- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Graphical Abstracts and Tidbit
- Author Guidelines
- Submission Site
- Open Access
- About American Journal of Hypertension
- Editorial Board
- Board of Directors
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- AJH Summer School
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Mean arterial pressure and urinary protein excretion responses to chronic reductions in uterine perfusion pressure (rupp) in pregnant rats. all data are expressed as mean ± sem., vascular responses to acetylcholine are reduced in pregnant rats with chronic reductions in uterine perfusion pressure (rupp). all data are expressed as mean ± sem., glomerular filtration rate and renal plasma flow responses to chronic reductions in uterine perfusion pressure (rupp) in pregnant rats. all data are expressed as mean ± sem., does a reduction in renal nitric oxide synthesis mediate the abnormal pressure natriuresis and elevation in arterial pressure during pih, does enhanced endothelin synthesis contribute to the elevation in arterial pressure during pih, does enhanced thromboxane and/or reduced prostacyclin synthesis mediate the renal and cardiovascular abnormalities in pih.
- < Previous
Pathophysiology of pregnancy-induced hypertension
- Article contents
- Figures & tables
- Supplementary Data
Joey P. Granger, Barbara T. Alexander, William A. Bennett, Raouf A. Khalil, Pathophysiology of pregnancy-induced hypertension, American Journal of Hypertension , Volume 14, Issue S3, June 2001, Pages 178S–185S, https://doi.org/10.1016/S0895-7061(01)02086-6
- Permissions Icon Permissions
Pregnancy-induced hypertension (PIH) is estimated to affect 7% to 10% of all pregnancies in the United States. Despite being the leading cause of maternal death and a major contributor of maternal and perinatal morbidity, the mechanisms responsible for the pathogenesis of PIH have not yet been fully elucidated. Studies during the past decade, however, have provided a better understanding of the potential mechanisms responsible for the pathogenesis of PIH. The initiating event in PIH appears to be reduced uteroplacental perfusion as a result of abnormal cytotrophoblast invasion of spiral arterioles. Placental ischemia is thought to lead to widespread activation/dysfunction of the maternal vascular endothelium that results in enhanced formation of endothelin and thromboxane, increased vascular sensitivity to angiotensin II, and decreased formation of vasodilators such as nitric oxide and prostacyclin. The quantitative importance of the various endothelial and humoral factors in mediating the reduction in renal hemodynamic and excretory function and elevation in arterial pressure during PIH is still unclear. Investigators are also attempting to elucidate the placental factors that are responsible for mediating activation/dysfunction of the maternal vascular endothelium. Microarray analysis of genes within the ischemic placenta should provide new insights into the link between placental ischemia and hypertension. More effective strategies for the prevention of preeclampsia should be forthcoming once the underlying pathophysiologic mechanisms that are involved in PIH are completely understood. Am J Hypertens 2001;14:178S–185S © 2001 American Journal of Hypertension, Ltd.
Pregnancy-induced hypertension (PIH) is estimated to affect 7% to 10% of all pregnancies in the United States. 1–4 Despite being one of the leading causes of maternal death and a major contributor of maternal and perinatal morbidity, the mechanisms responsible for the pathogenesis of PIH are unclear. Hypertension associated with preeclampsia develops during pregnancy and remits after delivery, implicating the placenta as a central culprit in the disease. An initiating event in PIH has been postulated to be reduced placental perfusion that leads to widespread dysfunction of the maternal vascular endothelium by mechanisms that remain to be defined. 1–4 The mechanisms leading to reduced placental perfusion in PIH may be multiple, but most studies in humans suggest abnormal cytotrophoblast invasion of spiral arterioles as an important factor. 1–5
Several lines of experimental evidence support this hypothesis. For example, studies in various animal models, including sheep, dog, rabbit, and rat have shown that reductions in uteroplacental blood flow leads to a hypertensive state that closely resembles PIH in women. 6 , 7 Additional support for this concept derives from studies in humans that indicate increased circulating fibronectin and factor VIII antigen, both markers of endothelial cell injury. 1–4 , 8–10 Decreases in the production of endothelial-derived relaxing factors, such as nitric oxide and prostacyclin, increase production of endothelin and thromboxane, and enhanced vascular reactivity to angiotensin II in women with PIH also suggest abnormal endothelial function. 1–4 , 11
During normal pregnancy, significant changes in cardiovascular and renal function occur to meet the metabolic needs of the mother and the fetus. 1–3 For example, maternal cardiac output and blood volume increase by approximately 40% to 50%, whereas total peripheral resistance and arterial blood pressure (BP) tend to decrease. 1–3 In addition, there are marked changes in renal function such as elevations in renal plasma flow and glomerular filtration rate of approximately 30% to 40%. 12 Renin concentration, renin activity, and angiotensin II levels are elevated; however, the vascular responsiveness to angiotensin II appears to be reduced. 13 The mechanisms that are involved in mediating these significant cardiovascular and renal changes during pregnancy have been studied extensively, and it appears that endothelial factors such as nitric oxide play an important role. 1–3 , 14 , 15
The marked hemodynamic and renal changes that normally occur during pregnancy do not manifest themselves in women who develop PIH. Pregnancy-induced hypertension is associated with significant elevations in total peripheral resistance, enhanced responsiveness to angiotensin II, and marked reductions in renal blood flow and glomerular filtration rate and proteinuria. 1–3 Although the physiologic mechanisms that mediate the alterations in cardiovascular and renal function have been extensively studied during normal pregnancy, information regarding the mediators of the reduction in renal and cardiovascular function during PIH has been limited because of the difficulty in performing mechanistic studies in pregnant women. Although several animal models have been developed to study PIH, information on the mechanisms involved in mediating the long-term reduction in kidney function and increase in arterial pressure is lacking. Experimental induction of chronic uteroplacental ischemia appears to be the most promising animal model to study potential mechanisms of PIH, as reductions in uteroplacental blood flow in a variety of animal models lead to a hypertensive state that closely resembles PIH in women. 1–3 , 6 , 7 , 16
Chronic reductions in uteroplacental perfusion pressure in gravid rats after day 14 of gestation, as reported by Eder and MacDonald 17 and Abitbol, 18 lead to significant increases in arterial pressure and proteinuria. We have recently begun to work with this model to examine potential pathophysiologic mechanisms that mediate the hypertension during chronic reductions in uteroplacental perfusion pressure. 19 We reduced uterine perfusion pressure in the gravid rat by approximately 40% by placing a silver clip around the aorta below the renal arteries. Because this procedure has been shown to cause an adaptive increase in uterine blood flow through the ovarian artery, we also placed a silver clip on both the right and left uterine arcade at the ovarian end just before the first segmental artery. 20 We found that reducing uteroplacental perfusion with this approach results in significant and consistent elevations in arterial pressure of 20 to 30 mm Hg as compared to control pregnant rats at day 19 of gestation (Fig. 1) . Our data also indicate that this hypertension is associated with proteinuria, reductions in renal plasma flow and glomerular filtration rate (Figs. 1 and 2 ), and a hypertensive shift in the pressure natriuresis relationship. 20 , 67 Moreover, our data indicate that endothelial function (Fig. 3) is significantly altered in response to chronic reductions in uteroplacental perfusion pressure in the pregnant rat. 21 , 22 Finally, we have found intrauterine growth restriction in response to chronic reductions in uteroplacental perfusion pressure in the pregnant rat, as the average pup size in this group is smaller than in normal pregnant rats. 20 Thus, a chronic reduction in uteroplacental perfusion pressure in the pregnant rat has many of the features of PIH in women. The role of various endothelial, autacoid, and hormonal factors in mediating the reduction in renal hemodynamic and excretory function and elevation in arterial pressure produced by chronic reductions in uteroplacental perfusion pressure will be the main focus of the remaining portion of this brief review.
02086-6/2/m_ajh.178S.f1.jpeg?Expires=1727251809&Signature=HU59qA1tgZM5buy2CyD6rS9d~QMuBoWUjiM~P4LzPso4xysxDPmbilnmunQuglkNX6U6wWv-0biLWd-gZ5-M00AfMgAsGEsb0qkZ4wqWKuIQe~EkmaZ7LRPls69LnkLly7QOB58TVMVr2ALnzKAZWzyZjmaqgjfTWDUi8g6FY50N4IWGZakyqECDwaLw5CttQ8OckoD9v7EEleJ47jlTZdYelXtKORUrYI~DbdodNyCRBCL4luDhqvW~nuGFmKLJ6fiYkeuk5LypyGHsofN~8jElyHEDOscspB~Jy08h0BOKaneKQeBiocbPGRmiwzo2jFF57jFHwQ0zw36YGWVUUg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
One potential mechanism for the reduction in pressure natriuresis and elevation in arterial pressure in response to a chronic reduction in uteroplacental perfusion pressure in the pregnant rat is a reduction in renal nitric oxide (NO) synthesis. 23–26 Nitric oxide is synthesized from L-arginine by a family of enzymes known as NO synthases (NOS). Nitric oxide synthase is readily inhibited by L-arginine analogs such as N -methyl-arginine (L-NMMA), N -nitro-L-arginine (L-NNA), and N -nitro-L-arginine methyl ester (L-NAME). Studies from our laboratories and others have indicated that NO plays an important role in the regulation of renal function and arterial pressure under various physiologic and pathophysiologic conditions. 24 , 27–33 Of particular relevance to PIH is the finding that reducing NO synthesis results in a hypertensive shift in the pressure natriuresis relationship. 28 , 33 This impairment in pressure natriuresis is also associated with reductions in renal plasma flow and glomerular filtration rate and an inability to transmit renal perfusion pressure into the renal interstitium. 28 , 33
Substantial evidence indicates that NO production is elevated in normal pregnancy. 14 , 15 Plasma and urinary levels of cGMP, the second messenger of NO, increase during pregnancy in rats. 14 , 15 Marked increases in 24-h urinary nitrate/nitrite excretion have also been reported to be normal during pregnancy in the rat. 14 , 15 Studies have also shown that pregnancy increases activity of calcium-dependent NOS in uterine artery and heart in early and late pregnancy. 14 , 15 Increased expression of mRNA levels for both constitutive NOS isoforms have been observed in a variety of tissues in late pregnancy. 14 , 15 Plasma arginine levels are also reduced in pregnancy. These findings presumably reflect increased utilization of substrate in response to increased formation of NO.
Increases in NO production appear to play an important role in the renal vasodilatation of pregnancy. 14 , 15 Recent studies by Conrad 14 and other researchers 15 clearly demonstrated that the renal vasodilatation in the pregnant rat is due to an increased NO production. Because NO appears be an important physiologic vasodilator in normal pregnancy, NO deficiency during preeclampsia might be involved in the disease process. Studies from several laboratories have found that chronic NOS inhibition in pregnant rats produces a hypertension associated with peripheral and renal vasoconstriction, proteinuria, intrauterine growth retardation, and increased fetal morbidity, a pattern that closely resembles the symptoms of human pregnancy-induced hypertension. 22 , 34 , 35 However, whether there is a reduction in NO production during pregnancy-induced hypertension is unclear. Much of the uncertainty originates from the difficulty in directly assessing the activity of the NO system in a clinical setting. 1–3 Assessment of whole body NO production by measurement of 24-h nitrate/nitrite excretion has yielded variable results due to difficulties in controlling for factors such as nitrate intake. We have recently reported that normal pregnancy in the rat is associated with significant increases in whole body NO production and renal protein expression of neuronal and inducible NOS. 36 We also recently determined whether whole body and renal NO production is reduced in a rat model of PIH produced by chronically reducing uterine perfusion pressure. 20 Chronic reductions in uterine perfusion pressure resulted in increases in arterial pressure of 20 to 25 mm Hg, decreases in renal plasma flow and glomerular filtration rate, but no difference in urinary nitrite/nitrate excretion relative to control pregnant rats. In contrast, reductions in uterine perfusion pressure in virgin rats resulted in no significant effects on arterial pressure. Renal endothelial and inducible NOS protein expression did not decrease significantly in the chronically reduced uterine perfusion pressure rats relative to normal pregnant rats; however, significant reductions in neuronal NOS were observed. The results of this study indicate that the increase in arterial pressure observed in response to chronic decreases in uterine perfusion pressure in pregnant rats is associated with no change in whole body NO production and a decrease in renal protein expression of neuronal NOS. Whether the reduction in renal protein expression of neuronal NOS occurs as a result of the hypertension or the reduction in renal protein expression of neuronal NOS plays a role in mediating the reduction in renal hemodynamics and elevation in arterial pressure remains to be determined.
Another endothelial-derived factor that may play a role in PIH is the vasoconstrictor endothelin. In 1988, Yanagisawa and co-workers 37 characterized an endothelial-derived vasoconstrictor, a 21-amino-acid peptide subsequently called endothelin. Endothelin is derived from a 23-amino-acid peptide precursor preproendothelin that is cleaved after translation to form proendothelin. In the presence of a converting enzyme located within the endothelial cells, proendothelin or big endothelin is cleaved to produce the 21-amino-acid peptide endothelin. Endothelin receptor-binding sites have been identified throughout the body with the greatest number of receptors in the kidneys and lungs. 38 The vasoconstrictor effects of endothelin are mediated by endothelin A receptors on the vascular smooth muscle. In addition, evidence is accumulating that endothelin B receptors located on vascular smooth muscle also contribute to the vasoconstrictor effects of this peptide. 39 Endothelin B receptors located on endothelium are thought to release NO and prostacyclin. Endothelin reduces renal hemodynamic and sodium excretory function and plays an important role in mediating the altered pressure natriuresis and other hemodynamic changes in several models of hypertension including the deoxycorticosterone salt hypertensive rat and the Dahl salt-sensitive hypertensive rat. 39–41
Because endothelial damage is a known stimulus for endothelin synthesis, increases in the production of endothelin may participate in PIH. Plasma concentration of endothelin has been measured in a number of studies involving normal pregnant women and women with pregnancy-induced hypertension. 42–45 Most investigators have found higher plasma concentrations of endothelin of approximately two- to threefold in women with PIH. 42–45 Typically, plasma levels of endothelin are highest during the latter stage of the disease, suggesting that endothelin may not be involved in the initiation of PIH, but rather in the progression of disease into a malignant phase. 42–45 Although the elevation in plasma levels of endothelin are only two- or threefold above normal during PIH, we found that this level of plasma endothelin can have significant long-term effects on systemic hemodynamics and arterial pressure regulation. 46 , 47 We found that increasing the plasma levels of endothelin within the two- to threefold range for 2 to 3 h had no effect on arterial pressure, whereas increasing endothelin levels for 7 days resulted in significant reductions in renal hemodynamics, renal pressure natriuresis, and significant elevations in mean arterial pressure. 46 , 47 The increase in mean arterial pressure was also associated with significant reductions in cardiac output and renal plasma flow and elevations in total peripheral resistance. 46 , 47 Thus, long-term elevations in plasma levels of endothelin comparable to those measured in patients with PIH could play a role in mediating the reductions in renal function and elevations in arterial pressure observed in women with PIH.
Although some studies have reported no significant changes in circulating levels of endothelin during PIH, a role for endothelin as a paracrine or autocrine agent in PIH remains worthy of consideration. Many of the experimental and genetic rat models of hypertension are not associated with elevations in plasma endothelin. 39 Yet, elevations in endothelin synthesis have been reported in specific tissues including the kidney. 39 For example, investigators have reported enhanced expression of preproendothelin in vascular tissues from various organ systems, including the kidney. 38 , 39 Several studies have also reported an increase in local production of endothelin in women with PIH. 42–44 Whether increased synthesis of endothelin occurs within the kidney during PIH remains uncertain, as some investigators have found no differences between preeclamptic and normal pregnant women in urinary excretion of endothelin—a measure of local renal synthesis. 42–44
We recently examined the role of endothelin in mediating the hypertension in response to chronic reductions in uterine perfusion pressure in conscious, chronically instrumented pregnant rats. 48 Renal expression of preproendothelin was significantly elevated in both the medulla and in the cortex of the pregnant rats with chronic reductions in uterine perfusion pressure as compared to control pregnant rats. Chronic administration of the selective endothelin type A receptor antagonist (ABT-627, 5 mg/kg/day for 10 days) markedly attenuated the increase in mean arterial pressure observed in the pregnant rats with chronic reductions in uterine perfusion pressure (Fig. 4) . However, endothelin type A receptor blockade had no significant effect on BP in the normal pregnant animals. These findings suggest that endothelin plays a major role in mediating the hypertension produced by chronic reductions in uterine perfusion pressure in pregnant rats.
Mean arterial pressure in response to chronic reductions in uterine perfusion pressure (RUPP) in control pregnant rats and pregnant rats pretreated with an ET A receptor antagonist (+ET A ). All data are expressed as mean ± SEM.
02086-6/2/m_ajh.178S.f4.jpeg?Expires=1727251809&Signature=DfTMRl0LFmLFpK1RLlL6OnrOLiky~-~DuwW6J6HJyxHE00qHRiQnRphemlQViW8a3VgUGITUeqEEUk1d5fJrAegTTCpuTaAhIB5d5SrBYiBbaBF8l4rtAV4Anb7PNxzYnJqIRzEsGgIQBtHOh5kn1yMYtpkZP8UrEp1abtIED6SvrBtyvCU-rxnd~2-BNuAT5b2cIyTaKttg-B-plfj6RALZ06LmB~DcbbSL9oa6ntlvNWHYgCNJWV09jrqQ5CqCeckkb6bfOUwLW-k8slErR-OosEzy6gkZ7j-Vog~kYbsHRzUJ2pfbQRpus45cYghyuoPWZ4A9DLNnAwnY4oT5Ig__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Several lines of evidence suggest that changes in the prostaglandin system may play a role in mediating the renal dysfunction and increase in arterial pressure during PIH. Significant alterations in prostacyclin and thromboxane production occur in women with PIH. 49–52 Plasma and urine levels of thromboxane are elevated in women with PIH, whereas syntheses of prostaglandins, such as prostacyclin, are reduced. 49–52 Additional evidence for a potential role of thromboxane in PIH derives from a study by Woods. 53 She demonstrated that short-term increases in systemic arterial pressure produced by acute reductions in uterine perfusion in pregnant dogs can be prevented by thromboxane receptor antagonism. Further evidence of a potential role for thromboxane is supported by studies in humans, indicating that low dose aspirin attenuates the development of PIH in women at risk for the disease. 1–3
Although some studies suggest a potential role for thromboxane in PIH, the quantitative importance of this substance in mediating the long-term reduction in renal hemodynamics and elevation in arterial pressure produced by chronic reductions in uterine perfusion pressure in pregnant rats is still uncertain. Thromboxane is not only produced by platelets and macrophages, but also by multiple renal cells. 54 , 55 Furthermore, the receptor for thromboxane appears to be abundant within the vasculature of the kidney. 54 , 55 Finally, there is considerable evidence that thromboxane-induced constriction contributes to the renal vasoconstriction in several experimental models of hypertension, 54 , 55 Whether thromboxane mediates the renal hemodynamic and arterial pressure changes observed in the rat model of PIH is unknown. In preliminary experiments, however, we found that urinary excretion of thromboxane B 2 was higher in the hypertensive pregnant rats with chronic reductions in uterine perfusion pressure than normal pregnant rats at day 19 of gestation. 56
Is the renin-angiotensin system important in mediating the reduction in renal function and increase in arterial pressure during PIH?
