Stack Exchange Network
Stack Exchange network consists of 183 Q&A communities including Stack Overflow , the largest, most trusted online community for developers to learn, share their knowledge, and build their careers.
Q&A for work
Connect and share knowledge within a single location that is structured and easy to search.

When is it appropriate to describe research as "recent"?
I want to write: "A recent study ..." ,
The particular study I want to cite was published two years ago. I don't think that this is very recent in terms of journal appearances. But it is the most recent I could find compared to similar studies, which is what I want to emphasize.
But what are the general semantics of "recent" when referencing sources?
- 10 If the date of the study matters, why not "A study from 2014...."? – mdd Commented Mar 9, 2016 at 0:44
- 8 It is an ineffective way of saying "This is important!" As a reviewer I would probably tolerate descriptions of anything from the past ten years as "recent." – Anonymous Physicist Commented Mar 9, 2016 at 1:32
- 3 In my mind recent is anything that is new enough that it hasn't been fully absorbed (worked its way into later research, publications and into people's minds). That might depend on recent to whom -- a 20 year-old mathematical theory might well be too recent to have fully worked its way into engineering practice, so if you're writing to the engineering audience it could be appropriate to call it recent. – Owen Commented Mar 9, 2016 at 3:40
- 4 Redundancy is not a bad thing in academic writing. – Dirk Commented Mar 9, 2016 at 5:23
- 4 Keep in mind that recent to you may not be recent to a future reader. If you have something more specific to convey ("most recent at the time of writing", "unsettled", "currently the hip and trendy thing that gets grants"), you'd be best served being more specific. Otherwise, your reader will have to look at your paper's publication date and try to work out what you meant from context. – Jeffrey Bosboom Commented Mar 9, 2016 at 10:26
4 Answers 4
Good question. The semantics of the word "recent", in general, and in academic writing, in particular, is not clearly defined (that is, fuzzy), which makes its practical use quite tricky, as evidenced by your question.
While @vonbrand's answer offers some valuable insights, such as considering the fluidity of a particular scientific field or domain, I would suggest a more practical solution to this problem, as follows. Consider literature that you reference in a particular paper. What is the temporal range of the sources? I think that this aspect could guide you in to where the word "recent" is appropriate and where not so much.
For example, if you cite sources from the current century as well as 1930s, then a paper from 2010 should be considered recent, but not one from 1950. If, on the other hand, your temporal range of references is rather narrow, say, recent 20 years, then you should refer to as "recent" for sources that are from approximately last 4-5 years. You can come up with your own rule of thumb (10-20% of the total range sounds pretty reasonable). The most important aspect would be not the actual value (for the rule of thumb), but rather your consistency in applying it throughout the paper.
- @thrau: My pleasure! Thank you for kind words and accepting. – Aleksandr Blekh Commented Mar 10, 2016 at 20:45
It depends on the area. If you are talking about slow moving areas, "recent" could be a decade ago; for something that moves fast, what was published last year is old hat.
Perhaps the easiest way out is to be more specific, "a study three years back..." (besides, the study might be several years back, or be a decade long study, but the journal issue just came out, so the publication date isn't necessarily telling).
As previously mentioned, the meaning of 'recent' depends on the topic of study. What is considered recent in mathematics may not be considered recent enough for computer science. My computer science professors have generally stuck with anything five years old as being the 'oldest' an article can be. Two to three years is generally better, especially in the tech field as things progress at a much higher rate. A good thing to look out for is when an article might pass the 5 year mark, someone will most likely have adapted the methodology or research findings in a more recent article. Best of luck!
It depends.
If you refer to something that has a precise date, you should be precise. I see no advantage in writing "A recent study showed..." over "The study X from 2010 showed..." The latter contains more information and reads as least as good (in my opinion even better, because it's more precise). A similar case is "The problem posed by X at the meeting Y in 2010..." (better than "The recently posed problem...").
One case in which "recent" could make sense is "The field X has attracted much attention recently" because usually one can not pin down an exact date for this event. However, in most cases this reads more like a self-perpetuating empty statement (if there is a simple reason why the reader should care about the field X then give that!). I have to admit that I myself also wrote sentences like this, but looking back it reads a bit weird. Nowadays, if I read "this field has attracted much attention recently" I really read that the authors do not know a good reason why their problem is interesting but feel that they should.
- On a slightly related note, how would you feel about "This field has attracted much attention recently because reasons "? – svavil Commented Mar 9, 2016 at 22:40
- I would say, the more precise the better. Probably in such a sentence just giving the reason that you feel that make the field exciting is enough. The additional information that these exciting facts resulted in "much attention recently" seems not so important. I would find it even better if the sentence would tell that the field is relevant and not that its fancy right now. – Dirk Commented Mar 10, 2016 at 10:51
You must log in to answer this question.
Not the answer you're looking for browse other questions tagged citations ..
- Featured on Meta
- Bringing clarity to status tag usage on meta sites
- Announcing a change to the data-dump process
Hot Network Questions
- Microsoft SQL In-Memory OLTP in SQL Express 2019/2022
- Pull up resistor question
- Escape from the magic prison
- Upstairs washer suds coming out of basement sink
- Why is a USB memory stick getting hotter when connected to USB-3 (compared to USB-2)?
- Fusion September 2024: Where are we with respect to "engineering break even"?
- Why does this theta function value yield such a good Riemann sum approximation?
- How to change upward facing track lights 26 feet above living room?
- Checklist of documents for visiting Poland if I already have a Schengen visa
- Thriller from the early to mid 1960's involving blackmailing an official over underage liason
- Referencing an other tikzpicture without overlay
- In roulette, is the frequency of getting long sequences of reds lower than that of shorter sequences?
- When can the cat and mouse meet?
- I'm a little embarrassed by the research of one of my recommenders
- Why didn't Air Force Ones have camouflage?
- Cohomological range of a perverse sheaf
- instance scaling based on another curve
- You find yourself locked in a room
- What was the first "Star Trek" style teleporter in SF?
- Find the global maxima over the interval [0,1]
- How to Interpret Statistically Non-Significant Estimates and Rule Out Large Effects?
- Why would autopilot be prohibited below 1000 AGL?
- Why doesn’t dust interfere with the adhesion of geckos’ feet?
- Choose your own adventure style: medieval post-demon apocalypse

When is the evidence too old?
A few weeks ago, when submitting an abstract to a nursing conference, I was suddenly faced with a dilemma about age. Not my own age, but the age of evidence I was using to support my work. One key element of the submission criteria was to provide five research citations to support the abstract, and all citations were to be less than ten years old. This requirement left me stumped for a while. The research I wanted to cite was more than ten years old, yet it was excellent research within a very small body of work on the topic. Suddenly I struggled to meet the criteria and almost gave up on the submission, thinking my abstract would not tick all of the boxes if I used research now deemed to be ‘out of date’. I suddenly thought about all of the work I had published more than ten years ago – all that hard work past its use-by date.
Way back in the mid 1990s, a colleague and I started to have conversations with Australian nurses about the importance of evidence based practice (EBP) for the future of Australian nursing. The movement away from the comfort of ‘ritual and routine’ to the uncertainty of EBP was challenging. At the time we described EBP according to the principle that “all interventions should be based on the best currently available scientific evidence” 1 . We had embraced the ideas of authors such as Ian Chalmers 2 and were keen to educate nurses and nursing students about “practices that had been clearly shown to work and question practices for which no evidence exists and discard those which have been shown to do harm” 1 It was very much about the importance of using the most ‘robust’ and ‘reliable’ evidence that we had available to guide us in clinical decision making, taking into account individual patients at the centre of care. It was also about teaching nurses and nursing students about how to ask the right questions, where to look for answers and how to recognize when you have found the right answer to support individualized patient care.
Definitions of evidence-based practice are quite varied and I have heard nurses talk about using “current best evidence” while others use the “most current evidence”. These are quite different approaches, with the latter statement suggesting that more recent is best. This is sometimes reinforced in nursing education, where students are graded according to the use of recent research, with limitations placed on the age of resources used to support their work. However, I wonder if we are losing something in this translation about the meaning of ‘best evidence’ to support care. When does the published evidence get too old and where do we draw the line and stop reading research from our past?
Personally I have always expected my students to use up to date research when supporting their recommendations for care. However, I have also encouraged them to look back to see where the new research has come from and to acknowledge the foundation it has been built on. I am always keen to hear about the latest developments in healthcare and work to support the readers of EBN who need and want to know about what is new and important in the health care literature. Keeping up to date with new evidence is critically important for change. But I wonder how we strike a balance between absorbing recent research and taking into account robust research that preceded its publication by more than a decade?
So, let’s think about these ideas for a minute. If we put our blinkers on and ignore important research from the recently ‘outdated’ literature from the 1990s (when I first became interested in doing research), we could miss some important foundational work that still influences practice today. The two references I have used below, both from the 1990s, would not be included in the discussion at all. If we only consider literature that is recent, and value that more highly than if it is robust, then we will be missing important evidence to inform practice. Researchers could start asking the same research questions over and over (I have seen some of this already in nursing literature) and even feel pressured to repeat previous studies all over again to check if the findings still hold true in the contemporary world. Perhaps that is something to watch for in the future.
It is important to keep up to date with current research findings, new innovations in care, recent trends in patient problems, trends in patient outcomes and changes in the social, political and system context of the care we provide. But it is also important to look back as we move forward, thinking about the strength of the evidence as well as its age.
Allison Shorten RN RM PhD
Yale University School of Nursing
References:
- Shorten A. & Wallace MC. ‘Evidence-based practice – The future is clear’. Australian Nurses Journal, 1996, Vol. 4, No. 6, pp. 22-24.
- Chalmers I. The Cochrane collaboration: Preparing, maintaining, and disseminating systematic reviews of the effects of health care, Annals New York Academy of Science, 1993, Vol. 703, pp. 156-165.
Comment and Opinion | Open Debate
The views and opinions expressed on this site are solely those of the original authors. They do not necessarily represent the views of BMJ and should not be used to replace medical advice. Please see our full website terms and conditions .
All BMJ blog posts are posted under a CC-BY-NC licence
BMJ Journals
Importance of staying up-to-date in Research topics

It’s vital to stay current in your field of Research to ensure that your study fits into the larger context of scientific knowledge and prevent duplicating work that’s already been done. Then, because you’re expected to follow those standards, staying on top of ever-changing legal and compliance duties is a business need.
Why is it critical to keep up with the most recent Research?
There are several reasons why it’s critical to stay current with your field of study changes.
Identifying fresh Research opportunities
Understanding the present state of knowledge on a topic, recognizing gaps, and focusing on a meaningful and responsive issue. A thorough literature search can help you find a research topic that is precise enough to be examined in the context of a specific test.
Also, to ensure that you don’t leave any important studies out of your literature review, staying current will assist you in defining your long-term research goals and career trajectory, not just the next topic to concentrate on.
The findings of the Research have an expiration date.
Time is a critical factor in the systematic review process and an important covariate in assessing study heterogeneity and a fundamental determinant of systematic review clinical relevance. Indeed, systematic reviews’ usefulness as a foundation for evidence-based practice depends on proper time considerations.
New Research-based on Previous works
Previous work can help you figure out which methodologies to utilize, what data or resources are already freely available to work with, and what Research limits to solve. Developing beneficial relationships with potential collaborators
As Research entails testing, verifying, and rejecting hypotheses regularly, keeping up with recent publications will assist you in defining building blocks for your study.
Guidance & Confirming that your Research is focused on a new topic
One of the key responsibilities for a doctorate adviser, department head, or field expertise is to advise students on relevant research subjects.
Staying current on literature in your line of work and learning how to do it effectively will help you better support them and guide their research careers. You will not only be assisting them in their career advancements, but you will also be contributing to the improvement of your discipline as a whole.
Observing what your competitors are doing
Research involves many activities. As a researcher, you rely on the information and insights of other researchers to help you understand specific elements of your profession or related disciplines.
How do I stay current with Research Topics?
Keeping upto date may appear daunting at first, but your sources can be divided into two categories: formal and informal. You’ll need to put up mechanisms for the many sources you deem to be relevant if you want to stay on top of newly published and emergent Research .
As your priorities may alter over time, you’ll need to go back and evaluate your notifications from time to time, especially if you’re performing your Research over several months or even years. To track changes in the direction of your original study interest, you have to create new alerts. As a result, keeping up with the Research Topics can help you uncover potential solutions or alternatives to problems you’re having with your study.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Science, health, and public trust.
September 8, 2021
Explaining How Research Works
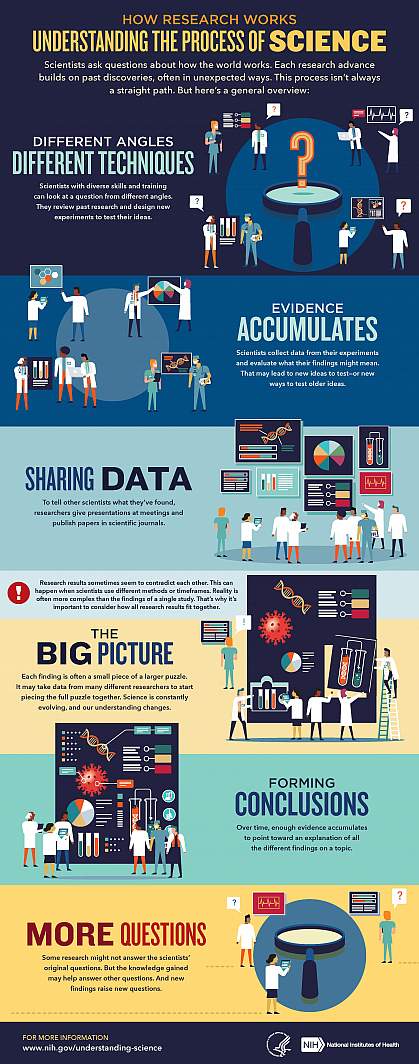
We’ve heard “follow the science” a lot during the pandemic. But it seems science has taken us on a long and winding road filled with twists and turns, even changing directions at times. That’s led some people to feel they can’t trust science. But when what we know changes, it often means science is working.

Explaining the scientific process may be one way that science communicators can help maintain public trust in science. Placing research in the bigger context of its field and where it fits into the scientific process can help people better understand and interpret new findings as they emerge. A single study usually uncovers only a piece of a larger puzzle.
Questions about how the world works are often investigated on many different levels. For example, scientists can look at the different atoms in a molecule, cells in a tissue, or how different tissues or systems affect each other. Researchers often must choose one or a finite number of ways to investigate a question. It can take many different studies using different approaches to start piecing the whole picture together.
Sometimes it might seem like research results contradict each other. But often, studies are just looking at different aspects of the same problem. Researchers can also investigate a question using different techniques or timeframes. That may lead them to arrive at different conclusions from the same data.
Using the data available at the time of their study, scientists develop different explanations, or models. New information may mean that a novel model needs to be developed to account for it. The models that prevail are those that can withstand the test of time and incorporate new information. Science is a constantly evolving and self-correcting process.
Scientists gain more confidence about a model through the scientific process. They replicate each other’s work. They present at conferences. And papers undergo peer review, in which experts in the field review the work before it can be published in scientific journals. This helps ensure that the study is up to current scientific standards and maintains a level of integrity. Peer reviewers may find problems with the experiments or think different experiments are needed to justify the conclusions. They might even offer new ways to interpret the data.
It’s important for science communicators to consider which stage a study is at in the scientific process when deciding whether to cover it. Some studies are posted on preprint servers for other scientists to start weighing in on and haven’t yet been fully vetted. Results that haven't yet been subjected to scientific scrutiny should be reported on with care and context to avoid confusion or frustration from readers.
We’ve developed a one-page guide, "How Research Works: Understanding the Process of Science" to help communicators put the process of science into perspective. We hope it can serve as a useful resource to help explain why science changes—and why it’s important to expect that change. Please take a look and share your thoughts with us by sending an email to [email protected].
Below are some additional resources:
- Discoveries in Basic Science: A Perfectly Imperfect Process
- When Clinical Research Is in the News
- What is Basic Science and Why is it Important?
- What is a Research Organism?
- What Are Clinical Trials and Studies?
- Basic Research – Digital Media Kit
- Decoding Science: How Does Science Know What It Knows? (NAS)
- Can Science Help People Make Decisions ? (NAS)
Connect with Us
- More Social Media from NIH
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Reliability and validity: Importance in Medical Research
Affiliations.
- 1 Al-Nafees Medical College,Isra University, Islamabad, Pakistan.
- 2 Fauji Foundation Hospital, Foundation University Medical College, Islamabad, Pakistan.
- PMID: 34974579
- DOI: 10.47391/JPMA.06-861
Reliability and validity are among the most important and fundamental domains in the assessment of any measuring methodology for data-collection in a good research. Validity is about what an instrument measures and how well it does so, whereas reliability concerns the truthfulness in the data obtained and the degree to which any measuring tool controls random error. The current narrative review was planned to discuss the importance of reliability and validity of data-collection or measurement techniques used in research. It describes and explores comprehensively the reliability and validity of research instruments and also discusses different forms of reliability and validity with concise examples. An attempt has been taken to give a brief literature review regarding the significance of reliability and validity in medical sciences.
Keywords: Validity, Reliability, Medical research, Methodology, Assessment, Research tools..
PubMed Disclaimer
Similar articles
- Principles and methods of validity and reliability testing of questionnaires used in social and health science researches. Bolarinwa OA. Bolarinwa OA. Niger Postgrad Med J. 2015 Oct-Dec;22(4):195-201. doi: 10.4103/1117-1936.173959. Niger Postgrad Med J. 2015. PMID: 26776330
- The measurement of collaboration within healthcare settings: a systematic review of measurement properties of instruments. Walters SJ, Stern C, Robertson-Malt S. Walters SJ, et al. JBI Database System Rev Implement Rep. 2016 Apr;14(4):138-97. doi: 10.11124/JBISRIR-2016-2159. JBI Database System Rev Implement Rep. 2016. PMID: 27532315 Review.
- Evaluation of research studies. Part IV: Validity and reliability--concepts and application. Fullerton JT. Fullerton JT. J Nurse Midwifery. 1993 Mar-Apr;38(2):121-5. doi: 10.1016/0091-2182(93)90146-8. J Nurse Midwifery. 1993. PMID: 8492191
- Validity and reliability of measurement instruments used in research. Kimberlin CL, Winterstein AG. Kimberlin CL, et al. Am J Health Syst Pharm. 2008 Dec 1;65(23):2276-84. doi: 10.2146/ajhp070364. Am J Health Syst Pharm. 2008. PMID: 19020196 Review.
- [Psychometric characteristics of questionnaires designed to assess the knowledge, perceptions and practices of health care professionals with regards to alcoholic patients]. Jaussent S, Labarère J, Boyer JP, François P. Jaussent S, et al. Encephale. 2004 Sep-Oct;30(5):437-46. doi: 10.1016/s0013-7006(04)95458-9. Encephale. 2004. PMID: 15627048 Review. French.
- Midwifery educators' knowledge of antenatal exercises in selected Nigerian midwifery schools. Ojong-Alasia MM, Moloko-Phiri SS, Matsipane MJ, Useh U. Ojong-Alasia MM, et al. Curationis. 2024 Aug 16;47(1):e1-e12. doi: 10.4102/curationis.v47i1.2495. Curationis. 2024. PMID: 39221715 Free PMC article.
- A psychometric assessment of a novel scale for evaluating vaccination attitudes amidst a major public health crisis. Cheng L, Kong J, Xie X, Zhang F. Cheng L, et al. Sci Rep. 2024 May 4;14(1):10250. doi: 10.1038/s41598-024-61028-z. Sci Rep. 2024. PMID: 38704420 Free PMC article.
- Test-Retest Reliability of Isokinetic Strength in Lower Limbs under Single and Dual Task Conditions in Women with Fibromyalgia. Gomez-Alvaro MC, Leon-Llamas JL, Melo-Alonso M, Villafaina S, Domínguez-Muñoz FJ, Gusi N. Gomez-Alvaro MC, et al. J Clin Med. 2024 Feb 24;13(5):1288. doi: 10.3390/jcm13051288. J Clin Med. 2024. PMID: 38592707 Free PMC article.
- Bridging, Mapping, and Addressing Research Gaps in Health Sciences: The Naqvi-Gabr Research Gap Framework. Naqvi WM, Gabr M, Arora SP, Mishra GV, Pashine AA, Quazi Syed Z. Naqvi WM, et al. Cureus. 2024 Mar 8;16(3):e55827. doi: 10.7759/cureus.55827. eCollection 2024 Mar. Cureus. 2024. PMID: 38590484 Free PMC article. Review.
- Reliability, validity, and responsiveness of the simplified Chinese version of the knee injury and Osteoarthritis Outcome Score in patients after total knee arthroplasty. Yao R, Yang L, Wang J, Zhou Q, Li X, Yan Z, Fu Y. Yao R, et al. Heliyon. 2024 Feb 21;10(5):e26786. doi: 10.1016/j.heliyon.2024.e26786. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38434342 Free PMC article.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Pakistan Medical Association

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

Why is Research Important in Healthcare?

- January 9, 2023
Table of Contents
Research is critical to improving patient outcomes and the quality of healthcare. It helps us to understand what works, what doesn’t work, and why. In addition, research is essential for developing new treatments and therapies. Without research, we would not have many of the lifesaving vaccines and medications that we take for granted today.
What Is Healthcare Research?
Research in healthcare is scientific and academic innovation aimed at answering medical and social questions. It typically involves observing, comparing, and sometimes testing people with different conditions; analyzing samples of blood or other tissues; examining patient records; surveying data from health and lifestyle surveys; and exploring the benefits, costs, acceptability, and wider impact of treatments.
Research in healthcare can also focus on improving the lives of people who receive care from our social care sector by introducing new devices or technologies, such as lifting equipment for residents or changing policies for better practice.
1. Research helps determine the best treatments and practices for various conditions.
Research helps to identify new and better ways to prevent, diagnose, and treat diseases and conditions. It provides insights into the causes of illnesses as well as potential treatments that can be applied in clinical practice.
By studying various factors such as lifestyle choices, diet patterns, social networks, and family support systems, researchers can provide information about what works best for different conditions. This helps doctors make informed decisions about their patients’ healthcare plans and provides them with the knowledge they need to provide effective treatments.
2. Research can identify risk factors for certain diseases and conditions
Research can help identify risk factors associated with specific diseases and conditions. These include physical , social , genetic , environmental , and lifestyle factors.
By studying groups of people and their patterns of disease occurrence, researchers can identify potential risk factors for a given condition and explore ways to reduce them or prevent them from occurring in the first place. This includes exploring potential preventive measures such as vaccines or lifestyle changes that may help reduce the risk of developing a given condition.
3. Research can identify early warning signs of certain diseases and conditions
Research can help identify early warning signs of certain diseases and conditions by examining new detection methods, conducting clinical trials, and performing studies on patients. This enables researchers to gain a better understanding of the causes, symptoms, and progression of diseases so they can develop more accurate diagnostic tools and effective treatments.
Research also provides insights into how certain conditions may affect individuals differently depending on their age or genetic background. Ultimately this leads to better outcomes for people with diagnosed illnesses by allowing them to take proactive steps to manage their condition or live a healthier lifestyle.
4. Research can identify the most effective treatments for certain conditions
Research can help to identify new and better ways to prevent, diagnose, and treat diseases and conditions. This enables researchers to study the effectiveness of different treatments for specific ailments in order to provide patients with information about what works best for them.
5. Research can help identify the best ways to care for patients
Research can help to identify the best ways to care for patients by providing new and improved treatments, information about what works and what does not, and an overall better understanding of diseases. This knowledge can be used to prevent, diagnose, treat, and prevent disease; provide better quality care; and improve patient outcomes.
Additionally, research helps medical professionals stay up-to-date on the latest advances in medical science so they can provide their patients with the best care possible, including health digitalization .
6. Research can help find ways to prevent certain conditions from developing
Research can help identify risk factors and explore potential methods of prevention for certain conditions. This may include vaccines, lifestyle changes, or medicines.
By understanding the causes of certain conditions and exploring ways to prevent them, researchers can help prevent people from developing those conditions in the first place. This can have a positive impact on communities by improving care and helping more people around the world.
7. Research can help find ways to prevent complications from developing in certain conditions
Research can help to identify risk factors and explore potential methods of prevention for conditions such as diseases and illnesses. By understanding the causes of a condition and its associated risks, researchers can develop strategies to reduce the likelihood of complications occurring. This will help improve patient care by allowing doctors to provide more effective treatments while also reducing the number of adverse side effects associated with them.
8. Research can help find better ways to communicate information about specific conditions and treatments to the public
Research can help improve public communication about certain conditions and treatments by providing evidence-based information. By disseminating this research-based knowledge to the public through various channels such as websites and publications, people can make more informed decisions about their healthcare. They will also have access to up-to-date information about current research trends in their area of interest or concern.
9. Research can help identify the best ways to engage with healthcare stakeholders
Research can help to identify the key stakeholders involved in healthcare, such as patients, healthcare providers, payers, and insurers, and analyze existing theories, models, and frameworks that govern these relationships to better understand how they work together to influence health outcomes and research effectiveness.
It can also identify ways to improve communication between stakeholders through research in order to ensure that everyone has access to the best possible care while reducing costs associated with treatments or hospital stays.
10. Research can help find ways to improve the quality and effectiveness of healthcare by generating new evidence .
Research can help improve the quality and effectiveness of healthcare by generating new evidence that can be applied to make healthcare affordable, safe, effective, equitable, accessible, and patient-centered .
By applying this evidence in practice, healthcare systems can be improved to ensure that patients receive the best possible care. This may include making decisions that are better informed by research findings or organizing care processes to improve safety and efficacy. It may also provide an opportunity to design healthcare benefits or inform policy changes that benefit patients across the board.
Research is essential in healthcare in order to improve the quality and effectiveness of care while also making it more affordable and accessible. By applying evidence-based practices and real-world evidence , healthcare systems can be improved to better serve patients and digitalize the field.
Health Analytics bridges the gap between sound scientific data and market access. Phone: (410) 997-3314 Email: [email protected]
- Health Care
- Evidence Generation Planning
- Evidence Generation
- Translating to Market Access
How recent is recent for good referencing?

Cited articles (i.e., references) in a research paper play a central role in demonstrating the necessity of the research and establishing the validity and significance of the research results.
Therefore, good referencing practices (e.g., citing relevant, critical, and recent research works on the topics) not only increase the quality of the research paper but also facilitate its peer review and availability to the right audience.
Citing or referencing recent articles in the research paper assures reviewers that an extensive literature review was undertaken while writing the paper and information in the paper is up to date. This builds trust between the authors of the paper and the reviewers, which may influence peer review reports.
How old is gold?
All being said, do we exactly know how old a research article can be before it gets the label of not being recent i.e., an old article not good for citing.
There is consensus among scientists and researchers that articles less than five years old are recent publications. However, it may vary from discipline to discipline. For example, researchers in fast-moving fields (e.g., nanotechnology or artificial intelligence) may feel 5-years is too old whereas those in biology may not have the same feeling.
How many recent references make a research paper contemporaneous?
Santini et al. (20018) suggested that if the most recent reference is more than 5 years or so, it can indicate that a full up to date review of the literature has not been undertaken.
However, the suggestion is a weak indicator of the comprehensiveness of the literature review done while writing a paper as it is based on the measure of only one reference.
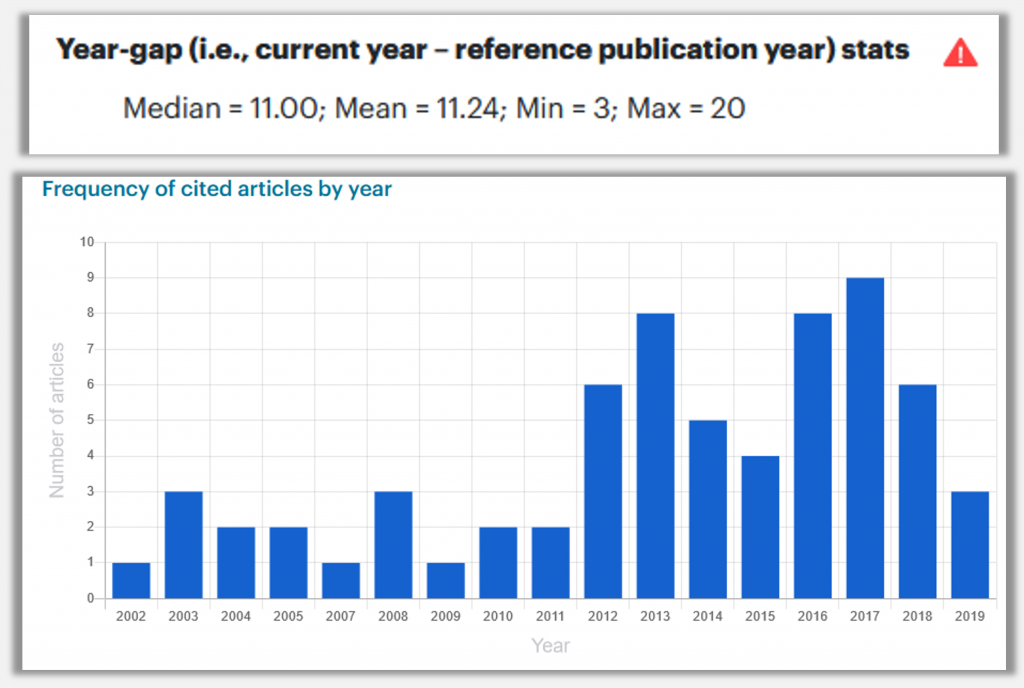
To build a robust understanding of the matter, nXr team analyzed how old references of 69 research papers (published in three highly acclaimed journals: Nature, Science, and Cell) were.

The graph clearly shows that 50% of references in the articles published in highly acclaimed journals are less than 6 years old. This indicates that well-written articles have the characteristic to cite more recent research papers.
How to get similar information for the references in the research paper you are writing?
No worries! When you cite using nXr reference manager and citation tool , nXr automatically creates a dashboard (accessible from your nXr.iLibrary) for the references in your research paper containing various data visualizations.
In one such visualization, you can see the publication year distribution of the references (see below). nXr also gives you an alert if 50% of the references are more than 5 years old so that you can check them.

- [email protected]
- Shapiro Library
- SNHU Library Frequently Asked Questions
FAQ: How old should or can a source be for my research?
- 7 Academic Integrity & Plagiarism
- 61 Academic Support, Writing Help, & Presentation Help
- 28 Access/Remote Access
- 7 Accessibility
- 8 Building/Facilities
- 6 Career/Job Information
- 25 Catalog/Print Books
- 25 Circulation
- 134 Citing Sources
- 14 Copyright
- 306 Databases
- 23 Directions/Location
- 19 Faculty Resources/Needs
- 7 Hours/Contacts
- 2 Innovation Lab & Makerspace/3D Printing
- 25 Interlibrary Loan
- 43 IT/Computer/Printing Support
- 3 Library Instruction
- 37 Library Technology Help
- 6 Multimedia
- 16 Online Programs
- 20 Periodicals
- 24 Policies
- 8 RefWorks/Citation Managers
- 4 Research Guides (LibGuides)
- 213 Research Help
- 22 University Services
Last Updated: Jul 18, 2024 Views: 130929
How old your research sources can be, using the publication date or date of creation as the defining criteria, is either stated in your assignment rubric or depends on your field of study or academic discipline. If it’s a requirement for your assignment, look for words like “sources must be published in the last 10 years” or words to that effect that specify the publication date or range required. If the currency of sources is not a requirement of your assignment, think about the course involved and what an appropriate age might be.
How fast-changing is the field of study?
Sources for a history paper might, by their very nature, be older if they are diaries, personal letters, or other documents created long ago and used as primary sources. Sources used for research in the sciences (health care, nursing, engineering), business and finance, and education and other social science fields require more “cutting edge” research, as these fields change quickly with the acquisition of new knowledge and the need to share it rapidly with practitioners in those fields.
A good rule of thumb is to use sources published in the past 10 years for research in the arts, humanities, literature, history, etc.
For faster-paced fields, sources published in the past 2-3 years is a good benchmark since these sources are more current and reflect the newest discoveries, theories, processes, or best practices.
Use the library’s Multi-Search search results page to limit your sources to those published within a date range you specify. Use the Publication Date custom setting seen on the left side of the search results page:

For further assistance with this or other search techniques, contact the Shapiro Library email at [email protected] or use our 24/7 chat service.
- Share on Facebook
Was this helpful? Yes 176 No 47
Frequently Asked Questions (FAQs) are a self-serve option for users to search and find answers to their questions.
Use the search box above to type your question to search for an answer or browse existing FAQs by group, topic, etc.
Tell Me More
Link to Question Form
More assistance.
Submit a Question
Related FAQs
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee on Health Research and the Privacy of Health Information: The HIPAA Privacy Rule; Nass SJ, Levit LA, Gostin LO, editors. Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research. Washington (DC): National Academies Press (US); 2009.

Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research.
- Hardcopy Version at National Academies Press
3 The Value, Importance, and Oversight of Health Research
The previous chapter reviewed the value of privacy, while this chapter examines the value and importance of health research. As noted in the introduction to Chapter 2 , the committee views privacy and health research as complementary values. Ideally, society should strive to facilitate both for the benefit of individuals as well as the public.
In addition to defining health research and delineating its value to individuals and society, this chapter provides an overview and historical perspective of federal research regulations that were in place long before the Privacy Rule was implemented. Because a great deal of medical research falls under the purview of multiple federal regulations, it is important to understand how the various rules overlap or diverge. The chapter also explains how the definition of research has become quite complex under the various federal regulations, which make a distinction between research and some closely related health practice activities that also use health data, such as quality improvement initiatives.
The chapter also reviews the available survey data regarding public perceptions of health research and describes the importance of effective communication about health research with patients and the public.
- CONCEPTS AND VALUE OF HEALTH RESEARCH
Definitions
Under both the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule and the Common Rule, “research” is defined as “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.” This is a broad definition that may include biomedical research, epidemiological studies, 1 and health services research, 2 as well as studies of behavioral, social, and economic factors that affect health.
Perhaps the most familiar form of health research is the clinical trial, in which patients volunteer to participate in studies to test the efficacy and safety of new medical interventions. But an increasingly large portion of health research is now information based. A great deal of research entails the analysis of data and biological samples that were initially collected for diagnostic, treatment, or billing purposes, or that were collected as part of other research projects, and are now being used for new research purposes. This secondary 3 use of data is a common research approach in fields such as epidemiology, health services research, and public health research, and includes analysis of patterns of occurrences, determinants, and natural history of disease; evaluation of health care interventions and services; drug safety surveillance; and some genetic and social studies ( Lowrance, 2002 ; Lowrance and Collins, 2007 ).
The Importance of Health Research
Like privacy, health research has high value to society. It can provide important information about disease trends and risk factors, outcomes of treatment or public health interventions, functional abilities, patterns of care, and health care costs and use. The different approaches to research provide complementary insights. Clinical trials can provide important information about the efficacy and adverse effects of medical interventions by controlling the variables that could impact the results of the study, but feedback from real-world clinical experience is also crucial for comparing and improving the use of drugs, vaccines, medical devices, and diagnostics. For example, Food and Drug Administration (FDA) approval of a drug for a particular indication is based on a series of controlled clinical trials, often with a few hundred to a few thousand patients, but after approval it may be used by millions of people in many different contexts. Therefore, tracking clinical experience with the drug is important for identifying relatively rare adverse effects and for determining the effectiveness in different populations or in various circumstances. It is also vital to record and assess experience in clinical practice in order to develop guidelines for best practices and to ensure high-quality patient care.
Collectively, these forms of health research have led to significant discoveries, the development of new therapies, and a remarkable improvement in health care and public health. 4 Economists have found that medical research can have an enormous impact on human health and longevity, and that the resulting increased productivity of the population contributes greatly to the national economy ( Hatfield et al., 2001 ; Murphy and Topel, 1999 ) in addition to the individual benefits of improved health. If the research enterprise is impeded, or if it is less robust, important societal interests are affected.
The development of Herceptin as a treatment for breast cancer is a prime example of the benefits of research using biological samples and patient records ( Box 3-1 ) ( Slamon et al., 1987 ). Many other examples of findings from medical records research have changed the practice of medicine as well. Such research underlies the estimate that tens of thousands of Americans die each year from medical errors in the hospital, and research has provided valuable information for reducing these medical errors by implementing health information technology, such as e-prescribing ( Bates et al., 1998 ; IOM, 2000b ). This type of research also has documented that disparities in health care and lack of access to care in inner cities and rural areas result in poorer health outcomes ( Mick et al., 1994 ). Furthermore, medical records research has demonstrated that preventive services (e.g., mammography) substantially reduce mortality and morbidity at reasonable costs ( Mandelblatt et al., 2003 ), and has established a causal link between the nursing shortage and patient health outcomes by documenting that patients in hospitals with fewer registered nurses are hospitalized longer and are more likely to suffer complications, such as urinary tract infections and upper gastrointestinal bleeding ( Needleman et al., 2002 ). These findings have all informed and influenced policy decisions at the national level. As the use of electronic medical records increases, the pace of this form of research is accelerating, and the opportunities to generate new knowledge about what works in health care are expanding ( CHSR, 2008 ).
Examples of Important Findings from Medical Database Research. Herceptin and breast cancer: Data were collected from a cohort of more than 9,000 breast cancer patients whose tumor specimens were consecutively received at the University (more...)
Advances in health information technology are enabling a transformation in health research that could facilitate studies that were not feasible in the past, and thus lead to new insights regarding health and disease. As noted by the National Committee on Vital and Health Statistics, “Clinically rich information is now more readily available, in a more structured format, and able to be electronically exchanged throughout the health and health care continuum. As a result, the information can be better used for quality improvement, public health, and research, and can significantly contribute to improvements in health and health care for individuals and populations” ( NCVHS, 2007a ). The informatics grid recently developed with support from the National Cancer Institute (Cancer Biomedical Informatics Grid, or caBIG) is an example of a how information technologies can facilitate health research by enabling broader sharing of health data while still ensuring regulatory compliance and protecting patient privacy ( Box 3-2 ).
caBIG (Cancer Biomedical Informatics Grid). The National Cancer Institute’s caBIG Data Sharing and Intellectual Capital Workspace’s mission is to enable all constituencies in the cancer community—including researchers, physicians, (more...)
Science today is also changing rapidly and becoming more complex, so no single researcher or single site can bring all the expertise to develop and validate medical innovations or to ensure their safety. Thus, efficient sharing of information between institutions has become even more important than in previous eras, when there were fewer new therapies introduced. The expansion of treatment options, as well as the escalating expense of new therapies, mandates greater scrutiny of true effectiveness, 5 once efficacy has been demonstrated. This requires registries of patient characteristics, outcomes, and adverse events. Large populations are required to facilitate comparison of patient populations and to calculate risk/benefit estimates. For example, INTERMACS 6 (Interagency Registry for Mechanically Assisted Circulatory Support) is a national registry for patients who are receiving mechanical circulatory support device therapy to treat advanced heart failure. This registry was devised as a joint effort of the National Heart, Lung and Blood Institute, Centers for Medicare & Medicaid Services, FDA, clinicians, scientists and industry representatives. Analysis of the data collected is expected to facilitate improved patient evaluation and management while aiding in better device development. Registry results are also expected to influence future research and facilitate appropriate regulation and reimbursement of such devices. Similarly, the Extracorporeal Life Support Organization (ELSO), 7 an international consortium of health care professionals and scientists who focus on the development and evaluation of novel therapies for support of failing organ systems, maintains a registry of extracorporeal membrane oxygenation and other novel forms of organ system support. Registry data are used to support clinical practice and research, as well as regulatory agencies. Another example is the database developed by the United Network for Organ Sharing (UNOS) for the collection, storage, analysis and publication of data pertaining to the patient waiting list, organ matching, and transplants. 8 Launched in 1999, this secure Internet-based system contains data regarding every organ donation and transplant event occurring in the United States since 1986.
Information-based research, such as research using health information databases has many advantages (reviewed by Lowrance, 2002 ). It is often faster and less expensive than experimental studies; it can analyze very large sets of data and may detect unexpected phenomena or differences among subpopulations that might not be included in a controlled experimental study; it can often be undertaken when controlled trials are simply not possible for ethical, technical, or other reasons, and it can be used to study effectiveness of a specific test or intervention in clinical practice, rather than just the efficacy as determined by a controlled experimental study. It can also reexamine data accrued in other research studies, such as clinical trials, to answer new questions quickly and inexpensively. However, information-based research does have limitations. Often it has less statistical rigor than controlled clinical studies because it lacks scientific control over the original data collection, quality, and format that prospective experimental research can dictate from the start. In addition to these scientific limitations, because of its relational and often distant physical separation from the data subjects, and the sheer volume of the records involved, obtaining individual consent for the research can be difficult or impossible.
Advances in information-based medical research could also facilitate the movement toward personalized medicine, which will make health research more meaningful to individuals. The goal of personalized medicine is to tailor prevention strategies and treatments to each individual based on his/her genetic composition and health history. In spite of the strides made in improving health through new treatments, it is widely known that most drugs are effective in only a fraction of patients who have the condition for which the drug is indicated. Moreover, a small percentage of patients are likely to have adverse reactions to drugs that are found to be safe for the majority of the population at the recommended dose. Both of these phenomena are due to variability in the patient population. Revolutionary advances in the study of genetics and other markers of health and disease are now making it possible to identify and study these variations, and are leading to more personalized approaches to health care—that is, the ability to give “the appropriate drug, at the appropriate dose, to the appropriate patient, at the appropriate time.” Achieving the goals of personalized medicine will lead to improvements in both the effectiveness and the safety of medical therapies.
Public Perceptions of Health Research
A number of studies have been undertaken to gauge the public’s attitude toward research and the factors that influence individuals’ willingness to participate in research. The surveys reviewed in this chapter focus on interventional clinical trials. A review of survey questions to gauge the public willingness to allow their medical records to be used in research can be found in Chapter 2 .
The Public Values Health Research
A number of studies suggest that most Americans have a positive view of medical research and believe that research is beneficial to society. A recent Harris poll found that nearly 80 percent of respondents were interested in health research findings, consistent with previous survey results ( Westin, 2007 ). A study in 2005 compiled data from 70 state surveys and 18 national surveys and found that the majority of Americans believe maintaining world leadership in health-related research is important. Seventy-eight percent of respondents said that it is very important, and 17 percent said that it is somewhat important. Only 4 percent of Americans reported that maintaining world leadership in health-related research is not impor tant ( Woolley and Propst, 2005 ). Similar results were found in a 2007 survey—76 percent of respondents reported that science plays a very important role in our health, and 78 percent reported that science plays a very important role in our competitiveness ( Research!America, 2007 ).
The Virginia Commonwealth University 2004 Life Sciences Survey also found that most Americans have a positive view of research. In this study, 90 percent of respondents agreed that developments in science have made society better; 92 percent reported that “scientific research is essential for improving the quality of human lives”; and 84 percent agreed that “the benefits of scientific research outweigh the harmful results” ( NSF, 2006 ).
Overall Experience When Participating in Research
Little is known about the attitudes of individuals who have actually participated in medical research. However, the available evidence suggests that most research participants have positive experiences. A recent Harris Poll found that 13 percent of respondents had participated in some form of health research, and 87 percent of those felt comfortable about their experience ( Westin, 2007 ). In a study focused on cancer, 93 percent of respondents who participated in research reported it as a very positive experience; 76 percent said they would recommend participation in a clinical trial to someone with cancer. Most physicians surveyed in this study stated that they believe clinical trial participants receive the best possible care, and have outcomes at least as good as patients receiving standard cancer treatment ( Comis et al., 2000 ). Another study found that 55 percent of individuals who participated in a research study would be willing to participate again in a future research study ( Trauth et al., 2000 ).
Willingness to Participate in Research
Public opinion surveys indicate that a majority of Americans are willing to participate in clinical research studies. In 2001, a compilation of studies commissioned by Research!America found that 63 percent of Americans would be willing to participate in a clinical research study ( Woolley and Propst, 2005 ). This percentage has remained stable over time. A 2007 Research!America survey also found that 63 percent of Americans would be very likely to participate in a clinical research study if asked ( Research!America, 2007 ); 68 percent of respondents reported that their desire to improve their own health or the health of others was a major factor in deciding whether to participate in a clinical research project ( Research!America, 2007 ).
Other surveys also suggest that willingness to participate in research focused on specific diseases is quite high. In one survey, the percentage of respondents indicating a willingness to participate in a medical research study was 88 percent for cancer, 86 percent for heart disease, 83 percent for a noncurable fatal disease, 79 percent for addiction, 78 percent for depression, and 76 percent for schizophrenia ( Trauth et al., 2000 ). Respondents with greater knowledge of how research is conducted were more willing to participate ( Trauth et al., 2000 ). Another study found that 8 of 10 Americans would consider participating in a clinical trial if faced with cancer. More than two-thirds of respondents said they would be willing to participate in a clinical trial designed to prevent cancer ( Comis et al., 2000 ).
Americans also seem to be very supportive of medical research that relies on genetic data. A 2007 survey found that 93 percent of Americans supported the use of genetic testing if the information collected is used by researchers to find new ways to diagnose, prevent, or treat disease ( Genetics & Public Policy Center, 2007 ). Two separate surveys found that 66 percent of Americans would be willing to donate their genetic material for medical research ( Genetics & Public Policy Center, 2007 ; Research!America, 2007 ). However, despite this apparent positive view of genetic research, 92 percent of Americans reported they were concerned about their genetic information being used in a “harmful way” ( Genetics & Public Policy Center, 2007 ).
Many factors, in addition to concerns about privacy and confidentiality ( Genetics & Public Policy Center, 2007 ; Research!America, 2007 ), may influence an individual’s willingness to participate in a medical research study. The Trauth survey found that individuals with higher income levels, with a college or graduate degree, or with children were more likely to participate in research. Age affected willingness to participate: 57 percent of respondents ages 18–34 were willing to participate in research, but only 31 percent of respondents ages 65 or older were willing ( Trauth et al., 2000 ).
Other factors that potentially influence an individual’s willingness to participate in research are race and ethnicity. It is well documented that minorities participate in health research at a much lower percentage than white Americans. Many cultural, linguistic, and socioeconomic barriers could be responsible for this difference ( Giuliano et al., 2000 ), and study results have been variable on this issue. Several studies suggest that the low participation rates by racial and ethnic minority groups are due to their strong distrust of the medical research community compared to the general population ( Braunstein et al., 2008 ; Corbie-Smith et al., 1999 ; Farmer et al., 2007 ; Grady et al., 2006 ; Shavers et al., 2002 ).
However, other evidence suggests that the low percentage of minorities participating in research is related to minority groups’ lack of access to the research community ( Brown et al., 2000 ; Wendler et al., 2006 ; Williams and Corbie-Smith, 2006 ). Thus, it is likely that the low number of minority individuals participating in medical research is at least partly due to recruitment techniques that are ineffective for minority populations.
The survey that focused on cancer research suggests that one of the main reasons why individuals do not participate in research is lack of knowledge about the availability of clinical trials. In a survey of nearly 6,000 cancer patients, 85 percent said they were unaware of the opportunity to participate in a clinical trial. Respondents who did participate said they did so because of one of the following beliefs: (1) trials provide access to the best quality of care (76 percent), (2) their participation would benefit future cancer patients (72 percent), (3) they would receive newer and better treatment (63 percent), and (4) participation would get them more care and attention (40 percent) ( Comis et al., 2000 ).
A recommendation from a physician can also impact participation. In the United States, 48 percent of respondents to one survey reported that a physicians’ recommendation would be a major factor in deciding whether to take part in a research study. Nearly three-fourths of respondents also cited an institution’s reputation as a key factor to consider when deciding whether to participate in a study ( Research!America, 2007 ). Twenty percent of respondents in an Italian public survey indicated that the presence of a physician as a reference during a research study influenced their willingness to participate ( Mosconi et al., 2005 ).
In sum, surveys indicate that the vast majority of Americans have a positive view of medical research, believe that research is beneficial to society, and are interested in health research findings. Although little is known about the attitudes of individuals who have actually participated in medical research, the available evidence suggests that most research participants have positive experiences. Surveys also suggest that a majority of Americans are willing to participate in clinical research studies. Similar to the findings in Chapter 2 , surveys indicate that many factors, in addition to concerns about privacy and confidentiality, can potentially influence an individual’s willingness to participate in medical research, including the type of research and personal characteristics such as health status, age, education, and race. Notably, respondents with greater knowledge of how research is conducted were more willing to participate in research.
- OVERSIGHT OF HEALTH RESEARCH
Historical Development of Federal Protections of Health Information in Research
The development of international codes, federal legislation, and federal regulation of human subjects often occurred in response to past abuses in biomedical experiments (reviewed by Pritts, 2008 ) ( Box 3-3 ). The most well-known examples included (1) reported abuses of concentration camp prisoners in Nazi experiments during World War II, and (2) the Tuskegee syphilis study begun in 1932, in which researchers withheld effective treatment from affected African American men long after a cure for syphilis was found. Most of the current principles and standards for conducting human subjects research were developed primarily to protect against the physical and mental harms that can result from these types of biomedical experiments. Therefore, they focus on the principles of autonomy and consent. Although the standards apply to research that uses identifiable health information, research based solely on information is not their primary focus.
The Basis for Human Subjects Protections in Biomedical Research. Nuremberg Code The Nuremberg Code, created by the international community after the Nazi War Crimes Trials, is generally seen as the first codification (more...)
In the United States, perhaps the most influential inquiry into the protection of human subjects in research was the Belmont Report. The Belmont principles have been elaborated on in many settings, and served as the basis for formal regulation of human subjects research in the United States. In general, states do not directly regulate the activity of most researchers ( Burris et al., 2003 ). However, the Belmont Commission’s recommendations were reflected in the Department of Health and Human Services’ (HHS’s) Policy for Protection of Human Subjects Research, Subpart A of 45 C.F.R. 46 (“Subpart A”) in 1979. 9 These protections were considered a benchmark policy for federal agencies, and in December 1981, the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research recommended 10 that all federal departments and agencies adopt the HHS regulations. 11
In 1982, the President’s Office of Science and Technology Policy appointed a Committee for the Protection of Human Research Subjects to respond to the recommendations of the President’s commission. The committee agreed that uniformity of federal regulations on human subjects protection is desirable to eliminate unnecessary regulations and to promote increased understanding by institutions that conduct federally supported or regulated research. As a result, in 1991, other federal departments and agencies joined HHS in adopting a uniform set of rules for the protection of human subjects of research, identical to Subpart A of 45 C.F.R. 46, which is now informally known as the “Common Rule.” Eighteen federal agencies have now adopted the Common Rule as their own respective regulations.
Overview of the Common Rule
The Common Rule governs most federally funded research conducted on human beings and aims to ensure that the rights of human subjects are protected during the course of a research project. The Common Rule stresses the importance of individual autonomy and consent; requires independent review of research by an Institutional Review Board (IRB); and seeks to minimize physical and mental harm. Privacy and confidentiality protections, although not defined in a detailed and prescriptive manner, are included as important components of risk in research.
The framework for achieving the goal of protecting human subjects is based on two foundational requirements: the informed consent of the research participant and the review of proposed research by an IRB. This section describes some of the basic parameters of the Common Rule (reviewed by Pritts, 2008 ). Particular provisions that interact with the HIPAA Privacy Rule are described in more detail in Chapter 4 .
Scope of the Common Rule
In general, the Common Rule applies only to research on human subjects that is supported by the federal government. 12 As noted previously, research is defined as “a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.” 13
Under the Common Rule, a “human subject” is defined as “a living individual about whom an investigator … conducting research obtains (1) Data through intervention or interaction with the individual, or (2) Identifiable private information.” Private information is considered to be personally identifiable if the identity of the subject is or may readily be ascertained by the investigator or associated with the information.
The Common Rule applies to most human subjects research conducted using federal funds, but its influence is broader because most institutions that accept federal funds sign an agreement (a Federalwide Assurance or FWA) with HHS to abide by the Common Rule requirements in all research, regardless of funding source. Nonetheless, some privately funded human subjects research is conducted outside the purview of federal regulation ( Goldman and Choy, 2001 ; Williams, 2005 ). Companies and other organizations may voluntarily choose to apply the Common Rule to their research projects, and many do. However, research projects in which compliance is voluntary are not subject to oversight or disciplinary action by HHS ( Goldman and Choy, 2001 ; Williams, 2005 ).
Informed Consent 14
The Common Rule requires that a researcher obtain informed consent (usually in writing) from a person before he/she can be admitted to a study ( Williams, 2005 ). Informed consent is sought through a process in which a person learns key facts about a research study, including the potential risks and benefits, so that he/she can then agree voluntarily to take part or decide against it.
The Common Rule informed consent regulations focus primarily on the elements and documentation of informed consent rather than on the process used to obtain it. As to the process, the regulations require that informed consent be sought only under circumstances that provide the prospective subject with adequate opportunity to consider whether to participate. The Common Rule requires that information pertaining to informed consent be given in language understandable to the subject, and that the consent does not imply that the subject is giving up his/her legal rights or that the investigator is released from liability for negligence during the conduct of the study. 15
The Common Rule also specifies a number of elements that must be provided when informed consent is sought. These elements include:
- an explanation of the purposes of the research,
- the expected duration of the subject’s participation,
- the potential risks and benefits of the research,
- how confidentiality will be maintained,
- the fact that participation is strictly voluntary, and
- who the subject can contact to answer questions about the study or about his/her rights as a research participant.
In certain limited circumstances, the Common Rule allows an informed consent to be for unspecified future research. For example, under the Common Rule an informed consent can be used to obtain a person’s permission to study personally identifiable information maintained in a repository for future, unspecified research purposes ( HHS, 2003 ).
For the most part, the required elements of an informed consent address all types of research, although some are more relevant to biomedical research (e.g., the consent must include a disclosure of appropriate alternative procedures or courses of treatment, if any, that might be advantageous to the subject). One required element of informed consent is particularly relevant to research involving personally identifiable health information. The Common Rule requires an informed consent to include a statement describing the extent, if any, to which confidentiality of records identifying the subject will be maintained. 16
Institutional Review Boards
Adopting the principles of the Belmont Report, the Common Rule requires that protocols for human subjects research be reviewed by an IRB ( Box 3-4 ) before research may begin. 17 The IRB must meet certain membership requirements, including having members with different expertise and at least one member who is not affiliated with the investigator’s institution. The Common Rule specifies which level of IRB review is needed for various types of research and provides criteria for the IRB to consider during the review. Although the Common Rule does not specify the procedures an IRB must follow in its review of protocols, it does require the IRB to have written procedures for how it will review protocols and document IRB decisions.
Institutional Review Boards. According to the Department of Health and Human Services (HHS) Institutional Review Board (IRB) guidebook, “the IRB is an administrative body established to protect the rights and welfare of human research subjects (more...)
The Common Rule requires that an IRB determine the following factors are satisfied to approve proposed research:
- Risks to subjects are minimized;
- Risks to subjects are reasonable in relation to anticipated benefits, if any, to subjects, and the importance of the knowledge that may reasonably be expected to result;
- The selection of subjects is equitable;
- Informed consent will be sought in accordance with the rules and will be documented;
- When appropriate, the research plan makes adequate provision for monitoring the data collected to ensure the safety of subjects; and
- When appropriate, adequate provisions are in place to protect the privacy of subjects and to maintain the confidentiality of data. 18
An IRB may waive the requirement to obtain informed consent or approve an alteration of the consent form for some minimal risk research. The IRB may also waive the requirement for signed consent in certain circumstances. 19
Anonymized Data
As noted above, the Common Rule considers use of “private identifiable information” to be human subjects research. Data are considered personally identifiable if the identity of the subject is or may be readily ascertained by the investigator or associated with the information accessed by the researcher. 20 However, the Common Rule exempts from its requirements research that involves:
[T]he collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. 21
Otherwise identifiable data may be deidentified or “anonymized” for purposes of the Common Rule if it is coded and certain other conditions are met ( HHS, 2004 ). Under Guidance issued by the Office for Human Research Protection, information is “coded” if identifying information (such as name or Social Security number) that would enable the investigator to readily ascertain the identity of the individual to whom the private information or specimens pertain has been replaced with a number, letter, symbol, or combination thereof (the code), and a key to decipher the code exists, enabling linkage of the identifying information to the private information or specimen.
Research involving only coded private information or specimens is not considered to involve human subjects under the Common Rule if the following conditions are met:
- The private information or specimens were not collected specifically for the currently proposed research project through an interaction or intervention with living individuals; and
- —The key to decipher the code is destroyed before the research begins;
- —The investigators and the holder of the key enter into an agreement prohibiting the release of the key to the investigators under any circumstances, until the individuals are deceased;
- —IRB-approved written policies and operating procedures for a repository or data management center prohibit the release of the key to investigators under any circumstances, until the individuals are deceased; or
- —Other legal requirements prohibit the release of the key to the investigators, until the individuals are deceased.
Under this standard, when a researcher accesses or receives data that have been coded and does not have access to the identifying key, the research is not considered human subjects research and is not subject to the Common Rule’s requirements of informed consent or IRB review and approval of protocol.
Enforcement of the Common Rule
The Common Rule requirements for informed consent do not preempt any applicable federal, state, or local laws that require additional information to be disclosed to a subject in order for informed consent to be legally effective. 22
Federal funding can be suspended or withdrawn from an institution when it is found to be in material violation of the Common Rule. 23 There is no authority to impose penalties directly on individual researchers for violations. Neither does the Common Rule expressly provide a research participant with a private right of action. It should be noted, however, that recent cases indicate that courts may be willing to hold an institution liable under common law negligence theories where the approved informed consent form is determined to be less than adequate ( Shaul et al., 2005 ). 24
FDA Protection of Human Research Subjects
Some health research is also subject to FDA regulations. The FDA is charged by statute with ensuring the protection of the rights, safety, and welfare of human subjects who participate in clinical investigations 25 involving articles subject to the Federal Food, Drug, and Cosmetic Act 26 (the Act), as well as clinical investigations that support applications for research or marketing permits for products regulated by the FDA, including drugs, medical devices, and biological products for human use ( Box 3-5 ).
FDA Protection of Human Subjects Regulations. The Food and Drug Administration (FDA) Protection of Human Subjects Regulations aim to protect the rights of human subjects enrolled in research involving products that the FDA regulates (i.e., drugs, medical (more...)
In January 1981, the FDA adopted regulations governing informed consent of human subjects 27 and regulations establishing standards for the composition, operation, and responsibilities of IRBs that review clinical investigations involving human subjects. 28 At the same time, HHS adopted the Common Rule regulations on the protection of human research subjects. 29 The FDA’s regulations were harmonized with the Common Rule in 1991 to the extent permitted by statute. Key differences between FDA and HHS regulations include that the FDA does not allow for waiver or alteration of informed consent and requires that subjects be informed that the FDA may inspect their medical records. In addition, studies of efficacy based solely on medical records research are not permitted to support registration. Remaining differences in the rules are due to differences in the statutory scope or requirements ( Lee, 2000 ).
- DISTINGUISHING HEALTH RESEARCH FROM PRACTICE
The Common Rule and Privacy Rule make a somewhat artificial distinction between health research and some closely related health care practices, such as public health practice, quality improvement activities, program evaluations, 30 and utilization reviews, 31 all of which may involve collection and analysis of personally identifiable health information. However, determining which activities meet the definition of “research” is a major challenge for IRBs, Privacy Boards, 32 investigators, and health care practitioners because neither the regulations nor their interpretations by HHS provide clear guidance on how to distinguish research from activities that use similar techniques to analyze health information ( IOM, 2000a ).
It is important for IRBs and Privacy Boards to correctly distinguish among activities that are or are not subject to the various provisions of the Privacy Rule and the Common Rule. Only research requires formal IRB or Privacy Board review and informed consent. 33 Inappropriate classification of an activity as research can make it difficult or impossible for important health care activities, such as public health practice and quality improvement, to be undertaken. On the other hand, failure to correctly identify an activity as research could potentially allow improper disclosure of personally identifiable health information without sufficient oversight.
Thus, standard criteria are urgently needed for IRBs and Privacy Boards to use when making distinctions between health research and related activities, and the committee recommends that HHS consult with relevant stake holders to develop such standard criteria. HHS is aware of this need, and created a working document titled “What Is Research?” However, the work on this project apparently has been delayed for unknown reasons ( NCURA, 2007 ). 34 As described below, a number of other models have already been proposed to help determine whether activities should be classified as research in the fields of public health and quality improvement, and these could be instructive for developing HHS guidance. Any criteria adopted by HHS should be regularly evaluated to ensure that they are helpful and producing the desired outcomes.
The following sections describe some ongoing efforts to develop such criteria in the fields of public health and quality improvement. The intent of the committee is not to endorse these particular models, but rather to illustrate the challenges associated with making these distinctions and establishing standard criteria.
Public Health Practice Versus Public Health Research
The Belmont Report defined health practice as “interventions designed solely to enhance the well-being of the person, patient or client, and which have reasonable expectation of success” ( CDC, 1999 ). To apply this definition to “public” health practice, the targeted beneficiary of the intervention must be expanded to include benefit to the community, rather than just a particular person. Neither the Common Rule nor the Privacy Rule provides a specific definition for public health research; rather public health research is included in the general definition of research. However, the Privacy Rule regulates public health practice differently from public health research (see Chapter 4 ).
An early model for distinguishing public health research from public health practice focused on the intent for which the activity was designed, noting that the intent of public health research is to “contribute to or generate generalizable knowledge,” while the intent of public health practice is to “conduct programs to prevent disease and injury and improve the health of communities” ( Snider and Stroup, 1997 ). The Centers for Disease Control and Prevention developed a similar method with an expanded assessment of intent. For example, the model posits that in public health research, the intended benefits of the project extend beyond the study participants, and the data collected exceed the requirements for the care of the study participants. But for public health practice, the intended benefits of the project are primarily for the participants in the activity, or for the participants’ community, and the only data collected are those needed to assess or improve a public health program or service, or the health of the participants and their community. The model also assumes that public health practice is based on well-established medical interventions and is nonexperimental ( CDC, 1999 ). However, these models both have been criticized as too subjective and too dependent on the opinion of the person conducting the activity ( Gostin, 2008 ; Hodge, 2005 ).
A new, more comprehensive model incorporating much of the previous two was recently proposed as a more objective checklist to be used by IRBs, Privacy Boards, and interested parties ( Hodge, 2005 ; Hodge and Gostin, 2004 ). The foundations for this model are specific definitions of public health research: “the collection and analysis of identifiable health data by a public health authority for the purpose of generating knowledge that will benefit those beyond the participating community who bear the risks of participation,” and public health practice: “the collection and analysis of identifiable health data by a public health authority for the purpose of protecting the health of a particular community, where the benefits and risks are primarily designed to accrue to the participating community.”
The model is based on two primary assumptions. First, the actor performing the activity in question is a governmental public health official, agent, agency, or entity at the federal, tribal, state, or local level. Second, the activity in question involves the acquisition, use, or disclosure of personally identifiable health data. The model is then divided into two stages. Stage 1 is applied to all activities, and can be used to distinguish practice from research in the easiest cases. Stage 2 is only applied to those cases that are hard to distinguish, and where Stage 1 failed to lead to a definitive IRB/Privacy Board decision ( Box 3-6 ).
A Model for Distinguishing Public Health Practice from Research. Stage 1 Public health practice:
Quality Improvement Versus Health Research
Quality improvement has been defined as “systematic, data-guided activities designed to bring about immediate, positive change in the delivery of health care in a particular setting” ( Baily, 2008 ). Quality improvement activities do not require IRB or Privacy Board approval under the Common Rule or the Privacy Rule, which classify quality improvement as a component of health care operations. 35
However, in many cases, it is difficult for health care providers, IRBs, and Privacy Boards to determine whether a particular activity is purely for quality improvement, or whether it also entails research. One survey 36 exploring opinions in the health care community about the need for IRBs to review various quality-related activities found that physicians conducting quality improvement were less likely than IRB chairs to believe that IRB review was required for a given hypothetical activity, or that informed consent was necessary ( Lindenauer et al., 2002 ). Recently, a highly publicized case has again brought the issue to the forefront for all the stakeholders ( Box 3-7 ).
A Case Study of Quality Improvement and Research. Peter Pronovost of Johns Hopkins University (JHU) led a quality improvement effort at 103 intensive care units (ICUs) in Michigan hospitals to reduce the number of catheter-related bloodstream infections. (more...)
Some members of the health care community have proposed requiring that all prospective quality improvement activities go through external review ( Bellin and Dubler, 2001 ), while others have outlined specific criteria to differentiate quality improvement activities from research.
For example, Casarett and colleagues developed a two-part test to identify quality improvement activities. The first test is whether the majority of patients are expected to benefit directly from “the knowledge to be gained” from the initiative. This means that the patients must actually benefit from the knowledge learned during the evaluation, not just from being a recipient of the protocol itself. If the patients are generally expected to directly benefit from the knowledge gained during the activity, then the activity is quality improvement. If not, the activity is research. The second test is whether the participants would be subjected to additional risks or burdens, including the risk of privacy breach, beyond the usual clinical practice in order to make the results of the initiative generalizable. If yes, then the initiative should be reviewed as research ( Casarett et al., 2000 ).
More recently, the Hastings Center published a report exploring the similarities and differences between research and quality improvement. The report emphasized three fundamental characteristics of quality improvement and three fundamental characteristics of research. The authors argue that individuals have a responsibility to participate in the quality improvement activities because all patients have an interest in receiving high-quality medical care, and the success of a quality improvement activity depends on the cooperation of all patients. In addition, the report notes that quality improvement activities are a low risk to the patient, so there is little justification for not participating. The report also assumes that quality improvement activities are based on existing knowledge about human health and should lead to immediate local improvements in the provision of medical care.
In contrast, the report notes that participation in research should be voluntary, and decisions to participate should be based on researchers’ full disclosure of all the potential risks and benefits. In addition, the authors assert that research is designed to create new knowledge about human health, rather than relying solely on existing knowledge, and that most research does not result in any direct benefit to the institution where the research is being conducted.
The authors concluded that IRBs are not the appropriate body for the ethical oversight of quality improvement activities. They argue that IRBs unnecessarily impose high transaction costs on these activities because of the difference in the way they are conducted compared to research. For example, in research, any changes in methodology require further IRB approval. In contrast, quality improvement activities involve frequent adjustments in the intervention, measurement, and goals of the activity based on the experience of the investigators. Requiring the investigator to revisit an IRB every time a small adjustment is needed in such an activity significantly increases the amount of time and effort required to conduct the initiative and to produce meaningful data. Also, the investigators involved in quality improvement activities ordinarily are already involved in the clinical care of participants and bear responsibility for the quality and safety of an intervention. Thus, the authors argue that there is no need for the additional oversight by an IRB to protect participant safety.
Rather, the report recommended integrating the ethical oversight of quality improvement activities into the ongoing management of an institution’s health care delivery system, suggesting that oversight of quality improvement could be left with the managers of clinical care organizations, and that consent to receive treatment should include consent to participate in any quality improvement project that is minimal risk. However, the report stated that if a project has the characteristics of both quality improvement and research, the project should be reviewed as both human subjects research and quality improvement ( Baily et al., 2006 ; Lynn et al., 2007 ).
In response to the ongoing confusion over when quality improvement rises to the level of research and requires IRB review, the IOM jointly hosted a meeting with the American Board of Internal Medicine in May 2008 to discuss this issue. Key members of the quality improvement community attended, and short- and long-term solutions to this problem were proposed. However, no written report from this meeting was produced and no general consensus was reached.
- THE IMPORTANCE OF EFFECTIVE COMMUNICATION WITH THE PUBLIC
As noted previously in this chapter, surveys indicate that the vast majority of Americans believe that health research is important and are interested in the findings of research studies. The majority of patients also appear to be willing to participate in health research, either by volunteering for a study to test a medical intervention or by allowing access to their medical records or stored biospecimens, under certain conditions. Their willingness to participate depends on trust in researchers to safeguard the rights and well-being of patients, including assurance of privacy and confidentiality, and the belief that it is a worthwhile endeavor that warrants their involvement. Yet patients often lack information about how research is conducted, and are rarely informed about research results that may have a direct impact on their health. The committee’s recommendations in this section are intended to address both the public’s desire for more information about health research and to help fulfill two of the committees overarching goals of the report: (1) improving the privacy and security of health information, and (2) improving the effectiveness of health research.
Disseminating Health Research Results
Ethicists have long suggested greater community involvement in health research studies, including more communication about research results (reviewed by Shalowitz and Miller, 2008a , b ). In addition, the IOM committee identified transparency—the responsibility to disclose clearly how and why personally identifiable information is being collected—as an important component of comprehensive privacy protections. A previous IOM report also recommended improved communication with the public and research participants to ensure that the protection process is open and accessible to all interested parties ( IOM, 2002 ). Effective communication would build the public’s trust of the research community and is consistent with the principles of fair information practices.
When patients consent to the use of their medical records in a particular study, health researchers should make greater efforts at the conclusion of the study to inform study participants about the results, and the relevance and importance of those results. Learning about clinically relevant findings from a study in which a patient has participated could make patients feel more integrated into the process and could encourage more to participate in future studies. A recent United Kingdom report on the use of personal data in health research concluded that public involvement in research is necessary for the success of information-based research, and that a public informed about the value of research is likely to have greater enthusiasm and confidence in research and the research community ( AMS, 2006 ). Moreover, direct feedback with study participants could lead to improved health care for the individuals if the results indicate that an altered course of care is warranted.
Nonetheless, there are multiple impediments, beyond cost, to providing meaningful feedback to participants. A summary of the results alone, while necessary and reasonable, can be seen as a token, and also raises questions about issues such as how best to write summaries, the stage at which results should be disseminated, and how to present research with uninformative outcomes. For example, one recent study found that sharing results directly with study participants was met with overwhelmingly favorable reactions from patients, but the study also revealed some obstacles ( Partridge et al., 2008 ). In a survey of women who had participated in a randomized trial of breast cancer therapy and had received a summary of the study results by mail, 95 percent reported that they were glad they received the results. Most respondents interpreted the results correctly, although incorrect interpretation of the results was associated with increased anxiety, as was dissatisfaction with treatment.
Although some guidelines for providing and explaining study results to research participants have been proposed, they differ in details because limited data are available on this subject, and thus standards are lacking ( Partridge and Winer, 2002 ; Partridge et al., 2008 ; Shalowitz and Miller, 2008b ; Zarin and Tse, 2008 ). Because transparency is best achieved by providing graded levels of information and guidance to interested parties ( IOM, 2002 ), it will be important to develop effective and efficient ways to communicate with various sectors of the population. A commitment to the principles of “plain language” 37 will be important. Broader adoption of electronic medical records may also be helpful in accomplishing this goal.
Research Registries
One way to make information about research studies more broadly available to the public is through registration of trials and other studies in public databases. HHS should encourage such registration of trials and other studies, particularly when research is conducted with an IRB/Privacy Board approved waiver of consent or authorization (see Chapter 4 ). Numerous clinical trial registries already exist, and registration has increased in recent years (reviewed by Zarin and Tse, 2008 ). In 2000, the National Library of Medicine established a clinical trials registry ( ClinicalTrials.gov ), which has expanded to include information from several other trial registries and to serve as the FDA-required site for submissions about clinical trials subject to the FDA databank requirement. The FDA Amendments Act of 2007 38 expanded the scope of required registrations at ClinicalTrials.gov and provided the first federally funded trials results database. It mandates registrations of controlled clinical investigations, except for Phase I trials, of drugs, biologics, and devices subject to FDA regulation.
A policy of the International Committee of Medical Journal Editors (ICMJE), adopted in fall 2005, also requires prospective trial registration as a precondition for publication ( DeAngelis et al., 2004 ). This policy led to a 73 percent increase in trial registrations of all intervention types from around the world ( Zarin et al., 2005 ). Nearly 45,000 trials had been registered by fall 2007.
However, although the development of such registries is an important first step toward providing high-quality clinical trial information to the public, no centralized system currently exists to disseminate information about clinical trials of drugs or other interventions, making it difficult for consumers and their health care providers to identify ongoing studies. The current statutory requirements for registration and data reporting in the United States are not as broad as the transnational policies of the ICMJE or the World Health Organization, which call for the registration of all interventional studies in human beings regardless of intervention type ( Laine et al., 2007 ; Sim et al., 2006 ). Moreover, noninterventional studies, such as observational studies that play an increasingly critical role in biomedical research, are not generally included in these databases. Because many noninterventional studies are conducted with an IRB/Privacy Board approved waiver of consent or authorization, including those studies in a registry could be an important method for increasing public knowledge of such studies.
Informing the Public About the Methods and Value of Research
As noted previously, clinical trials are the most visible of the various types of health research, but a great deal of information-based health research entails analysis of thousands of patient records to better understand human diseases, to determine treatment effectiveness, and to identify adverse side effects of therapies. This form of research is likely to increase in frequency as the availability of electronic records continues to expand. As we move toward the goal of personalized medicine, research results will be even more likely to be directly relevant to patients, but more study subjects will be necessary to derive meaningful results.
However, many patients probably are not aware that their medical records are being used in information-based research. For example, the recent study that used focus groups to examine the views of veterans toward the use of medical records in research found that the majority of participants (75 percent) were not aware that “under some circumstances, [their] medical records could be used in some research studies without [their] permission,” despite the fact that a notice of privacy practices, which included a statement that such research could occur, had been mailed to all participants less than a year prior to the study ( Damschroder et al., 2007 ).
Moreover, surveys show that many patients desire not only notice, but also the opportunity to decide whether to consent to such research with medical records. Those surveys further indicate that patients who wish to be asked for consent for each study are most concerned about the potentially detrimental affects of inappropriate disclosure of their personally identifiable health information, including discrimination in obtaining health or life insurance or employment.
As noted in Chapter 2 , strengthening security protections of health data should reduce the risk of security breaches and their potential negative consequences, and thus should help to alleviate patient concerns in this regard. But educating patients about how health research is conducted, monitored, and reported on could also help to ease patient concerns about privacy and increase patients’ trust in the research community, which as noted above is important for the public’s continued participation in health research. For example, datasets are most often provided to researchers without direct identifiers such as name and Social Security number. Furthermore, identifiers are not included in publications about research results. Also, under both the Privacy Rule and the Common Rule, a waiver of consent and authorization is possible only under the supervision of an IRB or Privacy Board, and a waiver is granted only when the research entails minimal risk and when obtaining individual consent and authorization is impracticable (see the previous section and also Chapter 4 ). Finally, professional ethics dictate that researchers safeguard data and respect privacy.
Conveying the value of medical records research to patients will be important. Surveys show that people are more supportive of research that is relevant to them and their loved ones. At the same time, educational efforts should stress the negative impact of incomplete datasets on research findings. Representative samples are essential to ensure the validity and generalizability of health research ( Box 3-8 ), but datasets will not represent the entire population if some people withhold access to their health information.
Selection Bias in Health Research. When researchers are required to obtain consent or authorization to access each individual’s medical record for a research study, it is likely that individuals’ willingness to grant access will not be (more...)
In addition, an educated public could also decrease the potential for biased research samples. A universal requirement for consent or authorization in medical records research leads to incomplete datasets, and thus to biased results and inaccurate conclusions. Some large medical institutions with a strong research history and reputation (e.g., Mayo Clinic) can obtain authorization and consent rates as high as 80 percent, but the 20 percent who refuse have distinct demographic and health characteristics. In fact, even a refusal rate of less than 5 percent can create selection bias in the data ( Jacobsen et al., 1999 ; see Chapter 5 for more detail). Conveying to the public the importance of health care improvements derived from medical records research and stressing the negative impact of incomplete datasets on research findings may increase the public’s participation in research and their willingness to support information-based research that is conducted with IRB or Privacy Board oversight, under a waiver of patient consent or authorization.
Numerous examples of important research findings from medical records research would not have been possible if direct patient consent and authorization were always required ( Box 3-1 ). For example, analysis of medical records showed that infants exposed to diethylstilbesterol (DES) during the first trimester of pregnancy had an increased risk of breast, vaginal, and cervical cancer as well as reproductive anomalies as adults. Similarly, studies of medical records led to the discovery that folic acid supplementation during pregnancy can prevent neural tube defects.
Thus, HHS and the health research community should work to edu cate the public about how research is done and the value it provides. All stakeholders, including professional organizations, nonprofit funders, and patient organizations, have different interests and responsibilities to make sure that their constituencies are well informed. For example, the American Society of Clinical Oncology and the American Heart Association already have some online resources to help patients gather information about research that may be relevant to their conditions. But coordination and identification of best practices by HHS would be helpful, and research is needed to identify which segments of the population would be receptive to and benefit from various types of information about how research is done and its value in order to create and implement an effective plan.
Greater use of community-based participatory research, in which community-based organizations or groups bring community members into the research process as partners to help design studies and disseminate the knowledge gained, 39 could help achieve this goal. These groups help researchers to recruit research participants by using the knowledge of the community to understand health problems and to design activities that the community is likely to value. They also inform community members about how the research is done and what comes out of it, with the goal of providing immediate community benefits from the results when possible.
- CONCLUSIONS AND RECOMMENDATIONS
Based on its review of the information described in this chapter, the committee agreed on a second overarching principle to guide the formation of recommendations. The committee affirms the importance of maintaining and improving health research effectiveness. Research discoveries are central to achieving the goal of extending the quality of healthy lives. Research into causes of disease, methods for prevention, techniques for diagnosis, and new approaches to treatment has increased life expectancy, reduced infant mortality, limited the toll of infectious diseases, and improved outcomes for patients with heart disease, cancer, diabetes, and other chronic diseases. Patient-oriented clinical research that tests new ideas makes rapid medical progress possible. Today, the rate of discovery is accelerating, and we are at the precipice of a remarkable period of investigative promise made possible by new knowledge about the genetic underpinnings of disease. Genomic research is opening new possibilities for preventing illness and for developing safer, more effective medical care that may eventually be tailored for specific individuals. Further advances in relating genetic information to predispositions to disease and responses to treatments will require the use of large amounts of existing health-related information and stored tissue specimens. The increasing use of electronic medical records will further facilitate the generation of new knowledge through research and accelerate the pace of discovery. These efforts will require broad participation of patients in research and broad data sharing to ensure that the results are valid and applicable to different segments of the population. Collaborative partnerships among communities of patients, their physicians, and teams of researchers to gain new scientific knowledge will bring tangible benefits for people in this country and around the world.
Surveys indicate that the majority of Americans believe that health research is important, are interested in the findings of research studies, and are willing to participate in health research. But patients often lack information about how research is conducted and are rarely informed about research results that may have a direct impact on their health. Effective communication could build the public’s trust of the research community, which is important because trust is necessary for the public’s continued participation in research. Moreover, direct feedback could lead to improved health care for study participants if the results indicate that an altered course of care is warranted.
Thus, the committee recommends that when patients consent to the use of their medical records in a particular study, health researchers should make greater efforts when the study ends to inform study participants about the results, and the relevance and importance of those results. Broader adoption of electronic health records may be helpful in accomplishing this goal, but standards and guidelines for providing and explaining study results to research participants or various sectors of the public are needed.
HHS should also encourage registration of trials and other studies in public databases, particularly when research is conducted with an IRB/Privacy Board approved waiver of consent or authorization, as a way to make information about research studies more broadly available to the public. Numerous clinical trial registries already exist, and registration has increased in recent years, but no centralized system currently exists for disseminating information about clinical trials of drugs or other interventions, making it difficult for consumers and their health care providers to identify ongoing studies. Moreover, noninterventional studies, such as observational studies that play an increasingly critical role in biomedical research, are not generally included in these databases. Because many noninterventional studies are conducted with an IRB/Privacy Board approved waiver of consent or authorization, including such studies in a registry could be an important method for increasing public knowledge of those studies.
Interventional clinical trials are the most visible of the various types of health research, but a great deal of information-based health research entails analysis of thousands of patient records to better understand human diseases, to determine treatment effectiveness, and to identify adverse side effects of therapies. This form of research is likely to increase in frequency as the availability of electronic health records continues to expand. As we move toward the goal of personalized medicine, research results will be even more likely to be directly relevant to patients, but more study participants will be necessary to derive meaningful results.
However, many patients are likely not aware that their medical records are being used in information-based research, and surveys show that many patients desire not only notice, but also the opportunity to decide about whether to consent to such research with medical records. As noted in Chapter 2 , strengthening security protections of health data should reduce the risk of security breaches and their potential negative consequences, and thus should help to alleviate patient concerns in this regard. But educating patients about how health research is conducted, monitored, and reported could also increase patients’ trust in the research community. Thus, HHS and the health research community should work to educate the public about how research is done.
It will also be important for HHS and researchers to convey the value of health care improvements derived from medical records research, and to stress the negative impact of incomplete datasets on research findings. Representative samples are essential to ensure the validity and generalizability of health research, but datasets will not be representative of the entire population if some people withhold access to their health information. A universal requirement for consent or authorization in information-based research may lead to incomplete datasets, and thus to biased results and inaccurate conclusions. Numerous examples of important research findings from medical records research would not have been possible if direct patient consent and authorization were always required.
To ensure that beneficial health research and related activities continue to be undertaken with appropriate oversight under federal regulations, it will be important for HHS to also provide more guidance on how to distinguish the various activities. The Privacy Rule makes a distinction between health research and some closely related endeavors, such as public health and quality improvement activities, which also may involve collection and analysis of personally identifiable health information. Under the Privacy Rule (as well as the Common Rule), these activities, which aim to protect the public’s health and improve the quality of patient care, are considered health care “practice” rather than health research. Therefore, they can be undertaken without consent or authorization, or an IRB/Privacy Board waiver of consent or authorization. However, it can be a challenge for IRBs and Privacy Boards to distinguish among activities that are or are not subject to the various provisions of the Privacy Rule and the Common Rule, and inappropriate decisions may prevent important activities from being undertaken or could potentially allow improper disclosure of personally identifiable health information.
To address these difficulties, a number of models have been proposed that outline the criteria IRBs and Privacy Boards should use to distinguish practice and research. For example, one recent model provides a detailed checklist for IRBs and Privacy Boards to use in determining whether an activity is public health research and required to comply with the research provisions of the Privacy Rule, or public health practice that does not need IRB/Privacy Board review. The committee believes that standardizing the criteria is essential to support the conduct of these important health care activities.
Thus, HHS should convene the relevant stakeholders to develop standard criteria for IRBs and Privacy Boards to use when making decisions about whether protocols entail research or practice. There should be flexibility in the regulation to allow important activities to go forward with appropriate levels of oversight. Also, it will be important to evaluate whether these criteria are effective in aiding IRB/Privacy Board reviews of proposed protocols, and whether they lead to appropriate IRB/Privacy Board decisions.
These changes suggested above could be accomplished without any changes to HIPAA by making them a condition of funding from HHS and other research sponsors and by providing some additional funds to cover the cost.
- AMS (Academy of Medical Sciences). Personal data for public good: Using health information in medical research. 2006. [accessed August 28, 2008]. http://www .acmedsci.ac .uk/images/project/Personal.pdf .
- Baily MA. Harming through protection? New England Journal of Medicine. 2008; 258 (8):768–769. [ PubMed : 18287599 ]
- Baily MA, Bottrell M, Lynn J, Jennings B. The ethics of using QI methods to improve health care quality and safety. A Hastings Center Special Report. 2006; 36 (4):S1–S40. [ PubMed : 16898359 ]
- Bates DW, Leape LL, Cullen DJ, Laird N, Petersen LA, Teich JM, Burdick E, Hickey M, Kleefield S, Shea B, Vander VM, Seger DL. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998; 280 (15):1311–1316. [ PubMed : 9794308 ]
- Bellin E, Dubler NN. The quality improvement-research divide and the need for external oversight. American Journal of Public Health. 2001; 91 (9):1512–1517. [ PMC free article : PMC1446813 ] [ PubMed : 11527790 ]
- Big Health. Big health consortium. 2008. [accessed October 29, 2008]. http://www .bighealthconsortium .org/about/
- Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine. 2008; 87 (1):1–9. [ PubMed : 18204365 ]
- Brown DR, Fouad MN, Basen-Engquist K, Tortolero-Luna G. Recruitment and retention of minority women in cancer screening, prevention and treatment trials. Annals of Epidemiology. 2000; 10 :S13–S21. [ PubMed : 11189088 ]
- Burris S, Gable L, Stone L, Lazzarini Z. The role of state law in protecting human subjects of public health research and practice. Journal of Law, Medicine & Ethics. 2003; 31 :654. [ PubMed : 14968667 ]
- Casarett D, Karlawish J, Sugarman J. Determining when quality improvement initiatives should be considered research: Proposed criteria and potential implications. JAMA. 2000; 284 (7):2275–2280. [ PubMed : 10807388 ]
- Casarett D, Karlawish J, Andrews E, Caplan A. Bioethical issues in pharmacoepidemiological research. In: Strom BL, editor. Pharmacoepidemiology. West Sussex, England: John Wiley & Sons, Ltd.; 2005. pp. 417–432.
- CDC (Centers for Disease Control and Prevention). Guidelines for defining public health research and public health non-research. 1999. [accessed March 4, 2008]. http://www .cdc.gov/od /science/regs/hrpp/researchdefinition .htm .
- CHSR (Coalition for Health Services Research). Framework for health services research policy for 2008. 2008. [accessed August 21, 2008]. http://www .chsr.org/Policy_Priorities .pdf .
- Comis RL, Aldige CR, Stovall EL, Krebs LU, Risher PJ, Taylor HJ. A quantitative survey of public attitudes towards cancer clinical trials. Philadelphia, PA: Coalition of National Cancer Cooperative Groups, Cancer Research Foundation of America, Cancer Leadership Council, and Oncology Nursing Society; 2000.
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans towards participation in medical research. Journal of General Internal Medicine. 1999; 14 :537–546. [ PMC free article : PMC1496744 ] [ PubMed : 10491242 ]
- Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: Patients’ willingness to allow researchers to access their medical records. Social Science & Medicine. 2007; 64 (1):223–235. [ PubMed : 17045717 ]
- DeAngelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, Kotzin S, Laine C, Marusic A, Overbeke AJPM, Schroeder TV, Sox HC, Van Der Weyden MB. Clinical trial registration: A statement from the International Committee of Medical Journal Editors. JAMA. 2004; 292 (11):1363–1364. [ PubMed : 15355936 ]
- Farmer D, Jackson SA, Camacho F, Hall MA. Attitudes of African American and low socioeconomic status white women toward medical research. Journal of Health Care for the Poor and Underserved. 2007; 18 :85–99. [ PubMed : 17337800 ]
- FDA (Food and Drug Administration). Certain estrogens for oral or parenteral use. Drugs for human use; drug efficacy study implementation. Federal Register. 1971; 36 (217):21537–21538.
- FDA. FDA public health advisory, deaths with antipsychotics in elderly patients with behavioral disturbances. 2005. [accessed August 18, 2008]. http://www .fda.gov/cder /drug/advisory/antipsychotics.htm .
- FDA. FDA alert [6/16/2008]: Information for healthcare professionals. Antipsychotics. 2008. [accessed August 18, 2008]. http://www .fda.gov/cder /drug/infosheets/hcp /antipsychotics_conventional.htm .
- Finkelstein EA, Fiebelkorn IC, Wang G. National medical spending attributable to overweight and obesity: How much, and who’s paying? Health Affairs Web Exclusive. 2003. [accessed August 21, 2008]. http://content .healthaffairs .org/cgi/content /abstract/hlthaff.w3.219v1 . [ PubMed : 14527256 ]
- Furrow BR, Greaney TL, Johnson SH, Jost TS, Schwartz RL. Bioethics: Health care law and ethics. St. Paul, MN: Thomson/West; 2004.
- GAO (Government Accountability Office). Scientific research: Continued vigilance critical to protecting human subjects. Washington, DC: GAO; 1996.
- Genetics & Public Policy Center. U.S. public opinion on uses of genetic information and genetic discrimination. 2007. [accessed August 21, 2008]. http://www .dnapolicy .org/resources/GINAPublic _Opinion_Genetic _Information_Discrimination.pdf .
- Gill S, Bronskill S, Normand S, Anderson G, Sykora K, Lam K, Bell C, Lee P, Fischer H, Herrmann N, Gurwitz J, Rochon P. Antipsychotic drug use and mortality in older adults with dementia. Annals of Internal Medicine. 2007; 146 :775–786. [ PubMed : 17548409 ]
- Giuliano AR, Mokuau N, Hughes C, Tortelero-Luna G, Risendal B, Ho RCS, Prewitt TE, McCaskill-Stevens WJ. Participation of minorities in cancer research: The influence of structural, cultural, and linguistic factors. Annals of Epidemiology. 2000; 10 :S22–S34. [ PubMed : 11189089 ]
- Goldman J, Choy A. Ethical and policy issues in research involving human participants. Bethesda, MD: National Bioethics Advisory Commission; 2001. Privacy and confidentiality in health research; pp. C1–C34.
- Gostin LO. Public health law: Power, duty, restraint. Berkeley, CA: University of California Press; 2008. Surveillance and public health research: Personal privacy and the “right to know.”
- Grady C, Hampson LA, Wallen GR, Rivera-Goba MV, Carrington KL, Mittleman BB. Exploring the ethics of clinical research in an urban community. American Journal of Public Health. 2006; 96 (11):1996–2001. [ PMC free article : PMC1751807 ] [ PubMed : 17018826 ]
- Hatfield M, Sonnenschein HF, Rosenberg LE. Exceptional returns: The economic value of America’s investment in medical research. 2001. [accessed August 21, 2008]. http://www .laskerfoundation .org/advocacy/pdf/exceptional.pdf .
- Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. New England Journal of Medicine. 1971; 284 (15):878–881. [ PubMed : 5549830 ]
- HEW (Department of Health, Education and Welfare). The Belmont Report: Ethical principles and guidelines for the protection of human subjects of research. 1979. [accessed August 21, 2008]. http://ohsr .od.nih.gov /guidelines/belmont.html . [ PubMed : 25951677 ]
- HHS (Department of Health and Human Services). Institutional review boards and the HIPAA Privacy Rule. 2003. [accessed August 21, 2008]. http: //privacyruleandresearch .nih.gov/pdf/IRB_Factsheet.pdf .
- HHS. Guidance on research involving coded private information or biological specimens. 2004. [accessed August 21, 2008]. http://www .hhs.gov/ohrp /humansubjects/guidance/cdebiol.pdf .
- Hodge JG Jr. An enhanced approach to distinguishing public health practice and human subjects research. Journal of Law, Medicine & Ethics. 2005; 33 (1):125–141. [ PubMed : 15934670 ]
- Hodge JG, Gostin LO. Public health practice vs. research: A report for public health practitioners including cases and guidance for making distinctions. Atlanta, GA: Council of State and Territorial Epidemiologists; 2004.
- IOM (Institute of Medicine). Health services research: Work force and educational issues. Washington, DC: National Academy Press; 1995.
- IOM. Protecting data privacy in health services research. Washington, DC: National Academy Press; 2000a. [ PubMed : 25057723 ]
- IOM. To err is human: Building a safer health system. Washington, DC: National Academy Press; 2000b. [ PubMed : 25077248 ]
- IOM. Responsible research: A systems approach to protecting research participants. Washington, DC: The National Academies Press; 2002. [ PubMed : 20669487 ]
- Jacobsen S, Xia Z, Campion M, Darby C, Plevak M, Seltman K, Melton L. Potential effect of authorization bias on medical record research. Mayo Clinic Proceedings. 1999; 74 :330–338. [ PubMed : 10221460 ]
- Laine C, Horton R, DeAngelis CD, Drazen JM, Frizelle FA, Godlee F, Haug C, Hébert PC, Kotzin S, Marusic A, Sahni P, Schroeder TV, Sox HC, Van Der Weyden MB, Verheugt FWA. Clinical trial registration—looking back and moving ahead. New England Journal of Medicine. 2007; 356 (26):2734–2736. [ PubMed : 17548427 ]
- Lee B. Comparison of FDA and HHS human subject protection regulations. 2000. [accessed August 21, 2008]. http://www .fda.gov/oc/gcp/comparison .html .
- Lindenauer PK, Benjamin EM, Naglieri-Prescod D, Fitzgerald J, Pekow P. The role of the institutional review board in quality improvement: A survey of quality officers, institutional review board chairs, and journal editors. The American Journal of Medicine. 2002; 113 :575–579. [ PubMed : 12459404 ]
- Lowrance WW. Learning from experience, privacy and the secondary use of data in health research. London: The Nuffield Trust; 2002.
- Lowrance WW, Collins FS. Identifiability in genomic research. Science. 2007; 317 :600–602. [ PubMed : 17673640 ]
- Lynn J, Baily MA, Bottrell M, Jennings B, Levine RJ, Davidoff F, Casarett D, Corrigan J, Fox E, Wynia MK, Agich GJ, Speroff T, Schyve P, Batalden P, Tunis S, Berlinger N, Cronenwett L, Fitzmaurice M, Dubler NN, James B. The ethics of using quality improvement methods in health care. Annals of Internal Medicine. 2007; 146 (6):666–674. [ PubMed : 17438310 ]
- Mandelblatt J, Saha S, Teutsch S, Hoerger T, Siu AL, Atkins D, Klein J, Helfand M. The cost-effectiveness of screening mammography beyond age 65: A systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2003; 139 :835–842. [ PubMed : 14623621 ]
- Mick S, Morlock LL, Salkever D, de Lissovoy G, Malitz F, Wise CG, Jones A. Strategic activity and financial performance of U.S. rural hospitals: A national study, 1983 to 1988. Journal of Rural Health. 1994; 10 (3):150–167. [ PubMed : 10138031 ]
- Mosconi P, Poli P, Giolo A, Apolone G. How Italian health consumers feel about clinical research: A questionnaire survey. European Journal of Public Health. 2005; 15 (4):372–379. [ PubMed : 16014662 ]
- Murphy K, Topel R. The economic value of medical research. Chicago, IL: University of Chicago Press; 1999.
- NCI (National Cancer Institute). Getting connected with caBIG: Data sharing and security framework. 2008. [accessed October 27, 2008]. https://cabig .nci.nih .gov/working_groups /DSIC_SLWG/data_sharing_policy/
- NCURA (National Council of University Research Administrators). Report on research compliance. 2007. [accessed March 4, 2008]. http://www .reportonresearchcompliance .com .
- NCVHS (National Committee on Vital and Health Statistics). Enhanced protections for uses of health data: A stewardship framework for “secondary uses” of electronically collected and transmitted health data. 2007a. [accessed December 19, 2007]. http://ncvhs .hhs.gov/071221lt.pdf .
- NCVHS, Ad Hoc Work Group on Secondary Uses of Health Data. Testimony of the cancer Biomedical Informatics Grid (caBIG) Data Sharing and Intellectual Capital (DSIC) workspace. 2007b August 1, 2007
- Needleman J, Buerhaus P, Mattke S, Stewart M, Zelevinsky K. Nurse-staffing levels and the quality of care in hospitals. New England Journal of Medicine. 2002; 346 (22):1715–1722. [ PubMed : 12037152 ]
- NSF (National Science Foundation). Science and Engineering Indicators 2006. Arlington, VA: National Science Foundation; 2006. Science and technology: Public attitudes and understanding. Chapter 7.
- OHRP (Office for Human Research Protections). IRB guidebook, part I.A. 2008a. [accessed August 21, 2008]. http://www .hhs.gov/ohrp /irb/irb_guidebook.htm .
- OHRP. OHRP concludes case regarding Johns Hopkins University research on hospital infections. 2008b. [accessed August 21, 2008]. http://www .hhs.gov/ohrp/news/recentnews .html#20080215 .
- Partridge A, Winer E. Informing clinical trial participants about study results. JAMA. 2002; 288 :363–365. [ PubMed : 12117402 ]
- Partridge AH, Wolff AC, Marcom PK, Kaufman PA, Zhang L, Gelman R, Moore C, Lake D, Fleming GF, Rugo HS, Atkins J, Sampson E, Collyar D, Winer EP. The impact of sharing results of a randomized breast cancer clinical trial with study participants. Breast Cancer Research and Treatment. 2008 June 10 [ PubMed : 18543100 ]
- Pitkin R. Folate and neural tube defects. American Journal of Clinical Nutrition. 2007; 85 (1):285S–288S. [ PubMed : 17209211 ]
- Pritts J. The importance and value of protecting the privacy of health information: Roles of HIPAA Privacy Rule and the Common Rule in health research. 2008. [accessed March 15, 2008]. http://www .iom.edu/CMS/3740/43729/53160 .aspx .
- Research!America. America speaks: Poll summary. Vol. 7. Alexandria, VA: United Health Foundation; 2007.
- Schneeweiss S, Setoguchi S, Brookhart A, Dormuth C, Wang P. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. Canadian Medical Association Journal. 2007; 176 :672–632. [ PMC free article : PMC1800321 ] [ PubMed : 17325327 ]
- Shalowitz DI, Miller FG. Communicating the results of clinical research to participants: Attitudes, practices, and future directions. PLoS Medicine. 2008a May 13; 5 (5):e91. [ PMC free article : PMC2375946 ] [ PubMed : 18479180 ]
- Shalowitz DI, Miller FG. The search for clarity in communicating research results to study participants. Journal of Medical Ethics. 2008b September; 34 (9):e17. [ PubMed : 18757617 ]
- Shaul RZ, Birenbaum S, Evans M. Legal liability in research: Early lessons from North America. BMC Medical Ethics. 2005; 6 (4):1–4. [ PMC free article : PMC1182131 ] [ PubMed : 15953387 ]
- Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Annals of Epidemiology. 2002; 12 :248–256. [ PubMed : 11988413 ]
- Sim I, Chan A-W, Gülmezoglu AM, Evans T, Pang T. Clinical trial registration: Transparency is the watchword. The Lancet. 2006; 367 (9523):1631–1633. [ PubMed : 16714166 ]
- Slamon D, Clark G, Wong S, Levin W, Ullrich A, McGuire W. Human breast cancer: Correlation of relapse and survival with amplification of the her-2/neu oncogene. Science. 1987; 235 (4785):177–182. [ PubMed : 3798106 ]
- Snider DE, Stroup DF. Defining research when it comes to public health. Public Health Reports. 1997; 112 :29–32. [ PMC free article : PMC1381834 ] [ PubMed : 9018284 ]
- Thorpe KE, Florence CS, Howard DH, Joski P. The impact of obesity in rising medical spending. Health Affairs Web Exclusive. 2004. [accessed August 21, 2008]. http://content .healthaffairs .org/cgi/content /abstract/hlthaff.w4.480v1 . [ PubMed : 15496437 ]
- Trauth JM, Musa D, Siminoff L, Jewell IK, Ricci E. Public attitudes regarding willingness to participate in medical research studies. Journal of Health & Social Policy. 2000; 12 (2):23–43. [ PubMed : 11184441 ]
- Veurink M, Koster M, Berg L. The history of DES, lessons to be learned. Pharmacy World & Science. 2005; 27 (3):139–143. [ PubMed : 16096877 ]
- Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt LA, Brawley OW, Gross CP, Emanuel E. Are racial and ethnic minorities less willing to participate in health research? PLoS Medicine. 2006; 3 (2):201–210. [ PMC free article : PMC1298944 ] [ PubMed : 16318411 ]
- Westin A. How the public views privacy and health research. 2007. [accessed November 11, 2008]. http://www .iom.edu/Object .File/Master/48 /528/%20Westin%20IOM %20Srvy%20Rept%2011-1107.pdf .
- Williams ED. Federal protection for human research subjects: An analysis of the Common Rule and its interactions with FDA regulations and the HIPAA Privacy Rule, CRS report for Congress. Washington, DC: Congressional Research Service; 2005.
- Williams IC, Corbie-Smith G. Investigator beliefs and reported success in recruiting minority participants. Contemporary Clinical Trials. 2006; 27 :580–586. [ PubMed : 16839822 ]
- Winston FK, Durbin DR, Kallan MJ, Moll EK. The danger of premature graduation to seat belts for young children. Pediatrics. 2000; 105 (6):1179–1183. [ PubMed : 10835054 ]
- WMA (World Medical Association). Declaration of Helsinki: Ethical principles for medical research involving human subjects. 1964. [accessed August 21, 2008]. http://ohsr .od.nih.gov /guidelines/helsinki.html . [ PubMed : 16010903 ]
- Woolley M, Propst SM. Public attitudes and perceptions about health-related research. JAMA. 2005; 294 (11):1380–1384. [ PubMed : 16174697 ]
- Zarin DA, Tse T. Moving toward transparency of clinical trials. Science. 2008; 319 :1340–1342. [ PMC free article : PMC2396952 ] [ PubMed : 18323436 ]
- Zarin DA, Tse T, Ide NC. Trial registration at ClinicalTrials.gov between May and October 2005. New England Journal of Medicine. 2005; 353 (26):2779–2787. [ PMC free article : PMC1568386 ] [ PubMed : 16382064 ]
Epidemiology is the study of the occurrence, distribution, and control of diseases in populations.
Health services research has been defined as a multidisciplinary field of inquiry, both basic and applied, that examines the use, costs, quality, accessibility, delivery, organization, financing, and outcomes of health care services to increase knowledge and understanding of the structure, processes, and effects of health services for individuals and populations ( IOM, 1995 ).
The National Committee on Vital and Health Statistics has noted that “secondary uses” of health data is an ill-defined term, and urges abandoning it in favor of precise description of each use ( NCVHS, 2007a ). Thus, the committee chose to minimize use of the term in this report.
See Standards for Privacy of Individually Identifiable Health Information, 64 Fed. Reg. 59918, 59967 (preamble to rule proposed November 3, 1999) for a discussion on the benefits of health records research.
Effectiveness can be defined as the extent to which a specific test or intervention, when used under ordinary circumstances, does what it is intended to do. Efficacy refers to the extent to which a specific test or intervention produces a beneficial result under ideal conditions (e.g., in a clinical trial).
See http://www .intermacs.org .
See http://www .elso.med.umich.edu .
See http://www .unos.org/Data .
The Department of Health, Education and Welfare (now HHS) had previously issued policy and guidance on the protection of human subjects. See Williams (2005) .
In its report “First Biennial Report on the Adequacy and Uniformity of Federal Rules and Policies, and their Implementation, for the Protection of Human Subjects in Biomedical and Behavioral Research, Protecting Human Subjects.”
45 C.F.R. part 46 (2005).
See 45 C.F.R. § 46.101 (2005).
See 45 C.F.R. § 46.102(d) (2005).
This section on informed consent is based largely on a Congressional Research Service report ( Williams, 2005 ), as adapted by Pritts (2008) .
See 45 C.F.R. § 46.116 (2005).
See 45 C.F.R. § 46.116(b) (2005).
See 45 C.F.R. § 46.103 (2005).
See 45 C.F.R. § 46.111 (2005). There are additional factors if the study includes subjects who are likely to be vulnerable to coercion or undue influence.
See 45 C.F.R. § 46.116(d); 46.117(c) (2005).
See 45 C.F.R. § 46.102(f) (2005).
See 45 C.F.R. § 46.101(b)(4) (2005).
See 45 C.F.R. § 46.116(e) (2005).
See 45 C.F.R. § 46.123 (2005).
See also Grimes v. Kennedy Krieger Institute , 782 A. 2d 807 (Md. Ct. App. 2001); Gelsinger v. University of Pennsylvania (Philadelphia County Court of Common Pleas filed September 18, 2000), available at http://www .sskrplaw.com /links/healthcare2.html .
The FDA has defined “clinical investigation” to be synonymous with “research.”
The Food, Drug, and Cosmetic Act Section 505(i), 507(d), or 520(g) of 21 U.S.C. 355(i), 357(d), or 360j(g) (1972).
See 21 C.F.R. part 50 (2008); 46 Fed. Reg. 8942 (1981).
See 21 C.F.R. part 56 (2008); 46 Fed. Reg. 8958 (1981).
See 45 C.F.R. part 46 (2005); 46 Fed. Reg. 8366 (1981).
The Centers for Disease Control and Prevention defines program evaluation as the “systematic investigation of the merit, worth, or significance of organized public health action,” noting that such evaluations are “systematic ways to improve and account for public health actions by involving procedures that are useful, feasible, ethical, and accurate.” They can be based on goals, processes, outcomes, or value ( http://www .cdc.gov/mmwr /preview/mmwrhtml/rr4811a1.htm ).
The Utilization Review Accreditation Commission defines utilization review as “the evaluation of the medical necessity, appropriateness, and efficiency of the use of health care services, procedures, and facilities under the provisions of the applicable health benefits plans” ( http://www .urac.org/about/ ).
Another type of oversight board defined by the Privacy Rule. See Chapter 4 .
Under the Privacy Rule, consent is referred to as authorization. See Chapter 4 .
Personal communication, C. Heide, Office for Civil Rights, HHS, May 29, 2008.
The Privacy Rule defines the term “health care operations” by listing a number of specific activities that qualify as health care operations. These include “conducting quality assessment and improvement activities, population-based activities relating to improving or reducing health care costs, and case management and care coordination.” See 45 C.F.R. § 164.501 (2006).
A total of 444 surveys were mailed to the medical directors of quality improvement and IRB chairs at hospitals with 400 or more beds that belong to the Council of Teaching Hospitals of the Association of American Medical Colleges, and to the editors of all U.S.-based medical journals that publish original research and appear in the Abridged Index Medicus. 236 surveys were returned, for a 53 percent response rate. The survey consisted of six brief scenarios that asked respondents to determine whether the described project needed IRB review and informed consent.
See http: //plainlanguage.gov/index.cfm .
FDA, Public Law 110–85 § 801 (2007).
See http://www .ahrq.gov/research/cbprrole .htm .
- Cite this Page Institute of Medicine (US) Committee on Health Research and the Privacy of Health Information: The HIPAA Privacy Rule; Nass SJ, Levit LA, Gostin LO, editors. Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research. Washington (DC): National Academies Press (US); 2009. 3, The Value, Importance, and Oversight of Health Research.
- PDF version of this title (1.6M)
In this Page
Other titles in this collection.
- The National Academies Collection: Reports funded by National Institutes of Health
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- The Value, Importance, and Oversight of Health Research - Beyond the HIPAA Priva... The Value, Importance, and Oversight of Health Research - Beyond the HIPAA Privacy Rule
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

