained for a longer period). The uncertainty in the number of deaths from the multivariate sensitivity analysis was used to estimate the mortality 95% UIs presented in Table 2 .
Malaria transmission model
A previously published model of malaria transmission dynamics was used to predict malaria deaths resulting from different COVID-19 scenarios 9 (the code is freely available at https://github.com/jamiegriffin/Malaria_simulation ). Simulations were run at the administrative 1-unit level (where, for each region, the model is calibrated to capture the seasonality, prevalence, vector composition, treatment coverage and vector control coverage, incorporating levels of pyrethroid resistance in each unit) and results are aggregated across regions according to the size of the population at risk of malaria. Results are presented for the high malaria burden country of Nigeria and for SSA as a whole. For Nigeria, administrative 1-unit level estimates of malaria prevalence, LLIN use, drug treatment, coverage of SMC and the timing of 2020 LLIN campaigns were made available by the National Malaria Elimination Program (NMEP) in Nigeria (see Extended Data Fig. 6 ). For other regions of SSA, models were parameterized using 2016 malaria prevalence from the Malaria Atlas Project (MAP, https://malariaatlas.org ). For all countries, modeled clinical cases were aligned with World Malaria Report median cases 18 , 21 . LLIN usage was estimated at the administrative 1-unit level also using MAP estimates, with LLIN usage after campaigns expected to be matched at each subsequent mass campaign. Malaria control depends on insecticide resistance in the local mosquitoes which diminishes the effectiveness of LLINs. This was estimated for each administrative unit from discriminating dose bioassays collated by the WHO over time (projecting forward to 2020) and combined with results from experimental hut trials to estimate the LLIN epidemiological impact 22 , 23 . Malaria transmission seasonality was estimated by local rainfall trends averaged over 8 years and offset by 35 d to reflect mosquito abundance (National Weather Service, Climate Prediction Center (cited 24 March 2016) 24 , 25 ). The estimated proportion of clinical cases receiving prompt treatment was based on Demographic Health Survey (DHS) data and is assumed to remain at estimated 2016 levels 26 . Malaria deaths across all ages were estimated using the modeled number of severe cases, scaled by the assumed proportion of severe cases resulting in mortality both in and outside the hospital setting, and adjusted by the location-specific proportion of clinical cases receiving treatment 9 . Estimates of malaria deaths in 2018 were scaled to align with World Malaria Report median deaths for 2018 for the same region 18 .
Different levels of malaria prevention and treatment interruption are considered together. The impact of the COVID-19 epidemic on malaria is determined solely by the duration of service interruption, which vary for malaria prevention and treatment activities according to which of the four different COVID-19 scenarios is considered. The duration of these different periods of interruption of malaria services is presented in Fig. 1 and is chosen to represent the range of durations observed in the multivariate sensitivity analysis of the COVID-19 model (see Extended Data Fig. 3d ). We assume that changing the human-to-human contact rate that influences the trajectory of the COVID-19 epidemic has no impact on malaria transmission other than through the duration of service interruption. The possible impact of COVID-19 on LLIN distribution is assumed to start at the beginning of the COVID-19 epidemic because most African countries initiated some mitigation or suppression activities. The increase in malaria cases caused by COVID-19 will depend on the time since the last LLIN campaign, because older nets are probably less effective due to loss of insecticide 23 . Aging of LLINs may be exacerbated by the spread of insecticide-resistant mosquitoes, because they may overcome the concentrations of insecticide on the LLINs earlier than susceptible mosquitoes 11 , 23 . All LLINs before 2020 are assumed to be standard pyrethroid-only LLINs, because the numbers of alternative LLINs procured in 2019 are very low. In Nigeria, the year and month of LLIN campaigns are known (or approximated for future mass distributions) at the administrative 1-unit level (state) providing greater resolution. For elsewhere in Africa, the Alliance for Malaria Prevention ( https://netmappingproject.allianceformalariaprevention.com ) estimates were used to calculate the timings of campaigns and the proportion of LLINs distributed in 2018 and 2019, and due in 2020 by country because it is unclear when or where the different campaigns were delivered at a subnational level. Different simulations were run for each administrative unit distributing LLINs at the appropriate year and season. Overall estimates of clinical cases in the administrative unit were weighted by the proportion of LLINs given out that year. LLIN campaigns due to occur before April 2020 were assumed to have occurred as planned. Those campaigns that were due at a time of COVID-19-induced disruption either went ahead as planned (achieving the same population coverage) or were delayed until a year after they were originally due. LLIN campaigns due in quarters 2–4 in 2020 were assumed to be delayed in the unmitigated, mitigated and suppression lift COVID-19 scenarios. This period of disruption is assumed to be longer than other control interventions, reflecting the high chance of disruption to the LLIN supply chain and difficulties in distributing LLINs to local communities. Standard pyrethroid LLINs were distributed in 2020 unless the region was due to have LLINs with the synergist piperonyl butoxide, and LLIN efficacy estimates were taken from Churcher et al. 23 . It is assumed that 80% of LLINs are distributed through mass campaigns and the remainder are distributed continually, and that these continual distributions cease if LLIN mass campaigns are delayed.
Uncertainty in the estimated number of clinical cases and deaths and life-years lost was investigated using a multivariate sensitivity analysis. It was not computationally possible to generate full posterior samples for all scenarios presented here. We therefore developed and tested a normal approximation to the posterior distribution for the output metrics.
First, 20 draws from the joint posterior distribution of the fitted transmission model parameters were used to generate 20 uncertainty runs for all 37 states in Nigeria, for each of the COVID-19 and malaria scenarios (see Extended Data Fig. 7 ). For each uncertainty run, we calculated the additional clinical cases and deaths to generate 95% UIs for Nigeria, and also calculated the coefficient of variation (CV). We then tested the applicability of a normal approximation for uncertainty in other regions, by undertaking a full uncertainty analysis across a smaller subset of 40 first administrative units (10 administrative units from each of Zambia (all provinces included), Mozambique, Democratic Republic of the Congo and Burkina Faso) and comparing the 95% UIs generated for each country to the intervals obtained using a normal distribution approximation with the CV for Nigeria. We found good agreement between the approximation and full uncertainty analysis for the regions tested (see Extended Data Fig. 7 ). We therefore applied this approach across all runs using the CV from the Nigeria simulations and the additional 40 administrative 1-unit levels to obtain 95% UIs across the results for SSA.
Results are highly sensitive to when mass LLIN campaigns are scheduled to occur. Multiple countries have subnational campaigns and it is unclear where, within the country, LLINs are due to be distributed. To illustrate this uncertainty caused by the timing of LLIN campaigns, we conducted a sensitivity analysis for countries where the location of mass campaigns is unknown by simulating distributions in either 2019 or 2020 (see Supplementary Table 12 ).
SMC was assumed to be undertaken in the same administrative units covered in 2019 and 70% of children aged <5 years are assumed to be treated, except in Senegal where children up to age 10 years are covered and we assume 70% coverage 18 . We simulated amodiaquine + sulfadoxine–pyrimethamine as the prophylactic drug delivered for SMC campaigns across three or four rounds, depending on the existing strategy of the country 27 . The proportion of clinical cases of malaria receiving the appropriate prompt treatment outside the COVID-19 epidemic was estimated based on data extracted from the DHS on the proportion of febrile children who were given medical treatment, and the type of treatment administered (DHS, https://dhsprogram.com ). Before 1 May 2020, indoor residual spraying was assumed to take place annually in the same administrative units covered historically (as per 2018) 28 . During the period of health system interruption, SMC and clinical treatment of cases can reduce to zero, reduce to 50% of the planned level (35% of the target age group are covered for SMC) or continue as before. IRS is assumed to be canceled.
The distribution of drugs either through existing SMC channels or through special MDA projects could be used to reduce the impact of COVID-19 on malaria. Bespoke methods of delivery are being considered to deliver drugs to households while maintaining social distancing. In regions where SMC is carried out, this intervention could be extended from the current target age of children aged <5 years to targeting children aged <10 or 15 years. All other aspects of the SMC campaigns are assumed to remain the same (that is, regions where it is deployed, seasonal timing, number of rounds and coverage within the targeted age group). Outside regions with SMC, a single round of MDA using a drug with a similar profile of prophylactic protection to sulfadoxine–pyrimethamine + amodiaquine (in the absence of resistance) is considered. It is assumed to be administered to 70% of the population (either age <5 years or to all ages) with the timing of the MDA aligned for each region to be optimally deployed at the start of the peak transmission season.
We simulated the number of malaria deaths from 1 May 2020 to 30 April 2021, for both the non-COVID-19 scenario and the four COVID-19 scenarios, and for a range of malaria intervention combination strategies. Care should be taken when directly comparing the relative impact of different malaria interventions because they vary in their period of disruption (other than in the suppression scenario). All possible treatment options are considered, although some, such as the halting of all case management for a year, are considered unlikely. Projected deaths were aggregated across regions and presented as the increase in deaths predicted for the different COVID-19 and malaria scenarios relative to the non-COVID-19 scenario for the year. Subnational differences outside Nigeria should be treated with caution. Many countries now have mass LLIN campaigns staggered over multiple years for logistical and financial reasons, and this information on subnational timing of LLIN campaigns was unavailable for all countries other than Nigeria, which can introduce substantial uncertainty (see Supplementary Table 12 ). Similarly, at a local level, the impact of service disruption would be greater for clinical treatment where clinics treat a high proportion of the local community relative to clinics serving a proportionally lower sample of the community.
The impact of the uncertainty in the R 0 for COVID-19 in Africa on malaria mortality is investigated for Nigeria by assuming that the period of service interruption increases from 6 months to 9 months, which is predicted to be required if the COVID-19 R 0 reduced from 3.0 to 2.5 (see Supplementary Table 6 ).
Fever in COVID-19 and malaria
A literature search was conducted to obtain the proportion of fever in COVID-19-positive patients, broken down by age and type of cohort. The search terms ‘covid’ OR ‘SARS-CoV-2’ AND ‘fever’ were used in the PubMed and MEDLINE (Ovid) databases, yielding 384 nonduplicate records. Titles and abstracts of these records were screened for the words ‘child’ or ‘children’, resulting in 28 hits.
Of the 28 papers, 9 were systematic reviews, which were screened for further references. With this, 36 papers were added for extraction. In all, 64 full texts were screened. Eight papers were rejected because either they were in Chinese ( n = 5) or they did not provide a breakdown of fever between adults and children ( n = 3). Data were extracted from 49 papers for this analysis 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 (see Supplementary Table 13 ). Each study examines a different cohort of patients, which may influence the prevalence of fever. In the present study we classify each cohort into those patients (1) admitted to hospital, (2) admitted to ICUs, (3) who are contacts of known cases or (4) in a mixed cohort.
Logistic regression is used to characterize how age influences the prevalence of fever in patients confirmed as having COVID-19. The reporting of fever increased substantially with age (likelihood ratio test P < 0.01), although there was no significant difference between the various cohorts examined in the present study (however, the number of data points investigating the presence of fever in contacts of known COVID-19 cases, which is more likely to represent community transmission, were relatively low; see Fig. 3a ). The percentage of people with malarial fever and how this varies with age are estimated from our malaria transmission dynamics model. Results of both models are then combined assuming that the prevalences of the two diseases are independent. The malaria model is also used to estimate the proportion of patent infections that are asymptomatic to determine the prevalence of asymptomatic malaria cases in COVID-19-infected individuals.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data used in this study are from publicly accessible sources accessed from the DHS ( https://dhsprogram.com ), PubMed ( https://pubmed.ncbi.nlm.nih.gov ) or Ovid MEDLINE ( https://ovidsp.ovid.com ). The results of the modeling work are available from the corresponding author for different regions and scenarios on reasonable request.
Code availability
Full details of the COVID-19 and malaria models, their code and parameterization are freely available at https://github.com/mrc-ide/squire and https://github.com/jamiegriffin/Malaria_simulation , respectively (accessed 22 April 2020). The malaria model was written in C++ code whereas the COVID-19 model was written in R using the ODIN package ( https://github.com/mrc-ide/odin ).
Massinga Loembé, M. et al. COVID-19 in Africa: the spread and response. Nat. Med. 26 , 999–1003 (2020).
PubMed Google Scholar
Brand, S. P. C. et al. Forecasting the scale of the COVID-19 epidemic in Kenya. Preprint at medRxiv https://doi.org/10.1101/2020.04.09.20059865 (2020).
Thompson, H. A. et al. The projected impact of mitigation and suppression strategies on the COVID-19 epidemic in Senegal: a modelling study. Preprint at medRxiv https://doi.org/10.1101/2020.07.03.20144949 (2020).
Walker, P. G. T. et al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science 369 , 413–422 (2020). https://doi.org/10.1126/science.abc0035
Bhatt, S. et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526 , 207–211 (2015).
CAS PubMed PubMed Central Google Scholar
The Potential Impact of Health Service Disruptions on the Burden of Malaria: A Modelling Analysis for Countries in Sub-Saharan Africa 1–44 (WHO, 2020); https://www.who.int/publications-detail/the-potential-impact-of-health-service-disruptions-on-the-burden-of-malaria
Hogan, A. B. et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Global Health https://doi.org/10.1016/S2214-109X(20)30288-6 (2020).
Global Malaria Programme: Tailoring Malaria Interventions in the COVID-19 Response (WHO, 2020); https://www.who.int/publications/m/item/tailoring-malaria-interventions-in-the-covid-19-response
Griffin, J. T. et al. Potential for reduction of burden and local elimination of malaria by reducing Plasmodium falciparum malaria transmission: a mathematical modelling study. Lancet Infect. Dis. 16 , 465–472 (2016).
PubMed PubMed Central Google Scholar
Global Malaria Programme: Achieving and Maintaining Universal Coverage with Long-lasting Insecticidal Nets for Malaria Control Vol. 4 (WHO, 2017); http://www.who.int/malaria/publications/atoz/who_recommendation_coverage_llin/en
Hemingway, J. et al. Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet 387 , 1785–1788 (2016).
Diop, B. Z., Ngom, M., Pougué Biyong, C. & Pougué Biyong, J. N. The relatively young and rural population may limit the spread and severity of COVID-19 in Africa: a modelling study. BMJ Global Health 5 , e002699 (2020).
Guidance on Temporary Malaria Control Measures in Ebola-affected Countries (WHO, 2014); https://www.who.int/malaria/publications/atoz/malaria-control-ebola-affected-countries/en
Dalrymple, U. et al. The contribution of non-malarial febrile illness co-infections to Plasmodium falciparum case counts in health facilities in sub-Saharan Africa. Malar. J. 18 , 195 (2019).
Ferguson, N. M. et al. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. Imperial College COVID-19 Response Team https://doi.org/10.25561/77482 (2020).
Verity, R. et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Dis. 20 , 669–677 (2020).
le Polain de Waroux, O. et al. Characteristics of human encounters and social mixing patterns relevant to infectious diseases spread by close contact: a survey in Southwest Uganda. BMC Infect. Dis. 18 , 172 (2018).
World Malaria Report 2019 (WHO, 2019); https://www.who.int/publications/i/item/world-malaria-report-2019
Murthy, S., Leligdowicz, A. & Adhikari, N. K. J. Intensive care unit capacity in low-income countries: a systematic review. PLoS ONE 10 , e0116949 (2015).
Flaxman, S. et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature https://doi.org/10.1038/s41586-020-2405-7 (2020).
World Malaria Report 2017 (WHO, 2017); https://www.who.int/malaria/publications/world-malaria-report-2017
Sherrard-Smith, E. et al. Systematic review of indoor residual spray efficacy and effectiveness against Plasmodium falciparum in Africa. Nat. Commun. 9 , 4982 (2018).
Churcher, T. S., Lissenden, N., Griffin, J. T., Worrall, E. & Ranson, H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. eLife 5 , e16090 (2016).
Walker, P. G. T., Griffin, J. T., Ferguson, N. M. & Ghani, A. C. Estimating the most efficient allocation of interventions to achieve reductions in Plasmodium falciparum malaria burden and transmission in Africa: a modelling study. Lancet Global Health 4 , e474–e484 (2016).
Garske, T., Ferguson, N. M. & Ghani, A. C. Estimating air temperature and its influence on malaria transmission across Africa. PLoS ONE 8 , e56487 (2013).
Demographic and Health Surveys (DHS Program, 2019); https://dhsprogram.com
Cairns, M. et al. Estimating the potential public health impact of seasonal malaria chemoprevention in African children. Nat. Commun. 3 , 881 (2012).
Tangena, J.-A. A. et al. Indoor residual spraying for malaria control in sub-Saharan Africa 1997 to 2017: an adjusted retrospective analysis. Malar. J. 19 , 150 (2020).
Cai, J. H. et al. [First case of 2019 novel coronavirus infection in children in Shanghai]. Chinese J. Pediatr. 58 , 86–87 (2020).
CAS Google Scholar
Jiehao, C. et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin. Infect. Dis . https://doi.org/10.1093/cid/ciaa198 (2020).
Cai, Q. et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy Eur. J. Allergy Clin. Immunol. https://doi.org/10.1111/all.14309 (2020).
Article Google Scholar
Bialek, S. et al. Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. MMWR Morb. Mortal. Wkly Rep. 69 , 422–426 (2020).
PubMed Central Google Scholar
Chan, J. F.-W. et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395 , 514–523 (2020).
Chang, D. et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA 323 , 1092 (2020).
Chen, D. et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw. Open 3 , e2011122 (2020).
Feng, C. et al. The first case of critically ill new coronavirus pneumonia in children in China. Chin. J. Pediatr. 58 , 179–182 (2020).
Google Scholar
Chen, G. et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 130 , 2620–2629 (2020).
Chen, N. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395 , 507–513 (2020).
Du, W. et al. Clinical characteristics of COVID-19 in children compared with adults in Shandong Province, China. Infection 48 , 445–452 (2020).
CAS PubMed Google Scholar
Feng, K. et al. Analysis of CT features of 15 children with 2019 novel coronavirus infection. Chin. J. Pediatr. 58 , E007 (2020).
Guan, W. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382 , 1708–1720 (2020).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 , 497–506 (2020).
Jiang, J. et al. Epidemiological and clinical characteristics of novel coronavirus infection in children: thoughts on the diagnostic criteria of suspected cases outside Hubei Province. Chin. Pediatr. Emerg. Med. 12 , E003 (2020).
Kam, K. et al. A well infant with coronavirus disease 2019 with high viral load. Clin. Infect. Dis . https://doi.org/10.1093/cid/ciaa201 (2020).
Kamali Aghdam, M., Jafari, N. & Eftekhari, K. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect. Dis. (Auckl.) 52 , 427–429 (2020).
Li, B., Shen, J., Li, L. & Yu, C. Radiographic and clinical features of children with coronavirus disease (COVID-19) pneumonia. Indian Pediatr. 57 , 423–426 (2020).
Li, W., Cui, H., Li, K., Fang, Y. & Li, S. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr. Radiol. 50 , 796–799 (2020).
Liu, J. et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 55 , 102763 (2020).
Liu, W. et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N. Engl. J. Med. 382 , 1370–1371 (2020).
Liu, Y. et al. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. Preprint at medRxiv https://doi.org/10.1101/2020.02.17.20024166 (2020).
Lu, X. et al. SARS-CoV-2 infection in children. N. Engl. J. Med. 382 , 1663–1665 (2020).
Yao-Ling, M. A. et al. Clinical features of children with SARS-CoV-2 infection: an analysis of 115 cases. Chin. J. Contemp. Pediatr. 22 , 290–293 (2020).
Qiu, H. et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect. Dis. 20 , 689–696 (2020).
Phan, L. T. et al. Importation and human-to-human transmission of a novel coronavirus in vietnam. N. Engl. J. Med. 382 , 872–874 (2020).
Schwartz, D. A. & Graham, A. L. Potential maternal and infant outcomes from coronavirus 2019-nCoV (SARS-CoV-2) infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses 12 , 194 (2020).
Shen, Q. et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr. Pulmonol. 55 , 1424–1429 (2020).
Sinha, I. P. et al. COVID-19 infection in children. Lancet Respir. Med. 8 , 446–447 (2020).
Song, F. et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295 , 210–217 (2020).
Su, L. et al. The different clinical characteristics of corona virus disease cases between children and their families in China—the character of children with COVID-19. Emerg. Microbes Infect. 9 , 707–713 (2020).
Sun, D. et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J. Pediatr. 16 , 251–259 (2020).
Tan, X. et al. Clinical features of children with SARS-CoV-2 infection: an analysis of 13 cases from Changsha, China. Zhongguo Dang Dai Er Ke Za Zhi 22, 294–298 (2020).
Tan, Y. et al. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J. Clin. Virol. 127 , 104353 (2020).
Tong, Z.-D. et al. Potential presymptomatic transmission of SARS-CoV-2, Zhejiang Province, China, 2020. Emerg. Infect. Dis. 26 , 1052–1054 (2020).
Wang, D. et al. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Chin. J. Pediatr. 58 , 269–274 (2020).
Wang, D. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323 , 1061 (2020).
Wang, H., Li, Y., Wang, F., Du, H. & Lu, X. Rehospitalization of a recovered coronavirus disease 19 (COVID-19) child with positive nucleic acid detection. Pediatr. Infect. Dis. J. 39 , e69–e70 (2020).
Wei, M. et al. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA 323 , 1313–1314 (2020).
CAS PubMed Central PubMed Google Scholar
Xia, W. et al. Clinical and CT features in pediatric patients with COVID‐19 infection: different points from adults. Pediatr. Pulmonol. 55 , 1169–1174 (2020).
Xing, Y.-H. et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J. Microbiol. Immunol. Infect. 53 , 473–480 (2020).
Xu, Y. et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 26 , 502–505 (2020).
Yang, P., Liu, P., Li, D. & Zhao, D. Corona virus disease 2019, a growing threat to children? J. Infect. 80 , 671–693 (2020).
Yang, X. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 8 , 475–481 (2020).
Zeng, L. K. et al. First case of neonate infected with novel coronavirus pneumonia in China. Chin. J. Pediatr. 58 , E009 (2020).
Zhang, J. et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy 75 , 1730–1741 (2020).
Zhang, M. Q. et al. Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing. Chin. J. Tuberc. Respir. Dis. 43 , E013 (2020).
Yuehua, Z. et al. 2019 novel coronavirus infection in a three-month-old baby. Chin. J. Pediatr. 58 , 182–184 (2020).
Zheng, F. et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr. Med. Sci. 40 , 275–280 (2020).
Zhou, Y. et al. Clinical features and chest CT findings of coronavirus disease 2019 in infants and young children. Zhongguo Dang Dai Er Ke Za Zhi 22 , 215–220 (2020).
Download references
Acknowledgements
This work was supported by funding from the UK Medical Research Council under a concordat with the UK Department for International Development (no. MR/R015600/1 to M.B, L.O, P.W., A.G. and T.C), the Wellcome Trust (no. 200222/Z/15/Z to T.C.) and the Bill and Melinda Gates Foundation (to A.G.). We thank all those who facilitated collation of the data provided by the NMEP in Nigeria. The maps in the figures were prepared using GADM v.3.6.
Author information
These authors contributed equally: Ellie Sherrard-Smith, Alexandra B. Hogan, Arran Hamlet, Oliver J. Watson, Charlie Whittaker.
Authors and Affiliations
MRC Centre for Global Infectious Disease Analysis, Imperial College London, London, UK
Ellie Sherrard-Smith, Alexandra B. Hogan, Arran Hamlet, Oliver J. Watson, Charlie Whittaker, Peter Winskill, Mara D. Kont, Joseph D. Challenger, Robert Verity, Ben Lambert, Marc Baguelin, Lilith K. Whittles, John A. Lees, Sangeeta Bhatia, Edward S. Knock, Lucy Okell, Hannah C. Slater, Azra C. Ghani, Patrick G. T. Walker & Thomas S. Churcher
National Malaria Elimination Programme, Abuja, Nigeria
Fatima Ali, Audu B. Mohammad, Perpetua Uhomoibhi, Ibrahim Maikore, Nnenna Ogbulafor, Jamilu Nikau & Okefu Oyale Okoko
Tropical Epidemiology Group, London School of Hygiene and Tropical Medicine, London, UK
Matthew Cairns
Manson Unit, Médecins Sans Frontières (Operational Centre Amsterdam), London, UK
Bhargavi Rao
Department of Infectious Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK
Marc Baguelin
PATH, Seattle, WA, USA
Hannah C. Slater
You can also search for this author in PubMed Google Scholar
Contributions
A.C.G., P.G.T.W., O.O.O. and T.S.C. conceived the study. E.S.-S., A.B.H., A.H., O.J.W., C.W. P.W., H.C.S., A.C.G., P.G.T.W. and T.S.C. designed the models. P.W., F.A., A.B.M., P.U., I.M., N.O., J.N., M.D.K., J.D.C., R.V., B.L., M.C., B.R., M.B., L.K.W., J.A.L., S.B., E.S.K., L.O. and H.C.S. provided input parameters and analyses. E.S.-S., A.B.H. and A.H. processed the data. E.S.-S., A.B.H., A.H., A.C.G., P.G.T.W., O.O.O. and T.S.C. wrote the first draft of the manuscript. All authors interpreted the results, contributed to writing and approved the final version for submission.
Corresponding author
Correspondence to Thomas S. Churcher .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information Jennifer Sargent was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 projected deaths due to covid-19 and malaria in sub-saharan africa (ssa) over time for different covid-19 scenarios..
The top row shows the COVID-19 epidemic and the number of people needing oxygen support per week for four different COVID-19 scenarios—an unmitigated epidemic (red), mitigation (blue), continued suppression (green), and suppression lift (purple). The thin dotted horizontal grey line indicates estimated healthcare capacity for a typical African country. The thick black horizontal line beneath each figure shows the period when COVID-19 mitigation or suppression activities are assumed in operation. The upper middle row indicates the assumed duration of interruption where COVID-19 interventions affect different malaria prevention activities (IRS = indoor residual spraying, LLINs = mass distribution of long-lasting insecticide treated nets, SMC = seasonal malaria chemoprevention) or case management of clinical cases with the level of this disruption presented in Table 2 . The lower middle row shows the predicted deaths due to COVID-19 per week in each scenario. The bottom row shows predicted malaria deaths per week for each scenario (coloured lines) and for the counter-factual with no COVID-19 induced disruption (black lines). The top coloured lines indicate a scenario when all services are reduced or cease (Table 2 , row 1) whereas the bottom dashed coloured lines show the most well-managed scenario (Table 2 , row 3). Grey lines in all rows show other scenarios to allow direct comparison.
Extended Data Fig. 2
Maps showing the impact of COVID-19 based interruption of malaria control activities for the a , Unmitigated, b , Suppression and c , Suppression lift scenarios. As shown in Fig. 2a for the COVID-19 mitigated scenario, estimated additional deaths per million people when all malaria interventions are ceased (long-lasting insecticide treated net distribution campaigns, seasonal malaria chemoprevention, and clinical treatment of cases) relative to normal service in the absence of COVID-19 for each administrative region. The different periods of service disruption are shown in Extended Data Fig. 1 . Maps illustrate how overall impact depend on the timing and duration of the period of service interruption and how this overlaps with malaria transmission seasons in different regions of Africa and were made in GADM 3.6 ( https://gadm.org/ ).
Extended Data Fig. 3 Univariate and multivariate sensitivity analyses of the effect of model parameters on the magnitude and duration of COVID-19 epidemics in sub-Saharan Africa.
a , Univariate sensitivity analysis showing the differences in the number of people needing supplemental oxygen, and the duration of the epidemic for four value of R 0 (2–3.5) across the four COVID-19 intervention scenarios (using default parameters shown in Supplementary Table 11 ). Note that in the mitigation scenario a 6-month period of social distancing results in a rebound epidemic for R 0 values < 3.0. In this plot the dotted lines show the same runs with 12 months of social distancing measures which prevents the rebound epidemic. b–e , Multivariate sensitivity analysis for the COVID-19 mitigation scenario using the range of parameters outlined in Supplementary Table 11 . b , Epidemic trajectories for the 500 different simulations showing the variability in the shape of the epidemic. Runs are coloured according to the potential period that malaria services might be interrupted which are estimated from the different individual epidemic curves. This period of service disruption starts from when mitigation measures are initiated and continues until the time healthcare capacity is no-longer over-burdened. c , the relationship between the assumed level of R 0 and level of social distancing during the mitigation period (% reduction in the contact rate) and the period of service interruption. Each point represents a single realisation of the 500 runs. Values where healthcare was still over capacity a year after the arrival of the epidemic are grouped at a year of service interruption. d , A histogram showing period of potential service interruption for the 500 runs. Bar colour indicates the numbers of deaths due to COVID-19 for the different simulations and show a high number of COVID-19 deaths can occur with a short period of interruption (for example, from a high R 0 ) or from an epidemic that causes a longer period of disruption (for example, a low R 0 and a rebound epidemic once mitigation measures are relaxed). The period of service disruption used in the default mitigation scenario in the main paper analysis is shown with a vertical dashed line in panel ( e ). Histogram showing the distribution of the number of COVID-19 deaths from the 500 runs of the multivariate sensitivity analysis. This distribution of was used to generate 95% uncertainty interval estimates for COVID-19 mitigation scenario deaths in Table 1 . Histogram colours show the R 0 values used in that simulation.
Extended Data Fig. 4 Maps showing how the impact malaria mitigation strategies are predicted to vary across sub-Saharan Africa.
a , Expansion of existing seasonal malaria chemoprevention (SMC) in regions where it occurred in the Sahel where it was conducted in 2019. Colours denote additional lives saved by expanding the age of those eligible from under 5 years to under 15 years. b , The predicted impact of mass drug administration (MDA) using a drug with a prophylactic profile of amodiaquine plus sulfadoxine-pyrimethamine for regions where SMC is not currently conducted. Both figures show scenarios where existing LLIN campaigns were maintained but routine treatment of clinical cases paused during the mitigated COVID-19 scenario.
Extended Data Fig. 5 Sub-national impact of how interruption of malaria services due to a mitigation COVID-19 scenario will influence the numbers of malaria deaths in different states of Nigeria.
The colour indicates the additional deaths predicted due to service interruption relative to normal service in the absence of COVID-19 for each state. Here the top row corresponds to the scenarios where net distribution is maintained and MDA is expanded, as per Supplementary Table 10 , and the bottom row corresponds to scenarios where net distribution is maintained and seasonal malaria chemoprevention is expanded as per Supplementary Table 8 . Grey areas denote states where this control intervention was not considered. Expansion of SMC was only evaluated in regions which undertook SMC in 2019 (bottom row) whilst MDA was considered in all other states (top row). All simulations assume that sufficient drugs are available. Maps were made in GADM 3.6 ( https://gadm.org/ ).
Extended Data Fig. 6 Nigeria-specific data inputs for the malaria model estimations.
a , Malaria prevalence by microscopy in children 6–59 months of age. b , Percentage of children sleeping under an insecticidal net the previous night. c , Estimates of seasonal malaria chemoprevention coverage calculated by dividing the number of doses administered by the proportion of the target age group. d , Estimates of the percentage of child malaria cases receiving artemisinin combination therapy (ACT). Figures ( a ), ( b ) and ( d ) were estimated from Demographic and Health Surveys (DHS) data. All estimates were at the state level other than ( d ) which was presented at the regional level. Maps were made in GADM 3.6 ( https://gadm.org/ ).
Extended Data Fig. 7 Multivariate uncertainty analysis for the malaria transmission model.
The true model uncertainty was quantified by calculating the additional clinical malaria cases, malaria deaths, and years of life lost due to malaria, using an additional 20 draws from the joint posterior distribution of the fitted model parameters. These simulations were performed for all 37 administrative 1 units in Nigeria, and 40 other units across four countries—Zambia (all provinces included), Mozambique, Democratic Republic of the Congo and Burkina Faso—and for each COVID-19 and malaria scenario. We used the outcomes for the Nigeria administrative units to calculate the coefficient of variation (CoV) and tested the application of a Normal approximation to compare the uncertainty intervals (UI) for the other countries. a , Shows the 95% UI for each of the four countries estimated from the different model runs (pink and purple error bars) with values estimated from the Normal approximation and fitted CoV values (red bars). Results indicate that the uncertainty generated using both methods was broadly similar. b , Illustration of how malaria parameter uncertainty influences estimates of the additional weekly deaths due to malaria in Nigeria over the year May 2020–April 2021 for each of the four COVID-19 scenarios. Two different levels of malaria service interruption are considered for each scenario, the first where LLINs and SMC are ceased and case management is reduced by 50% (pink line, Supplementary Table 1 row 1), and the second when only case management is reduced by 50% (purple line, Supplementary Table 1 , row 3). The solid dark lines represent best guess model predictions for the additional malaria deaths (difference between the levels of malaria service interruption and no COVID-19 induced disruption) whilst the shaded regions represent the 95% UIs generated by varying the input parameters within plausible ranges.
Supplementary information
Supplementary Tables 1–13.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Sherrard-Smith, E., Hogan, A.B., Hamlet, A. et al. The potential public health consequences of COVID-19 on malaria in Africa. Nat Med 26 , 1411–1416 (2020). https://doi.org/10.1038/s41591-020-1025-y
Download citation
Received : 07 May 2020
Accepted : 23 July 2020
Published : 07 August 2020
Issue Date : September 2020
DOI : https://doi.org/10.1038/s41591-020-1025-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Llin evaluation in uganda project (llineup): modelling the impact of covid-19-related disruptions on delivery of long-lasting insecticidal nets on malaria indicators in uganda.
- Jaffer Okiring
- Samuel Gonahasa
- Ellie Sherrard-Smith
Malaria Journal (2024)
Rapid classification of epidemiologically relevant age categories of the malaria vector, Anopheles funestus
- Emmanuel P. Mwanga
- Doreen J. Siria
- Fredros O. Okumu
Parasites & Vectors (2024)
The behaviour of adult Anopheles gambiae, sub-Saharan Africa’s principal malaria vector, and its relevance to malaria control: a review
- Willem Takken
- Derek Charlwood
- Steve W. Lindsay
Novel Plasmodium falciparum k13 gene polymorphisms from Kisii County, Kenya during an era of artemisinin-based combination therapy deployment
- Josephat Nyabayo Maniga
- Mong’are Samuel
- Saheed Adekunle Akinola
Malaria Journal (2023)
Effects of the COVID-19 pandemic on general health and malaria control in Ghana: a qualitative study with mothers and health care professionals
- Anna-Katharina Heuschen
- Alhassan Abdul-Mumin
- Olaf Müller
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pan Afr Med J
- v.41(Suppl 1); 2022
Malaria outbreak in Mbale: it´s the pits! a case study
Daniel kadobera.
1 Uganda National Institute of Public Health, P.O Box 7272, Kampala, Uganda,
2 Ministry of Health, Kampala, Uganda,
Gloria Bahizi
Lilian bulage, benon kwesiga, stephen ndugwa kabwama.
3 College of Health Sciences, Makerere University School of Public Health, Kampala, Uganda,
Alex Riolexus Ario
Julie roberts harris.
4 US Centers for Disease Control and Prevention, Kampala, Uganda,
5 Division of Global Health Protection, Center for Global Health, US Centers for Disease Control and Prevention, Atlanta, United States of America
Malaria is a leading cause of morbidity and mortality in Uganda. In June 2019, the Uganda Ministry of Health through routine surveillance data analysis was notified of an increase in malaria cases in Bumbobi and Nyondo Sub-counties, Mbale District, which exceeded the action thresholds. We investigated to assess outbreak magnitude, identify transmission risk factors, and recommend evidence-based control measures. We defined a confirmed case as a positive malaria result using malaria Rapid Diagnostic Test or microscopy from 1 Jan 2019 to 30 Jun 2019 in a resident or visitor of Bumbobi or Nyondo Sub-county, Mbale District. We reviewed medical records to develop a line list for descriptive epidemiology. In a case-control study, we compared exposures between 150 case-persons and 150 age- and village-matched asymptomatic controls. We conducted environmental and entomological assessments on vector dynamics and behavior. We identified 7,891 case-persons (attack rate [AR]=26%). Females (AR=36%) were more affected than males (AR=25%). The 5-18 year age group (AR=26%) was most affected. The epidemic curve showed steady increase in malaria cases from March following intermittent rainfall from January, with short spells of no rainfall up to June. In the matched pair case-control analysis, 95% (143/150) of case-patients and 49% (73/150) of controls had soil erosion control pits near their homes that held stagnant water for several days following rainfall (AOR=18, 95%CI=7-50); Active breeding sites were found near and within homesteads with Anopheles gambiaeas the predominant vector. Increased vector breeding sites due to erosion control pits sustained by the intermittent rainfall caused this outbreak. We recommended draining of pits immediately after the rains and increasing coverage for bed-nets.
How to use this case study
General instructions: case studies in applied epidemiology allow students to practice applying epidemiologic skills in the classroom to address real-world public health problems. The case studies are used as a vital component of an applied epidemiology curriculum, rather than as stand-alone tools. They are ideally suited to reinforce principles and skills already covered in a lecture or in background reading.
This case study has a facilitator guide and a participant guide. Each facilitator should review the Facilitator Guide, gain familiarity with the outbreak and investigation on which the case study is based, review the epidemiologic principles being taught, and think of examples in the facilitator´s own experience to further illustrate the points. Ideally, participants receive the case study one part at a time during the case study session. However, if the case study is distributed whole, participants should be asked not to look ahead.
During the case study session, one or two instructors facilitate the case study for 8 to 20 students in a classroom or conference room. The facilitator should hand out Part I and direct a participant to read one paragraph out loud, then progressing around the room and giving each participant a chance to read. Reading out loud and in turns has two advantages. First, all participants engage in the process and overcome any inhibitions by having her/his voice heard. Second, it keeps the all participants progressing through the case study at the same speed.
After a participant reads a question, the facilitator will direct participants to answer the question by perform calculations, construct graphs, or engage in a discussion of the answer. Sometimes, the facilitator can split the class to play different roles or take different sides in answering the question. As a result, participants learn from each other, not just from the facilitator. After the questions have been answered, the facilitator hands out the next part. At the end of the case study, the facilitator should direct a participant to once again read the objectives on page 1 to review and ensure that the objectives have been met.
Prerequisites: for this case study, participants should have received instruction or conducted readings in: Outbreak investigation; Intermediate epidemiology (interpreting odds ratio, epidemic curves, etc.); Basics of malaria epidemiology.
Target audience: trainees in the Uganda Field Epidemiology Training Program/Public Health Fellowship Program, other Field Epidemiology and Laboratory Training Programs (FELTPs), public health students, public health workers who may participate in rapid needs assessments and others who are interested in this topic.
Level of case study: intermediate or advanced
Time required : approximately 4 hours
Language : English
Case study material
- Download the case study student guide (PDF - 559 KB)
- Request the case study facilitator guide
Acknowledgement
We would like to appreciate key staff of the President´s Malaria Initiative, Dr. Mame Niang, and Dr. Kassahun Belay together with other officers from Makerere University School of Public Health and AFENET that made it possible for us to access funds for this case study development. We appreciate the Rapid Responders who worked tirelessly in the field to collect data that we used to develop the case study. We are grateful to the US CDC leadership, Dr. Lisa J. Nelson for the invaluable support.
Cite this article: Daniel Kadobera et al. Malaria outbreak in Mbale: it´s the pits! a case study. Pan African Medical Journal. 2022;41(1):3. 10.11604/pamj.supp.2022.41.1.31194
Competing interests
The authors declare no competing interests.
University of South Florida
Main Navigation

