An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager

Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults
Affiliations.
- 1 Thibodaux Regional Health System, Thibodaux, LA, USA. Electronic address: [email protected].
- 2 Unit of Innovation and Organization, Navarre Health Service, Spain. Electronic address: [email protected].
- 3 Institute of Evidence-Based Healthcare, Bond University, Gold Coast, QLD, Australia. Electronic address: [email protected].
- 4 Fielding School of Public Health and College of Letters and Science, University of California, Los Angeles, CA, USA. Electronic address: [email protected].
- 5 Geffen School of Medicine, University of California, Los Angeles, CA, USA. Electronic address: [email protected].
- 6 Clinical Excellence Research Center, School of Medicine, Stanford University, CA, USA. Electronic address: [email protected].
- 7 School of Pharmacy, University of Maryland, Baltimore, MD, USA. Electronic address: [email protected].
- PMID: 36055877
- PMCID: PMC9428332
- DOI: 10.1016/j.vaccine.2022.08.036
Introduction: In 2020, prior to COVID-19 vaccine rollout, the Brighton Collaboration created a priority list, endorsed by the World Health Organization, of potential adverse events relevant to COVID-19 vaccines. We adapted the Brighton Collaboration list to evaluate serious adverse events of special interest observed in mRNA COVID-19 vaccine trials.
Methods: Secondary analysis of serious adverse events reported in the placebo-controlled, phase III randomized clinical trials of Pfizer and Moderna mRNA COVID-19 vaccines in adults ( NCT04368728 and NCT04470427 ), focusing analysis on Brighton Collaboration adverse events of special interest.
Results: Pfizer and Moderna mRNA COVID-19 vaccines were associated with an excess risk of serious adverse events of special interest of 10.1 and 15.1 per 10,000 vaccinated over placebo baselines of 17.6 and 42.2 (95 % CI -0.4 to 20.6 and -3.6 to 33.8), respectively. Combined, the mRNA vaccines were associated with an excess risk of serious adverse events of special interest of 12.5 per 10,000 vaccinated (95 % CI 2.1 to 22.9); risk ratio 1.43 (95 % CI 1.07 to 1.92). The Pfizer trial exhibited a 36 % higher risk of serious adverse events in the vaccine group; risk difference 18.0 per 10,000 vaccinated (95 % CI 1.2 to 34.9); risk ratio 1.36 (95 % CI 1.02 to 1.83). The Moderna trial exhibited a 6 % higher risk of serious adverse events in the vaccine group: risk difference 7.1 per 10,000 (95 % CI -23.2 to 37.4); risk ratio 1.06 (95 % CI 0.84 to 1.33). Combined, there was a 16 % higher risk of serious adverse events in mRNA vaccine recipients: risk difference 13.2 (95 % CI -3.2 to 29.6); risk ratio 1.16 (95 % CI 0.97 to 1.39).
Discussion: The excess risk of serious adverse events found in our study points to the need for formal harm-benefit analyses, particularly those that are stratified according to risk of serious COVID-19 outcomes. These analyses will require public release of participant level datasets.
Keywords: Adverse events of special interest; Brighton Collaboration; COVID-19; COVID-19 vaccines; Coalition for Epidemic Preparedness Innovations; Moderna COVID-19 vaccine mRNA-1273; NCT04368728 ; NCT04470427 ; Pfizer-BioNTech COVID-19 vaccine BNT162b2; SARS-CoV-2; Safety Platform for Emergency vACcines; Serious adverse events; Vaccines; mRNA vaccines.
Copyright © 2022 Elsevier Ltd. All rights reserved.
PubMed Disclaimer
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
- Serious adverse events following mRNA vaccination in randomized trials in adults. Black S, Evans S. Black S, et al. Vaccine. 2023 May 26;41(23):3473-3474. doi: 10.1016/j.vaccine.2023.04.040. Epub 2023 Apr 28. Vaccine. 2023. PMID: 37121802 No abstract available.
Similar articles
- A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial. Akova M, Unal S. Akova M, et al. Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
- Use of COVID-19 Vaccines After Reports of Adverse Events Among Adult Recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 Vaccines (Pfizer-BioNTech and Moderna): Update from the Advisory Committee on Immunization Practices - United States, July 2021. Rosenblum HG, Hadler SC, Moulia D, Shimabukuro TT, Su JR, Tepper NK, Ess KC, Woo EJ, Mba-Jonas A, Alimchandani M, Nair N, Klein NP, Hanson KE, Markowitz LE, Wharton M, McNally VV, Romero JR, Talbot HK, Lee GM, Daley MF, Mbaeyi SA, Oliver SE. Rosenblum HG, et al. MMWR Morb Mortal Wkly Rep. 2021 Aug 13;70(32):1094-1099. doi: 10.15585/mmwr.mm7032e4. MMWR Morb Mortal Wkly Rep. 2021. PMID: 34383735 Free PMC article.
- Epidemiology of Myocarditis and Pericarditis Following mRNA Vaccination by Vaccine Product, Schedule, and Interdose Interval Among Adolescents and Adults in Ontario, Canada. Buchan SA, Seo CY, Johnson C, Alley S, Kwong JC, Nasreen S, Calzavara A, Lu D, Harris TM, Yu K, Wilson SE. Buchan SA, et al. JAMA Netw Open. 2022 Jun 1;5(6):e2218505. doi: 10.1001/jamanetworkopen.2022.18505. JAMA Netw Open. 2022. PMID: 35749115 Free PMC article.
- COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. Meo SA, et al. Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
- Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: living evidence syntheses and review. Pillay J, Gaudet L, Wingert A, Bialy L, Mackie AS, Paterson DI, Hartling L. Pillay J, et al. BMJ. 2022 Jul 13;378:e069445. doi: 10.1136/bmj-2021-069445. BMJ. 2022. PMID: 35830976 Free PMC article. Review.
- Reports of Batch-Dependent Suspected Adverse Events of the BNT162b2 mRNA COVID-19 Vaccine: Comparison of Results from Denmark and Sweden. Manniche V, Schmeling M, Gilthorpe JD, Hansen PR. Manniche V, et al. Medicina (Kaunas). 2024 Aug 19;60(8):1343. doi: 10.3390/medicina60081343. Medicina (Kaunas). 2024. PMID: 39202624 Free PMC article.
- Unlocking insights: Navigating COVID-19 challenges and Emulating future pandemic Resilience strategies with strengthening natural immunity. Wimalawansa SJ. Wimalawansa SJ. Heliyon. 2024 Jul 17;10(15):e34691. doi: 10.1016/j.heliyon.2024.e34691. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39166024 Free PMC article. Review.
- Comparison of Transient and Persistent Adverse Events After COVID-19 Vaccination: A Retrospective Analysis. Hikichi H, Fujioka Y, Saga A, Watanabe K, Hasegawa R, Moritoki Y, Ueki S. Hikichi H, et al. Cureus. 2024 Jun 28;16(6):e63410. doi: 10.7759/cureus.63410. eCollection 2024 Jun. Cureus. 2024. PMID: 39070394 Free PMC article.
- Adverse Events of COVID-19 Vaccines in the United States: Temporal and Spatial Analysis. Li Y, Li J, Dang Y, Chen Y, Tao C. Li Y, et al. JMIR Public Health Surveill. 2024 Jul 15;10:e51007. doi: 10.2196/51007. JMIR Public Health Surveill. 2024. PMID: 39008362 Free PMC article.
- Decoding trends in mRNA vaccine research: A comprehensive bibliometric study. Zhang C, Wang Y, Peng J, Wen X, Zhang Y, Li K, Du H, Hu X. Zhang C, et al. Hum Vaccin Immunother. 2024 Dec 31;20(1):2355037. doi: 10.1080/21645515.2024.2355037. Epub 2024 May 30. Hum Vaccin Immunother. 2024. PMID: 38813652 Free PMC article.
- Law B, Pim C. SO2-D2.1.3 Priority List of COVID-19 Adverse events of special interest [Internet]. 2021 Oct [cited 2022 Feb 17]. Available from: https://brightoncollaboration.us/wp-content/uploads/2021/11/SO2_D2.1.3_C... .
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. - PMC - PubMed
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. - PMC - PubMed
- Sadoff J., Gray G., Vandebosch A.n., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. - PMC - PubMed
- Health Canada. Search for clinical information on drugs and medical devices [Internet]. 2019 [cited 2021 Nov 9]. Available from: https://clinical-information.canada.ca/ .
Publication types
- Search in MeSH
Associated data
- Search in ClinicalTrials.gov
LinkOut - more resources
Full text sources.
- Elsevier Science
- Europe PubMed Central
- PubMed Central
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- News & Events
- FDA Newsroom
- Press Announcements
FDA Approves and Authorizes Updated mRNA COVID-19 Vaccines to Better Protect Against Currently Circulating Variants
FDA News Release
Today, the U.S. Food and Drug Administration approved and granted emergency use authorization (EUA) for updated mRNA COVID-19 vaccines (2024-2025 formula) to include a monovalent (single) component that corresponds to the Omicron variant KP.2 strain of SARS-CoV-2. The mRNA COVID-19 vaccines have been updated with this formula to more closely target currently circulating variants and provide better protection against serious consequences of COVID-19, including hospitalization and death. Today’s actions relate to updated mRNA COVID-19 vaccines manufactured by ModernaTX Inc. and Pfizer Inc.
In early June, the FDA advised manufacturers of licensed and authorized COVID-19 vaccines that the COVID-19 vaccines (2024-2025 formula) should be monovalent JN.1 vaccines. Based on the further evolution of SARS-CoV-2 and a rise in cases of COVID-19, the agency subsequently determined and advised manufacturers that the preferred JN.1-lineage for the COVID-19 vaccines (2024-2025 formula) is the KP.2 strain, if feasible.
“Vaccination continues to be the cornerstone of COVID-19 prevention,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “These updated vaccines meet the agency’s rigorous, scientific standards for safety, effectiveness, and manufacturing quality. Given waning immunity of the population from previous exposure to the virus and from prior vaccination, we strongly encourage those who are eligible to consider receiving an updated COVID-19 vaccine to provide better protection against currently circulating variants.”
The updated mRNA COVID-19 vaccines include Comirnaty and Spikevax, both of which are approved for individuals 12 years of age and older, and the Moderna COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, both of which are authorized for emergency use for individuals 6 months through 11 years of age.
What You Need to Know
- Unvaccinated individuals 6 months through 4 years of age are eligible to receive three doses of the updated, authorized Pfizer-BioNTech COVID-19 Vaccine or two doses of the updated, authorized Moderna COVID-19 Vaccine.
- Individuals 6 months through 4 years of age who have previously been vaccinated against COVID-19 are eligible to receive one or two doses of the updated, authorized Moderna or Pfizer-BioNTech COVID-19 vaccines (timing and number of doses to administer depends on the previous COVID-19 vaccine received).
- Individuals 5 years through 11 years of age regardless of previous vaccination are eligible to receive a single dose of the updated, authorized Moderna or Pfizer-BioNTech COVID-19 vaccines; if previously vaccinated, the dose is administered at least 2 months after the last dose of any COVID-19 vaccine.
- Individuals 12 years of age and older are eligible to receive a single dose of the updated, approved Comirnaty or the updated, approved Spikevax; if previously vaccinated, the dose is administered at least 2 months since the last dose of any COVID-19 vaccine.
- Additional doses are authorized for certain immunocompromised individuals ages 6 months through 11 years of age as described in the Moderna COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine fact sheets.
Individuals who receive an updated mRNA COVID-19 vaccine may experience similar side effects as those reported by individuals who previously received mRNA COVID-19 vaccines and as described in the respective prescribing information or fact sheets. The updated vaccines are expected to provide protection against COVID-19 caused by the currently circulating variants. Barring the emergence of a markedly more infectious variant of SARS-CoV-2, the FDA anticipates that the composition of COVID-19 vaccines will need to be assessed annually, as occurs for seasonal influenza vaccines.
For today’s approvals and authorizations of the mRNA COVID-19 vaccines, the FDA assessed manufacturing and nonclinical data to support the change to include the 2024-2025 formula in the mRNA COVID-19 vaccines. The updated mRNA vaccines are manufactured using a similar process as previous formulas of these vaccines. The mRNA COVID-19 vaccines have been administered to hundreds of millions of people in the U.S., and the benefits of these vaccines continue to outweigh their risks.
On an ongoing basis, the FDA will review any additional COVID-19 vaccine applications submitted to the agency and take appropriate regulatory action.
The approval of Comirnaty (COVID-19 Vaccine, mRNA) (2024-2025 Formula) was granted to BioNTech Manufacturing GmbH. The EUA amendment for the Pfizer-BioNTech COVID-19 Vaccine (2024-2025 Formula) was issued to Pfizer Inc.
The approval of Spikevax (COVID-19 Vaccine, mRNA) (2024-2025 Formula) was granted to ModernaTX Inc. and the EUA amendment for the Moderna COVID-19 Vaccine (2024-2025 Formula) was issued to ModernaTX Inc.
Related Information
- Comirnaty (COVID-19 Vaccine, mRNA) (2024-2025 Formula)
- Spikevax (COVID-19 Vaccine, mRNA) (2024-2025 Formula)
- Moderna COVID-19 Vaccine (2024-2025 Formula)
- Pfizer-BioNTech COVID-19 Vaccine (2024-2025 Formula)
- FDA Resources for the Fall Respiratory Illness Season
- Updated COVID-19 Vaccines for Use in the United States Beginning in Fall 2024
- June 5, 2024, Meeting of the Vaccines and Related Biological Products Advisory Committee
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, radiation-emitting electronic products, and for regulating tobacco products.
- Scoping Review
- Open access
- Published: 14 November 2021
Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis
- Qiao Liu 1 na1 ,
- Chenyuan Qin 1 , 2 na1 ,
- Min Liu 1 &
- Jue Liu ORCID: orcid.org/0000-0002-1938-9365 1 , 2
Infectious Diseases of Poverty volume 10 , Article number: 132 ( 2021 ) Cite this article
58k Accesses
220 Citations
369 Altmetric
Metrics details
To date, coronavirus disease 2019 (COVID-19) becomes increasingly fierce due to the emergence of variants. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance. We aimed to systematically evaluate the effectiveness and safety of COVID-19 vaccines in the real world and to establish a reliable evidence-based basis for the actual protective effect of the COVID-19 vaccines, especially in the ensuing waves of infections dominated by variants.
We searched PubMed, Embase and Web of Science from inception to July 22, 2021. Observational studies that examined the effectiveness and safety of SARS-CoV-2 vaccines among people vaccinated were included. Random-effects or fixed-effects models were used to estimate the pooled vaccine effectiveness (VE) and incidence rate of adverse events after vaccination, and their 95% confidence intervals ( CI ).
A total of 58 studies (32 studies for vaccine effectiveness and 26 studies for vaccine safety) were included. A single dose of vaccines was 41% (95% CI : 28–54%) effective at preventing SARS-CoV-2 infections, 52% (31–73%) for symptomatic COVID-19, 66% (50–81%) for hospitalization, 45% (42–49%) for Intensive Care Unit (ICU) admissions, and 53% (15–91%) for COVID-19-related death; and two doses were 85% (81–89%) effective at preventing SARS-CoV-2 infections, 97% (97–98%) for symptomatic COVID-19, 93% (89–96%) for hospitalization, 96% (93–98%) for ICU admissions, and 95% (92–98%) effective for COVID-19-related death, respectively. The pooled VE was 85% (80–91%) for the prevention of Alpha variant of SARS-CoV-2 infections, 75% (71–79%) for the Beta variant, 54% (35–74%) for the Gamma variant, and 74% (62–85%) for the Delta variant. The overall pooled incidence rate was 1.5% (1.4–1.6%) for adverse events, 0.4 (0.2–0.5) per 10 000 for severe adverse events, and 0.1 (0.1–0.2) per 10 000 for death after vaccination.
Conclusions
SARS-CoV-2 vaccines have reassuring safety and could effectively reduce the death, severe cases, symptomatic cases, and infections resulting from SARS-CoV-2 across the world. In the context of global pandemic and the continuous emergence of SARS-CoV-2 variants, accelerating vaccination and improving vaccination coverage is still the most important and urgent matter, and it is also the final means to end the pandemic.
Graphical Abstract
Since its outbreak, coronavirus disease 2019 (COVID-19) has spread rapidly, with a sharp rise in the accumulative number of infections worldwide. As of August 8, 2021, COVID-19 has already killed more than 4.2 million people and more than 203 million people were infected [ 1 ]. Given its alarming-spreading speed and the high cost of completely relying on non-pharmaceutical measures, we urgently need safe and effective vaccines to cover susceptible populations and restore people’s lives into the original [ 2 ].
According to global statistics, as of August 2, 2021, there are 326 candidate vaccines, 103 of which are in clinical trials, and 19 vaccines have been put into normal use, including 8 inactivated vaccines and 5 protein subunit vaccines, 2 RNA vaccines, as well as 4 non-replicating viral vector vaccines [ 3 ]. Our World in Data simultaneously reported that 27.3% of the world population has received at least one dose of a COVID-19 vaccine, and 13.8% is fully vaccinated [ 4 ].
To date, COVID-19 become increasingly fierce due to the emergence of variants [ 5 , 6 , 7 ]. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance [ 6 , 8 ]. Several reviews systematically evaluated the effectiveness and/or safety of the three mainstream vaccines on the market (inactivated virus vaccines, RNA vaccines and viral vector vaccines) based on random clinical trials (RCT) yet [ 9 , 10 , 11 , 12 , 13 ].
In general, RNA vaccines are the most effective, followed by viral vector vaccines and inactivated virus vaccines [ 10 , 11 , 12 , 13 ]. The current safety of COVID-19 vaccines is acceptable for mass vaccination, but long-term monitoring of vaccine safety is needed, especially in older people with underlying conditions [ 9 , 10 , 11 , 12 , 13 ]. Inactivated vaccines had the lowest incidence of adverse events and the safety comparisons between mRNA vaccines and viral vectors were controversial [ 9 , 10 ].
RCTs usually conduct under a very demanding research circumstance, and tend to be highly consistent and limited in terms of population characteristics and experimental conditions. Actually, real-world studies differ significantly from RCTs in terms of study conditions and mass vaccination in real world requires taking into account factors, which are far more complex, such as widely heterogeneous populations, vaccine supply, willingness, medical accessibility, etc. Therefore, the real safety and effectiveness of vaccines turn out to be a major concern of international community. The results of a mass vaccination of CoronaVac in Chile demonstrated a protective effectiveness of 65.9% against the onset of COVID-19 after complete vaccination procedures [ 14 ], while the outcomes of phase 3 trials in Brazil and Turkey were 50.7% and 91.3%, reported on Sinovac’s website [ 14 ]. As for the Delta variant, the British claimed 88% protection after two doses of BNT162b2, compared with 67% for AZD1222 [ 15 ]. What is surprising is that the protection of BNT162b2 against infection in Israel is only 39% [ 16 ]. Several studies reported the effectiveness and safety of the COVID-19 vaccine in the real world recently, but the results remain controversial [ 17 , 18 , 19 , 20 ]. A comprehensive meta-analysis based upon the real-world studies is still in an urgent demand, especially for evaluating the effect of vaccines on variation strains. In the present study, we aimed to systematically evaluate the effectiveness and safety of the COVID-19 vaccine in the real world and to establish a reliable evidence-based basis for the actual protective effect of the COVID-19 vaccines, especially in the ensuing waves of infections dominated by variants.
Search strategy and selection criteria
Our methods were described in detail in our published protocol [PROSPERO (Prospective register of systematic reviews) registration, CRD42021267110]. We searched eligible studies published by 22 July 2021, from three databases including PubMed, Embase and Web of Science by the following search terms: (effectiveness OR safety) AND (COVID-19 OR coronavirus OR SARS-CoV-2) AND (vaccine OR vaccination). We used EndNoteX9.0 (Thomson ResearchSoft, Stanford, USA) to manage records, screen and exclude duplicates. This study was strictly performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
We included observational studies that examined the effectiveness and safety of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines among people vaccinated with SARS-CoV-2 vaccines. The following studies were excluded: (1) irrelevant to the subject of the meta-analysis, such as studies that did not use SARS-CoV-2 vaccination as the exposure; (2) insufficient data to calculate the rate for the prevention of COVID-19, the prevention of hospitalization, the prevention of admission to the ICU, the prevention of COVID-19-related death, or adverse events after vaccination; (3) duplicate studies or overlapping participants; (4) RCT studies, reviews, editorials, conference papers, case reports or animal experiments; and (5) studies that did not clarify the identification of COVID-19.
Studies were identified by two investigators (LQ and QCY) independently following the criteria above, while discrepancies reconciled by a third investigator (LJ).
Data extraction and quality assessment
The primary outcome was the effectiveness of SARS-CoV-2 vaccines. The following data were extracted independently by two investigators (LQ and QCY) from the selected studies: (1) basic information of the studies, including first author, publication year and study design; (2) characteristics of the study population, including sample sizes, age groups, setting or locations; (3) kinds of the SARS-CoV-2 vaccines; (4) outcomes for the effectiveness of SARS-CoV-2 vaccines: the number of laboratory-confirmed COVID-19, hospitalization for COVID-19, admission to the ICU for COVID-19, and COVID-19-related death; and (5) outcomes for the safety of SARS-CoV-2 vaccines: the number of adverse events after vaccination.
We evaluated the risk of bias using the Newcastle–Ottawa quality assessment scale for cohort studies and case–control studies [ 21 ]. and assess the methodological quality using the checklist recommended by Agency for Healthcare Research and Quality (AHRQ) [ 22 ]. Cohort studies and case–control studies were classified as having low (≥ 7 stars), moderate (5–6 stars), and high risk of bias (≤ 4 stars) with an overall quality score of 9 stars. For cross-sectional studies, we assigned each item of the AHRQ checklist a score of 1 (answered “yes”) or 0 (answered “no” or “unclear”), and summarized scores across items to generate an overall quality score that ranged from 0 to 11. Low, moderate, and high risk of bias were identified as having a score of 8–11, 4–7 and 0–3, respectively.
Two investigators (LQ and QCY) independently assessed study quality, with disagreements resolved by a third investigator (LJ).
Data synthesis and statistical analysis
We performed a meta-analysis to pool data from included studies and assess the effectiveness and safety of SARS-CoV-2 vaccines by clinical outcomes (rates of the prevention of COVID-19, the prevention of hospitalization, the prevention of admission to the ICU, the prevention of COVID-19-related death, and adverse events after vaccination). Random-effects or fixed-effects models were used to pool the rates and adjusted estimates across studies separately, based on the heterogeneity between estimates ( I 2 ). Fixed-effects models were used if I 2 ≤ 50%, which represented low to moderate heterogeneity and random-effects models were used if I 2 > 50%, representing substantial heterogeneity.
We conducted subgroup analyses to investigate the possible sources of heterogeneity by using vaccine kinds, vaccination status, sample size, and study population as grouping variables. We used the Q test to conduct subgroup comparisons and variables were considered significant between subgroups if the subgroup difference P value was less than 0.05. Publication bias was assessed by funnel plot and Egger’s regression test. We analyzed data using Stata version 16.0 (StataCorp, Texas, USA).
A total of 4844 records were searched from the three databases. 2484 duplicates were excluded. After reading titles and abstracts, we excluded 2264 reviews, RCT studies, duplicates and other studies meeting our exclude criteria. Among the 96 studies under full-text review, 41 studies were excluded (Fig. 1 ). Ultimately, with three grey literatures included, this final meta-analysis comprised 58 eligible studies, including 32 studies [ 14 , 15 , 17 , 18 , 19 , 20 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 ] for vaccine effectiveness and 26 studies [ 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 ] for vaccine safety. Characteristics of included studies are showed in Additional file 1 : Table S1, Additional file 2 : Table S2. The risk of bias of all studies we included was moderate or low.
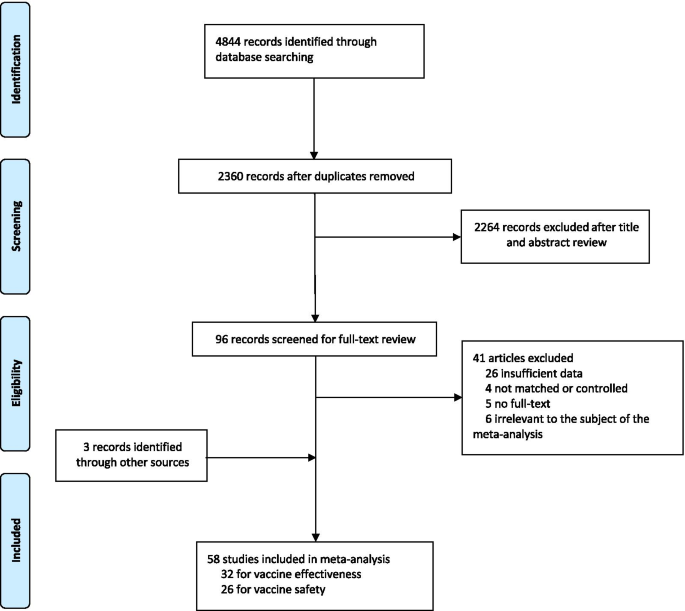
Flowchart of the study selection
Vaccine effectiveness for different clinical outcomes of COVID-19
We separately reported the vaccine effectiveness (VE) by the first and second dose of vaccines, and conducted subgroup analysis by the days after the first or second dose (< 7 days, ≥ 7 days, ≥ 14 days, and ≥ 21 days; studies with no specific days were classified as 1 dose, 2 dose or ≥ 1 dose).
For the first dose of SARS-CoV-2 vaccines, the pooled VE was 41% (95% CI : 28–54%) for the prevention of SARS-CoV-2 infection, 52% (95% CI : 31–73%) for the prevention of symptomatic COVID-19, 66% (95% CI : 50–81%) for the prevention of hospital admissions, 45% (95% CI : 42–49%) for the prevention of ICU admissions, and 53% (95% CI : 15–91%) for the prevention of COVID-19-related death (Table 1 ). The subgroup, ≥ 21 days after the first dose, was found to have the highest VE in each clinical outcome of COVID-19, regardless of ≥ 1 dose group (Table 1 ).
For the second dose of SARS-CoV-2 vaccines, the pooled VE was 85% (95% CI : 81–89%) for the prevention of SARS-CoV-2 infection, 97% (95% CI : 97–98%) for the prevention of symptomatic COVID-19, 93% (95% CI: 89–96%) for the prevention of hospital admissions, 96% (95% CI : 93–98%) for the prevention of ICU admissions, and 95% (95% CI : 92–98%) for the prevention of COVID-19-related death (Table 1 ). VE was 94% (95% CI : 78–98%) in ≥ 21 days after the second dose for the prevention of SARS-CoV-2 infection, higher than other subgroups, regardless of 2 dose group (Table 1 ). For the prevention of symptomatic COVID-19, VE was also relatively higher in 21 days after the second dose (99%, 95% CI : 94–100%). Subgroups showed no statistically significant differences in the prevention of hospital admissions, ICU admissions and COVID-19-related death (subgroup difference P values were 0.991, 0.414, and 0.851, respectively).
Vaccine effectiveness for different variants of SARS-CoV-2 in fully vaccinated people
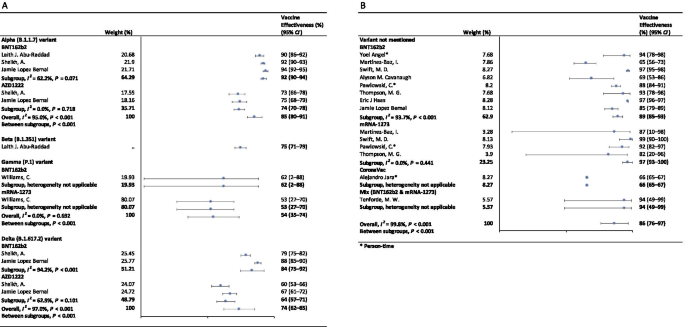
In the fully vaccinated groups (over 14 days after the second dose), the pooled VE was 85% (95% CI: 80–91%) for the prevention of Alpha variant of SARS-CoV-2 infection, 54% (95% CI : 35–74%) for the Gamma variant, and 74% (95% CI : 62–85%) for the Delta variant. There was only one study [ 23 ] focused on the Beta variant, which showed the VE was 75% (95% CI : 71–79%) for the prevention of the Beta variant of SARS-CoV-2 infection. BNT162b2 vaccine had the highest VE in each variant group; 92% (95% CI : 90–94%) for the Alpha variant, 62% (95% CI : 2–88%) for the Gamma variant, and 84% (95% CI : 75–92%) for the Delta variant (Fig. 2 ).
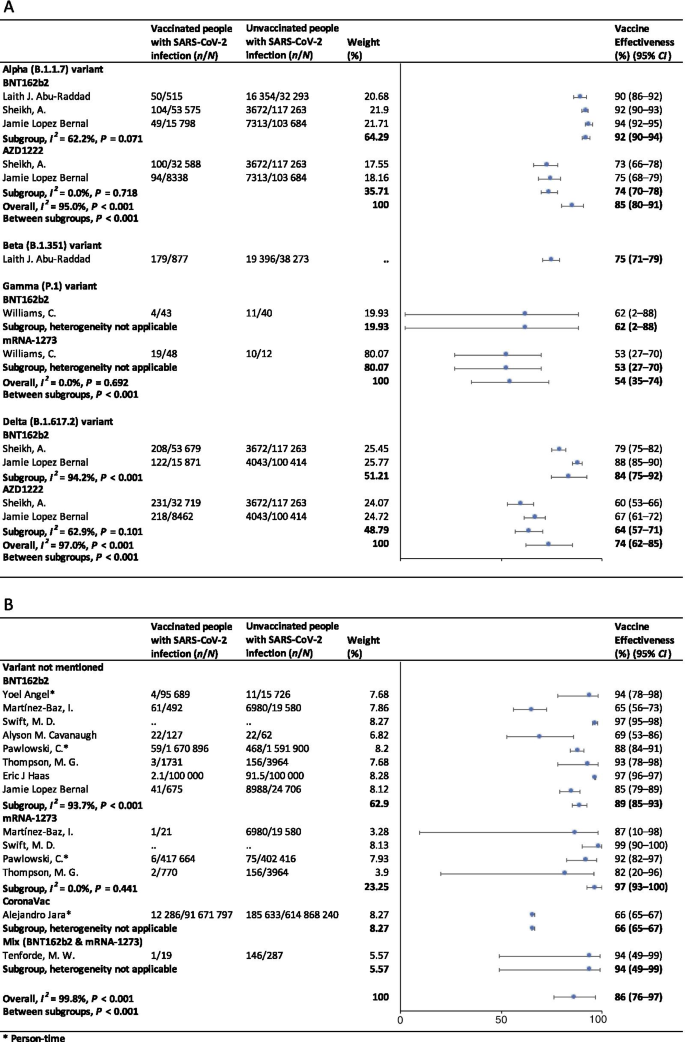
Forest plots for the vaccine effectiveness of SARS-CoV-2 vaccines in fully vaccinated populations. A Vaccine effectiveness against SARS-CoV-2 variants; B Vaccine effectiveness against SARS-CoV-2 with variants not mentioned. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, COVID-19 coronavirus disease 2019, CI confidence interval
For studies which had not mentioned the variant of SARS-CoV-2, the pooled VE was 86% (95% CI: 76–97%) for the prevention of SARS-CoV-2 infection in fully vaccinated people. mRNA-1273 vaccine had the highest pooled VE (97%, 95% CI: 93–100%, Fig. 2 ).
Safety of SARS-CoV-2 vaccines
As Table 2 showed, the incidence rate of adverse events varied widely among different studies. We conducted subgroup analysis by study population (general population, patients and healthcare workers), vaccine type (BNT162b2, mRNA-1273, CoronaVac, and et al.), and population size (< 1000, 1000–10 000, 10 000–100 000, and > 100 000). The overall pooled incidence rate was 1.5% (95% CI : 1.4–1.6%) for adverse events, 0.4 (95% CI : 0.2–0.5) per 10 000 for severe adverse events, and 0.1 (95% CI : 0.1–0.2) per 10 000 for death after vaccination. Incidence rate of adverse events was higher in healthcare workers (53.2%, 95% CI : 28.4–77.9%), AZD1222 vaccine group (79.6%, 95% CI : 60.8–98.3%), and < 1000 population size group (57.6%, 95% CI : 47.9–67.4%). Incidence rate of sever adverse events was higher in healthcare workers (127.2, 95% CI : 62.7–191.8, per 10 000), Gam-COVID-Vac vaccine group (175.7, 95% CI : 77.2–274.2, per 10 000), and 1000–10 000 population size group (336.6, 95% CI : 41.4–631.8, per 10 000). Incidence rate of death after vaccination was higher in patients (7.6, 95% CI : 0.0–32.2, per 10 000), BNT162b2 vaccine group (29.8, 95% CI : 0.0–71.2, per 10 000), and < 1000 population size group (29.8, 95% CI : 0.0–71.2, per 10 000). Subgroups of general population, vaccine type not mentioned, and > 100 000 population size had the lowest incidence rate of adverse events, severe adverse events, and death after vaccination.
Sensitivity analysis and publication bias
In the sensitivity analyses, VE for SARS-CoV-2 infections, symptomatic COVID-19 and COVID-19-related death got relatively lower when omitting over a single dose group of Maria et al.’s work [ 33 ]; when omitting ≥ 14 days after the first dose group and ≥ 14 days after the second dose group of Alejandro et al.’s work [ 14 ], VE for SARS-CoV-2 infections, hospitalization, ICU admission and COVID-19-related death got relatively higher; and VE for all clinical status of COVID-19 became lower when omitting ≥ 14 days after the second dose group of Eric et al.’s work [ 34 ]. Incidence rate of adverse events and severe adverse events got relatively higher when omitting China CDC’s data [ 74 ]. P values of Egger’s regression test for all the meta-analysis were more than 0.05, indicating that there might not be publication bias.
To our knowledge, this is a comprehensive systematic review and meta-analysis assessing the effectiveness and safety of SARS-CoV-2 vaccines based on real-world studies, reporting pooled VE for different variants of SARS-CoV-2 and incidence rate of adverse events. This meta-analysis comprised a total of 58 studies, including 32 studies for vaccine effectiveness and 26 studies for vaccine safety. We found that a single dose of SARS-CoV-2 vaccines was about 40–60% effective at preventing any clinical status of COVID-19 and that two doses were 85% or more effective. Although vaccines were not as effective against variants of SARS-CoV-2 as original virus, the vaccine effectiveness was still over 50% for fully vaccinated people. Normal adverse events were common, while the incidence of severe adverse events or even death was very low, providing reassurance to health care providers and to vaccine recipients and promote confidence in the safety of COVID-19 vaccines. Our findings strengthen and augment evidence from previous review [ 75 ], which confirmed the effectiveness of the BNT162b2 mRNA vaccine, and additionally reported the safety of SARS-CoV-2 vaccines, giving insight on the future of SARS-CoV-2 vaccine schedules.
Although most vaccines for the prevention of COVID-19 are two-dose vaccines, we found that the pooled VE of a single dose of SARS-CoV-2 vaccines was about 50%. Recent study showed that the T cell and antibody responses induced by a single dose of the BNT162b2 vaccine were comparable to those naturally infected with SARE-CoV-2 within weeks or months after infection [ 76 ]. Our findings could help to develop vaccination strategies under certain circumstances such as countries having a shortage of vaccines. In some countries, in order to administer the first dose to a larger population, the second dose was delayed for up to 12 weeks [ 77 ]. Some countries such as Canada had even decided to delay the second dose for 16 weeks [ 78 ]. However, due to a suboptimum immune response in those receiving only a single dose of a vaccine, such an approach had a chance to give rise to the emergence of variants of SARS-CoV-2 [ 79 ]. There remains a need for large clinical trials to assess the efficacy of a single-dose administration of two-dose vaccines and the risk of increasing the emergence of variants.
Two doses of SARS-CoV-2 vaccines were highly effective at preventing hospitalization, severe cases and deaths resulting from COVID-19, while the VE of different groups of days from the second vaccine dose showed no statistically significant differences. Our findings emphasized the importance of getting fully vaccinated, for the fact that most breakthrough infections were mild or asymptomatic. A recent study showed that the occurrence of breakthrough infections with SARS-CoV-2 in fully vaccinated populations was predictable with neutralizing antibody titers during the peri-infection period [ 80 ]. We also found getting fully vaccinated was at least 50% effective at preventing SARS-CoV-2 variants infections, despite reduced effectiveness compared with original virus; and BNT162b2 vaccine was found to have the highest VE in each variant group. Studies showed that the highly mutated variants were indicative of a form of rapid, multistage evolutionary jumps, which could preferentially occur in the milieu of partial immune control [ 81 , 82 ]. Therefore, immunocompromised patients should be prioritized for anti-COVID-19 immunization to mitigate persistent SARS-CoV-2 infections, during which multimutational SARS-CoV-2 variants could arise [ 83 ].
Recently, many countries, including Israel, the United States, China and the United Kingdom, have introduced a booster of COVID-19 vaccine, namely the third dose [ 84 , 85 , 86 , 87 ]. A study of Israel showed that among people vaccinated with BNT162b2 vaccine over 60 years, the risk of COVID-19 infection and severe illness in the non-booster group was 11.3 times (95% CI: 10.4–12.3) and 19.5 times (95% CI: 12.9–29.5) than the booster group, respectively [ 84 ]. Some studies have found that the third dose of Moderna, Pfizer-BioNTech, Oxford-AstraZeneca and Sinovac produced a spike in infection-blocking neutralizing antibodies when given a few months after the second dose [ 85 , 87 , 88 ]. In addition, the common adverse events associated with the third dose did not differ significantly from the symptoms of the first two doses, ranging from mild to moderate [ 85 ]. The overall incidence rate of local and systemic adverse events was 69% (57/97) and 20% (19/97) after receiving the third dose of BNT162b2 vaccine, respectively [ 88 ]. Results of a phase 3 clinical trial involving 306 people aged 18–55 years showed that adverse events after receiving a third dose of BNT162b2 vaccine (5–8 months after completion of two doses) were similar to those reported after receiving a second dose [ 85 ]. Based on V-safe, local reactions were more frequently after dose 3 (5323/6283; 84.7%) than dose 2 (5249/6283; 83.5%) among people who received 3 doses of Moderna. Systemic reactions were reported less frequently after dose 3 (4963/6283; 79.0%) than dose 2 (5105/6283; 81.3%) [ 86 ]. On August 4, WHO called for a halt to booster shots until at least the end of September to achieve an even distribution of the vaccine [ 89 ]. At this stage, the most important thing we should be thinking about is how to reach a global cover of people at risk with the first or second dose, rather than focusing on the third dose.
Based on real world studies, our results preliminarily showed that complete inoculation of COVID-19 vaccines was still effective against infection of variants, although the VE was generally diminished compared with the original virus. Particularly, the pooled VE was 54% (95% CI : 35–74%) for the Gamma variant, and 74% (95% CI : 62–85%) for the Delta variant. Since the wide spread of COVID-19, a number of variants have drawn extensive attention of international community, including Alpha variant (B.1.1.7), first identified in the United Kingdom; Beta variant (B.1.351) in South Africa; Gamma variant (P.1), initially appeared in Brazil; and the most infectious one to date, Delta variant (B.1.617.2) [ 90 ]. Israel recently reported a breakthrough infection of SARS-CoV-2, dominated by variant B.1.1.7 in a small number of fully vaccinated health care workers, raising concerns about the effectiveness of the original vaccine against those variants [ 80 ]. According to an observational cohort study in Qatar, VE of the BNT162b2 vaccine against the Alpha (B.1.1.7) and Beta (B.1.351) variants was 87% (95% CI : 81.8–90.7%) and 75.0% (95% CI : 70.5–7.9%), respectively [ 23 ]. Based on the National Immunization Management System of England, results from a recent real-world study of all the general population showed that the AZD1222 and BNT162b2 vaccines protected against symptomatic SARS-CoV-2 infection of Alpha variant with 74.5% (95% CI : 68.4–79.4%) and 93.7% (95% CI : 91.6–95.3%) [ 15 ]. In contrast, the VE against the Delta variant was 67.0% (95% CI : 61.3–71.8%) for two doses of AZD1222 vaccine and 88% (95% CI : 85.3–90.1%) for BNT162b2 vaccine [ 15 ].
In terms of adverse events after vaccination, the pooled incidence rate was very low, only 1.5% (95% CI : 1.4–1.6%). However, the prevalence of adverse events reported in large population (population size > 100 000) was much lower than that in small to medium population size. On the one hand, the vaccination population in the small to medium scale studies we included were mostly composed by health care workers, patients with specific diseases or the elderly. And these people are more concerned about their health and more sensitive to changes of themselves. But it remains to be proved whether patients or the elderly are more likely to have adverse events than the general. Mainstream vaccines currently on the market have maintained robust safety in specific populations such as cancer patients, organ transplant recipients, patients with rheumatic and musculoskeletal diseases, pregnant women and the elderly [ 54 , 91 , 92 , 93 , 94 ]. A prospective study by Tal Goshen-lag suggests that the safety of BNT162b2 vaccine in cancer patients is consistent with those previous reports [ 91 ]. In addition, the incidence rate of adverse events reported in the heart–lung transplant population is even lower than that in general population [ 95 ]. On the other hand, large scale studies at the national level are mostly based on national electronic health records or adverse event reporting systems, and it is likely that most mild or moderate symptoms are actually not reported.