1 Chapter 1: The Importance of Research Methods and Becoming an Informed Consumer of Research
Case study : student apprehension regarding research methods.
Research Study
Understanding and Measuring Student Apprehension in Criminal Justice Research Methods Courses 1
Research Question
How do we measure disinterest, relevance argumentation, and math anxiety experienced by students enrolled in research methods courses?
Methodology
It is said that �misery loves company,� so you are not alone in your apprehension and anxiety regarding your research methods course. The problem of student apprehension and anxiety related to taking a research methods course is not new and has been studied for over 25 years. Previously, such apprehension and anxiety appeared to be caused by math anxiety, especially as it applies to statistics. The authors of this article believe that student apprehension goes beyond math anxiety; that math anxiety is too simplistic of an explanation of student fear of research methods courses. Besides math anxiety, the researchers think that apprehension is caused by student indifference to the subject matter and irrelevance of the course because it does not apply to the �real world.� They state that student apprehension in research methods and statistics courses is due to three main factors:
Disinterest (D.);
Relevance Argumentation (RA.), and;
Math Anxiety (MA.).
Taken together, the reconceptualization is known as D.RA.MA., and the combination of these three factors constitutes the D.RA.MA. scale for research methods and statistics courses.
The researchers developed the D.RA.MA. scale by constructing survey questions to measure each factor in the scale (i.e., disinterest, relevance argumentation, and math anxiety). After they developed the survey, they tested it by distributing the survey to three criminal justice classes, totaling 80 students, from a midsized regional comprehensive university in the southern region of the United States. Higher scale scores demonstrate more disinterest, more relevance argumentation, or more math anxiety.
The D.RA.MA. scale consists of 20 survey questions. Ten questions were borrowed from an existing Math Anxiety scale developed by Betz 2 . The researchers then created five items to assess Disinterest and five items intended to measure Relevance Argumentation. The items for the D.RA.MA. scale are illustrated below.
Math Anxiety 3
I usually have been at ease in math classes.
Math does not scare me at all.
I am no good at math.
I don�t think that I could do advanced math.
Generally, I have been secure about attempting math.
For some reason, even though I study, math seems unusually hard for me.
Math has been my worst subject.
My mind goes blank and I am unable to think clearly when working in mathematics.
I think I could handle more difficult math.
I am not the type to do well in mathematics.
Relevance Argumentation 4
I will need research methods for my future work.
I view research methods as a subject that I will rarely use.
Research methods is not really useful for students who intend to work in Criminal Justice.
Knowing research methods will help me earn a living.
Research methods does not reflect the �real world.�
Research Disinterest 5
I am excited about taking research methods.
It would not bother me at all to take more research methods courses.
I expect a research methods class to be boring.
I don�t expect to learn much in research methods.
I really don�t care if I learn anything in research methods, as long as I get the requirement completed.
The Math Anxiety Scale responses for the 80 students ranged from 0 to 30 with a mean of 14, demonstrating a moderate level of math anxiety among the study participants. The responses for Relevance Argumentation ranged from 0 to 12 with a mean of 5.4 while those for Disinterest ranged from 1 to 15 with a mean of 7.0, demonstrating a moderate level of disinterest and relevance argumentation among students regarding research methods. Based on these findings, the study demonstrated that student apprehension regarding research methods courses goes beyond math anxiety and includes two additional factors; disinterest in the subject matter and irrelevance of research methods to the �real world.�
Limitations with the Study Procedure
This research study was designed to develop a broader measure of student apprehension in criminal justice research methods courses. Moving beyond just math anxiety, the researchers accomplished their objective by developing the D.RA.MA. scale; adding disinterest and relevance argumentation to the understanding of student apprehension regarding research methods. As is true for all research, this study is not without limitations. The biggest limitation of this study is the limited sample size. Only 80 students completed the survey. Although this is certainly a good start, similar research (i.e., replication) needs to be completed with larger student samples in different locations throughout the country before the actual quality of the D.RA.MA. scale can be determined.
Impact on Criminal Justice
The D.RA.MA. scale developed in this study identifies disinterest and relevance argumentation, in addition to math anxiety, as part of student apprehension and resistance to research methods. A variety of instructional strategies can be inferred from the D.RA.MA. survey. However, it is important for professors to recognize that no single approach will reduce research methods resistance and apprehension for all students. For example, discussing research methods in a popular culture framework may resonate with students and lead to engaged students who are more interested in the subject matter and identify with the relevance of research methods to criminal justice in general and the future careers of students, in particular. This approach may provide an effective means for combating student disinterest and relevance argumentation in criminal justice research methods courses. At a minimum, it is critical for professors to explain the relevance of research methods to the policies and practices of police, courts, and corrections. Students need to realize that research methods are essential tools for assessing agency policies and practices. Professors will always have D.RA.MA.-plagued students, but recognizing the problem and then developing effective strategies to connect with these students is the challenge all professors face. Experimenting with a multitude of teaching strategies to alleviate the math anxiety, relevance argumentation, and disinterest of criminal justice research methods students will result in more effective teaching and learning.
In This Chapter You Will Learn
What research is and why it is important to be an informed consumer of research
The sources of knowledge development and problems with each
How research methods can dispel myths about crime and the criminal justice system
The steps in the research process
How research has impacted criminal justice operations
Introduction
As noted in the chapter opening case study, it is expected that you have some anxiety and apprehension about taking this criminal justice research methods course. But, you have taken a significant step toward success in this course by opening up your research methods book, so congratulations are in order. You might have opened this book for a number of reasons. Perhaps it is the first day of class and you are ready to get started on the course material. Perhaps you have a quiz or exam soon. Perhaps the book has been gathering dust on your shelf since the first day of class and you are not doing well in your research methods class and are looking for the book to help with course improvement. Perhaps you are taking a research methods class in the future and are seeing if all the chatter among students is true.
No matter how you got here, two things are probably true. First, you are taking this research methods course because it is a requirement for your major. The bottom line is that most of the students who read this text are required to take a research methods course. While you may think studying research methods is irrelevant to your career goals, unnecessary, overly academic, or perhaps even intimidating, you probably must finish this course in order to graduate. Second, you have heard negative comments about this course. The negative comments mention the difficulty of the course and the relevance of the course (e.g., �I am going to be a police officer, so why do I need to take a research methods course?�). If you are like most students we have experienced in our research methods courses in the past, you are not initially interested in this course and are concerned about whether you will do well in it.
If you are concerned about the course, realize that you are not alone because most students are anxious about taking a research methods course. Also realize that your professor is well aware of student anxiety and apprehension regarding research methods. So, relax and do not think about the entire course and the entire book. Take the course content one chapter, one week at a time. One of the advantages of taking a research methods course is that you learn about the process of research methods. Each chapter builds upon the previous chapters, illustrating and discussing more about the research process. This is certainly an advantage, but it is also critical that you understand the initial chapters in this book so you are not confused with the content discussed in later chapters. In addition to anxiety and apprehension over the course material, research methods can be boring if you only read and learn about it with no particular purpose in mind. Although examples are prevalent throughout the book, as you read this material, it is recommended that you think about the relevancy and application of the topics covered in this book to your specific criminal justice interests. As you continue to read the book, think about how you might use the information you are reading in your current position or your intended profession.
The goal of this research methods book is to develop you into an informed consumer of research. Most, if not all, of your fellow classmates will never conduct their own research studies. However, every one of you will be exposed to research findings in your professional and personal lives for the remainder of your lives. You are exposed to research findings in the media (e.g., television, newspapers, and online), in personal interaction with others (e.g., friends and family, doctors, and professors), as well as in class. You should challenge yourself for this semester to keep a journal and document exposure to research in your daily life outside of college whether through the nightly news, newspapers, magazine articles, Internet, personal conversations, or other means. At the end of the semester, you will be amazed at the amount of research you are exposed to in a short period of time. This book is focused on research exposure and assisting you to become an educated consumer of research by providing you the skills necessary to differentiate between good and not so good research. Why should you believe research findings if the study is faulty? Without being an educated consumer of research, you will not be able to differentiate between useful and not useful research. This book is designed to remedy this problem.
This book was written to make your first encounter with research methods relevant and successful while providing you the tools necessary to become an educated consumer of research. Therefore, this book is written with the assumption that students have not had a prior class on research methods. In addition, this book assumes that practical and evaluative knowledge of research methods is more useful than theoretical knowledge of the development of research methods and the relationship between theory and research. Since the focus of this book is on consumerism, not researcher training, practical and evaluative knowledge is more useful than theoretical knowledge.
It is also important to understand that the professors who design academic programs in criminal justice at the associate and bachelor level believe that an understanding of research methods is important for students. That is why, more than likely, this research methods course is a required course in your degree program. These professors understand that a solid understanding of research methods will enrich the qualifications of students for employment and performance in their criminal justice careers.
As previously stated, the basic goal of this book is to make students, as future and possibly even current practitioners in the criminal justice system, better informed and more capable consumers of the results of criminal justice research. This goal is based on the belief that an understanding of research methods allows criminal justice practitioners to be better able to make use of the results of research as it applies to their work-related duties. In fact, thousands of research questions are asked and answered each year in research involving criminal justice and criminological topics. In addition, thousands of articles are published, papers presented at conferences, and reports prepared that provide answers to these questions. The ability to understand research gives practitioners knowledge of the most current information in their respective fields and the ability to use this knowledge to improve the effectiveness of criminal justice agencies.
How Do We Know What We Know? Sources of Knowledge
The reality is the understanding of crime and criminal justice system operations by the public is frequently the product of misguided assumptions, distorted interpretations, outright myths, and hardened ideological positions. 6 This is a bold statement that basically contends that most people�s knowledge of crime and criminal justice is inaccurate. But, how do these inaccuracies occur? Most people have learned what they know about crime and criminal justice system operations through some other means besides scientific research results and findings. Some of that knowledge is based on personal experience and common sense. Much of it is based on the information and images supported by politicians, governmental agencies, and especially the media. This section will discuss the mechanisms used to understand crime and criminal justice operations by the public. It is important to note that although this section will focus on the failings of these knowledge sources, they each can be, and certainly are, accurate at times, and thus are valuable sources of knowledge.
Knowledge from Authority
We gain knowledge from parents, teachers, experts, and others who are in positions of authority in our lives. When we accept something as being correct and true just because someone in a position of authority says it is true, we are using what is referred to as authority knowledge as a means of knowing. Authorities often expend significant time and effort to learn something, and we can benefit from their experience and work.
However, relying on authority as a means of knowing has limitations. It is easy to overestimate the expertise of other people. A person�s expertise is typically limited to a few core areas of significant knowledge; a person is not an expert in all areas. More specifically, criminal justice professors are not experts on all topics related to criminal justice. One professor may be an expert on corrections but know little about policing. If this professor discusses topics in policing in which he is not an expert, we may still assume he is right when he may be wrong. Authority figures may speak on fields they know little about. They can be completely wrong but we may believe them because of their status as an expert. Furthermore, an expert in one area may try to use his authority in an unrelated area. Other times, we have no idea of how the experts arrived at their knowledge. We just know they are experts in the topic area.
As I am writing this, I recall an example of authority knowledge that was wrong during my police academy training in the late 1980s. My academy training was about four years after the U.S. Supreme Court decision in Tennessee v. Garner. 7 In this case, the Court limited the use of deadly force by police to defense of life situations and incidents where the suspect committed a violent offense. Prior to the decision, the police in several states could use deadly force on any fleeing suspect accused of a felony offense. One day, the academy class was practicing mock traffic stops. During one of my mock traffic stops, I received information that the vehicle I stopped was stolen. The driver and passenger exited the vehicle and fled on foot. I did not use deadly force (this was a training exercise so was not real) against the suspects and was chastised by my instructor who insisted that I should have shot the suspects as they were fleeing. Training instructors, just like professors, convey authority knowledge but, in this case, the instructor was wrong. I was not legally authorized to use deadly force in the traffic stop scenario despite the insistence of my instructor to the contrary.
Politicians are sometimes taken as a source of authority knowledge about the law, crime, and criminal justice issues. Since they enact laws that directly impact the operations of the criminal justice system, we may assume they are an authority on crime and criminal justice. More specifically, we may assume that politicians know best about how to reduce crime and increase the effectiveness of the criminal justice system. However, history is rife with laws that sounded good on paper but had no impact on crime. For example, there is little evidence that sex offender registration protects the public from sexual predators or acts as a deterrent to repeat sex offenders even though every state has a law requiring convicted sex offenders to register with local authorities. Perhaps politicians are not the criminal justice experts some perceive them to be.
History is also full of criminal justice authorities that we now see as being misinformed. For example, Cesare Lombroso is the father of the positivist school of criminology. He is most readily recognized for his idea that some individuals are born criminal. He stated that criminals have certain unique biological characteristics, including large protruding jaws, high foreheads, flattened noses, and asymmetrical faces, to name a few. 8 These characteristics were similar to those found in primitive humans. Therefore, Lombroso argued that some individuals were genetic �throwbacks� to a more primitive time and were less evolved than other people and thus, were more likely to be criminals. Lombroso�s research has been discredited because he failed to compare criminals with noncriminals. By studying only criminals, he found characteristics that were common to criminals. However, if Lombroso had studied a group of noncriminals, he would have discovered that these biological characteristics are just as prevalent among noncriminals. This example involves authority knowledge that is supported by research but the research methods used were flawed. The errors of Lombroso seem obvious now, but what do we know today through authority knowledge that is inaccurate or will be proven wrong in the future?
Knowledge from Tradition
In addition to authority knowledge, people often rely on tradition for knowledge. Tradition knowledge relies on the knowledge of the past. Individuals accept something as true because that is the way things have always been so it must be right. A good example of tradition knowledge is preventive/random patrol. Ever since vehicles were brought into the police patrol function, police administrators assumed that having patrol officers drive around randomly in the communities they serve, while they are not answering calls for service, would prevent crime. If you were a patrol officer in the early 1970s and asked your supervisor, �Why do I drive around randomly throughout my assigned area when I am not answering a call for service?� the answer would have been, �That is the way we have always done patrol and random patrol reduces crime through deterrence.� The Kansas City Preventive Patrol Experiment challenged the tradition knowledge that preventive/random patrol reduces crime. The results of the study made it clear that the traditional practice of preventive/random patrol had little to no impact on reducing crime. This allowed police departments to develop other patrol deployment strategies such as directed patrol and �hot spots� policing since preventive patrol was seen as ineffective. The development of effective patrol deployment strategies continues today.
Knowledge from Common Sense
We frequently rely on common sense knowledge for what we know about crime and the criminal justice system because it �just makes sense.� For example, it �just makes sense� that if we send juvenile delinquents on a field trip to prison where they will see first hand the prison environment as well as be yelled at by actual prisoners, they will refrain from future delinquency. That is exactly what the program Scared Straight, originally developed in the 1970s, is designed to do. Scared Straight programs are still in existence today and are even the premise for the television show Beyond Scared Straight on the A&E television network. As originally created, the program was designed to decrease juvenile delinquency by bringing at-risk and delinquent juveniles into prison where they would be �scared straight� by inmates serving life sentences. Participants in the program were talked to and yelled at by the inmates in an effort to scare them. It was believed that the fear felt by the participants would lead to a discontinuation of their delinquent behavior so that they would not end up in prison themselves. This sounds like a good idea. It makes sense, and the program was initially touted as a success due to anecdotal evidence based on a few delinquents who turned their lives around after participation in the program.
However, evaluations of the program and others like it showed that the program was in fact unsuccessful. In the initial evaluation of the Scared Straight program, Finckenauer used a classic experimental design (discussed in Chapter 5), to evaluate the original �Lifer�s Program� at Rahway State Prison in New Jersey where the program was initially developed. 13 Juveniles were randomly assigned to an experimental group that attended the Scared Straight program and a control group that did not participate in the program. Results of the evaluation were not positive. Post-test measures revealed that juveniles who were assigned to the experimental group and participated in the program were actually more seriously delinquent afterwards than those who did not participate in the program. Also using an experimental design with random assignment, Yarborough evaluated the �Juvenile Offenders Learn Truth� (JOLT) program at the State Prison of Southern Michigan at Jackson. 14 This program was similar to that of the �Lifer�s Program,� only with fewer obscenities used by inmates. Post-test measurements were taken at two intervals, three and six months after program completion. Again, results were not positive. Findings revealed no significant differences in delinquency between those juveniles who attended the program and those who did not. Other experiments conducted on Scared Straight- type programs further revealed their inability to deter juveniles from further delinquency. 15 Despite the common sense popularity of these programs, the evaluations showed that Scared Straight programs do not reduce delinquency and, in some instances, may actually increase delinquency. The programs may actually do more harm than good. I guess that begs the question, �Why do we still do these types of programs?�
Scared Straight programs and other widely held common sense beliefs about crime and the criminal justice system are questionable, based on the available research evidence. Common sense is important in our daily lives and is frequently correct, but, at times, it also contains inaccuracies, misinformation, and even prejudice.
CLASSICS IN CJ RESEARCH
Is It Safe to Put Felons on Probation?
Research Study 9
In the mid-1970s, the number of offenders on probation began to significantly increase. By the mid-1980s, probation was the most frequently used sentence in most states and its use was becoming more common for felons, whereas previously, probation was typically limited to misdemeanor crimes and offenses committed by juveniles. Increasing numbers of felony offenders were being placed on probation because judges had no other alternative forms of punishment. Prisons were already operating above capacity due to rising crime rates. Despite the increase in the use of probation in the 1980s, few empirical studies of probation (particularly its use with felony offenders) had been published. In the early 1980s, the Rand Corporation conducted an extensive study of probation to learn more about the offenders sentenced to probation and the effectiveness of probation as a criminal sanction. At the time the study began, over one-third of California�s probation population were convicted felons. 10 This was the first large-scale study of felony probation.
Is it safe to put felons on probation?
Data for the study were obtained from the California Board of Prison Terms (CBPT). The Board had been collecting comprehensive data on all offenders sentenced to prison since 1978 and on a sample of adult males from 17 counties who received probation. From these two data sources, researchers selected a sample of male offenders who had been convicted of the following crimes: robbery, assault, burglary, theft, forgery, and drug offenses. These crimes were selected because an offender could receive either prison or probation if convicted. Approximately 16,500 male felony offenders were included in the study. For each offender, researchers had access to their personal characteristics, information on their crimes, court proceedings, and disposition.
Two main research questions were answered in this study. First, what were the recidivism rates for felony offenders who received probation? When assessing recidivism rates, the study found that the majority of offenders sentenced to probation recidivated during the follow-up period, which averaged 31 months. Overall, 65% of the sample of probationers were re-arrested and 51 % were charged with and convicted of another offense. A total of 18% were convicted of a violent crime.
The second research question asked, what were the characteristics of the probationers who recidivated? Property offenders were more likely to recidivate compared to violent or drug offenders. Researchers also discovered that probationers tended to recidivate by committing the same crime that placed them on probation. Rand researchers included time to recidivism in their analysis and found that property and violent offenders recidivated sooner than drug offenders. The median time to the first filed charge was five months for property offenders and eight months for violent offenders.
The issue of whether or not the findings would generalize to other counties in California and to other states was raised. Data for the study came from probation and prison records from two counties in California. These two counties were not randomly selected, but were chosen because of their large probation populations and the willingness of departments to provide information. Further, the probation departments in these counties had experienced significant budget cuts. Supervision may have become compromised as a result and this could have explained why these counties had high rates of recidivism. Studies of probation recidivism in other states have found recidivism rates to be much lower, suggesting the Rand results may not have applied elsewhere. 11 Several studies examining the effectiveness of probation and the factors correlated with probation outcomes were published after 1985. Much of this research failed to produce results consistent with the Rand study.
The Rand study of felony probation received a considerable amount of attention within the field of corrections. According to one scholar, the study was acclaimed as �the most important criminological research to be reported since World War II.� 12 The National Institute of Justice disseminated the report to criminal justice agencies across the country and even highlighted the study in their monthly newsletter. Today, the study remains one of the most highly cited pieces of corrections research.
According to Rand researchers, these findings raised serious doubts about the effectiveness of probation for felony offenders. Most of the felons sentenced to probation recidivated and researchers were unable to develop an accurate prediction model to improve the courts� decision-making. The continued use of probation as a sanction for felony offenders appeared to be putting the public at risk. However, without adequate prison space, the courts had no other alternatives besides probation when sentencing offenders.
The researchers made several recommendations to address the limitations of using probation for felony offenders. First, it was recommended that states formally acknowledge that the purpose of probation had changed. Probation was originally used as a means of furthering the goal of rehabilitation in the correctional system. As the United States moved away from that goal in the late 1960s, the expectations of probation changed. Probation was now used as a way to exercise �restrictive supervision� over more serious offenders. Second, probation departments needed to redefine the responsibilities of their probation officers. Probation officers were now expected to be surveillance officers instead of treatment personnel, which required specialized training. In addition, states needed to explore the possibility of broadening the legal authority of its probation officers by allowing them to act as law enforcement officers if necessary. Third, states were advised to adopt a formal client management system that included risk/need assessments of every client. Such a system would help establish uniform, consistent treatment of those on probation and would also help departments allocate their resources efficiently and effectively. Fourth, researchers encouraged states to develop alternative forms of community punishment that offered more public protection than regular probation, which led to the development and use of intensive supervision probation, house arrest, electronic monitoring, day reporting centers, and other intermediate punishments.
Knowledge from Personal Experience
If you personally see something or if it actually happens to you, then you are likely to accept it as true and gain knowledge from the experience. Gaining knowledge through actual experiences is known as personal experience knowledge, and it has a powerful and lasting impact on everyone. Personal experiences are essential building blocks of knowledge and of what we believe to be true. The problem with knowledge gained from personal experiences is that personal experiences can be unique and unreliable, which can distort reality and lead us to believe things that are actually false.
How can events that someone personally experienced be wrong? The events are not wrong. Instead, the knowledge gained from the experience is wrong. For example, the research consistently shows that a person�s demeanor significantly impacts the decision-making of police officers. During a traffic stop, if a person is rude, disrespectful, and uncooperative to the officer, then the driver is more likely to receive a traffic citation than a warning. That is what the research on police discretion shows. However, if a person was rude and uncooperative to a police officer during a traffic stop and was let go without a citation, the person will gain knowledge from this personal experience. The knowledge gained may include that being disrespectful during future traffic stops will get this person out of future tickets. Not likely. The event is not wrong. Instead, the knowledge gained from the experience is wrong because being disrespectful to the police usually leads to more enforcement action taken by the police, not less.
As a student in criminal justice, you have probably experienced something similar in interaction with friends, relatives, and neighbors. Your knowledge of criminal justice that you have developed in your criminal justice classes is trumped by one experience your friend, relative, or neighbor had with the criminal justice system. They believe they are right because they experienced it. However, there are four errors that occur in the knowledge gained from personal experiences: overgeneralization, selective observation, illogical reasoning, and resistance to change.
Overgeneralization happens when people conclude that what they have observed in one or a few cases is true for all cases. For example, you may see that a wealthy businesswomen in your community is acquitted of bribery and may conclude that �wealthy people, especially women, are never convicted in our criminal justice system,� which is an overgeneralization. It is common to draw conclusions about people and society from our personal interactions, but, in reality, our experiences are limited because we interact with just a small percentage of people in society.
The same is true for practitioners in the criminal justice system. Practitioners have a tendency to believe that because something was done a particular way in their agency, it is done that way in all agencies. That may not be true. Although there are certainly operational similarities across criminal justice agencies, there are also nuances that exist across the over 50,000 criminal justice agencies in the United States. Believing that just because it was that way in your agency, it must be that way in all agencies leads to overgeneralization.
Selective observation is choosing, either consciously or unconsciously, to pay attention to and remember events that support our personal preferences and beliefs. In fact, with selective observation, we will seek out evidence that confirms what we believe to be true and ignore the events that provide contradictory evidence. We are more likely to notice pieces of evidence that reinforce and support our ideology. As applied to the criminal justice system, when we are inclined to be critical of the criminal justice system, it is pretty easy to notice its every failing and ignore its successes. For example, if someone believes the police commonly use excessive force, the person is more likely to pay attention to and remember a police brutality allegation on the nightly news than a police pursuit that led to the apprehension of the suspect without incident on the same nightly news. As another example, if you believe treatment efforts on sex offenders are futile, you will pay attention to and remember each sex offender you hear about that recidivates but will pay little attention to any successes. It is easy to find instances that confirm our beliefs, but with selective observation, the complete picture is not being viewed. Therefore, if we only acknowledge the events that confirm our beliefs and ignore those that challenge them, we are falling victim to selective observation.
Besides selective observation, some of our observations may simply be wrong. Consider eyewitness identification. It is a common practice in the criminal justice system, but research has consistently demonstrated inaccuracies in eyewitness identification. The witness feels certain that the person viewed is the person who committed the offense, but sometimes the witness is wrong. Even when our senses of sight, hearing, taste, touch, and smell are fully operational, our minds have to interpret what we have sensed, which may lead to an inaccurate observation.
RESEARCH IN THE NEWS
When Your Criminal Past Isn�t Yours 16
The business of background checks on prospective employees is increasing significantly. According to the Society for Human Resource Management, since the events of September 11, 2001, the percentage of companies that conduct criminal history checks during the hiring process has risen past 90%. Employers spend at least $2 billion a year to look into the pasts of their prospective employees. Problems with the business of background checks were identified through research that included a review of thousands of pages of court filings and interviews with dozens of court officials, data providers, lawyers, victims, and regulators.
The business of background checks is a system weakened by the conversion to digital files and compromised by the significant number of private companies that profit by amassing public records and selling them to employers. The private companies create a system in which a computer program scrapes the public files of court systems around the country to retrieve personal data. Basically, these are automated data-mining programs. Today, half the courts in the United States put criminal records on their public websites. So, the data are there for the taking, but the records that are retrieved typically are not checked for errors�errors that would be obvious to human eyes.
The errors can start with a mistake entered into the logs of a law enforcement agency or a court file. The biggest culprits, though, are companies that compile databases using public information. In some instances, their automated formulas misinterpret the information provided them. Other times, records wind up assigned to the wrong people with a common name. Furthermore, when a government agency erases a criminal conviction after a designated period of good behavior, many of the commercial databases don�t perform the updates required to purge offenses that have been removed from public record. It is clear that these errors can have substantial ramifications, including damaged reputations and loss of job opportunities.
Illogical reasoning occurs when someone jumps to premature conclusions or presents an argument that is based on invalid assumptions. Premature conclusions occur when we feel we have the answer based on a few pieces of evidence and do not need to seek additional information that may invalidate our conclusion. Think of a detective who, after examining only a few pieces of evidence, quickly narrows in on a murder suspect. It is common for a detective to assess the initial evidence and make an initial determination of who committed the murder. However, it is hoped that the detective will continue to sort through all the evidence for confirmation or rejection of his original conclusion regarding the murder suspect. Illogical reasoning by jumping to premature conclusions is common in everyday life. We look for evidence to confirm or reject our beliefs and stop when a small amount of evidence is present; we jump to conclusions. If a person states, �I know four people who have dropped out of high school, and each one of them ended up addicted to drugs, so all dropouts abuse drugs,� the person is jumping to conclusions.
Illogical reasoning also occurs when an argument, based on invalid assumptions, is presented. Let�s revisit the Scared Straight example previously discussed. Program developers assumed that brief exposure to the harsh realities of prison would deter juveniles from future delinquency. The Scared Straight program is an example of illogical reasoning. Four hours of exposure to prison life is not going to counteract years of delinquency and turn a delinquent into a nondelinquent. The program is based on a false assumption and fails to recognize the substantial risk factors present in the lives of most delinquents that must be mediated before the juvenile can live a crime-free lifestyle. A fear of prison, developed through brief exposure, is not enough to counteract the risk factors present in the lives of most delinquents. Although the Scared Straight program sounds good, it is illogical to assume that a brief experience with prison life will have a stronger impact on the decisions made by delinquents than peer support for delinquency, drug abuse, lack of education, poor parental supervision, and other factors that influence delinquency.
Resistance to change is the reluctance to change our beliefs in light of new, accurate, and valid information to the contrary. Resistance to change is common and it occurs for several reasons. First, even though our personal experience may be counter to our belief system, it is hard to admit we were wrong after we have taken a position on an issue. Even when the research evidence shows otherwise, people who work within programs may still believe they are effective. As previously stated, even though the research evidence shows otherwise, Scared Straight programs still exist and there is even a television show devoted to the program. Second, too much devotion to tradition and the argument that this is the way it has always been done inhibits change and hinders our ability to accept new directions and develop new knowledge. Third, uncritical agreement with authority inhibits change. Although authority knowledge is certainly an important means of gaining knowledge, we must critically evaluate the ideas, beliefs, and statements of those in positions of authority and be willing to challenge those statements where necessary. However, people often accept the beliefs of those in positions of authority without question, which hinders change.
Knowledge from Media Portrayals
Television shows, movies, websites, newspapers, and magazine articles are important sources of information. This is especially true for information about crime and the criminal justice system since most people have not had much contact with criminals or the criminal justice system. Instead of gaining knowledge about the criminal justice system through personal experience, most people learn about crime and the operations of the criminal justice system through media outlets. Since the primary goal of many of these media outlets is to entertain, they may not accurately reflect the reality of crime and criminal justice. Despite their inaccuracies, the media has a substantial impact on what people know about crime and the criminal justice system. Most people know what they know about crime and criminal justice through the media, and this knowledge even has an impact on criminal justice system operations.
An example of the potential impact of the media on the actual operations of the criminal justice system involves the CSI: Crime Scene Investigation television shows. The shows have been criticized for their unrealistic portrayal of the role of forensic science in solving criminal cases. Critics claim that CSI viewers accept what they see on the show as an accurate representation of how forensic science works. When summoned for jury duty, they bring with them unrealistic expectations of the forensic evidence they will see in trial. When the expected sophisticated forensic evidence is not presented in the real trial, the juror is more likely to vote to acquit the defendant. This phenomenon is known as the CSI Effect. Has the research shown that the CSI Effect exists and is impacting the criminal justice system? Most of the research shows that the CSI Effect does not exist and thus does not impact juror decision-making, but other research has shown that viewers of CSI have higher expectations related to evidence presented at trial. 17
There are several instances in which media attention on a particular topic created the idea that a major problem existed when it did not. An example is Halloween sadism. Halloween sadism is the practice of giving contaminated treats to children during trick or treating. 18 In 1985, Joel Best wrote an article entitled, �The Myth of the Halloween Sadist.� 19 His article reviewed press coverage of Halloween sadism in the leading papers in the three largest metropolitan areas ( New York Times, Los Angeles Times, and Chicago Tribune ) from 1958�1984. Although the belief in Halloween sadism is widespread, Best found few reported incidents and few reports of children being injured by Halloween sadism. Follow-ups on these reported incidents led to the conclusion that most of these reports were hoaxes. Best concluded, �I have been unable to find a substantiated report of a child being killed or seriously injured by a contaminated treat picked up in the course of trick or treating.� 20 Since 1985, Best has kept his research up to date and has come to the same conclusion. Halloween sadism is an urban legend; it is a story that is told as true, even though there is little or no evidence that the events in the story ever occurred.
Dispelling Myths: The Power of Research Methods
In the prior section, sources of knowledge were discussed along with the limitations of each. A researcher (e.g., criminologist), ideally, takes no knowledge claim for granted, but instead relies on research methods to discover the truth. In the attempt to generate new knowledge, a researcher is skeptical of knowledge that is generated by the sources discussed in the prior section, and this skepticism leads to the questioning of conventional thinking. Through this process, existing knowledge claims are discredited, modified, or substantiated. Research methods provide the researcher with the tools necessary to test current knowledge and discover new knowledge.
Although knowledge developed through research methods is by no means perfect and infallible, it is definitely a more systematic, structured, precise, and evidence-based process than the knowledge sources previously discussed. However, researchers should not dismiss all knowledge from the prior sources discussed, because, as mentioned, these sources of knowledge are sometimes accurate and certainly have their place in the development of knowledge. Researchers should guard against an elitist mind-set in which all knowledge, unless it is research-based knowledge, is dismissed.
To further discuss the importance of research methods in the development of knowledge, this section will discuss myths about crime and criminal justice. Myths are beliefs that are based on emotion rather than rigorous analysis. Take the myth of the Halloween sadist previously discussed. Many believe that there are real examples of children being harmed by razor blades, poison, or other nefarious objects placed in Halloween candy. This belief has changed the practices of many parents on Halloween; not allowing their children to trick-or-treat in their neighborhood and forbidding them from going to the doors of strangers. After careful analysis by Best, there is not a single, known example of children being seriously injured or killed by contaminated candy given by strangers. The Halloween sadist is a myth but it is still perpetuated today, and as the definition states, it is a belief based upon emotion rather than rigorous analysis. People accept myths as accurate knowledge of reality when, in fact, the knowledge is false.
The power of research is the ability to dispel myths. If someone were to assess the research literature on a myth or do their own research, she would find that the knowledge based on the myth is wrong. Perceived reality is contradicted by the facts developed through research. But that does not mean that the myth still doesn�t exist. It is important to keep in mind that the perpetuation and acceptance of myths by the public, politicians, and criminal justice personnel has contributed to the failure of criminal justice practices and policies designed to reduce crime and improve the operations of the criminal justice system. In this section, a detailed example of a myth about crime, police, courts, and corrections will be presented to demonstrate how the myth has been dispelled through research. In addition, several additional myths about crime, police, courts, and corrections will be briefly presented.
The Health Benefits of Alcohol Consumption 21
The press release from Oregon State University is titled �Beer Compound Shows Potent Promise in Prostate Cancer Battle.� The press release leads to several newspaper articles throughout the country written on the preventative nature of drinking beer on prostate cancer development with titles such as �Beer Protects Your Prostate� and �Beer May Help Men Ward Off Prostate Cancer.� By the titles alone, this sounds great; one of the main ingredients in beer appears to thwart prostate cancer.
The study that generated these headlines was conducted by a group of researchers at Oregon State University using cultured cells with purified compounds in a laboratory setting. The research showed that xanthohumol, a compound found in hops, slowed the growth of prostate cancer cells and also the growth of cells that cause enlarged prostates. But you would have to drink more than 17 pints of beer to consume a medically effective dose of xanthohumol, which is almost a case of beer. In addition, although the research is promising, further study is necessary to determine xanthohumol�s true impact on prostate cancer.
These are the types of headlines that people pay attention to and want to believe as true, even if disproven by later research. People want to believe that there are health benefits to alcohol consumption. You have probably heard about the health benefits of drinking red wine, but here is something you should consider. Recently, the University of Connecticut released a statement describing an extensive research misconduct investigation involving a member of its faculty. The investigation was sparked by an anonymous allegation of research irregularities. The comprehensive report of the investigation, which totals approximately 60,000 pages, concludes that the professor is guilty of 145 counts of fabrication and falsification of data. The professor had gained international notoriety for his research into the beneficial properties of resveratrol, which is found in red wine, especially its impact on aging. Obviously, this throws his research conclusions, that red wine has a beneficial impact on the aging process, into question.
Myths about Crime�Drug Users Are Violent
The myth of drug users as violent offenders continues to be perpetuated by media accounts of violent drug users. The public sees drug users as violent offenders who commit violent crimes to get money for drugs or who commit violent crimes while under the intoxicating properties of drugs. The public also recognizes the violent nature of the drug business with gangs and cartels using violence to protect their turf. In May 2012, extensive media attention was given to the case of the Miami man who ate the face of a homeless man for an agonizing 18 minutes until police shot and killed the suspect. The police believed that the suspect was high on the street drug known as �bath salts.� This horrific case definitely leaves the image in the public�s mind about the relationship between violence and drug use.
In recent years, media reports have focused on the relationship between methamphetamine use and violence; before then it was crack cocaine use and violence. 32 However, media portrayals regarding the violent tendencies of drug users date back to the 1930s and the release of Reefer Madness. In 1985, Goldstein suggested that drugs and violence could be related in three different ways:
1. violence could be the direct result of drug ingestion;
2. violence could be a product of the instability of drug market activity; and
3. violence could be the consequence of people having a compulsive need for drugs or money for drugs. 33
So, what does the research show? Studies have found that homicides related to crack cocaine were usually the product of the instability of drug market activity (i.e., buying and selling drugs can be a violent activity) and rarely the result of drug ingestion. 34 After an extensive review of research studies on alcohol, drugs, and violence, Parker and Auerhahn concluded, �Despite a number of published statements to the contrary, we find no significant evidence suggesting that drug use is associated with violence. There is substantial evidence to suggest that alcohol use is significantly associated with violence of all kinds.� 35 The reality is not everyone who uses drugs becomes violent and users who do become violent do not do so every time they use drugs; therefore, the relationship between violence and drug use is a myth.
MYTHS ABOUT CRIME
Some additional myths about crime that research does not support include:
�Crime statistics accurately show what crimes are being committed and what crimes are most harmful. 22
�Most criminals�especially the dangerous ones�are mentally ill. 23
�White-collar crime is only about financial loss and does not hurt anyone. 24
�Serial murderers are middle-aged, white males. 25
�Criminals are significantly different from noncriminals. 26
�People are more likely to be a victim of violent crime committed by a stranger than by someone they know. 27
�Older adults are more likely to be victimized than people in any other age group. 28
�Sex offender registration protects the public from sexual predators. 29
�Juvenile crime rates are significantly increasing. 30
�Only the most violent juveniles are tried as adults. 31
Myths about Police�Female Police Officers Do Not Perform as Well as Males
Female police officers still face the myth that they cannot perform as well as male police officers. Throughout history, females have faced significant difficulties even becoming police officers. In the past, it was common for police agencies to require all police applicants to meet a minimum height requirement to be considered for employment. The minimum height requirement was 5?8? for most agencies, which limited the ability of females to successfully meet the minimum standards to become a police officer. Even if women could meet the minimum height requirements, they were typically faced with a physical-abilities test that emphasized upper body strength (e.g., push-ups and bench presses). Women failed these tests more often than men, and thus were not eligible to be police officers. Minimum height requirements are no longer used in law enforcement, but the perception that female police officers are not as good as males still exists. Today, the myth that women cannot be effective police officers is based largely on the belief that the need to demonstrate superior physical strength is a daily, common occurrence in law enforcement along with the belief that police work is routinely dangerous, violent, and crime-related.
So, what does the research show? On occasion, it is useful for police officers to be able to overpower suspects by demonstrating superior physical strength, but those types of activities are rare in law enforcement. In addition, it is fairly rare for a police officer to have to deal with a dangerous and violent encounter or even an incident involving a crime. The Police Services Study conducted in the 1970s analyzed 26,418 calls for service in three metropolitan areas and found that only 19% of calls for service involve crime and only 2% of the total calls for service involve violent crime. 43 This research study was among the first to assess the types of calls for service received by police agencies.
Despite the belief that women do not make good police officers, consistent research findings show that women are extremely capable as police officers, and in some respects, outperform their male counterparts. 44 Research has demonstrated several advantages to the hiring, retention, and promotion of women in law enforcement. First, female officers are as competent as their male counterparts. Research does not show any consistent differences in how male and female patrol officers perform their duties. Second, female officers are less likely to use excessive force. Research has shown that female patrol officers are less likely to be involved in high-speed pursuits, incidents of deadly force, and the use of excessive force. Female officers are more capable at calming potentially violent situations through communication and also demonstrate heightened levels of caution. Third, female officers can help implement community-oriented policing. Studies have shown that female officers are more supportive of the community-policing philosophy than are their male counterparts. Fourth, female officers can improve law enforcement�s response to violence against women. Studies have shown that female officers are more patient and understanding in handling domestic violence calls, and female victims of domestic violence are more likely to provide positive evaluations of female officers than their male counterparts. 52
MYTHS ABOUT POLICE
Some additional myths about the police that research does not support include:
�Police target minorities for traffic stops and arrests. 36
�Most crimes are solved through forensic science. 37
�COMPSTAT reduces crime. 38
�Intensive law enforcement efforts at the street level will lead to the control of illicit drug use and abuse. 39
�Police work primarily entails responding to crimes in progress or crimes that have just occurred. 40
�Police presence reduces crime. 41
�Detectives are most responsible for solving crimes and arresting offenders. 42