Rays H.Y. Jiang, PhD
In new study, USF researchers connect Florida malaria outbreak with South and Central America
- July 19, 2024
- Research & Innovation
For people in the United States, an outbreak of malaria might seem exotic, a health issue in a remote or far-away part of the world. But malaria − an insidious, mosquito-borne disease carried by a parasite − knows no boundaries and refuses to leave the global stage. In fact, the deadly Plasmodium falciparum kills an estimated 600,000 people a year, 95 percent of them children under age 5.
That’s one reason a University of South Florida College of Public Health researcher and her colleagues are concerned about any appearance of malaria in the United States, and they diligently identified and tracked a recent outbreak here in the Sunshine State.
“Despite malaria having been eradicated from the United States more than 70 years ago, and local transmissions having ceased for about 20 years, the risk persists,’’ said Rays H.Y. Jiang , PhD, an associate professor with the USF Genomics Program . “Malaria remains a significant global infectious disease that could potentially affect the United States again.’’
Jiang and a dozen researchers from Florida published new findings in the Emerging Infectious Diseases Journal of the Centers for Disease Control and Prevention in Atlanta. Titled “ Autochthonous Plasmodium vivax Infections , Florida,” the paper underscores the usefulness and power of genomic tools in epidemiologic investigations, such as tracing the origins of malaria infection.