The renin-angiotensin system plays an important role in the long-term regulation of renal function and arterial pressure during a variety of physiologic and pathophysiologic conditions. 57 During normal pregnancy, plasma renin concentration, renin activity, and angiotensin II (Ang II) levels are all elevated; however, the vascular responsiveness to Ang II appears to be reduced. 1–3 The importance of the renin-angiotensin in the regulation of renal function and arterial pressure during PIH is unclear. Although some studies have reported that reductions in uterine perfusion pressure enhances uteroplacental renin release, most animal studies have reported decreased or normal plasma renin activity and Ang II concentrations. 1–3 In addition, most investigators have observed that in established human preeclampsia, plasma renin activity and Ang II levels are usually low or normal. 1–3 Although circulating levels of Ang II may be normal during PIH, it is possible that reducing uteroplacental perfusion pressure could increase the renal sensitivity to Ang II through reductions in NO or prostacyclin synthesis or by enhanced formation of thromboxane. Consistent with this suggestion are studies indicating enhanced vascular responsiveness to Ang II in vessels from animals or humans with PIH. 1–3 Furthermore, previous studies from our laboratory and others have found that, unlike normal conditions, the preglomerular vessels of the renal circulation become extremely sensitive to the vasoconstrictor actions of Ang II when the renal synthesis of NO or prostacyclin is reduced or when thromboxane synthesis is elevated. 29 , 30 , 57 Increased vascular Ang II responsiveness during PIH, however, does not prove Ang II as an important endogenous mediator of the vasoconstriction or hypertension in experimental models of PIH, as increased responsiveness may only reflect low endogenous Ang II formation. Thus, the importance of increased Ang II to the control of renal function and BP during PIH is unclear. A previous study by Woods and Brooks, 58 however, indicates that Ang II may not be important in mediating the acute rise in arterial pressure during short-term reductions in uterine perfusion pressure in dogs. They demonstrated that the increase in arterial pressure in response to reduced uterine perfusion pressure was unaltered in animals whose renin-angiotensin system had been fixed by prior infusion of captopril plus Ang II infusion. Although the results from this acute study suggest that the renin-angiotensin system might not be involved in mediating increases in systemic arterial pressure during acute reductions in uteroplacental blood flow, the mechanisms causing hypertension under acute conditions may not necessarily be the same as those that contribute to the chronic hypertension induced by long-term reductions in uteroplacental perfusion pressure.
We recently determined the importance of Ang II in mediating the long-term reduction in renal hemodynamic and the hypertension produced by chronic reductions in uterine perfusion pressure in pregnant rats. 59 Chronic oral administration of a converting enzyme inhibitor (enalapril, 250 mg/L for 6 days) decreased mean arterial pressure to a similar extent in pregnant rats with reduced uterine perfusion pressure (RUPP) and normal pregnant rats. Blockade of the renin-angiotensin system (RAS), however, had no significant effect on the BP response to chronic reductions in uterine perfusion pressure as the differences in BP between the normal pregnant and RUPP rats were similar in control and converting enzyme inhibitor-treated groups. These findings suggest that the RAS does not play a major role in mediating the hypertension produced by chronic reductions in uterine perfusion pressure in pregnant rats. 59
Is maternal endothelial activation/dysfunction in preeclampsia due to enhanced cytokine production in response to placental ischemia?
Although reductions in blood flow to the uteroplacental unit are known to result in cardiovascular and renal abnormalities consistent with the pathophysiologic features of human PIH, the physiologic mechanisms linking placental ischemia with the abnormalities in the maternal circulation are unclear. 60 Several lines of evidence support the hypothesis that the ischemic placenta contributes to endothelial cell activation/dysfunction of the maternal circulation by enhancing the synthesis of cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1). 60 TNF-α and IL-1 are inflammatory cytokines that have been shown to induced structural as well as functional alterations in endothelial cells. 61 These inflammatory cytokines also enhance the formation of a number of endothelial cell substances such as endothelin and reduce acetylcholine-induced vasodilatation. 60–62 Also supporting a potential role of TNF-α in preeclampsia are findings that plasma levels of TNF-α are significantly elevated in women with preeclampsia by approximately twofold. 60 , 63 Furthermore, IL-6, which is activated by TNF-α, has also been reported to be elevated in preeclamptic women. 60 Although high levels of TNF-α, as observed during septic shock or after lipopolysaccharide administration, activate gene expression of inducible nitric oxide synthase, modest levels of TNF-α have been shown to destabilize the mRNA of endothelial nitric oxide synthase. 64
Whether chronic and modest increases in plasma TNF-α can activate the endothelium during pregnancy and lead to reduced kidney function, high BP, and other features of PIH is unknown. Consistent with a potential role of cytokine activation in PIH is the recent study by Faas and colleagues. 65 They reported that an intravenous infusion of a high dose of lipopolysaccharide (LPS) decreased BP in pregnant rats, whereas a very low dose infusion of the endotoxin resulted in significant and long-term increases in BP and urinary albumin excretion and significant platelet aggregation in conscious pregnant rats. Although LPS is known to activate TNF-α, it is unclear whether the effects of low dose LPS on cardiovascular and kidney function were mediated through TNF-α or IL-1, as these cytokines were not measured in that study.
Although plasma levels of TNF-α are elevated by two- to threefold in women with PIH, the importance of TNF-α in mediating the systemic and renal hemodynamic changes associated with this disease is unclear. To determine the long-term effects of a two- to threefold elevation in plasma TNF-α on renal and systemic hemodynamics in pregnant rats we recently infused TNF-α for 5 days at a rate of 50 ng/day during days 14 to 19 of gestation in pregnant rats. 66 Plasma levels doubled in the TNF-α-treated pregnant rats. Arterial pressure was significantly higher in the TNF-α-treated pregnant rat as compared to pregnant controls at day 19 of gestation. A twofold elevation in plasma TNF-α in pregnant rats also caused a significant reduction in renal hemodynamics. These data suggest that elevated plasma levels of TNF-α observed in preeclamptic women may play an important role in the pathogenesis of PIH.
Although these preliminary findings with TNF-α support the cytokine hypothesis, finding the link between placental ischemia and maternal endothelial and vascular abnormalities remains an important area of investigation. Microarray analysis of genes within the ischemic placenta of women with preeclampsia and in animal models of chronic reductions in uterine perfusion pressure should provide new insights into the link between placental ischemia and hypertension. More effective strategies for the prevention of preeclampsia should be forthcoming once the underlying pathophysiologic mechanisms that are involved in PIH are completely understood.
Studies during the past decade have provided a better understanding of the potential mechanisms responsible for the pathogenesis of PIH. The initiating event in PIH has been postulated to be reduced uteroplacental perfusion as a result of abnormal cytotrophoblast invasion of spiral arterioles (Fig. 5) . Placental ischemia is thought to lead to widespread activation/dysfunction of the maternal vascular endothelium that results in enhanced formation of endothelin and thromboxane, increased vascular sensitivity to Ang II, and decreased formation of vasodilators such as NO and prostacyclin. These endothelial abnormalities, in turn, cause chronic hypertension by impairing renal pressure natriuresis and increasing total peripheral resistance. The quantitative importance of the various endothelial and humoral factors in mediating the reduction in renal hemodynamic and excretory function and elevation in arterial pressure during PIH is still unclear. Results from ongoing basic and clinical studies, however, should provide new and important information regarding the physiologic mechanisms responsible for the elevation in arterial pressure in women with preeclampsia. More effective strategies for the prevention of preeclampsia should be forthcoming once the underlying pathophysiologic mechanisms that are involved in PIH are completely understood.
Potential mechanism whereby chronic reductions in uteroplacental perfusion may lead to hypertension. ET = endothelin; TBX = thromboxane; PGI 2 = prostacyclin; NO = nitric oxide; ANG II = angiotensin II.
02086-6/2/m_ajh.178S.f5.jpeg?Expires=1727251810&Signature=Pu~pIdARix~HrpbjmJqHBIrgPs8kfgGFtS7Z-rzTefF5v4SbgAetUS-oDANgTCzhjalY77UxwW1flUrVhOvzAxfMwWROiuP~1PgVwn0sK-M8A8URUdxqBrBz2ttKm6EIsR3dss5BOysV4EHglc80PV4IqS312ybu29jURoGFyLg7fMRQxiPxONV0aBwGPWQM2bZ8SlGThPIRnrNxEcMachLm4fpzbotARECdxfWGQL-i7n0Jt00cTcIQ0WQbALG80gOaQFQ7ETLmLlbyj3QpU5kyg1W3j9y6q5Sz-daUzLgKNFKVjPhWEMxQ68Mto7IIqc5XctLc6tb-SZxw2YlhCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
1. August P , Lindheimer MD : Pathophysiology of preeclampsia . Hypertension 1995 ; 142 : 2407 – 2426 .
Google Scholar
2. Lindheimer MD , Katz AI : Renal physiology and disease in pregnancy , in Seldin D.W. and Giebisch G. (Eds). The Kidney: Physiology and Pathophysiology . 2nd ed. Raven Press : New York , 1992 . 3371 – 3431 .
Google Preview
3. Chesley LC Hypertensive disorders in pregnancy . Appleton-Century-Crofts : New York , 1978 .
4. Saftlas AF , Olson DR , Franks AL , Atrash HK , Pokras R : Epidemiology of preeclampsia and eclampsia in the United States, 1979–1986 . Am J Obstet Gynecol 1990 ; 163 : 460 – 465 .
5. Gerretsen G , Huisjes HJ , Elema JD : Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation . Br J Obstet Gynecol 1981 ; 88 : 876 – 881 .
6. Conrad KP : Animal models of pre-eclampsia: do they exist? . Fetal Med Rev 1990 ; 2 : 67 – 88 .
7. Douglas BH : The rat as a model for preeclampsia , in Lindheimer M.D., Katz A.I. and Zuspan F.P. (Eds). Hypertension in Pregnancy . John Wiley : New York , 1976 . 411 – 419 .
8. Roberts JM , Taylor RN , Goldfien A : Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia . Am J Hypertens 1991 ; 4 : 700 – 708 .
9. Morris NH , Eaton BM , Dekker G : Nitric oxide, the endothelium, pregnancy and pre-eclampsia . Br J Obstet Gynecol 1996 ; 103 : 4 – 15 .
10. Rodgers GM , Taylor RN , Roberts JM : Preeclampsia is associated with a serum factor cytotoxic to human endothelial cells . Am J Obstet Gynecol 1988 ; 159 : 908 – 914 .
11. Keith JC Jr , Thatcher CD , Schaub RG : Beneficial effects of U-63, 557A, a thromboxane synthetase inhibitor, in an ovine model of pregnancy-induced hypertension . Am J Obstet Gynecol 1987 ; 157 : 199 – 203 .
12. Davison JM , Hytten FE : Glomerular filtration during and after pregnancy . J Obstet Gynecol (Brit Common) 1974 ; 81 : 588 – 595 .
13. Gant NF , Daley GL , Chand S , Whalley PJ , McDonald PC : A study of angiotensin II pressor response throughout primigravid pregnancy . J Clin Invest 1973 ; 52 : 2682 – 2689 .
14. Conrad KP : Possible mechanisms for changes in renal hemodynamics during pregnancy: studies from animal models . Am J Kidney Dis 1987 ; 9 : 253 – 263 .
15. Baylis C , Suto T , Conrad K : Importance of nitric oxide in control of systemic and renal hemodynamics during normal pregnancy: studies in the rat and implications for preeclampsia . Hypertens Pregnancy 1996 ; 15 : 147 – 169 .
16. Losonczy G , Brown G , Venuto RC : Increased peripheral resistance during reduced uterine perfusion pressure hypertension in pregnant rabbits . Am J Med Sci 1992 ; 303 : 233 – 240 .
17. Eder DJ , McDonald MT : A role for brain angiotensin II in experimental pregnancy-induced hypertension in laboratory rats . Clin Exp Hyper Hyper Preg 1987 ; B6 : 431 – 451 .
18. Abitbol MM : Simplified technique to produce toxemia in the rat: consideration on cause of toxemia . Clin Exp Hyper Hyper Preg 1982 ; B1 : 93 – 103 .
19. Alexander BT , Kassab SE , Miller MT , Abram SR , Reckelhoff JF , Bennett WA , Granger JP : Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide . Hypertension 2001 ; 37 : 1191 – 1195 .
20. Nienartowicz A , Link S , Moll W : Adaptation of the uterine arcade in rats during pregnancy . J Develop Physiol 1989 ; 21 : 101 – 108 .
21. Crews JK , Herrington JN , Granger JP , Khalil RA : Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rats . Hypertension 2000 ; 35 : 71 – 76 .
22. Khalil RA , Crews JK , Novak J , Kassab S , Granger JP : Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats . Hypertension 1998 ; 31 : 1065 – 1069 .
23. Granger JP , Alexander BT : Pathophysiology of pregnancy-induced hypertension . Curr Concepts Hypertens 1999 ; 3 : 5 – 6 .
24. Granger JP , Alexander BT : Abnormal pressure natriuresis in hypertension: role of nitric oxide . Acta Physiol Scand 2000 ; 168 : 161 – 168 .
25. Seligman SP , Buyon JP , Clancy RM , Young BK , Abramson SB : The role of nitric oxide in the pathogenesis of preeclampsia . Am J Obstet Gynecol 1994 ; 171 : 944 – 948 .
26. Baylis C , Engels K : Adverse interactions between pregnancy and a new model of systemic hypertension produced by chronic blockade of endothelial derived relaxing factor (EDRF) in the rat . Clin Exp Hypertens 1992 ; B11 : 117 – 129 .
27. Bachmann S , Mundel P : NO in the kidney: synthesis, localization and function . Am J Kidney Dis 1994 ; 24 : 112 – 129 .
28. Nakamura T , Alberola A , Granger JP : Role of renal interstitial pressure as a mediator of sodium retention during blockade of endothelium derived nitric oxide hypertension . Hypertension 1993 ; 21 : 956 – 960 .
29. Alberola A , Salazar FJ , Nakamura T , Granger JP : Renal hemodynamic effects of angiotensin II (AII): interactions with endothelium derived nitric oxide . Am J Physiol 1994 ; 267 : R1472 – R1478 .
30. Schnackenberg C , Wilkins C , Granger JP : Role of nitric oxide in modulating the vasoconstrictor actions of angiotensin II in preglomerular and postglomerular vessels in dogs . Hypertension 1995 ; 26 : 1024 – 1029 .
31. Novak J , Reckelhoff JF , Bumgarner L , Cockrell K , Kassab SE , Granger JP : Role of nitric oxide in mediating the reduced sensitivity of the renal circulation to angiotensin II in pregnant rats . Hypertension 1997 ; 30 : 580 – 584 .
32. Kassab S , Miller T , Novak J , Reckelhoff JF , Hester RL , Granger JP : Systemic hemodynamics and regional blood flows during chronic nitric oxide synthesis inhibition in pregnancy . Hypertension 1998 ; 30 : 315 – 320 .
33. Nakamura T , Salazar FJ , Alberola A , Granger JP : Effect of renal perfusion pressure on renal interstitial hydrostatic pressure and Na excretion: role of endothelium-derived nitric oxide . Nephron 1998 ; 78 : 104 – 111 .
34. Yallampalli C , Garfield RE : Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia . Am J Obstet Gynecol 1993 ; 169 : 1316 – 1320 .
35. Molnar M , Suto T , Toth T , Hertelendy F : Prolonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation . Am J Obstet Gynecol 1994 ; 170 : 1458 – 1466 .
36. Alexander BT , Reckelhoff JF , Kassab S , Granger JP : Differential expression of renal nitric oxide synthase isoforms during pregnancy in rats . Hypertension 1999 ; 33 : 435 – 439 .
37. Yanagisawa M , Kurihara H , Kimura S : A novel potent vasoconstrictor peptide produced by vascular endothelial cells . Nature 1988 ; 332 : 411 – 415 .
38. Kohan DE : Endothelins in the normal and diseased kidney . Am J Kidney Dis 1997 ; 29 : 2 – 26 .
39. Schiffrin EL : Endothelin: potential role in hypertension and vascular hypertrophy . Hypertension 1995 ; 25 : 1135 – 1143 .
40. Kato T , Kassab S , Wilkins FC , Kirchner K , Keiser J , Granger JP : Endothelin antagonist improve renal function in spontaneously hypertensive rats . Hypertension 1995 ; 25 : 883 – 887 .
41. Kassab S , Novak J , Miller T , Granger J : Cardiovascular and renal actions of endothelin receptor antagonism in Dahl salt-sensitive hypertension . Hypertension 1997 ; 30 : 682 – 686 .
42. Dekker GA , Kraayenbrink AA , Zeeman GG , van Kamp GJ : Increased plasma levels of the novel vasoconstrictor peptide endothelin in severe pre-eclampsia . Eur J Obstet Gynecol Reprod Biol 1991 ; 40 : 215 – 220 .
43. Clark BA , Halvorson L , Sachs B , Epstein FH : Plasma endothelin levels in preeclampsia: elevation and correlation with uric acid levels and renal impairment . Am J Obstet Gynecol 1992 ; 166 : 962 – 968 .
44. Roberts JM , Taylor RN , Musci TJ , Rogers GM , Hubel CA , McLaughlin MK : Preeclampsia: an endothelial cell disorder . Am J Obstet Gynecol 1989 ; 161 : 1200 – 1204 .
45. Taylor RN , Varma M , Teng NNH , Roberts JM : Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies . J Clin Endocrinol Metab 1990 ; 71 : 1675 – 1677 .