Myths about Courts�The Death Penalty Is Administered Fairly
According to a recent Gallup poll, 52% of Americans say the death penalty is applied fairly in the United States, the lowest mark in almost 40 years. 53 The issue of fairness and the death penalty typically concerns whether the punishment is equally imposed on offenders who are equally deserving based on legal factors (i.e., similar offense, similar prior criminal history, similar aggravating circumstances, and similar mitigating circumstances). 54 Unfairness can be shown if similarly situated offenders are more or less likely to receive death sentences based on age, gender, and race.
So, what does the research show? First, has research shown that a defendant�s age influences his or her chances of being sentenced to death? A study of about 5,000 homicides, controlling for legally relevant variables, found that defendants over the age of 25 were more than twice as likely to receive the death penalty in comparison to those 25 years of age or younger. 55
Second, has research shown that a defendant�s gender influences his or her chance of being sentenced to death? Capital punishment is almost exclusively reserved for male defendants. On December 31, 2010, there were 3,158 prisoners under a sentence of death in the United States: 58 were women, or 1.8%. 56 However, women account for 10�12% of all murders in the United States. 57 One research study found that male defendants were 2.6 times more likely than females to receive a death sentence after controlling for legally relevant factors. 58
Third, has research shown that a defendant�s race influences his or her chance of being sentenced to death? Most of the research on the biased nature of the death penalty has focused on racial inequities in the sentence. Although some research has shown that a defendant�s race has an impact on the likelihood of receiving a death sentence, a significant amount of research has shown that the race of the victim has the most substantial impact on death sentences. The research evidence clearly shows that offenders who murder white victims are more likely to receive a death sentence than offenders who murder black victims. 59 When assessing the race of both the victim and offender, the composition most likely to receive the death penalty is when a black offender murders a white victim. 60
MYTHS ABOUT COURTS
Some additional myths about courts that research does not support include:
�Many criminals escape justice because of the exclusionary rule. 45
�Subjecting juvenile offenders to harsh punishments can reduce crime committed by juveniles. 46
�Public opinion is overwhelmingly in favor of imprisonment and harsh punishment for offenders. 47
�The death penalty brings closure and a sense of justice to the family and friends of murder victims. 48
�Insanity is a common verdict in criminal courts in the United States. 49
�Eyewitness identification is reliable evidence. 50
�Most people who commit crimes based on hatred, bias, or discrimination face hate crime charges and longer sentencing. 51
Myths about Corrections�Imprisonment Is the Most Severe Form of Punishment
It seems clear that besides the death penalty, the most severe punishment available in our criminal justice system is to lock up offenders in prison. On a continuum, it is perceived that sentence severity increases as one moves from fines, to probation, to intermediate sanctions such as boot camps, and finally, to incarceration in prison. The public and politicians support this perception as well.
So, what does the research show? What do criminals think is the most severe form of punishment? A growing body of research has assessed how convicted offenders perceive and experience the severity of sentences in our criminal justice system. 61 Research suggests that alternatives to incarceration in prison (i.e., probation and intermediate sanctions) are perceived by many offenders as more severe due to a greater risk of program failure (e.g., probation revocation). In comparison, serving prison time is easier. 62 �
For example, one study found that about one-third of nonviolent offenders given the option of participating in an Intensive Supervision Probation (ISP) program, chose prison instead because the prospects of working every day and submitting to random drug tests was more punitive than serving time in prison. 73 Prisoners also stated that they would likely be caught violating probation conditions (i.e., high risk of program failure) and be sent to prison anyway. 74 In another research study involving survey responses from 415 inmates serving a brief prison sentence for a nonviolent crime, prison was considered the eighth most severe sanction, with only community service and probation seen as less punitive. Electronic monitoring (seventh), intensive supervision probation (sixth), halfway house (fifth), intermittent incarceration (fourth), day reporting (third), county jail (second), and boot camp (first) were all rated by inmates as more severe sanctions than prison. 75
MYTHS ABOUT CORRECTIONS
Some additional myths about corrections that research does not support include:
�Punishing criminals reduces crime. 63
�Prisons are too lenient in their day-to-day operations (prisons as country clubs). 64
�Prisons can be self-supporting if only prisoners were forced to work. 65
�Private prisons are more cost effective than state-run prisons. 66
�Focus of community corrections is rehabilitation rather than punishment. 67
�Correctional rehabilitation does not work. 68
�Drug offenders are treated leniently by the criminal justice system. 69
�Most death row inmates will be executed eventually. 70
�If correctional sanctions are severe enough, people will think twice about committing crimes. 71
�Sexual violence against and exploitation of inmates of the same gender are primarily the result of lack of heterosexual opportunities. 72
What is Research and Why is It Important to be an Informed Consumer of Research?
We probably should have started the chapter with the question �What is research?� but we wanted to initially lay a foundation for the question with a discussion of the problems with how knowledge is developed and the power of research in discovering the truth. Research methods are tools that allow criminology and criminal justice researchers to systematically study crime and the criminal justice system. The study of research methods is the study of the basic rules, appropriate techniques, and relevant procedures for conducting research. Research methods provide the tools necessary to approach issues in criminal justice from a rigorous standpoint and challenge opinions based solely on nonscientific observations and experiences. Similarly, research is the scientific investigation of an issue, problem, or subject utilizing research methods. Research is a means of knowledge development that is designed to assist in discovering answers to research questions and leads to the creation of new questions.
How Is Knowledge Development through Research Different?
Previously, sources of knowledge development were discussed, including authority, tradition, common sense, personal experience, and media portrayals. The problems generated by each knowledge source were also discussed. Research is another source of knowledge development, but it is different than those previously discussed in several ways. First, research relies on logical and systematic methods and observations to answer questions. Researchers use systematic, well-established research practices to seek answers to their questions. The methods and observations are completed in such a way that others can inspect and assess the methods and observations and offer feedback and criticism. Researchers develop, refine, and report their understanding of crime and the criminal justice system more systematically than the public does through casual observation. Those who conduct scientific research employ much more rigorous methods to gather the information/knowledge they are seeking.
Second, in order to prove that a research finding is correct, a researcher must be able to replicate the finding using the same methods. Only through replication can we have confidence in our original finding. For researchers, it may be important to replicate findings many times over so that we are assured our original finding was not a coincidence or chance occurrence. The Minneapolis Domestic Violence Experiment is an example of this and will be discussed in detail in Chapter 5. In the experiment, the researchers found that arrests for domestic violence lead to fewer repeat incidences in comparison to separation of the people involved and mediation. Five replication studies were conducted and none were able to replicate the findings in the Minneapolis study. In fact, three of the replications found that those arrested for domestic violence had higher levels of continued domestic violence, so arrest did not have the deterrent effect found in the Minneapolis study.
Third, research is objective. Objectivity indicates a neutral and nonbiased perspective when conducting research. Although there are examples to the contrary, the researcher should not have a vested interest in what findings are discovered from the research. The researcher is expected to remain objective and report the findings of the study regardless of whether the findings support their personal opinion or agenda. In addition, research ensures objectivity by allowing others to examine and be critical of the methodology, findings, and results of research studies.
It should be clear that using research methods to answer questions about crime and the criminal justice system will greatly reduce the errors in the development of knowledge previously discussed. For example, research methods reduces the likelihood of overgeneralization by using systematic procedures for selecting individuals or groups to study that are representative of the individuals or groups that we wish to generalize. This is the topic of Chapter 3, which covers sampling procedures. In addition, research methods reduces the risk of selective observation by requiring that we measure and observe our research subjects systematically.
Being an Informed Consumer of Research
Criminal justice and criminological research is important for several reasons. First, it can provide better and more objective information. Second, it can promote better decision-making. Today, more than ever, we live in a world driven by data and in which there is an increasing dependence on the assessment of data when making decisions. As well as possible, research ensures that our decisions are based on data and not on an arbitrary or personal basis. Third, it allows for the objective assessment of programs. Fourth, it has often been the source of innovation within criminal justice agencies. Fifth, it can be directly relevant to criminal justice practice and have a significant impact on criminal justice operations.
Before we apply research results to practices in the criminal justice system, and before we even accept those research results as reasonable, we need to be able to know whether or not they are worthwhile. In other words, should we believe the results of the study? Research has its own limitations, so we need to evaluate research results and the methods used to produce them, and we do so through critical evaluation. Critical evaluation involves identifying both positive and negative aspects of the research study�both the good and the bad. Critical evaluation involves comparing the methodology used in the research with the standards established in research methods.
Through critical evaluation, consumers of research break studies down into their essential elements. What are the research questions and hypotheses? What were the independent and dependent variables? What research design was used? Was probability sampling used? What data-gathering procedures were employed? What type of data analysis was conducted and what conclusions were made? These are some of the questions that are asked by informed consumers of research. The evaluation of research ranges from the manner in which one obtains an idea to the ways in which one writes about the research results, and understanding each step in the research process is useful in our attempts to consume research conducted by others. Located between these two activities are issues concerning ethics, sampling, research design, data analyses, and interpretations.
The research design and procedures are typically the most critically evaluated aspects of research and will likewise receive the greatest amount of attention in this text. Informed consumers of research don�t just take the results of a research study at face value because the study is in an academic journal or written by someone with a Ph.D. Instead, informed consumers critically evaluate research. Taking what is learned throughout this text, critical evaluation of research is covered in Chapter 8, and upon completing this text, it is hoped that you will be an informed consumer of research and will put your research knowledge to use throughout your career.
Although many students will never undertake their own research, all will be governed by policies based upon research and exposed to research findings in their chosen professional positions. Most government agencies, including the criminal justice system, as well as private industry, routinely rely on data analysis. Criminal justice students employed with these agencies will be challenged if not prepared for quantitative tasks. Unfortunately, it is not unusual to find students as well as professionals in criminal justice who are unable to fully understand research reports and journal articles in their own field.
Beyond our criminal justice careers, we are all exposed to and use research to help us understand issues and to make personal decisions. For example, we know that cigarette smoking causes lung cancer and has other significant health impacts, so we don�t smoke. Your doctor tells you that your cholesterol is too high and you need to limit your red meat intake because research shows that consumption of red meat raises cholesterol; so, you quit eating red meat. That is why not all the examples in this text are criminal justice research examples. Some come from the medical field while others come from psychology and other disciplines. This is to remind you that you are probably exposed to much more research than you thought on day one of this class.
Overall, knowledge of research methods will allow you to more appropriately consider and consume information that is important to your career in criminal justice. It will help you better understand the process of asking and answering a question systematically and be a better consumer of the kind of information that you really need to be the best criminal justice professional you can. Once familiar with research methods, your anxiety about reviewing technical reports and research findings can be minimized. As discussed in the next section, research methods involve a process and once you understand the process, you can apply your knowledge to any research study, even those in other disciplines.
The Research Process
One of the nice things about studying research methods is it is about learning a process. Research methods can be seen as a sequential process with the first step being followed by the second step, and so on. There are certainly times when the order of the steps may be modified, but researchers typically follow the same process for each research study they complete regardless of the research topic (as depicted in Figure 2.1 in Chapter 2). Very simply, a research problem or question is identified, and a methodology is selected, developed, and implemented to answer the research question. This sequential process is one of the advantages of understanding research methods, because once you understand the process, you can apply that process to any research question that interests you. In addition, research methods are the same across disciplines. So, sampling is the same in business as it is in health education and as it is in criminal justice. Certainly the use of a particular method will be more common in one discipline in comparison to another, but the protocol for implementing the method to complete the research study is the same. For example, field research (discussed in Chapter 6) is used much more frequently in anthropology than in criminal justice. However, the research protocol to implement field research is the same whether you are studying an indigenous Indian tribe in South America in anthropology or a group of heroin users in St. Louis in criminal justice.
Some authors have presented the research process as a wheel or circle, with no specific beginning or end. Typically, the research process begins with the selection of a research problem and the development of research questions or hypotheses (discussed further in Chapter 2). It is common for the results of previous research to generate new research questions and hypotheses for the researcher. This suggests that research is cyclical, a vibrant and continuous process. When a research study answers one question, the result is often the generation of additional questions, which plunges the researcher right back into the research process to complete additional research to answer these new questions.
In this section, a brief overview of the research process will be presented. The chapters that follow address various aspects of the research process, but it is critical that you keep in mind the overall research process as you read this book, which is why is it presented here. Although you will probably not be expected to conduct a research study on your own, it is important for an educated consumer of research to understand the steps in the research process. The steps are presented in chronological order and appear neatly ordered. In practice, the researcher can go back and forth between the steps in the research process.
Step 1: Select a Topic and Conduct a Literature Review
The first step in the research process is typically the identification of a problem or topic that the researcher is interested in studying. Research topics can arise from a wide variety of sources, including the findings of a current study, a question that a criminal justice agency needs to have answered, or the result of intellectual curiosity. Once the researcher has identified a particular problem or topic, the researcher assesses the current state of the literature related to the problem or topic. The researcher will often spend a considerable amount of time in determining what the existing literature has to say about the topic. Has the topic already been studied to the point that the questions in which the researcher is interested have been sufficiently answered? If so, can the researcher approach the subject from a previously unexamined perspective? Many times, research topics have been previously explored but not brought to completion. If this is the case, it is certainly reasonable to examine the topic again. It is even appropriate to replicate a previous study to determine whether the findings reported in the prior research continue to be true in different settings with different participants. This step in the research process is also discussed in Chapter 2.
Step 2: Develop a Research Question
After a topic has been identified and a comprehensive literature review has been completed on the topic, the next step is the development of a research question or questions. The research question marks the beginning of your research study and is critical to the remaining steps in the research process. The research question determines the research plan and methodology that will be employed in the study, the data that will be collected, and the data analysis that will be performed. Basically, the remaining steps in the process are completed in order to answer the research question or questions established in this step. The development of research questions is discussed in more detail in Chapter 2.
Step 3: Develop a Hypothesis
After the research questions have been established, the next step is the formulation of hypotheses, which are statements about the expected relationship between two variables. For example, a hypothesis may state that there is no relationship between heavy metal music preference and violent delinquency. The two variables stated in the hypothesis are music preference and violent delinquency. Hypothesis development is discussed in more detail in Chapter 2.
Step 4: Operationalize Concepts
Operationalization involves the process of giving the concepts in your study a working definition and determining how each concept in your study will be measured. For example, in Step 3, the variables were music preference and violent delinquency. The process of operationalization involves determining how music preference and violent delinquency will be measured. Operationalization is further discussed in Chapter 2.
Step 5: Develop the Research Plan and Methodology
The next step is to develop the methodology that will be employed to answer the research questions and test the hypotheses. The research methodology is the blueprint for the study, which outlines how the research is to be conducted. The research questions will determine the appropriate methodology for the study. The research design selected should be driven by the research questions asked. In other words, the research questions dictate the methods used to answer them. The methodology is basically a research plan on how the research questions will be answered and will detail:
1. What group, subjects, or population will be studied and selected? Sampling will be discussed in Chapter 3.
2 . What research design will be used to collect data to answer the research questions? Various research designs will be covered in Chapters 4�7.
You need to have familiarity with all research designs so that you can become an educated consumer of research. A survey cannot answer all research questions, so knowing a lot about surveys but not other research designs will not serve you well as you assess research studies. There are several common designs used in criminal justice and criminology research. Brief descriptions of several common research designs are presented below, but each is discussed in detail in later chapters.
Survey research is one of the most common research designs employed in criminal justice research. It obtains data directly from research participants by asking them questions and is often conducted through self-administered questionnaires and personal interviews. For example, a professor might have her students complete a survey during class to understand the relationship between drug use and self-esteem. Survey research is discussed in Chapter 4.
Experimental designs are used when researchers are interested in determining whether a program, policy, practice, or intervention is effective. For example, a researcher may use an experimental design to determine if boot camps are effective at reducing juvenile delinquency. Experimental design is discussed in Chapter 5.
Field research involves researchers studying individuals or groups of individuals in their natural environment. The researcher is observing closely or acting as part of the group under study and is able to describe in depth not only the subject�s behaviors, but also consider the motivations that drive those behaviors. For example, if a researcher wanted to learn more about gangs and their activities, he may �hang out� with a gang in order to observe their behavior. Field research is discussed in Chapter 6.
A case study is an in-depth analysis of one or a few illustrative cases. This design allows the story behind an individual, a particular offender, to be told and then information from cases studies can be extrapolated to a larger group. Often these studies require the review and analysis of documents such as police reports and court records and interviews with the offender and others. For example, a researcher may explore the life history of a serial killer to try and understand why the offender killed. Case studies are discussed in Chapter 6.
Secondary data analysis occurs when researchers obtain and reanalyze data that was originally collected for a different purpose. This can include reanalyzing data collected from a prior research study, using criminal justice agency records to answer a research question, or historical research. For example, a researcher using secondary data analysis may analyze inmate files from a nearby prison to understand the relationship between custody level assignment and disciplinary violations inside prison. Secondary data analysis is discussed in Chapter 7.
Content analysis requires the assessment of content contained in mass communication outlets such as newspapers, television, magazines, and the like. In this research design, documents, publications, or presentations are reviewed and analyzed. For example, a researcher utilizing content analysis might review true crime books involving murder to see how the characteristics of the offender and victim in the true crime books match reality as depicted in the FBI�s Supplemental Homicide Reports. Content analysis is discussed in Chapter 7.
Despite the options these designs offer, other research designs are available and will be discussed later in the text. Ultimately, the design used will depend on the nature of the study and the research questions asked.
Step 6: Execute the Research Plan and Collect Data
The next step in the research process is the collection of the data based on the research design developed. For example, if a survey is developed to study the relationship between gang membership and violent delinquency, the distribution and collection of surveys from a group of high school students would occur in this step. Data collection is discussed in several chapters throughout this text.
Step 7: Analyze Data
After the data have been collected, the next phase in the research process involves analyzing the data through various and appropriate statistical techniques. The most common means for data analysis today is through the use of a computer and statistically oriented software. Data analysis and statistics are discussed in Chapter 9.
Step 8: Report Findings, Results, and Limitations
Reporting and interpreting the results of the study make up the final step in the research process. The findings and results of the study can be communicated through reports, journals, books, or computer presentations. At this step, the results are reported and the research questions are answered. In addition, an assessment is made regarding the support or lack of support for the hypotheses tested. It is also at this stage that the researcher can pose additional research questions that may now need to be answered as a result of the research study. In addition, the limitations of the study, as well as the impact those limitations may have on the results of the study, will be described by the researcher. All research has limitations, so it is incumbent on the researcher to identify those limitations for the reader. The process of assessing the quality of research will be discussed in Chapter 8.
Research in Action: Impacting Criminal Justice Operations
Research in the criminal justice system has had significant impacts on its operations. The following sections provide an example of research that has significantly impacted each of the three main components of the criminal justice system: police, courts, and corrections. The purpose of this section is to demonstrate that research has aided the positive development and progression of the criminal justice system.
Police Research Example 76
The efforts of criminal justice researchers in policing have been important and have created the initial and critical foundation necessary for the further development of effective and productive law enforcement. One seminal study asked: How important is it for the police to respond quickly when a citizen calls? The importance of rapid response was conveyed in a 1973 National Commission on Productivity Report despite the fact that there was very little empirical evidence upon which to base this assumption. In fact, the Commission stated �there is no definitive relationship between response time and deterrence, but professional judgment and logic do suggest that the two are related in a strong enough manner to make more rapid response important.� 77 Basically the Commission members were stating that we don�t have any research evidence that response times are important, but we �know� that they are. Police departments allocated substantial resources to the patrol function and deployed officers in an effort to improve response time through the use of the 9-1-1 telephone number, computer-assisted dispatch, and beat assignment systems. Officers were typically assigned to a patrol beat. When the officers were not answering calls for service, they remained in their assigned beats so they could immediately respond to an emergency.
The data for the project were collected as part of a larger experiment on preventive patrol carried out in Kansas City, Missouri, between October 1972 and September 1973. 78 To determine the impact of response time, researchers speculated that the following variables would be influenced by response time: 1) the outcome of the response, 2) citizen satisfaction with response time, and 3) citizen satisfaction with the responding officer. Several data sources were used in the study. First, surveys were completed after all citizen-initiated calls (excluding automobile accidents) that involved contact with a police officer. The survey instrument consisted of questions to assess the length of time to respond to a call and the outcome of the call (i.e., arrest). Over 1,100 surveys were completed. Second, a follow-up survey was mailed to citizens whom the police had contacted during their response. These surveys asked questions to assess citizen satisfaction with response time and outcome. Over 425 of these surveys were returned.
The data collected during the study showed that response time did not determine whether or not the police made an arrest or recovered stolen property. This was the most surprising finding from the study because it challenged one of the basic underlying principles of police patrol. Researchers attributed the lack of significance to the fact that most citizens waited before calling the police. Rapid response simply did not matter in situations where citizens delayed in reporting the crime.
Rapid response time was not only believed to be important in determining the outcome of a response (i.e., more likely to lead to an arrest), it was also considered an important predictor of citizen satisfaction. Data from the study showed that when the police arrived sooner than expected, citizens were more satisfied with response time. However, subsequent research has shown that citizens are also satisfied with a delayed response as long as the dispatcher sets a reasonable expectation for when the patrol officer will arrive. Response time was also the best predictor of how satisfied a citizen was with the responding officer. It was further revealed that citizens became dissatisfied with the police when they were not informed of the outcome (i.e., someone was arrested). Again, these findings indicate the need for dispatchers and patrol officers to communicate with complainants regarding when they should expect an officer to arrive and the outcome of the call.
Based on the results of the response time study, the researchers concluded that rapid response was not as important as police administrators had thought. Response time was not related to an officer�s ability to make an arrest or recover stolen property. Results from the response time study challenged traditional beliefs about the allocation of patrol in our communities. Based on tradition knowledge, as previously discussed, rapidly responding to calls for service is what the police had always done since they started using patrol vehicles. In addition, common sense, as previously discussed, played a role in the practice of rapid response to calls for service; it just made sense that if a patrol officer arrives sooner, she will be more likely to make an arrest.
Prior to the research, police departments operated under the assumption that rapid response was a crucial factor in the ability of an officer to solve a crime and an important predictor of citizen satisfaction. In response to the research on rapid response, many police departments changed the way they responded to calls for service. Many departments adopted a differential police response approach. Differential police response protocols allow police departments to prioritize calls and rapidly dispatch an officer only when an immediate response is needed (i.e., crimes in progress). For crimes in progress, rapid response is critical and may reduce the injuries sustained by the victim as well, but these emergency calls usually account for less than 2% of all 9-1-1 calls for police service. For nonemergency calls, an officer is either dispatched at a later time when the officer is available or a report is taken over the phone or through some other means. Differential police response has been shown to save departments money and give patrol officers more time to engage in community-oriented and proactive policing activities. The benefits for a department are not at the expense of the public. In fact, a study by Robert Worden found a high degree of citizen satisfaction with differential police response. 79
Courts Research Example 80
Research on the courts component of the criminal justice system, while far from complete, has produced direct effects on the operations of the criminal justice system. The study reviewed in this section asked the following research question: Are jurors able to understand different legal rules for establishing a defendant�s criminal responsibility? The study described below explored the issue of criminal responsibility as it applies to the insanity defense in the United States. For several years, the M � Naghten rule was the legal rule applied in all courts of the United States. Under M � Naghten, criminal responsibility was absent when the offender did not understand the nature of his actions due to failure to distinguish �right� from �wrong.� This is known as the �right/wrong test� for criminal responsibility. The case of Durham v. United States was heard in the U.S. Court of Appeals for the District of Columbia and offered an alternative test for criminal responsibility and insanity. The legal rule emerging from Durham was that criminal responsibility was absent if the offense was a product of mental disease or defect. This ruling provided psychiatrists with a more important role at trial because of the requirement that the behavior be linked to a mental disorder that only a psychiatrist could officially determine.
At the time of Simon�s 1967 study, most courts across the country still followed the M � Naghten rule. Questions arose, however, regarding whether juries differed in their understanding of M � Naghten versus Durham and, in turn, whether this resulted in differences in their ability to make informed decisions regarding criminal responsibility in cases involving the insanity defense. The study was designed to determine the effect of different legal rules on jurors� decision-making in cases where the defense was insanity. There was a question of whether there was a difference between the rules to the extent that jurors understood each rule and could capably apply it.
Simon conducted an experimental study on jury deliberations in cases where the only defense was insanity. 81 Utilizing a mock jury approach, Simon took the transcripts of two actual trials with one reflecting the use of the M � Naghten rule and the other the Durham rule. Both cases were renamed and the transcripts were edited to constitute a trial of 60�90 minutes in length. These edited transcripts were then recorded, with University of Chicago Law School faculty as the attorneys, judges, and witnesses involved in each case. Groups of 12 jurors listened to each trial with instruction provided at the end regarding the particular rule of law ( M � Naghten or Durham) for determining criminal responsibility. Each juror submitted a written statement with his or her initial decision on the case before jury deliberations, and the juries� final decisions after deliberation were also reported.
Simon found significant differences in the verdicts across the two groups ( M � Naghten rule applied and Durham rule applied) even when the case was the same. For the M � Naghten version of the case, the psychiatrists stated that the defendant was mentally ill yet knew right from wrong during the crime. These statements/instructions should have led to a guilty verdict on the part of the mock jury. As expected, the M � Naghten juries delivered guilty verdicts in 19 of the 20 trials, with one hung jury. For the Durham version of the case, the psychiatrists stated that the crime resulted from the defendant�s mental illness, which should have lead to acquittal. However, the defendant was acquitted in only five of the 26 Durham trials. Twenty-six groups of 12 jurors were exposed to the Durham version of the trial and the case was the same each time. Simon interpreted these results as suggesting that jurors were unambiguous in their interpretations and applications of M � Naghten (due to the consistency in guilty verdicts), but they were less clear on the elements of Durham and how to apply it (reflected by the mix of guilty, not guilty, and hung verdicts). 82
After Simon�s study, most states rejected the Durham test. Recall her finding that the Durham rule produced inconsistent verdicts. She interpreted this finding as Durham being no better than providing no guidance to jurors on how to decide the issue of insanity. The observation helped to fuel arguments against the use of Durham, which, in turn, contributed to its demise as a legal rule. Today, only New Hampshire uses a version of the Durham rule in insanity cases.
WHAT RESEARCH SHOWS: IMPACTING CRIMINAL JUSTICE OPERATIONS
The Punishment Cost of Being Young, Black, and Male
Steffensmeier, Ulmer, and Kramer 83 hypothesized that African Americans overall were not likely to be treated more harshly than white defendants by the courts because it was only particular subgroups of minority defendants that fit with court actors� stereotypes of �more dangerous� offenders. In particular, they argued that younger African American males not only fulfilled this stereotype more than any other age, race, and gender combination, they were also more likely to be perceived by judges as being able to handle incarceration better than other subgroups.
In order to test their hypotheses, the researchers examined sentencing data from Pennsylvania spanning four years (1989�1992). Almost 139,000 cases were examined. The sentences they examined included whether a convicted defendant was incarcerated in prison or jail, and the length of incarceration in prison or jail. The researchers found that offense severity and prior record were the most important predictors of whether a convicted defendant was incarcerated and the length of incarceration. The authors found that the highest likelihood of incarceration and the longest sentences for males were distributed to African Americans aged 18�29 years. Their analysis of females revealed that white females were much less likely than African American females to be incarcerated, regardless of the age group examined. Taken altogether, the analysis revealed that African American males aged 18�29 years maintained the highest odds of incarceration and the longest sentences relative to any other race, sex, and age group.
Overall, this research showed that judges focused primarily on legal factors (offense severity and prior record) when determining the sentences of convicted offenders. These are the factors we expect judges to consider when making sentencing decisions. However, the research also found that judges base their decisions in part on extralegal factors, particularly the interaction of a defendant�s age, race, and gender. This research expanded our knowledge beyond the impact of singular factors on sentencing to expose the interaction effects of several variables (race, gender, and age). Court personnel are aware of these interaction effects based on this study, and others that followed, as well as their personal experiences in the criminal justice system. Identification and recognition of inequities in our justice system (in this case that young, African American males are punished more severely in our justice system) is the first step in mitigating this inequity.
Corrections Research Example 84
Although the research in corrections is far from complete, it has contributed greatly to the development of innovative programs and the professional development of correctional personnel. The contributions of academic and policy-oriented research can be seen across the whole range of correctional functions from pretrial services through probation, institutional corrections, and parole.
Rehabilitation remained the goal of our correctional system until the early 1970s, when the efficacy of rehabilitation was questioned. Violent crime was on the rise, and many politicians placed the blame on the criminal justice system. Some believed the system was too lenient on offenders. Interest in researching the effectiveness of correctional treatment remained low until 1974 when an article written by Robert Martinson and published in Public Interest titled �What Works? Questions and Answers about Prison Reform� generated enormous political and public attention to the effectiveness of correctional treatment. 85
Over a six-month period, Martinson and his colleagues reviewed all of the existing literature on correctional treatment published in English from 1945 to 1967. Each of the articles was evaluated according to traditional standards of social science research. Only studies that utilized an experimental design, included a sufficient sample size, and could be replicated were selected for review. A total of 231 studies examining a variety of different types of treatment were chosen, including educational and vocational training, individual and group counseling, therapeutic milieus, medical treatment, differences in length and type of incarceration, and community corrections. All of the treatment studies included at least one measure of offender recidivism, such as whether or not offenders were rearrested or violated their parole. The recidivism measures were used to examine the success or failure of a program in terms of reducing crime.
After reviewing all 231 studies, Martinson reported that there was no consistent evidence that correctional treatment reduced recidivism. Specifically, he wrote, �with few and isolated exceptions, the rehabilitative efforts that have been reported so far have had no appreciable effect on recidivism.� 86 Martinson further indicated that the lack of empirical support for correctional treatment could be a consequence of poorly implemented programs. If the quality of the programs were improved, the results may have proved more favorable, but this conclusion was for the most part ignored by the media and policy-makers.
Martinson�s report became commonly referred to as �nothing works� and was subsequently used as the definitive study detailing the failures of rehabilitation. The article had implications beyond questioning whether or not specific types of correctional treatment reduced recidivism. The entire philosophy of rehabilitation was now in doubt because of Martinson�s conclusion that �our present strategies … cannot overcome, or even appreciably reduce, the powerful tendencies of offenders to continue in criminal behavior.� 87
Martinson�s article provided policy makers the evidence to justify spending cuts on rehabilitative programs. Furthermore, it allowed politicians to respond to growing concerns about crime with punitive, get-tough strategies. States began implementing strict mandatory sentences that resulted in more criminals being sent to prison and for longer periods of time. Over the next several years, Martinson�s article was used over and over to support abandoning efforts to treat offenders until rehabilitation became virtually nonexistent in our correctional system.
Chapter Summary
This chapter began with a discussion of sources of knowledge development and the problems with each. To depict the importance of research methods in knowledge development, myths about crime and the criminal justice system were reviewed along with research studies that have dispelled myths. As the introductory chapter in this text, this chapter also provided an overview of the steps in the research process from selecting a topic and conducting a literature review at the beginning of a research study to reporting findings, results, and limitations at the end of the study. Examples of actual research studies in the areas of police, courts, and corrections were also provided in this chapter to demonstrate the research process in action and to illustrate how research has significantly impacted practices within the criminal justice system. In addition, this chapter demonstrated the critical importance of becoming an informed consumer of research in both your personal and professional lives.
Critical Thinking Questions
1. What are the primary sources of knowledge development, and what are the problems with each?
2. How is knowledge developed through research methods different from other sources of knowledge?
3. What myths about crime and criminal justice have been dispelled through research? Give an example of a research study that dispelled a myth.
4. Why is it important to be an informed consumer of research?
5. What are the steps in the research process, and what activities occur at each step?
authority knowledge: Knowledge developed when we accept something as being correct and true just because someone in a position of authority says it is true
case study: An in-depth analysis of one or a few illustrative cases
common sense knowledge: Knowledge developed when the information �just makes sense�
content analysis: A method requiring the analyzing of content contained in mass communication outlets such as newspapers, television, magazines, and the like
CSI Effect: Due to the unrealistic portrayal of the role of forensic science in solving criminal cases in television shows, jurors are more likely to vote to acquit a defendant when the expected sophisticated forensic evidence is not presented
differential police response: Methods that allow police departments to prioritize calls and rapidly dispatch an officer only when an immediate response is needed (i.e., crimes in progress)
experimental designs: Used when researchers are interested in determining whether a program, policy, practice, or intervention is effective
field research: Research that involves researchers studying individuals or groups of individuals in their natural environment
Halloween sadism: The practice of giving contaminated treats to children during trick or treating
hypotheses: Statements about the expected relationship between two concepts
illogical reasoning: Occurs when someone jumps to premature conclusions or presents an argument that is based on invalid assumptions
myths: Beliefs that are based on emotion rather than rigorous analysis
operationalization: The process of giving a concept a working definition; determining how each concept in your study will be measured
overgeneralization: Occurs when people conclude that what they have observed in one or a few cases is true for all cases
personal experience knowledge: Knowledge developed through actual experiences
research: The scientific investigation of an issue, problem, or subject utilizing research methods
research methods: The tools that allow criminology and criminal justice researchers to systematically study crime and the criminal justice system and include the basic rules, appropriate techniques, and relevant procedures for conducting research
resistance to change: The reluctance to change our beliefs in light of new, accurate, and valid information to the contrary
secondary data analysis: Occurs when researchers obtain and reanalyze data that were originally collected for a different purpose
selective observation: Choosing, either consciously or unconsciously, to pay attention to and remember events that support our personal preferences and beliefs
survey research: Obtaining data directly from research participants by asking them questions, often conducted through self-administered questionnaires and personal interviews
tradition knowledge: Knowledge developed when we accept something as true because that is the way things have always been, so it must be right
variables: Concepts that have been given a working definition and can take on different values
1 Briggs, Lisa T., Stephen E. Brown, Robert B. Gardner, and Robert L. Davidson. (2009). �D.RA.MA: An extended conceptualization of student anxiety in criminal justice research methods courses.� Journal of Criminal Justice Education 20 (3), 217�226.
2 Betz, N. E. (1978). �Prevalence, distribution, and correlates of math anxiety in college students. Journal of Counseling Psychology 25 (5), 441�448.
3 Briggs, et al., 2009, p. 221.
4 Ibid, p. 221.
5 Ibid, p. 221.
6 Kappeler, Victor E., and Gary W. Potter. (2005). The mythology of crime and criminal justice. Prospect Heights, IL: Waveland.
7 Tennessee v. Gamer, 471 U.S. 1 (1985).
8 Lombroso-Ferrero, Gina. (1911). Criminal man, according to the classification of Cesare Lombroso. New York: Putnam.
9 This study was included in Amy B. Thistlethwaite and John D. Wooldredge. (2010). Forty studies that changed criminal justice: Explorations into the history of criminal justice research. Upper Saddle River, NJ: Prentice Hall.
10 Petersilia, J., S. Turner, J. Kahan, and J. Peterson. (1985). Granting felons probation: Public risks and alternatives. Santa Monica, CA: Rand.
11 Vito, G. (1986). �Felony probation and recidivism: Replication and response.� Federal Probation 50, 17�25.
12 Conrad, J. (1985). �Research and development in corrections.� Federal Probation 49, 69�71.
13 Finckenauer, James O. (1982). Scared straight! and the panacea phenomenon. Englewood Cliffs, NJ: Prentice Hall.
14 Yarborough, J.C. (1979). Evaluation of JOLT (Juvenile Offenders Learn Truth) as a deterrence program. Lansing, MI: Michigan Department of Corrections.
15 Petrosino, Anthony, Carolyn Turpin-Petrosino, and James O. Finckenauer. (2000). �Well-meaning programs can have harmful effects! Lessons from experiments of programs such as Scared Straight,� Crime & Delinquency 46, 354�379.
16 Robertson, Jordan. �I�m being punished for living right�: Background check system is haunted by errors. December 20, 2011. http://finance.yahoo.com/news /ap-impact-criminal-past-isnt-182335059.html. Retrieved on December 29, 2011.
17 Shelton, D. E. (2008). �The �CSI Effect�: Does it really exist?� NIJ Journal 259 [NCJ 221501].
18 Best, Joel. (2011). �Halloween sadism: The evidence.� http://dspace.udel.edu:8080/dspace/bitstream/handle/ 19716/726/Halloween%20sadism.revised%20thru%20201l.pdf?sequence=6. Retrieved on May 7, 2012.
19 Best, Joel. (1985, November). �The myth of the Halloween sadist. Psychology Today 19 (11), p. 14.
21 �Beer compound shows potent promise in prostate cancer battle.� Press release from Oregon State University May 30, 2006. http://oregonstate.edu/ua/ncs/archives/2006/ may/beer-compound-shows-potent-promise-prostate-cancer-battle. Retrieved on January 6, 2012; Colgate, Emily C., Cristobal L. Miranda, Jan F. Stevens, Tammy M. Bray, and Emily Ho. (2007). �Xanthohumol, a prenylflavonoid derived from hops induces apoptosis and inhibits NF-kappaB activation in prostate epithelial cells,� Cancer Letters 246, 201�209; �Health benefits of red wine exaggerated� http://health.yahoo.net/articles /nutrition/health-benefits-red-wine-exaggerated. Retrieved on January 14, 2012; �Scientific journals notified following research misconduct investigation.� January 11, 2012. http://today.uconn.edu/blog/2012/01/scientific-journals -notified-following-research-misconduct-investigation/. Retrieved on January 14, 2012.
22 Pepinsky, Hal. �The myth that crime and criminality can be measured.� 3�11 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
23 Bullock, Jennifer L., and Bruce A. Arrigo. �The myth that mental illness causes crime.� 12�19 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
24 Friedrichs, David O. �The myth that white-collar crime is only about financial loss.� 20�28 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
25 Kuhns III, Joseph B., and Charisse T. M. Coston. �The myth that serial murderers are disproportionately white males.� 37�44 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
26 Longmire, Dennis R., Jacqueline Buffington-Vollum, and Scott Vollum. �The myth of positive differentiation in the classification of dangerous offenders.� 123�131 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
27 Masters, Ruth E., Lori Beth Way, Phyllis B. Gerstenfeld, Bernadette T. Muscat, Michael Hooper, John P. J. Dussich, Lester Pincu, and Candice A. Skrapec. (2013). CJ realities and challenges, 2nd ed. New York: McGraw-Hill.
32 Brownstein, Henry H. �The myth of drug users as violent offenders.� 45�53 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
33 Goldstein, P. (1985). �The drugs/violence nexus: A tripartite conceptual framework.� Journal of Drug Issues 15, 493�506.
34 Goldstein, P, H. Brownstein, and P. Ryan. (1992). �Drug-related homicide in New York City: 1984 and 1988.� Crime & Delinquency 38, 459�476.
35 Parker, R., and K. Auerhahn. (1998). �Alcohol, drugs, and violence.� Annual Review of Sociology 24, 291�311, p. 291.
36 Buerger, Michael. �The myth of racial profiling.� 97�103 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
37 Cordner, Gary, and Kathryn E. Scarborough. �The myth that science solves crimes.� 104�110 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
38 Willis, James J., Stephen D. Mastrofski, and David Weisburd. �The myth that COMPSTAT reduces crime and transforms police organizations.� 111�119 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
39 Masters, et al., 2013.
43 Scott, Eric J. (1981). Calls for service: Citizen demand and initial police response. Washington, DC: Government Printing Office.
44 Lersch, Kim. �The myth of policewomen on patrol.� 89�96 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
45 Janikowski, Richard. �The myth that the exclusionary rule allows many criminals to escape justice.� 132�139 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
46 Bishop, Donna M. �The myth that harsh punishments reduce juvenile crime.� 140�148 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
47 Immarigeon, Russ. �The myth that public attitudes are punitive.� 149�157 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
48 Acker, James R. �The myth of closure and capital punishment.� 167�175 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
49 Masters, et al., 2013.
52 Lersch, 2006.
53 Newport, Frank. �In U.S., support for death penalty falls to 39-year low.� October 13, 2011. http://www.gallup .com/poll/150089/support-death-penalty-falls-year-low.aspx. Retrieved on April 16, 2012.
54 Applegate, Brandon. �The myth that the death penalty is administered fairly.� 158�166 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
55 Williams, M. R., and J. E. Holcomb. (2001). �Racial disparity and death sentences in Ohio.� Journal of Criminal Justice 29, 207�218.
56 Snell, Tracy L. (2011, December). Capital punishment, 2010�statistical tables. Washington, DC: Bureau of Justice Statistics.
57 Applegate, 2006.
58 Williams and Holcomb, 2001.
59 Applegate, 2006.
61 Wood, Peter B. �The myth that imprisonment is the most severe form of punishment.� 192�200 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
63 Michalowski, Raymond. �The myth that punishment reduces crime.� 179�191 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
64 McShane, Marilyn, Frank P. Williams III, and Beth Pelz. �The myth of prisons as country clubs.� 201�208 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
65 Parker, Mary. �The myth that prisons can be self-supporting.� 209�213 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
66 Blakely, Curtis, and John Ortiz Smykla. �Correctional privatization and the myth of inherent efficiency.� 214�220 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
67 Jones, G. Mark. �The myth that the focus of community corrections is rehabilitation.� 221�226 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
68 Cullen, Francis T., and Paula Smith. �The myth that correctional rehabilitation does not work.� 227�238 in Bohm, Robert M., and Jeffrey T. Walker. (2006). Demystifying crime and criminal justice. Los Angeles: Roxbury.
69 Masters, et al., 2013.
73 Petersilia, Joan. (1990). �When probation becomes more dreaded than prison. Federal Probation 54, 23�27.
75 Wood, P. B., and H. G. Grasmick. (1999). �Toward the development of punishment equivalencies: Male and female inmates rate the severity of alternative sanctions compared to prison.� Justice Quarterly 16, 19�50.
76 Example is excerpted from Amy B. Thistlethwaite and John D. Wooldredge. (2010). Forty studies that changed criminal justice: Explorations into the history of criminal justice research. Upper Saddle River, NJ: Prentice Hall. This is an excellent book that demonstrates the impact research has had on criminal justice operations.
77 National Commission on Productivity. (1973). Opportunities for improving productivity in police services. Washington, DC: United States Government Printing Office, p. 19.
78 Pate, T., A. Ferrara, R. Bowers, and J. Lorence. (1976). Police response time: Its determinants and effects. Washington, DC: Police Foundation.
79 Worden, R. (1993). �Toward equity and efficiency in law enforcement: Differential police response. American Journal of Police 12, 1�32.
80 Example is excerpted from Amy B. Thistlethwaite and John D. Wooldredge. (2010). Forty studies that changed criminal justice: Explorations into the history of criminal justice research. Upper Saddle River, NJ: Prentice Hall.
81 Simon, R. (1967). The jury and the defense of insanity. Boston: Little, Brown.
83 Steffensmeier, D., J. Ulmer, & J. Kramer. (1998). �The interaction of race, gender, and age in criminal sentencing: The punishment cost of being young, black, and male. Criminology 36, 763�797.
84 Example is excerpted from Amy B. Thistlethwaite and John D. Wooldredge. (2010). Forty studies that changed criminal justice: Explorations into the history of criminal justice research. Upper Saddle River, NJ: Prentice Hall.
85 Martinson, R. (1974). �What works? Questions and answers about prison reform.� The Public Interest 10, 22�54.
86 Ibid, p. 25.
87 Ibid, p. 49.
Applied Research Methods in Criminal Justice and Criminology Copyright © 2022 by University of North Texas is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
What Is Research, and Why Do People Do It?
- Open Access
- First Online: 03 December 2022
Cite this chapter
You have full access to this open access chapter