“It’s important to note that the risk of malaria in Florida remains low, and local transmission events like these are promptly stopped with all patients recovering,’’ Jiang added. “However, raising awareness, particularly among physicians in Florida, is crucial. This ensures early detection and appropriate treatment.’’
The CDC paper focused on a cluster of seven patients at hospitals around Florida who in 2023 were diagnosed with Plasmodium vivax malaria. Experts believe they were infected while visiting Central and South America.
All seven patients were concentrated within a four-mile radius, raising concern about potential local transmission cycles. To trace the origin of the infections, researchers isolated DNA from blood samples and ran it though highly sophisticated screening and comparison tests.
Jiang and her team examined the genomic characteristics, probable transmission dynamics, and likely origins of the 2023 strain in Florida, demonstrating the role of genomic epidemiology (tracking pathogens) and the scale of intervention required to prevent infection or outbreak.
“In sum, our phylogenomic analysis support the interpretation of a single, limited introduction event from Central/South America into Florida,’’ the study states. “Although the risk for autochthonous (local) malaria in the U.S. remains low, the potential threat of imported P. vivax setting off and establishing local transmission … in conducive environments is a public health concern.’’
Malaria is most prevalent in parts of Africa, where strained health care systems struggle with prevention techniques and treatment. While malaria isn’t endemic in Florida, the state is home to the Anopheles mosquito, which is responsible for spreading the disease. Untreated malaria caused by P. falciparum , a protozoan parasite, can be life threatening.