46. Wilkins FC Jr , Alberola A , Mizelle HL , Opgenorth TJ , Granger JP : Chronic hypertension produced by long-term pathophysiological increases in circulating endothelin levels in conscious dogs . J Cardiovasc Res 1993 ; 22 : 325 – 328 .
47. Wilkins FC Jr , Alberola A , Mizelle HL , Opgenorth TJ , Granger JP : Systemic hemodynamics and renal function during long-term pathophysiological increases in circulating endothelin . Am J Physiol 1995 ; 268 : R375 – R381 .
48. Alexander BT , Rinewalt AN , Cockrell KL , Bennett WA , Granger JP : Endothelin-A receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure . Hypertension 2001 ; 37 : 485 – 489 .
49. Wang Y , Walsh S , Kay H : Placenta lipid peroxides and thromboxane are increased and prostacyclin is decreased in women with preeclampsia . Am J Obstet Gynecol 1992 ; 167 : 946 – 949 .
50. Friedman SA : Preeclampsia: a review of the role of prostaglandins . Obstet Gynecol 1988 ; 71 : 122 – 137 .
51. Conrad KP , Dunn MJ : Renal synthesis and urinary excretion of eicosanoids during pregnancy in rats . Am J Physiol 1987 ; 253 : F1197 .
52. Wang Y , Walsh SW , Guo J , Zhang J : The imbalance between thromboxane and prostacyclin in preeclampsia is associated with an imbalance between lipid peroxides and vitamin E in maternal blood . Am J Obstet Gynecol 1991 ; 165 : 1695 – 1700 .
53. Woods LL : Importance of prostaglandins in hypertension during reduced uteroplacental perfusion pressure . Am J Physiol 1989 ; 257 : R1558 – R1561 .
54. Remuzzi G , Fitzgerald GA , Patrono C : Thromboxane synthesis and action within the kidney . Kidney Int 1992 ; 41 : 1483 – 1493 .
55. Ogletree ML : Overview of physiological and pathophysiological effects of thromboxane A2 . Fed Proc 1987 ; 46 : 133 – 138 .
56. Llinas MT , Alexander BT , Abram SR , Sedeek M , Granger JP : Enhanced production of thromboxane A2 in response to chronic reductions in uterine perfusion pressure in pregnant rats . FASEB J 2001 ; 15 : A288 . (Abstract)
57. Hall JE , Granger JP : Role of sodium and fluid excretion in hypertension , in Swales J.D. (Ed). Textbook of Hypertension . Blackwell Scientific Pubs : Oxford , 1994 . 360 – 387 .
58. Woods LL , Brooks VL : Role of the renin-angiotensin system in hypertension during reduced uteroplacental perfusion pressure . Am J Physiol 1989 ; 257 : R204 – R209 .
59. Alexander BT , Cockrell KL , Sedeek M , Granger JP : Role of the renin-angiotensin system in mediating the hypertension produced by chronic reductions in uterine perfusion pressure in the pregnant rat . Hypertension 2001 ; 37 : 986 . (Abstract)
60. Conrad KP , Benyo DF : Placental cytokines and the pathogenesis of preeclampsia . Am J Reprod Immunol 1997 ; 37 : 240 – 249 .
61. Pober JS , Cotran RS : Cytokines and endothelial cell biology . Physiol Rev 1990 ; 70 : 427 – 451 .
62. Marsden PA , Brenner BM : Transcriptional regulation of the endothelin-1 gene by TNFα . Am J Physiol 1992 ; 262 : C854 – C861 .
63. Kupferminc MJ , Peaceman AM , Wigton TR , Rehnberg KA , Socol ML : Tumor necrosis factor-alpha I is elevated in plasma and amniotic fluid of patients with severe preeclampsia . Am J Obstet Gynecol 1994 ; 170 : 1752 – 1759 .
64. Yoshizumi M , Perrella MA , Burnett JC , Lee ME : Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life . Circ Res 1993 ; 73 : 205 – 209 .
65. Faas MM , Schulling GA , Baller JFW , Visscher CA , Bakker WW : A new animal model for human preeclampsia: ultra-low-dose endotoxin infusion in pregnant rats . Am J Obstet Gynecol 1994 ; 171 : 158 – 164 .
66. Granger JP , Bennett WM , Alexander BT , Cockrell KL , Whitworth NS : Long-term elevation of plasma TNF-alpha increases arterial pressure and reduces kidney function in pregnant rats . Hypertension 1999 ; 34 : 337 . (abst)
67. Granger JP , Alexander BT , Abram SR , Reckelhoff JF , Wilson J , Rinewalt AN : Chronic reductions in uterine perfusion pressure in the pregnant rat produces hypertension and reduces pressure-natriuresis . Hypertension 2001 ; 37 : 682 . (Abstract)
- epoprostenol
- hypertension, pregnancy-induced
- nitric oxide
- pre-eclampsia
- hypertension
- hemodynamics
- excretory function
- angiotensin ii
- thromboxane
- vascular endothelium
- vasodilators
- endothelins
- endothelium
- maternal mortality
- arterial pressure
- perinatal period
- cytotrophoblast
| Month: | Total Views: |
|---|---|
| December 2016 | 3 |
| January 2017 | 20 |
| February 2017 | 38 |
| March 2017 | 26 |
| April 2017 | 25 |
| May 2017 | 22 |
| June 2017 | 45 |
| July 2017 | 37 |
| August 2017 | 25 |
| September 2017 | 93 |
| October 2017 | 126 |
| November 2017 | 126 |
| December 2017 | 576 |
| January 2018 | 655 |
| February 2018 | 790 |
| March 2018 | 1,146 |
| April 2018 | 1,134 |
| May 2018 | 964 |
| June 2018 | 980 |
| July 2018 | 1,136 |
| August 2018 | 1,366 |
| September 2018 | 1,227 |
| October 2018 | 1,292 |
| November 2018 | 1,219 |
| December 2018 | 1,030 |
| January 2019 | 983 |
| February 2019 | 1,270 |
| March 2019 | 1,616 |
| April 2019 | 1,566 |
| May 2019 | 1,611 |
| June 2019 | 1,340 |
| July 2019 | 1,385 |
| August 2019 | 1,169 |
| September 2019 | 1,421 |
| October 2019 | 1,637 |
| November 2019 | 1,817 |
| December 2019 | 1,081 |
| January 2020 | 1,327 |
| February 2020 | 1,787 |
| March 2020 | 1,404 |
| April 2020 | 1,262 |
| May 2020 | 980 |
| June 2020 | 1,290 |
| July 2020 | 1,248 |
| August 2020 | 1,486 |
| September 2020 | 1,953 |
| October 2020 | 2,058 |
| November 2020 | 1,787 |
| December 2020 | 1,171 |
| January 2021 | 1,422 |
| February 2021 | 2,556 |
| March 2021 | 2,747 |
| April 2021 | 2,118 |
| May 2021 | 1,780 |
| June 2021 | 1,317 |
| July 2021 | 1,264 |
| August 2021 | 1,180 |
| September 2021 | 1,412 |
| October 2021 | 1,626 |
| November 2021 | 1,523 |
| December 2021 | 1,076 |
| January 2022 | 1,190 |
| February 2022 | 2,210 |
| March 2022 | 2,325 |
| April 2022 | 2,212 |
| May 2022 | 1,687 |
| June 2022 | 1,099 |
| July 2022 | 1,055 |
| August 2022 | 938 |
| September 2022 | 1,190 |
| October 2022 | 1,377 |
| November 2022 | 1,375 |
| December 2022 | 845 |
| January 2023 | 1,104 |
| February 2023 | 1,843 |
| March 2023 | 1,603 |
| April 2023 | 1,274 |
| May 2023 | 1,146 |
| June 2023 | 1,037 |
| July 2023 | 1,172 |
| August 2023 | 869 |
| September 2023 | 1,063 |
| October 2023 | 1,190 |
| November 2023 | 1,402 |
| December 2023 | 889 |
| January 2024 | 1,060 |
| February 2024 | 1,921 |
| March 2024 | 1,673 |
| April 2024 | 1,309 |
| May 2024 | 1,406 |
| June 2024 | 847 |
| July 2024 | 876 |
| August 2024 | 428 |
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1941-7225
- Copyright © 2024 American Journal of Hypertension, Ltd.
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Review Series - Hypertension under specific condition
- Published: 20 June 2022
Hypertensive disorders of pregnancy: definition, management, and out-of-office blood pressure measurement
- Hirohito Metoki 1 , 2 ,
- Noriyuki Iwama 2 , 3 ,
- Hirotaka Hamada 3 ,
- Michihiro Satoh 1 , 2 ,
- Takahisa Murakami 1 , 2 ,
- Mami Ishikuro 2 &
- Taku Obara 2 , 4
Hypertension Research volume 45 , pages 1298–1309 ( 2022 ) Cite this article
18k Accesses
37 Citations
37 Altmetric
Metrics details
Hypertensive disorders of pregnancy increase the risk of adverse maternal and fetal outcomes. In 2018, the Japanese classification of hypertensive disorders of pregnancy was standardized with those of other countries, and a hypertensive disorder of pregnancy was considered to be present if hypertension existed during pregnancy and up to 12 weeks after delivery. Strategies for the prevention of hypertensive disorders of pregnancy have become much clearer, but further research is needed on appropriate subjects and methods of administration, and these have not been clarified in Japan. Although guidelines for the use of antihypertensive drugs are also being studied and standardized with those of other countries, the use of calcium antagonists before 20 weeks of gestation is still contraindicated in Japan because of the safety concerns that were raised regarding possible fetal anomalies associated with their use at the time of their market launch. Chronic hypertension is now included in the definition of hypertensive disorders of pregnancy, and blood pressure measurement is a fundamental component of the diagnosis of hypertensive disorders of pregnancy. Out-of-office blood pressure measurements, including ambulatory and home blood pressure measurements, are important for pregnant and nonpregnant women. Although conditions such as white-coat hypertension and masked hypertension have been reported, determining their occurrence in pregnancy is complicated by the gestational week. This narrative review focused on recent reports on hypertensive disorders of pregnancy, including those related to blood pressure measurement and classification.
Similar content being viewed by others

Chronic hypertension diagnosed before or during pregnancy and its effects on pregnancy outcomes

Time in therapeutic range and risk of preeclampsia in chronic hypertensive pregnant women

Optimal blood pressure target to prevent severe hypertension in pregnancy: A systematic review and meta-analysis
Introduction.
In Japan, “pregnancy toxemia”, with three main features, “hypertension,” “proteinuria,” and “edema”, was defined and classified in 1982 [ 1 ] and then again in 1984 [ 2 ]. This term was widely used until 2005, when it was changed to “pregnancy-induced hypertension.” In 2018, the classification was standardized with those of other countries, and “hypertensive disorders of pregnancy (HDP)” were considered to be present if hypertension existed during pregnancy and up to 12 weeks after delivery [ 3 ]. High blood pressure before pregnancy (chronic hypertension) is now included in the definition of HDP. Among the various hypotheses explaining the etiology of HDP, the two-stage theory and angiogenesis imbalance are the most plausible. The two-stage theory of the etiology of HDP may have led to the novel possibility of treatment/prevention for HDP. Furthermore, assessing the circulating levels of angiogenic factors may have diverse clinical roles in preventing adverse outcomes in HDP [ 4 ]. This narrative review focused on recent reports on HDP, including those related to blood pressure measurement and classification.
Classification and definition of hypertensive disorders of pregnancy
HDP are classified into four types: preeclampsia, gestational hypertension, superimposed preeclampsia, and chronic hypertension [ 5 ]. Preeclampsia is defined as hypertension after 20 gestational weeks with proteinuria, organ damage, or uteroplacental dysfunction. Gestational hypertension is similar to preeclampsia; however, the condition is defined as hypertension alone after 20 gestational weeks. Based on the Japan Society for the Study of Hypertension in Pregnancy (JSSHP), superimposed preeclampsia is defined as hypertension accompanied by organ damage or proteinuria [ 3 , 5 ].
In normal pregnancy, spiral artery remodeling occurs, where trophoblastic cells invade the decidua and replace the endothelial cells and vascular smooth muscle of the decidua spiral artery. As a result, the maternal blood vessels begin to perfuse into the interchorionic space, which increases the partial pressure of oxygen in the placenta and reduces systemic vascular resistance (Fig. 1A ). Angiogenic factors, vascular endothelial growth factors (VEGFs), and placental growth factors (PlGFs) affect angiogenesis intracellularly through the receptor VEGFR-1 (Fig. 1B ). Uterine natural killer (uNK) cells and regulatory T cells are essential for maintaining pregnancy and inhibiting allogeneic responses toward the fetus [ 6 , 7 ]. Decidual uNK cells control trophoblast invasion by producing interleukin-8 and interferon-inducible protein-10 chemokines and secrete a series of angiogenic factors [ 8 ]. Early vascular changes resulting from desquamation, such as intimal vacuolation and disintegration, and thinning of the tunica media occur before trophoblastic cells are present near the spiral arteries of the uterus [ 9 ].
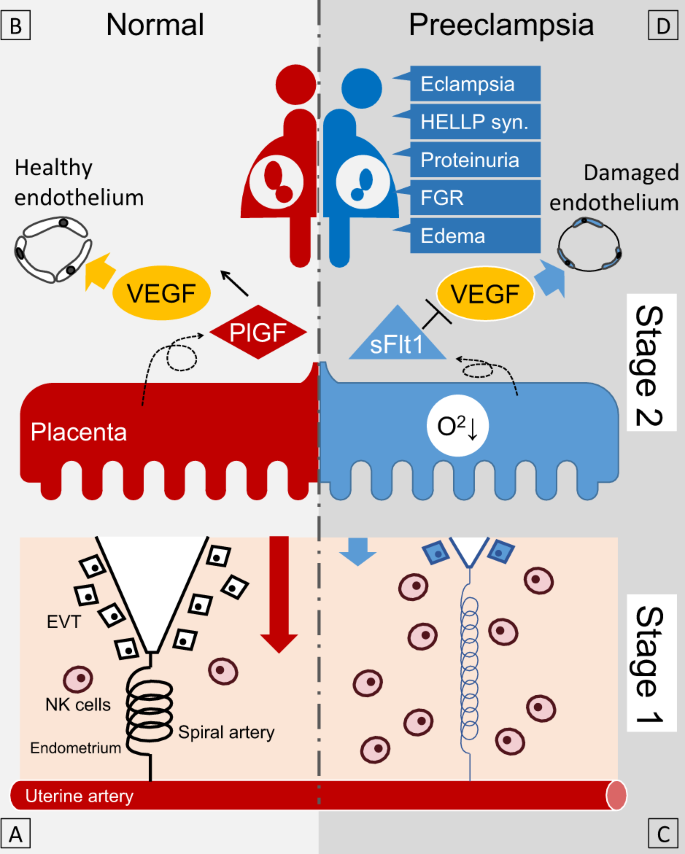
Schematic diagram of the two-stage theory of preeclampsia. In normal pregnancy, appropriate EVT invasion into the maternal endometrium (red arrow) leads to sufficient maternal blood flow from the spiral artery ( A ). PlGF, which is secreted from the placenta, activates VEGF and maintains a healthy endothelium ( B ). On the other hand, in preeclamptic pregnancy, incomplete invasion of the EVT (blue arrow) leads to insufficient maternal blood flow from the spiral artery and subsequent placental hypoxia ( C ). sFlt1 is then secreted from the placenta, which suppresses VEGF, resulting in systemic endothelial dysfunction and the appearance of various clinical symptoms ( D ). HELLP syn. hemolysis, elevated liver enzymes, low platelet count syndrome, FGR fetal growth restriction, NK cells natural killer cells, EVT extravillous trophoblast, PlGF placental growth factor, sFlt1 soluble fms-like tyrosine kinase-1, VEGF vascular endothelial growth factor
Preeclampsia
Preeclampsia is a complex medical disorder [ 10 ]. According to recent guidelines in Japan [ 5 ] and other countries [ 10 , 11 , 12 , 13 , 14 ], preeclampsia is characterized by hypertension with maternal acute kidney injury, liver dysfunction, neurological features, hemolysis or thrombocytopenia, or fetal growth restriction. Preeclampsia is thought to originate from the placenta because of the rapid improvement of clinical symptoms of preeclampsia after placenta delivery [ 15 ], while retained placenta leads to the development of preeclampsia; the removal of the placenta by intrauterine curettage results in disappearance of the symptoms [ 16 ].
Insufficient angiogenesis and remodeling cause an incomplete increase in the partial pressure of oxygen in the fetal placental circulation (Fig. 1C ), resulting in placental ischemia and damage [ 17 ]. Stimulated soluble VEGFR-1 (sFlt-1) production in trophoblast cells inhibits PlGF production and soluble endoglin (sEng) production [ 18 ]. Inhibition of VEGF and PlGF by sFlt-1 suppresses the invasion of trophoblastic cells into the shed membrane and damages vascular endothelial cells (Fig. 1D ). By binding and antagonizing TGF-β, sEng inhibits the invasion of cytotrophoblast cells [ 19 ]. The transition of these factors into maternal circulation causes the maternal symptoms of preeclampsia [ 20 , 21 ]. Placental abnormalities in early pregnancy may cause chronic uteroplacental insufficiency, local ischemia, and the release of inflammatory cytokines, resulting in earlier maternal hypertension in early-onset preeclampsia [ 22 , 23 , 24 ]. In contrast, late-onset preeclampsia is more frequently based on placental dysfunction associated with chronic oxidative stress due to maternal metabolic abnormalities such as obesity and insulin resistance [ 22 , 23 , 25 ]. At the same time, there is much overlap in placental pathology and continuous features in desmoplastic vascular lesion pathology among the four HDP subtypes [ 26 ].
Superimposed preeclampsia
Superimposed preeclampsia is defined as chronic hypertension or kidney disease that progresses to preeclampsia [ 3 ]. It should be noted that in countries other than Japan, it basically refers only to superimposed chronic hypertension [ 10 , 11 , 12 , 13 , 14 ]. Vascular endothelial dysfunction is reported to predict the development of superimposed preeclampsia in chronic hypertension [ 27 ]. In preeclampsia following de novo gestational hypertension, early placental calcification and weight gain precede preeclampsia [ 28 ]. Pregnant women with IgA nephropathy [ 29 ] and chronic kidney disease [ 30 ] had 7.3- and 10.4-fold greater risks of preeclampsia than others, respectively.