- James Hiebert 6 ,
- Jinfa Cai 7 ,
- Stephen Hwang 7 ,
- Anne K Morris 6 &
- Charles Hohensee 6
Part of the book series: Research in Mathematics Education ((RME))
22k Accesses
Abstractspiepr Abs1
Every day people do research as they gather information to learn about something of interest. In the scientific world, however, research means something different than simply gathering information. Scientific research is characterized by its careful planning and observing, by its relentless efforts to understand and explain, and by its commitment to learn from everyone else seriously engaged in research. We call this kind of research scientific inquiry and define it as “formulating, testing, and revising hypotheses.” By “hypotheses” we do not mean the hypotheses you encounter in statistics courses. We mean predictions about what you expect to find and rationales for why you made these predictions. Throughout this and the remaining chapters we make clear that the process of scientific inquiry applies to all kinds of research studies and data, both qualitative and quantitative.
You have full access to this open access chapter, Download chapter PDF
Part I. What Is Research?
Have you ever studied something carefully because you wanted to know more about it? Maybe you wanted to know more about your grandmother’s life when she was younger so you asked her to tell you stories from her childhood, or maybe you wanted to know more about a fertilizer you were about to use in your garden so you read the ingredients on the package and looked them up online. According to the dictionary definition, you were doing research.
Recall your high school assignments asking you to “research” a topic. The assignment likely included consulting a variety of sources that discussed the topic, perhaps including some “original” sources. Often, the teacher referred to your product as a “research paper.”
Were you conducting research when you interviewed your grandmother or wrote high school papers reviewing a particular topic? Our view is that you were engaged in part of the research process, but only a small part. In this book, we reserve the word “research” for what it means in the scientific world, that is, for scientific research or, more pointedly, for scientific inquiry .
Exercise 1.1
Before you read any further, write a definition of what you think scientific inquiry is. Keep it short—Two to three sentences. You will periodically update this definition as you read this chapter and the remainder of the book.
This book is about scientific inquiry—what it is and how to do it. For starters, scientific inquiry is a process, a particular way of finding out about something that involves a number of phases. Each phase of the process constitutes one aspect of scientific inquiry. You are doing scientific inquiry as you engage in each phase, but you have not done scientific inquiry until you complete the full process. Each phase is necessary but not sufficient.
In this chapter, we set the stage by defining scientific inquiry—describing what it is and what it is not—and by discussing what it is good for and why people do it. The remaining chapters build directly on the ideas presented in this chapter.
A first thing to know is that scientific inquiry is not all or nothing. “Scientificness” is a continuum. Inquiries can be more scientific or less scientific. What makes an inquiry more scientific? You might be surprised there is no universally agreed upon answer to this question. None of the descriptors we know of are sufficient by themselves to define scientific inquiry. But all of them give you a way of thinking about some aspects of the process of scientific inquiry. Each one gives you different insights.