Compared with the usual local adverse events (such as pain at the injection site, redness at the injection site, etc.) and normal systemic reactions (such as fatigue, myalgia, etc.), serious and life-threatening adverse events were rare due to our results. A meta-analysis based on RCTs only showed three cases of anaphylactic shock among 58 889 COVID-19 vaccine recipients and one in the placebo group [ 11 ]. The exact mechanisms underlying most of the adverse events are still unclear, accordingly we cannot establish a causal relation between severe adverse events and vaccination directly based on observational studies. In general, varying degrees of adverse events occur after different types of COVID-19 vaccination. Nevertheless, the benefits far outweigh the risks.
Our results showed the effectiveness and safety of different types of vaccines varied greatly. Regardless of SARS-CoV-2 variants, vaccine effectiveness varied from 66% (CoronaVac [ 14 ]) to 97% (mRNA-1273 [ 18 , 20 , 45 , 46 ]). The incidence rate of adverse events varied widely among different types of vaccines, which, however, could be explained by the sample size and population group of participants. BNT162b2, AZD1222, mRNA-1273 and CoronaVac were all found to have high vaccine efficacy and acceptable adverse-event profile in recent published studies [ 96 , 97 , 98 , 99 ]. A meta-analysis, focusing on the potential vaccine candidate which have reached to the phase 3 of clinical development, also found that although many of the vaccines caused more adverse events than the controls, most were mild, transient and manageable [ 100 ]. However, severe adverse events did occur, and there remains the need to implement a unified global surveillance system to monitor the adverse events of COVID-19 vaccines around the world [ 101 ]. A recent study employed a knowledge-based or rational strategy to perform a prioritization matrix of approved COVID-19 vaccines, and led to a scale with JANSSEN (Ad26.COV2.S) in the first place, and AZD1222, BNT162b2, and Sputnik V in second place, followed by BBIBP-CorV, CoronaVac and mRNA-1273 in third place [ 101 ]. Moreover, when deciding the priority of vaccines, the socioeconomic characteristics of each country should also be considered.
Our meta-analysis still has several limitations. First, we may include limited basic data on specific populations, as vaccination is slowly being promoted in populations under the age of 18 or over 60. Second, due to the limitation of the original real-world study, we did not conduct subgroup analysis based on more population characteristics, such as age. When analyzing the efficacy and safety of COVID-19 vaccine, we may have neglected the discussion on the heterogeneity from these sources. Third, most of the original studies only collected adverse events within 7 days after vaccination, which may limit the duration of follow-up for safety analysis.
Based on the real-world studies, SARS-CoV-2 vaccines have reassuring safety and could effectively reduce the death, severe cases, symptomatic cases, and infections resulting from SARS-CoV-2 across the world. In the context of global pandemic and the continuous emergence of SARS-CoV-2 variants, accelerating vaccination and improving vaccination coverage is still the most important and urgent matter, and it is also the final means to end the pandemic.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional information files.
Abbreviations
Coronavirus disease 2019
Severe Acute Respiratory Syndrome Coronavirus 2
Vaccine effectiveness
Confidence intervals
Intensive care unit
Random clinical trials
Preferred reporting items for systematic reviews and meta-analyses
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2021. https://coronavirus.jhu.edu/map.html . Accessed 20 Aug 2021.
Barranco R, Rocca G, Molinelli A, Ventura F. Controversies and challenges of mass vaccination against SARS-CoV-2 in Italy: medico-legal perspectives and considerations. Healthcare (Basel). 2021. https://doi.org/10.3390/healthcare9091163 .
Article Google Scholar
COVID-19 vaccine tracker. 2021. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/ . Accessed 20 Aug 2021.
Coronavirus (COVID-19) Vaccinations. 2021. https://ourworldindata.org/covid-vaccinations . Accessed 20 Aug 2021.
Kirby T. New variant of SARS-CoV-2 in UK causes surge of COVID-19. Lancet Respir Med. 2021;9(2):e20–1. https://doi.org/10.1016/s2213-2600(21)00005-9 .
Article CAS PubMed PubMed Central Google Scholar
Callaway E. Fast-spreading COVID variant can elude immune responses. Nature. 2021;589(7843):500–1. https://doi.org/10.1038/d41586-021-00121-z .
Article CAS PubMed Google Scholar
Reardon S. How the Delta variant achieves its ultrafast spread. Nature. 2021. https://doi.org/10.1038/d41586-021-01986-w .
Article PubMed Google Scholar
Li R, Liu J, Zhang H. The challenge of emerging SARS-CoV-2 mutants to vaccine development. J Genet Genomics. 2021;48(2):102–6. https://doi.org/10.1016/j.jgg.2021.03.001 .
Article PubMed PubMed Central Google Scholar
Chen M, Yuan Y, Zhou Y, Deng Z, Zhao J, Feng F, Zou H, Sun C. Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials. Infect Dis Poverty. 2021;10(1):94. https://doi.org/10.1186/s40249-021-00878-5 .
Ling Y, Zhong J, Luo J. Safety and effectiveness of SARS-CoV-2 vaccines: a systematic review and meta-analysis. J Med Virol. 2021. https://doi.org/10.1002/jmv.27203 .
Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Razizadeh MH, Turner DL, Turner RJ. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9050467 .
Sathian B, Asim M, Banerjee I, Roy B, Pizarro AB, Mancha MA, van Teijlingen ER, Kord-Varkaneh H, Mekkodathil AA, Subramanya SH, et al. Development and implementation of a potential coronavirus disease 2019 (COVID-19) vaccine: a systematic review and meta-analysis of vaccine clinical trials. Nepal J Epidemiol. 2021;11(1):959–82. https://doi.org/10.3126/nje.v11i1.36163 .
Yuan P, Ai P, Liu Y, Ai Z, Wang Y, Cao W, Xia X, Zheng JC. Safety, tolerability, and immunogenicity of COVID-19 vaccines: a systematic review and meta-analysis. medRxiv. 2020. https://doi.org/10.1101/2020.11.03.20224998 .
Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2107715 .
Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2108891 .
Israel says Pfizer Covid vaccine is just 39% effective as delta spreads, but still prevents severe illness. 2021. https://www.cnbc.com/2021/07/23/delta-variant-pfizer-covid-vaccine-39percent-effective-in-israel-prevents-severe-illness.html . Accessed 20 Aug 2021.
Zacay G, Shasha D, Bareket R, Kadim I, Hershkowitz Sikron F, Tsamir J, Mossinson D, Heymann AD. BNT162b2 vaccine effectiveness in preventing asymptomatic infection with SARS-CoV-2 virus: a nationwide historical cohort study. Open Forum Infect Dis. 2021;8(6): ofab262. https://doi.org/10.1093/ofid/ofab262 .
Martínez-Baz I, Miqueleiz A, Casado I, Navascués A, Trobajo-Sanmartín C, Burgui C, Guevara M, Ezpeleta C, Castilla J. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Eurosurveillance. 2021. https://doi.org/10.2807/1560-7917.Es.2021.26.21.2100438 .
Tenforde MW, Olson SM, Self WH, Talbot HK, Lindsell CJ, Steingrub JS, Shapiro NI, Ginde AA, Douin DJ, Prekker ME, et al. Effectiveness of Pfizer-BioNTech and moderna vaccines against COVID-19 among hospitalized adults aged ≥65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):674–9. https://doi.org/10.15585/mmwr.mm7018e1 .
Pawlowski C, Lenehan P, Puranik A, Agarwal V, Venkatakrishnan AJ, Niesen MJM, O’Horo JC, Virk A, Swift MD, Badley AD, et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med (N Y). 2021. https://doi.org/10.1016/j.medj.2021.06.007 .
Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . Accessed 20 Aug 2021.
Rostom A, Dubé C, Cranney A, et al. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep. (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. Available from: https://www.ncbi.nlm.nih.gov/books/NBK35156/ . Accessed 20 Aug 2021
Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 COVID-19 vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;385(2):187–9. https://doi.org/10.1056/NEJMc2104974 .
Angel Y, Spitzer A, Henig O, Saiag E, Sprecher E, Padova H, Ben-Ami R. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325(24):2457–65. https://doi.org/10.1001/jama.2021.7152 .
Azamgarhi T, Hodgkinson M, Shah A, Skinner JA, Hauptmannova I, Briggs TWR, Warren S. BNT162b2 vaccine uptake and effectiveness in UK healthcare workers—a single centre cohort study. Nat Commun. 2021;12(1):3698. https://doi.org/10.1038/s41467-021-23927-x .
Bianchi FP, Germinario CA, Migliore G, Vimercati L, Martinelli A, Lobifaro A, Tafuri S, Stefanizzi P. BNT162b2 mRNA COVID-19 vaccine effectiveness in the prevention of SARS-CoV-2 infection: a preliminary report. J Infect Dis. 2021. https://doi.org/10.1093/infdis/jiab262 .
Britton A, Jacobs Slifka KM, Edens C, Nanduri SA, Bart SM, Shang N, Harizaj A, Armstrong J, Xu K, Ehrlich HY, et al. Effectiveness of the Pfizer-BioNTech COVID-19 vaccine among residents of two skilled nursing facilities experiencing COVID-19 outbreaks—Connecticut, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(11):396–401. https://doi.org/10.15585/mmwr.mm7011e3 .
Cavanaugh AM, Fortier S, Lewis P, Arora V, Johnson M, George K, Tobias J, Lunn S, Miller T, Thoroughman D, et al. COVID-19 outbreak associated with a SARS-CoV-2 R1 lineage variant in a skilled nursing facility after vaccination program—Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):639–43. https://doi.org/10.15585/mmwr.mm7017e2 .
Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, Malek JA, Coyle P, Ayoub HH, Al Kanaani Z, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021. https://doi.org/10.1038/s41591-021-01446-y .
Chodick G, Tene L, Patalon T, Gazit S, Ben Tov A, Cohen D, Muhsen K. Assessment of effectiveness of 1 dose of BNT162b2 vaccine for SARS-CoV-2 infection 13 to 24 days after immunization. JAMA Netw Open. 2021;4(6): e2115985. https://doi.org/10.1001/jamanetworkopen.2021.15985 .
Chodick G, Tene L, Rotem RS, Patalon T, Gazit S, Ben-Tov A, Weil C, Goldshtein I, Twig G, Cohen D, et al. The effectiveness of the TWO-DOSE BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab438 .
Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. https://doi.org/10.1056/NEJMoa2101765 .
Flacco ME, Soldato G, Acuti Martellucci C, Carota R, Di Luzio R, Caponetti A, Manzoli L. Interim estimates of COVID-19 vaccine effectiveness in a mass vaccination setting: data from an Italian Province. VacCInes (Basel). 2021. https://doi.org/10.3390/vaccines9060628 .
Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–29. https://doi.org/10.1016/s0140-6736(21)00947-8 .
Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, Wellington E, Stowe J, Gillson N, Atti A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–35. https://doi.org/10.1016/s0140-6736(21)00790-x .
Hyams C, Marlow R, Maseko Z, King J, Ward L, Fox K, Heath R, Tuner A, Friedrich Z, Morrison L, et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis. 2021. https://doi.org/10.1016/s1473-3099(21)00330-3 .
Khan N, Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. 2021. https://doi.org/10.1053/j.gastro.2021.05.044 .
Knobel P, Serra C, Grau S, Ibañez R, Diaz P, Ferrández O, Villar R, Lopez AF, Pujolar N, Horcajada JP, et al. COVID-19 mRNA vaccine effectiveness in asymptomatic healthcare workers. Infect Control Hosp Epidemiol. 2021. https://doi.org/10.1017/ice.2021.287 .
Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O’Doherty M, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373: n1088. https://doi.org/10.1136/bmj.n1088 .
Mazagatos C, Monge S, Olmedo C, Vega L, Gallego P, Martín-Merino E, Sierra MJ, Limia A, Larrauri A. Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, Spain, weeks 53, 2020 to 13 2021. Eurosurveillance. 2021. https://doi.org/10.2807/1560-7917.Es.2021.26.24.2100452 .
Pilishvili T, Fleming-Dutra KE, Farrar JL, Gierke R, Mohr NM, Talan DA, Krishnadasan A, Harland KK, Smithline HA, Hou PC, et al. Interim estimates of vaccine effectiveness of Pfizer-BioNTech and Moderna COVID-19 vaccines among health care personnel—33 US Sites, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):753–8. https://doi.org/10.15585/mmwr.mm7020e2 .
Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2. https://doi.org/10.1016/s0140-6736(21)01358-1 .
Shrotri M, Krutikov M, Palmer T, Giddings R, Azmi B, Subbarao S, Fuller C, Irwin-Singer A, Davies D, Tut G, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021. https://doi.org/10.1016/s1473-3099(21)00289-9 .
Skowronski DM, Setayeshgar S, Zou M, Prystajecky N, Tyson JR, Galanis E, Naus M, Patrick DM, Sbihi H, El Adam S, et al. Single-dose mRNA vaccine effectiveness against SARS-CoV-2, including Alpha and Gamma variants: a test-negative design in adults 70 years and older in British Columbia,Canada. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab616 .
Swift MD, Breeher LE, Tande AJ, Tommaso CP, Hainy CM, Chu H, Murad MH, Berbari EF, Virk A. Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab361 .
Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, Olsho LEW, Caban-Martinez AJ, Fowlkes AL, Lutrick K, et al. Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2107058 .
Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, Bedston S, Beggs J, Bradley D, Chuter A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–57. https://doi.org/10.1016/s0140-6736(21)00677-2 .
Williams C, Al-Bargash D, Macalintal C, Stuart R, Seth A, Latham J, Gitterman L, Fedsin S, Godoy M, Kozak R, et al. COVID-19 outbreak associated with a SARS-CoV-2 P.1 lineage in a long-term care home after implementation of a vaccination program—Ontario, April–May 2021. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab617 .
Alhazmi A, Alamer E, Daws D, Hakami M, Darraj M, Abdelwahab S, Maghfuri A, Algaissi A. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060674 .
Andrzejczak-Grządko S, Czudy Z, Donderska M. Side effects after COVID-19 vaccinations among residents of Poland. Eur Rev Med Pharmacol Sci. 2021;25(12):4418–21. https://doi.org/10.26355/eurrev_202106_26153 .
Baldolli A, Michon J, Appia F, Galimard C, Verdon R, Parienti JJ. Tolerance of BNT162b2 mRNA COVI-19 vaccine in patients with a medical history of COVID-19 disease: a case control study. Vaccine. 2021;39(32):4410–3. https://doi.org/10.1016/j.vaccine.2021.06.054 .
Cherian S, Paul A, Ahmed S, Alias B, Manoj M, Santhosh AK, Varghese DR, Krishnan N, Shenoy P. Safety of the ChAdOx1 nCoV-19 and the BBV152 vaccines in 724 patients with rheumatic diseases: a post-vaccination cross-sectional survey. Rheumatol Int. 2021;41(8):1441–5. https://doi.org/10.1007/s00296-021-04917-0 .
Chevallier P, Coste-Burel M, Le Bourgeois A, Peterlin P, Garnier A, Béné MC, Imbert BM, Drumel T, Le Gouill S, Moreau P, et al. Safety and immunogenicity of a first dose of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic stem-cells recipients. EJHaem. 2021. https://doi.org/10.1002/jha2.242 .
Connolly CM, Ruddy JA, Boyarsky BJ, Avery RK, Werbel WA, Segev DL, Garonzik-Wang J, Paik JJ. Safety of the first dose of mRNA SARS-CoV-2 vaccines in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-220231 .
Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, Zisapel M, Elalouf O, Kaufman I, Meidan R, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-220647 .
Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, Nair N, Martin S, Clark T, Markowitz L, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–8. https://doi.org/10.15585/mmwr.mm7008e3 .
Hashimoto T, Ozaki A, Bhandari D, Sawano T, Sah R, Tanimoto T. High anaphylaxis rates following vaccination with the Pfizer BNT162b2 mRNA vaccine against COVID-19 in Japanese health care workers; a secondary analysis of initial post-approval safety data. J Travel Med. 2021. https://doi.org/10.1093/jtm/taab090 .
Lv G, Yuan J, Xiong X, Li M. Mortality rate and characteristics of deaths following COVID-19 vaccination. Front Med (Lausanne). 2021;8: 670370. https://doi.org/10.3389/fmed.2021.670370 .
McMurry R, Lenehan P, Awasthi S, Silvert E, Puranik A, Pawlowski C, Venkatakrishnan AJ, Anand P, Agarwal V, O’Horo JC, et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA COVID-19 vaccines. Med (N Y). 2021. https://doi.org/10.1016/j.medj.2021.06.006 .
Monin L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, Domingo-Vila C, Hayday TS, Graham C, Seow J, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–78. https://doi.org/10.1016/s1470-2045(21)00213-8 .
Pagotto V, Ferloni A, Mercedes Soriano M, Díaz M, Braguinsky Golde N, González MI, Asprea V, Staneloni MI, Zingoni P, Vidal G, et al. Active monitoring of early safety of Sputnik V vaccine in Buenos Aires, Argentina. MediCIna (B Aires). 2021;81(3):408–14.
Google Scholar
Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder-Finesod A, Mandelboim M, Indenbaum V, Levy I, Raanani E, et al. BNT162b2 vaccination in heart transplant recipients: Clinical experience and antibody response. J Heart Lung Transplant. 2021. https://doi.org/10.1016/j.healun.2021.04.003 .
Quiroga B, Sánchez-Álvarez E, Goicoechea M, de Sequera P. COVID-19 vaccination among Spanish nephrologists: acceptance and side effects. J Healthc Qual Res. 2021. https://doi.org/10.1016/j.jhqr.2021.05.002 .
Ram R, Hagin D, Kikozashvilli N, Freund T, Amit O, Bar-On Y, Beyar-Katz O, Shefer G, Moshiashvili MM, Karni C, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy—a single center prospective cohort study. Transplant Cell Ther. 2021. https://doi.org/10.1016/j.jtct.2021.06.024 .
Revon-Riviere G, Ninove L, Min V, Rome A, Coze C, Verschuur A, de Lamballerie X, André N. The BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults with cancer: a monocentric experience. Eur J Cancer. 2021;154:30–4. https://doi.org/10.1016/j.ejca.2021.06.002 .
Riad A, Pokorná A, Mekhemar M, Conrad J, Klugarová J, Koščík M, Klugar M, Attia S. Safety of ChAdOx1 nCoV-19 vaccine: independent evidence from two EU states. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060673 .
Riad A, Sağıroğlu D, Üstün B, Pokorná A, Klugarová J, Attia S, Klugar M. Prevalence and risk factors of CoronaVac Side effects: an independent cross-sectional study among healthcare workers in Turkey. J Clin Med. 2021. https://doi.org/10.3390/jcm10122629 .
Rosman Y, Lavi N, Meir-Shafrir K, Lachover-Roth I, Cohen-Engler A, Mekori YA, Confino-Cohen R. Safety of BNT162b2 mRNA COVID-19 vaccine in patients with mast cell disorders. J Allergy Clin Immunol Pract. 2021. https://doi.org/10.1016/j.jaip.2021.06.032 .
Signorelli C, Odone A, Gianfredi V, Capraro M, Kacerik E, Chiecca G, Scardoni A, Minerva M, Mantecca R, Musarò P, et al. Application of the “immunization islands” model to improve quality, efficiency and safety of a COVID-19 mass vaccination site. Ann Ig. 2021;33(5):499–512. https://doi.org/10.7416/ai.2021.2456 .
Vallée A, Chan-Hew-Wai A, Bonan B, Lesprit P, Parquin F, Catherinot É, Choucair J, Billard D, Amiel-Taieb C, Camps È, et al. Oxford-AstraZeneca COVID-19 vaccine: need of a reasoned and effective vaccine campaign. Public Health. 2021;196:135–7. https://doi.org/10.1016/j.puhe.2021.05.030 .
Wang J, Hou Z, Liu J, Gu Y, Wu Y, Chen Z, Ji J, Diao S, Qiu Y, Zou S, et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol. 2021. https://doi.org/10.1016/j.jhep.2021.04.026 .
Zhang MX, Zhang TT, Shi GF, Cheng FM, Zheng YM, Tung TH, Chen HX. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021. https://doi.org/10.1080/14760584.2021.1925112 .
Shay DK, Gee J, Su JR, Myers TR, Marquez P, Liu R, Zhang B, Licata C, Clark TA, Shimabukuro TT. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine—United States, March–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):680–4. https://doi.org/10.15585/mmwr.mm7018e2 .
Prevention CCfDCa. Information analysis of COVID-19 vaccine adverse reaction monitoring in China. 2021-5-28. http://www.chinacdc.cn/jkzt/ymyjz/ymyjjz_6758/202105/t20210528_230908.html . Accessed 20 Aug 2021.
Kow CS, Hasan SS. Real-world effectiveness of BNT162b2 mRNA vaccine: a meta-analysis of large observational studies. Inflammopharmacology. 2021;29(4):1075–90. https://doi.org/10.1007/s10787-021-00839-2 .
Angyal A, Longet S, Moore S, Payne RP, Harding A et al. T-Cell and Antibody Responses to First BNT162b2 Vaccine Dose in Previously SARS-CoV-2-Infected and Infection-Naive UK Healthcare Workers: A Multicentre, Prospective, Observational Cohort Study. Available at SSRN: https://ssrn.com/abstract=3820576 or https://doi.org/10.2139/ssrn.3820576 . Accessed 20 Aug 2021.
Pimenta D, Yates C, Pagel C, Gurdasani D. Delaying the second dose of covid-19 vaccines. BMJ. 2021;372: n710. https://doi.org/10.1136/bmj.n710 .
Tauh T, Mozel M, Meyler P, Lee SM. An updated look at the 16-week window between doses of vaccines in BC for COVID-19. BC Med J. 2021;63(3):102–3.
Kadire SR, Wachter RM, Lurie N. Delayed second dose versus standard regimen for COVID-19 vaccination. N Engl J Med. 2021;384(9): e28. https://doi.org/10.1056/NEJMclde2101987 .
Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, Mandelboim M, Gal Levin E, Rubin C, Indenbaum V, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2109072 .
Truong TT, Ryutov A, Pandey U, Yee R, Goldberg L, Bhojwani D, Aguayo-Hiraldo P, Pinsky BA, Pekosz A, Shen L, et al. Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity. medRxiv. 2021. https://doi.org/10.1101/2021.02.27.21252099 .
Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, Solomon IH, Kuo HH, Boucau J, Bowman K, et al. Persistence and evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med. 2020;383(23):2291–3. https://doi.org/10.1056/NEJMc2031364 .
Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med. 2021;385(6):562–6. https://doi.org/10.1056/NEJMsb2104756 .
Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, Mizrahi B, Alroy-Preis S, Ash N, Milo R, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–400. https://doi.org/10.1056/NEJMoa2114255 .
Hause AM, Baggs J, Gee J, Marquez P, Myers TR, Shimabukuro TT, Shay DK. Safety monitoring of an additional dose of COVID-19 vaccine—United States, August 12–September 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(39):1379–84. https://doi.org/10.15585/mmwr.mm7039e4 .
Furlow B. Immunocompromised patients in the USA and UK should receive third dose of COVID-19 vaccine. Lancet Rheumatol. 2021. https://doi.org/10.1016/s2665-9913(21)00313-1 .
Flaxman A, Marchevsky NG, Jenkin D, Aboagye J, Aley PK, Angus B, Belij-Rammerstorfer S, Bibi S, Bittaye M, Cappuccini F, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet. 2021;398(10304):981–90. https://doi.org/10.1016/s0140-6736(21)01699-8 .
Peled Y, Ram E, Lavee J, Segev A, Matezki S, Wieder-Finesod A, Halperin R, Mandelboim M, Indenbaum V, Levy I, et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: immunogenicity and clinical experience. J Heart Lung Transplant. 2021. https://doi.org/10.1016/j.healun.2021.08.010 .
WHO. WHO press conference on coronavirus disease (COVID-19)—4 August 2021. 2021. https://www.who.int/multi-media/details/who-press-conference-on-coronavirus-disease-(covid-19)---4-august-2021 . Accessed 20 Aug 2021.
Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls. edn. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
Goshen-Lago T, Waldhorn I, Holland R, Szwarcwort-Cohen M, Reiner-Benaim A, Shachor-Meyouhas Y, Hussein K, Fahoum L, Baruch M, Peer A, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021. https://doi.org/10.1001/jamaoncol.2021.2675 .
Ou MT, Boyarsky BJ, Motter JD, Greenberg RS, Teles AT, Ruddy JA, Krach MR, Jain VS, Werbel WA, Avery RK, et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021. https://doi.org/10.1097/tp.0000000000003780 .
Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben-David A, Asraf K, Doolman R, Sapir E, Regev Yochay G, et al. Short-term outcome of pregnant women vaccinated by BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021. https://doi.org/10.1002/uog.23729 .
Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang KT, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–82. https://doi.org/10.1056/NEJMoa2104983 .
Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder-Finesod A, Mandelboim M, Indenbaum V, Levy I, Raanani E, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40(8):759–62. https://doi.org/10.1016/j.healun.2021.04.003 .
Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2110345 .
Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, Neuzil KM, Hahn W, Hunt J, Mulligan MJ, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2105290 .
El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, Campbell TB, Clark J, Jackson LA, Fichtenbaum CJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2113017 .
Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç F, Akalın EH, Tabak ÖF, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–22. https://doi.org/10.1016/s0140-6736(21)01429-x .
Kumar S, Saurabh MK, Maharshi V. Efficacy and safety of potential vaccine candidates against coronavirus disease 2019: a systematic review. J Adv Pharm Technol Res. 2021;12(3):215–21. https://doi.org/10.4103/japtr.JAPTR_229_20 .
Burgos-Salcedo J. A rational strategy to support approved COVID-19 vaccines prioritization. Hum Vaccin Immunother. 2021;17(10):3474–7. https://doi.org/10.1080/21645515.2021.1922060 .
Download references
Acknowledgements
This study was funded by the National Natural Science Foundation of China (72122001; 71934002) and the National Science and Technology Key Projects on Prevention and Treatment of Major infectious disease of China (2020ZX10001002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper. No payment was received by any of the co-authors for the preparation of this article.
Author information
Qiao Liu and Chenyuan Qin are joint first authors
Authors and Affiliations
Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, 100191, China
Qiao Liu, Chenyuan Qin, Min Liu & Jue Liu
Institute for Global Health and Development, Peking University, Beijing, 100871, China
Chenyuan Qin & Jue Liu
You can also search for this author in PubMed Google Scholar
Contributions
LQ and QCY contributed equally as first authors. LJ and LM contributed equally as correspondence authors. LJ and LM conceived and designed the study; LQ, QCY and LJ carried out the literature searches, extracted the data, and assessed the study quality; LQ and QCY performed the statistical analysis and wrote the manuscript; LJ, LM, LQ and QCY revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Min Liu or Jue Liu .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Supplementary Information
Additional file 1: table s1..
Characteristic of studies included for vaccine effectiveness.
Additional file 2: Table S2.
Characteristic of studies included for vaccine safety.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, Q., Qin, C., Liu, M. et al. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty 10 , 132 (2021). https://doi.org/10.1186/s40249-021-00915-3
Download citation
Received : 07 September 2021
Accepted : 01 November 2021
Published : 14 November 2021
DOI : https://doi.org/10.1186/s40249-021-00915-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Effectiveness
- Meta-analysis
Infectious Diseases of Poverty
ISSN: 2049-9957
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Ethical Considerations of Offering Benefits to COVID-19 Vaccine Recipients
- 1 Sturm College of Law, University of Denver, Denver, Colorado
- 2 Medical Ethics and Health Policy, Perelman School of Medicine, University of Pennsylvania, Philadelphia
- Viewpoint The Ethics of COVID-19 Immunity-Based Licenses (“Immunity Passports”) Govind Persad, JD, PhD; Ezekiel J. Emanuel, MD, PhD JAMA
- Viewpoint Mandating COVID-19 Vaccines—Ethical and Legal Considerations Lawrence O. Gostin, JD; Daniel A. Salmon, MPH, PhD; Heidi J. Larson, PhD JAMA
- Viewpoint Problems With Paying People to Be Vaccinated Against COVID-19 Emily A. Largent, JD, PhD, RN; Franklin G. Miller, PhD JAMA
- Research Letter Lottery-Based Incentive in Ohio and COVID-19 Vaccination Rates Allan J. Walkey, MD, MSc; Anica Law, MD, MS; Nicholas A. Bosch, MD, MSc JAMA
Entry into a million-dollar lottery for getting vaccinated against COVID-19 is Ohio’s offer to adults. Teens who get vaccinated receive a lottery ticket for state college tuition, room, board, and more. Other states are offering gift cards. Now many employers are offering rewards for COVID-19 vaccination. Businesses ranging from Krispy Kreme and Sam Adams beer to the Cincinnati Reds have announced discounts or prizes for vaccinated individuals. Are these benefit programs ethical? Are they useful? Are they better than mandates?
Incentives for Vaccination Are Ethical
Benefits or incentives for becoming vaccinated are not new. The Centers for Disease Control and Prevention now recommends exempting vaccinated people from mask requirements. Businesses like Target and Safeway have long offered coupons to customers who receive flu vaccines on site.
The ethical case for instituting vaccine benefit programs is justified by 2 widely recognized values: (1) reducing overall harm from COVID-19 and (2) protecting disadvantaged individuals. 1 If benefit programs increase vaccine uptake, they directly protect recipients. By reducing transmission, increased uptake also protects the population, including ineligible children and adults, unvaccinated adults, and individuals with conditions reducing vaccine efficacy ( Table ). Because transmission has been higher and outcomes worse in less-advantaged communities, stemming transmission especially protects those in disadvantaged communities. In addition, costs, such as time off work for getting a vaccine or dealing with vaccine-related adverse effects, finding daycare for children, and transportation to a vaccine site, hamper access for poorer and marginalized people. Benefit programs, especially in the form of guaranteed cash payments, could improve access and increase uptake by offsetting these costs.
Many benefit programs reimburse or compensate for costs related to vaccine receipt and incentivize vaccine receipt. Encouraging healthy choices through generous reimbursement is viewed as unproblematic in other health care contexts. For instance, the Affordable Care Act provides free preventive services such as other vaccines or cancer screening tests. However, just as some insurance designs go beyond zero out-of-pocket costs to affirmatively reward choices such as getting preventive care, payments that go beyond restoring the prevaccination status quo need not raise special concerns. 2 Rewards often serve the dual function of incentivizing socially valuable choices and offsetting cost barriers.
Responding to Arguments Against Incentives for Vaccines
Some might argue that benefit programs coerce or exploit. This is mistaken. Offering a benefit cannot coerce because, unlike a threat, an offered benefit does not threaten to deprive someone of anything they are otherwise entitled to, a fundamental requirement to constitute coercion. 3
Some argue offers of benefits exploit persons who are poor. Individuals who are less well-off may have more need for the offered benefits. But the charge of exploitation is only plausible if poor individuals are incentivized to increase their personal risks to enrich others. 4 This is clearly not the case with COVID-19 vaccination, which protects recipients rather than heightening risk. Recipients get a “double benefit”: protection from disease alongside a government bond, gift card, or lottery ticket. Recognizing that incentives may be particularly compelling to poor people does not constitute taking unfair advantage of their poverty. Encouraging vaccination by offering benefits helps mitigate inequity, unlike refusing to provide benefits due to concerns about exploitation. Throughout the pandemic, some employers have offered hazard pay to recruit workers in severely affected occupations, such as bus drivers and health workers. Promising benefits for vaccinated people raises fewer concerns.
Others argue that offering benefits distorts or corrupts medical decision-making by introducing inappropriate or irrelevant motivations. 5 But modest cash benefits are more likely to clear away distractions (eg, concerns about lost wages or transportation costs) and allow individuals to focus on protecting themselves and their families. Even if larger benefits were offered, financial motivations need not undermine the “moral significance” of vaccination, and in any event, such concerns should not override the imperative of stemming the pandemic. In this situation, it is important to not be hypocritical: people are paid generously for other socially valuable or personally meaningful activities like providing medical care. (And the COVID-19 pandemic illustrated that many who provide essential services are not sufficiently compensated for the value of their work.)
Lotteries more credibly raise distortion concerns because they may appeal to psychological biases. Leveraging psychological biases to encourage uptake of a safe, socially beneficial, and effective vaccine, however, seems no more an objection to a lottery than to messaging campaigns that harness biases such as loss aversion, which leads people to perceive a loss as more significant than an equally sized gain.
Others have a more fundamental objection: that medical decisions should be made without reference to an individual’s financial interests. 6 Financial benefits, however, could help to focus vaccine decisions on medical factors by offsetting other costs, such as the need to take time off of work because of vaccine adverse effects. Furthermore, many medical decisions beyond vaccination have prompted efforts to promote socially preferable outcomes through financial incentives. Vaccination seems no different in this respect from smoking cessation or healthier diets, which are choices society also promotes through benefits and penalties.
Is offering benefits only to current recipients unfair to people who were vaccinated earlier? 7 Entering both early and late recipients into a lottery obviates this worry. But focusing on currently unvaccinated people can also be appropriate. Earlier recipients have already enjoyed the far greater benefit of longer protection from COVID-19. Latecomers are often treated differently from early adopters. For instance, a bakery may discount bread prices before closing time to sell bread rather than give it away. Patients who appear at a clinic at a slow time may be able to make a walk-in appointment rather than having to navigate waiting lists. These practices respond to varying demand, but are not unfair.
Could people offering benefits undermine public willingness to receive future vaccines without pay? With respect to modest benefits that offset barriers to vaccination, this concern seems doubtful. Even if it has empirical merit, it must be weighed against the large benefits to society of increasing COVID-19 vaccine receipt now.
While offering benefits to COVID-19 vaccine recipients is typically ethical, it may not always be optimal. For health workers or prison guards who interact with vulnerable people and have a duty to protect them, vaccine mandates may be more ethically appropriate than leaving vaccination optional while offering incentives.
Additionally, even though benefit programs likely increase overall willingness to be vaccinated, offers of benefits may decrease willingness among specific individuals or subpopulations. 8 Benefit designs should target individuals who would be responsive to benefits and avoid decreasing others’ willingness. In addition, the effectiveness of some incentives, such as vaccine lotteries, may quickly wane. In Ohio, the announcement of a lottery was associated with an increase from 15 104 people vaccinated the day before the lottery to 32 941 people vaccinated the day after, the highest number for the next 4 weeks. On June 15, only 7061 people were vaccinated. 9
Despite the large social benefits of vaccination, unnecessarily large benefit payments may waste public funds. 4 If almost as many people would change their position and agree to be vaccinated for incentives of $50 as $200, there is no good reason to start with $200. Overly large benefits for vaccine recipients may also invite distrust by making vaccination seem especially risky or burdensome. 10 To avoid waste, benefit programs should also be evaluated for efficacy and cost-effectiveness and be rigorously compared with alternative options. Even though incentives appear to prompt surges in vaccination, programs that establish vaccination as a social norm may better sustain high vaccination rates.
It is common to thank those who perform socially valuable actions, such as by offering recognition, awards, payment, or other benefits. People who choose to be vaccinated against COVID-19 help society end the pandemic. While benefit programs that recognize or encourage their choice to be vaccinated may not always be the right approach, and may not be a sustainable approach, they are neither fundamentally ethically objectionable nor ethically unique.
Corresponding Author: Ezekiel J. Emanuel, MD, PhD, Medical Ethics and Health Policy, Perelman School of Medicine, University of Pennsylvania, 423 Guardian Dr, Blockley Hall, Ste 1412, Philadelphia, PA 19104 ( [email protected] ).
Published Online: July 1, 2021. doi:10.1001/jama.2021.11045
Conflict of Interest Disclosures: Dr Persad reported grants from Greenwall Foundation and personal fees from ASCO Post and the World Health Organization. Dr Emanuel reported personal fees, nonfinancial support, or both from companies, organizations, and professional health care meetings. Dr Emanuel is also a venture partner at Oak HC/FT; a partner at Embedded Healthcare LLC, ReCovery Partners LLC, and COVID-19 Recovery Consulting; and an unpaid board member of Village MD and Oncology Analytics.
See More About
Persad G , Emanuel EJ. Ethical Considerations of Offering Benefits to COVID-19 Vaccine Recipients. JAMA. 2021;326(3):221–222. doi:10.1001/jama.2021.11045
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
COVID-19 vaccine: A 2021 analysis of perceptions on vaccine safety and promise in a U.S. sample
Roles Conceptualization, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Global Health, Indiana University School of Medicine, Indianapolis, Indiana, United States of America
Roles Investigation, Methodology, Project administration, Validation, Visualization, Writing – review & editing
Affiliation Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia, United States of America
Roles Investigation, Methodology, Validation, Visualization, Writing – review & editing
Affiliation Department of Psychiatry, Harvard Medical School, Boston, Massachusetts, United States of America
Roles Data curation, Formal analysis, Software, Visualization, Writing – review & editing
Affiliation Department of Biostatistics and Health Data Science, Indiana University School of Medicine, Indianapolis, Indiana, United States of America
Roles Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing
Affiliation Department of Global Health, Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, Indiana, United States of America
- Vitalis C. Osuji,
- Eric M. Galante,
- David Mischoulon,
- James E. Slaven,
- Gerardo Maupome