Chronic hypertension in pregnancy
Blood pressure during early pregnancy seems important in pregnancies complicated by hypertension [ 31 , 32 ]. A systolic blood pressure <130 mmHg within 14–15 weeks of gestation was reported to reduce the risk of early-onset superimposed preeclampsia in women with chronic hypertension [ 33 ]. As described in a later section on white-coat hypertension, it is essential to diagnose whether a patient has sustained or white-coat hypertension. Because chronic hypertension is a risk factor for perinatal mortality in both early and late gestation, a planned delivery at 37 to 38 weeks of gestation is reported to be a superior balance of risk [ 34 ].
Maternal outcomes
Regarding the risk of developing cardiovascular diseases later in life, although there are differences among HDP subtypes, Veerbeek et al. [ 35 ] reported that all types of HDP seem to be associated with high risks. Gestational hypertension is reported to be associated with a 4.2-fold higher risk for future chronic hypertension [ 36 ] and a greater risk of cardiovascular disease, coronary heart disease, and heart failure [ 37 ]. Preeclampsia is associated with a fourfold increased risk of future heart failure and a twofold increased risk of coronary heart disease, stroke, and death due to coronary heart or cardiovascular disease [ 38 ]. Women with HDP were reported to have a 6.3-fold higher risk for future hypertension within 2 years postpartum compared to controls [ 39 ] and a 4.9-fold higher risk of chronic kidney disease in later life [ 40 ].
Birth outcomes
Maternal cardiac output in early pregnancy has been associated with being small for gestational age (SGA) [ 41 ]. Maternal hypertension-related factors were associated with infant growth via placental factors based on the genome wide association study summary statistics of BioBank Japan data and compared with cohort data [ 42 ]. The Hokkaido study showed that women with HDP had 2.1-, 3.5-, and 3.6-fold higher risks of having SGA infants, preterm birth, and infants with low birth weight than those with normotensive pregnancy [ 43 ]. Home [ 44 ] and ambulatory [ 45 ] blood pressure measurements have been shown to be more associated with birth weight than clinic blood pressure; these are reviewed in subsequent sections. The trajectory of maternal blood pressure during pregnancy is also an indicator of infant birth weight [ 46 , 47 , 48 ].
Long-term outcomes of offspring
According to a meta-analysis of eight studies, HDP were associated with a 1.2-fold higher risk of asthma in offspring [ 49 ]. In a study, offspring exposed to HDP had a 1.4- and 1.3-fold higher risk for autism spectrum disorders and attention-deficit hyperactivity disorder, respectively [ 50 ]. The Helsinki Birth Cohort Study reported that offspring exposed to maternal gestational hypertension in utero had an increased risk of type 2 diabetes in late adulthood after adjustment for low birth weight or small for gestational age infants [ 51 ].
The results of studies in Japan on the long-term prognosis of pregnant women with HDP and their children exposed to HDP are now being reported. The TMM BirThree Cohort Study reported that women with superimposed preeclampsia had a 1.8-fold increased risk of having children with autistic behavior at 2 years old compared to normotensive women [ 52 ]. The Hokkaido Birth Cohort Study reported that male children exposed to HDP caught up with their growth and gained more weight by 7 years of age than male children who were not exposed to HDP [ 53 ]. According to observations in the Japan Environment and Children’s Study (JECS), HDP were not a risk factor for offspring regardless of the sensitivity analyses using possible mediating factors such as cesarean delivery, birth weight, and gestational age [ 54 ].
When examining the association between HDP and prognosis, there is no need to adjust for preterm birth and low birth weight because they are included in HDP outcomes and are mediators rather than confounders when considering their impact on the long-term prognosis of offspring [ 49 , 50 , 52 , 53 ]. On the other hand, as mentioned earlier, several studies have performed sensitivity analyses considering the role of HDP as mediators [ 51 , 54 ]. Based on the results of the ongoing mediator analysis and other studies, future studies need to examine possible intervention points for the association between HDP and child outcomes and develop better intervention methods.
Prediction, prevention, and treatment
Associated factors and prediction.
The Fetal Medicine Foundation (FMF) first-trimester prediction model (the FMF triple test) has high detection rates of 90% and 75% for the prediction of early and preterm preeclampsia, respectively, with a 10% false-positive rate [ 55 ]. This FMF triple test consists of a combination of maternal factors and measurements of mean arterial pressure, the uterine artery pulsatility index, and serum placental growth factor. An Asia-wide study using an algorithm developed by the FMF in Asian people confirmed the validity of the FMF triple test with a detection rate of 64% for the prediction of preterm preeclampsia with a 10% false-positive rate [ 56 ].
In addition to the FMF triple test, several predictors have been reported in individual studies, and those presented in this study are listed in Table 1 .
The JECS is a cohort study that started in 2011 to investigate the relationship between environmental exposure and child health. Several studies on HDP have been conducted with the JECS cohort. Higher levels of HbA1c at a nondiabetic level [ 57 ], both lower and higher Na intake before pregnancy [ 58 ], elevated serum IgE levels during the first trimester [ 59 ], higher caffeine intake [ 60 ], working a schedule of ≥36 h per week with night shifts [ 61 ], smoking [ 62 ], alcohol consumption [ 63 ], and becoming pregnant with in vitro fertilization and embryo transfer [ 64 ] were associated with the risk of hypertensive disorders of pregnancy. Moreover, coffee intake was associated with a decreased risk of HDP [ 60 ]. Although this is a large cohort study, some studies reported that no association between the exposures and outcomes can be found, such as calcium intake and HDP among primiparas [ 65 ]. The JECS involves a novel approach to adjunct studies. The peak areas of N-dimethylglycine and S-methylcysteine were significantly higher in the first-trimester serum of patients with early-onset HDP than in controls [ 66 ].
Sleep quality in early pregnancy may help predict elevated systolic blood pressure in the first trimester [ 67 ], and overnight oxygen saturation screening ~1 month before the due date may be useful in predicting late-onset gestational hypertension [ 68 ]. Unmodifiable factors include twin pregnancy [ 69 , 70 ] and residing in a high-altitude area (>2500 m) [ 71 , 72 ]. Blood pressure is known to be elevated in twin pregnancy [ 69 ], regardless of whether the pregnancy is a dichorionic or monochorionic diamniotic twin pregnancy [ 70 ]; therefore, pregnant women with unmodifiable factors should be followed up as high-risk pregnancies.
Several efforts to perform comprehensive metabolomic analysis in samples of pregnant women have been reported, such as the C-MATCH [ 73 ] and HELIX studies [ 74 ]. The metabolite profiles of women who developed HDP were comparable to those of women with normal pregnancies with longer gestation in the Maternity Log study, which is an adjunct to the BirThree cohort study [ 75 ].
Aspirin administration has been described in various guidelines as effective in preventing the onset of preeclampsia. The ASPRE study showed that aspirin treatment for pregnant women at high risk for preeclampsia reduced the incidence of preeclampsia to 0.38 [ 76 ]. The NICE [ 11 ], ACOG [ 12 ], USPSTF [ 77 ], SOGC [ 13 ], SOMANZ [ 14 ], and ISSHP [ 10 ] guidelines state that aspirin should be administered to high-risk pregnant women. However, the Japanese guidelines from the JSSHP that were issued in 2015 state that aspirin should be given to a limited number of women [ 78 ], while those that were issued in 2021 state that aspirin should be considered for women with preeclampsia to prevent recurrence in subsequent pregnancy [ 5 ]. In Asian women, the dose-dependent efficacy of low-dose aspirin [ 79 ] and its efficacy in women with blood pressure of 130–139/80–89 mmHg, which is included in the American College of Cardiology/American Heart Association definition of Stage 1 hypertension or mild hypertension [ 80 ], have also been reported. However, a study reported that aspirin has poor efficacy when started at 12–20 weeks gestation [ 81 ]. The ADA guidelines also strongly recommended aspirin for women with diabetic pregnancies until 2020 [ 82 ]; moreover, the recommendations became weaker in the 2021 edition and later editions [ 83 ]. A recent study reported limited efficacy of aspirin in preventing preeclampsia among women with diabetic pregnancies [ 84 ]. Future studies are warranted on eligible subjects and administration methods.
In principle, in Japan, inpatient management is recommended for HDP patients with blood pressure of 160/110 mmHg or higher, antihypertensive treatment should be given if a patient’s blood pressure is repeatedly found to be 160/110 mmHg or higher, and antihypertensive treatment is considered if a patient’s blood pressure is 140/90 mmHg or higher. Furthermore, if a patient has recurrent blood pressure of 160/110 mmHg or higher or has preeclamptic symptoms, magnesium sulfate should be administered to prevent eclampsia, and if management at the patient’s own facility is difficult, referral to a higher-level medical facility should be considered [ 5 ].
There are concerns that antihypertensive treatment during pregnancy may increase the risk of placental abruption and preterm delivery [ 85 ]. Data from Scotland showed a 2.3-fold increase in congenital defects with the use of antihypertensive drugs [ 86 ]. However, untreated hypertension, not antihypertensive medication, is a risk to the child [ 87 ]. The CHIPS study reported no significant group differences in the risk of pregnancy loss, high-level neonatal care, or overall maternal complications between less-tight (target office diastolic blood pressure of 100 mmHg) and tight (target office diastolic blood pressure of 85 mmHg) control of hypertension in pregnancy [ 88 ]. A recent meta-analysis showed that blood pressure-lowering treatment significantly prevented not only severe hypertension, preeclampsia, and severe preeclampsia but also placental abruption and preterm birth, while the risk of SGA was increased [ 89 ].
Currently, Japanese guidelines refer to methyldopa, hydralazine, and labetalol as oral antihypertensive drugs that can be used during pregnancy, while nifedipine can only be used after 20 weeks of pregnancy [ 5 ]. Guidelines for the use of different antihypertensive drugs have not been developed. There is a possibility of improved maternal prognosis with physiological nomogram-guided care and tailored pharmacological intervention [ 90 ]. In Japan, the use of calcium antagonists in early pregnancy is still not approved on the package label, and deviation from the guidelines is a concern [ 91 ]. However, in Japan, the most frequently prescribed oral antihypertensive drug during pregnancy is nifedipine, followed by methyldopa, hydralazine, and furosemide [ 92 ]. It has been reported that the risk of birth defects due to amlodipine use in the first trimester was not significantly different compared to the risk of the use of other antihypertensives in a case–control study in Japan [ 93 ]; more extensive observation is urgently needed.
Similar concerns have been raised regarding long-term prognosis. A comparison of the long-term prognosis of infants between treatment groups in a historical cohort study also reported the possibility of attention-deficit hyperactivity disorder and sleep disorders in infants whose mothers received drug interventions for gestational hypertension [ 94 ]. On the other hand, studies examining the effects of antihypertensive medications may not have examined baseline blood pressure levels [ 86 ], or baseline blood pressure levels may be obviously different [ 94 ]; hence, the risk of antihypertensive medication use must be carefully assessed. Such concerns are expected to be clarified by national-scale cohort studies.
Blood pressure measurement during pregnancy
There are several debates regarding how blood pressure should be measured during pregnancy [ 95 ]. Reports suggest that blood pressure values in pregnant women with preeclampsia vary depending on the measurement environment [ 96 ]. Hurrell et al. conducted a detailed review of blood pressure measurements in pregnant women [ 97 ]. A recent meta-analysis also confirmed that both systolic and diastolic blood pressure decrease by ~4 mmHg in the second trimester [ 98 ]; the results were very similar to those of a single cohort study investigating the use of home blood pressure [ 99 ].
Ambulatory blood pressure measurement
Ambulatory blood pressure measurement is valuable for diagnosing masked or white-coat hypertension (16) and assessing diurnal variations in blood pressure in pregnant women [ 100 , 101 ]. Normal daytime values for ambulatory blood pressure monitoring in pregnant women have been reported to be less than 130/77 mmHg at ≤22 weeks, 133/81 mmHg at 26–30 weeks, and 135/86 mmHg after 30 weeks [ 102 ]. Diurnal variations in blood pressure during pregnancy have been reported to be nocturnal declines of 12–14%/18–19% in systolic/diastolic blood pressure [ 100 ]. It has also been reported that nocturnal declines in blood pressure are attenuated before gestational hypertension nephropathy becomes apparent [ 101 ]. Among 146 Japanese pregnant women with suspected HDP, ambulatory blood pressure monitoring was more strongly associated with SGA infants, with an odds ratio of 1.74 times for every 10 mmHg increase (95% CI: 1.28–2.38; P = 0.001) compared with office blood pressure measurement (OR: 1.40; 95% CI: 0.92–2.13; P = 0.11) [ 45 ].
Home blood pressure measurement
Home blood pressure measurement is suitable for detecting long-term and seasonal variations in blood pressure. In a 2008 statement on home blood pressure measurement, the American Heart Association noted that “Home blood pressure measurement is theoretically ideal for monitoring changes in blood pressure during pregnancy because it is the best technique for providing multiple readings recorded at the same time of day over prolonged periods of time.” [ 103 ]. Furthermore, a report from the consensus meeting of the European Council on Hypertension issued around the same time stated that “Home blood pressure monitoring, although at present not commonly practiced in this setting, has considerable potential in improving the management of pregnant women.” [ 104 ]. According to the Japanese Society of Hypertension guidelines, for general (nonpregnant) patients, if the results of office blood pressure and home blood pressure measurements are different, the home blood pressure result has priority for treatment [ 91 ]. In pregnancy, home blood pressure measurements may be taken by pregnant women before recommendations are made by health care providers. Using home blood pressure monitoring, seasonal blood pressure [ 99 ] and hemodynamic changes are well observed. In a study that simultaneously included both home and clinic blood pressure levels in early pregnancy, the adjusted odds ratios for having a baby that was 500 g smaller per standard deviation increase in mean and diastolic blood pressure were 1.29 (95% CI: 1.04–1.59) and 1.28 (95% CI: 1.04–1.58) for home blood pressure and 1.02 (95% CI: 0.83–1.24) and 1.06 (95% CI: 0.87–1.30) for clinic blood pressure, respectively, with only home blood pressure measurements having a significant association [ 44 ]. Furthermore, the maternal blood pressure trajectory during pregnancy was an indicator of infant birth weight [ 46 ]. However, no study has determined whether interventions based on home blood pressure measurements improve outcomes.
Several values have been proposed as the diagnostic threshold of home blood pressure based on population distribution and regression with office blood pressure values. Using the standard major axis method, the home blood pressure values reported to be equivalent to a clinical blood pressure of 140/90 mmHg were 120.8/83.5 mmHg, 126.0/85.2 mmHg, and 136.3/89.3 mmHg in the first, second, and third trimesters, respectively [ 105 ]. However, no consensus value has been established [ 106 ].
A meta-analysis reported in 2020 summarized nine studies and noted that the use of home blood pressure measurements in the antenatal period was associated with a reduced risk of induction of labor, hospitalization before delivery, and diagnosis of preeclampsia and that the number of prenatal visits was significantly lower in the home blood pressure group, but there was no significant difference in the combined maternal, fetal, or neonatal outcomes compared to conventional care [ 107 ].
Clinical significance of white-coat hypertension
White-coat hypertension is a condition in which a patient has high blood pressure in the office but normal blood pressure outside the office. Generally, 24-h ambulatory blood pressure monitoring or home blood pressure monitoring may be used to identify white-coat hypertension. Ishikuro et al. reported that among pregnant women who were normotensive, the white-coat effect during pregnancy was 4.1/3.8, 3.4/1.6, and 1.8/2.4 mmHg in early, mid-, and late pregnancy, respectively [ 108 ]. When the factors affecting the white-coat effect were examined in the same population, no significant differences were found for body mass in sex, age, or family history of hypertension. However, the effect was significantly greater in primiparas than in multiparas in early pregnancy for systolic blood pressure and in late pregnancy for diastolic blood pressure [ 109 ]. A meta-analysis of 16 studies on the white-coat effect showed that office blood pressure measurements were 4/3 (3–6/2–4) mmHg higher than home blood pressure measurements [ 106 ]. White-coat hypertension is prevalent in women with preexisting diabetes and may indicate an increased risk of developing pregnancy-induced hypertensive disorders later in life [ 110 ].
Based on ambulatory blood pressure monitoring in early pregnancy, it has been reported that 22% of pregnant women have sustained hypertension, and 8% of those with white-coat hypertension develop preeclampsia; thus, white-coat hypertension in pregnancy may have a relatively good prognosis [ 102 ]. It is essential to recognize that hypertension in the office may not necessarily require antihypertensive treatment if the blood pressure outside the office is normal. However, 42% of pregnant women with white-coat hypertension in early pregnancy showed hypertension both in the office and out of the office until delivery [ 102 ]; therefore, careful follow-up is necessary in such cases.
Clinical significance of masked hypertension
There are few studies on masked hypertension in pregnant women. Salazar et al. reported that masked hypertension is a prevalent and high-risk condition. An office blood pressure of ≥125/75 mmHg in the second half of gestation seems appropriate for indicating out-of-office measurements in women with high-risk pregnancies [ 111 ]. Pregnant women with masked hypertension had a 7.8 times higher risk of preeclampsia than those who were normotensive [ 112 ]. Unlike white-coat hypertension, masked hypertension cannot be detected unless all pregnant women who are at risk receive out-of-office blood pressure measurements. Therefore, further study is needed to determine which women should undergo blood pressure measurement outside the office.
Present blood pressure monitoring situations in clinical practice
According to a survey reported in 2021, 89.3% of obstetricians took blood pressure measurements in an outpatient setting only once per occasion if a woman’s blood pressure was normal. However, if the pregnant women had hypertension, 54.8% took a second measurement, and 40.3% repeated blood pressure measurements until a stable reading was obtained [ 113 ]. Furthermore, 62.8% of the obstetricians recorded the lowest value when they measured blood pressure twice, and 69.0% of the physicians recorded the last measurement when the blood pressure was measured until it stabilized [ 113 ]. Therefore, when conducting research based on databases, researchers need to recognize some variation in the measurements recorded on each measurement occasion. On the other hand, blood pressure measurements for research purposes at a venue different from that of the antenatal checkup was reported to be equivalent to home blood pressure measurements and significantly lower than those at antenatal checkups, and the possibility of a stressful environment during the antenatal checkup causing an increase in blood pressure should be considered [ 114 ].
A survey of family medicine and obstetrics/gynecology physicians providing prenatal care at a tertiary obstetrics hospital in Canada found that obstetricians were more likely to use home blood pressure monitoring. In contrast, family physicians were more likely to use 24 h ambulatory blood pressure monitoring as a diagnostic aid. While obstetricians were more likely than family physicians to use effective home blood pressure monitoring during pregnancy and monitor hypertension with home blood pressure monitoring, family physicians were significantly more likely than obstetricians to target “tight” blood pressure control [ 115 ].