Half the world’s population is at risk for malaria, a mosquito-borne disease becoming increasingly resistant to the drug artemisinin. (Photo courtesty of USF Health Communications)
In addition, Florida’s long summers and mild winters allow mosquitoes more time to breed. This could be exacerbated by global warming, experts believe, as entire populations of insects might migrate from their normal home.
“Climate change could create more suitable habitats for Anopheles mosquitoes, which thrive in warm and wet environments,’’ Jiang said. “This could potentially lead to an expansion of their geographic range, increasing the risk of disease transmission.’’
Jiang collaborated on the CDC paper with researchers from USF, Tampa General Hospital and the Florida Department of Health, including co-senior authors Drs. Liwang Cui and Kami Kim, along with lead author Swamy Adapa and contributing author Dr. John Adams. The team hopes their research leads to new pathways in solving the riddle of malaria on a world-wide scale.
“Malaria remains a significant global health challenge, particularly in regions where it is endemic,’’ Jiang said. “With no highly effective vaccines currently available, early detection and treatment are crucial.’’
Return to article listing
Rays H.J. Jiang
Explore More Categories
- Alumni & Development
- Awards & Honors
- College News
- Community Engagement
- In the Media
- Student Life
- USF SafetyFlorida

About Department News
Welcome to the USF COPH news page. Our marketing and communications team is entrusted with storytelling. Through written stories, photography, video and social media we highlight alumni, faculty, staff and students who are committed to passionately solving problems and creating conditions that allow every person the universal right to health and well-being. These are our stories.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Case-control studies of severe malaria
Affiliation.
- 1 Tropical Health Epidemiology Unit, London School of Hygiene and Tropical Medicine, UK.
- PMID: 1597871
The majority of children infected with Plasmodium falciparum in areas of stable endemicity do not develop severe, life-threatening disease. It is important to identify risk factors for the minority who do. Case-control studies in which children with severe disease are compared with children with non-severe disease and with community controls, avoid some of the ethical and logistical problems inherent in such an undertaking. This paper discusses methodological aspects of case-control studies of severe malaria including case and control definitions, selection of cases and controls, potential risk factors, sample size calculations and analysis. Although specifically concerned with malaria, many of these issues are equally relevant to case-control studies of other infectious and parasitic diseases in a tropical environment.
PubMed Disclaimer
Similar articles
- Epidemiological features of severe paediatric malaria in north western Ethiopia. Seboxa T, Snow RW. Seboxa T, et al. East Afr Med J. 1997 Dec;74(12):780-3. East Afr Med J. 1997. PMID: 9557422
- [Pertinence of the 2000 WHO criteria in non-immune children with severe malaria in Dakar, Senegal ]. Imbert P, Gérardin P, Rogier C, Jouvencel P, Brousse V, Guyon P, Ka AS. Imbert P, et al. Bull Soc Pathol Exot. 2003 Aug;96(3):156-60. Bull Soc Pathol Exot. 2003. PMID: 14582287 French.
- Childhood malaria in East London. Ladhani S, El Bashir H, Patel VS, Shingadia D. Ladhani S, et al. Pediatr Infect Dis J. 2003 Sep;22(9):814-9. doi: 10.1097/01.inf.0000086401.13592.79. Pediatr Infect Dis J. 2003. PMID: 14506374
- Clinical and parasitological studies on immunity to Plasmodium falciparum malaria in children. Høgh B. Høgh B. Scand J Infect Dis Suppl. 1996;102:1-53. Scand J Infect Dis Suppl. 1996. PMID: 9060051 Review.
- Methodologic issues of case-control studies: a review of established and newly recognized limitations. D'Agata EM. D'Agata EM. Infect Control Hosp Epidemiol. 2005 Apr;26(4):338-41. doi: 10.1086/502548. Infect Control Hosp Epidemiol. 2005. PMID: 15865267 Review. No abstract available.
- Risk Associated with Malaria Infection in Tihama Qahtan, Aseer Region, Kingdom of Saudi Arabia: 2006-2007. Alshahrani AM, Abdelgader TM, Mohya M, Jubran S, Abdoon A, Daffalla AA, Babiker A, Kyalo D, Noor AM, Al-Zahrani MH, Snow RW. Alshahrani AM, et al. Malar Control Elimin. 2019 Jun 25;5(2):144. doi: 10.4172/2470-6965/1000144. Epub 2016 Apr 30. Malar Control Elimin. 2019. PMID: 31286096 Free PMC article.
- Malaria risk in young male travellers but local transmission persists: a case-control study in low transmission Namibia. Smith JL, Auala J, Haindongo E, Uusiku P, Gosling R, Kleinschmidt I, Mumbengegwi D, Sturrock HJ. Smith JL, et al. Malar J. 2017 Feb 10;16(1):70. doi: 10.1186/s12936-017-1719-x. Malar J. 2017. PMID: 28187770 Free PMC article.
- G6PD gene variants and its association with malaria in a Sri Lankan population. Dewasurendra RL, Rockett KA, Fernando SD, Carter R, Kwiatkowski DP, Karunaweera ND; MalariaGEN Consortium. Dewasurendra RL, et al. Malar J. 2015 Feb 22;14:93. doi: 10.1186/s12936-015-0603-9. Malar J. 2015. PMID: 25885177 Free PMC article.
- Risk factors for border malaria in a malaria elimination setting: a retrospective case-control study in Yunnan, China. Xu JW, Liu H, Zhang Y, Guo XR, Wang JZ. Xu JW, et al. Am J Trop Med Hyg. 2015 Mar;92(3):546-51. doi: 10.4269/ajtmh.14-0321. Epub 2015 Jan 19. Am J Trop Med Hyg. 2015. PMID: 25601994 Free PMC article.
- Increased risk for severe malaria in HIV-1-infected adults, Zambia. Chalwe V, Van geertruyden JP, Mukwamataba D, Menten J, Kamalamba J, Mulenga M, D'Alessandro U. Chalwe V, et al. Emerg Infect Dis. 2009 May;15(5):749; quiz 858. doi: 10.3201/eid1505.081009. Emerg Infect Dis. 2009. PMID: 19402961 Free PMC article.
- Search in MeSH
Related information
Linkout - more resources.
- Genetic Alliance
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Research and Innovation
- The Abstract
- Audio Abstract Podcast
- Centennial Campus
- Campus Life
- Faculty and Staff
- Awards and Honors
- HR and Finance
- Resilient Pack
- We Are the Wolfpack
- Service and Community
- Red Chair Chats
- News Releases
- In the News
- NC State Experts on 2024 Elections
- NC State Experts Available on Climate
- NC State Experts on Roe v. Wade
- NC State Supply Chain Experts
- Experts on COVID-19
- Hurricane Experts
- About NC State News
- Faculty Support
- Training Program
The Unintended Consequences of Success Against Malaria