Exercise 1.2
As you read about each descriptor below, think about what would make an inquiry more or less scientific. If you think a descriptor is important, use it to revise your definition of scientific inquiry.
Creating an Image of Scientific Inquiry
We will present three descriptors of scientific inquiry. Each provides a different perspective and emphasizes a different aspect of scientific inquiry. We will draw on all three descriptors to compose our definition of scientific inquiry.
Descriptor 1. Experience Carefully Planned in Advance
Sir Ronald Fisher, often called the father of modern statistical design, once referred to research as “experience carefully planned in advance” (1935, p. 8). He said that humans are always learning from experience, from interacting with the world around them. Usually, this learning is haphazard rather than the result of a deliberate process carried out over an extended period of time. Research, Fisher said, was learning from experience, but experience carefully planned in advance.
This phrase can be fully appreciated by looking at each word. The fact that scientific inquiry is based on experience means that it is based on interacting with the world. These interactions could be thought of as the stuff of scientific inquiry. In addition, it is not just any experience that counts. The experience must be carefully planned . The interactions with the world must be conducted with an explicit, describable purpose, and steps must be taken to make the intended learning as likely as possible. This planning is an integral part of scientific inquiry; it is not just a preparation phase. It is one of the things that distinguishes scientific inquiry from many everyday learning experiences. Finally, these steps must be taken beforehand and the purpose of the inquiry must be articulated in advance of the experience. Clearly, scientific inquiry does not happen by accident, by just stumbling into something. Stumbling into something unexpected and interesting can happen while engaged in scientific inquiry, but learning does not depend on it and serendipity does not make the inquiry scientific.
Descriptor 2. Observing Something and Trying to Explain Why It Is the Way It Is
When we were writing this chapter and googled “scientific inquiry,” the first entry was: “Scientific inquiry refers to the diverse ways in which scientists study the natural world and propose explanations based on the evidence derived from their work.” The emphasis is on studying, or observing, and then explaining . This descriptor takes the image of scientific inquiry beyond carefully planned experience and includes explaining what was experienced.
According to the Merriam-Webster dictionary, “explain” means “(a) to make known, (b) to make plain or understandable, (c) to give the reason or cause of, and (d) to show the logical development or relations of” (Merriam-Webster, n.d. ). We will use all these definitions. Taken together, they suggest that to explain an observation means to understand it by finding reasons (or causes) for why it is as it is. In this sense of scientific inquiry, the following are synonyms: explaining why, understanding why, and reasoning about causes and effects. Our image of scientific inquiry now includes planning, observing, and explaining why.