In a survey of 128 patients conducted in the United States, postpartum women perceived a telehealth technology remote intervention as a safe and easy-to-use method, with an acceptable burden of care and an overall satisfactory method of monitoring blood pressure in the postpartum period [ 116 ]. A survey conducted in Belgium reported that 80% of midwives and 67% of obstetricians who used remote blood pressure monitoring in pregnancy perceived digital technologies as an important component of prenatal monitoring [ 117 ]. The results of an online survey of obstetricians in the United Kingdom showed that the percentage of obstetricians who thought that home blood pressure measurements and urinalysis were helpful indicators of clinical diagnosis rose from 88% before the COVID-19 pandemic to 96% after the pandemic. In addition, 47% of the obstetricians agreed that pregnant women would change their predetermined medications based on their measured blood pressure levels [ 118 ].
Novel attempt at blood pressure telemonitoring
In 2008, the transmission of home blood pressure measurements was discussed [ 119 ], but the explorations of remote monitoring are rapidly progressing during the COVID-19 pandemic. A study examined current practices and attitudes concerning home-based blood pressure and cardiotocography monitoring and telemonitoring in high-risk pregnancies requiring maternal and fetal monitoring and reported that home-based monitoring and telemonitoring were offered in 26% and 23% of hospitals, respectively, in the Netherlands [ 120 ]. In a retrospective comparison, the digital platform among high-risk pregnancies significantly reduced prenatal visits, ultrasounds, and hypertension-related hospitalizations compared to usual care without self-monitoring.
As mentioned earlier, the Maternity Log study [ 75 ] attempted to collect life logs, including home blood pressure measurements. There have been text-based attempts at blood pressure management in the postpartum period [ 121 ]. A study attempted to use a smartphone application to monitor home blood pressure in Belgium [ 122 ]. A prospective, randomized, controlled trial, called BP-PRESELF, using home blood pressure measurements is ongoing to assess whether home blood pressure monitoring in women with a history of preeclampsia/HELLP syndrome during pregnancy is a valuable tool for the early detection of chronic hypertension [ 123 ].
Blood pressure measurement during pregnancy is crucial in diagnosing HDP. The precise measurement and evaluation of blood pressure, including its variability, will continue to play an essential role in determining the prognosis and elucidating the pathogenesis of HDP.
Suzuki M, Ichijo M, Ichinoe K, Okada H, Kuji N, Kobori T, et al. Report of the Committee on Pregnancy Toxicosis. Acta Obst Gynaec Jpn. 1982;34:837–40.
Google Scholar
Suzuki M, Ichijo M, Ichinoe K, Iwasaki H, Okada H, Kawabe S, et al. Report of the Committee on Pregnancy Toxicosis. Acta Obst Gynaec Jpn. 1984;36:983–9.
Watanabe K, Matsubara K, Nakamoto O, Ushijima J, Ohkuchi A, Koide K, et al. Outline of the new definition and classification of “Hypertensive Disorders of Pregnancy(HDP)”: a revised JSSHP statement of 2005. Hypertens Res Pregnancy. 2018;6:33–7.
Article Google Scholar
Ngene NC, Moodley J. Role of angiogenic factors in the pathogenesis and management of pre-eclampsia. Int J Gynaecol Obstet. 2018;141:5–13.
Article CAS PubMed Google Scholar
Japan Society for the Stuy of HYPERTENSION IN PREGNANCY. Best Practise Guide 2021 for Care and Treatment of Hypertension in Pregnancy. Tokyo: Medical View Co., Ltd.; 2021.
Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80:1036–45.
Article CAS PubMed PubMed Central Google Scholar
Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71.
Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–74.
Sava RI, March KL, Pepine CJ. Hypertension in pregnancy: taking cues from pathophysiology for clinical practice. Clin Cardiol. 2018;41:220–7.
Article PubMed PubMed Central Google Scholar
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43.
Hypertension in pregnancy: diagnosis and management. London; 2019. https://www.nice.org.uk/guidance/ng133 .
Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet Gynecol. 2020;135:1492–95.
Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P, Canadian Hypertensive Disorders of Pregnancy Working G. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014;4:105–45.
Article PubMed Google Scholar
Lowe SA, Bowyer L, Lust K, McMahon LP, Morton MR, North RA, et al. The SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust NZ J Obstet Gynaecol. 2015;55:11–6.
Hunter CA Jr, Howard WF, Mc CC Jr. Amelioration of the hypertension of toxemia by postpartum curettage. Am J Obstet Gynecol. 1961;81:884–9.
Magann EF, Martin JN Jr., Isaacs JD, Perry KG Jr., Martin RW, Meydrech EF. Immediate postpartum curettage: accelerated recovery from severe preeclampsia. Obstet Gynecol. 1993;81:502–6.
CAS PubMed Google Scholar
Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension. 2013;62:1046–54.
Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension. 2009;53:399–403.
Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology. 2009;24:147–58.
Article PubMed CAS Google Scholar
Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Investig. 2003;111:649–58.
Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–9.
Vatten LJ, Skjaerven R. Is pre-eclampsia more than one disease? BJOG. 2004;111:298–302.
Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG. 2006;113:580–9.
Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol. 2003;189:1173–7.
Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. 1996;175:1365–70.
Stanek J. Placental pathology varies in hypertensive conditions of pregnancy. Virchows Arch. 2018;472:415–23.
Bramham K, Villa PM, Joslin JR, Laivuori H, Hamalainen E, Kajantie E, et al. Predisposition to superimposed preeclampsia in women with chronic hypertension: endothelial, renal, cardiac, and placental factors in a prospective longitudinal cohort. Hypertens Pregnancy. 2020;39:326–35.
Chen KH, Seow KM, Chen LR. Progression of gestational hypertension to pre-eclampsia: a cohort study of 20,103 pregnancies. Pregnancy Hypertens. 2017;10:230–7.
Liu Y, Ma X, Zheng J, Liu X, Yan T. A systematic review and meta-analysis of kidney and pregnancy outcomes in IgA nephropathy. Am J Nephrol. 2016;44:187–93.
Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol. 2015;10:1964–78.
Nzelu D, Dumitrascu-Biris D, Nicolaides KH, Kametas NA. Chronic hypertension: first-trimester blood pressure control and likelihood of severe hypertension, preeclampsia, and small for gestational age. Am J Obstet Gynecol. 2018;218:e331–7.
Nzelu D, Dumitrascu-Biris D, Kay P, Nicolaides KH, Kametas NA. Severe hypertension, preeclampsia and small for gestational age in women with chronic hypertension diagnosed before and during pregnancy. Pregnancy Hypertens. 2018;14:200–4.
Ueda A, Hasegawa M, Matsumura N, Sato H, Kosaka K, Abiko K, et al. Lower systolic blood pressure levels in early pregnancy are associated with a decreased risk of early-onset superimposed preeclampsia in women with chronic hypertension: a multicenter retrospective study. Hypertens Res. 2022;45:135–45.
Grover S, Brandt JS, Reddy UM, Ananth CV. Chronic hypertension, perinatal mortality and the impact of preterm delivery: a population-based study. BJOG. 2022;129:572–9.
Veerbeek JH, Hermes W, Breimer AY, van Rijn BB, Koenen SV, Mol BW, et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension. 2015;65:600–6.
Watanabe M, Sairenchi T, Nishida K, Uchiyama K, Haruyama Y, Satonaka H, et al. Gestational hypertension as risk factor of hypertension in middle-aged and older women. Int J Environ Res Public Health. 2020;17:4052.
Article PubMed Central Google Scholar
Lo CCW, Lo ACQ, Leow SH, Fisher G, Corker B, Batho O, et al. Future cardiovascular disease risk for women with gestational hypertension: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9:e013991.
Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003497.
Giorgione V, Ridder A, Kalafat E, Khalil A, Thilaganathan B. Incidence of postpartum hypertension within 2 years of a pregnancy complicated by pre-eclampsia: a systematic review and meta-analysis. BJOG. 2021;128:495–503.
Oishi M, Iino K, Tanaka K, Ishihara K, Yokoyama Y, Takahashi I, et al. Hypertensive disorders of pregnancy increase the risk for chronic kidney disease: A population-based retrospective study. Clin Exp Hypertens. 2017;39:361–5.
De Paco C, Kametas N, Rencoret G, Strobl I, Nicolaides KH. Maternal cardiac output between 11 and 13 weeks of gestation in the prediction of preeclampsia and small for gestational age. Obstet Gynecol. 2008;111:292–300.
Sato N, Fudono A, Imai C, Takimoto H, Tarui I, Aoyama T, et al. Placenta mediates the effect of maternal hypertension polygenic score on offspring birth weight: a study of birth cohort with fetal growth velocity data. BMC Med. 2021;19:260.
Poudel K, Kobayashi S, Miyashita C, Ikeda-Araki A, Tamura N, Ait Bamai Y, et al. Hypertensive disorders during pregnancy (HDP), maternal characteristics, and birth outcomes among Japanese Women: a Hokkaido Study. Int J Environ Res Public Health. 2021;18:3342.
Iwama N, Metoki H, Ohkubo T, Ishikuro M, Obara T, Kikuya M, et al. Maternal clinic and home blood pressure measurements during pregnancy and infant birth weight: the BOSHI study. Hypertens Res. 2016;39:151–7.
Eguchi K, Ohmaru T, Ohkuchi A, Hirashima C, Takahashi K, Suzuki H, et al. Ambulatory BP monitoring and clinic BP in predicting small-for-gestational-age infants during pregnancy. J Hum Hypertens. 2016;30:62–7.
Iwama N, Oba MS, Satoh M, Ohkubo T, Ishikuro M, Obara T, et al. Association of maternal home blood pressure trajectory during pregnancy with infant birth weight: the BOSHI study. Hypertens Res. 2020;43:550–9.
Guo Q, Feng P, Yu Q, Zhu W, Hu H, Chen X, et al. Associations of systolic blood pressure trajectories during pregnancy and risk of adverse perinatal outcomes. Hypertens Res. 2020;43:227–34.
Teng H, Wang Y, Han B, Liu J, Cao Y, Wang J, et al. Gestational systolic blood pressure trajectories and risk of adverse maternal and perinatal outcomes in Chinese women. BMC Pregnancy Childbirth. 2021;21:155.
Li P, Xiong T, Hu Y. Hypertensive disorders in pregnancy and risk of asthma in offspring: a systematic review and meta-analysis. BMJ Open. 2021;11:e046769.
Maher GM, O’Keeffe GW, Kearney PM, Kenny LC, Dinan TG, Mattsson M, et al. Association of hypertensive disorders of pregnancy with risk of neurodevelopmental disorders in offspring: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:809–19.
Kajantie E, Osmond C, Eriksson JG. Gestational hypertension is associated with increased risk of type 2 diabetes in adult offspring: the Helsinki Birth Cohort Study. Am J Obstet Gynecol. 2017;216:e281–7.
Ishikuro M, Murakami K, Yokozeki F, Onuma T, Noda A, Ueno F, et al. Hypertension in pregnancy as a possible factor for child autistic behavior at two years old. Pregnancy Hypertens. 2021;25:88–90.
Poudel K, Kobayashi S, Miyashita C, Yamaguchi T, Tamura N, Ikeda-Araki A, et al. Hypertensive disorders during pregnancy and anthropometric measurement of children up to 7 Years of age: the Hokkaido Birth Cohort Study in Japan. Int J Environ Res Public Health. 2021;18:10951.
Yang L, Sato M, Saito-Abe M, Irahara M, Nishizato M, Sasaki H, et al. Hypertensive disorders of pregnancy and risk of allergic conditions in children: Findings from the Japan Environment and Children’s study (JECS). World Allergy Organ J. 2021;14:100581.
Chaemsaithong P, Sahota DS, Poon LC. First trimester preeclampsia screening and prediction. Am J Obstet Gynecol. 2022;226:S1071-S1097.e2. https://doi.org/10.1016/j.ajog.2020.07.020 .
Chaemsaithong P, Pooh RK, Zheng M, Ma R, Chaiyasit N, Tokunaka M, et al. Prospective evaluation of screening performance of first-trimester prediction models for preterm preeclampsia in an Asian population. Am J Obstet Gynecol. 2019;221:650.e1–6.
Iwama N, Sugiyama T, Metoki H, Saito M, Hoshiai T, Watanabe Z, et al. Associations between glycosylated hemoglobin level at less than 24 weeks of gestation and adverse pregnancy outcomes in Japan: The Japan Environment and Children’s Study (JECS). Diabetes Res Clin Pract. 2020;169:108377.
Kyozuka H, Fukusda T, Murata T, Yamaguchi A, Kanno A, Yasuda S, et al. Impact of preconception sodium intake on hypertensive disorders of pregnancy: The Japan Environment and Children’s study. Pregnancy Hypertens. 2021;23:66–72.
Kyozuka H, Murata T, Fukuda T, Endo Y, Yamaguchi A, Yasuda S, et al. Immunoglobulin E levels and pregnancy-induced hypertension: Japan Environment and Children’s Study. Sci Rep. 2021;11:8664.
Kawanishi Y, Kakigano A, Kimura T, Ikehara S, Sato T, Tomimatsu T, et al. Hypertensive disorders of pregnancy in relation to coffee and tea consumption: the Japan Environment and Children’s Study. Nutrients. 2021;13:343.
Suzumori N, Ebara T, Matsuki T, Yamada Y, Kato S, Omori T, et al. Effects of long working hours and shift work during pregnancy on obstetric and perinatal outcomes: a large prospective cohort study-Japan Environment and Children’s Study. Birth. 2020;47:67–79.
Tanaka K, Nishigori H, Watanabe Z, Iwama N, Satoh M, Murakami T, et al. Higher prevalence of hypertensive disorders of pregnancy in women who smoke: the Japan environment and children’s study. Hypertens Res. 2019;42:558–66.
Iwama N, Metoki H, Nishigori H, Mizuno S, Takahashi F, Tanaka K, et al. Association between alcohol consumption during pregnancy and hypertensive disorders of pregnancy in Japan: the Japan Environment and Children’s Study. Hypertens Res. 2019;42:85–94.
Nagata C, Yang L, Yamamoto-Hanada K, Mezawa H, Ayabe T, Ishizuka K, et al. Complications and adverse outcomes in pregnancy and childbirth among women who conceived by assisted reproductive technologies: a nationwide birth cohort study of Japan environment and children’s study. BMC Pregnancy Childbirth. 2019;19:77.
Kyozuka H, Murata T, Fukuda T, Yamaguchi A, Kanno A, Yasuda S, et al. Association between pre-pregnancy calcium intake and hypertensive disorders during the first pregnancy: the Japan environment and children’s study. BMC Pregnancy Childbirth. 2020;20:424.
Kyozuka H, Fukuda T, Murata T, Endo Y, Kanno A, Yasuda S, et al. Comprehensive metabolomic analysis of first-trimester serum identifies biomarkers of early-onset hypertensive disorder of pregnancy. Sci Rep. 2020;10:13857.
Okada K, Saito I, Katada C, Tsujino T. Influence of quality of sleep in the first trimester on blood pressure in the third trimester in primipara women. Blood Press. 2019;28:345–55.
Watanabe M, Shinohara H, Kodama H. Nocturnal oxygen desaturation in the late third trimester of uncomplicated pregnancy for prediction of late-onset gestational hypertension. J Obstet Gynaecol Res. 2020;46:1735–43.
Mikami Y, Takai Y, Era S, Ono Y, Saitoh M, Baba K, et al. Differences in home blood pressure and pulse rates between singleton and twin pregnancies. J Int Med Res. 2018;46:1496–504.
Iwama N, Metoki H, Nishigori H, Mizuno S, Takahashi F, Tanaka K, et al. Blood pressure changes during twin pregnancies: the Japan Environment and Children’s Study. J Hypertens. 2019;37:206–15.
Grant ID, Giussani DA, Aiken CE. Blood pressure and hypertensive disorders of pregnancy at high altitude: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2021;3:100400.
Bailey B, Euser AG, Bol KA, Julian CG, Moore LG. High-altitude residence alters blood-pressure course and increases hypertensive disorders of pregnancy. J Matern Fetal Neonatal Med. 2020. https://doi.org/10.1080/14767058.2020.1745181 .
Sakurai K, Miyaso H, Eguchi A, Matsuno Y, Yamamoto M, Todaka E, et al. Chiba study of Mother and Children’s Health (C-MACH): cohort study with omics analyses. BMJ Open. 2016;6:e010531.
Maitre L, de Bont J, Casas M, Robinson O, Aasvang GM, Agier L, et al. Human Early Life Exposome (HELIX) study: a European population-based exposome cohort. BMJ Open. 2018;8:e021311.
Yamauchi T, Ochi D, Matsukawa N, Saigusa D, Ishikuro M, Obara T, et al. Machine learning approaches to predict gestational age in normal and complicated pregnancies via urinary metabolomics analysis. Sci Rep. 2021;11:17777.
Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–22.
Force USPST, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, et al. Aspirin use to prevent preeclampsia and related morbidity and mortality: US preventive services task force recommendation statement. JAMA. 2021;326:1186–191.
Japan Society for the Stuy of HYPERTENSION IN PREGNANCY. Best Practise Guide 2015 for Care and Treatment of Hypertension in Pregnancy. Tokyo: Medical View Co., Ltd.; 2015.
Gu W, Lin J, Hou YY, Lin N, Song MF, Zeng WJ, et al. Effects of low-dose aspirin on the prevention of preeclampsia and pregnancy outcomes: a randomized controlled trial from Shanghai, China. Eur J Obstet Gynecol Reprod Biol. 2020;248:156–63.
Huai J, Lin L, Juan J, Chen J, Li B, Zhu Y, et al. Preventive effect of aspirin on preeclampsia in high-risk pregnant women with stage 1 hypertension. J Clin Hypertens. 2021;23:1060–7.
Article CAS Google Scholar
Lin L, Huai J, Li B, Zhu Y, Juan J, Zhang M, et al. A randomized controlled trial of low-dose aspirin for the prevention of preeclampsia in women at high risk in China. Am J Obstet Gynecol. 2022;226:251.e251–12.
American Diabetes A. 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl 1)1:S183–92.
American Diabetes Association Professional Practice C, American Diabetes Association Professional Practice C, Draznin B, Aroda VR, Bakris G, Benson G, et al. 15. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1)1:S232–43.