- Published: May 19, 2022
- https://doi.org/10.1371/journal.pone.0268784
- Reader Comments
Despite reliable evidence-based research supporting the COVID-19 vaccines, population-wide confidence and trust remain limited. We sought to expand prior knowledge about COVID-19 vaccine perceptions, while determining which population groups are at greatest risk for not getting a vaccine.
Study participants in the U.S. (79% female, median age group 46–60 years) were recruited through an online Qualtrics survey distributed as a Facebook advertisement from 3/19/21–4/30/21. We assumed that every participant is at risk of COVID-19 infection and should be able to get the vaccine with proper access. Bivariate and multivariable models were performed. Collinearity between variables was assessed.
A total of 2,626 responses were generated and 2,259 were included in data analysis. According to our multivariate model analysis, vaccines were perceived as safe by those who had or planned to obtain full vaccination (adjusted odds ratio (aOR) (95% confidence interval) = 40.0 (19.0, 84.2); p< 0.0001) and those who indicated trust in science (aOR = 10.5 (5.1, 21.8); p< 0.0001); vaccines were perceived as not safe by those who self-identified as Republicans vs. self-identified Democrats (aOR = 0.2 (0.1, 0.5); p = 0.0020) and those with high school or lower education (aOR = 0.2 (0.1, 0.4); p = 0.0007). Similarly, according to our multivariate model analysis, the following groups were most likely to reject vaccination based on belief in vaccinations: those with lower income (aOR = 0.8 (0.6, 0.9); p = 0.0106), those who do not know anyone who had been vaccinated (aOR = 0.1 (0.1, 0.4); p< 0.0001), those who are unwilling to get vaccinated even if family and friends had done so (aOR = 0.1 (<0.1, 0.2); p< 0.0001), those who did not trust science (aOR < 0.1 (<0.1, 0.1); p< 0.0001), those who believe that vaccination was unnecessary if others had already been vaccinated (aOR = 2.8 (1.5, 5.1); p = 0.0007), and those who indicate refusal to vaccinate to help others (aOR = 0.1 (0.1, 0.2); p< 0.0001). An alpha of p<0.05 was used for all tests.
Level of education and partisanship, but not race/ethnicity, were the most likely factors associated with vaccine hesitancy or likelihood to vaccinate. Also, low vaccination rates among underrepresented minorities may be due to distrust for healthcare industries. Population sub-groups less likely to be vaccinated and/or receptive to vaccines should be targeted for vaccine education and incentives.
Citation: Osuji VC, Galante EM, Mischoulon D, Slaven JE, Maupome G (2022) COVID-19 vaccine: A 2021 analysis of perceptions on vaccine safety and promise in a U.S. sample. PLoS ONE 17(5): e0268784. https://doi.org/10.1371/journal.pone.0268784
Editor: Weijing He, University of Texas Health Science Center at San Antonio, UNITED STATES
Received: July 26, 2021; Accepted: May 8, 2022; Published: May 19, 2022
Copyright: © 2022 Osuji et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting information files.
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
In early 2020, the SARS-CoV-2 (COVID-19) pandemic unmasked the many flaws that healthcare systems faced worldwide. While some of these issues were difficult to predict, such as the feasibility of pandemic response protocols or federal government regulations to be activated [ 1 ], other healthcare issues were to be expected, especially in the United States. For example, disparities in healthcare treatment and outcomes derived from different socioeconomic factors. Studies published in 2020 showed that the pandemic had much higher infection rates in minority populations such as Black and Hispanic/Latinx compared to their white counterparts; American Indians/ Alaska Natives (AI/ ANs), Black and Hispanic/Latinx communities also experienced significantly higher mortality rates [ 2 , 3 ]. The Centers for Disease Control and Prevention (CDC) released information relating social determinants of health to poorer COVID-19 outcomes, stating that “factors such as discrimination, neighborhood and physical environment, housing, occupation, education, income, and wealth gaps put some racial and ethnic minority groups at increased risk of severe illness from COVID-19, including death” [ 4 ]. Many factors play a role in disparities relevant to the COVID-19 pandemic. These include limited access to health services, education, and transportation, which tend to affect more severely communities of color and people of low socioeconomic status [ 5 ].
Just under one year after the first identification of COVID-19 in China [ 6 , 7 ], the PfizerBioNTech and Moderna COVID-19 vaccines were approved by the US Food and Drug Administration (FDA) under Emergency Use Authorization [ 8 , 9 ]. Ultimately, the Pfizer vaccine was fully approved as of August 23 rd , 2021. These vaccines represented a major milestone in vaccine production history, as no other vaccine had ever been created so rapidly with such positive results [ 10 ]. Although mistrust of vaccines is not uncommon in American culture, hesitation regarding the COVID-19 vaccines may be among the strongest yet [ 11 ]. Despite substantial evidence-based research supporting the vaccines’ safety and efficacy, there are lay public concerns regarding the vaccine rollout. For instance, an analysis [ 12 ] from March 2021 in individuals getting vaccines showed that white Americans were receiving vaccinations at a rate two times that of Black Americans, and the gap for Hispanic/Latinx was even larger. The rationale behind these gaps between racial/ethnic groups remains uncertain and highlights the importance of characterizing the factors and mechanisms underlying potential associations amongst demographic and socioeconomic groups.
With the current vaccines showing 95% efficacy, the estimated percentage of Americans needing vaccination to reach herd immunity ranges from 60 to 72% [ 13 ]. However, according to a November 2020 survey [ 14 ], 40% of Americans said that they will “definitely not” or “probably not” get the COVID-19 vaccine when it becomes available to them. Therefore, more needs to be done to bolster interest and trust in the vaccines. While companies and governmental organizations attempt to convey the necessary strategies to ease vaccine uncertainty and hesitation, a large segment of the lay public remains skeptical. As of May 2021, there were state-level COVID-19 vaccine incentives developed to increase vaccination rates across the United States. Irrespective of these incentives, only 48.6% of the US population was fully vaccinated as of July 2021, while 56% had received at least one dose [ 15 ]. Given these data, reasons surrounding vaccination hesitancy needed to be further explored. We aimed to expand current knowledge about COVID-19 vaccine perceptions through a characterization of sociocultural, socioeconomic, and demographic features in the context of opinions about receiving a COVID-19 vaccine. The objectives of the present survey were to establish:
- What segments of the population believe the COVID-19 vaccines to be safe?
- What are the perceived barriers to obtaining the COVID-19 vaccine—for self and others?
- Is there an association between individual sociocultural characteristics and either acceptance or rejection of the vaccine?
- Is there an association between individual demographic characteristics and either acceptance or rejection of the vaccine?
Materials and methods
This research project was granted IRB approval by Indiana University (protocol #10670).
Data collection was done using an online survey distributed to the general public, and our methodology followed criteria from the CHERRIES checklist [ 16 ]. The survey was created using Qualtrics and piloted with 15 respondents. Based on responses and feedback from our iterative process to pilot the survey, questions were added, rephrased, or deleted. The final survey had 37 questions, with 1–6 questions per page. Question format included 28 multiple choices, with the remainder as yes/no questions. Both English and Spanish versions of the survey were available. A description of the ethical approval, anonymity, and data utilization was provided and acknowledged at the beginning of the survey. Personal information was not required, and participants were offered the option to enter an email address if they wished to participate in an optional raffle draw for five $20 Walmart gift cards. All data were stored in a secure password protected website, to which only study investigators had access. A completeness check prior to submission was not implemented, but a forced response feature on Qualtrics was used for all questions except those involving zip code and email address, to ensure that no significant questions were left unanswered. A link to the final version of the survey was posted to a Facebook page created for the study, and Facebook advertisements were used to promote the study. The survey was made available on March 19 th , 2021 and was closed on April 30 th , 2021. The final data collection survey is available as an attachment ( S1 File ).
This was a survey open to every Facebook user in the United States, based on the assumption that every adult was at risk of COVID-19 infection and should theoretically be able to get the vaccine. We limited responses to people stating they were at least 18-years old and able to read, understand, and agree to the terms of the online survey. Bivariate associations were evaluated using Mantel-Haenszel chi-square tests for questions where one or both variables had ordered categorical responses, and Pearson chi-square tests if both variables had nominal categories. Multivariable models were also performed, using an a priori p-value cut point of 0.20 for inclusion in the model. Collinearity between variables was assessed, leading to the exclusion of several variables from each multivariable model, retaining those based on statistical analysis and the team’s clinical experience. For ease of analysis, race was grouped into 2 categories: white and underrepresented minority. Low income was categorized based on respondents who indicated making less than $40,000 in annual income. The final level of significance for these multivariable models was set at p < 0.05.
All analytic assumptions were verified, and the analyses were performed using SAS/STAT software ® v9.4 [ 17 ].
A total of 2,626 responses were obtained. Based on a total of 3,743 potential participants who clicked our survey link on Facebook, our completion rate was 70.2%. Following data cleaning and exclusion of incomplete responses, a total of 2,259 responses were evaluable.
As outlined in Table 1 , most participants were under 60 years of age (61.5%; median age in the 46–60 years group), female (79.2%) and white (89.6%). Most had never been employed in the healthcare field (63.4%), some were employed full time (44.5%), many had at least some college education (93.1%), about half were affiliated with the Democratic party (54.7%), and many lived within family households (75.7%).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0268784.t001
To determine what groups perceived the vaccine as safe, bivariate and multivariable models were created. Table 2 shows that subjects who perceived the vaccination as being safe were more likely to have already obtained their second dose or planned on getting it (we allowed for single shot vaccines in our analyses) (97% vs. 12%; p< 0.0001), did not have a prior health condition (98% vs. 86%; p< 0.0001), trusted science (97.1% vs. 21%; p< 0.0001)/vaccines (97% vs. 17%; p< 0.0001)/doctors (97% vs. 21%; p< 0.0001), believed in the effectiveness of hand washing (94% vs. 88%; p = 0.0056)/social distancing (96% vs. 59%; p< 0.0001)/wearing a mask (95% vs. 43%; p< 0.0001), were female (88% vs. 66%; p = 0.0005), were white (90% vs. 82%; p = 0.0063), had higher levels of education (94% vs. 79%; p< 0.0001), and identified as Democrats (58% vs. 7%; p< 0.0001). In the multivariate model, subjects who were still independently associated with the perception of the vaccines being safe were those more likely to have received their second dose (or planned on it) (p< 0.0001), who trusted science (p< 0.0001), had higher levels of education (p = 0.0007), or were Democrats (p = 0.0020).
https://doi.org/10.1371/journal.pone.0268784.t002
To determine what groups were likely to perceive the most barriers to vaccination, bivariate and multivariable models were created ( Table 3 ). By analyzing subjects who were actively seeking vaccination versus those who were not, we found the former were more likely to have had their second dose (or were likely to get it) (92% vs. 20%; p< 0.0001), did not have a prior health condition (94% vs. 85%; p = 0.0283), trusted science (96% vs. 34%; p< 0.0001)/vaccines (95% vs. 31%; p< 0.0001)/doctors (93% vs. 35%; p< 0.0001), believed in the effectiveness of social distancing (91% vs. 68%; p< 0.0001)/wearing a mask (97% vs. 52%; p< 0.0001), were younger (p< 0.0001), were not male (72% vs. 68%; p = 0.0326), were an under-represented minority (40% vs. 23%; p = 0.0043), had a higher median income ($56,000 vs. $49,000; p = 0.0053), or were Democrats (48% vs. 12%; p< 0.0001). In the multivariate model, subjects that were still independently associated with actively seeking a vaccination were those with their second dose already received (or planned on it) (p< 0.0001) and who trusted in science (p = 0.0006).
https://doi.org/10.1371/journal.pone.0268784.t003
Data for the final two objectives were aggregated and analyzed together ( Table 4 ). For those who “do not believe in vaccines”, the variables more likely associated with such outcome included not having a high-risk medical condition (42% vs. 53%; p = 0.0111), not knowing someone who is vaccinated (87% vs. 98%; p< 0.0001), not trusting vaccines (21% vs. 97%; p< 0.0001)/science (26% vs. 97%; p< 0.0001)/doctors (28% vs. 97%; p< 0.0001), not believing in the effectiveness of hand washing (90% vs. 94%; p = 0.0410)/ social distancing (65% vs. 96%; p< 0.0001)/wearing a mask (51% vs. 94%; p< 0.0001), not receiving an annual flu shot (21% vs. 83%; p< 0.0001), thinking there is no need if others have been vaccinated (58% vs. 8%; p< 0.0001), and not wanting to get vaccinated to help others (27% vs. 96%; p< 0.0001). In the multivariate model, subjects that were still independently associated with not believing in vaccines did not know someone who was vaccinated (p< 0.0001), did not trust science (p< 0.0001), believed vaccination is unnecessary if others were vaccinated (p = 0.0007), and would not get vaccinated to help others (p< 0.0001).
https://doi.org/10.1371/journal.pone.0268784.t004
Additionally, the variables associated with subjects who “do not believe in vaccines” included not getting vaccinated even if friends and family had been vaccinated (26% vs. 89%; p< 0.0001), being male (30% vs. 19%; p = 0.0053), being an underrepresented minority (25% vs. 9%; p< 0.0001), not being employed full time (65% vs. 55%; p = 0.0260), having a lower median income ($ 49, 000 vs. $51, 000; p = 0.0020), having lower levels of educational attainment (21% vs. 6%; p< 0.0001), and not being a Democrat (89% vs. 43%; p< 0.0001). In the multivariate model, subjects who were still independently associated with not believing in vaccines were those not getting vaccinated even if friends and family had done so (p< 0.0001), and having a lower median income (p = 0.0106).
Our study is not the first to examine the relationship between various demographics and vaccine hesitancy. Kini and colleagues explored 39 studies regarding demographics of vaccine acceptance and hesitation. Their systematic review suggests that vaccine acceptance increases with age and is higher for males and white individuals [ 18 ]. While our study reports different significant findings (see below), this is likely attributed to the context and sample of the studies, along with possible confounding variables as discussed later. Our results pertain to the time when data were collected: given the long and haphazard evolution of the pandemic and associated perceptions, the relevance of our results must be contextualized to the time and the stage of the pandemic. Our data show some disparities in perception and opinions regarding the COVID-19 vaccines based on the following key variables: age, race, income, educational level, underlying health conditions, and political partisanship. Participants who had received the first of two doses of the COVID-19 vaccine at the time of our study may already have been convinced of the safety of the vaccines. Additionally, during the early stages of vaccine promotion, there was emphasis from the CDC on possible worsening of underlying pulmonary, cardiac, and other health conditions, such as chronic obstructive pulmonary disease, heart failure, and asthma [ 19 ]. This could explain why individuals with underlying health conditions were likely to regard the vaccines as protective and safe.
Our results also showed that those who identifies as white, compared to members of underrepresented minorities, were more likely to consider the vaccine as safe. Based on an assumption of a positive correlation between perceiving the vaccine as safe and actually getting the vaccine, the CDC has shown that as of July 4 th , 2021, of those who had received at least one dose of the vaccine, 59% were white, 9% were black, 16% were Hispanic/Latinx, and 6% were Asian Americans [ 20 ]. However, it is unclear whether such disparity is affected by the communities in which vaccines are most readily available, or if such disparity in fact represents an individual decision due to distrust that might exist between underrepresented minorities and the healthcare industry. As such, it is vital to review past literature as it pertains to recent findings during the pandemic. Regarding vaccine hesitancy of underrepresented minorities, there has been clear evidence of disparities in healthcare treatment for Black and white patients. Davidio et al reviewed multiple papers that describe physician perceptions and treatment of Black vs. white patients with clear significance regarding the negative handling of Black patients [ 21 ]. Armstrong et al point out that experience of discrimination was strongly associated with healthcare system distrust (HCSD) in their study comparing African American and white survey respondents [ 22 ]. Additionally, Balasuriya et al explored factors associated with COVID-19 acceptance and access among Black and Latinx communities, and identified the pervasive mistreatment of Black and Latinx communities, rooted in structural racism, to be a key influence on vaccine acceptance [ 23 ]. Results such as this provide a strong basis to argue why underrepresented minorities may have been less eager to seek out vaccinations. Regarding vaccine hesitancy and political affiliation, other studies corroborate these results. In one study, it was found that US Republican counties consistently had lower general vaccination rates than Democratic counties [ 24 ]. In a polling done by Kaiser Family Foundation in May 2020, it was found that Republicans were less likely to report wearing masks, social distancing or getting vaccinated against COVID-19 [ 25 ].
Level of education has a strong effect on willingness to receive a COVID-19 vaccine: having a college degree has been associated with a 43% increase in likelihood of getting the vaccine [ 26 ]. Assuming the likelihood of obtaining the COVID-19 vaccine is positively correlated with perception that the vaccine is safe, it is worthwhile posing the question whether level of education outweighs other effects of race, gender, political affiliation, and underlying health conditions. Delay in COVID-19 vaccination notwithstanding (earlier in 2021 when our data were collected), the CDC has pointed to a divide in communities based on political party affiliation. To ultimately determine the prime factors in safety perception, we conducted a multivariable analysis and found that the following groups were most likely to perceive vaccines as being safe: 99.3% Democrats (vs. 86.0% Republicans, specifically) and 93.1% with higher educational attainment (vs. 6.8% with high school level specifically). It is important to correlate these results with previous studies that examined similar topics. Regarding results about education impacting vaccine rates, previous studies would support this. Suryadevara and colleagues collaborated with their county health department to educate high-risk, resource-poor families regarding vaccination concerns. Their results showed a drastic increase for general vaccine completion and annual influenza vaccine rates [ 27 ]. Another study showed that when providing low-literacy educational materials to resource-poor families regarding the pneumococcal vaccine, the test group was four times more likely to discuss the vaccination in appointments and five times more likely to receive the vaccine than control group [ 28 ]. Even more recent studies with COVID-19 support our findings. For instance, a recent study indicated that lack of high school education positively correlates with increased vaccine hesitancy and decreased vaccination levels [ 29 ].
Our multivariable model outcome also suggests that race and ethnicity are not necessarily the primary determinants of vaccine hesitancy and likelihood of vaccination, because low vaccination rates among underrepresented populations may be explained by the historical distrust within some members of underrepresented minorities toward health care organizations and providers, as well as suspicion about clinical research studies, in view of past atrocities such as the Tuskegee Syphilis experiment [ 30 ], or similar experiments with STD infections in Guatemala [ 31 ]. Our multivariable results support this possibility by indicating those being potential factors in rejecting the COVID-19 vaccine. Specifically, after adjusting for variables, one of the groups found to be independently associated and most likely to reject vaccination according to socioeconomic and demographic factors were individuals with lower income. Considering that low-income populations usually consist of groups that identify as underrepresented minorities [ 32 ], slow rates of vaccination in these groups might reflect individual distrust of health care providers. However, this finding does not rule out the possibility of low distributions in low-income locations (e.g., rural), which could be a barrier by itself for vaccination opportunities. As pointed out by DeMaria-Ghalili and colleagues, “health inequalities are most acute among those living in rural and low resourced areas of the state, and among underrepresented populations (particularly Black/African American and Latino), who lack access to health care, experience digital divide, and face persistent local healthcare workforce shortages.” The report further discusses that people in areas of lower socio-economic status or fewer resources (usually rural areas) have a more difficult time scheduling and going to appointments for vaccinations, noting “pharmacy deserts” to be an issue in having access to appropriate healthcare resources such as vaccines [ 33 ]. Economic precarity and poor technological advancements may be obstacles to both registering for and getting the vaccine, possibly associated with sparse information among low-income populations [ 34 ]. Therefore, to bolster vaccination, efforts should be made to target groups who are most likely to encounter barriers to COVID-19 vaccination, through governmental incentives, including free childcare and rides to vaccination sites, lottery tickets or cash vouchers, complimentary food and drinks at the vaccination sites, and tax credit [ 35 ], rather than privately offered incentives that may vary greatly throughout the country.
Our successful recruitment for this survey was helped by the ever-increasing prevalence of social media in peoples’ lives. This highlights the need for proper, scientific-based information regarding the pandemic to reach the lay public before opinions appear on social media newsfeeds. On the other hand, only 2.1% of our sample thought that social media sites were reliable sources for vaccine information. While this would appear to suggest limited influence of social media with regard to COVID vaccines, we have to interpret this with caution in view of a small, self-selected sample that may not reflect the U.S. population as a whole. While some individuals may have legitimate reasons for declining vaccination, e.g. allergies to some ingredients in the preparation or other medical contraindications, misperceptions about vaccines as presented by some members of the media can lead to vaccine refusal for inappropriate reasons [ 36 ]. Therefore, it is important to disseminate the scientific basis for vaccines whenever possible. Negative press about variant viruses and the possibility of ineffective vaccines lead to further public distrust of the otherwise monumental feat of creating and distributing the COVID-19 vaccines [ 37 ]. Education of the public is essential for the continued success of vaccination efforts in general. As an example, in one study [ 38 ], Human Papilloma Virus (HPV) vaccine education sessions were held for parents, healthcare and school staff who had little knowledge regarding HPV vaccines. After the sessions, results showed that over 90% of respondents felt vaccine education was important and 85–97% were supportive of school-based vaccine clinics. In another study on flu vaccination during pregnancy [ 39 ], pregnant women refused flu vaccines due to likely susceptibility to influenza and concerns for vaccine safety. The study intervention was a brief educational video by the CDC, which addressed vaccination health beliefs in a clear and easy to understand format. The primary outcome was receipt of the flu vaccine on the next prenatal visit, and suggested that appropriate education and reassurance were influential in vaccination. We must do the same for the COVID-19 vaccine, seeing that our findings suggest that educational attainment is one of the two most important factors that determine the likelihood that one will perceive the vaccine as safe and be likely to accept vaccination. Given that an overwhelming majority of our respondents indicated that they considered doctors, nurses, and other healthcare workers as reliable sources of vaccination information, it is imperative to begin incorporating COVID-19 vaccine questions and education during health care visits. Moreover, training healthcare professionals in cultural competency, defined as “the ability of individuals and systems to work or respond effectively across cultures in a way that acknowledges and respects the culture of the person or organization being served” [ 40 ] would help them navigate this conversation with knowledge and transparency to promote mutual trust and possibly increased likelihood of vaccination [ 41 , 42 ]. Unfortunately, cultural competency training is still limited in medical schools and residency programs [ 43 ], and broader implementation is needed. This will be critical for engaging minority/underrepresented groups, though we acknowledge that these groups may have general difficulties accessing any medical care and this in turn may contribute to lower vaccination rates. Some respondents chose “no access” as a reason for not receiving the vaccine. The term “no access” is admittedly broad and could have included decreased vaccination distribution to impoverished neighborhoods, or it could mean that individuals do not know where to go to get their vaccine. We kept our questionnaire concise so as not to overburden respondents, and consequently could not necessarily qualify the specific reasons for perception about no or limited access. Further investigation is needed to characterize the specific obstacles experienced by people seeking the vaccination. As health literacy regarding the still relatively new COVID-19 pandemic remains a challenge [ 44 ], our present survey can hopefully act as a compass to inform providers on the underlying rationale that their patients have for being skeptical about vaccines or medical advice.
In addition, we need steps to encourage the population to get vaccinated irrespective of political affiliation. Per our findings, those who identify as Democrats are more likely to perceive the vaccine as being safe. Partisanship and vaccination status continue to play a role in both U.S. vaccination efforts and the government’s response to the pandemic in general. Other studies have shown similar results [ 45 ], where 65% of Democrats and 51% of vaccinated adults say that the surge in COVID cases makes them angry at people who have not gotten a vaccine, while 59% of Republicans and 56% of unvaccinated adults say that the federal government should be blamed. Our study shows that Republicans less likely to become vaccinated trust information that comes directly from their health care team, more than information that originates from the government. Therefore, ensuring that all personnel on the health care team are culturally competent to facilitate conversations brought on by patients regarding the COVID-19 vaccine will be instrumental in ensuring vaccination acceptance across spectra. Finally, incentives must be focused on core groups that we believe are more likely to reject the vaccine. These include underrepresented minorities, people with lower educational level, those who identify as young, males, and those with high risk underlying medical conditions.
Our study has limitations, especially regarding data collection. Given the current pandemic and difficulty with in-person survey distribution, it was decided that an online distribution would be preferable, based on the assumption that every individual is at risk of contracting the virus and becoming affected by the pandemic. We used Facebook due to its wide reach. However, we recognize that not everyone has access to computers or Facebook, so this survey may favor those of higher socioeconomic status. Likewise, we did not seek parity since the sample was largely one of convenience, based on who responded to the questionnaire. Although forced responses were used for our survey to ensure completion and prevent answers, we could not determine other potential factors that may have caused incomplete responses in cases where respondents were allowed to select up to three options, e.g. for trusted sources of information. Obstacles to completion might have included feeling pressed for time, concerns about privacy in view of the open nature of social media, or rejection based on personally held political views. This could result in a self-selection bias due to differences between respondents and non-respondents, therefore skewing the findings. For example, many participants were white, female, and/or Democrat voters, which is not representative of the U.S. population per se and could bias the results in favor of opting for vaccination, perhaps due to stronger belief in vaccines. Obviously, given the enormous number of Facebook users in the U.S., and the fact that users are allowed to protect their privacy by restricting access to personal data (including by omitting it in their profiles), it would be difficult to assess the “typical” Facebook user in the context of these factors. Along those lines, about 87% of respondents were already vaccinated, which suggests that most considered the benefits greater than the risks. This may therefore result in under-reporting and under-characterizing negative views of the vaccine that we sought to capture in the survey. Another limitation of this study is that it only represents a snapshot in time of opinions of COVID-19 vaccine perceptions, which can be fluid. Because the vaccine data are rapidly changing and information provided to the public may evolve as days progress, our results can only be applicable to this specific point in time. Ideally, the present study should be repeated in the future to ascertain trends over time. From a methodological standpoint, future studies should focus on obtaining a wider and more diverse set of respondents, including individuals that do not have access to computers or Facebook. One feasible alternative could be the distribution of both online and paper surveys to the same group of respondents during the same wave of data collection, thus allowing for estimation of changes across strategies for survey contact.
While our findings are in line with some existing perspectives in the field, as they relate to the role of socioeconomic factors [ 26 , 32 ], educational influence [ 38 , 39 ], and partisanship [ 45 ], we have contributed a more robust and elaborate perception of the U.S population on COVID vaccines, while identifying specific groups at risk for not getting a vaccine. In conclusion, level of education and partisanship, but not race/ethnicity, were the most likely factors associated with vaccine hesitancy or likelihood to vaccinate. This suggests that improved education, not just about vaccines per se, but with regard to formal schooling in general, may be at the heart of promoting greater acceptability of vaccination. Likewise, low vaccination rates among underrepresented minorities may be due to distrust for healthcare industries, but further research is needed to fully characterize the relative contributions of low access vs. distrust. Many white people and many with a Republican party affiliation also expressed reluctance about vaccination, suggesting that mistrust of the healthcare industry, vaccinations in general, and/or the government is not limited to minorities and/or economically challenged populations. Regardless, population sub-groups less likely to be vaccinated and/or receptive to vaccines should be targeted for vaccine education and incentives, and outcomes of these interventions need to be closely studied for determination of efficacy.
Supporting information
S1 file. qualtrics survey questionnaire..
https://doi.org/10.1371/journal.pone.0268784.s001
S1 Data. Inclusion criteria.
https://doi.org/10.1371/journal.pone.0268784.s002
Acknowledgments
The authors would like to thank our collaborators at Qualtrics and Facebook for helping facilitate the successful completion of this study.
- View Article
- PubMed/NCBI
- Google Scholar
- 4. Centers for Disease Control and Prevention (CDC): COVID-19 Racial and Ethnic Health Disparities . https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/racial-ethnic-disparities/index.html . Accessed on 10 th , Dec 2020.
- 5. Johnson, Akilah: Lack of Health Services and Transportation Impede Access to Vaccine in Communities of Color . https://www.washingtonpost.com/health/2021/02/13/covid-racial-ethnic-disparities/ . Accessed on 13 th , Feb 2021.
- 8. U.S Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine?s_cid=11700:covid%20vaccine%20fda%20approval:sem.ga:p:RG:GM:gen:PTN:FY22 Accessed on 11th, December, 2020.
- 9. U.S Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid Accessed on 18 th , December 2020
- 12. The New York Times: Pandemics Racial Disparities Persist in Vaccine Rollout . https://www.nytimes.com/interactive/2021/03/05/us/vaccine-racial-disparities.html?campaign_id=37&emc=edit_rr_20210306&instance_id=27792&nl=race%2Frelated®i_id=76131559&segment_id=52913&te=1&user_id=4fc6fb065b9e912a39a1a6408b1e81c2 . Accessed on 5 th , March, 2021.
- 14. Pew Research Center. https://www.pewresearch.org/science/2020/12/03/intent-toget-a-covid-19-vaccine-rises-to-60-as-confidence-in-research-and-development-processincreases Accessed on 3 rd , December, 2020.
- 15. Mayo Clinic: US COVID-19 Vaccine Tracker. https://www.mayoclinic.org/coronavirus-covid-19/vaccine-tracker Accessed on: 17 th , July 2021.
- 17. All result output were created using SAS. Copyright © 2021 SAS Institute Inc., Cary, NC, USA.
- 19. Centers for Disease Control: Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19 . https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html Accessed on: 18 th , October 2021.
- 20. Centers for Disease Control: Latest Data on COVID-19 vaccination by Race/ Ethnicity . https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-on-covid-19-vaccinations-race-ethnicity/ Accessed on: 18 th , October 2021.
- 25. Hamel, L., Kearney, A., Kirzinger, A., Lopes, L., Munana, C., Brodie, M. KFF Health Tracking Poll — May 2020 . Kaiser Family Foundation . https://www.kff.org/coronavirus-covid-19/report/kff-health-tracking-poll-may-2020/ Accessed on 19 th , January 2022.
- 26. Thomas, K., Darling, J. Education is now a Bigger Factor than Race in Desire for COVID-19 Vaccine . https://healthpolicy.usc.edu/evidence-base/education-is-now-a-bigger-factor-than-race-in-desire-for-covid-19-vaccine/ Accessed on 17 th , July 2021.
- 30. Ada McVean. McGill. 40 Years of Human Experimentation in America : The Tuskegee Study . https://www.mcgill.ca/oss/article/history/40-years-human-experimentation-america-tuskegee-study Accessed on 19th January, 2022.
- 31. Amy Gutmann. Presidential Commission for the Study of Bioethical Issues. “Ethically Impossible” STD Research in Guatemala from 1946 to 1948. https://bioethicsarchive.georgetown.edu/pcsbi/node/654.html Accessed on 19 th January, 2022.
- 32. Simms, M. The Urban Institute. Racial and Ethnic Disparities among low-income Families . https://www.urban.org/sites/default/files/publication/32976/411936-racial-and-ethnic-disparities-among-low-income-families.pdf Accessed on 18th October, 2021
- 33. DiMaria-Ghalili, R., Foreshaw Rouse, A., Coates, M., Hathaway, Z., Hirsch, J., Wetzel, S., et al. Disrupting Disparities in Pennsylvania: Retooling for Geographic, Racial and Ethnic Growth [White paper]. https://aarp-states.brightspotcdn.com/6f/b6/de161f3a4a63a23e811693d90b68/aarp-drexel-pennsylvania-disrupting-disparities-design-0421-final.pdf Accessed on 19th January, 2022.
- 35. Devon Delfino. Incentives for CoVID-19 Vaccination : Food , Cash , & Other Perks . https://www.goodrx.com/health-topic/vaccines/covid-19-vaccination-incentives Accessed on 20th, January, 2022.
- 37. The Atlantic. 5 Pandemic Mistakes We Keep Repeating . https://www.theatlantic.com/ideas/archive/2021/02/how-public-health-messaging-backfired/618147/ Accessed on 26 th , February 2021.
Study: Highlighting harms of skipping COVID vaccines more effective than promoting benefits