For Immediate Release
For decades, insecticide-treated bed nets and indoor insecticide spraying regimens have been important – and widely successful – treatments against mosquitoes that transmit malaria, a dangerous global disease. Yet these treatments also – for a time – suppressed undesirable household insects like bed bugs, cockroaches and flies.
Now, a new North Carolina State University study reviewing the academic literature on indoor pest control shows that as the household insects developed resistance to the insecticides targeting mosquitoes, the return of these bed bugs, cockroaches and flies into homes has led to community distrust and often abandonment of these treatments – and to rising rates of malaria.
In short, the bed nets and insecticide treatments that were so effective in preventing mosquito bites – and therefore malaria – are increasingly viewed as the causes of household pest resurgence.
“These insecticide-treated bed nets were not intended to kill household pests like bed bugs, but they were really good at it,” said Chris Hayes, an NC State Ph.D. student and co-corresponding author of a paper describing the work. “It’s what people really liked, but the insecticides are not working as effectively on household pests anymore.”
“Non-target effects are usually harmful, but in this case they were beneficial,” said Coby Schal, Blanton J. Whitmire Distinguished Professor of Entomology at NC State and co-corresponding author of the paper.
“The value to people wasn’t necessarily in reducing malaria, but was in killing other pests,” Hayes added. “There’s probably a link between use of these nets and widespread insecticide resistance in these house pests, at least in Africa.”
The researchers add that other factors – famine, war, the rural/city divide, and population displacement, for example – also could contribute to rising rates of malaria.
To produce the review, Hayes combed through the academic literature to find research on indoor pests like bed bugs, cockroaches and fleas, as well as papers on malaria, bed nets, pesticides and indoor pest control. The search yielded more than 1,200 papers, which, after an exhaustive review process, was whittled down to a final count of 28 peer-reviewed papers fulfilling the necessary criteria.
One paper – a 2022 survey of 1,000 households in Botswana – found that while 58% were most concerned with mosquitoes in homes, more than 40% were most concerned with cockroaches and flies.
Hayes said a recent paper – published after this NC State review was concluded – showed that people blamed the presence of bed bugs on bed nets.
“There is some evidence that people stop using bed nets when they don’t control pests,” Hayes said.
The researchers say that all hope is not lost, though.
“There are, ideally, two routes,” Schal said. “One would be a two-pronged approach with both mosquito treatment and a separate urban pest management treatment that targets pests. The other would be the discovery of new malaria-control tools that also target these household pests at the same time. For example, the bottom portion of a bed net could be a different chemistry that targets cockroaches and bed bugs.
“If you offer something in bed nets that suppresses pests, you might reduce the vilification of bed nets.”
The study appears in Proceedings of the Royal Society B . The review was supported in part by the Blanton J. Whitmire Endowment at NC State, and grants from the U.S. Department of Housing and Urban Development Healthy Homes program (NCHHU0053-19), the Department of the Army, U.S. Army Contracting Command, Aberdeen Proving Ground, Natick Contracting Division, Ft. Detrick, Maryland (W911QY1910011), and the Triangle Center for Evolutionary Medicine (257367).
-kulikowski-
Note to editors : The abstract of the paper follows.
“Review on the impacts of indoor vector control on domiciliary pests: good intentions challenged by harsh realities”
Authors: Chris Hayes and Coby Schal, NC State University
Published: July 24, 2024 in Proceedings of the Royal Society B
DOI: 10.1098/rspb.2024.0609
Abstract : Arthropod vectored diseases have been a major impediment to societal advancements globally. Strategies to mitigate transmission of these diseases include preventative care (e.g., vaccination), primary treatment, and most notably the suppression of vectors in both indoor and outdoor spaces. The outcomes of indoor vector control (IVC) strategies, such as long-lasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS), are heavily influenced by individual and community-level perceptions and acceptance. These perceptions, and therefore product acceptance, are largely influenced by the successful suppression of non-target nuisance pests such as bed bugs and cockroaches. Adoption and consistent use of LLINs and IRS is responsible for immense reductions in the prevalence and incidence of Malaria. However, recent observations suggest that failed control of indoor pests, leading to product distrust and abandonment, may threaten vector control program success and further derail already slowed progress towards malaria elimination. We review the evidence of the relationship between IVC and nuisance pests and discuss the dearth of research on this relationship. We make the case that the ancillary control of indoor nuisance and public health pests needs to be considered in the development and implementation of new technologies for malaria elimination.
- Designing Healthy and Resilient Societies
- college of agriculture and life sciences
- Department of Entomology and Plant Pathology
- pesticide resistance
More From NC State News

Climate is most important factor in where mammals choose to live, study finds

Array Pinpoints Imprinted Genes with Potential Links to Disease

How a ‘Digital Twin’ Can Make Wireless Networks Faster, More Reliable
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
July 25, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Exploring the dynamics of combating market-driven epidemics
by Public Library of Science

A case definition of market-driven epidemics (MDEs) could help address critical barriers to timely, effective prevention and mitigation, according to a study published this week in PLOS Global Public Health by Jonathan Quick from Duke University School of Medicine, U.S., and colleagues.
The misuse and overconsumption of certain consumer products have become major global risk factors for premature deaths at all ages, with their total costs in trillions of dollars. Progress in reducing such deaths has been difficult, slow, and too often unsuccessful. To address this challenge, Quick and colleagues introduced a case definition of MDEs, which arise when companies aggressively market products with proven harms, deny these harms, and actively oppose mitigation efforts.
To demonstrate the application of this concept, the researchers selected three MDE products: cigarettes, sugar, and prescription opioids. Based on the histories of these three epidemics, the researchers described five MDE phases: market expansion, evidence of harm, corporate resistance, mitigation, and market adaptation.
From the peak of consumption to the most recent available data, U.S. cigarette sales fell by 82%, sugar consumption by 15%, and prescription opioid prescriptions by 62%. In each case, the consumption tipping point occurred when compelling evidence of harm, professional alarm, and an authoritative public health voice or public mobilization overcame the impact of corporate marketing and resistance efforts.
Among the three epidemics, the gap between suspicion of harm and the consumption tipping point ranged from one to five decades—much of which was attributable to the time required to generate sufficient evidence of harm. Market adaptation to the reduced consumption of target products had both negative impacts (e.g., geographical shift of corporate marketing efforts) and positive impacts (e.g., consumer shift away from sugar-sweetened beverages).
According to the authors, this is the first comparative analysis of three successful efforts to change the product consumption patterns of millions of people—and over time, some of the associated adverse health impacts of these products. The MDE epidemiological approach of shortening the latent time between phases provides the global health community with a new method to address existing and emerging potentially harmful products and their health, social, and economic impacts.
While the specific product and circumstances are unique to each MDE, understanding the epidemiology of consumption and health impacts , and epidemic milestones, should help public health leaders combat current MDEs and more swiftly recognize future MDEs. Given the similar patterns among different MDEs, public health leaders, researchers, civil society and others can apply the mitigation strategies presented in the review article to save lives and lessen the impact of continuing and emerging MDEs.
The authors add, "The use of cigarettes and other unhealthy products costs the world millions of lives and trillions of dollars each year. An analysis of U.S. progress against three such market-driven epidemics demonstrates that we can save lives through earlier, more decisive action by public health leaders, researchers, and public mobilization."
They conclude, "The use of cigarettes and other unhealthy products often follows patterns similar to infectious disease epidemics, causing widespread harm before any public health response. We can save lives by recognizing these market-driven epidemics earlier and acting more decisively to control them."
Explore further
Feedback to editors

Short-term vegan diet associated with reductions in biological age estimates
42 minutes ago

Study finds big disparities in stroke services across the US
Jul 27, 2024
Study identifies biomarker that could predict whether colon cancer patients benefit from chemotherapy
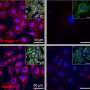
New study shows 'dancing molecules' can regenerate cartilage in 3 days
Jul 26, 2024
Study uncovers key immune cells for combating aggressive Merkel cell carcinoma

Study finds unhealthy air quality from wildfires may impact fertility treatments

Higher CEO pay in large health care systems linked to hospital consolidations, study suggests
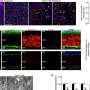
Researchers discover potential therapeutic target for degenerative eye disease
Researchers move a step closer to developing at-home test to detect dementia

Double mastectomy may offer no survival benefit to women with breast cancer
Related stories.

As federal menthol ban languishes, Black smokers are left to the mercy of marketers: Study
May 2, 2024

Experts recommend caution on the use of non-sugar sweeteners
Jan 22, 2024

Health care providers should talk with adult smokers about relative risks of tobacco products, including e-cigarettes
Apr 16, 2024

Increased e-cigarette sales elevate concern about continued availability of flavored products
Jun 23, 2023

Study shows public perception of e-cigarettes vs. cigarettes harms changed sharply during pandemic
Jun 8, 2022