We need to add a final note about this descriptor. We have phrased it in a way that suggests “observing something” means you are observing something in real time—observing the way things are or the way things are changing. This is often true. But, observing could mean observing data that already have been collected, maybe by someone else making the original observations (e.g., secondary analysis of NAEP data or analysis of existing video recordings of classroom instruction). We will address secondary analyses more fully in Chap. 4 . For now, what is important is that the process requires explaining why the data look like they do.
We must note that for us, the term “data” is not limited to numerical or quantitative data such as test scores. Data can also take many nonquantitative forms, including written survey responses, interview transcripts, journal entries, video recordings of students, teachers, and classrooms, text messages, and so forth.

Exercise 1.3
What are the implications of the statement that just “observing” is not enough to count as scientific inquiry? Does this mean that a detailed description of a phenomenon is not scientific inquiry?
Find sources that define research in education that differ with our position, that say description alone, without explanation, counts as scientific research. Identify the precise points where the opinions differ. What are the best arguments for each of the positions? Which do you prefer? Why?
Descriptor 3. Updating Everyone’s Thinking in Response to More and Better Information
This descriptor focuses on a third aspect of scientific inquiry: updating and advancing the field’s understanding of phenomena that are investigated. This descriptor foregrounds a powerful characteristic of scientific inquiry: the reliability (or trustworthiness) of what is learned and the ultimate inevitability of this learning to advance human understanding of phenomena. Humans might choose not to learn from scientific inquiry, but history suggests that scientific inquiry always has the potential to advance understanding and that, eventually, humans take advantage of these new understandings.
Before exploring these bold claims a bit further, note that this descriptor uses “information” in the same way the previous two descriptors used “experience” and “observations.” These are the stuff of scientific inquiry and we will use them often, sometimes interchangeably. Frequently, we will use the term “data” to stand for all these terms.
An overriding goal of scientific inquiry is for everyone to learn from what one scientist does. Much of this book is about the methods you need to use so others have faith in what you report and can learn the same things you learned. This aspect of scientific inquiry has many implications.
One implication is that scientific inquiry is not a private practice. It is a public practice available for others to see and learn from. Notice how different this is from everyday learning. When you happen to learn something from your everyday experience, often only you gain from the experience. The fact that research is a public practice means it is also a social one. It is best conducted by interacting with others along the way: soliciting feedback at each phase, taking opportunities to present work-in-progress, and benefitting from the advice of others.
A second implication is that you, as the researcher, must be committed to sharing what you are doing and what you are learning in an open and transparent way. This allows all phases of your work to be scrutinized and critiqued. This is what gives your work credibility. The reliability or trustworthiness of your findings depends on your colleagues recognizing that you have used all appropriate methods to maximize the chances that your claims are justified by the data.
A third implication of viewing scientific inquiry as a collective enterprise is the reverse of the second—you must be committed to receiving comments from others. You must treat your colleagues as fair and honest critics even though it might sometimes feel otherwise. You must appreciate their job, which is to remain skeptical while scrutinizing what you have done in considerable detail. To provide the best help to you, they must remain skeptical about your conclusions (when, for example, the data are difficult for them to interpret) until you offer a convincing logical argument based on the information you share. A rather harsh but good-to-remember statement of the role of your friendly critics was voiced by Karl Popper, a well-known twentieth century philosopher of science: “. . . if you are interested in the problem which I tried to solve by my tentative assertion, you may help me by criticizing it as severely as you can” (Popper, 1968, p. 27).
A final implication of this third descriptor is that, as someone engaged in scientific inquiry, you have no choice but to update your thinking when the data support a different conclusion. This applies to your own data as well as to those of others. When data clearly point to a specific claim, even one that is quite different than you expected, you must reconsider your position. If the outcome is replicated multiple times, you need to adjust your thinking accordingly. Scientific inquiry does not let you pick and choose which data to believe; it mandates that everyone update their thinking when the data warrant an update.
Doing Scientific Inquiry
We define scientific inquiry in an operational sense—what does it mean to do scientific inquiry? What kind of process would satisfy all three descriptors: carefully planning an experience in advance; observing and trying to explain what you see; and, contributing to updating everyone’s thinking about an important phenomenon?
We define scientific inquiry as formulating , testing , and revising hypotheses about phenomena of interest.
Of course, we are not the only ones who define it in this way. The definition for the scientific method posted by the editors of Britannica is: “a researcher develops a hypothesis, tests it through various means, and then modifies the hypothesis on the basis of the outcome of the tests and experiments” (Britannica, n.d. ).

Notice how defining scientific inquiry this way satisfies each of the descriptors. “Carefully planning an experience in advance” is exactly what happens when formulating a hypothesis about a phenomenon of interest and thinking about how to test it. “ Observing a phenomenon” occurs when testing a hypothesis, and “ explaining ” what is found is required when revising a hypothesis based on the data. Finally, “updating everyone’s thinking” comes from comparing publicly the original with the revised hypothesis.
Doing scientific inquiry, as we have defined it, underscores the value of accumulating knowledge rather than generating random bits of knowledge. Formulating, testing, and revising hypotheses is an ongoing process, with each revised hypothesis begging for another test, whether by the same researcher or by new researchers. The editors of Britannica signaled this cyclic process by adding the following phrase to their definition of the scientific method: “The modified hypothesis is then retested, further modified, and tested again.” Scientific inquiry creates a process that encourages each study to build on the studies that have gone before. Through collective engagement in this process of building study on top of study, the scientific community works together to update its thinking.
Before exploring more fully the meaning of “formulating, testing, and revising hypotheses,” we need to acknowledge that this is not the only way researchers define research. Some researchers prefer a less formal definition, one that includes more serendipity, less planning, less explanation. You might have come across more open definitions such as “research is finding out about something.” We prefer the tighter hypothesis formulation, testing, and revision definition because we believe it provides a single, coherent map for conducting research that addresses many of the thorny problems educational researchers encounter. We believe it is the most useful orientation toward research and the most helpful to learn as a beginning researcher.
A final clarification of our definition is that it applies equally to qualitative and quantitative research. This is a familiar distinction in education that has generated much discussion. You might think our definition favors quantitative methods over qualitative methods because the language of hypothesis formulation and testing is often associated with quantitative methods. In fact, we do not favor one method over another. In Chap. 4 , we will illustrate how our definition fits research using a range of quantitative and qualitative methods.
Exercise 1.4
Look for ways to extend what the field knows in an area that has already received attention by other researchers. Specifically, you can search for a program of research carried out by more experienced researchers that has some revised hypotheses that remain untested. Identify a revised hypothesis that you might like to test.
Unpacking the Terms Formulating, Testing, and Revising Hypotheses
To get a full sense of the definition of scientific inquiry we will use throughout this book, it is helpful to spend a little time with each of the key terms.
We first want to make clear that we use the term “hypothesis” as it is defined in most dictionaries and as it used in many scientific fields rather than as it is usually defined in educational statistics courses. By “hypothesis,” we do not mean a null hypothesis that is accepted or rejected by statistical analysis. Rather, we use “hypothesis” in the sense conveyed by the following definitions: “An idea or explanation for something that is based on known facts but has not yet been proved” (Cambridge University Press, n.d. ), and “An unproved theory, proposition, or supposition, tentatively accepted to explain certain facts and to provide a basis for further investigation or argument” (Agnes & Guralnik, 2008 ).
We distinguish two parts to “hypotheses.” Hypotheses consist of predictions and rationales . Predictions are statements about what you expect to find when you inquire about something. Rationales are explanations for why you made the predictions you did, why you believe your predictions are correct. So, for us “formulating hypotheses” means making explicit predictions and developing rationales for the predictions.
“Testing hypotheses” means making observations that allow you to assess in what ways your predictions were correct and in what ways they were incorrect. In education research, it is rarely useful to think of your predictions as either right or wrong. Because of the complexity of most issues you will investigate, most predictions will be right in some ways and wrong in others.
By studying the observations you make (data you collect) to test your hypotheses, you can revise your hypotheses to better align with the observations. This means revising your predictions plus revising your rationales to justify your adjusted predictions. Even though you might not run another test, formulating revised hypotheses is an essential part of conducting a research study. Comparing your original and revised hypotheses informs everyone of what you learned by conducting your study. In addition, a revised hypothesis sets the stage for you or someone else to extend your study and accumulate more knowledge of the phenomenon.
We should note that not everyone makes a clear distinction between predictions and rationales as two aspects of hypotheses. In fact, common, non-scientific uses of the word “hypothesis” may limit it to only a prediction or only an explanation (or rationale). We choose to explicitly include both prediction and rationale in our definition of hypothesis, not because we assert this should be the universal definition, but because we want to foreground the importance of both parts acting in concert. Using “hypothesis” to represent both prediction and rationale could hide the two aspects, but we make them explicit because they provide different kinds of information. It is usually easier to make predictions than develop rationales because predictions can be guesses, hunches, or gut feelings about which you have little confidence. Developing a compelling rationale requires careful thought plus reading what other researchers have found plus talking with your colleagues. Often, while you are developing your rationale you will find good reasons to change your predictions. Developing good rationales is the engine that drives scientific inquiry. Rationales are essentially descriptions of how much you know about the phenomenon you are studying. Throughout this guide, we will elaborate on how developing good rationales drives scientific inquiry. For now, we simply note that it can sharpen your predictions and help you to interpret your data as you test your hypotheses.

Hypotheses in education research take a variety of forms or types. This is because there are a variety of phenomena that can be investigated. Investigating educational phenomena is sometimes best done using qualitative methods, sometimes using quantitative methods, and most often using mixed methods (e.g., Hay, 2016 ; Weis et al. 2019a ; Weisner, 2005 ). This means that, given our definition, hypotheses are equally applicable to qualitative and quantitative investigations.
Hypotheses take different forms when they are used to investigate different kinds of phenomena. Two very different activities in education could be labeled conducting experiments and descriptions. In an experiment, a hypothesis makes a prediction about anticipated changes, say the changes that occur when a treatment or intervention is applied. You might investigate how students’ thinking changes during a particular kind of instruction.
A second type of hypothesis, relevant for descriptive research, makes a prediction about what you will find when you investigate and describe the nature of a situation. The goal is to understand a situation as it exists rather than to understand a change from one situation to another. In this case, your prediction is what you expect to observe. Your rationale is the set of reasons for making this prediction; it is your current explanation for why the situation will look like it does.
You will probably read, if you have not already, that some researchers say you do not need a prediction to conduct a descriptive study. We will discuss this point of view in Chap. 2 . For now, we simply claim that scientific inquiry, as we have defined it, applies to all kinds of research studies. Descriptive studies, like others, not only benefit from formulating, testing, and revising hypotheses, but also need hypothesis formulating, testing, and revising.
One reason we define research as formulating, testing, and revising hypotheses is that if you think of research in this way you are less likely to go wrong. It is a useful guide for the entire process, as we will describe in detail in the chapters ahead. For example, as you build the rationale for your predictions, you are constructing the theoretical framework for your study (Chap. 3 ). As you work out the methods you will use to test your hypothesis, every decision you make will be based on asking, “Will this help me formulate or test or revise my hypothesis?” (Chap. 4 ). As you interpret the results of testing your predictions, you will compare them to what you predicted and examine the differences, focusing on how you must revise your hypotheses (Chap. 5 ). By anchoring the process to formulating, testing, and revising hypotheses, you will make smart decisions that yield a coherent and well-designed study.
Exercise 1.5
Compare the concept of formulating, testing, and revising hypotheses with the descriptions of scientific inquiry contained in Scientific Research in Education (NRC, 2002 ). How are they similar or different?
Exercise 1.6
Provide an example to illustrate and emphasize the differences between everyday learning/thinking and scientific inquiry.
Learning from Doing Scientific Inquiry
We noted earlier that a measure of what you have learned by conducting a research study is found in the differences between your original hypothesis and your revised hypothesis based on the data you collected to test your hypothesis. We will elaborate this statement in later chapters, but we preview our argument here.
Even before collecting data, scientific inquiry requires cycles of making a prediction, developing a rationale, refining your predictions, reading and studying more to strengthen your rationale, refining your predictions again, and so forth. And, even if you have run through several such cycles, you still will likely find that when you test your prediction you will be partly right and partly wrong. The results will support some parts of your predictions but not others, or the results will “kind of” support your predictions. A critical part of scientific inquiry is making sense of your results by interpreting them against your predictions. Carefully describing what aspects of your data supported your predictions, what aspects did not, and what data fell outside of any predictions is not an easy task, but you cannot learn from your study without doing this analysis.

Analyzing the matches and mismatches between your predictions and your data allows you to formulate different rationales that would have accounted for more of the data. The best revised rationale is the one that accounts for the most data. Once you have revised your rationales, you can think about the predictions they best justify or explain. It is by comparing your original rationales to your new rationales that you can sort out what you learned from your study.
Suppose your study was an experiment. Maybe you were investigating the effects of a new instructional intervention on students’ learning. Your original rationale was your explanation for why the intervention would change the learning outcomes in a particular way. Your revised rationale explained why the changes that you observed occurred like they did and why your revised predictions are better. Maybe your original rationale focused on the potential of the activities if they were implemented in ideal ways and your revised rationale included the factors that are likely to affect how teachers implement them. By comparing the before and after rationales, you are describing what you learned—what you can explain now that you could not before. Another way of saying this is that you are describing how much more you understand now than before you conducted your study.
Revised predictions based on carefully planned and collected data usually exhibit some of the following features compared with the originals: more precision, more completeness, and broader scope. Revised rationales have more explanatory power and become more complete, more aligned with the new predictions, sharper, and overall more convincing.
Part II. Why Do Educators Do Research?
Doing scientific inquiry is a lot of work. Each phase of the process takes time, and you will often cycle back to improve earlier phases as you engage in later phases. Because of the significant effort required, you should make sure your study is worth it. So, from the beginning, you should think about the purpose of your study. Why do you want to do it? And, because research is a social practice, you should also think about whether the results of your study are likely to be important and significant to the education community.
If you are doing research in the way we have described—as scientific inquiry—then one purpose of your study is to understand , not just to describe or evaluate or report. As we noted earlier, when you formulate hypotheses, you are developing rationales that explain why things might be like they are. In our view, trying to understand and explain is what separates research from other kinds of activities, like evaluating or describing.
One reason understanding is so important is that it allows researchers to see how or why something works like it does. When you see how something works, you are better able to predict how it might work in other contexts, under other conditions. And, because conditions, or contextual factors, matter a lot in education, gaining insights into applying your findings to other contexts increases the contributions of your work and its importance to the broader education community.
Consequently, the purposes of research studies in education often include the more specific aim of identifying and understanding the conditions under which the phenomena being studied work like the observations suggest. A classic example of this kind of study in mathematics education was reported by William Brownell and Harold Moser in 1949 . They were trying to establish which method of subtracting whole numbers could be taught most effectively—the regrouping method or the equal additions method. However, they realized that effectiveness might depend on the conditions under which the methods were taught—“meaningfully” versus “mechanically.” So, they designed a study that crossed the two instructional approaches with the two different methods (regrouping and equal additions). Among other results, they found that these conditions did matter. The regrouping method was more effective under the meaningful condition than the mechanical condition, but the same was not true for the equal additions algorithm.
What do education researchers want to understand? In our view, the ultimate goal of education is to offer all students the best possible learning opportunities. So, we believe the ultimate purpose of scientific inquiry in education is to develop understanding that supports the improvement of learning opportunities for all students. We say “ultimate” because there are lots of issues that must be understood to improve learning opportunities for all students. Hypotheses about many aspects of education are connected, ultimately, to students’ learning. For example, formulating and testing a hypothesis that preservice teachers need to engage in particular kinds of activities in their coursework in order to teach particular topics well is, ultimately, connected to improving students’ learning opportunities. So is hypothesizing that school districts often devote relatively few resources to instructional leadership training or hypothesizing that positioning mathematics as a tool students can use to combat social injustice can help students see the relevance of mathematics to their lives.
We do not exclude the importance of research on educational issues more removed from improving students’ learning opportunities, but we do think the argument for their importance will be more difficult to make. If there is no way to imagine a connection between your hypothesis and improving learning opportunities for students, even a distant connection, we recommend you reconsider whether it is an important hypothesis within the education community.
Notice that we said the ultimate goal of education is to offer all students the best possible learning opportunities. For too long, educators have been satisfied with a goal of offering rich learning opportunities for lots of students, sometimes even for just the majority of students, but not necessarily for all students. Evaluations of success often are based on outcomes that show high averages. In other words, if many students have learned something, or even a smaller number have learned a lot, educators may have been satisfied. The problem is that there is usually a pattern in the groups of students who receive lower quality opportunities—students of color and students who live in poor areas, urban and rural. This is not acceptable. Consequently, we emphasize the premise that the purpose of education research is to offer rich learning opportunities to all students.
One way to make sure you will be able to convince others of the importance of your study is to consider investigating some aspect of teachers’ shared instructional problems. Historically, researchers in education have set their own research agendas, regardless of the problems teachers are facing in schools. It is increasingly recognized that teachers have had trouble applying to their own classrooms what researchers find. To address this problem, a researcher could partner with a teacher—better yet, a small group of teachers—and talk with them about instructional problems they all share. These discussions can create a rich pool of problems researchers can consider. If researchers pursued one of these problems (preferably alongside teachers), the connection to improving learning opportunities for all students could be direct and immediate. “Grounding a research question in instructional problems that are experienced across multiple teachers’ classrooms helps to ensure that the answer to the question will be of sufficient scope to be relevant and significant beyond the local context” (Cai et al., 2019b , p. 115).
As a beginning researcher, determining the relevance and importance of a research problem is especially challenging. We recommend talking with advisors, other experienced researchers, and peers to test the educational importance of possible research problems and topics of study. You will also learn much more about the issue of research importance when you read Chap. 5 .
Exercise 1.7
Identify a problem in education that is closely connected to improving learning opportunities and a problem that has a less close connection. For each problem, write a brief argument (like a logical sequence of if-then statements) that connects the problem to all students’ learning opportunities.
Part III. Conducting Research as a Practice of Failing Productively
Scientific inquiry involves formulating hypotheses about phenomena that are not fully understood—by you or anyone else. Even if you are able to inform your hypotheses with lots of knowledge that has already been accumulated, you are likely to find that your prediction is not entirely accurate. This is normal. Remember, scientific inquiry is a process of constantly updating your thinking. More and better information means revising your thinking, again, and again, and again. Because you never fully understand a complicated phenomenon and your hypotheses never produce completely accurate predictions, it is easy to believe you are somehow failing.
The trick is to fail upward, to fail to predict accurately in ways that inform your next hypothesis so you can make a better prediction. Some of the best-known researchers in education have been open and honest about the many times their predictions were wrong and, based on the results of their studies and those of others, they continuously updated their thinking and changed their hypotheses.
A striking example of publicly revising (actually reversing) hypotheses due to incorrect predictions is found in the work of Lee J. Cronbach, one of the most distinguished educational psychologists of the twentieth century. In 1955, Cronbach delivered his presidential address to the American Psychological Association. Titling it “Two Disciplines of Scientific Psychology,” Cronbach proposed a rapprochement between two research approaches—correlational studies that focused on individual differences and experimental studies that focused on instructional treatments controlling for individual differences. (We will examine different research approaches in Chap. 4 ). If these approaches could be brought together, reasoned Cronbach ( 1957 ), researchers could find interactions between individual characteristics and treatments (aptitude-treatment interactions or ATIs), fitting the best treatments to different individuals.
In 1975, after years of research by many researchers looking for ATIs, Cronbach acknowledged the evidence for simple, useful ATIs had not been found. Even when trying to find interactions between a few variables that could provide instructional guidance, the analysis, said Cronbach, creates “a hall of mirrors that extends to infinity, tormenting even the boldest investigators and defeating even ambitious designs” (Cronbach, 1975 , p. 119).
As he was reflecting back on his work, Cronbach ( 1986 ) recommended moving away from documenting instructional effects through statistical inference (an approach he had championed for much of his career) and toward approaches that probe the reasons for these effects, approaches that provide a “full account of events in a time, place, and context” (Cronbach, 1986 , p. 104). This is a remarkable change in hypotheses, a change based on data and made fully transparent. Cronbach understood the value of failing productively.
Closer to home, in a less dramatic example, one of us began a line of scientific inquiry into how to prepare elementary preservice teachers to teach early algebra. Teaching early algebra meant engaging elementary students in early forms of algebraic reasoning. Such reasoning should help them transition from arithmetic to algebra. To begin this line of inquiry, a set of activities for preservice teachers were developed. Even though the activities were based on well-supported hypotheses, they largely failed to engage preservice teachers as predicted because of unanticipated challenges the preservice teachers faced. To capitalize on this failure, follow-up studies were conducted, first to better understand elementary preservice teachers’ challenges with preparing to teach early algebra, and then to better support preservice teachers in navigating these challenges. In this example, the initial failure was a necessary step in the researchers’ scientific inquiry and furthered the researchers’ understanding of this issue.
We present another example of failing productively in Chap. 2 . That example emerges from recounting the history of a well-known research program in mathematics education.
Making mistakes is an inherent part of doing scientific research. Conducting a study is rarely a smooth path from beginning to end. We recommend that you keep the following things in mind as you begin a career of conducting research in education.
First, do not get discouraged when you make mistakes; do not fall into the trap of feeling like you are not capable of doing research because you make too many errors.
Second, learn from your mistakes. Do not ignore your mistakes or treat them as errors that you simply need to forget and move past. Mistakes are rich sites for learning—in research just as in other fields of study.
Third, by reflecting on your mistakes, you can learn to make better mistakes, mistakes that inform you about a productive next step. You will not be able to eliminate your mistakes, but you can set a goal of making better and better mistakes.
Exercise 1.8
How does scientific inquiry differ from everyday learning in giving you the tools to fail upward? You may find helpful perspectives on this question in other resources on science and scientific inquiry (e.g., Failure: Why Science is So Successful by Firestein, 2015).
Exercise 1.9
Use what you have learned in this chapter to write a new definition of scientific inquiry. Compare this definition with the one you wrote before reading this chapter. If you are reading this book as part of a course, compare your definition with your colleagues’ definitions. Develop a consensus definition with everyone in the course.
Part IV. Preview of Chap. 2
Now that you have a good idea of what research is, at least of what we believe research is, the next step is to think about how to actually begin doing research. This means how to begin formulating, testing, and revising hypotheses. As for all phases of scientific inquiry, there are lots of things to think about. Because it is critical to start well, we devote Chap. 2 to getting started with formulating hypotheses.
Agnes, M., & Guralnik, D. B. (Eds.). (2008). Hypothesis. In Webster’s new world college dictionary (4th ed.). Wiley.
Google Scholar
Britannica. (n.d.). Scientific method. In Encyclopaedia Britannica . Retrieved July 15, 2022 from https://www.britannica.com/science/scientific-method
Brownell, W. A., & Moser, H. E. (1949). Meaningful vs. mechanical learning: A study in grade III subtraction . Duke University Press..
Cai, J., Morris, A., Hohensee, C., Hwang, S., Robison, V., Cirillo, M., Kramer, S. L., & Hiebert, J. (2019b). Posing significant research questions. Journal for Research in Mathematics Education, 50 (2), 114–120. https://doi.org/10.5951/jresematheduc.50.2.0114
Article Google Scholar
Cambridge University Press. (n.d.). Hypothesis. In Cambridge dictionary . Retrieved July 15, 2022 from https://dictionary.cambridge.org/us/dictionary/english/hypothesis
Cronbach, J. L. (1957). The two disciplines of scientific psychology. American Psychologist, 12 , 671–684.
Cronbach, L. J. (1975). Beyond the two disciplines of scientific psychology. American Psychologist, 30 , 116–127.
Cronbach, L. J. (1986). Social inquiry by and for earthlings. In D. W. Fiske & R. A. Shweder (Eds.), Metatheory in social science: Pluralisms and subjectivities (pp. 83–107). University of Chicago Press.
Hay, C. M. (Ed.). (2016). Methods that matter: Integrating mixed methods for more effective social science research . University of Chicago Press.
Merriam-Webster. (n.d.). Explain. In Merriam-Webster.com dictionary . Retrieved July 15, 2022, from https://www.merriam-webster.com/dictionary/explain
National Research Council. (2002). Scientific research in education . National Academy Press.
Weis, L., Eisenhart, M., Duncan, G. J., Albro, E., Bueschel, A. C., Cobb, P., Eccles, J., Mendenhall, R., Moss, P., Penuel, W., Ream, R. K., Rumbaut, R. G., Sloane, F., Weisner, T. S., & Wilson, J. (2019a). Mixed methods for studies that address broad and enduring issues in education research. Teachers College Record, 121 , 100307.
Weisner, T. S. (Ed.). (2005). Discovering successful pathways in children’s development: Mixed methods in the study of childhood and family life . University of Chicago Press.
Download references
Author information
Authors and affiliations.
School of Education, University of Delaware, Newark, DE, USA
James Hiebert, Anne K Morris & Charles Hohensee
Department of Mathematical Sciences, University of Delaware, Newark, DE, USA
Jinfa Cai & Stephen Hwang
You can also search for this author in PubMed Google Scholar
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions
Copyright information
© 2023 The Author(s)
About this chapter
Hiebert, J., Cai, J., Hwang, S., Morris, A.K., Hohensee, C. (2023). What Is Research, and Why Do People Do It?. In: Doing Research: A New Researcher’s Guide. Research in Mathematics Education. Springer, Cham. https://doi.org/10.1007/978-3-031-19078-0_1
Download citation
DOI : https://doi.org/10.1007/978-3-031-19078-0_1
Published : 03 December 2022
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-19077-3
Online ISBN : 978-3-031-19078-0
eBook Packages : Education Education (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Search Menu
- Sign in through your institution
- CNS Injury and Stroke
- Epilepsy and Sleep
- Movement Disorders
- Multiple Sclerosis/Neuroinflammation
- Neuro-oncology
- Neurodegeneration - Cellular & Molecular
- Neuromuscular Disease
- Neuropsychiatry
- Pain and Headache
- Advance articles
- Editor's Choice
- Author Guidelines
- Submission Site
- Why publish with this journal?
- Open Access
- About Brain
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Why we need a revolution in clinical research.
- Article contents
- Figures & tables
- Supplementary Data
Masud Husain, Why we need a revolution in clinical research, Brain , Volume 147, Issue 9, September 2024, Pages 2897–2898, https://doi.org/10.1093/brain/awae265
- Permissions Icon Permissions
We are at a pivotal moment for clinical research. In the UK, the system is fundamentally broken as recent reports have alluded to. 1 , 2 In other parts of the world too there are similar issues that are, at the very least, slowing down innovation and research. There are many factors that have been identified as contributing to this sad state of affairs in the UK. One important issue that has not attracted so much attention recently—though it was the subject of a report 3 in 2020—is the relationship between higher educational institutions (mostly universities) and healthcare providers (largely the National Health Service, NHS).
The vast majority of research activity in the UK occurs within the higher education sector, while most patient-related research such as clinical trials relies on NHS infrastructure. And this is where there is a massive disconnect. Each of these systems are huge, cumbersome behemoths, with their own local lumbering administrations focused on aims that are not aligned to the mission of producing rapid results in clinical research. In the university sector, the priorities of leaders are to keep the system financially afloat and minimize potential legal risks. Many institutions in the UK are on the cusp of fiscal ruin and so require grant and other research income to subsidize their existence. In the NHS on the other hand the aim is to cut waiting lists which, post pandemic and doctors’ industrial action, are now very lengthy, and to provide adequate service delivery. Making healthcare research effective and efficient is the last thing on the minds of the leadership of either sector.
But who can blame them? Surely, it’s difficult enough to run either a university or an NHS hospital? Indeed, this seems sufficient explanation—an adequate excuse—for some leaders of both these types of institution for the huge delays in getting any useful research done. Many teams are now waiting over a year to get their grant-funded research off the ground. Remarkably, some trials are failing because they never start, several years after the funding has been awarded. Material or data transfer agreements between universities; slothful legal reviews of contracts and agreements with third parties; calculating overheads to be charged; multiple reviews of research protocols by R&D departments; dragging of feet over costings independently for the university and hospital; sluggish reviews by research services; signing off contracts with the NHS; obtaining honorary contracts for non-clinical personnel; and many other procedures may take months, if not years, to complete. The system is both Byzantine and exasperating to navigate. No wonder that pharmaceutical companies are balking at initiating trials in the UK, their gaze turning instead to countries where they are more serious about getting things done sooner, not later. 1
So how do we get out of this mess? Given the narrow goals that the leaderships of universities and NHS hospitals have, we cannot expect a great deal more from them on this front— unless they are compelled to make changes. In the UK, when the National Institute for Health and Care Research (NIHR) was formed in 2006, many of us were under the impression that its mission really was to ‘create a health research system in which the NHS supports outstanding individuals, working in world-class facilities, conducting leading-edge research focused on the needs of patients and the public’. 4 Clearly though this just hasn’t happened. Otherwise, why the need for recent reports? 1 , 2
One of the key reasons for this failure (we cannot refer it to it as anything else) is the simple fact that universities and NHS infrastructure are not joined up. Many pretend to be, but it is obvious to anyone who works at even the best centres in the UK that this is a sham. At Oxford, one of the hospital networks calls itself the Oxford University Health NHS Foundation Trust, but there really is very little to suggest why ‘University’ should be in its title. The levels of duplication of work and contracting between the university and the hospital make a mockery of the concept of seamless integration between these institutions. It is the same elsewhere too. The result is a growing duopoly of administrations that negotiate with each other, waste time and slow the pace of progress. Even when a research proposal has been approved by a ‘joint’ R&D unit, there needs to be a costings agreement between university and NHS trust.
From a national perspective this makes little sense, either economically or for governance. We are in the bizarre situation where two sets of institution—universities and hospitals—both largely funded by taxpayers are independently setting their (growing) administrative staffs to scrutinize research protocols or haggle over costings on projects that are mostly funded by government or charities. It is even worse for multicentre studies when many different universities and NHS trusts each want a share of the pie. This has a hidden cost in numbers of people employed, researchers’ time dealing with paperwork, and an opportunity cost in terms of time taken to get studies off the ground. Furthermore, there is no incentive to do things better or faster. There is simply a parochial incentive to make money locally and mitigate risks locally . Until the day that universities and hospitals associated with them are compelled to work as one integrated unit, there is very little hope for change. We will be left in the current quagmire of structural indolence. And that is why we need a revolution. Writing more reports on the matter will not help.
It is interesting to reflect on the fact that it was also radical change that was necessary to bring medicine into the modern era—to make it based on observation, clinical examination and the scientific method—in the first place. From the confusing and sometimes bizarre practices that characterized medicine in the 18th century, there emerged a new way of doing things which came about within one generation and in perhaps one of the least advanced places in Europe for clinical science at that time: Paris. From being a backwater, the ‘Paris School of Medicine’ instigated such dramatic change that within 50 years it became the leading international centre for clinical practice, attracting physicians from around the world to learn about the ‘new medicine’. 5
The rise of scientific medicine in Paris depended on systematic correlation of physical examination findings on hundreds of patients with pathological findings at post-mortem; flexibility to revise diagnoses on the basis of these assessments; deployment of statistics, including data on mortality; and most of all on conducting this work and teaching it to medical students in hospitals. 5 What made this possible was reform. Before the French Revolution, control of medical care rested largely with the Church. With the reform of medical education that came after the Revolution, hospitals were centralized and their administration was overseen by the state. Fundamental changes in the way in which faculties of medicine were organized in France led the way for dramatic new ways of learning from patients and disseminating knowledge to clinicians. Medical education was transformed but it needed the convulsive change of a Revolution to make this happen. 6 It required top-down edicts to bring about change because there was no incentive for the old institutions to make those changes themselves.
We are now confronted with a similar problem. The old institutions—universities and hospitals—are used to doing things their way. There is no incentive for them to change unless the state or its organs of power intervene. In the UK, NIHR funds now support Biomedical Research Centres (BRCs) which supposedly cross universities and NHS hospital trusts, but in truth the fiscal support helps to prop up university research personnel with very little going to the NHS. Most importantly, the NIHR has not insisted on BRCs having joined up (i.e. single) integrated, university-NHS systems in place, or for seamless national transfer of approvals across sites without the need for new sets of contractual agreements. Nothing fundamental will change unless it or the new government compels this change. The pursuit of national interests requires national leadership to intervene; we can't rely on local, devolved institutions to make the obvious decisions that are required. This is why we need a revolution in healthcare research.
O’Shaughnessy J . Commercial clinical trials in the UK: The Lord O’Shaughnessy review - final report. Accessed 13 August 2024. https://www.gov.uk/government/publications/commercial-clinical-trials-in-the-uk-the-lord-oshaughnessy-review/commercial-clinical-trials-in-the-uk-the-lord-oshaughnessy-review-final-report
The Academy of Medical Sciences . Future-proofing UK health research: A people-centred, coordinated approach. https://acmedsci.ac.uk/file-download/23875189
The Academy of Medical Sciences . Transforming health through innovation: integrating the NHS and academia. https://acmedsci.ac.uk/file-download/23932583
Department of Health and Social Care . Best research for best health: a new national health research strategy. https://www.gov.uk/government/publications/best-research-for-best-health-a-new-national-health-research-strategy
La Berge A , Hannaway C . Paris medicine: Perspectives past and present . Clio Med . 1998 ; 50 : 1 – 69 .
Google Scholar
Ackerknecht EH . Medicine at the Paris hospital, 1794–1848 . Johns Hopkins University Press ; 1967 .
Google Preview
Email alerts
Citing articles via, looking for your next opportunity.
- Contact the editorial office
- Guarantors of Brain
- Recommend to your Library
Affiliations
- Online ISSN 1460-2156
- Print ISSN 0006-8950
- Copyright © 2024 Guarantors of Brain
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Advertisement
Supported by
Why This Jobs Report Could Be the Most Pivotal One in Years
It’s tough to overstate how much hinges on Friday’s employment update, from the path for interest rates to the economic outlook.
- Share full article