A new study in the Journal of Public Health suggests that it is more effective to emphasize the harms that come from not getting vaccinated rather than emphasizing the benefits of vaccination at the individual or community level.
The study was based on findings from three experimental messages given to 1,085 participants from China who were randomly assigned to one of four groups, including one control group.
All groups were given a message about COVID-19 vaccination; the first group was told, "Vaccination can make you develop antibodies against COVID-19, thus reducing the likelihood of contracting COVID- 19 and developing severe symptoms after infection."
The second group’s message was "Vaccination can promote the formation of community herd immunity, thereby reducing the likelihood of community members getting infected with COVID-19 and developing severe symptoms after infection," while the third group’s message emphasized harm: "If you are not vaccinated, you will not develop antibodies to COVID-19, and thus you will be more susceptible to COVID-19 and more likely to develop severe symptoms after infection."
73% of harm group willing to get vaccinated
When asked about intent to receive the COVID-19 vaccine after hearing the group’s message, 72.6%. of the harm group said they would get vaccinated, compared to 62% in the community-benefit group. In the personal-benefit group, 65.5% said they likely to get vaccinated.
" The findings of the study can provide valuable insights for improving the ability of governments to respond to pandemics, ” said senior author Ke Feng, PhD, of the University of Electronic Science and Technology of China, in a press release from Oxford University Press.
FDA approves use of Novavax's updated COVID vaccine
The US Food and Drug Administration (FDA) today announced that it has granted emergency use authorization for Novavax's updated COVID-19 vaccine.
Approval of the protein-based vaccine comes about a week after the FDA green-lighted the two updated mRNA vaccines—made by Moderna and Pfizer-BioNTech—which target the KP.2 variant. The Novavax vaccine targets JN.1, the parent of KP.2.
Novavax's updated vaccine is authorized for people ages 12 and older.
A third COVID vaccine option
In a statement, Peter Marks, MD, PhD, who directs the FDA's Center for Biologics Evaluation and Research, said COVID vaccines continue to have a major positive impact on public health and that vaccination continues to be the most effective method for COVID prevention. "Today's authorization provides an additional COVID-19 vaccine option that meets the FDA's standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization."
Today's authorization provides an additional COVID-19 vaccine option that meets the FDA's standards.
The company has said the vaccine will be available in prefilled syringes and that it will promptly deliver it as soon as the FDA authorizes the vaccine. Its data suggest the JN.1 monovalent vaccine induces broad neutralization responses against JN.1 lineage viruses, including ones such as KP.2 and KP.3
New Mexico reports more H5N1 in dairy cows as California probes possible outbreaks on 3 farms