Do NC, Vestgaard M, Asbjornsdottir B, Norgaard SK, Andersen LLT, Jensen DM, et al. Unchanged Prevalence of Preeclampsia After Implementation of Prophylactic Aspirin for All Pregnant Women With Preexisting Diabetes: A Prospective Cohort Study. Diabetes Care. 2021. https://doi.org/10.2337/dc21-1182 .
Banhidy F, Acs N, Puho EH, Czeizel AE. The efficacy of antihypertensive treatment in pregnant women with chronic and gestational hypertension: a population-based study. Hypertens Res. 2010;33:460–6.
Fitton CA, Fleming M, Aucott L, Pell JP, Mackay DF, McLay JS. Congenital defects and early childhood outcomes following in-utero exposure to antihypertensive medication. J Hypertens. 2021;39:581–8.
Fitton CA, Fleming M, Steiner MFC, Aucott L, Pell JP, Mackay DF, et al. In utero antihypertensive medication exposure and neonatal outcomes: a Data Linkage Cohort Study. Hypertension. 2020;75:628–33.
Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407–17.
Abe M, Arima H, Yoshida Y, Fukami A, Sakima A, Metoki H, et al. Optimal blood pressure target to prevent severe hypertension in pregnancy: a systematic review and meta-analysis. Hypertens Res. 2022;45:887–99.
Mulder EG, Ghossein-Doha C, Cauffman E, Lopes van Balen VA, Schiffer V, Alers RJ, et al. Preventing recurrent preeclampsia by tailored treatment of nonphysiologic hemodynamic adjustments to pregnancy. Hypertension. 2021;77:2045–53.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Ishikawa T, Obara T, Nishigori H, Miyakoda K, Ishikuro M, Metoki H, et al. Antihypertensives prescribed for pregnant women in Japan: prevalence and timing determined from a database of health insurance claims. Pharmacoepidemiol Drug Saf. 2018;27:1325–34.
Mito A, Murashima A, Wada Y, Miyasato-Isoda M, Kamiya CA, Waguri M, et al. Safety of amlodipine in early pregnancy. J Am Heart Assoc. 2019;8:e012093.
Pasker-de Jong PC, Zielhuis GA, van Gelder MM, Pellegrino A, Gabreels FJ, Eskes TK. Antihypertensive treatment during pregnancy and functional development at primary school age in a historical cohort study. BJOG. 2010;117:1080–6.
Pickering TG. Reflections in hypertension. How should blood pressure be measured during pregnancy? J Clin Hypertens. 2005;7:46–9.
Rey E, Morin F, Boudreault J, Pilon F, Vincent D, Ouellet D. Blood pressure assessments in different subtypes of hypertensive pregnant women: office versus home patient- or nurse-measured blood pressure. Hypertens Pregnancy. 2009;28:168–77.
Hurrell A, Webster L, Chappell LC, Shennan AH. The assessment of blood pressure in pregnant women: pitfalls and novel approaches. Am J Obstet Gynecol. 2021. https://doi.org/10.1016/j.ajog.2020.10.026 .
de Haas S, Mulder E, Schartmann N, Mohseni Z, Abo Hasson F, Alsadah F, et al. Blood pressure adjustments throughout healthy and hypertensive pregnancy: a systematic review and meta-analysis. Pregnancy Hypertens. 2022;27:51–8.
Metoki H, Ohkubo T, Watanabe Y, Nishimura M, Sato Y, Kawaguchi M, et al. Seasonal trends of blood pressure during pregnancy in Japan: the babies and their parents’ longitudinal observation in Suzuki Memorial Hospital in Intrauterine Period study. J Hypertens. 2008;26:2406–13.
Brown MA, Robinson A, Bowyer L, Buddle ML, Martin A, Hargood JL, et al. Ambulatory blood pressure monitoring in pregnancy: what is normal? Am J Obstet Gynecol. 1998;178:836–42.
Cugini P, Di Palma L, Battisti P, Leone G, Pachi A, Paesano R, et al. Describing and interpreting 24-hour blood pressure patterns in physiologic pregnancy. Am J Obstet Gynecol. 1992;166:54–60.
Brown MA, Mangos G, Davis G, Homer C. The natural history of white coat hypertension during pregnancy. BJOG. 2005;112:601–6.
Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D, et al. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:10–29.
Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, et al. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens. 2008;26:1505–26.
Mikami Y, Takai Y, Era S, Ono Y, Saitoh M, Baba K, et al. Provisional criteria for the diagnosis of hypertension in pregnancy using home blood pressure measurements. Hypertens Res. 2017;40:679–84.
Tran K, Padwal R, Khan N, Wright MD, Chan WS. Home blood pressure monitoring in the diagnosis and treatment of hypertension in pregnancy: a systematic review and meta-analysis. CMAJ Open. 2021;9:E642–50.
Kalafat E, Benlioglu C, Thilaganathan B, Khalil A. Home blood pressure monitoring in the antenatal and postpartum period: A systematic review meta-analysis. Pregnancy Hypertens. 2020;19:44–51.
Ishikuro M, Obara T, Metoki H, Ohkubo T, Yamamoto M, Akutsu K, et al. Blood pressure measured in the clinic and at home during pregnancy among nulliparous and multiparous women: the BOSHI study. Am J Hypertens. 2013;26:141–8.
Ishikuro M, Obara T, Metoki H, Ohkubo T, Iwama N, Katagiri M, et al. Parity as a factor affecting the white-coat effect in pregnant women: the BOSHI study. Hypertens Res. 2015;38:770–5.
Vestgaard M, Asbjornsdottir B, Ringholm L, Andersen LLT, Jensen DM, Damm P, et al. White coat hypertension in early pregnancy in women with pre-existing diabetes: prevalence and pregnancy outcomes. Diabetologia. 2019;62:2188–99.
Salazar MR, Espeche WG, Balbin E, Leiva Sisnieguez CE, Leiva Sisnieguez BC, Stavile RN, et al. Office blood pressure values and the necessity of out-of-office measurements in high-risk pregnancies. J Hypertens. 2019;37:1838–44.
Salazar MR, Espeche WG, Leiva Sisnieguez BC, Balbin E, Leiva Sisnieguez CE, Stavile RN, et al. Significance of masked and nocturnal hypertension in normotensive women coursing a high-risk pregnancy. J Hypertens. 2016;34:2248–52.
Suzuki H, Takagi K, Tanaka K, Ichihara A, Seki H. A survey on the measurement of blood pressure in pregnant women and management of hypertensive disorders of pregnancy by the Japan Society for the Study of Hypertension in Pregnancy (JSSHP). Hypertens Res Pregnancy. 2021;9:30–9.
Usuzaki T, Ishikuro M, Metoki H, Murakami K, Noda A, Ueno F, et al. Comparison among research, home, and office blood pressure measurements for pregnant women: The TMM BirThree Cohort Study. J Clin Hypertens. 2020;22:2004–13.
Nash CM, Shetty N. Current state of affairs: a study regarding diagnosis, treatment and use of home blood pressure monitoring for hypertension in pregnancy. Pregnancy Hypertens. 2021;24:96–9.
Thomas NA, Drewry A, Racine Passmore S, Assad N, Hoppe KK. Patient perceptions, opinions and satisfaction of telehealth with remote blood pressure monitoring postpartum. BMC Pregnancy Childbirth. 2021;21:153.
Lanssens D, Vandenberk T, Lodewijckx J, Peeters T, Storms V, Thijs IM, et al. Midwives’, Obstetricians’, and recently delivered mothers’ perceptions of remote monitoring for prenatal care: retrospective survey. J Med Internet Res. 2019;21:e10887.
Fletcher B, Chappell LC, Lavallee L, Wilson HM, Stevens R, Mackillop L, et al. Changes to management of hypertension in pregnancy, and attitudes to self-management: an online survey of obstetricians, before and following the first wave of the COVID-19 pandemic. Pregnancy Hypertens. 2021;26:54–61.
Denolle T, Weber JL, Calvez C, Getin Y, Daniel JC, Lurton O, et al. Diagnosis of white coat hypertension in pregnant women with teletransmitted home blood pressure. Hypertens Pregnancy. 2008;27:305–13.
van den Heuvel JFM, Ayubi S, Franx A, Bekker MN. Home-based monitoring and telemonitoring of complicated pregnancies: Nationwide Cross-Sectional Survey of Current Practice in the Netherlands. JMIR Mhealth Uhealth. 2020;8:e18966.
Triebwasser JE, Janssen MK, Hirshberg A, Srinivas SK. Successful implementation of text-based blood pressure monitoring for postpartum hypertension. Pregnancy Hypertens. 2020;22:156–9.
Vandenberk T, Storms V, Lanssens D, De Canniere H, Smeets CJ, Thijs IM, et al. A vendor-independent mobile health monitoring platform for digital health studies: development and usability study. JMIR Mhealth Uhealth. 2019;7:e12586.
Muijsers HEC, van der Heijden OWH, de Boer K, van Bijsterveldt C, Buijs C, Pagels J, et al. Blood pressure after PREeclampsia/HELLP by SELF monitoring (BP-PRESELF): rationale and design of a multicenter randomized controlled trial. BMC Womens Health. 2020;20:41.
Download references
Acknowledgements
All authors have significantly contributed to and agree with the content of the manuscript. We would like to thank Editage ( www.editage.com ) for English language editing.
This study was supported by Grants for Scientific Research [16H05243, 19H03905, 10632242, 19K18659] from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Japan Agency for Medical Research and Development (AMED) Birthday [grant number: JP21gk0110039]; and a Grant-in-Aid (19DA1001) from the Ministry of Health, Labor and Welfare, Health Research on Children, Youth and Families, Japan.
Author information
Authors and affiliations.
Division of Public Health, Hygiene and Epidemiology, Tohoku Medical and Pharmaceutical University, Sendai, Japan
Hirohito Metoki, Michihiro Satoh & Takahisa Murakami
Department of Preventive Medicine and Epidemiology, Tohoku Medical Megabank Organization, Tohoku University, Sendai, Japan
Hirohito Metoki, Noriyuki Iwama, Michihiro Satoh, Takahisa Murakami, Mami Ishikuro & Taku Obara
Department of Obstetrics and Gynecology, Tohoku University Hospital, Sendai, Japan
Noriyuki Iwama & Hirotaka Hamada
Department of Pharmaceutical Sciences, Tohoku University Hospital, Sendai, Japan
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Hirohito Metoki .
Ethics declarations
Conflict of interest.
HM concurrently holds the noncompensated subdirectorship at the Tohoku Institute for Management of Blood Pressure, which is supported by Omron Health Care Co. Ltd., and is involved in collaborative research with Omron Health Care in another study. HM has also received grants or scholarships from Academic Contributions from Pfizer Japan Inc., Astellas Research Support, Daiichi Sankyo Co. Ltd., Bayer Academic Support, Otsuka Pharmaceutical Co., Ltd, Takeda Research Support, Eli Lilly Japan K.K., Baxter Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Chugai Pharmaceutical Co., Ltd., and Teijin Pharma Limited. These companies were not involved in this review article.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Metoki, H., Iwama, N., Hamada, H. et al. Hypertensive disorders of pregnancy: definition, management, and out-of-office blood pressure measurement. Hypertens Res 45 , 1298–1309 (2022). https://doi.org/10.1038/s41440-022-00965-6
Download citation
Received : 18 January 2022
Revised : 11 May 2022
Accepted : 27 May 2022
Published : 20 June 2022
Issue Date : August 2022
DOI : https://doi.org/10.1038/s41440-022-00965-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Antihypertensive Agents
- Blood Pressure
- Hypertension
- Pregnancy-Induced
- Masked Hypertension
- White-Coat Hypertension
This article is cited by
Relationship between parity and the prevalence of chronic kidney disease in japan considering hypertensive disorders of pregnancy and body mass index.
- Hongxin Wang
- Noriyuki Iwama
- Masatoshi Saito
BMC Nephrology (2024)
Hypertensive disorders of pregnancy and the risk of dementia: a systematic review and meta-analysis of cohort studies
- Ahmed Arafa
- Rena Kashima
- Yoshihiro Kokubo
Hypertension Research (2024)
Associations of maternal liver biomarkers in the first trimester with the risk of hypertensive disorders of pregnancy
Self-management system for postpartum women with hypertension disorders: an ehealth application intervention study.
- Chung-Wei Chang
- Yi-Jing Tsai
- Ting-Wei Hou
BMC Pregnancy and Childbirth (2023)
Effective gestational weight gain advice to optimize infant birth weight in Japan based on quantile regression analysis
- Noriko Sato
- Rei Haruyama
- Naoyuki Miyasaka
Scientific Reports (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Pregnancy-Induced hypertension
Affiliations.
- 1 Unit of Reproductive Endocrinology and Unit of Human Reproduction, First Department of Obstetrics and Gynecology, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece.
- 2 Third Department of Internal Medicine, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece.
- PMID: 26158653
- DOI: 10.14310/horm.2002.1582
Pregnancy-induced hypertension (PIH) complicates 6-10% of pregnancies. It is defined as systolic blood pressure (SBP) >140 mmHg and diastolic blood pressure (DBP) >90 mmHg. It is classified as mild (SBP 140-149 and DBP 90-99 mmHg), moderate (SBP 150-159 and DBP 100-109 mmHg) and severe (SBP ≥ 160 and DBP ≥ 110 mmHg). PIH refers to one of four conditions: a) pre-existing hypertension, b) gestational hypertension and preeclampsia (PE), c) pre-existing hypertension plus superimposed gestational hypertension with proteinuria and d) unclassifiable hypertension. PIH is a major cause of maternal, fetal and newborn morbidity and mortality. Women with PIH are at a greater risk of abruptio placentae, cerebrovascular events, organ failure and disseminated intravascular coagulation. Fetuses of these mothers are at greater risk of intrauterine growth retardation, prematurity and intrauterine death. Ambulatory blood pressure monitoring over a period of 24 h seems to have a role in predicting deterioration from gestational hypertension to PE. Antiplatelet drugs have moderate benefits when used for prevention of PE. Treatment of PIH depends on blood pressure levels, gestational age, presence of symptoms and associated risk factors. Non-drug management is recommended when SBP ranges between 140-149 mmHg or DBP between 90-99 mmHg. Blood pressure thresholds for drug management in pregnancy vary between different health organizations. According to 2013 ESH/ESC guidelines, antihypertensive treatment is recommended in pregnancy when blood pressure levels are ≥ 150/95 mmHg. Initiation of antihypertensive treatment at values ≥ 140/90 mmHg is recommended in women with a) gestational hypertension, with or without proteinuria, b) pre-existing hypertension with the superimposition of gestational hypertension or c) hypertension with asymptomatic organ damage or symptoms at any time during pregnancy. Methyldopa is the drug of choice in pregnancy. Atenolol and metoprolol appear to be safe and effective in late pregnancy, while labetalol has an efficacy comparable to methyldopa. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II antagonists are contraindicated in pregnancy due to their association with increased risk of fetopathy.
PubMed Disclaimer
Similar articles
- Role of antihypertensive therapy in mild to moderate pregnancy-induced hypertension: a prospective randomized study comparing labetalol with alpha methyldopa. Molvi SN, Mir S, Rana VS, Jabeen F, Malik AR. Molvi SN, et al. Arch Gynecol Obstet. 2012 Jun;285(6):1553-62. doi: 10.1007/s00404-011-2205-2. Epub 2012 Jan 15. Arch Gynecol Obstet. 2012. PMID: 22249781 Clinical Trial.
- [Association between gestational blood pressure and pregnancy induced hypertension or pre-eclampsia]. Wang Y, Tang HR, Wang Y, Zheng MM, Ye XD, Dai YM, Hu YL. Wang Y, et al. Zhonghua Fu Chan Ke Za Zhi. 2021 Nov 25;56(11):767-773. doi: 10.3760/cma.j.cn112141-20210601-00297. Zhonghua Fu Chan Ke Za Zhi. 2021. PMID: 34823289 Chinese.
- [Hypertension in pregnancy]. Cífková R. Cífková R. Cas Lek Cesk. 2009;148(2):65-71. Cas Lek Cesk. 2009. PMID: 19637440 Review. Czech.
- [Hypertension in pregnancy]. Cífková R. Cífková R. Vnitr Lek. 2006 Mar;52(3):263-70. Vnitr Lek. 2006. PMID: 16722158 Review. Czech.
- Ambulatory blood pressure monitoring for the early identification of hypertension in pregnancy. Ayala DE, Hermida RC. Ayala DE, et al. Chronobiol Int. 2013 Mar;30(1-2):233-59. doi: 10.3109/07420528.2012.714687. Epub 2012 Sep 24. Chronobiol Int. 2013. PMID: 23006127
- Pregnancy-Induced Hypertension Pathophysiology and Contemporary Management Strategies: A Narrative Review. Agarwal GS, Agrawal AK, Singhal D, Bawiskar D, Shedge SS. Agarwal GS, et al. Cureus. 2024 Jul 6;16(7):e63961. doi: 10.7759/cureus.63961. eCollection 2024 Jul. Cureus. 2024. PMID: 39105037 Free PMC article. Review.
- BMA-based Mendelian randomization identifies blood metabolites as causal candidates in pregnancy-induced hypertension. Guo J, Zheng X, Du X, Li W, Lu L. Guo J, et al. Hypertens Res. 2024 Jul 1. doi: 10.1038/s41440-024-01787-4. Online ahead of print. Hypertens Res. 2024. PMID: 38951678
- The effect of histo-blood group ABO system transferase (BGAT) on pregnancy related outcomes:A Mendelian randomization study. Sun Y, Zheng H, Wang M, Gu R, Wu X, Yang Q, Zhao H, Bi Y, Zheng J. Sun Y, et al. Comput Struct Biotechnol J. 2024 Apr 29;23:2067-2075. doi: 10.1016/j.csbj.2024.04.040. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 38800635 Free PMC article.
- Efficacy of Magnesium Sulfate and Labetalol in the Treatment of Pregnancy-Induced Hypertension and Its Effect on Anxiety and Depression: A Retrospective Cohort Study. Wang S, Zhang J, Zhu T, Xie X, Xia X, Li Y. Wang S, et al. Alpha Psychiatry. 2024 Mar 1;25(2):243-248. doi: 10.5152/alphapsychiatry.2024.231342. eCollection 2024 Mar. Alpha Psychiatry. 2024. PMID: 38798818 Free PMC article.
- Cardiovascular effect of preeclampsia upon offspring development: Are (Pro) renin-renin receptor ((P)RR) and gender related? Baeza-Pérez LG, Cabrera-Becerra SE, Romero-Nava R, Ramos-Tovar E, Hernández-Campos ME, López-Sánchez P. Baeza-Pérez LG, et al. Iran J Basic Med Sci. 2024;27(5):621-629. doi: 10.22038/IJBMS.2024.72486.15790. Iran J Basic Med Sci. 2024. PMID: 38629095 Free PMC article.