Heated tobacco: The risks and benefits
Jan 7, 2022
Recommended for you

New study highlights global disparities in activity limitations and assistive device use
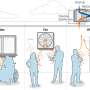
Scientists sets out seven steps to achieve clean indoor air post-pandemic
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Open access
- Published: 25 July 2024
Retrospective study on the dengue fever outbreak in Puntland State, Somalia
- Saaid Said Jama 1 , 2 , 3 , 4 ,
- Said Nuriye Abshir 1 , 2 , 3 , 4 ,
- Jibril Said Jama 4 &
- Mohamed Mohamud Abdi 3
BMC Infectious Diseases volume 24 , Article number: 735 ( 2024 ) Cite this article
19 Accesses
Metrics details
Dengue infection is a mosquito-borne, endemic viral disease, particularly in developing countries. Here, we report the results of the clinicodemographic, serologic profile and the monthly occurrence of a recent dengue fever outbreak in Puntland State (Somalia).
Methodology
We analyzed the data of 956 dengue-suspected patients who were investigated using the rapid diagnostic testing (RDT) method for detecting NS1 (dengue virus non-structural protein) and IgM antibodies employing the SD Biosensor Dengue Dou NS Ag and IgM test kit (Germany) at the Puntland Public Health Referral Laboratory from November 21, 2022, to May 27, 2023.
We found that 118 cases were positive for dengue among the suspected patients enrolled in the present study. Of these cases, 76.2% were dengue NSI positive, 13.6% were dengue IgM positive, and 10.2% were both NSI and IgM positive. The number of females and males in the confirmed cases was equal, and most (48.3%) were aged 20 years or less. 43.1% of them lived in the Nugal region, particularly in Garowe. Clinically, fever was the most frequent symptom (88.9%). The cases peaked in December 2022 but dropped from January to March, with a slight rise in February, and then increased in April and May 2023.
This study highlights the clinicodemographic characteristics, seroprevalence, and monthly occurrence of dengue in Puntland. We recommend improving vector control measures, enhancing case management, strengthening dengue surveillance, developing an early warning system, and conducting future studies to characterize the circulating strains.
Peer Review reports
Dengue, one of the relevant public health issues, especially in tropical and subtropical countries, is a systemic febrile illness caused by the dengue virus (DENV), a single-stranded RNA virus that belongs to the genus Flavivirus and consists of four serotypes (DEN 1–4), transmitted to humans by the bite of the Aedes aegypti mosquito [ 1 , 2 ]. The spread of the mosquito has been significantly aided by major demographic shifts, including population expansion, unplanned urbanization leading to poor housing, and the need for water storage [ 3 ].
Clinically, the illness can be asymptomatic or have manifestations such as fever, headache, retro-orbital pain, muscle and joint pain, rash, lymphadenopathy, hepatomegaly, nausea, and vomiting. In addition, some patients experience severe abdominal pain, persistent vomiting, tachypnea, hemorrhagic presentations, and neurological and mildly altered mental status features, which are considered to be warning signs [ 4 , 5 , 6 ].
Currently, serological tests used for diagnosing dengue infection rely on detecting either dengue non-structural protein 1 (NS1) antigen or dengue immunoglobulin M (IgM) through the enzyme-linked immunosorbent assay (ELISA) method [ 7 ]. Unfortunately, these tests might not be available in resource-limited settings. Therefore, less-sensitive, rapid diagnostic tests (RDTs) detecting dengue NS1 antigen or IgM are frequently used in these areas. Combining the two RDT tests raises their sensitivity for detecting dengue to 88.7% [ 8 ].
About 2.5 billion (> 40% of the world’s population) are at risk of dengue. According to World Health Organization (WHO) estimates, there may be 50–100 million dengue infections annually worldwide [ 9 ]. Epidemics of dengue have been reported in several African nations [ 10 ]. In Somalia, an outbreak of the dengue disease has been documented as early as 1985 in Hargeisa [ 11 ]. Also, infections of DENV-1, DENV-2, and DENV-3 and co-infections of DENV-1/2 and DENV-2/3 serotypes were identified in the Mogadishu outbreak in 2011 [ 12 ]. Recently, dengue was reported in the country in October 2022 [ 13 ]. In Puntland State (Somalia), the first case of dengue fever from the Sol region (Las Anod district) was confirmed on 21 November 2022. To our knowledge, no onset of dengue has been previously reported in the Puntland State. The present study, therefore, aims to describe the clinicodemographic, serologic profile, and monthly occurrence of a recent dengue fever outbreak in Puntland State (Somalia).
Study design
A descriptive observational, retrospective study was conducted using secondary data from the Puntland Public Health Referral Laboratory in the period from November 21, 2022, to May 27, 2023.
This study was carried out in the Puntland State of Somalia, which is located in the northeastern part of the country and bordered by the Indian Ocean to its east and the Gulf of Aden to the north. The Puntland State consists of nine regions, namely, Bari, Karkar, Sanag, Nugal, Mudug, Sool, Ayn, Haylan, and Gardafue.
Study population and sample size
All dengue-suspected cases tested and registered in the computerized record of the Puntland Public Health referral laboratory during the study period were included in the study. Patients with incomplete data on demographic, clinical, and serological profiles were excluded. A total of 956 dengue-suspected patients were included in this study.
Diagnostic tool
All patients were tested for dengue rapid diagnostics of both NS1 and IgM using the SD Biosensor Dengue Dou NS Ag and IgM test kit (Germany) at the Puntland Public Health Lab. The SD Biosensor DENV NS1 has a sensitivity and specificity of 90.0%/90.2%, whereas the anti-DENV IgM has a sensitivity and specificity of 71.8%/83.5%.
Data collection tool
Data were obtained from the laboratory in an Excel sheet. The data regarding demographic characteristics (sex, age, region, and district), clinical symptoms, testing dates, and results of dengue IgM and NS1 RDTs were collected.
Statistical analysis
The data, obtained in a Microsoft Excel sheet, was checked for repetition. The data were complete regarding demographic characteristics, testing dates, and serologic results. Data were then imported into Statistical Package for the Social Sciences (SPSS) 20, and descriptive analysis was carried out by frequencies, and percentages and presented in tables and figures.
Demographic characteristics of the dengue cases
Of the 956 dengue-suspected patients examined, we found that 118 were dengue-positive. The number of dengue-positive females and males was equal. Most of the dengue cases (48.3%) were aged 20 years or younger, and the age group (21–40 years) represented 42.4%, while the remaining 9.3% were 41 years of age or older (Table 1 ).
Regarding the geographic distribution of the dengue-positive cases, inhabitants of the Nugal region represented 43.1%, of which 38.1% were living in the Garowe district and 5% were in the Burtinle district. Residents of Mudug region (Galkayo district), Sool region (Las Anod district), and Karkar region (Gardo district) infected with dengue were 16.1%, 15.3%, and 13.6%, respectively, whereas 9.3% of the cases were from Bari region (Bossaso district). Finally, 2.5% of the cases were residents of the Sanag region (Dhahar district), as shown in Table 1 .
Clinical profile of dengue cases
Furthermore, the clinical symptoms observed in the dengue-infected patients and their frequencies after we excluded 10 cases with missing clinical symptoms are summarized in Table 2 .
Severity of the dengue patients
In this study, only 7 patients aged 18 years or less were hospitalized; of them, 4 were females and 3 were males (Table 3 ).
Serology profile of the dengue-positive cases
Finally, as reported in Table 4 , out of the 956 dengue-suspected patients examined in the present study, 118 cases were confirmed dengue-positive. The current study showed that 90 (76.2%) of the dengue cases were dengue NSI positive, 16 (13.6%) of them were dengue IgM positive, and the remaining 12 (10.2%) were both dengue IgM and NSI positive ( Table 4 ).
Monthly distribution of dengue cases
The frequency of occurrence of the dengue cases varied during the study period. The monthly distribution of the dengue cases in Puntland is reported in Fig. 1 .
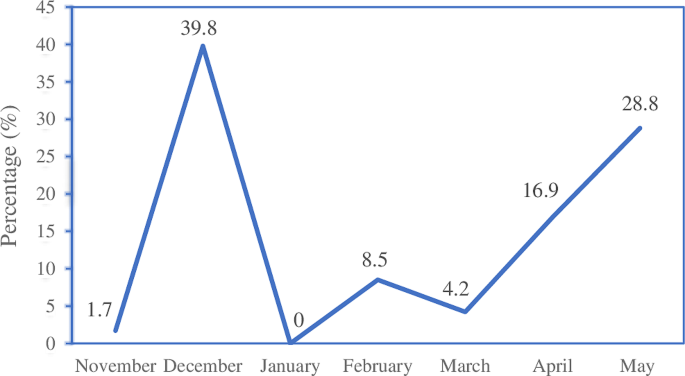
Monthly occurrence of dengue cases
Currently, dengue infection is endemic in many parts of the world [ 14 ]. Patients with this infection may present with various clinical features that resemble other acute febrile illnesses, making its diagnosis difficult [ 15 ]. Therefore, efficient clinical and laboratory evaluations are cornerstones of care for dengue-suspected patients.
Of the 956 dengue-suspected patients examined in this study, we found 118 dengue positive cases. The number of males and females infected was equal, contrasting the results of earlier reports, where female preponderance was observed [ 16 , 17 , 18 , 19 ]. This observation also contrasts with other studies that found a higher rate of dengue infection among males [ 20 , 21 , 22 ]. Further, we found that most (48.3%) of the infected cases were 20 years of age or less. This result is comparable to that (52%) reported in Darfur, Sudan [ 23 ]. The higher vulnerability of this age group to the disease might be due to their exposure to mosquito bites in schools, during outdoor activities, and indoor biting since many of them stay home longer. Also, the clinical features are known to be more apparent in children [ 24 ]. In the present study, we investigated the distribution of the disease in all regions of Puntland State (Somalia) (see Table 1 ). Most of the dengue-positive cases were in the Nugal region, particularly in Garowe, the capital city of Puntland. This city has experienced rapid population growth, fast urbanization, an increase in internally displaced persons (IDPs) camps, and the influx of large returnees and migrants [ 25 ]. The higher prevalence of dengue in Garowe could be related to these demographic factors and climate changes.
Clinically, dengue patients frequently present with a triad of fever, pain, and rash. Nevertheless, gastrointestinal and bleeding symptoms might occur in variable proportions [ 26 ]. Our study showed that fever was the most common clinical symptom (88.9%), which is consistent with earlier studies [ 27 , 28 ]. The second most frequent symptom (35.2%) was myalgia (Table 1 ). A higher frequency of myalgia among dengue patients was described before [ 5 , 29 , 30 ]. This was followed by skin rash and gastrointestinal symptoms, consistent with a study conducted in Saudi Arabia [ 31 ].
In our study, most of the dengue-positive cases were mild and treated as outpatients. Only seven pediatric cases required hospital admission. The increased risk of severe forms of dengue resulting in hospitalization and mortality among children in tropical areas has been documented [ 32 ]. Moreover, in pediatric patients, dengue frequently causes profound vascular leakage and abrupt shock [ 33 ].
In this study, the overall seroprevalence of dengue was found to be 118 (12.3%), which is lower than the results of other studies carried out in Kenya (61.2%) and Sudan (42%) [ 34 , 35 ]. The study also showed that 13.6% of dengue cases were dengue IgM antibody positive, which is in line with the 14.7% reported from Eastern Italy [ 36 ]. This result was lower than the 21% reported in northwest Ethiopia [ 37 ]. Furthermore, the present study revealed a higher positive rate (76.2%) of dengue NS1 among the study participants (Table 3 ). It is known that NS1 antibody is useful in the early stages of the disease, especially in the days 3–5 after the onset of the illness, when viremia levels might be undetectable and anti-IgM antibody levels have yet to rise [ 38 ]. Thus, the higher positive rates of NS1 in this study could be attributed to patients presenting in the acute phase of the illness. The discrepancies in the seroprevalence of dengue might be explained by the differences in environmental conditions, the sample sizes, and the diagnostic methods employed.
Rainfall and temperature play an important role in mosquito proliferation and the incidence of dengue illness [ 39 ]. The temperature in Puntland varies from 27 °C to 37 °C, and the spring season (April–June) is regarded as the principal rainy season, while the autumn season (October–December) is a short rainy season [ 40 ]. The seasonal distribution of dengue throughout the study period is reported in Fig. 1. Dengue cases peaked in December 2022, fell from January to March with a slight rise in February, and then increased in April and May 2023. The monthly variation in dengue cases could be due to weather changes and rainfall. A previous study revealed the impact of climate variability on the occurrence of dengue [ 29 ].
Although this study presents evidence of the dengue fever outbreak in Puntland State, Somalia, some limitations should be considered. Firstly, the study used secondary data subjected to incomplete or missing information. As discussed earlier, there were cases with missing clinical features. Secondly, we were unable to characterize the virus since we used serologic tests for the diagnosis. Thirdly, we were unable to identify the source of dengue infection or provide detailed information related to the disease transmission. Lastly, data on dengue case outcomes was not recorded, so assessing the patients’ outcomes was impossible.
In conclusion, we found that of the 956 dengue-suspected patients investigated, 118 cases were dengue-positive. Patients aged 20 years or younger were the most infected. Fever was the most frequent clinical symptom in the patients. Cases were highest in December 2022, followed by May 2023. We recommend improving vector control measures, enhancing case management, strengthening dengue surveillance, developing an early warning system, and conducting future studies to characterize the circulating strains.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
Rapid diagnostic tests
Dengue virus
Ribonucleic acid
Non-structural protein 1 antigen
Immunoglobulin M
Enzyme-linked immunosorbent assay
World Health Organization
Internally displaced persons
Unnikrishnan R, Faizal BP, Vijayakumar P, et al. A retrospective study on the socio-demographic factors and clinical parameters of dengue disease and their effects on the clinical course and recovery of the patients in a tertiary care hospital of Bangladesh. Ngondi JM, editor. J Fam Med Prim Care. 2015;4:369–72. https://doi.org/10.1371/journal.pntd.0010297 .
Article Google Scholar
Unnikrishnan R, Faizal BP, Vijayakumar P, et al. Clinical and laboratory profile of dengue in the elderly. J Fam Med Prim Care. 2015;4:369–72.
Maheshwari V, Gupta D, Parmar D. Clinico–epidemiological profile of confirmed cases of dengue infection: a tertiary care teaching hospital based study of central India. Indian J Pathol Oncol. 2020;5:531–5.
Castilho BM, Silva MT, Freitas ARR, et al. Factors associated with thrombocytopenia in patients with dengue fever: a retrospective cohort study. BMJ Open. 2020;10:e035120. https://doi.org/10.1136/bmjopen-2019-035120 . https://bmjopen.bmj.com/lookup/doi/ .
Article PubMed PubMed Central Google Scholar
Mohan K, Malaiyan J, Nasimuddin S, et al. Clinical profile and atypical manifestation of dengue fever cases between 2011 and 2018 in Chennai, India. J Fam Med Prim Care. 202AD;9:1119–23.
Alvarado-Castro VM, Ramírez-Hernández E, Paredes-Solís S, et al. Clinical profile of dengue and predictive severity variables among children at a secondary care hospital of Chilpancingo, Guerrero, Mexico: case series. Boletín Médico Del Hosp Infant México (English Ed. 2016;73:237–42.
Google Scholar
Chaloemwong J, Tantiworawit A, Rattanathammethee T, et al. Useful clinical features and hematological parameters for the diagnosis of dengue infection in patients with acute febrile illness: a retrospective study. BMC Hematol. 2018;18:1–10.
Luvira V, Thawornkuno C, Lawpoolsri S, et al. Diagnostic performance of Dengue NS1 and antibodies by serum concentration technique. Trop Med Infect Dis. 2023;8:1–12.
Mehta SR, Bafna TA, Aarati B. Pokale. Demographic and clinical spectrum of dengue patients admitted in a tertiary care hospital. Med J Dr DY Patil Vidyapeeth. 2018;11:128–31.
Were F. The dengue situation in Africa. Paediatr Int Child Health. 2012;32:18–21.
Botros BA, Watts DM, Soliman AK, et al. Serological evidence of dengue fever among refugees, Hargeysa, Somalia. J Med Virol. 1989;29:79–81.
Article CAS PubMed Google Scholar
Bosa HK, Montgomery JM, Kimuli I, et al. Dengue fever outbreak in Mogadishu, Somalia 2011: co-circulation of three dengue virus serotypes. Int J Infect Dis. 2014;21:3. https://doi.org/10.1016/j.ijid.2014.03.412 .
Somalia World Health Emergency Programme Update – Somalia. 2023;(Jan):1–5. https://reliefweb.int/attachments/bc3d1897-6915-4165-88c8-ca4549e5b584/Health-Emergency-Programme-January-2023.pdf .
World Health Organization (WHO). Dengue and severe dengue. 2023; https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue .
Prashanth E, Adnan I. Clinical profile of dengue infection at a center in north Karnataka, India. Glob J Infect Dis Clin Res. 2019;5:006–9.
Hussien HH. Epidemiological Descriptive Analysis of Disease Outbreaks in 2019 in Sudan. Open J Epidemiol. 2020;10:419–31.
Bello OA, Aminu M, Jatau ED. Seroprevalence of IgM Antibodies to Dengue Fever Virus among patients presenting with symptoms of Fever in some hospitals in Kaduna State, Nigeria. Int J Sci Res. 2016;5(3):1255–9.
Aniakwaa-Bonsu E, Amoako-Sakyi D, Dankwa K, et al. Seroprevalence of Dengue viral infection among adults attending the University of Cape Coast Hospital. Adv Infect Dis. 2021;11:60–72.
Fujimoto DE, Koifman S. Clinical and laboratory characteristics of patients with dengue hemorrhagic fever manifestations and their transfusion profile. Rev Bras Hematol Hemoter. 2014;36:115–20.
Woyessa AB, Mengesha M, Kassa W, et al. The first acute febrile illness investigation associated with dengue fever in Ethiopia, 2013: a descriptive analysis. Ethiop J Heal Dev. 2014;28:155–61.
Kumar A, Sarwari S, Ahmed Arain W, et al. A retrospective study on Laboratory Profile and Clinical parameters of Dengue Fever patients. Pakistan J Med Heal Sci. 2021;15:3357–9.
Riccò M, Peruzzi S, Balzarini F et al. Dengue fever in Italy: the eternal return of an emerging Arboviral Disease. Trop Med Infect Dis. 2022;7.
Ahmed A, Ali Y, Elmagboul B, et al. Dengue fever in the Darfur area, Western Sudan. Emerg Infect Dis. 2019;25:2125–6.
Article PubMed Central Google Scholar
Alera MT, Srikiatkhachorn A, Velasco JM, et al. Incidence of Dengue Virus infection in adults and children in a prospective longitudinal cohort in the Philippines. PLoS Negl Trop Dis. 2016;10:1–14.
UN-HABITAT. Puntland’s Ministry of Public Transport and Work. Garowe Urban Profile. www.unhabitat.org/somalia .
Hasan MJ, Tabassum T, Sharif M, et al. Comparison of clinical manifestation of dengue fever in Bangladesh: an observation over a decade. BMC Infect Dis. 2021;21:1–10. https://doi.org/10.1186/s12879-021-06788-z .
Article CAS Google Scholar
Obonyo M, Fidhow A, Ofula V. Investigation of laboratory confirmed dengue outbreak in north-eastern Kenya, 2011. PLoS ONE. 2018;13:1–11.
Islam QT, Sagor HB, Tuli TC. Et. Changing clinical pattern of Dengue Fever and its unusual Manifestations- 9 2019 outbreak in Dhaka, Bangladesh. J Bangladesh Coll Physicians Surg. 2020;39:9–18.
Al Awaidy ST, Khamis F, Al-Zakwani I et al. Epidemiological and clinical characteristics of patients with Dengue Fever in a recent outbreak in Oman: a single center retrospective-cohort study. Oman Med J. 2022;37.
Aziz BAA, Hassanien SEA, Abdou AM. Clinical and Hematological Effects of Dengue Viruses Infection. Am J Infect Dis Microbiol Vol 4, 2016, Pages 74–78. 2016;4:74–8. http://pubs.sciepub.com/ajidm/4/4/2/index.html , http://pubs.sciepub.com/ajidm/4/4/2/abstract.html .
Shahin W, Nassar A, Kalkattawi M, et al. Dengue fever in a tertiary hospital in Makkah, Saudi Arabia. Dengue Bull. 2009;33:34–44.
Tsheten T, Clements ACA, Gray DJ, Adhikary RK, Furuya-Kanamori L, Wangdi K. Clinical predictors of severe dengue: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10(1):1–10. https://doi.org/10.1186/s40249-021-00908-2 .
Idrus NL, Jamalid SM, Bakar AA, Embong H, Ahmad NS. Comparison of clinical and laboratory characteristics between severe and non-severe dengue in paediatrics. PLoS Negl Trop Dis. 2023;2023–December:1–10. https://doi.org/10.1371/journal.pntd.0011839 .
Lim JK, Matendechero SH, Alexander N, Lee JS, Lee KS, Namkung S, et al. Clinical and epidemiologic characteristics associated with dengue fever in Mombasa, Kenya. Int J Infect Dis. 2020;100:207–15. https://doi.org/10.1016/j.ijid.2020.08.074 .
Elaagip A, Alsedig K, Altahir O, et al. Seroprevalence and associated risk factors of dengue fever in Kassala state, eastern Sudan. PLoS Negl Trop Dis. 2020;14:e0008918. https://doi.org/10.1371/journal.pntd.0008918 .
Loconsole D, Metallo A, De Robertis AL, et al. Seroprevalence of Dengue Virus, West Nile Virus, Chikungunya Virus, and Zika Virus in International Travelers attending a travel and Migration Center in 2015–2017, Southern Italy. Vector-Borne Zoonotic Dis. 2018;18:331–4.
Ferede G, Tiruneh M, Abate E, et al. A serologic study of dengue in northwest Ethiopia: suggesting preventive and control measures. PLoS Negl Trop Dis. 2018;12:1–17.
Moi ML, Omatsu T, Tajima S, et al. Detection of dengue virus nonstructural protein 1 (NS1) by using ELISA as a useful laboratory diagnostic method for dengue virus infection of international travelers. J Travel Med. 2013;20:185–93.
Article PubMed Google Scholar
Morales I, Salje H, Saha S, et al. Seasonal distribution and climatic correlates of dengue disease in Dhaka, Bangladesh. Am J Trop Med Hyg. 2016;94:1359–61.
Puntland W. 2023. http://de.wikipedia.org/wiki/Puntland .
Download references
Acknowledgements
The authors would like to thank the Ministry of Health, (Puntland), and the Puntland Public Health Lab.
Not applicable.
Author information
Authors and affiliations.
Faculty of Medicine, University of Health Sciences, Bosaso, Puntland, Somalia
Saaid Said Jama & Said Nuriye Abshir
Health emergencies program, World Health Organization, Garowe, Puntland, Somalia
Ministry of Health (Puntland), Alula, Puntland, Somalia
Saaid Said Jama, Said Nuriye Abshir & Mohamed Mohamud Abdi
Ministry of Health (Puntland), Bosaso, Puntland, Somalia
Saaid Said Jama, Said Nuriye Abshir & Jibril Said Jama
You can also search for this author in PubMed Google Scholar
Contributions
SSJ designed the study, performed data analysis and interpretation, and drafted the manuscript. SNA and JSJ contributed to the collection, analysis, and interpretation of the data and the writing of the manuscript. MMA was involved in laboratory tests, the collection, analysis, and interpretation of data, and writing the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Correspondence to Saaid Said Jama .
Ethics declarations
Consent for publication, competing interests.
The authors declare no competing interests.
Ethical declaration
This study was approved by the ethical review committee (ERC) for the Ministry of Health of Puntland State (Somalia), and the need for consent to participate was waived by the ERC under approval number MOH-PL/ERC/2206. Permission was obtained from the General Directorate office of the Ministry. The study was performed according to the relevant guidelines and regulations.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Jama, S.S., Abshir, S.N., Jama, J.S. et al. Retrospective study on the dengue fever outbreak in Puntland State, Somalia. BMC Infect Dis 24 , 735 (2024). https://doi.org/10.1186/s12879-024-09552-1
Download citation
Received : 14 January 2024
Accepted : 21 June 2024
Published : 25 July 2024
DOI : https://doi.org/10.1186/s12879-024-09552-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Dengue fever
- Dengue outbreak
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]