The US Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) today confirmed another H5N1 avian flu outbreak in a dairy herd in New Mexico, the state’s first since the middle of April.
The outbreak pushes the nation's total confirmed outbreaks in dairy facilities to 194 in 13 states.
Though the pace of H5N1 outbreaks on dairy farms has slowed over the summer months, federal officials said at a briefing earlier this month that it was too soon to say if the virus was ebbing in cattle. They noted that cattle movement between states, which played a role in initial spread, picks back up in the fall due to greater demand for milk, partly due to schools resuming.
Suspected outbreaks in Central Valley
The California Department of Food and Agriculture yesterday announced that it is investigating the possible introduction of the virus into three dairy farms in the state’s Central Valley. It said samples have been sent for testing to California Animal Health and Food Safety laboratory. If positive, they will be considered "presumptive" and submitted to the USDA for confirmation.
In early June, WastewaterSCAN launched an H5 wastewater dashboard to help track undetected H5 spread, and, since then, traces of the virus have been found in nine states, seven of which have reported H5N1 outbreaks in dairy cows. California and Arkansas were the only two states with positive H5 wastewater findings that hadn't reported recent outbreaks on dairy or poultry farms.
California is the nation's top milk production state and accounts for one fifth of the nation's supply. The state has about 1,100 dairy farms, and herds in Tulare, Merced, and Stanislaus counties make up just more than half of the state's milk output.
COVID still on the rise in parts of US
As the United States heads into the Labor Day holiday weekend and with schools resuming, COVID-19 activity continues to rise in many areas, but it's showing early signs of decline in others, the Centers for Disease Control and Prevention (CDC) said today in its data updates.
Wastewater SARS-CoV-2 detections are still at the very high level and are highest in the South. Though levels are dropping in the West, they are rising in the South, Midwest, and Northeast, the CDC said.
Meanwhile, wastewater tracking from WastewaterSCAN shows that detections nationally are still at the high level, with no clear trend up or down over the past 3 weeks. The group, however, noted an upward trend in the Midwest. WastewaterSCAN is a national wastewater monitoring system based at Stanford University in partnership with Emory University.
KP.3.1.1 levels climb; hospitalizations elevated
In updated variant proportion estimates today, the CDC said the level of KP.3.1.1—a JN.1 offshoot thought to more easily evade immunity from earlier infection and vaccination—continues to rise sharply and is at 42.2%, up from 29.5% 2 weeks ago.
Among the CDC’s other COVID indicators, national test positivity is at 17%, down a hair since the previous week. Positivity is higher in Texas and surrounding states and in the lower Midwestern states than in other parts of the country. Emergency department visits declined from the previous week and are highest in parts of the South and Southeast.
Hospitalizations from COVID remain elevated, especially in seniors and in children younger than 2 years old, the CDC said in its weekly respiratory illness summary .
Deaths from COVID-19, though still low, rose last week and were at the highest level in Kentucky.
Chinese researchers warn of rise in Acinetobacter strains carrying multiple carbapenemase genes
A study by Chinese researchers indicates a global rise in Acinetobacter species carrying multiple carbapenem-resistance genes.
In a letter published yesterday in Clinical Microbiology and Infection, a team led by researchers from Zhejiang University School of Medicine said analysis of 30,713 Acinetobacter genomes from the National Center for Biotechnology Information Pathogen Detection Database revealed that 1,409 (5.1%) of 27,487 A baumannii isolates and 216 (6.7%) of 3,226 non-baumannii Acinetobacter spp. (nAB) isolates co-harbored at least two carbapenemases, with notable increases from 2018 through 2023. Multiple carbapenemase-positive Acinetobacter genomes were detected in 61 countries across six continents, with the highest prevalence found in the United States (33.7%), India (13.3%), and China (8.6%).
Carbapenem-resistant A baumannii is already considered an urgent public health threat by the World Health Organization and the US Centers for Disease Control and Prevention because it's so difficult to treat. Some studies have found mortality rates as high as 72% in patients with carbapenem-resistant A baumannii infections.
But among the 54 distinct combinations of double carbapenemases identified, the analysis also found the co-existence of metallo-beta-lactamase and tetracycline-inactivating genes, which confer resistance to many of the recently approved beta-lactam/beta-lactamase inhibitor combinations (such as ceftazidime/avibactam and meropenem/vaborbactam) and tigecycline, all of which have been considered potential treatments for carbapenem-resistant A baumannii infections. These combinations, the study authors said, further restrict the antibiotic treatment options for Acinetobacter infections.
Strains found in clinical settings, livestock, and the environment
While nearly all the A baumannii strains originated from clinical settings, 33.5% of the nAB strains were isolated from environmental or livestock sources, the researchers added.
"In summary, the prevalence of Acinetobacter species co-harboring multiple carbapenemases is increasing globally, and the emergence of novel combinations of carbapenemases and their coexistence with Tet(X) enzymes requires further investigation" they wrote. "Moreover, the global emergence of carbapenem-resistant Acinetobacter species in diverse sources emphasizes the critical importance for a One Health approach for the investigation and control of its spread."
Investors can play 'pivotal role' in addressing antimicrobial resistance, report says
A new report lays out the financial risks posed by antimicrobial resistance (AMR) and how investors might be able to mitigate them.
The health risks posed by drug-resistant pathogens are already well known. A 2022 study published in The Lancet estimated that AMR was directly responsible for 1.27 million deaths, and contributed to an additional 3.7 million deaths, in 2019. That's more than the number of deaths caused by HIV/AIDS, malaria, and many cancers.
But the report published this week by the Farm Animal Investment Risk & Return (FAIRR) initiative, the MSCI Sustainability Institute, and Investor Action on AMR highlights the significant financial costs. According to World Bank estimates, unchecked drug-resistance could cause annual gross domestic product losses ranging from US $1 trillion to $3.4 trillion by 2030, driven by increased human and veterinary healthcare costs, reduced productivity, and declines in global livestock production. Those losses could rise to $100 trillion by 2050 if the weak pipeline for new antibiotics continues to falter.
Incorporating an 'AMR lens' into investment decisions
But investors can play a role in addressing AMR by incorporating an "AMR lens" into investment decisions, the report suggests. This means identifying opportunities to invest in companies that are part of the solution to AMR and avoiding investments that exacerbate it. One clear area of opportunity is antibiotic research and development
"Investors can play a pivotal role in driving the research and development of new antibiotics, diagnostics, and alternative treatments," the report states. "By providing the necessary funding, investors can help accelerate the pace of innovation and commercialization of solutions."
Investors can play a pivotal role in driving the research and development of new antibiotics, diagnostics, and alternative treatments.
In addition, the report suggests that investors can work with companies in the livestock sector to encourage them to reduce inappropriate antibiotic use and adopt alternative treatment strategies, invest in global AMR surveillance systems that will help companies with global risk planning, and support healthcare companies that are using tools and databases to evaluate appropriate antibiotic use.
"By working collaboratively, investors can address existing gaps and promote sustainable practices," the report states. "Understanding the economic impact, integrating AMR into investment decisions, and supporting research and innovation are crucial."
In case you missed it
This week's top reads, study puts understanding of long covid and vaccination into question.
Seven percent of patients were diagnosed as having long COVID.
Ahead of a holiday weekend and as schools resume, COVID activity is still elevated with wastewater levels are rising in all regions but the West.
Listeria outbreak tied to deli meats grows; death toll reaches 9
It is now the largest listeriosis outbreak in the US since the 2011 outbreak linked to cantaloupe.

New Hampshire reports fatal EEE case
The state's epidemiologist says New England may be at elevated risk for eastern equine encephalitis this year, based on mosquito surveillance.
The authorization of Novavax's updated monovalent vaccine gives consumers a protein-based immunization option.
UK studies highlight prevalence, severity, and impact of long-COVID symptoms
A study of healthcare workers finds persistent fatigue, tiredness, and shortness of breath have a significant impact on work and life, while another highlights pain as a persistent symptom.

Survey reveals growing American distrust in vaccines for COVID, other infectious diseases
Twenty-two percent believe the falsity that it's less risky to get infected with COVID-19 than to get the vaccine, up from 10% in 2021.

Wearable activity trackers could offer early clues on COVID-19
For COVID-19 diagnosis, wearables were accurate 88% of the time.
Weight-loss drug tied to lower risk of death from COVID, heart disease, all causes
A commentator likened the drug's effects to those of a vaccine against the indirect effects of a pathogen, without waning over time.

Cambodia's recent H5N1 case involved novel reassortant
Earlier this year, animal health officials warned that the reassortant is spreading in the Greater Mekong subregion.
Our underwriters
Unrestricted financial support provided by.

- Antimicrobial Resistance
- Chronic Wasting Disease
- All Topics A-Z
- Resilient Drug Supply
- Influenza Vaccines Roadmap
- CIDRAP Leadership Forum
- Roadmap Development
- Coronavirus Vaccines Roadmap
- Antimicrobial Stewardship
- Osterholm Update
- Newsletters
- About CIDRAP
- CIDRAP in the News
- Our Director
- Osterholm in the Press
- Shop Merchandise
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clin Exp Vaccine Res
- v.10(2); 2021 May
Inside the story about the research and development of COVID-19 vaccines
Shrina p. patel.
Ramanbhai Patel College of Pharmacy, Charusat University, Anand, India.
Gayatri S. Patel
Jalpa v. suthar.
The ongoing coronavirus threat from China has spread rapidly to other nations and has been declared a global health emergency by the World Health Organization (WHO). The pandemic has resulted in over half of the world's population living under conditions of lockdown. Several academic institutions and pharmaceutical companies that are in different stages of development have plunged into the vaccine development race against coronavirus disease 2019 (COVID-19). The demand for immediate therapy and potential prevention of COVID-19 is growing with the increase in the number of individuals affected due to the seriousness of the disease, global dissemination, lack of prophylactics, and therapeutics. The challenging part is a need for vigorous testing for immunogenicity, safety, efficacy, and level of protection conferred in the hosts for the vaccines. As the world responds to the COVID-19 pandemic, we face the challenge of an overabundance of information related to the virus. Inaccurate information and myths spread widely and at speed, making it more difficult for the public to identify verified facts and advice from trusted sources, such as their local health authority or WHO. This review focuses on types of vaccine candidates against COVID-19 in clinical as well as in the preclinical development platform.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) originated in Hubei Province, China, in December 2019 (and possibly earlier, though unrecognized), as a pneumonia-causing disorder [ 1 ], most likely the result of natural selection in animal hosts (bats, pangolins) before the zoonotic transition [ 2 ]. Seven members of this viral family are now known to infect humans, three of whom have the potential to cause severe respiratory diseases [ 3 ]. Coronaviruses (CoVs) are positive-sense, single-stranded Coronaviridae family (subfamily Coronavirinae) RNA viruses that infect a broad range of hosts to produce diseases ranging from the common cold to severe/fatal diseases [ 4 ]. The novel virus was initially named “2019-nCoV” by the International Committee on Virus Taxonomy. It was changed to “SARS-CoV-2” since it was found to be the sister virus of an extreme acute respiratory syndrome (SARS-CoV) [ 5 ]. The ongoing threat of coronavirus emerging in China has spread rapidly to other countries and has been declared by the World Health Organization (WHO) as a global health emergency [ 6 ].
Virus genetic sequencing shows that it is a beta coronavirus that is closely related to the SARS virus [ 7 ]. Currently, immunization prevents 2–3 million deaths from more than 20 life-threatening diseases that are now being controlled by vaccinations, and work is underway at an unprecedented pace to make coronavirus disease 2019 (COVID-19) a vaccine-preventable illness [ 8 ]. To accelerate the research and development process and to establish new standards and standards to prevent the spread of the coronavirus pandemic and care for those affected, WHO brings together the world's scientists and public health practitioners [ 7 ]. In human medical intervention, vaccines are one of the monumental achievements in mitigating the dispersion and effects of infectious diseases [ 9 ]. Vaccines are the most useful method for contagious disease prevention because they are more cost-effective than treatment and reduce morbidity and mortality without long-lasting effects [ 10 ]. Preventive and therapeutic vaccines will be of fundamental significance as the most obvious way to safeguard public health [ 11 ]. Since the coronavirus shares substantial sequence homology with two other lethal coronaviruses, SARS and Middle East respiratory syndrome (MERS), the vaccines identified could potentially promote the design of anti-SARS-CoV-2 vaccines. It is essential to establish safe and effective vaccines to contain the COVID-19 pandemic, eradicate its spread, and eventually prevent its future recurrence [ 12 ]. By exposing individuals to antigens, vaccination can produce long-lasting immunity to drive the production of immunological memory before meeting live pathogens. Thus the resulting immunity can be mediated by the activation of humoral antibodies and the effector function of cellular T-cells [ 13 ]. The full development path for an effective SARS-CoV-2 vaccine will involve th e cooperation of industry, government, and academia in unprecedented ways, each contributing its strengths [ 14 ].
It is a difficult task to develop a SARS-CoV-2 vaccine to control its spread and help remove it from the human population since there is a lack of knowledge on its biological properties, epidemiology, individual immune responses to it, and so forth [ 15 ]. The S protein is the critical target of vaccine production since it includes a receptor-binding domain (RBD) and viral functions. It will be essential to confirm the clinical significance of the SARS-CoV-2 binding and neutralizing antibody titers and their ability to predict efficacy [ 16 ]. Only in a significant clinical efficacy study would it be possible to confirm the association between antibody titers and defense against COVID-19 [ 17 ]. For any frequently used vaccine, there is a theoretical risk that vaccination could cause subsequent infection with SARS-CoV-2 more severe. This has been confirmed in feline coronaviruses and has been observed in some SARS-CoV-1 animal vaccine challenge models [ 18 ].
The key benefit of next-generation vaccines is that they can be produced based on sequence data alone [ 19 ]. If the viral protein(s) that are essential for the defense against infection or disease and therefore for inclusion in the vaccine is established, the availability of coding sequences for the viral protein(s) is sufficient to start the production of the vaccine rather than to rely on the ability to grow the virus [ 20 ]. This makes these platforms extremely adaptable and dramatically accelerates the production of vaccines, as is evident from the fact that the majority of currently underway clinical trials of COVID-19 vaccines include a next-generation platform [ 19 ]. A prospective pharmaceutical manufacturer must send an application to a regulatory authority such as the Food and Drug Administration (FDA) to examine the new vaccine after a possible vaccine has been announced by a researcher [ 21 ].
The demand for immediate therapy and potential prevention of COVID-19 is growing [ 22 ] with the increase in the number of individuals affected due to the seriousness of the disease, global dissemination, lack of prophylactics, and therapeutics [ 23 ]. Attempts are being made to establish secure and successful methods for prophylactics [ 24 , 25 ]. Several vaccines are in different phases of clinical trials [ 6 ], but there is a lack of prophylactics in the present scenario [ 26 ]. Several attempts have been made to create COVID-19 vaccines to avoid the pandemic condition as well as the S-protein SARS-CoV-2 has been used for most of the emerging vaccine candidates. In Fig. 1 , the overview of vaccine candidates in their respective ongoing clinical phases depicts the percentage of vaccine candidates amongst which the majority of developing vaccines is in phase 1/2. The data shown below in the graph is assessed until 15 October 2020, in the pipeline of vaccine development and registered clinical trials globally.

In Fig. 2 , the overview of the global COVID-19 vaccine landscape in clinical development depicts that there are seven major types of vaccine candidates for COVID-19 is illustrated as (inactivated, non-replicating viral vectors, replicating viral vectors, protein subunit, nucleic acid-based, and virus-like particles [VLP]), showing the percentage of candidate vaccines that are currently under clinical development. The nucleic acid-based platform includes both RNA vaccines and DNA vaccines. Among the seven types of vaccine candidates, protein subunit-based vaccines constitute the highest 31% in clinical development. In contrast, VLP-based vaccine and replicating viral vectors comprises the lowest as 5% in the clinical development.

In Fig. 3 , the overview of global COVID-19 vaccine landscape in preclinical development depicts that there are 10 significant types of vaccine candidates for COVID-19 is illustrated as (inactivated, replicating bacteria vector, DNA, live attenuated virus, non-replicating viral vectors, protein subunit, t-cell based, replicating viral vectors, RNA, and VLP), showing the percentage of candidate vaccines that are currently under preclinical development. Among the 10 types of vaccine candidates, protein subunit-based vaccines constitute the highest 36% in clinical development whereas T-cell based vaccine and replicating bacteria vector comprises the lowest at 1% in the preclinical development globally.

RNA-Based Vaccine
As a result of considerable developments in biotechnology, due to their higher potency, short development cycles, low-cost product, and safe administration, mRNA vaccines represent a substantial improvement over traditional vaccine strategies [ 27 ]. The mRNA is an evolving platform that is non-infectious and non-integrated and has almost no possible risk of insertional mutagenesis. Antigen discovery, sequence analysis, and optimization, screening of modified nucleotides, delivery system discovery, and immune response and safety assessment tests are the sequential events in the mRNA vaccine production process [ 28 ]. In vaccines, two primary forms of RNA are investigated: virally derived, RNA self-replicating, and mRNA non-replicating. The antigen and the necessary viral replication machinery are typically self-replicating RNAs, whereas conventional mRNA-based vaccines encode only the antigen of interest with 50 and 30 untranslated regions (UTRs) [ 27 ].
The immunogenicity of mRNA can be decreased, and changes can be made to enhance the stability of these vaccines [ 29 ]. Furthermore, anti-vector immunity is also resisted as mRNA is the minimally immunogenic genetic vector, allowing repeated administration of the vaccine [ 30 ]. This platform has empowered the rapid vaccine development program due to its flexibility and ability to reproduce the structure and expression of the antigen as seen in the course of natural infection [ 31 ]. A possible benefit of mRNA vaccines is the convenient availability of a portable mRNA “printing” facility for large-scale production of mRNA [ 32 ].
mRNA-1273 (Moderna TX Inc.)
It is a vaccine composed of lipid nanoparticle (LNP) encapsulated synthetic mRNA that codes for SARS-CoV-2 full-length, pre-fusion stabilized spike protein (S) [ 33 ]. It has the potential to induce an antiviral response that is highly S-protein specific. Also, it is known to be relatively harmless since it is neither composed of the inactivated pathogen nor of the live pathogen sub-units [ 34 ]. To perform the phase II trials, the vaccine has received FDA fast-track approval. The company published the interim antibody data for phase I of eight participants who received different levels of dose [ 33 ]. For the participants receiving 100 µg dose, neutralizing antibody levels were significantly higher than those observed in convalescent sera. In the 25 µg and 100 µg dose cohorts, the vaccine was found to be primarily safe and well-tolerated. In comparison, three participants reported systemic symptoms of grade 3 following administration of the second 250 µg dose level [ 26 ]. The possible benefits of a prophylactic vaccine mRNA strategy include the ability to replicate natural infection to induce a more effective immune response and the ability to incorporate multiple mRNAs into a single vaccine [ 12 ].
On 24 February 2020, Moderna declared that it had released the first batch of mRNA-1273 against SARS-CoV-2 for human use, prepared using the methods and strategies outlined in its previous patents. mRNA-1273 vials have been shipped to the National Institute of Allergy and Infectious Diseases (NIAID), a division of the National Institutes of Health (NIH), to be used in the United States in the proposed phase 1 study [ 35 ]. In collaboration with researchers at the NIAID Vaccine Research Centre, Moderna reports that mRNA-1273 is an mRNA vaccine targeting a prefusion stabilized form of the S protein associated with SARS-CoV-2, which was chosen by Moderna [ 32 ]. Patent application WO2018115527 describes vaccines consisting of mRNA encoding at least one MERS coronavirus antigen, preferably an S protein or an S protein fragment (S1), an envelope protein (E), a membrane protein (M), or a nucleocapsid protein (N), all of which have been successful in inducing an immune response unique to the antigen [ 33 ]. Intradermal administration of a LNP-encapsulated mRNA mixture encoding MERS-CoV S proteins into mice has been shown to result in vivo translation and humoral immune response induction [ 12 ].
BNT162b1 (BioNTech, Fosun Pharma, Pfizer)
BNT162b1 is a codon-optimized mRNA vaccine that codes for the essential target of the neutralizing antibody virus, trimerized SARS-CoV-2 RBD [ 29 ]. The vaccine shows improved immunogenicity due to the addition of the foldon trimerization domain of T4 fibrin-derived to the RBD antigen. In 80 nm ionizable cationic LNPs, the mRNA is encapsulated, which guarantees its efficient delivery [ 31 ]. In phase 1/2 clinical trials, elevated levels of RBD-specific immunoglobulin G (IgG) antibodies with a geometric mean concentration were found to be 8 to 46.3 times the convalescent serum titer. Whereas, the SARS-CoV-2 neutralizing antibody geometric mean titers were found to be 1.8 to 2.8 times the convalescent serum panel [ 29 ]. With no adverse effects, mild and transient local reactions and systemic events were observed. The data review did not, however, assess the protection and immune response beyond 2 weeks after the second dose administration [ 31 ].
Report of available effectiveness, tolerability, and immunogenicity results from an ongoing placebo-controlled, observer-blinded dose-escalation study in healthy adults 18–55 years of age, randomized to receive two 21-day separate doses of 10 µg, 30 µg, or 100 µg of BNT162b1, a nucleoside-modified LNP mRNA vaccine encoding trimerized SARS-CoV-2 spike glycoprotein dose-dependent, usually mild to moderate, and temporary, was the local reactions and systemic events [ 29 ]. The BNT162b1 vaccine candidate now being clinically studied integrates such nucleoside modified RNA and encodes the SARS-CoV-2 spike protein RBD, a primary target of virus-neutralizing antibodies [ 31 ]. Sera's RBD-binding IgG and SARS-CoV-2 neutralizing titers increased both at the dose level and after the second dose. Geometric mean neutralizing titers were 1.8 to 2.8 times those of a panel of human sera convalescent COVID-19. These findings help further evaluation of this candidate for the mRNA vaccine [ 33 ]. By adding a T4 fibritin-derived “foldon” trimerization domain, the RBD antigen expressed by BNT162b1 is modified to improve its immunogenicity by a multivalent display. The RNA vaccine is formulated in LNPs for more effective delivery to cells after intramuscular injection [ 31 ]. In Table 1 , potential RNA-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ].
| No. | Title | Description | Vaccine candidate | Phase trial | Sponsor and collaboration | Reference |
|---|---|---|---|---|---|---|
| 1 | Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19) | This clinical trial is designed to assess the safety, reactogenicity, and immunogenicity of mRNA-1273. It encodes for a full-length, prefusion stabilized spike (S) protein of SARS-CoV-2. | LNP-encapsulated mRNA | Phase 1 | NIAID | NCT04283461 [ ] |
| 2 | Dose-confirmation study to evaluate the safety, reactogenicity, and immunogenicity of mRNA-1273 COVID-19 vaccine in adults aged 18 years and older | This clinical study will assess the safety, reactogenicity, and immunogenicity of 2 dose levels of mRNA-1273 SARS-CoV-2 vaccine. | LNP-encapsulated mRNA | Phase 2 | Sponsor: Moderna TX Inc. | NCT04405076 [ ] |
| Collaborator: Biomedical Advanced Research and Development Authority | ||||||
| 3 | A study to evaluate efficacy, safety, and immunogenicity of mRNA-1273 vaccine in adults aged 18 years and older to prevent COVID-19 | The study was designed to primarily evaluate the efficacy, safety, and immunogenicity of mRNA-1273 to prevent COVID-19 for up to 2 years after the second dose of mRNA-1273. | LNP-encapsulated mRNA | Phase 3 | Sponsor: Moderna TX Inc. | NCT04470427 [ ] |
| Collaborator: Biomedical Advanced Research and Development Authority & NIAID | ||||||
| 4 | A phase I clinical trial of novel coronavirus pneumonia (COVID-19) mRNA vaccine (BNT162b1) in China | To evaluate the safety and tolerability profiles of BNT162b1 P/B immunization given 21 days apart on healthy Chinese subjects through 28 days after boost vaccination. | 3 LNP-mRNAs | Phase 1 | Jiangsu Provincial Center for Disease Prevention and Control | ChiCTR2000034825 [ ] |
| 5 | A trial investigating the safety and effects of one BNT162 vaccine against COVID-19 in healthy adults | The vaccine BNT162b3 will be administered using a P/B regimen. This trial has been divided into two parts for dose-escalation cohorts in older subjects. | 3 LNP-mRNAs | Phase 1/2 | BioNTech RNA Pharmaceuticals GmbH | NCT04537949 [ ] |
| 6 | Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals | This study is a phase 1/2/3, randomized, placebo-controlled, observer-blind, dose-finding, vaccine candidate-selection, and efficacy study in healthy individuals. | 3 LNP-mRNAs | Phase 3 | Sponsor: BioNTech SE | NCT04368728 [ ] |
| Collaborator: Pfizer | ||||||
| 7 | A study to evaluate the safety, reactogenicity, and immunogenicity of vaccine CVnCoV in healthy adults | This study aims to evaluate the safety and reactogenicity profile after 1 and 2 dose administrations of CVnCoV at different dose levels. | mRNA | Phase 1 | Sponsor: CureVac AG | NCT04449276 [ ] |
| Collaborator: Coalition for Epidemic Preparedness Innovations (CEPI) | ||||||
| 8 | Ascending dose study of investigational SARS-CoV-2 vaccine ARCT-021 in healthy adult | To determine safety and tolerability and immunogenicity of investigational vaccine ARCT-021 in healthy adult volunteers. | mRNA | Phase 1/2 | Arcturus Therapeutics Inc. | NCT04480957 [ ] |
| 9 | A clinical trial to assess the safety of a coronavirus vaccine in healthy men and women | The main aim of the study is to assess the safety of the vaccine and its effects on the immune system. | LNP-nCoVsaRNA | Phase 1 | Imperial College London | ISRCTN17072692 [ ] |
| 10 | A phase I clinical trial to evaluate the safety, tolerance, and preliminary immunogenicity of different doses of a SARS-CoV-2 mRNA vaccine in population aged 18–59 years and 60 years and above | To explore the immune persistence of the investigational vaccine at the recommended dose and the specific cellular immune response to the RBD of S protein. | mRNA | Phase 1 | People's Liberation Army (PLA) Academy of Military Sciences, Walvax Biotech. | ChiCTR2000034112 [ ] |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; LNP, lipid nanoparticle; NIAID, National Institute of Allergy and Infectious Diseases; P/B, prime/boost; RBD, receptor-binding domain.
Viral Vector-Based Vaccines
Viral vector-based vaccines have a high degree of protein expression and long-term stability, inducing strong immune responses [ 46 ]. These include vaccines focused on chemically weakened viruses used to bear antigens or pathogens of concern for immune response induction [ 47 ]. A possible prophylactic strategy against a pathogen is a viral vector-based vaccine. These vaccines are highly selective in transmitting genes to the target cells, are highly effective in gene transduction, and are useful in inducing immune responses [ 48 ]. They have a long-term and high level of antigenic protein expression and thus have an excellent potential for prophylactic use as these vaccines activate and facilitate cytotoxic T cells, eventually contributing to the elimination of infected virus cells [ 46 ]. The generation of immunity to the vector is an essential consideration for the development of virus vectored vaccines, which could impede the antigen-specific response to boost vaccination [ 49 ]. Reports from preclinical and clinical trials suggested that adequate safety can be obtained from a single dose [ 50 ].