Publication types
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- Genetic Alliance
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Diagnosis and...
Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance
Visual summary: Resumen visual
Spanish version: Versión en español
- Related content
- Peer review
- Katie Webster , senior systematic reviewer 1 ,
- Sarah Fishburn , chair of guideline committee 1 ,
- Mike Maresh , clinical adviser 1 ,
- Sarah C Findlay , lay member ,
- Lucy C Chappell , topic advisor and NIHR research professor in obstetrics 2
- on behalf of the Guideline Committee
- 1 National Guideline Alliance, Royal College of Obstetricians and Gynaecologists, London
- 2 King’s College London
- Correspondence to: L C Chappell [email protected]

What you need to know
Hypertension affects about 10% of pregnant women, including those with pre-existing hypertension, chronic hypertension that is first diagnosed during pregnancy, and hypertension related to pregnancy (gestational hypertension and pre-eclampsia)
Target blood pressure during the antenatal period should be 135/85 mm Hg for women with hypertension during pregnancy
Hypertension during pregnancy is associated with an increased risk of hypertension and cardiovascular disorders in later life. Women should be offered appropriate lifestyle and dietary advice to minimise this risk
Hypertension in pregnancy is a common condition, affecting about 10% of pregnant women. This includes women with chronic hypertension—which may be diagnosed before pregnancy or in the early stages of pregnancy (<20 weeks’ gestation)—and women with hypertension related to pregnancy (gestational hypertension and pre-eclampsia) (see box 1). If not identified and treated, hypertension can lead to adverse events for both the woman and her baby, including increased risk of maternal stroke, lower birth weight, and increased risk of the baby requiring neonatal intensive care.
Definitions for hypertensive disorders of pregnancy
Chronic hypertension— Hypertension that is present at the booking visit or before 20 weeks’ gestation, or if the woman is already taking antihypertensive medication when starting maternity care. It can be primary or secondary in aetiology
Gestational hypertension— New hypertension presenting after 20 weeks of pregnancy without significant proteinuria
Pre-eclampsia— New onset hypertension (>140 mm Hg systolic or >90 mm Hg diastolic) after 20 weeks of pregnancy and the coexistence of one or both of the following new-onset conditions:
Proteinuria (urine protein:creatinine ratio ≥30 mg/mmol, or albumin:creatinine ratio ≥8 mg/mmol, or ≥1 g/L [2+] on dipstick testing)
Other maternal organ dysfunction, including features such as renal or liver involvement, neurological or haematological complications, or uteroplacental dysfunction (such as fetal growth restriction, abnormal umbilical artery Doppler waveform analysis, or stillbirth)
General practitioners and specialists other than obstetricians play a vital role in the identification of hypertension during pregnancy, first …
Log in using your username and password
BMA Member Log In
If you have a subscription to The BMJ, log in:
- Need to activate
- Log in via institution
- Log in via OpenAthens
Log in through your institution
Subscribe from £184 *.
Subscribe and get access to all BMJ articles, and much more.
* For online subscription
Access this article for 1 day for: £50 / $60/ €56 ( excludes VAT )
You can download a PDF version for your personal record.
Buy this article
Pregnancy-Induced Hypertension: Preeclampsia and Eclampsia Essay
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
Pregnancy Induced Hypertension
Pre-eclampsia.
A woman with pregnancy induced hypertension (PIH) experiences high blood pressure and protein deposits are found in her urine. In most cases, this condition occurs after twenty weeks of pregnancy and is common among first time mothers, teenagers and old mothers above forty years and who have had multiple pregnancies.
- Rushes or spots on the face.
- Protein in the urine.
- Sudden weight gain
- Sharp pain in the stomach especially on upper right side and around the ribs
The condition can be detected through urine tests that check protein levels in the urine and using a Doppler scan that monitors flow of blood in the placenta. If the PIH is mild, the doctor recommends less salt consumption and resting by lying on left side so as to suppress the weight of the baby acting on the blood vessels. For severe cases, the doctor may use medications such as a magnesium sulfate injection to lower the blood pressure. Other medications include methyldopa, labetalol, and calcium channel blockers. Among these medications, research has shown methyldopa is the best of all because it has very few side effects to both the mother and fetus (Seneviratne, 1998, p. 167).
Pre-eclampsia is a form of pregnancy induced hypertension that is associated with presence of proteins in the urine commonly known as proteinuria. It occurs at about twenty weeks of pregnancy but it is mostly common beyond twenty four weeks. It affects women who are having their first pregnancy as well as those who get pregnant in the course of pre-existing hypertension conditions.
The condition is characterized by a rise in blood pressure that can go above 140/90mmHg. It is usually diagnosed during a routine antenatal checkup and in some cases the condition may warrant admission of the patient for close monitoring. Medical researchers have not fully discovered the pathophysiology of pre-eclampsia. However, it is believed to be a placental disorder that could result from poor perfusion in the placenta. It could also result from poor nutrition and high body fat. The underlying effect is poor development of the fetus, which is normally smaller than usual, mainly due inadequate flow of blood in the placenta. Severe pre-eclampsia may be experienced by a pregnant woman who previously had a mild type of this disease. The most dangerous thing about this condition is that it often appears with little or no warning. The blood pressure rises to about 160/110mmHg and there is a high quantity of protein deposits in the urine. The patient may have one or a combination of the following symptoms: severe headache, blurred vision, epigastric sharp pain similar to a heartburn, nausea and vomiting, muscle twitching and swelling of limbs (Wickham, 2008, p. 212).
Treatment for pre-eclampsia in particular focuses on the high blood pressure. Doctors usually advise bed rest and antihypertensive medication may be administered to lower the blood pressure if the patient is in critical condition. In cases where the patient has convulsions, drugs to counter convulsions may be given. Doctors believe the best treatment for pre-eclampsia is induced premature birth, which is usually done through caesarean section. The following medications are used for reducing blood pressure:
- Magnesium sulfate, which prevents the risk of developing eclampsia
- Calcium channel blockers
Methyldopa, which is administered orally, is considered the best medication among these since it has fewer side effects.
One of the complications that could results from pre-eclampsia is where the pregnant woman develops seizures and eventually goes into coma. The symptoms for eclampsia are similar to those of pre-eclampsia. However, the most common symptom for eclampsia is seizures. The tests for both pre-eclampsia and eclampsia include, blood platelet count, protein check in the urine and kidney function analysis.
An intravenous injection of magnesium sulfate helps to reduce the chances of seizures recurring. Other medications may also be given to manage the level of blood pressure. These medications include hydralazine and labetalol. Premature birth may also be induced by use of oxytocin or prostaglandins, which can induce labor pains and hence prepare the cervix for delivery.
Side Effects of the Various Medications
Magnesium sulfate has adverse effects on muscles, as it makes them to grow weaker. It may also cause dizziness and slow breathing. Hydralazine may cause loss of appetite, mild diarrhea and vomiting. He patient could experience severe side effects such as yellowing of eyes, irregular heartbeat, joint pains and swelling of the mouth. Labetalol could induce side effects such as nose stuffiness, fatigue, indigestion, wheezing, persistent cough, chest pains and yellowing of the eyes. Oxytocin’s side effects include abrupt uterus contraction, vomiting, heavy bleeding during childbirth and blood clotting problems. Calcium channel blockers could cause side effects such as reduced heart rate and constipation. The use of Nifedipine has been known to have side effects such as blurred vision, heartburn, swelling of gums and limbs and constipation (Lyall & Belfort, 2007, p. 250).
Lyall, F., & Belfort, M. A. (2007). Pre-eclampsia: Etiology and clinical practice . New York, NY: Cambridge University Press.
Seneviratne, H. (1998). Pregnancy induced hypertension. Himayatnagar, Hyderabad: Orient Longman.
Wickham, S. (2008). Midwifery: Best practice, volume 5. New York, NY: New York Press.
- Sickle Cell Anaemia and its Molecular Diagnosis
- How to Clean a Wound During First Aid
- The Major Medical Causes of Maternal Deaths and Ways to Reduce It
- Supporting the Health Needs of Patients With Parkinson’s, Preeclampsia, and Postpartum Depression
- Placenta Previa: A Literature Review
- Male Sexual Performance: Complementary and Alternative Medicine
- Column Agglutination Technology (CAT) in Blood Bank
- Bardet-Bield Syndrome (BBS): Overview
- Raising Awareness on Food Poisoning Among Children Riyadh
- Spinal Anatomy: A Discussion of Cases of Spinal Defects
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, March 28). Pregnancy-Induced Hypertension: Preeclampsia and Eclampsia. https://ivypanda.com/essays/pregnancy-induced-hypertension-preeclampsia-and-eclampsia/
"Pregnancy-Induced Hypertension: Preeclampsia and Eclampsia." IvyPanda , 28 Mar. 2022, ivypanda.com/essays/pregnancy-induced-hypertension-preeclampsia-and-eclampsia/.
IvyPanda . (2022) 'Pregnancy-Induced Hypertension: Preeclampsia and Eclampsia'. 28 March.
IvyPanda . 2022. "Pregnancy-Induced Hypertension: Preeclampsia and Eclampsia." March 28, 2022. https://ivypanda.com/essays/pregnancy-induced-hypertension-preeclampsia-and-eclampsia/.
1. IvyPanda . "Pregnancy-Induced Hypertension: Preeclampsia and Eclampsia." March 28, 2022. https://ivypanda.com/essays/pregnancy-induced-hypertension-preeclampsia-and-eclampsia/.
Bibliography
IvyPanda . "Pregnancy-Induced Hypertension: Preeclampsia and Eclampsia." March 28, 2022. https://ivypanda.com/essays/pregnancy-induced-hypertension-preeclampsia-and-eclampsia/.
- Nutritional Requirements During Pregnancy Words: 1137
- Main Risk Factors of the First Period of Pregnancy Words: 644
- Cardiac Disease During Pregnancy Words: 2254
- Development of Protocols for Early Pregnancy Words: 2067
- Arterial Hypertension: Complications, Treatment and Impact Words: 1164
- Ectopic Pregnancy: Causes, Diagnosis, Treatment Words: 626
- Hypertension: Diagnosis and Treatment Words: 514
- Pregnancy and Reproductive Health in Public Views Words: 577
- Nutritional Support During Pregnancy Words: 1121
- Problem of People with Hypertension Words: 559
- Diet and Lifestyle Before and During Pregnancy Words: 554
- Pregnancy as an Adolescent Health Risk Words: 972
- Adolescent Pregnancy and Nursing Role in Prevention Words: 902
- Diet, Nutrition and Prevention of Hypertension Words: 2488
- Teenage Pregnancy Objectives and Causes Words: 2445
- Effects of Teen Pregnancy on Mother and Child Words: 1124
- Teen Pregnancy and Single Young Mothers Words: 1711
Complication of Pregnancy: Pregnancy Induced Hypertension (PIH)
Teaching plan.
Pregnant women are prone to complications, which threaten their lives and that of the infants. Pregnancy-induced hypertension is one of the complications that pregnant women experience. To prevent or manage complications, parents usually attend childbirth classes.
The childbirth class, which was held at Sibley Memorial Hospital taught parents how to handle experiences that they undergo during the first, second, and third trimesters. Despite the fact that the instructor covered extensive topics, she did not cover the topic of pregnancy-induced hypertension. Hence, the teaching plan focused on signs and symptoms, the nature of the complication, risk factors, and applicable interventions.
On February 7, 2015, I attended a boot camp for childbirth class that was hosted at Sibley Memorial Hospital. The session of the childbirth class started at 9am and ended at 5pm. The instructor’s name was Rosemarie Antunes, a registered nurse with the experience of 30 years in obstetric, labor, and delivery nursing acquired from different hospitals in different states.
She obtained her licenses as a registered nurse (RN) from the Virginia Department of Health Professions in 2004 and State of Connecticut, Department of Public Health in 1980. She has also received professional certification from Prepared Childbirth Educators in 2006 and Certified Labor Doula in 2009. Rosemarie took her diploma education for RN at Saint Francis Hospital School in 1977-1980.
Currently, she works for Fauquier Health System, a family birthing center, since 2004. She also works for Labor and Delivery, Postpartum, and Normal Newborn as a certified childbirth educator with experience of 10 years in preparing expectant mothers and their partners for what is ahead of them. Rosemarie is a mother of six children with 10 grandchildren (R. Antunes, personal communication, February 7, 2015).
The instructor employed constructivism as a teaching philosophy because she aided the participants to understand experiences that they expect during pregnancy and delivery by using questions and demonstrations. To construct the foundation of teaching, the instructor told the participants to ask questions that they might have before she commences each session.
Moreover, the instructor informed the participants that she would stop at any time during presentation to allow them to ask questions. To enhance understanding among the participants, the instructor demonstrated her teachings using various methods. The teaching methods that the instructor employed in demonstrations are videos, PowerPoint presentation, handouts, dolls, and birth balls.
The childbirth class took place on February 7, 2015 at Sibley Memorial Hospital in one of their lecture halls from 9am to 5pm. The childbirth class comprised of Caucasian couples, African couples, and Asians couples. Interestingly, during their introduction, all of them indicated that they were expecting their babies in March 2015.
From the introduction, it became apparent that half of the couples were married while the remaining couples were just partners. The participants were young adults between the ages of 20 to 30 years, who were expecting their babies for the first time. The couples were ready for the childbirth lessons as they brought with them pillows, birth balls, and blankets.
The topics covered in childbirth class aimed at enhancing the understanding of pregnancy (3 rd trimester), labor, Christina Birth story, comfort techniques, medical procedures, cesarean birth, newborn procedures, postpartum, and labor rehabilitation. The instructor covered anatomy and physiology of pregnancy from first trimester to the third trimester, and hormonal proliferations that happen during pregnancy.
To improve their health, the instructor encouraged the pregnant women to eat food high in fiber, drink water at all times, and call HCP whenever they experience pain during urination. The instructor also covered signs of labor and expected medical emergencies such as induction and cesarean births, which are essential in saving babies and mothers.
She taught the participants how to employ exercise, relaxation, massage, and music in improving the birth of the newborn in a natural way. The instructor also mentioned postpartum and gave healthcare instructions for newborn, such as safety and breastfeeding techniques.
Pregnancy-Induced Hypertension (PIH)
Although the instructor extensively covered diverse areas of childbirth, I noted that she did not delve deep into complications of pregnancy, and thus, she should have examined the topic of pregnancy-induced hypertension (PIH).
Pregnant women are susceptible to PIH or gestational hypertension owing to changes in their bodies. Essentially, PIH has medical importance because it threatens the lives of the baby and the mother. Therefore, I will explore the topic of PIH with a view of equipping the participants with the appropriate knowledge that is critical in prevention, treatment, and management of the complication.
Summary of Teaching
The first objective of teaching pregnant women is to enable them to identify signs and symptoms of PIH. As PIH requires early detection for treatment and management interventions to be effective, pregnant women need to understand how to identify the signs and symptoms of PIH very early.
Excessive swelling of hands and feet, dizziness, excessive nausea, rapid heartbeat, severe headaches, drowsiness, fever, blurred vision, and pain in the abdomen are some of the signs and symptoms of PIH, which pregnant women need to watch so that they can seek early medical attention.
According to Jwa et al. (2013), early detection of PIH is critical for fetal and maternal health because it enhances the effectiveness of treatment and management interventions. As teaching methods, I will employ PowerPoint presentation, brochures, handouts, and discussion.
In teaching about PIH, the second objective is to enable the participating couples to understand the nature of PIH. Given that pregnant women experience diverse forms of hypertension, PIH is a unique form of hypertension because it only happens after the 20th week of pregnancy and can be either transient or chronic (Sajith et al., 2014).
When blood pressure of a pregnant woman is higher than 140/90 in two different occasions, and her urine contains no proteins, the differential diagnosis indicates PIH. As a teaching method, I will demonstrate diagnosis of PIH by measuring blood pressure of the pregnant women and undertaking urinalysis to determine the presence of proteins in urine.
The third objective is to enable the participating couples to understand risks of PIH. The common risk factors for PIH are women with the first-time pregnancy, increased maternal age, family history, multiple gestations, proteinuria, hypertension, and diabetes mellitus (Jwa et al., 2013).
Moreover, nutrition also has other risk factors for PIH because an increased consumption of vitamin E and mono- and poly-unsaturated fatty acids increases the risk for PIH, while an increased consumption of magnesium, potassium, and vitamin C reduces the risk for PIH (Kazemian et al., 2012).
Sleep disturbance is also a possible risk factor for PHI because it correlates with hypertension (Haney, Buysse, & Okun, 2011). To expose these findings, I will employ PowerPoint presentation, brochures, handouts, and discussion.
The fourth objective of teaching is to enhance understanding of available treatment and management interventions of PIH. When pregnant women know the nature of available interventions, they can discuss with their doctors and choose the best intervention that fits them, hence, promote therapeutic adherence.
Sajith et al. (2014) state that both mono- and combined therapies of antihypertensive drugs are used in the treatment and management of PIH because they are safe for mothers and infants. Kazemian et al., (2012) recommends the application of nutrition in the prevention, treatment, and management of PIH.
Moreover, Haney, Buysse, and Okun (2011) recommend that alleviation of sleep disturbance reduces blood pressure, and hence, prevents the occurrence of PIH. The methods of teaching will comprise the use of the PowerPoint presentation, brochures, handouts, and discussion.
Pregnancy-Induced Hypertension
What is pregnancy-induced hypertension.
Pregnancy-induced hypertension refers to the high blood pressure, which women experience when they are pregnant.
Why is it important for pregnant women?
Pregnancy-induced hypertension affects pregnant women because their body changes during pregnancy. If doctors do not detect and treat pregnancy-induced hypertension, the mother and the baby will die. Therefore, pregnant women need to understand this disease so that they can seek medical attention whenever they experience signs and symptoms and save themselves and the unborn babies.
Signs and Symptoms
The common signs and symptoms of pregnancy-induced hypertension are excessive swelling of hands and feet, severe morning sickness, dizziness, fast heartbeat, severe headaches, drowsiness, high temperature, poor vision, and pain in the abdomen.
Nature of Pregnancy-Induced Hypertension
Pregnancy-induced hypertension is different from other types of hypertensions because it affects women only, occurs after 20 weeks of pregnancy, and there are no proteins in the urine. However, when not treated, it progresses into a disease called preeclampsia, which causes urine to appear in urine.