- About UNAIDS
- Global AIDS Strategy 2021-2026
- United Nations declarations and goals
- UNAIDS governance
- UNAIDS Programme Coordinating Board
- Results and transparency portal
- UNAIDS Cosponsors
- UNAIDS ambassadors and global advocates
- UNAIDS leadership
- UNAIDS evaluation office
- UNAIDS ethics office
- UNAIDS transformation
- Sustainability of the HIV response
- Community pandemic response
- Education Plus Initiative
- Global alliance to end AIDS in children
- Equal access to cutting edge HIV technologies
- Save lives: Decriminalize
- Global council on inequality, AIDS and pandemics
- Resources and financing
- Global HIV Prevention Coalition
- Global Partnership to Eliminate Stigma and Discrimination
- 2025 AIDS targets
- AIDS and SDGs
- Community mobilization
- Fast-Track cities
- H6 partnership
- HIV prevention
- HIV treatment
- Human rights
- Key populations
- Private sector and the AIDS response
- Security and humanitarian affairs
- Social protection
- Universal health coverage
- Young people
Press centre
- Publications
- Infographics
- FAQ on HIV and AIDS
- World AIDS Day
- Zero Discrimination Day
- Latest data on HIV
- Data on key populations
- Laws and policies
- HIV financial resources
- Technical Support Mechanism
- Learn about HIV and AIDS
- Take action
- Become a donor
- Investment Book
Press Release