Ad5-nCoV (CanSino Biologics Inc., Beijing Institute of Biotechnology)
A four-fold increase in RBD and S protein-specific neutralizing antibodies was observed within 14 days [ 51 ]. Ad5-nCoV is a recombinant type-5 adenovirus (Ad5) replication-defective vector expressing the recombinant SARS-CoV-2 spike protein. It was prepared by cloning, together with the plasminogen activator signal peptide gene, an optimized full-length gene of the S protein in the Ad5 vector devoid of genes E1 and E3 [ 29 ]. The vaccine was constructed from the Microbix Biosystem using the Admax system. A positive antibody reaction or seroconversion of immunization was identified in phase I clinical trials and peaked at day 28, post-vaccination. Also, the response of CD4+T cells and CD8+T cells peaked at day 14 post-vaccination. However, the pre-existing anti-Ad5 immunity has partially restricted the reaction of both the antibody and the T cell [ 51 ]. The study would further assess the antibody response in recipients between 18 and 60 years of age who received one of three doses in the study, with follow-up at 3- and 6-months post-vaccination [ 29 ].
Coroflu (University of Wisconsin-Madison, FluGen, Bharat Biotech)
M2SR, a self-limiting variant of the influenza virus that is modified by spike protein sequence insertion of the SARS-CoV-2 gene. Besides, the vaccine expresses the influenza virus' hemagglutinin protein, thereby triggering an immune response to both viruses [ 52 ]. The M2SR is self-limiting and, since it lacks the M2 gene, does not undergo replication. It is capable of entering the cell, thereby causing immunity to the virus [ 32 ]. It is delivered intra-nasally, mimicking the normal viral infection pathway. Compared to intramuscular injections, this route stimulates many immune system modes and has higher immunogenicity [ 52 ].
LV-SMENP-DC (Shenzhen Geno-Immune Medical Institute)
By using SMENP minigenes to engineer dendritic cells (DC) with a lentiviral vector expressing the conserved domains of the structural proteins SARS-CoV-2 and protease [ 29 ], the LV-SMENP-DC vaccine is prepared. Subcutaneous vaccine inoculation introduces antigen-presenting cell antigens, which eventually cause cytotoxic T cells and produce an immune response [ 48 ].
ChAdOx1 (University of Oxford)
The recombinant adenovirus vaccine ChAdOx1 was developed using codon-optimized S glycoprotein and synthesized at the 5 ends with the leading tissue plasminogen activator (tPA) sequence [ 50 ]. The SARS-CoV-2 amino acid coding sequence (2 to 1273) and the tPA leader have been propagated in the shuttle plasmid. This shuttle plasmid is responsible for the coding between the Gateway recombination cloning site of the main immediate-early genes of the human cytomegalovirus (IE CMV) along with tetracycline operator sites and polyadenylation signal from bovine growth hormone (BGH) [ 29 ]. In the bacterial artificial chromosome, the adenovirus vector genome is built by inserting the SARS-CoV-2 S gene into the ChAdOx1 adenovirus genome's E1 locus. In the T-Rex human embryonic kidney 293 (HEK-293) cell lines, the virus was then allowed to replicate and purified by ultracentrifugation of the CsCl gradient [ 53 ]. The absence of any subgenomic RNA from preclinical trials in intra-muscularly vaccinated animals is suggestive of improved immunity to the virus [ 50 ]. Previous studies have proposed that the immune response should be marshalled by a single shot [ 53 ]. In Table 2 , potential viral vector-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 45 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 ].
| No. | Title | Description | Vaccine candidate | Phase trial | Sponsor and collaboration | Reference |
|---|---|---|---|---|---|---|
| 1 | Clinical trial to evaluate the safety and immunogenicity of the COVID-19 vaccine (COVID-19-101) | This is a randomized, placebo-controlled, two-center, trial in healthy adult volunteers to investigate the safety, tolerability, and immunogenicity of a novel measles-vector-based vaccine candidate against SARS-CoV-2 infection (TMV-083). | Measles-vector based | Phase 1 | Sponsor: Institute Pasteur | NCT04497298 [ ] |
| Collaborator: Themis Bioscience GmbH, Coalition for Epidemic Preparedness Innovations | ||||||
| 2 | A phase I clinical trial of influenza virus vector COVID-19 vaccine for intranasal spray (DelNS1-2019-nCoV-RBD-OPT1) | The effect of pre-existing antibodies against influenza A (H1N1) virus on the immunogenicity of Influenza virus vector COVID-19 vaccine for intranasal spray (DelNS1-2019-nCoVRBD-OPT1) in a healthy population for safety. | Intranasal flu-based-RBD | Phase 1 | Sponsor: Beijing Wantai Biological Pharmacy | ChiCTR2000037782 [ ] |
| Collaborator: Xiamen University | ||||||
| 3 | A phase I/II study to determine efficacy, safety, and immunogenicity of the candidate coronavirus disease (COVID-19) vaccine ChAdOx1 nCoV-19 in UK healthy adult volunteers | To assess the efficacy of ChAdOx1 nCoV-19 against COVID-19. To assess the safety of the candidate vaccine ChAdOx1 nCoV. | ChAdOx1-S | Phase 1/2 | Sponsor: University of Oxford | 2020-001072-15 [ ] |
| Collaborator: AstraZeneca | ||||||
| 4 | A phase III study to investigate a vaccine against COVID-19 | This study aims to assess whether healthy people in Brazil can be protected from COVID-19 with a new vaccine called ChAdOx1 nCoV-19. | ChAdOx1-S | Phase 3 | Sponsor: University of Oxford | ISRCTN89951424 [ ] |
| Collaborator: AstraZeneca | ||||||
| 5 | Study of AZD1222 for the prevention of COVID-19 in Japan | A safe and effective vaccine for COVID-19 prevention would have a significant global public health impact because currently, there are no licensed preventions available against COVID-19. | AZD1222 | Phase 1/2 | Sponsor: AstraZeneca | NCT04568031 [ ] |
| Collaborator: Iqvia Pty. Ltd. | ||||||
| 6 | Phase III double-blind, placebo-controlled study of AZD1222 for the prevention of COVID-19 in adults | The study aims to assess the safety, efficacy, and immunogenicity of AZD1222 for the prevention of COVID-19. | AZD1222 | Phase 3 | Sponsor: AstraZeneca | NCT04516746 [ ] |
| Collaborator: Iqvia Pty. Ltd. | ||||||
| 7 | Replication defective simian adenovirus (GRAd) encoding S | RT-CoV-2 is an open-label, dose-escalation multicenter clinical trial to assess the safety and immunogenicity of the candidate COVID-19 vaccine GRAd-CoV-2 in healthy Italian volunteers aged 18–55 years and 65–85 years inclusive. | Replication defective simian adenovirus (GRAd) encoding S | Phase 1 | Sponsor: ReiThera Srl | NCT04528641 [ ] |
| Collaborator: Istituto Nazionale per le Malattie Infettive Lazzaro Spallanzani | ||||||
| 8 | A clinical trial of a recombinant adenovirus 5 vectored COVID-19 vaccine (Ad5-nCoV) with two doses in healthy adults | This is a clinical trial to evaluate the safety and immunogenicity of a recombinant Ad5-nCoV with two doses and with different administration routes in healthy adults aged 18 years and older. | Ad5-nCoV | Phase 1 | Sponsor: Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China | NCT04552366 [ ] |
| Collaborator: Zhongnan Hospital | ||||||
| 9 | Safety and immunogenicity trial of an oral SARS-CoV-2 vaccine (VXA-CoV2-1) for prevention of COVID-19 in healthy adults | VXA-CoV2-1 is a non-replicating Ad5 vector adjuvanted oral tableted vaccine being developed to prevent COVID-19. | Ad5 adjuvanted oral vaccine platform | Phase 1 | Vaxart | NCT04563702 [ ] |
| 10 | Safety, tolerability, and immunogenicity of the candidate vaccine MVA-SARS-2-S against COVID-19 | In this clinical trial, healthy volunteers in two different dose cohorts will be vaccinated twice with the candidate vaccine MVA-SARS-2-S. | MVA-SARS-2-S | Phase 1 | Sponsor: Universitätsklinikum Hamburg-Eppendorf | NCT04569383 [ ] |
| Collaborator: German Center for Infection Research, Philipps University Marburg Medical Center, Ludwig-Maximilians–University of Munich |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RBD, receptor-binding domain; MVA, modified vaccinia Ankara.
Protein Subunit-Based Vaccines
Subunit vaccines, safer and more straightforward to manufacture, present a host with high immunogenicity with one or few antigens, but need adjuvants to evoke a strong defensive immune response [ 62 ]. A subunit vaccine is a synthetic peptide or recombinant antigenic protein-dependent vaccine which is essential for long-term protection and a therapeutic invigoration of the immune response [ 63 ]. The subunit vaccine exhibits low immunogenicity and requires an adjuvant's additional assistance to potentiate the vaccine-induced immune responses. An adjuvant may improve the biological half-life of the antigenic material, or the immunomodulatory cytokine response may be improved. The use of an adjuvant, therefore, helps to overcome the shortcomings of the protein subunit vaccines [ 64 ]. Subunit vaccines may be designed to concentrate the immune response on the neutralization of epitopes, thus preventing the development of non-neutralizing antibodies that may encourage disease-related antibody-dependent enhancement [ 65 ]. Antigenic proteins thought to cause a defensive immune response are used in protein subunit vaccines. The S protein of SARS-CoV-2 is the most appropriate antigen to induce neutralizing antibodies against the pathogen [ 13 ]. The S protein is comprised of two subunits. In the S1 subunit, the N-terminal domain, RBD, and receptor-binding motif (RBM) domains are found, while the S2 subunit consists of FP, HR 1, and 2 [ 62 ]. The virus reaches the cell by endocytosis using S-protein mediated binding to the human angiotensin-converting enzyme 2 (hACE2) receptor. Therefore, S-protein and its antigenic fragments are key objectives for the establishment of a subunit vaccine [ 63 ]. A complex protein with two conformation states, i.e., a pre-fusion and post-fusion state, is the S glycoprotein [ 62 ]. Therefore, the antigen must maintain its surface chemistry and the profile of the initial pre-fusion spike protein to retain the epitopes for igniting good quality antibody responses. Also, targeting the masked RBM as an antigen, it will increase the neutralizing antibody response and enhance the overall efficacy of the vaccine [ 66 ].
NVX-CoV2373 (Novavax Inc., Emergent BioSolutions)
NVX-CoV2373 is a nano-particle-mediated immunogenic vaccine-mediated on coronavirus S-protein, the recombinant expression of stable pre-fusion [ 67 ]. In the baculovirus system, the protein has been stably expressed. By inducing high levels of neutralizing antibodies, the company aims to use the matrix-M adjuvant to strengthen the immune response against the SARS-CoV-2 spike protein [ 35 ]. A single immunization in animal models resulted in a high level of anti-spike protein antibodies that blocked the binding domain of the hACE2 receptor and could elicit SARS-CoV-2 wild-type virus-neutralizing antibodies [ 68 ].
Molecular clamp stabilized spike protein vaccine candidate
It is being developed in partnership with GSK and Dynavax by the University of Queensland [ 29 ]. The University will have access to the vaccine adjuvant (AS03 Adjuvant) platform technology, which is believed to enhance the response of the vaccine and reduce the amount of vaccine needed per dose [ 69 ]. The University is developing a stabilized pre-fusion, recombinant viral protein subunit vaccine based on the molecular clamp technology. It has been established that this technology induces the development of neutralizing antibodies [ 34 ].
PittCoVacc (University of Pittsburgh)
It is a recombinant SARS-CoV-2 vaccine based on the micro-needle array (MNA) that involves administering rSARS-CoV-2 S1 and rSARS-CoV-2-S1fRS09 (recombinant immunogens) [ 70 ]. A significant increase in statistically significant antigen-specific antibodies was found in the mice models in preclinical studies at the end of 2 weeks [ 29 ]. Furthermore, even after sterilization using gamma rays, the immunogenicity of the vaccine was maintained. Statistically, relevant antibody titers confirm the feasibility of the MNA-SARS-CoV-2 vaccine at the early stage and even before boosting [ 70 ].
Triple antigen vaccine (Premas Biotech, India)
It is a multi-antigenic VLP vaccine prototype in which an engineered Saccharomyces cerevisiae expression platform (D-CryptTM) co-expresses the recombinant spike, membrane, and envelope protein of SARS-CoV-2 [ 71 ]. The proteins then, like the VLP, undergo self-assembly. The biophysical characterization of the VLP was simultaneously given by the transmission electron microscopy and allied analytical data [ 29 ]. After more research and development, this prototype has the potential to engage in preclinical trials as a vaccine candidate. Besides, cost-effectively, it is assumed to be safe and easy to produce on a mass scale [ 71 ]. In Table 3 , potential protein subunit-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 45 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 ].
| No. | Title | Description | Vaccine candidate | Phase trial | Sponsor and collaboration | Reference |
|---|---|---|---|---|---|---|
| 1 | Evaluation of the safety and immunogenicity of a SARS-CoV-2 rS nanoparticle vaccine with/without matrix-M adjuvant | The study is designed to evaluate the safety and immunogenicity in 131 healthy participants ≥18 to 59 (inclusive) years of age at two sites in Australia. | Full-length recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvanted with matrix M | Phase 1/2 | Sponsor: Novavax | NCT04368988 [ ] |
| Collaborator: Coalition for Epidemic Preparedness Innovations | ||||||
| 2 | A study looking at the effectiveness and safety of a COVID-19 vaccine in South African adults | This is a study to evaluate the effectiveness and safety of healthy HIV-negative (HIV−) adult participants and in medically stable HIV-positive (HIV+) adult participants in up to 10 sites across South Africa. | Full-length recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvanted with matrix M | Phase 2 | Novavax | NCT04533399 [ ] |
| 3 | Phase I clinical study of recombinant novel coronavirus vaccine | In this trial, a total of 50 subjects were recruited; the test vaccines were divided into three groups, low-dose, high-dose vaccine groups, and placebo groups. | Adjuvanted recombinant protein (RBD-Dimer) | Phase 1 | Sponsor: Anhui Zhifei Longcom Biologic Pharmacy Co. Ltd. | NCT04445194 [ ] |
| Collaborator: Beijing Chao Yang Hospital | ||||||
| 4 | Recombinant new coronavirus vaccine (CHO cells) to prevent SARS-CoV-2 phase i clinical trial (≥60 years old) | To evaluate the safety and tolerability of recombinant new coronavirus vaccine (CHO cells) to explore the immunogenicity and durability of different doses. | Adjuvanted recombinant protein (RBD-Dimer) | Phase 1/2 | Anhui Zhifei Longcom Biologic Pharmacy Co. Ltd. | NCT04550351 [ ] |
| 5 | KBP-201 COVID-19 vaccine trial in healthy volunteers | This is a FIH, observer-blinded, randomized, placebo-controlled, parallel-group study to evaluate the safety and immunogenicity of the KBP-COVID-19 vaccine. | RNA-based protein subunit | Phase 1/2 | Kentucky Bioprocessing Inc. | NCT04473690 [ ] |
| 6 | Study of recombinant protein vaccine formulations against COVID-19 in healthy adults 18 years of age and older | The objective of the study is to describe the neutralizing antibody profile and safety profile of all participants in each group up to 12 months post-last injection. | S protein (baculovirus production) | Phase 1/2 | Sponsor: Sanofi Pasteur, a Sanofi Company | NCT04537208 [ ] |
| Collaborator: GlaxoSmithKline | ||||||
| 7 | A study to evaluate the safety, tolerability, and immunogenicity of UB-612 COVID-19 vaccine | This is an open-label, dose-escalation clinical study of 3 ascending doses of UB-612 COVID-19 vaccine in healthy adults, aged from 20 to 55 years old. | S1-RBD-protein | Phase 1 | Sponsor: United Biomedical Inc., Asia | NCT04545749 [ ] |
| Collaborator: COVAXX | ||||||
| 8 | SCB-2019 as COVID-19 vaccine | This is a randomized, double-blind, placebo-controlled, FIH study to assess safety, reactogenicity, and immunogenicity of SCB-2019 at multiple dose levels. | Native like trimeric subunit spike protein vaccine | Phase 1 | Clover Biopharmaceuticals AUS Pty. Ltd. | NCT04405908 [ ] |
| 9 | Monovalent recombinant COVID19 vaccine (COVAX19) | This is a study to test a new vaccine (Covax-19) against COVID-19 | Recombinant spike protein with Advax adjuvant | Phase 1 | Sponsor: Vaxine Pty. Ltd. | NCT04453852 [ ] |
| Collaborator: Central Adelaide Local Health Network Incorporated | ||||||
| 10 | An interventional study to evaluate the safety and immune response of a vaccine against SARS-CoV-2, when given to healthy adult participants | To assess the safety and tolerability of SARS-CoV-2 Sclamp vaccine compared to placebo by evaluating solicited local adverse events will be evaluated by severity score, frequency, duration, and intensity by FDA toxicity scoring. | Molecular clamp stabilized spike protein with MF59 adjuvant | Phase 1 | University of Queensland, CSL, Seqirus | ACTRN12620000674932 [ ] |
| 11 | A study to evaluate the safety and immunogenicity of MVC-COV1901 against COVID-19 | This is a prospective, open-labelled, single-center study to evaluate the safety and immunogenicity of MVC-COV1901. | S-2P protein+CpG 1018 | Phase 1 | Medigen Vaccine Biologics Corp. | NCT04487210 [ ] |
| 12 | Study of the safety, reactogenicity, and immunogenicity of “EpiVacCorona” vaccine for the prevention of COVID-19 (EpiVacCorona) | The research tasks are to evaluate the safety, reactogenicity of the EpiVacCorona vaccine when administered twice intramuscularly and to identify the development of adverse. | Peptide | Phase 1 | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology “Vector” | NCT04527575 [ ] |
| 13 | A randomized, double-blind, placebo-controlled phase I trial for anti-novel coronavirus pneumonia (COVID-19) recombinant vaccine (Sf9) | The aim is to evaluate the safety, tolerability, and immunogenicity of a recombinant SARS-CoV-2 vaccine (Sf9 cell) in a healthy Chinese population aged 18 years and older. | RBD (baculovirus production expressed in Sf9 cells) | Phase 1 | West China Hospital, Sichuan University | ChiCTR2000037518[ ] |
| 14 | Safety and immunogenicity trial of multi-peptide vaccination to prevent COVID-19 infection in adults (pVAC) | To evaluate the safety and immunogenicity of a single use of a SARS-CoV-2-derived multi-peptide vaccine in combination with the toll-like receptor 1/2 ligand XS15 in adults. | SARS-CoV-2 HLA-DR peptides | Phase 1 | University Hospital Tuebingen | NCT04546841 [ ] |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; HIV, human immunodeficiency virus; RBD, receptor-binding domain; FIH, first-in-human; FDA, U.S. Food and Drug Administration.
DNA-Based Vaccines
A typical DNA vaccine is a plasmid DNA molecule that codes for the host immune system to be presented with one or more antigens [ 62 ]. They have the advantages of stability and successful delivery over mRNA vaccines [ 84 ]. Still, since they are needed to reach the nucleus, they have the risk of vector mutations and incorporation into the host genome [ 85 ]. DNA vaccines reflect a revolutionary approach, followed by a wide variety of immune responses, by the direct injection of plasmids encoding antigens [ 86 ]. The most groundbreaking approach to vaccination is the introduction of the DNA vaccine that codes for the antigen and an adjuvant that stimulates the adaptive immune response [ 87 ]. The transfected cells express the transgene, which gives a steady supply of transgene-specific proteins very similar to the live virus [ 84 ]. Also, immature DCs, which eventually present the antigen on the cell surface to the CD4 + and CD8 + T cells in combination with the major histocompatibility complex (MHC) 2 and MHC 1 antigens, endocytose the antigen material, thereby stimulating both successful humoral and cell-mediated immune systems [ 87 ]. DNA vaccines are considered safe and stable and can be developed easily, but their immunogenicity and immune response efficiency in humans have not yet been demonstrated [ 21 ].
INO-4800 (Inovio Pharmaceuticals)
It is an anti-SARS-CoV-2 prophylactic DNA vaccine. It uses the SARS-CoV-2 codon-optimized S protein sequence to which an immunoglobulin E (IgE) leader sequence is attached [ 29 ]. Using BamHI and XhoI, the SARS-CoV-2 IgE-spike sequence was synthesized and digested. Under the management of IE CMV, and BGH polyadenylation signal, the digested DNA was incorporated into the expression plasmid pGX0001 [ 85 ]. In preclinical studies, the existence of functional antibodies and the response of T cells indicate that the vaccine will produce an efficient immune response within seven days after vaccination [ 88 ]. The vaccine has entered phase I clinical trials (phase I: NCT04336410) and it is anticipated that this phase of clinical trials will be completed by July, with participants receiving 1.0 mg of INO-4800 by electroporation with CELLECTRA 2000 per dosing visit. The research will assess the immunological profile, efficacy, and tolerability of the candidate vaccine in healthy human adults upon intradermal injection and electroporation [ 29 ]. INO-4800 and the previous Inovio vaccine INO-4700 express either SARS-CoV-2-S or MERS-CoV-S inside the same DNA vector, respectively [ 85 ]. The vaccine is delivered by intramuscular injection, accompanied by injection site electroporation. The need for electroporation could restrict INO-4800's ability to be expanded to the scales necessary for a global pandemic and may be difficult to handle globally [ 13 ].
bacTRL (Symvivo Corporation)
Symvivo Corporation's bacTRL platform uses the engineered probiotic Bifidobacterium longum to deliver a SARS-CoV-2-S expressing DNA vaccine into intestinal cells. The first-in-man study of the bacTRL platform will also be a phase I study of the COVID-19 vaccine, so no prior immunological results are available [ 13 ]. In Table 4 , DNA-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 89 , 90 , 91 , 92 , 93 , 94 ].
| No. | Title | Description | Vaccine candidate | Phase trial | Sponsor and collaboration | Reference |
|---|---|---|---|---|---|---|
| 1 | Safety, tolerability, and immunogenicity of INO-4800 followed by electroporation in healthy volunteers for COVID-19 | INO-4800 was administered by intradermal injection followed by electroporation using the CELLECTRA 2000 device in healthy adults aged 19 to 64 years of Korea. | DNA plasmid vaccine with electroporation | Phase 1/2 | Sponsor: International Vaccine Institute | NCT04447781 [ ] |
| Collaborator: Inovio Pharmaceuticals | ||||||
| 2 | Safety, tolerability, and immunogenicity of INO-4800 for COVID-19 in healthy volunteers | This is an open-label trial of INO-4800 which contains the plasmid pGX9501, which encodes for the full length of the Spike glycoprotein of SARS-CoV-2. | DNA plasmid vaccine with electroporation | Phase 1 | Sponsor: Inovio Pharmaceuticals | NCT04336410 [ ] |
| Collaborator: Coalition for Epidemic Preparedness Innovations | ||||||
| 3 | Study of COVID-19 DNA vaccine (AG0301-COVID19) | This is a single-center, non-randomized, open-label, non-controlled trial. 30 healthy volunteers aged 20–65, will be enrolled for low and high dose group. | DNA plasmid vaccine+adjuvant | Phase 1/2 | Sponsor: AnGes Inc. | NCT04463472 [ ] |
| Collaborator: Japan Agency for Medical Research and Development | ||||||
| 4 | Study of COVID-19 DNA vaccine (AG0302-COVID19) | This study will assess the safety and immunogenicity of AG0302-COVID19 in healthy adult volunteers. | DNA plasmid vaccine+adjuvant 2 | Phase 1/2 | AnGes Inc. | NCT04527081 [ ] |
| 5 | Novel corona virus-2019-nCov vaccine by intradermal route in healthy subjects | A prospective, randomized, adaptive clinical study to evaluate the safety and immunogenicity of novel corona virus-2019-nCov vaccine candidate. | DNA plasmid vaccine | Phase 1/2 | Cadila Healthcare Limited | CTRI/2020/07/026352 [ ] |
| 6 | Safety and immunogenicity study of GX-19, a COVID-19 preventive DNA vaccine in healthy adults | This clinical study is to evaluate the safety, tolerability, and immunogenicity of the COVID-19 preventive vaccine by intramuscular administration in healthy volunteers. | DNA Vaccine (GX-19) | Phase 1/2 | Genexine Inc. | NCT04445389 [ ] |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Virus-Like Particles Vaccine
VLPs are particles that form spontaneously, consisting of many co-expressed or mixed structural viral proteins. Several commercial vaccines are based on VLPs, such as hepatitis B and human papillomavirus vaccines [ 95 ]. Without the need for adjuvants, these vaccines can be constructed and used. Only when antigens with neutralizing epitopes are extensively investigated is the production of such vaccines possible [ 22 ]. A VLP is a self-assembled nanostructure incorporating essential viral structural proteins. VLP is similar to true viruses' molecular and morphological features but is non-infectious and non-replicating due to the absence of genetic materials [ 26 ]. Successful applications of VLP have been proved by vaccinological and virological study [ 95 ]. In the ongoing battle against the COVID-19 pandemic, the development of SARS-CoV-2 VLPs is highly in demand as an accessibly safe and relevant substitute for naturally pathogenic viruses [ 26 ]. A study suggested the possible use of plant biotechnology for the development of low-cost COVID-19 vaccines and plant-made antibodies for diagnosis, prophylaxis, and therapy [ 22 ].
In the current research, we have established SARS-CoV-2 VLPs effectively, using the mammalian expression system [ 47 ], which helps maintain specific patterns of protein glycosylation [ 22 ]. For the efficient formation and release of SARS-CoV2 VLPs among the four SARS-CoV-2 structural proteins, we have shown that membrane protein (M) expression and small envelope protein (E) are essential [ 47 ]. Also, the corona-like structure presented in SARS-CoV-2 VLPs from Vero E6 cells is more stable and unified in comparison with those from HEK-293 T cells. Our data show that the molecular and morphological characteristics of native virion particles in SARS-CoV-2 VLPs make SARS-CoV-2 VLPs a promising candidate vaccine and a powerful tool for research into SARS-CoV-2 [ 96 ]. The immunogenic composition composed of MERS-CoV nanoparticle VLPs containing at least one trimer of S protein formed by baculovirus overexpression in Sf9 cells was disclosed in patent application WO2015042373 by Novavax in 2015 [ 35 ]. When administered along with their patented adjuvant Matrix M, this VLP preparation induced a neutralizing antibody response in mice and transgenic cattle. Sera preparations from vaccinated cattle (SAB-300 or SAB-301) were also injected into Ad5-hDPP4 transduced BALB/c mice before the MERS-CoV challenge [ 22 ]. With a single prophylactic injection, both SAB-300 and SAB-301 were able to protect these mice from MERS-CoV infection [ 96 ]. On 26 February, Novavax announced that due to their prior experience dealing with other coronaviruses, including both MERS and SARS, animal testing of possible COVID-19 vaccine candidates had begun. Using their recombinant nanoparticle vaccine technology along with their proprietary adjuvant matrix-M, their COVID-19 candidate vaccines targeting the S protein of SARS-CoV-2 were created [ 35 ].
UMass Medical School researchers have developed a framework to create vaccines using VLPs, which one scientist claims may be a successful and safer alternative to a COVID-19 vaccine. Trudy Morrison, Ph.D., professor of Microbiology & Physiological Systems, said her work on a VLP-based respiratory syncytial virus vaccine that can be modified to COVID-19 causes severe lower respiratory tract disease in young children and the elderly. And some of the problems inherent in the production of vaccines from inactivated or live viruses will be avoided [ 97 ].
Medicago, a biopharmaceutical company, headquartered in Quebec City, announced the successful development of a coronavirus VLP only 20 days after the SARS-CoV-2 (COVID-19 disease virus) gene was obtained [ 29 ]. The manufacturing of VLP is the first step in the development of the COVID-19 vaccine, which will now undergo preclinical protection and efficacy testing. They plan to negotiate clinical testing of the vaccine with the relevant health authorities by summer (July/August) 2020 once this is done. Medicago uses its technology platform to create antibodies against SARS-CoV-2. These antibodies to SARS-CoV-2 might theoretically be used to treat people who are infected by the virus. In part, this study is sponsored by the Canadian Institutes for Health Research [ 98 ]. In Table 5 , potential VLPs-based vaccine candidates are listed below for COVID-19 which are in the clinical development phase and registered globally [ 81 , 99 ].
| No. | Title | Description | Vaccine candidate | Phase trial | Sponsor and collaboration | Reference |
|---|---|---|---|---|---|---|
| 1 | A phase 1/2 randomized, placebo-controlled, multicentre study to evaluate the safety and immunogenicity of COVID-19 vaccine in healthy adults | RBD SARS-CoV-2 HBsAg VLP vaccine, administered at two dose amounts 5 mcg and 25 mcg, by intramuscular injection by investigators during an in-clinic visit. | RBD-HBsAg VLPs | Phase 1/2 | Sponsor: SpyBiotech | ACTRN12620000817943 [ ] |
| Collaborator: Serum Institute of India | ||||||
| 2 | Safety, tolerability, and immunogenicity of a coronavirus-like particle COVID-19 vaccine in adults aged 18–55 years | The study will be a randomized, partially-blinded, prime-boost, staggered dose-escalation study at three dose levels (3.75 µg, 7.5 µg, and 15 µg VLP). | Plant-derived VLP was adjuvanted with GSK or Dynavax adjs. | Phase 1 | Medicago | NCT04450004 [ ] |
VLP, virus-like particle; COVID-19, coronavirus disease 2019; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; HBsAg, Hepatitis B surface antigen.
Current Updates
To bring this pandemic to an end, a large share of the world needs to be immune to the virus. The safest way to achieve this is with a vaccine. Vaccines are a technology that humanity has often relied on in the past to bring down the death toll of infectious diseases. Within less than 12 months after the beginning of the COVID-19 pandemic, several research teams rose to the challenge and developed vaccines that protect from SARS-CoV-2, the virus that causes COVID-19. Now the challenge is to make these vaccines available to people around the world.
To resume a normal lifestyle, free from government lockdowns, and fear of continuing pandemic waves over the coming months, the world is anxiously awaiting a safe, successful vaccine to protect against COVID-19. Innovative ties with both pharmaceutical companies and medical start-ups are joining hands with scientists across the continents to repurpose medications, create vaccines, and devices to hinder the progress of this overwhelming pandemic. A large number of vaccine candidates for COVID-19 based on different platforms have already been identified. Current review shows preclinical as well as in clinical development of vaccine candidates, wherein, five major vaccine platforms for COVID-19 namely RNA, DNA, viral vector, protein subunit, and VLP which constitutes 10, 2, 10, 14, and 2 vaccine candidates globally in clinical development as of 15 October 2020. Among all the vaccine platforms, extensive research and development are going on protein subunit-based vaccine which has the maximum candidates in the clinical development.
A significant amount of hindrance to the rapid production of vaccines is the length of clinical trials. With several phases, including the preclinical stage and clinical development, which is a three-phase process, the vaccine development process is very laborious. However, if adequate data is already available, it has been proposed that a few stages be skipped to accelerate the achievement of a vaccine faster with a rapid regulatory review, approval, development, and quality control. By looking towards pandemic conditions, the scientific fraternity will be ready for any harmful situation to overwhelmed opportunities. Therefore, the current situation has given the world a new perspective to facilitate research in the worst circumstances and hasten the drug development process.
No potential conflict of interest relevant to this article was reported.
Our websites may use cookies to personalize and enhance your experience. By continuing without changing your cookie settings, you agree to this collection. For more information, please see our University Websites Privacy Notice .
UConn Today
September 4, 2024 | Anna Zarra Aldrich, College of Agriculture, Health and Natural Resources
New Study Provides Insight to Why Covid Vaccines Hit Some Harder than Others
From exercise to birth control, researchers found many factors contribute to vaccine side effects

Students receiving the COVID 19 vaccine at Hawley Armory on April 8, 2021. The rollout of the vaccines across the state in the spring helped set up a return to a more familiar university experience. (Sean Flynn/UConn Photo)
When you got the SARS-CoV2 vaccine to protect against COVID-19, you may have experienced severe side effects. Or maybe you didn’t.