By Jeanna Smialek
Jeanna Smialek is an economics journalist who reported this article from New York.
A fresh jobs report set for release on Friday could mark a turning point for the American economy, making it one of the most important and closely watched pieces of data in years.
The employment numbers will shed crucial light on whether a recent jump in the unemployment rate, which tracks the share of people who are looking for work but have not yet found it, was a blip or the start of a problematic trend.
The jobless rate rose notably in July after a year of creeping higher. If that continued in August, economists are likely to increasingly worry that the United States may be in — or nearing — the early stages of a recession. But if the rate stabilized or ticked down, as economists forecast, July’s weak numbers are likely to be viewed as a false alarm.
The answer is coming at a pivotal moment, as the Federal Reserve moves toward its first rate cut since the 2020 pandemic.
Central bankers have been clear that they will lower interest rates at their meeting on Sept. 17-18. Whether that cut is a normal quarter-point reduction or a larger half-point move could hinge on how well the job market is holding up. It is rare for so much to ride on a single data point.
“It matters a lot,” said Julia Coronado, founder of MacroPolicy Perspectives, a research firm. “It’s going to set the tone for the Fed, and that’s going to set the tone for global monetary policy and markets.”
Unemployment and Underemployment
The jobless rate historically jumps during recessions.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .
- Website search
Why is it important to handle grievances properly?
5th September 2024
What might happen if grievances are not handled properly? The recent Employment Appeal Tribunal case of Nelson v Renfrewshire Council relates to a complaint of constructive unfair dismissal under section 95(1) of the Employment Rights Act 1996 following a two-stage grievance process carried out by the respondent. Constructive dismissal is a complex area of law. Although the employee has resigned, for employment law purposes they have been “dismissed”. However, the employee’s resignation must be in response to a sufficiently serious and fundamental breach of contract by the employer.
The claimant was employed as a teacher from 2012 until 2022 and the Employment Tribunal claim arose out of events between 7 October 2021 and the claimant’s resignation on 7 November 2022. The initiating incident occurred during a work-related discussion between the claimant and head teacher during which the claimant felt that the head teacher had behaved in an aggressive and intimidating way towards her.
The head teacher’s voice was described by witnesses as raised and having an “angry” tone when speaking to the claimant in her office. The claimant was seen leaving the office visibly upset and was followed by the head teacher who pointed at her and said words to the effect of “If you’ve got something to say, say it to my face” and “what we were discussing is confidential”.
The claimant subsequently lodged a grievance which alleged the head teacher had treated her in a way that was “threatening, insensitive and aggressive”, contrary to the respondent’s “Respect at Work” policy. An investigation was carried out and a stage-one hearing was convened. Despite two witness statements supporting the claimant’s grievance, the investigator found no evidence that the head teacher had treated the claimant in the manner that she had alleged. This finding was based on the grounds that the head teacher and another witness had denied the claims. No statements had been taken or provided by the respondent.
The claimant appealed the outcome to a stage-two hearing. The conclusion was that the claimant experienced the incident to be “threatening, insensitive and aggressive” but it was again found that the head teacher had not behaved in that way.
The claimant did not appeal to the stage-three hearing as she had lost faith in the system and resigned with immediate effect. The respondent’s grievance procedure expressly stated that employees were normally expected to exhaust these grievance procedures if they wished to take their grievance to an Employment Tribunal.
Employment Tribunal (ET)
The claimant made a claim for constructive unfair dismissal arising from the alleged mistreatment by the head teacher and the way her grievance was handled, which constituted a breach of the implied term of trust and confidence.
The ET held that the head teacher had pointed at the claimant, that her words were aggressive and that it was “not professional behaviour”. The essential allegation was therefore proved, and the head teacher’s conduct was found to be “threatening, insensitive and aggressive”.
The ET found that this incident was likely to, and did, undermine trust and confidence without reasonable and proper cause. However, on its own, it would not come close to a breach of the implied term because it did not reach the level of destruction of, or causing serious damage to, the relationship of trust and confidence. It was regrettable and inappropriate behaviour but it was a one-off incident of relatively brief duration. Although the incident caused “some damage to the relationship of trust and confidence, that relationship was certainly not seriously damaged or destroyed.”
The stage-one hearing and the approach to the investigation was found to be unsatisfactory for several reasons. There was no proper attempt to gather evidence from the head teacher, no statement was obtained and she did not attend any minuted hearing or face questions. Her evidence appeared to have been gathered in an informal discussion which was inadequate for a fair and thorough investigation. This also meant there was a different process and level of scrutiny to the claimant’s evidence and the head teacher’s evidence.
The ET also found that the stage-one investigator showed bias and took into account her own prior knowledge and assessment of the head teacher, giving more weight to the evidence of the head teacher and another witness because of their rank within the school. The ET held this undermined trust and confidence without reasonable and proper cause.
The ET also found that the approach to evidence at the stage-two hearing was problematic and was not a re-hearing in any meaningful sense. The decision maker received significant evidence from the stage-one investigator rather than from primary sources and received no direct evidence or statement from the head teacher. The head teacher did not attend the hearing and the outcome letter failed to deal with the central conflict of evidence and was inadequate to detect and correct the earlier bias. There were significant failings throughout the respondent’s process and the claimant was entitled to be distressed about those. Objectively, the relationship of trust and confidence had been damaged.
However, the ET found that a stage-three hearing was an entirely viable option with a realistic chance of righting the wrongs of the previous stages. The ET therefore found that there was no breach of the implied term of trust and confidence because although the relationship had been damaged, the situation had not reached the level of serious damage to, or destruction of, the relationship of trust and confidence.
This meant the claimant’s resignation was not in response to a fundamental breach of contract and her claim was dismissed.
Employment Appeal Tribunal (EAT)
The claimant appealed on three grounds:
- if in light of the findings of fact made by the ET, whether its decision to dismiss the claim was perverse;
- whether in any event the ET failed properly to apply established legal principles to the facts; and
- that the decision was not Meek compliant, in that the reasoning in key aspects was inadequate.
The threshold for perversity is a high one, and an appeal on this ground will only succeed where an “overwhelming” case is made that the ET reached a decision which no reasonable ET, on a proper appreciation of the evidence and the law would have reached.
The EAT found that within the ET judgment there was an explicit link between the claimant’s failure to exhaust the grievance process and a conclusion that the relationship had not been damaged to the extent necessary to find a claim for constructive dismissal. The EAT held that the claimant’s failure to engage with the third-stage of the grievance process was irrelevant and accordingly the appeal in this aspect was allowed. Only the employer’s conduct is relevant when considering constructive dismissal claims.
The claimant identified six instances where the ET made findings as to whether the relationship of trust and confidence had actually been damaged as opposed to whether, looked at objectively, the conduct would be likely to seriously damage or destroy the relationship of trust and confidence. The EAT concluded that the language used by the ET indicated that its approach amounted to an error or law. The appeal was upheld on this basis.
The EAT also held that there was an error of law in the ET’s failure to address whether or not the conduct in question, looked at cumulatively, amounted in and of itself to a breach of the implied term.
The appeal in regards of Meek compliance was dismissed. There was not a lack of adequate reasoning by the ET, rather a misapplication of the law from the facts established.
The EAT remitted the matter back to the ET to consider whether, in light of its findings in fact, and without regard to the claimant’s failure to exhaust the grievance process, the respondent’s conduct amounted individually or cumulatively, to a repudiatory breach of the implied term of trust and confidence.
The judgment in this case serves as a reminder to employers of the importance of carrying out a fair and thorough grievance procedure. Any investigator appointed should proceed without bias to enable an independent, thorough and transparent investigation process, leading to a clear and well-reasoned outcome letter. This is important throughout all stages of the grievance procedure. A second-stage hearing, where there is one, should never be predetermined by the result of the first-stage, even unconsciously. Employers should ensure that they follow their own grievance procedures and comply with any time limits referred to and of course, be mindful of the Acas Code of Practice on Disciplinary and Grievance procedures.
For a different aspect of constructive dismissal see our recent article about affirmation of the contract .
Seeking advice on employment law issues?
Speak to one of our employment law specialists
HOW CAN WE HELP?
To get in touch with one of our legal experts please fill in your details.

- Nearest Office * Nearest Office London Cardiff Reading Southampton Oxford
- First Name *
- Last Name *
Privacy Notice
Blake Morgan Privacy Policy
We do not use any enquiries to the [email protected] email address or any completed forms for marketing purposes.
- Phone This field is for validation purposes and should be left unchanged.
Enjoy That? You Might Like These:
The importance of considering reasonable adjustments, new rules on tips imminent: what do they mean for employers, the king’s speech – what is next for employment law.
- Reason for Enquiry * Reason for Enquiry Banking and Finance Business Owners and Entrepreneurs Charities Commercial, Debt & Asset Recoveries Commercial Property Construction and Development Corporate Education Employment Families Government Healthcare International Tax and Succession Planning Litigation Dispute Resolution Manufacturing Notarial Services for Business Notarial Services Private Client Property Regulatory Residential Property Resolving Disputes Retail and Leisure Social Housing Technology Travel
- Nearest Office * Nearest Office Cardiff London Oxford Reading Southampton
- Comments This field is for validation purposes and should be left unchanged.
The Role of Research: To Learn, to Solve, to Inspire

- Click to share on LinkedIn (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to share on X (Opens in new window)
- Click to email a link to a friend (Opens in new window)
- Click to print (Opens in new window)
Taking a peek into the unknown is the job of professors. With their research, they ask the big, knotty questions, the questions at the limits of human understanding for which answers are not easily found.
“It is challenging,” says Joanna Carey , associate professor of environmental science and the Debi and Andy Butler Term Chair. “Basically, our job is to figure out knowledge that nobody knows yet.”
Babson College may not be a large research institution, but its professors still produce a sizable amount of research in a wide range of fields, from medicine and the environment, to history and culture, to technology and innovation, to business and entrepreneurship. In this article, five Babson professors discuss their diverse work, giving a greater appreciation of the breadth and significance of research at the College.
BABSON MAGAZINE : Read the complete Summer 2024 issue .
Their research, along with that of their colleagues, flourishes in a supportive environment. The College helps fund research endeavors and trips to academic conferences, while giving professors the freedom to pursue their scholarly interests.
Alumni donors, meanwhile, have funded numerous term chairs, which allow professors to spend more time on their research, and professors at the tight-knit school frequently collaborate across disciplines. “We challenge each other to solve problems in different ways,” says Dessislava Pachamanova , professor of analytics and computational finance and the Zwerling Family Endowed Term Chair.
The end result is research that influences and inspires, that makes an impact around the globe, that helps us understand the world and our place in it.
Professors’ research also makes its way into the classroom. “The research they’re doing outside of the classroom informs and strengthens their approach within it,” says Babson President Stephen Spinelli Jr. MBA’92, PhD . “That value proposition enhances our academic rigor and ensures Babson remains at the forefront of emerging trends in entrepreneurship and beyond.”
‘I Have Always Been Fascinated by Nature.’
Imagine a stream trickling down a mountain, as it makes its way to a river, which widens as it reaches the sea. That water is majestic and immense, and it carries with it many things on its journey. “Every time I see a river, I think, ‘That’s a lot of material being moved,’ ” says Joanna Carey, associate professor of environmental science and the Debi and Andy Butler Term Chair.
One of those things in the water is silicon, the second most abundant element in Earth’s crust and a frequent subject of Carey’s research. Silicon moves from the crust, into plants, into rivers, and finally, into the ocean.
Following that path and examining watery places such as rivers, marshes, and estuaries, Carey’s research demonstrates how human activity is causing drastic changes in the amount of silicon as it cycles through the world. Those changes have a story to tell about land use, about the food chain, about carbon dioxide levels, and about our planet as it warms.

Consider the microscopic but mighty diatoms, for instance, an abundant alga that requires silicon to grow. What will the changing levels of silicon mean for these organisms responsible for more than 20% of the oxygen produced every year on Earth?
“They are really important,” Carey says. “Their importance on a global scale can’t be overestimated.”
For the last few years, Carey has led a team that created and examined the largest data set in the world on river silicon chemistry with funding from the National Center for Ecological Analysis and Synthesis. She’ll soon be looking at data from all seven continents for a project funded by the United States Geological Survey.
To be a scientist now, trying to bring a clearer understanding of climate change’s formidable and far-reaching impact, is to perform critical work.
“It is very fundamental science,” Carey says. “There is an urgency in what we are doing.”
Much is at stake, including the future of the students she teaches in the classroom. “This is an issue they will deal with their whole lives,” Carey says.
‘Research Has Always Felt Natural to Me.’

Technology evolves fast. For those in the workplace, that speed can feel overwhelming. “There is a lot of risk of people falling behind,” says Ruben Mancha , associate professor of information systems.
Those workers falling behind are a major concern of Mancha’s research. He looks at how organizations can adopt technology and transform how they operate in a responsible way, by considering the many human implications of the changes they’re implementing. “What is different about my framing is that responsibility,” he says. “It’s focused on the human side. It’s a people-first approach.”
New tech, such as an artificial intelligence tool like ChatGPT, actually can have a positive effect on employees’ workdays, taking on tedious tasks and freeing them to focus on more essential matters.

This benefit only works, though, if workers are confident using these tools. The line between those who are tech literate and those who are not is a stark one. “Those who can work with the technology will use it,” Mancha says. “Those who can’t will be replaced.”
In his research, Mancha examines two ways that organizations can not only guide employees through technological changes but also empower them. One way is by introducing them to low-code development platforms, which offer a much easier way to code, thus enabling many more employees to become developers. The second way is to launch a sustained and effective program for upskilling, creating a workforce that is competent and confident with tech.
Such measures do more than train an employee in the latest and greatest. They also change a workforce’s mindset. Give employees a new program they know how to use, and suddenly they have greater power to transform and innovate. “It changes how people see themselves and how they use technology,” Mancha says. “It’s about bringing the innovation culture to the enterprise.”
Mancha hopes decision makers in business will take his research to heart, and he’s excited to share it with students in the classroom. Before becoming a professor, he worked as a lab scientist in biotech. Research is something that comes naturally to him.
“It is my way of thinking,” he says.
‘I Like to Solve Problems.’
Unfortunately, the work of the International Committee of the Red Cross is seemingly never-ending. The essential organization operates in war zones—in Ukraine, in the Gaza Strip, and in conflict areas far removed from the world’s spotlight. “There are fires everywhere,” says Dessislava Pachamanova, professor of analytics and computational finance and the Zwerling Family Endowed Term Chair.
The work is not only relentless, but it is also costly and logistically challenging. Because of the alarming number of conflicts around the world in recent years, the organization faced a substantial funding shortfall. As a result, a team composed of Pachamanova, other researchers, and supply chain coordinators within the organization sought to determine how to best allocate medical supplies for where they need to go.

This was a tricky thing to figure out. Ship too many supplies, and costly medications may sit unused and expire. Ship too little, and people may not receive the critical, lifesaving supplies they need. For more than a year, Pachamanova and the group looked at the issue.
Ultimately, they developed an inventory management decision support system that was rolled out across a dozen medical distribution centers in Africa, the Middle East, and Ukraine in 2023. By reducing the inventory levels of medical supplies by nearly a quarter with virtually no negative effect on service, the system saved the Red Cross a significant amount of money while facilitating a collaborative planning process across the organization. “The Red Cross considers it a great success,” Pachamanova says.
This is exactly the type of result she is seeking with her research. “I am looking for impact. That is the main thing that drives me,” says Pachamanova, who has applied her expertise in optimization, analytics, machine learning, and simulation to fields as diverse as finance, logistics, and health care.
Pachamanova wants to incorporate her experience working with the Red Cross in a new Babson class she is designing. “I want to introduce students to this kind of experience,” she says, “where you go in, you understand the big problem, but identifying how to start a solution is very hard, and you won’t know where you’ll end up.”

‘I Like Asking Questions and Looking for Answers.’
A company is not an island. Its actions are not secluded. Rather, they ripple outward. A company’s supply chain, its partners, its manufacturing, its customers—all of these relationships, all of these connections, operate within one intertwined system that has an impact on communities and the environment.
The research of Sinan Erzurumlu , professor of innovation and operations management, concerns itself with these systems in which organizations operate. Lying at the intersection of business, society, and the environment, his work focuses on how companies can make decisions that are both sustainable and innovative.
When looking at a company’s actions in his research, Erzurumlu typically asks a direct question: Who does this benefit? “It could benefit a community. It could benefit the planet,” he says. “That perspective drives me a lot.”

The goal is to build a more sustainable future, but talking and researching about sustainability, such an immense, complex, and daunting challenge, is not easy. “I think sustainability is a human mindset problem,” Erzurumlu says. “It’s not just reducing carbon emissions. It’s about changing that mindset. We need to make that transition to a sustainable future. Convincing people to do that is a hard job.”
Large organizations also can’t simply transition into sustainable businesses overnight. Making integral changes is like trying to turn a cargo ship. “It’s a process,” Erzurumlu says. “They can’t make the turn immediately.”
In one recent research article he co-authored, Erzurumlu looked at the systems-thinking approach that three companies—retailers of household products, fashion, and beverages, respectively—took to sustainability. The companies, among other measures, sought to limit the water they used in their operations, reduce the use of hazardous chemicals, and collect waste to recycle and remake into new products.
He hopes other companies can learn from their efforts. He also hopes such research will give his students real-world insights about sustainability. “I think teaching is as important as being a researcher,” he says. “I see the classroom as an outlet for my research. Teaching about my research is an extension of my scholarly identity.”
‘Research Is a Great Opportunity to Better Understand Hard Problems.’

Hospitals are full of caring, smart people striving to deliver the best treatment possible. Helping them with that mission is what Wiljeana Glover tries to achieve in her research.
To conduct her research, Glover likes to leave her desk and work on site, embedded with those on the front lines of health care. “When I can, I am physically going into hospitals, observing, getting to know clinicians,” says the Stephen C. and Carmella R. Kletjian Foundation Distinguished Professor of Global Healthcare Entrepreneurship.
“That is fun for me and helps me understand how they do the work they do.”
Glover often works with hospitals and clinics to understand how they identify and implement improvements, whether a new procedure or innovation. The goal is to make sure that these improvements support patients equitably. Equity in care, for people of color, for women, can remain elusive.

Clinicians typically see her as a partner. “In some cases, they see me as part of their innovation or improvement team,” Glover says. “They appreciate the insights they are receiving along the way.”
In a recent research article she co-authored, Glover looked at quality improvement efforts at medical centers and how those organizations can make a sustained commitment to addressing equity. Data measurement, team composition, and the need for ongoing actions were all examined. “How do we not think of equity as a one off?” Glover says. “How do we build in equitable practice? How does it become part of the way we do things?”
Glover also serves as the founding faculty director of Babson’s Kerry Murphy Healey Center for Health Innovation and Entrepreneurship. In addition to studying equity, the center’s affiliated faculty conducts research on healthcare startups, the impact of artificial intelligence and analytics, and entrepreneurial training for clinicians and scientists.
Glover praises the spirit of collaboration she sees in her fellow faculty members, who share ideas with one another and work together on research. “It is one of my favorite things about doing research at Babson,” she says. “It really is a part of the secret sauce of research here.”
Posted in Insights , Outcomes
Tagged Entrepreneurship Education , Faculty , Research , Babson Magazine
More from Babson Magazine »
Related Stories
Latest stories.

Young Entrepreneurs Drive ‘Bright Future,’ GEM U.S. Report Shows

Babson College Climbs to No. 2 in Wall Street Journal’s Rankings of the Best Colleges in the U.S.

Finding Loyal Customers for Long-Lasting Success Is Easier than You Think
More babson.

IMAGES
VIDEO
COMMENTS
Some archival data sets offer important insights but are not updated to the recent past. For example, the U.S. census is updated every 10 years. A scholar asking a research question requiring census data for an answer might be forced to use data up to 10 years old. A second example is the Pew religious data, which are updated every 7 years.
Research investment has responded to this, and other health challenges in recent years. For example, there has been a significant move towards greater collaboration between researchers, nurses, patients and family caregivers to address the needs identified as important by the community.
For example, if you cite sources from the current century as well as 1930s, then a paper from 2010 should be considered recent, but not one from 1950. If, on the other hand, your temporal range of references is rather narrow, say, recent 20 years, then you should refer to as "recent" for sources that are from approximately last 4-5 years.
A few weeks ago, when submitting an abstract to a nursing conference, I was suddenly faced with a dilemma about age. Not my own age, but the age of evidence I was using to support my work. One key element of the submission criteria was to provide five research citations to support the abstract, and all citations were to be less than ten years old.
Why is it critical to keep up with the most recent Research? There are several reasons why it's critical to stay current with your field of study changes. ... Time is a critical factor in the systematic review process and an important covariate in assessing study heterogeneity and a fundamental determinant of systematic review clinical ...
Our objective in this series is to describe how a clinical researcher can adopt an evidence-based research approach—and why it is important to do so. 2 Need for evidence-based research Unnecessary clinical research is unethical as it puts patients at avoidable risk, limits the funding available for important and relevant research, and may ...
Placing research in the bigger context of its field and where it fits into the scientific process can help people better understand and interpret new findings as they emerge. A single study usually uncovers only a piece of a larger puzzle. Questions about how the world works are often investigated on many different levels.
a calls-for-papers service.BooksBooks will be of more use i. some research areas than others. A few databases include books but the best way to keep up to date is t. rough the publishers' websites. The major academic publishers, as listed under 'Journal issues' above, offer this, but also check the websites of publishers who specialise in ...
MeSH terms. Reliability and validity are among the most important and fundamental domains in the assessment of any measuring methodology for data-collection in a good research. Validity is about what an instrument measures and how well it does so, whereas reliability concerns the truthfulness in the data obtain ….
information. An important "pain point" that future infor-mation tools should address is helping researchers filter information at the point of need. Introduction Conducting and delivering up-to-date research is key to academic work, but keeping up to date is becoming more challenging: Researchers have to locate relevant information
Research can help improve the quality and effectiveness of healthcare by generating new evidence that can be applied to make healthcare affordable, safe, effective, equitable, accessible, and patient-centered. By applying this evidence in practice, healthcare systems can be improved to ensure that patients receive the best possible care.
Nursing Research: What It Is and Why It Matters. When people think about medical research, they often think about cutting-edge surgical procedures and revolutionary new medications. As important as those advancements are, another type of research is just as vital: nursing research. This type of research informs and improves nursing practice.
Peer review is a mutual responsibility among fellow scientists, and scientists are expected, as part of the academic community, to take part in peer review. If one is to expect others to review their work, they should commit to reviewing the work of others as well, and put effort into it. 2) Be pleasant. If the paper is of low quality, suggest ...
All being said, do we exactly know how old a research article can be before it gets the label of not being recent i.e., an old article not good for citing. There is consensus among scientists and researchers that articles less than five years old are recent publications. However, it may vary from discipline to discipline.
A good rule of thumb is to use sources published in the past 10 years for research in the arts, humanities, literature, history, etc. For faster-paced fields, sources published in the past 2-3 years is a good benchmark since these sources are more current and reflect the newest discoveries, theories, processes, or best practices. Use the ...
Discuss how scientific research guides public policy. Appreciate how scientific research can be important in making personal decisions. Scientific research is a critical tool for successfully navigating our complex world. Without it, we would be forced to rely solely on intuition, other people's authority, and blind luck.
The stamp of a good research worker is attention to detail at all levels of his/her research. Attention to detail cultivates good habits and the detail required in referencing and preparing a bibliography focuses attention on the whole research procedure. ... If the most recent reference is more than five years or so, this may indicate that a ...
A recent United Kingdom report on the use of personal data in health research concluded that public involvement in research is necessary for the success of information-based research, and that a public informed about the value of research is likely to have greater enthusiasm and confidence in research and the research community . Moreover ...
Keeping up with the fast-growing and ever-changing medical literature is necessary to safeguard the quality of patient care, and it helps researchers to remain at the forefront of their field. There are numerous existing and emerging tools to help clinicians and researchers keep track of new knowledge more efficiently.
What research is and why it is important to be an informed consumer of research. The sources of knowledge development and problems with each. How research methods can dispel myths about crime and the criminal justice system. The steps in the research process. How research has impacted criminal justice operations. Introduction
The following section will seek to provide some answers to why research impact has become so important in the past decade. ... However, in recent years, research impact has begun to eclipse outputs with regard to importance, at least in the UK. Steven Hill, Director of Research Policy for Research England, has argued that the justification for ...
cept of critical inquiry. Research grows out of the primary human tendency or need to learn, to know, to solve problems, to question received wisdom and take. -for-granted assumptions. These impulses are fundamentally critical; the need to know is the counterpoint to the sense that what is already know.
In academia, intellectual contribution to one's field of study through publication of research defines impact. Yet, impact is not just about academic scholarship and productivity but also about real-world influence which speaks to whether research endeavors and their findings hold sufficient meaning to make a difference to human life (Abhimalla et al., 2014).
Abstractspiepr Abs1. Every day people do research as they gather information to learn about something of interest. In the scientific world, however, research means something different than simply gathering information. Scientific research is characterized by its careful planning and observing, by its relentless efforts to understand and explain ...
We are at a pivotal moment for clinical research. In the UK, the system is fundamentally broken as recent reports have alluded to. 1, 2 In other parts of the world too there are similar issues that are, at the very least, slowing down innovation and research. There are many factors that have been identified as contributing to this sad state of affairs in the UK.
A fresh jobs report set for release on Friday could mark a turning point for the American economy, making it one of the most important and closely watched pieces of data in years.
This is important throughout all stages of the grievance procedure. A second-stage hearing, where there is one, should never be predetermined by the result of the first-stage, even unconsciously. Employers should ensure that they follow their own grievance procedures and comply with any time limits referred to and of course, be mindful of the ...
He hopes other companies can learn from their efforts. He also hopes such research will give his students real-world insights about sustainability. "I think teaching is as important as being a researcher," he says. "I see the classroom as an outlet for my research. Teaching about my research is an extension of my scholarly identity."
"Research experience can help make students more competitive for graduate or professional schools by preparing them for graduate thesis or capstone projects," Rawlinson said. "Ultimately, involvement in research makes students more well-rounded learners." 3. The chance to learn from the faculty who generate the science that informs practice
Why do you need to rotate credentials regularly? Rotating credentials regularly prevents prolonged exposure of passwords, reducing the chances of successful attacks if a password is compromised. It ensures that any stolen credentials are quickly rendered useless. Should you rotate service account passwords?