Press release
New unaids report shows aids pandemic can be ended by 2030, but only if leaders boost resources and protect human rights now.
GENEVA/MUNICH, 22 July 2024— A new report released today by UNAIDS shows that the
GENEVA/MUNICH, 22 July 2024— A new report released today by UNAIDS shows that the world is at a critical moment that will determine whether world leaders meet their commitment to end AIDS as a public health threat by 2030. The report, The Urgency of Now: AIDS at a Crossroads , brings together new data and case studies which demonstrate that the decisions and policy choices taken by world leaders this year will decide the fate of millions of lives and whether the world’s deadliest pandemic is overcome.
Whilst the end of AIDS is within our grasp, this decade, currently the world is off track. Globally, of the 39.9 million people living with HIV, 9.3 million, nearly a quarter, are not receiving life-saving treatment. As a consequence, a person dies from AIDS-related causes every minute.
Leaders pledged to reduce annual new infections to below 370 000 by 2025, but new HIV infections are still more than three times higher than that, at 1.3 million in 2023. And now cuts in resourcing and a rising anti-rights push are endangering the progress that has been made.
“World leaders pledged to end the AIDS pandemic as a public health threat by 2030, and they can uphold their promise, but only if they ensure that the HIV response has the resources it needs and that the human rights of everyone are protected,” said UNAIDS Executive Director, Winnie Byanyima . “Leaders can save millions of lives, prevent millions of new HIV infections, and ensure that everyone living with HIV can live healthy, full lives.”
The report finds that if leaders take the bold actions needed now to ensure sufficient and sustainable resourcing and protect everyone’s human rights, the number of people living with HIV, requiring life-long treatment, will settle at around 29 million by 2050 but if they take the wrong path, the number of people who will need life-long support will rise to 46 million (compared to 39.9 million in 2023).
The report shows continued (although slower) progress in rolling out medicines to people living with HIV with 30.7 million people now on treatment, more than 3 in 4 people living with HIV. As recently as 2010 treatment coverage stood at just 47%. The expansion of people accessing treatment is a landmark public health achievement that has seen AIDS-related deaths halved since 2010—from 1.3 million to 630 000 in 2023.
However, the world is off track to meet the 2025 target of reducing AIDS-related deaths to below 250 000.
Although tremendous progress has been made in preventing new HIV infections which have fallen by 39% since 2010 globally, and by 59% in eastern and southern Africa, the report shows that new HIV infections are rising in three regions, the Middle East and North Africa, Eastern Europe and central Asia and Latin America, and gaps and inequalities persist.
“Countries are making enormous progress to end the AIDS epidemic by 2030, however there have been many challenges that could slow our efforts,” said Dr Anthony Fauci, Former Scientific Advisor to the US President. “We must do everything we can to be continually vocal and proactive. Failure is not an option here. In fact, it is unthinkable. If we all work together, we shall meet our common goal. I for one will continue to work with all of my strength to make sure that we do indeed end the AIDS epidemic and I implore all of you to commit to the same.”
Gender inequality is exacerbating the risks faced by girls and women and driving the pandemic. HIV incidence among adolescent girls and young women is still extraordinarily high in parts of eastern and southern Africa and western and central Africa.
Because stigma and discrimination against marginalized communities create barriers to vital prevention and treatment services, key populations, including sex workers, men who have sex with men and people who inject drugs, represent an increased proportion at (55%) of new infections globally compared to 2010 (45%).
The report demonstrates that HIV prevention and treatment services will only reach people if human rights are upheld, if unfair laws against women and against marginalized communities are scrapped, and if discrimination and violence are tackled head on.
UNAIDS calculations show that whilst 20% of HIV resources should be dedicated towards HIV prevention for populations most affected by HIV, just 2.6% of total HIV spending went towards interventions for key populations in 2023.
Around the world funding is shrinking, holding back progress and even leading to rising epidemics in certain regions. In 2023, total resources available for HIV (US$ 19.8 billion) dropped by 5% from 2022 and were US$ 9.5 billion short of the amount needed by 2025 (US$ 29.3 billion). Domestic funding in low- and middle-income countries—which make up 59% of total resources for HIV—is being constrained by the debt crisis and fell for the fourth consecutive year, with a 6% decline from 2022 to 2023.
Increased resource mobilization is needed, especially in Asia and the Pacific—where the numbers of people living with HIV are projected to almost double by 2050—and in Eastern Europe and Central Asia, Latin America and the Middle East and North Africa, regions with growing epidemics, but where funding for HIV has decreased significantly. Around half of the total resources needed by 2025, and 93% of the current HIV funding gap, are outside of sub-Saharan Africa.
The Urgency of Now: AIDS at a Crossroads shows that decisions taken this year will determine if global targets are met, AIDS is ended as a public health threat by 2030, and a sustainable HIV response is built.
“The fraying of solidarity between and within countries is putting progress in danger, but the path that ends AIDS is a path that has been proven, and is a path that leaders have promised to take. Whether leaders fulfill their pledge to end AIDS is a political and financial choice. The time to choose the right path is now,” said Ms Byanyima.
The Joint United Nations Programme on HIV/AIDS (UNAIDS) leads and inspires the world to achieve its shared vision of zero new HIV infections, zero discrimination and zero AIDS-related deaths. UNAIDS unites the efforts of 11 UN organizations—UNHCR, UNICEF, WFP, UNDP, UNFPA, UNODC, UN Women, ILO, UNESCO, WHO and the World Bank—and works closely with global and national partners towards ending the AIDS epidemic by 2030 as part of the Sustainable Development Goals. Learn more at unaids.org and connect with us on Facebook , Twitter , Instagram and YouTube .
Watch report launch

crossroads.unaids.org

Executive summary
Video playlist

Core epidemiology slides

Data on HIV
Press release in other languages, opening remarks by winnie byanyima.

Regional profiles
- Asia and the Pacific
- Eastern Europe and Central Asia
- Eastern and Southern Africa
- Latin America
- Middle East and North Africa
- Western and Central Africa
- Western and Central Europe and North America

IMAGES
COMMENTS
A community-based case-control study with a 1:1 ratio was employed at Waghemra Zone from September 14 to November 27, 2022. ... the study findings showed the presence of a malaria outbreak in ...
1. Introduction. Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [].Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous ...
This case-control study verified the occurrence of a malaria outbreak in the Waghemra zone. Age, the availability of artificial water-holding bodies, nearby stagnant water, sleeping outside overnight, and a lack of knowledge about malaria transmission and prevention all contributed to the epidemic's existence.
An unmatched 1:1 case-control study was conducted in October 2017. This study employed the stages used in the investigation of an outbreak guidelines recommended by the Centre for Disease Control and Prevention (CDC) [].This is a guide to real time stepwise investigation of acute public health events at a local, national, or multinational level.
This documentary video discusses the epidemiology of malaria; strategies for prevention, including vector control and vaccines; and the pipeline of promising new drugs for the fight to eliminate ma...
We investigated to assess outbreak magnitude, identify transmission risk factors, and recommend evidence-based control measures. We defined a confirmed case as a positive malaria result using malaria Rapid Diagnostic Test or microscopy from 1 Jan 2019 to 30 Jun 2019 in a resident or visitor of Bumbobi or Nyondo Sub-county, Mbale District.
Global Malaria Epidemiology. The number of total malaria cases globally increased in 2021 (from 245 million in 2020 to 247 million in 2021), with most of the increase occurring in Africa. However, case incidence remained stable from 2020 to 2021 (59 cases per 1000 population at risk) following an increase from 2019 (57 cases/1000 population ...
The estimated number of global malaria cases in 2022 exceeded pre-COVID-19 pandemic levels in 2019, according to WHO's 2023 World malaria report. Several threats to the malaria global response are highlighted in the report, including climate change. The report, an annual assessment of global trends in malaria control and elimination, noted ...
Here, using a prospective case-control design, we investigated the role of An. stephensi in transmission following a malaria outbreak in Dire Dawa, Ethiopia in April-July 2022.
6. Early warning, detection and response to malaria outbreaks and epidemics 106 6.1 Definition and classification of epidemics 106 6.2 Epidemic curves of P. falciparum and P. vivax malaria 107 6.3 Factors that may contribute to epidemics 108 6.4 Definition of areas that are prone to epidemics 110 6.5 Surveillance system for epidemics 111
Overview. Malaria is a life-threatening disease spread to humans by some types of mosquitoes. It is mostly found in tropical countries. It is preventable and curable. The infection is caused by a parasite and does not spread from person to person. Symptoms can be mild or life-threatening. Mild symptoms are fever, chills and headache.
A 1:1 unmatched case-control study involving 80 cases and 80 controls was conducted using interviewer-administered questionnaires at household level. ... The malaria outbreak at Tongogara refugee camp reemphasizes the role of behavioural factors in malaria transmission. Intensified health education to address human behaviours that expose ...
Malaria is a leading cause of morbidity and mortality in Uganda. In June 2019, the Uganda Ministry of Health through routine surveillance data analysis was notified of an increase in malaria cases in Bumbobi and Nyondo Sub-counties, Mbale District, which exceeded the action thresholds. We investigated to assess outbreak magnitude, identify ...
The malaria elimination case‑study series ... epidemic Occurrence of cases in excess of the number expected in a given place and time. eradication Permanent reduction to zero of the worldwide incidence of infection caused by human malaria parasites as a result of deliberate efforts. Intervention measures are no longer needed once eradication ...
African highlands often suffer of devastating malaria epidemics, sometimes in conjunction with complex emergencies, making their control even more difficult. In 2000, Burundian highlands experienced a large malaria outbreak at a time of civil unrest, constant insecurity and nutritional emergency. ... The objective of this case study is to ...
Forecasting, warning, and detection of malaria epidemics: a case study Lancet. 2003 May 17;361(9370):1705-6. doi: 10.1016/S0140-6736(03)13366-1. ... Central and Gucha led to typical resurgent outbreaks whereas exceptional rainfall in Nandi and Kericho led to true malaria epidemics. Management of malaria in the highlands, including improved ...
A summary of the main findings, limitations and policy implications of our study is shown in Table 1. The pervasive and potentially large consequences of COVID-19 on African communities, such as ...
This case-study is part of a series of malaria elimination case-studies conducted by the WHO Global Malaria Programme and the University of California, San Francisco (UCSF), Global Health Group. The WHO Global Malaria Programme and the UCSF Global Health Group wish to acknowledge the financial support of the Bill & Melinda Gates Foundation in
Abstract. Malaria is a leading cause of morbidity and mortality in Uganda. In June 2019, the Uganda Ministry of Health through routine surveillance data analysis was notified of an increase in malaria cases in Bumbobi and Nyondo Sub-counties, Mbale District, which exceeded the action thresholds. We investigated to assess outbreak magnitude ...
of malaria epidemics: a case study Simon I Hay, Eric C Were, Melanie Renshaw, Abdisalan M Noor, Sam A Ochola, Iyabode Olusanmi, Nicholas Alipui, Robert W Snow Our aim was to assess whether a combination of seasonal climate forecasts, monitoring of meteorological conditions, and early detection of cases could have helped to prevent the 2002
In new study, USF researchers connect Florida malaria outbreak with South and Central America Kurt Loft; July 19, 2024; Research & Innovation; For people in the United States, an outbreak of malaria might seem exotic, a health issue in a remote or far-away part of the world. But malaria − an insidious, mosquito-borne disease carried by a ...
Our aim was to assess whether a combination of seasonal climate forecasts, monitoring of meteorological conditions, and early detection of cases could have helped to prevent the 2002 malaria emergency in the highlands of western Kenya. Seasonal climate forecasts did not anticipate the heavy rainfall. Rainfall data gave timely and reliable early warnings; but monthly surveillance of malaria out ...
Case-control studies in which children with severe disease are compared with children with non-severe disease and with community controls, avoid some of the ethical and logistical problems inherent in such an undertaking. This paper discusses methodological aspects of case-control studies of severe malaria including case and control definitions ...
The study appears in Proceedings of the Royal Society B. The review was supported in part by the Blanton J. Whitmire Endowment at NC State, and grants from the U.S. Department of Housing and Urban Development Healthy Homes program (NCHHU0053-19), the Department of the Army, U.S. Army Contracting Command, Aberdeen Proving Ground, Natick ...
The Report [1] also indicated that there was an increase in the number of malaria cases and deaths due to the interruption of malaria control strategies (for example, distribution of bed nets, testing, and treatment) attributable to COVID-19 pandemic; health units were overwhelmed by COVID-19 patients and malaria-infected individuals were less ...
Study teams abstracted malaria case management indicators from registers for January to November for 2013 and 2014 and interviewed health-care workers. Nationwide weekly surveillance data for suspect malaria cases reported between 2011 and 2014 were analysed independently. ... and to document the effect of the Ebola-virus-disease epidemic on ...
A case definition of market-driven epidemics (MDEs) could help address critical barriers to timely, effective prevention and mitigation, according to a study published this week in PLOS Global ...
The study appears in Proceedings of the Royal Society B. The review was supported in part by the Blanton J. Whitmire Endowment at NC State, and grants from the U.S. Department of Housing and Urban ...
Background Dengue infection is a mosquito-borne, endemic viral disease, particularly in developing countries. Here, we report the results of the clinicodemographic, serologic profile and the monthly occurrence of a recent dengue fever outbreak in Puntland State (Somalia). Methodology We analyzed the data of 956 dengue-suspected patients who were investigated using the rapid diagnostic testing ...
GENEVA/MUNICH, 22 July 2024—A new report released today by UNAIDS shows that the world is at a critical moment that will determine whether world leaders meet their commitment to end AIDS as a public health threat by 2030.The report, The Urgency of Now: AIDS at a Crossroads, brings together new data and case studies which demonstrate that the decisions and policy choices taken by world ...