This study was published in the Journal of Agriculture and Food Research .
Concerns about potential side effects were a major barrier for some people to getting the vaccine at all, yet little research had been done on what could make someone more vulnerable to experiencing side effects.
One of Andersen’s collaborators on the paper is Christa Palancia Esposito at Fairfield University. Esposito is a nurse practitioner and midwife who noticed in both her practice and in emerging literature that COVID-19 infection was impacting women’s health differently from men’s.
Andersen and Esposito had previously collaborated on a study looking at how women respond to certain dietary interventions based on whether they use hormonal birth control.
“We thought that was interesting because there was a lot of research coming out about sex-specific differences and COVID-19 illness severity but less about responsiveness and side effects to vaccines,” Andersen says.
Given all of this, they decided to see if sex, hormonal birth control use, diet, body mass index (BMI), or exercise impacted someone’s experience of post-vaccine symptoms.
“It just got us thinking more about personalized health and whether certain characteristics could be playing a role here,” Andersen says.
For this pilot study, the researchers surveyed 82 people who received any of the three vaccines available in 2021.
They found that stress, BMI, exercise, and use of hormonal birth control all played a role.
The researchers found a significant correlation between stress and one’s perception of the intensity of the side effects from the vaccine.
“Whether stress influences psychological perception of side effects, or whether stress responses lead to biological changes that result in side effects and impact efficacy of SARS-CoV2 vaccines, as it has been shown to do with other vaccines, is worth studying,” Andersen says.
While there were no sex-dependent differences in the experience of side effects, women did generally report higher levels of stress and less regular exercise than men in the study.
Some of the associations between individual characteristics and perceived vaccine side effects were dose-dependent for the Moderna and Pfizer vaccines, which had two initial doses. For example, people who exercise regularly reported experiencing a lower severity of side effects for their first dose than those who did not exercise regularly. But for the second dose, they experienced a greater severity of side effects.
“Exercise in itself, especially acutely, can be inflammatory and certainly can impact the immune system,” Andersen says, with growing evidence that exercise impacts vaccine efficacy.
Women who were on hormonal birth control also had an increased experience of side effects, especially those using the birth control pill. People with a higher BMI also reported greater severity of side effects.
The researchers also looked at what kinds of supplements people took and what dietary patterns they followed. But, due to the small sample size, they were not able to find any significant associations between these characteristics and perceived vaccine side effects.
Andersen says she will use data from this study to support future work in her lab which focuses on the connections between diet and lifestyle factors, metabolic health, and immune function.
“My lab will be able to immediately take these factors and use the knowledge we gained to better understand individualized responses to lifestyle and dietary interventions that are aimed at achieving specific immune outcomes,” Andersen says.
As more data from SARS-CoV2 vaccinations become available, this study will provide a foundation for more work looking at these and other factors to improve vaccine delivery and reduce side effects through an individualized approach to health.
“The long-term goal is to make vaccines more effective and at the same time minimize side effects or adverse responses that may influence acceptance of potentially lifesaving, preventative health measures.”
This work relates to CAHNR’s Strategic Vision area focused on Enhancing Health and Well-Being Locally, Nationally, and Globally.
Follow UConn CAHNR on social media
Recent Articles

September 5, 2024
The School of Nursing’s Largest First-Year Class
Read the article

Podcast: What Aerosmith Can Teach Us About Vocal Injury

UConn Law Welcomes New Faculty
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Brief Communication
- Open access
- Published: 29 April 2024
Influence of COVID-19 on trust in routine immunization, health information sources and pandemic preparedness in 23 countries in 2023
- Jeffrey V. Lazarus ORCID: orcid.org/0000-0001-9618-2299 1 , 2 , 3 ,
- Trenton M. White 1 , 2 ,
- Katarzyna Wyka 1 ,
- Scott C. Ratzan 1 ,
- Kenneth Rabin 1 ,
- Heidi J. Larson ORCID: orcid.org/0000-0002-8477-7583 4 , 5 ,
- Federico Martinon-Torres ORCID: orcid.org/0000-0002-9023-581X 6 ,
- Ernest Kuchar 7 ,
- Salim S. Abdool Karim ORCID: orcid.org/0000-0002-4986-2133 8 , 9 ,
- Tamara Giles-Vernick 10 ,
- Selina Müller ORCID: orcid.org/0000-0003-0886-3487 11 ,
- Carolina Batista 12 , 13 ,
- Nellie Myburgh 14 ,
- Beate Kampmann 15 &
- Ayman El-Mohandes 1
Nature Medicine volume 30 , pages 1559–1563 ( 2024 ) Cite this article
6472 Accesses
6 Citations
182 Altmetric
Metrics details
- Communication
- Public health
It is unclear how great a challenge pandemic and vaccine fatigue present to public health. We assessed perspectives on coronavirus disease 2019 (COVID-19) and routine immunization as well as trust in pandemic information sources and future pandemic preparedness in a survey of 23,000 adults in 23 countries in October 2023. The participants reported a lower intent to get a COVID-19 booster vaccine in 2023 (71.6%), compared with 2022 (87.9%). A total of 60.8% expressed being more willing to get vaccinated for diseases other than COVID-19 as a result of their experience during the pandemic, while 23.1% reported being less willing. Trust in 11 selected sources of vaccine information each averaged less than 7 on a 10-point scale with one’s own doctor or nurse and the World Health Organization, averaging a 6.9 and 6.5, respectively. Our findings emphasize that vaccine hesitancy and trust challenges remain for public health practitioners, underscoring the need for targeted, culturally sensitive health communication strategies.
Similar content being viewed by others
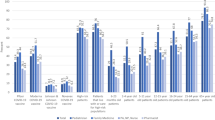
Changes in vaccine attitudes and recommendations among US Healthcare Personnel during the COVID-19 pandemic
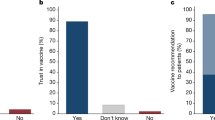
Communicating doctors’ consensus persistently increases COVID-19 vaccinations

Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021
The emergence of the severe acute respiratory syndrome coronavirus 2 virus in late 2019 precipitated a global health emergency that contributed to more than 7 million reported deaths globally as of 19 January 2024 (ref. 1 ) and an estimated 18.2 million excess deaths between 1 January 2020 and 31 December 2021 (ref. 2 ). The coronavirus disease 2019 (COVID-19) pandemic, requiring urgent international intervention, led to an accelerated pace of research and development of multiple safe, effective COVID-19 vaccines, which were first authorized for emergency use in December 2020 3 . The expeditious vaccine development and limited availability resulted in serious challenges in the equitable global distribution of vaccines, coupled with vaccine-related misinformation and mistrust of the science behind vaccine safety 4 .
Vaccine hesitancy 5 , pandemic fatigue 6 and vaccine fatigue, defined as the ‘inertia or inaction toward vaccine information or instruction due to perceived burden and burnout’ 7 , continue to present challenges to vaccine uptake in 2023. Although COVID-19 has been deprioritized as a substantial public health threat since 2023, the virus strains continue to circulate and, in some settings, lead to new increases in hospitalization and intensive care unit admission 1 . The potential impact of vaccine hesitancy on confidence in booster doses remains substantial 8 . In addition, documented spillover effects on routine immunization pose a threat for the reemergence of some childhood and adult vaccine-preventable diseases 9 , 10 .
In this Brief Communication, the fourth study in a series of annual global surveys across 23 countries (Brazil, Canada, China, Ecuador, France, Germany, Ghana, India, Italy, Kenya, Mexico, Nigeria, Peru, Poland, Russia, Singapore, South Africa, South Korea, Spain, Sweden, Türkiye, the United Kingdom and the United States) 11 , 12 , 13 , we report perspectives of adults in the general public on COVID-19 and routine immunization in late 2023, trust in pandemic information sources and collective preparedness to address any possible future pandemic. We also compare COVID-19 vaccine acceptance in 2023 to that in previous years to promote a better understanding of the current and future challenges public health authorities may face in encouraging vaccine uptake.
The reported uptake of at least one COVID-19 vaccine dose rose to 87.8% in 2023 across the 23 countries (Fig. 1a ), as compared with 36.9% in 2021 ( P < 0.001) and 70.4% in 2022 ( P = 0.002). The reported uptake of at least one COVID-19 vaccine was similar in middle-income countries (MICs; 86.9%) and high-income countries (HICs; 87.5%) ( P = 0.381). COVID-19 vaccine booster acceptance among those vaccinated decreased from 87.9% in 2022 to 71.6% in 2023 ( P < 0.001) (Fig. 1b ). This decrease was most profound in HICs (from 85.1% to 63.3%, P < 0.001), compared with MICs (from 90.5% to 78.9%, P = 0.010). The perspectives on willingness to get vaccinated against diseases other than COVID-19 (for example, influenza, measles and hepatitis B) indicate that 60.8% of respondents may be more and 23.1% less willing to get vaccinated in 2023, following their experience during the COVID-19 pandemic (Fig. 1c ). Individual country analyses on vaccine acceptance are available in Extended Data Fig. 1 .
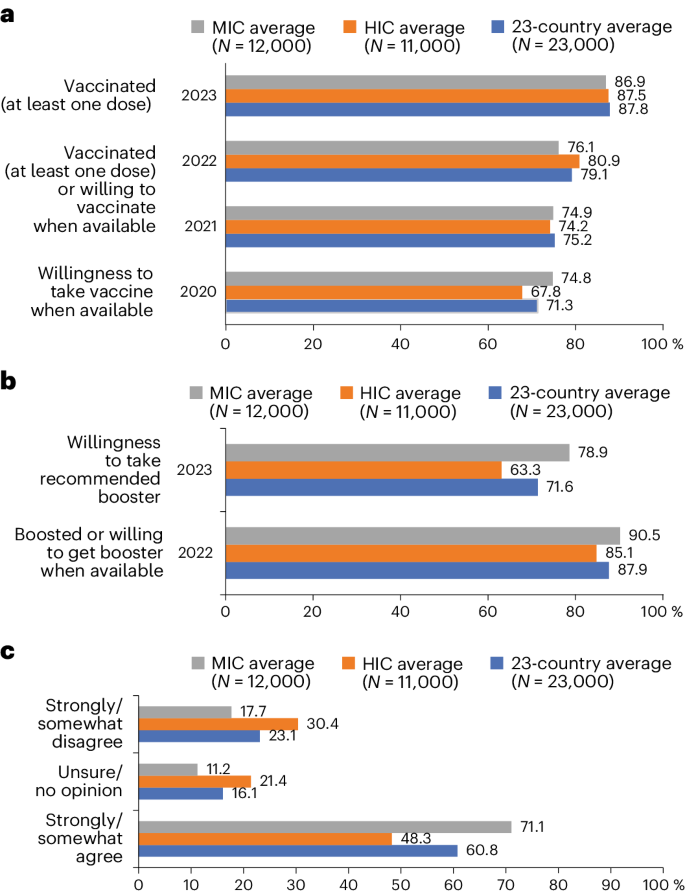
a , COVID-19 vaccine acceptance among 23 countries, HICs and MICs. b , COVID-19 booster vaccine acceptance among 23 countries, HICs and MICs. c , Reported pandemic influence toward routine immunization. Four countries (Ghana, Kenya, Peru and Türkiye) were not included in the 2020 global survey. HICs: Canada, France, Germany, Italy, Poland, Singapore, South Korea, Spain, Sweden, the United Kingdom and the United States. ‘Routine immunization’ referrs to ‘other diseases (for example, flu, measles and viral hepatitis B)’ in the survey item.
The COVID-19 pandemic led to widespread disruptions in routine immunization services globally, including for childhood doses, resulting in delayed and reduced vaccine uptake 10 . The results of this study demonstrate that 23.1% of respondents are less likely to accept vaccines for diseases other than COVID-19. Experience from the diversion of healthcare resources during the pandemic, along with lockdown measures and concerns about infection, highlights the need for resilient primary care systems, especially in maintaining access to crucial prevention interventions, such as routine childhood and adult vaccination. Other challenges, including disruptions to vaccine supply chains, underscore the importance of strengthening immunization systems and services to prevent future outbreaks 14 , 15 . Moreover, the extension of COVID-19 vaccine skepticism to other vaccines, including among parents who make vaccination decisions for their children 10 , signals a crucial need for ongoing efforts in vaccine education and trust building. Looking ahead, these insights should inform strategies to fortify healthcare systems against similar challenges to minimize disruptions and ensure continuity of essential health services, including routine vaccinations. Meanwhile, many communities are facing increased vulnerability to vaccine-preventable diseases 10 , highlighting the need for innovative strategies to ensure the continuity of routine immunization and COVID-19 vaccination campaigns to improve vaccine confidence.
The survey responses on trust in sources that provide information or guidance on pandemic interventions revealed generally high levels of trust in those close to the individual, although all 11 studied sources averaged less than seven points on a ten-point scale. For example, ‘my doctor or nurse’ ranked highest at 6.9 and ‘my family and friends’ ranked at 6.4 (Extended Data Fig. 2d ). Similarly, established health institutions such as the World Health Organization (WHO) (6.5) and the US Centers for Disease Control and Prevention (6.4) ranked high. Social media platforms (5.0) and religious leaders (5.0) each ranked neutrally (Extended Data Fig. 2d ). There was variability across countries, for example, ‘religious leaders’ ranked 3.16 in Sweden and 3.19 in Germany but 6.57 in Nigeria and 6.72 in India, whereas ‘my doctor or nurse’ ranked 4.95 in Russia and 7.70 in Kenya (Extended Data Fig. 2e ). Trust in health authorities that recommended COVID-19 vaccination was higher than trust in governments’ management of the COVID-19 pandemic at 65.4% and 56.4%, respectively (Extended Data Fig. 3 ). General trust in health authorities was 66.8% and 63.9% in MICs and HICs, respectively ( P = 0.542), while general trust in government was 60.7% and 51.7% in MICs and HICs, respectively ( P = 0.073). A decrease in perceived trust in science as a result of COVID-19 vaccine development was reported by 13.9% of respondents (MICs 13.4% and HICs 14.3%, P = 0.674). A decrease in perceived trust in the pharmaceutical industry as a result of COVID-19 vaccine development was reported by 18.7% of respondents (MICs 18.4% and HICs 19.1%, respectively, P = 0.772) (Extended Data Fig. 3 ). Trust in the science behind available COVID-19 vaccines was reported by 71.6% of respondents on average, with this value being 74.5% and 68.4% among MICs and HICs, respectively ( P = 0.115) (Extended Data Fig. 3 ). The unprecedented speed of development, the novel application of mRNA technology and the proliferation of misinformation, particularly on social media, raised concerns among some about the thoroughness of testing and long-term safety of COVID-19 vaccines and contributed to increased skepticism regarding science generally, as well as its application to preventive and therapeutic applications in particular 16 , 17 , 18 . Moreover, factors such as prepandemic vaccine-related controversies and mistrust in pharmaceutical companies, governments and health institutions, sometimes the result of cultural beliefs or past negative experiences, have further complicated public health communication 16 , 19 .
Perspectives on future pandemic preparedness reveal a mixed picture of confidence and trust among global populations. Approximately three-quarters (74.9%) of respondents are confident that society collectively will manage the next health crisis better than the COVID-19 pandemic, yet only 63.3% reported trusting a hypothetical WHO recommendation to vaccinate if such a crisis was announced (Fig. 2 ). Approximately a quarter of respondents in Russia (26.6%) and the United States (25.5%) express low trust in the WHO as a reliable source of information to announce a new pandemic threat (Extended Data Fig. 2a ). Approximately half of respondents in Ghana (51.5%), India (51.3%) and Kenya (49.2%) report a high level of confidence in our collective ability to better manage the next potential health crisis (Extended Data Fig. 2c ). A 2023 analysis in Kenya reporting 49.6% of respondents rating their own government’s management of the pandemic as very good or excellent may inform public confidence in future management capabilities 20 . Confidence in Ghana may be attributable to the government’s approach in preparing early readiness assessments, strategic and substantial investments in response planning and the effective use of surveillance technology 21 . India’s confidence in pandemic preparedness might be higher due to vaccine production capacity and public health investments in massive awareness campaigns and the rapid expansion of testing and contact tracing capabilities, despite having a large population and fragmented health system 22 . By contrast, 30.2% of respondents to our survey in France and 28.9% of respondents in Poland are ‘not at all confident’ in our collective ability, the highest percentages among the countries studied. These findings are comparable to panel data in France and Poland demonstrating low and decreasing trust in scientists among these populations during COVID-19 23 . Trust in the collective scientific and health communities to respond effectively to pandemic threats will require country-specific approaches that consider relevant sociocultural factors. How much individuals trust scientists and governments, respectively, has been observed as weakly related in Brazil and the United States, suggesting populations in these countries distinguish between these two health communicator groups, whereas the relationship was stronger in France, and populations view them as more closely aligned 23 . For example, in the United States and Brazil, a trend toward privatization and the erosion of the government’s role in mitigating public health threats exacerbated racial inequities and contributed to a fragmented response to the COVID-19 pandemic 24 , 25 . Ongoing global efforts to prepare for future global health threats promote a comprehensive ‘vaccines plus’ approach that incorporates social and behavioral preventive measures alongside rigorous testing and treatment 26 . Heightened vaccine hesitancy relative to COVID-19, pandemic fatigue and concerted disinformation campaigns have strong implications for plans to prevent or manage future pandemics, as well as a degree of spillover effect on our collective ability to control other vaccine-preventable diseases 27 . This may be particularly important as it pertains to routine childhood immunizations.
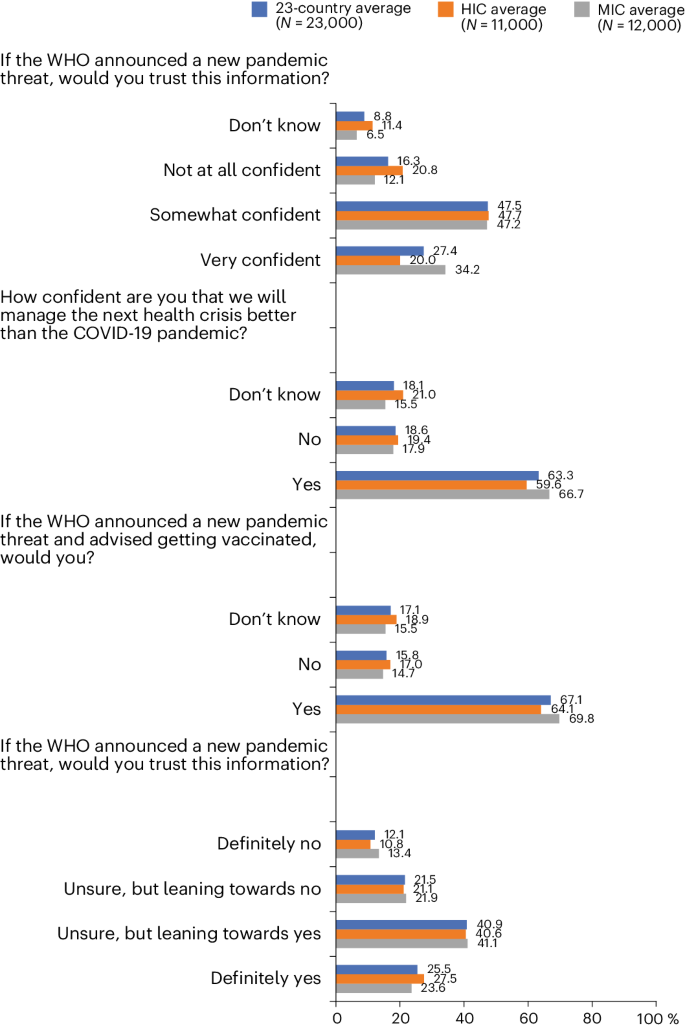
MICs: Brazil, China, Ecuador, Ghana, India, Kenya, Mexico, Nigeria, Peru, Russia, South Africa and Türkiye. Four countries (Ghana, Kenya, Peru and Türkiye) were not included in the 2020 global survey. HICs: Canada, France, Germany, Italy, Poland, Singapore, South Korea, Spain, Sweden, the United Kingdom and the United States.
A vocal minority of vaccine-resistant populations continue to believe inaccurate and disproven claims, such as the effectiveness of ivermectin as a treatment for COVID-19 and some conspiracy theories, that drive resistance to vaccination 28 , 29 . Disinformation aiming to influence public opinion poses major challenges for communication campaigns that require heterogeneous data-driven precision public health approaches 30 , 31 . These strategies should focus on delivering clear, accurate and culturally sensitive information to specific communities through their preferred information channels and via trusted sources and on exposing the motivation of those behind disinformation. It is important to acknowledge that individuals often show a preference for information that aligns with their existing beliefs and perceive such information as more credible 32 . This biased selection and perception is more pronounced among those with higher health literacy 32 , which is a factor that health communication professionals must consider.
The critical need to catch up on routine immunizations and prepare for potential new pandemic threats, coupled with the continued spread of COVID-19, requires maintaining vigilance in addressing vaccine hesitancy globally. The varying degrees of hesitancy observed across different demographic groups and countries emphasize the importance of culturally and contextually relevant strategies that include the selection of welcomed credible sources as primary conduits of information to address and mitigate vaccine hesitancy. The findings of this study demonstrate that the WHO and the US Centers for Disease Control and Prevention, as well as the respondents’ personal doctor, were more highly trusted as sources of pandemic information. The communication of accurate and timely information, as well as countering misinformation, are pivotal in guiding public perception and behavior toward COVID-19 vaccination acceptance.
Furthermore, whole-of-society action has been recommended by pandemic researchers to address the thus far fragmented approaches seen in relation to pandemic preparedness and response 33 , 34 . Such an approach involves various sectors and actors in decision-making processes to build resilient systems and takes life risks other than health, such as employment, housing and food security status, into consideration. A proposed pandemic agreement is currently being debated in advance of the May 2024 World Health Assembly. It aims to strengthen global collaboration between countries and global health organizations, including the WHO, around improving One Health data monitoring and sharing, toward ensuring equitable access to preventive and therapeutic measures and strengthening health systems 35 . The intent of such an agreement would signal to Member States and their populations that pandemic preparedness to address the shortcomings of the COVID-19 pandemic response is being taken seriously, including the rapid, real-time country collaboration on surveillance and the equitable distribution of vaccines and other mitigation and elimination efforts.
Limitations to interpreting these data include the recognition of a fundamental discrepancy that may exist between the respondents’ reported willingness to receive the vaccine and their actual vaccination behavior. What people express in surveys can differ meaningfully from their actions 27 . Therefore, the findings regarding vaccine acceptance and hesitancy should not be directly equated with actual vaccine uptake; rather, the reported responses reflect attitudes and opinions at a specific point in time. As public perceptions of the COVID-19 pandemic and vaccination evolves, so too might their willingness to be vaccinated. This temporal aspect suggests that the acceptance levels reported in our study are subject to change due to a variety of factors, including new information about the virus and the vaccine, changes in public health recommendations and shifts in societal norms and attitudes toward vaccination. While our study assessed individuals’ perceptions of trust in sources of pandemic information, including governments and health authorities, we did not investigate the quality of country responses to the pandemic, which may be an important determinant of such trust, given its independent association with COVID-19 vaccination 20 . Our study’s design did not allow for a detailed analysis of the nuanced relationship between language, trust and cultural context, while early research on the impact of health communication language on vaccine hesitancy in bilingual settings may be mediated by cultural factors regarding trust in health and governing institutions 36 . We permitted participants to respond using their preferred language within their country.
This study reveals that a substantial proportion of individuals express resistance to vaccination and that concerns about COVID-19 vaccination appear to have spilled over to affect other vaccine-preventable diseases. This underscores the increasingly urgent necessity for sustained vaccine education and trust-building efforts. Moreover, although we found that people were generally confident that society will handle future health crises better, there remains a notable lack of trust and potential adherence to the recommendations of public health authorities. Health system preparedness for future outbreaks and global health threats should include improving vaccine accessibility and vaccine demand through effective, culturally and contextually relevant public communication strategies and innovative use of digital and social media in health education employing infodemic countermeasures.
Study design and sample
This study employed random stratified sampling in a 23-panel cross-sectional design (Extended Data Table 1 and Reporting Summary). A target quota was established for four strata (that is, age, gender, country-specific statistical regions and country-specific levels of education) according to the latest available country data for these strata and with a minimum quota of 50 participants per strata 37 , 38 , 39 , 40 , 41 . There were 23,000 participants, 1,000 from each country, the populations for which collectively represent nearly 60% of the world’s population 42 . MICs consisted of Brazil, China, Ecuador, Ghana, India, Kenya, Mexico, Nigeria, Peru, Russia, South Africa and Türkiye and HICs consisted of Canada, France, Germany, Italy, Poland, Singapore, South Korea, Spain, Sweden, the United Kingdom and the United States 43 . The details on participant recruitment are described in Reporting Summary.
Survey instrument
The instrument ( Supplementary Information ) included 30 items from previous study iterations, 9 new items on misinformation and pandemic preparedness and 11 items on trusted sources of information selected by the authors following a scoping review of peer-reviewed primary research that used survey methodologies to assess these topics 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 . The selected items aimed to cover a broad spectrum of information channels that people might rely on for pandemic-related information. They include formal and informal sources, spanning from international health organizations to personal acquaintances, attempting to capture a comprehensive view of trust in different information environments and applicable for a global sample. The questionnaire was cross-culturally translated from English to the two most widely spoken lanugages in each country.
Statistical analysis
Descriptive statistics were used to report COVID-19 vaccine uptake and booster acceptance. In 2022, COVID-19 booster acceptance was defined as having received at least one dose of a booster and if not, willingness to take the booster when it is available (answer options ‘strongly agree’ or ‘somewhat agree’ to question ‘I will take the COVID-19 booster dose(s) when it is available to me’). We also report the descriptive statistics for items related to reported attitudes toward routine immunization, trusted sources of information and future pandemic preparedness. The participants ranked the trustworthiness of these sources on a scale of 1 to 10, where 1 indicated ‘no trust at all’ and 10 represented ‘complete trust’. For each source of information, individual scores from participants within a country were aggregated to produce a single mean score for that source in that country. This method allowed for a concise representation of the collective trust level in each information source per country. The country-specific weighted estimates were used to compute 23-country average as well as averages for MIC and HIC country groupings. Independent sample t -tests were used to compare average estimates over time as well as for HIC and MIC country groups. All the analyses were conducted in SAS version 9.4 software. All the estimates reported in the paper have a maximum credibility interval of error of ±3.1 percentage points. The country-specific standard errors for each estimate are provided in Extended Data Table 2 .
Ethics and inclusion statement
This study was approved and the survey administered by Emerson College, Boston, USA under institutional review board protocol no. 20–023-F-E-6/12, which employed online data collection panels not requiring local review. Informed consent was obtained from participants after describing the study purpose and expected risks and benefits before participants were permitted to advance to the study questionnaire. We fully endorse the Nature Portfolio journals’ guidance on MIC authorship and inclusion. In this fourth-round study, the author list has expanded from 8 to 12 with stronger regional and gender balances. These authors (two authors from South Africa, one from Brazil, three from Spain, four from the United States and four from Poland, Germany and France) provided insights into the translation of the survey to local languages and interpretation and discussion of the results for their respective countries. We reviewed relevant studies from among the 23 studied countries in preparing the survey instrument and manuscript.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw data generated in this study are available for download via Zenodo at https://doi.org/10.5281/zenodo.10568581 (ref. 58 ). All authors had access to the raw data.
Code availability
All code for data analysis associated with the paper is available for download via Zenodo at https://doi.org/10.5281/zenodo.10568594 (ref. 59 ).
COVID-19 epidemiological update—19 January 2024. World Health Organization https://www.who.int/publications/m/item/covid-19-epidemiological-update–19-january-2024 (2024).
Wang, H. et al. Lancet 399 , 1513–1536 (2022).
Carvalho, T., Krammer, F. & Iwasaki, A. Nat. Rev. Immunol. 21 , 245–256 (2021).
Adu, P. et al. J. Infect. Public Health 16 , 441–466 (2023).
Larson, H. J. Nat. Hum. Behav. 6 , 1609–1610 (2022).
Jørgensen, F., Bor, A., Rasmussen, M. S., Lindholt, M. F. & Petersen, M. B. Proc. Natl Acad. Sci. USA 119 , e2201266119 (2022).
Su, Z., Cheshmehzangi, A., McDonnell, D., da Veiga, C. P. & Xiang, Y. T. Front Immunol. 13 , 839433 (2022).
Limbu, Y. B. & Gautam, R. K. Vaccines 11 , 816 (2023).
Grills, L. A. & Wagner, A. L. Vaccine 41 , 6127–6133 (2023).
The State of the World’s Children 2023. UNICEF https://www.unicef.org/reports/state-worlds-children-2023 (2023).
Lazarus, J. V. et al. Nat. Med. 27 , 225–228 (2020).
Lazarus, J. V. et al. Nat. Commun. 13 , 1–14 (2022).
Lazarus, J. V. et al. Nat. Med. 29 , 366–375 (2023).
Shet, A. et al. Lancet Glob. Health 10 , e186–e194 (2022).
Alam, S. T. et al. Int. J. Prod. Econ. 239 , 108193 (2021).
Zhao, S. et al. JMIR Public Health Surveill. 9 , e40201 (2023).
Porter, E., Velez, Y. & Wood, T. J. R. Soc. Open Sci. 10 , 221097 (2023).
Pierri, F. et al. J. Med. Internet Res. 25 , e42227 (2023).
Wan, M., Su, Q., Xiang, R. & Huang, C. R. Int J. Data Sci. Anal. 15 , 313–327 (2023).
Arsenault, C. et al. Lancet Glob. Health 12 , e156–e165 (2024).
Kenu, E., Frimpong, J. A. & Koram, K. A. Ghana Med. J. 54 , 72 (2020).
Venkata-Subramani, M. & Roman, J. Am. J. Med. Sci. 360 , 742–748 (2020).
Algan, Y., Cohen, D., Davoine, E., Foucault, M. & Stantcheva, S. Proc. Natl Acad. Sci. USA 118 , e2108576118 (2021).
Feldman, J. M. & Bassett, M. T. Br. Med. J. 384 , e076969 (2024).
Muniz, R. C., Ferradas, F. M., Gomez, G. M. & Pegler, L. J. Disasters 45 , S97–S118 (2021).
Greenhalgh, T., Griffin, S., Gurdanski, D. & Hamdy, A. Br. Med. J . 376 , o1 (2022).
Zhang, V., Zhu, P. & Wagner, A. L. Int J. Environ. Res. Public Health 20 , 3376 (2023).
Annenberg Public Policy Center Vaccine Confidence Falls as Belief in Health Misinformation Grows (Annenberg School for Communication, 2023). https://www.asc.upenn.edu/news-events/news/vaccine-confidence-falls-belief-health-misinformation-grows
Romer, D. & Jamieson, K. H. Front. Psychol. 14 , 1175571 (2023).
Czerniak, K. et al. J. Am. Med. Inform. Assoc. 30 , 752–760 (2023).
Nat. Med. 25 , 1177 (2019).
Meppelink, C. S., Smit, E. G., Fransen, M. L. & Diviani, N. J. Health Commun. 24 , 129–140 (2019).
Lazarus, J. V. et al. Nature 611 , 332–345 (2022).
Make it the last pandemic by the Independent Panel for Pandemic Preparedness and Response. The Independent Panel for Pandemic Preparedness and Response https://theindependentpanel.org/mainreport/ (2021).
Hanbali, L., Hannon, E., Lehtimaki, S., McNab, C. & Schwalbe, N. R. BMJ Glob. Health 8 , e013348 (2023).
Geipel, J., Grant, L. H. & Keysar, B. Sci. Rep. 12 , 253 (2022).
American community survey demographic and housing estimates. United States Census Bureau https://data.census.gov/cedsci/table?d=ACS5-Year Estimates Data Profiles&table=DP05&tid=ACSDP5Y2018.DP05 (2018).
UNESCO Institute for Statistics. Demographic and socio-economic data. UIS.Stat (2023) http://data.uis.unesco.org/
Population data. Organisation for Economic Co-operation and Development https://stats.oecd.org/Index.aspx?DataSetCode=EDU_DEM (2022).
World Bank data: population, total. World Bank (2023) https://data.worldbank.org/indicator/SP.POP.TOTL
The World Factbook. U.S. Central Intelligence Agency https://www.cia.gov/the-world-factbook/about/archives/ (2021).
Population, total—Brazil, Canada, China, Ecuador, France, Germany, Ghana, India, Italy, Kenya, Mexico, Nigeria, Peru, Poland, Russian Federation, South Africa, Korea, Rep., Singapore, Spain, Sweden, Turkiye, United Kingdom, United States data. The World Bank https://data.worldbank.org/indicator/SP.POP.TOTL?locations=BR-CA-CN-EC-FR-DE-GH-IN-IT-KE-MX-NG-PE-PL-RU-ZA-KR-SG-ES-SE-TR-GB-US (2022).
World Bank Country and Lending Groups. World Bank Group (2023) https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
Lin, T. H., Chang, M. C., Chang, C. C. & Chou, Y. H. Soc. Sci. Med 296 , 114744 (2022).
Gesser-Edelsburg, A., Cohen, R., Hijazi, R. & Shahbari, N. A. E. J. Med. Internet Res. 22 , e19370 (2020).
Chen, X., Lee, W. & Lin, F. Int. J. Environ. Res. Public Health 19 , 8033 (2022).
U.S. Census Bureau. Household Pulse Survey: Measuring Social and Economic Impacts during the Coronavirus Pandemic (2023).
Laganà, A. S. et al. Open Med. 17 , 475–484 (2022).
Beatty, A. L. et al. JAMA Netw. Open 4 , e2140364 (2021).
American COVID-19 Vaccine Poll (African American Research Collaborative, 2023); https://covidvaccinepoll.com/app/aarc/covid-19-vaccine-messaging/#/
Oladeji, O. Asian J. Res. Infect. Dis. 9 ,1-10 (2022).
Rikard-Bell, M. et al. Aust. N. Z. J. Obstet. Gynaecol. 63 , 335–343 (2023).
U-Report. UNICEF https://ureport.in/opinion/5601/
Biezen, R. et al. Hum. Vaccin. Immunother. 18 , 2147770 (2022).
Yousaf, A. R. et al. Pediatr. Infect. Dis. J. 42 , 252–259 (2023).
Verma, S. S. et al. J. Clin. Transl. Sci. 5 , 1–5 (2021).
Eagan, R. L., Larson, H. J. & de Figueiredo, A. Hum. Vaccin Immunother. 19 , 2237374 (2023).
COVID-19 influence on trust in routine immunisation, health information sources, and pandemic preparedness in 23 countries in 2023. Zenodo (2023) https://doi.org/10.13039/10.5281/zenodo.10568581
Code COVID-19 influence on trust in routine immunisation, health information sources, and pandemic preparedness in 23 countries in 2023. Zenodo (2023) https://doi.org/10.13039/10.5281/zenodo.10568594
Download references
Acknowledgements
J.V.L. and T.M.W. acknowledge support to ISGlobal from the Spanish Ministry of Science, Innovation and Universities through the ‘Centro de Excelencia Severo Ochoa 2019–2023’ program (CEX2018-000806-S) funded by MCIN/AEI/10.13039/501100011033 and from the ‘Generalitat de Catalunya’ through the CERCA program.
Author information
Authors and affiliations.
Graduate School of Public Health and Health Policy, City University of New York, New York City, NY, USA
Jeffrey V. Lazarus, Trenton M. White, Katarzyna Wyka, Scott C. Ratzan, Kenneth Rabin & Ayman El-Mohandes
Barcelona Institute for Global Health, Barcelona, Spain
Jeffrey V. Lazarus & Trenton M. White
Hospital Clínic, University of Barcelona, Barcelona, Spain
Jeffrey V. Lazarus
London School of Hygiene and Tropical Medicine, London, UK
Heidi J. Larson
Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, USA
University Clinic Hospital of Santiago de Compostela, Healthcare Research Institute of Santiago (IDIS), Santiago de Compostela, Spain
Federico Martinon-Torres
Department of Pediatrics with Clinical Assessment Unit, Medical University of Warsaw, Warsaw, Poland
Ernest Kuchar
Centre for the AIDS Program of Research in South Africa, Durban, South Africa
Salim S. Abdool Karim
Mailman School of Public Health, Columbia University, New York City, NY, USA
Anthropology and Ecology of Disease Emergence Unit, Institut Pasteur, Université Paris Cité, Paris, France
Tamara Giles-Vernick
Heidelberg Institute of Global Health, Heidelberg University Hospital, Heidelberg, Germany
Selina Müller
Baraka Impact Finance, Geneva, Switzerland
Carolina Batista
Movement Health Foundation, Rio de Janeiro, Brazil
Wits Health Consortium, Johannesburg, South Africa
Nellie Myburgh
Charité Centre for Global Health, Berlin, Germany
Beate Kampmann
You can also search for this author in PubMed Google Scholar
Contributions
J.V.L., A.E.-M. and T.M.W. conceived of the study. K.W. was responsible for coding and data analyses, with input from T.M.W. J.V.L. and T.M.W. A.E.-M. wrote the first draft of the paper. T.M.W., K.W., K.R., J.V.L., A.E.-M., S.C.R., H.J.L., F.M-T., E.K., S.A.K., T.G.-V., S.M., C.B., N.M. and B.K. edited subsequent revisions of the draft and approved the final paper.
Corresponding author
Correspondence to Jeffrey V. Lazarus .
Ethics declarations
Competing interests.
Study funding was provided by Moderna, to the City University of New York Research Foundation. The authors retained full autonomy in the design of the study; the development of the survey instrument; the collection, analysis and interpretation of data; the presentation of results; and the decision to submit the article for publication. J.V.L. has received speaker fees from Echosens, Gilead Sciences, Moderna, Novo Nordisk, Novovax, Pfizer and ViiV and grants from Gilead Sciences, GSK, Madrigal Pharmaceuticals and Roche Diagnostics outside the submitted work. The other authors declare no competing interests.
Peer review
Peer review information.
Nature Medicine thanks Godfrey Bwire, Tanja Stamm and Joseph Wu for their contribution to the peer review of this work. Primary Handling Editor: Ming Yang, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 reported willingness for routineimmunization, covid-19 vaccine and booster acceptance and hesitancy in october 2023 by country..
Sample size for each individual country n = 1,000. Middle-Income Countries (MIC): Brazil, China, Ecuador, Ghana, India, Kenya, Mexico, Nigeria, Peru, Russia, South Africa, Turkiye. High-Income Countries (HIC): Canada, France, Germany, Italy, Poland, Singapore, South Korea, Spain, Sweden, United Kingdom, United States. ( a )“I am more willing to get vaccinated against other disease (e g, flu, measles,viral hepatitis B)”, ( b ) COVID-19 vaccine acceptance and hesitancy, October 2023, COVID-19 vaccine acceptance was defined as having received at least one dose of a COVID-19 vaccine. COVID-19 vaccine hesitancy was defined as not having received at least one dose of a COVID-19 vaccine. ( c ) COVID-19 booster acceptance and hesitancy among vaccinated respondents, October 2023. COVID-19 booster acceptance among vaccinated respondents was defined as willingness to take future recommended boosters (answer options “strongly agree” or “somewhat agree” to question “I will take the recommended COVID-19 booster”. COVID-19 booster hesitancy among vaccinated respondents was defined as having reportedeither “unsure/ no opinion” or “somewhat disagree” or “strongly disagree” to the same question.
Extended Data Fig. 2 Reportedattitudes about future potential pandemic preparedness.
Sample size for each individual country n = 1,000. Middle-Income Countries (MIC): Brazil, China, Ecuador, Ghana, India, Kenya, Mexico, Nigeria, Peru, Russia, South Africa, Turkiye. High-Income Countries (HIC): Canada, France, Germany, Italy, Poland, Singapore, South Korea, Spain, Sweden, United Kingdom, United States. ( a ) “If the World Health Organization (WHO) announced a new pandemic threat, would you trust thisinformation?”, ( b ) “If the World Health Organization (WHO) announced a newpandemic threat and advised getting vaccinated, would you?”, ( c ) “How confidentare you that we will manage the next health crisis better than the COVID-19 pandemic?”, ( d ) Reported trust in the sources of information about COVID-19 vaccines, ( e ) Reported trustin the sources of information about COVID-19 vaccines by country.
Extended Data Fig. 3 Trust in science, health authorities, government and pharmaceutical industry.
Sample size for each individual country n = 1,000. Middle- Income Countries (MIC): Brazil, China, Ecuador, Ghana, India, Kenya, Mexico, Nigeria, Peru, Russia, South Africa, Turkiye. High-Income Countries (HIC): Canada, France, Germany, Italy, Poland, Singapore, South Korea, Spain, Sweden, United Kingdom, United States. Survey items: ( a ) ‘I trust the science behind the COVID-19 vaccines available to me,’ ( b ) ‘How much do you trust the health authorities that recommended you get a COVID-19 vaccine?,’ ( c ) ‘How much did you trust your government’s management of the COVID-19 pandemic in your country?,’ ( d ) ‘Did the development of COVID-19 vaccines affect your trust in science generally?,’ ( e ) ‘Did the development of the COVID-19 vaccines affect your trust in the pharmaceutical industry?’.
Supplementary information
Supplementary information, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Lazarus, J.V., White, T.M., Wyka, K. et al. Influence of COVID-19 on trust in routine immunization, health information sources and pandemic preparedness in 23 countries in 2023. Nat Med 30 , 1559–1563 (2024). https://doi.org/10.1038/s41591-024-02939-2
Download citation
Received : 19 December 2023
Accepted : 21 March 2024
Published : 29 April 2024
Issue Date : June 2024
DOI : https://doi.org/10.1038/s41591-024-02939-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Public trust in vaccines shows a dip.
Nature India (2024)
Routine Immunization During the Covid-19 Pandemic: Need for Improved Strategies for Future Threats
- Vipin M. Vashishtha
Indian Pediatrics (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
September 4, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
New study provides insight to why COVID vaccines hit some harder than others
by Anna Zarra Aldrich, University of Connecticut

When you got the SARS-CoV2 vaccine to protect against COVID-19, you may have experienced severe side effects. Or maybe you didn't.
Catherine Andersen, associate professor of nutritional sciences in the College of Agriculture, Health and Natural Resources (CAHNR), published a paper highlighting factors that may explain why some people perceived more side effects than others including stress, exercise , and use of hormonal birth control.
This study was published in the Journal of Agriculture and Food Research .
Concerns about potential side effects were a major barrier for some people to getting the vaccine at all, yet little research had been done on what could make someone more vulnerable to experiencing side effects.
One of Andersen's collaborators on the paper is Christa Palancia Esposito at Fairfield University. Esposito is a nurse practitioner and midwife who noticed in both her practice and in emerging literature that COVID-19 infection was impacting women's health differently from men's.
Andersen and Esposito had previously collaborated on a study looking at how women respond to certain dietary interventions based on whether they use hormonal birth control.
"We thought that was interesting because there was a lot of research coming out about sex-specific differences and COVID-19 illness severity but less about responsiveness and side effects to vaccines," Andersen says.
Given all of this, they decided to see if sex, hormonal birth control use, diet, body mass index (BMI), or exercise impacted someone's experience of post-vaccine symptoms.
"It just got us thinking more about personalized health and whether certain characteristics could be playing a role here," Andersen says.
For this pilot study , the researchers surveyed 82 people who received any of the three vaccines available in 2021. They found that stress, BMI, exercise, and use of hormonal birth control all played a role.
The researchers found a significant correlation between stress and one's perception of the intensity of the side effects from the vaccine.
"Whether stress influences psychological perception of side effects, or whether stress responses lead to biological changes that result in side effects and impact efficacy of SARS-CoV2 vaccines, as it has been shown to do with other vaccines, is worth studying," Andersen says.
While there were no sex-dependent differences in the experience of side effects, women did generally report higher levels of stress and less regular exercise than men in the study.
Some of the associations between individual characteristics and perceived vaccine side effects were dose-dependent for the Moderna and Pfizer vaccines, which had two initial doses. For example, people who exercise regularly reported experiencing a lower severity of side effects for their first dose than those who did not exercise regularly. But for the second dose, they experienced a greater severity of side effects.
"Exercise in itself, especially acutely, can be inflammatory and certainly can impact the immune system ," Andersen says, with growing evidence that exercise impacts vaccine efficacy.
Women who were on hormonal birth control also had an increased experience of side effects, especially those using the birth control pill. People with a higher BMI also reported greater severity of side effects.
The researchers also looked at what kinds of supplements people took and what dietary patterns they followed. But, due to the small sample size , they were not able to find any significant associations between these characteristics and perceived vaccine side effects.
Andersen says she will use data from this study to support future work in her lab which focuses on the connections between diet and lifestyle factors, metabolic health, and immune function.
"My lab will be able to immediately take these factors and use the knowledge we gained to better understand individualized responses to lifestyle and dietary interventions that are aimed at achieving specific immune outcomes," Andersen says.
As more data from SARS-CoV2 vaccinations become available, this study will provide a foundation for more work looking at these and other factors to improve vaccine delivery and reduce side effects through an individualized approach to health.
"The long-term goal is to make vaccines more effective and at the same time minimize side effects or adverse responses that may influence acceptance of potentially lifesaving, preventative health measures."
Explore further
Feedback to editors

Excessive light pollution may increase risk of Alzheimer's, especially in younger people
13 minutes ago

100-fold improvement in sight seen after gene therapy trial
5 hours ago

Autoimmune disease researchers find immune cells escape therapy due to 'exhausted' state
7 hours ago

Researchers develop rapid test to detect dopamine

Study: First sustained remission of HIV infection following a bone marrow transplant in absence of protective mutation

Brain scans reveal that mindfulness meditation for pain is not a placebo

Innovative computational approach yields novel cancer targets

AI-driven tool could improve brain pressure monitoring in intensive care patients
8 hours ago

Risky combinations of psychiatric drugs prescribed for young patients

New study reveals the signals in your brain that initiate spontaneous actions
Related stories.

How mindset could affect the body's response to vaccination
Aug 27, 2024

Video: Why do some people have side effects with COVID-19 vaccines while others do not?
Mar 31, 2021
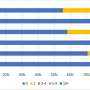
New research sheds light on the side effects of COVID-19 vaccination
Aug 29, 2023

COVID-19 vaccination may increase risk for urinary tract symptoms
Aug 1, 2024

No side effects from your COVID vaccine? Don't worry, it's still working
Jan 19, 2022

Why some people don't experience vaccine side effects, and why it's not a problem
Apr 26, 2021
Recommended for you

Study shows that vaccination against RSV lowers the risk of hospitalization in people over 60
13 hours ago

Vaccination skepticism and esoteric attitudes are linked, study finds
9 hours ago

Investigational mpox mRNA vaccine more effectively reduces disease severity in primates compared to available vaccines
Sep 4, 2024

H5 influenza vaccines: What needs to be done to reduce the risk of a pandemic

Childhood HIV vaccination strategy shows promise in study
Sep 2, 2024

Fentanyl vaccine heads for clinical trials, with goal of saving lives
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
VIDEO
COMMENTS
Discussion. A two-dose regimen of BNT162b2 (30 μg per dose, given 21 days apart) was found to be safe and 95% effective against Covid-19. The vaccine met both primary efficacy end points, with ...
In this rapid living systematic evidence synthesis and meta-analysis, we searched EMBASE and the US National Institutes of Health's iSearch COVID-19 Portfolio, supplemented by manual searches of COVID-19-specific sources, until Dec 1, 2022, for studies that reported vaccine effectiveness immediately and at least 112 days after a primary vaccine series or at least 84 days after a booster dose.
The Coronavirus Efficacy (COVE) phase 3 trial was launched in late July 2020 to assess the safety and efficacy of the mRNA-1273 vaccine in preventing SARS-CoV-2 infection. An independent data and ...
Introduction. The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 192 million cases and 4.1 million deaths as of July 22, 2021. 1 This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems. Despite the implementation of stringent public health measures, there ...
Community‐based studies in five countries show consistent strong benefits from early rollouts of COVID‐19 vaccines. By the beginning of June 2021, almost 11% of the world's population had received at least one dose of a coronavirus disease 2019 (COVID‐19) vaccine. 1 This represents an extraordinary scientific and logistic achievement — in 18 months, researchers, manufacturers and ...
Participants were enrolled from December 28, 2020 (2 weeks after the introduction of a Covid-19 vaccine), through May 19, 2021, at 33 sites across 25 U.S. states, representing more than 500,000 ...
Safety and adverse effects of current COVID-19 vaccines. As shown in Table I, current vaccines have demonstrated considerable efficacy in diminishing mild, moderate and severe cases with a low risk of adverse events 21.For some of these vaccines [such as Convidicea (AD5-nCoV), Janssen (Ad26.COV2.S), Sinopharm (BBIBP-CorV), Covaxin (BBV152) and Sinovac (CoronaVac)], there is the information ...
No vaccine was statistically significantly associated with a decreased risk for severe COVID-19 than other vaccines, although mRNA-1273 and Gam-COVID-Vac have the highest P-scores (0.899 and 0.816 ...
Since the outbreak of the Coronavirus disease 2019 (COVID-19) pandemic caused by the SARS-CoV-2 virus in late 2019, substantial research has been undertaken to uncover the health consequences ...
There is no question that the current vaccines are effective and safe. The risk of severe reaction to a COVID-19 jab, say researchers, is outweighed by the protection it offers against the deadly ...
The effectiveness of the mRNA vaccines in preventing COVID-19 disease progression in 2021 set new expectations about the role of prevention interventions for the disease. Efficacy observed in the trials was more than 90%.1,2 The efficacy of other vaccines evaluated in large randomised trials, such as the Oxford-AstraZeneca (70%) and Sputnik V (91%) vaccines, have been criticised for elements ...
Implications for research Future research should evaluate the long-term effect of vaccines, compare different vaccines and vaccine schedules, assess vaccine efficacy and safety in specific populations, and include outcomes such as preventing long COVID-19. Ongoing evaluation of vaccine efficacy and effectiveness against emerging variants of ...
6 Clinical Excellence Research Center, School of Medicine, Stanford University, CA, USA. Electronic address: [email protected]. ... Results: Pfizer and Moderna mRNA COVID-19 vaccines were associated with an excess risk of serious adverse events of special interest of 10.1 and 15.1 per 10,000 vaccinated over placebo baselines of 17.6 and ...
This document stated that symptomatic laboratory-confirmed COVID-19 might be an acceptable primary endpoint for a COVID-19 vaccine efficacy trial with an efficacy level of at least 50% required. Moreover, other secondary criteria might be used to determine the possible efficacy on asymptomatic or severe infections.
2020 has been a difficult year for all, but has seen 58 vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) be developed and in clinical trials,1 with some vaccines reportedly having more than 90% efficacy against COVID-19 in clinical trials. This remarkable achievement is much-needed good news as COVID-19 cases are currently at their highest daily levels globally.2 ...
Two doses of BNT162b2 are highly effective across all age groups (≥16 years, including older adults aged ≥85 years) in preventing symptomatic and asymptomatic SARS-CoV-2 infections and COVID-19-related hospitalisations, severe disease, and death, including those caused by the B.1.1.7 SARS-CoV-2 variant. There were marked and sustained declines in SARS-CoV-2 incidence corresponding to ...
We matched vaccine recipients and controls on variables associated with the probability of both vaccination and infection or severity of Covid-19: age, sex, sector (general Jewish, Arab, or ultra ...
The updated mRNA COVID-19 vaccines include Comirnaty and Spikevax, both of which are approved for individuals 12 years of age and older, and the Moderna COVID-19 Vaccine and Pfizer-BioNTech COVID ...
To date, coronavirus disease 2019 (COVID-19) becomes increasingly fierce due to the emergence of variants. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance. We aimed to systematically evaluate the effectiveness and safety of COVID-19 vaccines in the real world and to establish a ...
The protective effects of vaccination and prior infection against severe Covid-19 are reviewed, with proposed directions for future research, including mucosal immunity and intermittent vaccine boo...
With respect to modest benefits that offset barriers to vaccination, this concern seems doubtful. Even if it has empirical merit, it must be weighed against the large benefits to society of increasing COVID-19 vaccine receipt now. While offering benefits to COVID-19 vaccine recipients is typically ethical, it may not always be optimal.
Background Despite reliable evidence-based research supporting the COVID-19 vaccines, population-wide confidence and trust remain limited. We sought to expand prior knowledge about COVID-19 vaccine perceptions, while determining which population groups are at greatest risk for not getting a vaccine. Methods Study participants in the U.S. (79% female, median age group 46-60 years) were ...
The two-dose COVID-19 vaccination campaign substantially reduced hospitalisations and deaths despite high infection rates. 1-3 However, the effectiveness against infection, as happens also for other vaccines, wanes within months of the second dose. 4,5 Studies in Qatar showed substantial waning 6,7 in effectiveness against SARS-CoV-2 ...
In a statement, Peter Marks, MD, PhD, who directs the FDA's Center for Biologics Evaluation and Research, said COVID vaccines continue to have a major positive impact on public health and that vaccination continues to be the most effective method for COVID prevention. "Today's authorization provides an additional COVID-19 vaccine option that ...
1. A phase 1/2 randomized, placebo-controlled, multicentre study to evaluate the safety and immunogenicity of COVID-19 vaccine in healthy adults. RBD SARS-CoV-2 HBsAg VLP vaccine, administered at two dose amounts 5 mcg and 25 mcg, by intramuscular injection by investigators during an in-clinic visit. RBD-HBsAg VLPs.
Study author Havlir disclosed that she receives non-financial support from Abbott that is not related to the paper. Moderna's vaccination against COVID-19 is administered in Silver Spring, Maryland.
Students receiving the COVID 19 vaccine at Hawley Armory on April 8, 2021. ... Concerns about potential side effects were a major barrier for some people to getting the vaccine at all, yet little research had been done on what could make someone more vulnerable to experiencing side effects. One of Andersen's collaborators on the paper is ...
The coronavirus disease 2019 (COVID-19) pandemic, requiring urgent international intervention, led to an accelerated pace of research and development of multiple safe, effective COVID-19 vaccines ...
Students receiving the COVID 19 vaccine at Hawley Armory on April 8, 2021. The rollout of the vaccines across the state in the spring helped set up a return to a more familiar university experience.
Background paper on Covid-19 disease and vaccines: prepared by the Strategic Advisory Group of Experts (SAGE) on immunization working group on COVID-19 vaccines. December 22, 2020 ( https://apps ...