Risk Factors
The risk factors for pregnancy-induced hypertension are first-time pregnancy, age of the mother, bloodline with this disease, proteins in urine, many pregnancies, diabetes, nutrition, high blood pressure, and sleep disturbance.
Treatment and Management Interventions
- Use medications that reduce high blood pressure (antihypertensive drugs).
- Control food intake by reducing the amount of oils while increasing the amount of potassium, magnesium, and vitamin C.
- Avoid disturbance during sleep and have peace of mind.
Haney, A., Buysse, D., & Okun, M. (2011). Sleep and pregnancy-induced hypertension: A possible target for intervention? Journal of Clinical Sleep Medicine, 9 (12), 1349-1356.
Jwa, S., Arata, N., Sakamoto, N., Watanabe, N., Aoki, H., Kurauchi-Mito, A., Dongmei, Q., Ohya, Y., Ichihara, A., & Kitagawa. (2011). Prediction of pregnancy-induced hypertension by shift of blood pressure class according to the JSH 2009 guidelines. Hypertension Research, 34 (1), 1203-1208.
Kazemian, E., Dorosti-Motiagh, A., Sotoudeh, G., Eshraghian, M., & Ansary, S. (2012). The nutritional status of women with gestational hypertension compared to normal pregnant women. Women’s Health Care, 1 (10), 1-6.
Sajith, M., Nimbargi, V., Modi, A., Sumariya, R., & Pawar, A. (2014). Incidence of pregnancy induced hypertension and prescription pattern of antihypertensive drugs in pregnancy. International Journal of Pharma Sciences and Research, 5 (4), 163-170.
Cite this paper
- Chicago (N-B)
- Chicago (A-D)
StudyCorgi. (2020, January 15). Complication of Pregnancy: Pregnancy Induced Hypertension (PIH). https://studycorgi.com/complication-of-pregnancy-pregnancy-induced-hypertension-pih/
"Complication of Pregnancy: Pregnancy Induced Hypertension (PIH)." StudyCorgi , 15 Jan. 2020, studycorgi.com/complication-of-pregnancy-pregnancy-induced-hypertension-pih/.
StudyCorgi . (2020) 'Complication of Pregnancy: Pregnancy Induced Hypertension (PIH)'. 15 January.
1. StudyCorgi . "Complication of Pregnancy: Pregnancy Induced Hypertension (PIH)." January 15, 2020. https://studycorgi.com/complication-of-pregnancy-pregnancy-induced-hypertension-pih/.
Bibliography
StudyCorgi . "Complication of Pregnancy: Pregnancy Induced Hypertension (PIH)." January 15, 2020. https://studycorgi.com/complication-of-pregnancy-pregnancy-induced-hypertension-pih/.
StudyCorgi . 2020. "Complication of Pregnancy: Pregnancy Induced Hypertension (PIH)." January 15, 2020. https://studycorgi.com/complication-of-pregnancy-pregnancy-induced-hypertension-pih/.
This paper, “Complication of Pregnancy: Pregnancy Induced Hypertension (PIH)”, was written and voluntary submitted to our free essay database by a straight-A student. Please ensure you properly reference the paper if you're using it to write your assignment.
Before publication, the StudyCorgi editorial team proofread and checked the paper to make sure it meets the highest standards in terms of grammar, punctuation, style, fact accuracy, copyright issues, and inclusive language. Last updated: April 30, 2024 .
If you are the author of this paper and no longer wish to have it published on StudyCorgi, request the removal . Please use the “ Donate your paper ” form to submit an essay.

- Pregnancy Classes

Gestational Hypertension: Pregnancy Induced Hypertension (PIH)
Gestational Hypertension also referred to as Pregnancy-Induced Hypertension (PIH) is a condition characterized by high blood pressure during pregnancy . Gestational Hypertension can lead to a serious condition called Preeclampsia , also referred to as Toxemia. Hypertension during pregnancy affects about 6-8% of pregnant women .
The different types of hypertension during pregnancy:
High blood pressure can present itself in a few different ways during pregnancy. The following are the 3 common types of gestational hypertension:
- Chronic Hypertension – Women who have high blood pressure (over 140/90) before pregnancy, early in pregnancy (before 20 weeks), or continue to have it after delivery .
- Gestational Hypertension – High blood pressure that develops after week 20 in pregnancy and goes away after delivery.
- Preeclampsia – Both chronic hypertension and gestational hypertension can lead to this severe condition after week 20 of pregnancy . Symptoms include high blood pressure and protein in the urine. This can lead to serious complications for both mom and baby if not treated quickly.
Who is at risk?
The following women may have an increased risk of developing gestational hypertension:
- First-time moms
- Women whose sisters and mothers had PIH
- Women carrying multiples
- Women younger than age 20 or older than age 40
- Women who had high blood pressure or kidney disease prior to pregnancy
How do I know if I have Gestational Hypertension?
At each prenatal checkup, your healthcare provider will check your blood pressure and urine levels. Your doctor may also check your kidney and blood-clotting functions, order blood tests, perform an ultrasound scan to check your baby’s growth, and use a Doppler Scan to measure the efficiency of blood flow to the placenta.
How is it treated?
Treatment depends on how close you are to your due date. If you are close to your due date and the baby is developed enough, your health care provider may want to deliver your baby as soon as possible. If you have mild hypertension and your baby is not fully developed, your doctor will probably recommend the following:
- Rest, lying on your left side to take the weight of the baby off your major blood vessels.
- Increase prenatal checkups.
- Consume less salt.
- Drink 8 glasses of water a day.
If you have severe Hypertension, your doctor may try to treat you with blood pressure medication until you are far enough along to deliver safely.
How will this affect my baby?
Hypertension can prevent the placenta from getting enough blood. If the placenta doesn’t get enough blood, your baby gets less oxygen and food. This can result in low birth weight. Most women still can deliver a healthy baby if hypertension is detected and treated early. If your hypertension is severe, it can lead to Preeclampsia, which can have much more serious effects on mom and baby.
How can I prevent Gestational Hypertension:
Currently, there is no sure way to prevent hypertension. Some contributing factors to high blood pressure can be controlled, while others cannot. Follow your doctor’s instruction about diet and exercise . Some ways you can help prevent gestational hypertension include the following:
- Use salt as needed for taste
- Drink at least 8 glasses of water a day
- Increase the amount of protein you take in, and decrease the number of fried foods and junk food you eat
- Get enough rest.
- Exercise regularly
- Elevate your feet several times during the day
- Avoid drinking alcohol
- Avoid beverages containing caffeine
- Your doctor may suggest you take the prescribed medicine and additional supplements
Want to Know More?
- Exercise During Pregnancy
Compiled using information from the following sources: National Heart Lung and Blood Institute, https://www.nhlbi.nih.gov/index.htm Mayo Clinic, https://www.mayoclinic.com Medscape; Hypertension and Pregnancy, https://www.emedicine.medscape.com
BLOG CATEGORIES
- Pregnancy Symptoms 5
- Can I get pregnant if… ? 3
- Paternity Tests 2
- The Bumpy Truth Blog 7
- Multiple Births 10
- Pregnancy Complications 68
- Pregnancy Concerns 62
- Cord Blood 4
- Pregnancy Supplements & Medications 14
- Pregnancy Products & Tests 8
- Changes In Your Body 5
- Health & Nutrition 2
- Labor and Birth 65
- Planning and Preparing 24
- Breastfeeding 29
- Week by Week Newsletter 40
- Is it Safe While Pregnant 55
- The First Year 41
- Genetic Disorders & Birth Defects 17
- Pregnancy Health and Wellness 149
- Your Developing Baby 16
- Options for Unplanned Pregnancy 18
- Child Adoption 19
- Fertility 54
- Pregnancy Loss 11
- Uncategorized 4
- Women's Health 34
- Prenatal Testing 16
- Abstinence 3
- Birth Control Pills, Patches & Devices 21
- Thank You for Your Donation
- Unplanned Pregnancy
- Getting Pregnant
- Healthy Pregnancy
- Privacy Policy
- Pregnancy Questions Center
Share this post:
Similar post.

First-Trimester Screening

Can I Give Birth Safely if I have Coronavirus?

Ectopic Pregnancy
Track your baby’s development, subscribe to our week-by-week pregnancy newsletter.
- The Bumpy Truth Blog
- Fertility Products Resource Guide
Pregnancy Tools
- Ovulation Calendar
- Baby Names Directory
- Pregnancy Due Date Calculator
- Pregnancy Quiz
Pregnancy Journeys
- Partner With Us
- Corporate Sponsors
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Endothelin: Key Mediator of Hypertension in Preeclampsia
Eric m george.
1 Department of Physiology and Biophysics, University of Mississippi Medical Center, Jackson, Mississippi, USA
Joey P Granger
Preeclampsia is a pregnancy-induced hypertensive disorder characterized by proteinuria and widespread maternal endothelial dysfunction. It remains one of the most common disorders in pregnancy and remains one of the leading causes of maternal and fetal morbidity. Recent research has revealed that placental insufficiency, resulting in hypoxia and ischemia, is a central causative pathway in the development of the disorder. In response, the placenta secretes soluble substances into the maternal circulation which are responsible for the symptomatic phase of the disease. Among the most well characterized factors in the disease pathology are the anti-angiogenic protein soluble fms-like tyrosine kinase-1 (sFlt-1), inflammatory cytokines, and agonistic angiotensin II type-1 receptor autoantibodies. Each of these factors has been shown to induce hypertension experimentally through the production of endothelin-1 (ET-1), a powerful vasoconstrictor. Antagonism of the endothelin-A receptor has proved beneficial in numerous animal models of gestational hypertension, and it remains an intriguing target for pharmacological intervention in preeclampsia.
One of the most common complications of pregnancy is preeclampsia. Preeclampsia occurs in ~8% of all pregnancies, and is significantly more prevalent in specific ethnic subpopulations. 1 Despite an increasing awareness of the condition, and aggressive therapeutic intervention, preeclampsia remains a leading cause of both fetal and maternal perinatal morbidity and mortality, with ~15% of all preterm pregnancies attributable to preeclampsia. 2 The disease is primarily seen in nulliparous women, with a significantly decreased incidence in succeeding pregnancies. 3 Traditionally, the disorder has been defined by hypertension (systolic >140 mm Hg), proteinuria (300 mg/24 h), and edema. 4 However, current guidelines omit edema as a necessary symptom for diagnosis, and there is increasing discussion as to whether the absolute values of proteinuria are the correct metrics by which to guide treatment, or whether the protein/creatine ratio would be a more diagnostic measurement. 5
Treatment of preeclampsia is problematic, and is mainly aimed to minimize the symptomatic complication of the disease. Prophylactic administration of magnesium sulfate to counteract the possibility of seizure in the most severe cases is common. Hypertension in more severe cases is managed by any of several antihypertensive agents, though full normalization of hypertension by pharmacological means is not feasible. In all cases the only means of full remission is delivery, not only of the fetus, but more importantly, the placenta. To illustrate, there have been reported cases in which delivery of the fetus without total delivery of the placenta failed to remit the maternal symptoms, implicating the placenta as the central causative agent in disease progression. Therefore, the ultimate goal of the treatment regimen is to prolong gestation as long as possible for maturity of the fetus and safe delivery. In the severest cases induction prior to 37 weeks gestation is necessary, with considerably increased risk of complications for the newborn. 4
Origins of Preeclampsia
While the exact etiology which underlies preeclampsia is not clear, it is believed to originate from a fundamental error in the normal process of placentation. During a normal healthy pregnancy, a highly coordinated program ensures that the placenta and fetus are supplied with an adequate blood supply necessary for proper oxygen and nutrient delivery. Cytotrophoblasts originating from the fetus migrate into the maternal vasculature, and invade the maternal spiral arteries. These invasive trophoblasts gradually replace the endothelial lining of the vessels, undergoing a shift to an endothelial-like cell in the process. This normal program of invasive remodeling converts the normally low-capacitance, high-resistance vessels into highcapacitance, low-resistance vessels. This ensures adequate delivery of blood to the placenta, and thus the fetus. 6 , 7
In the preeclamptic patient this coordinated program is significantly interrupted. Clues to the nature of the developmental abnormalities come from examination of the spiral arteries in the uterine myometrium. The normal remodeling of the spiral arteries does not occur, resulting in small, highly resistant, low-capacity vessels. 8 As a result of this failure to remodel, insufficient blood flow to the uteroplacental unit results. The placenta, which even in a normal pregnancy is a comparatively hypoxic organ, becomes extremely hypoxic; resulting in a dramatic increase in expression of hypoxia-inducible genes. 9 – 11 An interesting caveat to this model of the origin of preeclampsia is that there are reported instances in which deficient spiral artery remodeling fails to lead to a preeclamptic state. 12 The intriguing possibility is that there are additional factors which must be present in conjunction with the hypoxic placenta in order for full expression of the disease. Preeclampsia is therefore to be regarded as occurring in two distinct phases. In the first phase, the abovementioned errors in vascular remodeling set the stage for the placental ischemia/hypoxia which provides the stimulus for release of primary and secondary factors which lead to the second symptomatic phase, hallmarked by widespread by maternal endothelial dysfunction.
One area of intensive research in recent years has been the vascular endothelial growth factor (VEGF) signaling pathway, which is necessary for the maintenance of proper endothelial cell function and health. Specifically, much study has been given to the soluble secreted form of the VEGF receptor, soluble fms-like tyrosine kinase-1 (sFlt-1), which acts as a VEGF antagonist by binding free VEGF and making it unavailable for signaling to membrane bound receptors. 13 Circulating levels of sFlt-1 are significantly elevated in preeclamptic women when compared to healthy pregnant women, and elevated tissue levels of sFlt-1 are found in the placenta of preeclamptic women. 14 , 15 Significantly, in an in vitro context, sFlt-1 is released from both placental explants and trophoblasts in response to decreased oxygen tension, suggesting a mechanism by which placental ischemia/hypoxia might lead to increased circulating sFlt-1. 16 – 18 Perhaps most importantly, infusion of sFlt-1 in rodents leads to preeclampsia-like phenotypes, with hypertension, renal injury, and proteinuria, and is now a commonly used experimental model of hypertension. 15 , 19 – 25
It is now commonly understood that placental hypoxia and ischemia are the underlying source for vasoactive factors which are at the root of the symptomatic phase of the disorder. However, the mechanisms of endothelial dysfunction are still under investigation. One factor which has repeatedly been shown to play a crucial role in the development of hypertension in experimental animal models of placental hypoxia/ischemia is the protein endothelin-1 (ET-1).
Et-1 in Preeclampsia
First characterized over 20 years ago, ET-1 was first identified as a potent endothelium-derived vasoconstrictor, the most potent vasoconstrictor known. 26 Derived from a longer 203-amino acid precursor known as preproendothelin, the active peptide proteolytically cleaved into its final 21-amino acid form. Numerous cardiovascular diseases have been shown to be associated with elevated ET-1 production, including hypertension, congestive heart failure, and chronic renal failure. 27 – 30 Much of the research on ET-1 has focused on the role of the endothelin type A (ET A ) receptor, which is found in the vascular smooth muscle and are important regulators of ET-1 dependent vasoconstriction and cellular proliferation. 30 , 31 However, there is another ET-1 receptor, the endothelin type B (ET B ) receptor which is found, among other locations, on vascular endothelial and renal epithelial cells. 28 , 32 , 33 In contrast to ET A receptor activation, agonism of ET B receptors conveys a vasodilatory response through the production of nitric oxide (NO) and cyclooxygenase metabolites. 34 The exact role of endothelin and the relative contributions of the ET A and ET B receptors to human disease are not fully elucidated though the system has shown great promise as a target for the treatment of cardiovascular disease. 35
Several lines of evidence, summarized in Table 1 , suggest a role for ET-1 as a pathophysiological factor in the development of preeclampsia. Multiple studies have examined circulating levels of ET-1 in normal pregnant and preeclamptic cohorts, and found elevated levels of plasma ET-1 in the preeclamptic group, with some studies indicating that the level of circulating ET-1 correlates with the severity of the disease symptoms, though this is not a universal finding. 36 – 39 Coincidental with the increase in circulating ET-1, at least one group measured a significant negative correlation between ET-1 levels and the levels of the vasodilators NO and cGMP in preeclamptic women. 38 This increase in ET-1 can be partially attributed to an increased activity of the endothelin-converting enzyme in the circulation of preeclamptic women, which persists well into the postpartum period. 40 Concurrent infusion of ET-1 with the endothelin-converting enzyme inhibitor phosphoramidon shows differential effects in nonpregnant and normal pregnant women vs. those with diagnosed preeclampsia. 41 There are also indications that at least in vitro , a secondary VEGF-dependent pathway for ET-1 induction occurs through the activity of matrix metalloproteinase-2 independently of endothelin-converting enzyme, though whether this pathway is physiologically relevant in preeclampsia is yet to be determined. 42
Findings in human preeclampsia
| Findings in Human Preeclampsia | References |
|---|---|
| Elevated circulating ET-1 | Taylor , Baksu Nishikawa |
| Increased circulating endothelin converting enzyme (ECE) activity | Ajne |
| Differential effect of systemic ECE inhibition | Ajne |
| Elevated localized ET-1 production in maternal tissues | Napolitano , Faxen |
The endothelin-1 (ET-1) system is altered in human preeclampsia. Numerous studies have examined the ET-1 system in preeclamptic women. Among the reported findings are elevated circulating levels of ET-1, increased endothelin converting enzyme activity in the circulation, elevated tissue production of preproET-1 mRNA, and differential response to ECE antagonism when compared to nonpregnant or healthy pregnant women. Relevant citations are reported here and in the text.
These differences in ET-1 levels have been implicated in increased contractility of the uterine arteries in an ex vivo experimental model. 39 In addition to increased circulating ET-1, there is a clear autocrine/paracrine role for ET-1, and examination of preproendothelin message levels in a variety of tissue beds from preeclamptic women has demonstrated increased local production, suggesting a further role in the etiology of the disease. 43 , 44 Finally, when placental explants from healthy pregnancies are incubated with exogenously applied ET-1, they demonstrate a significant increase in the expression of markers for oxidative stress, which has been shown to be an important effector for the widespread maternal endothelial dysfunction and hypertension associated with preeclampsia. Furthermore, ET-1 caused a decrease in the proliferation of JEG-3 cells, a commonly used trophoblast cell line, leaving open the possibility that ET-1 plays a role in the failure of trophoblast invasion in the early stages of preeclampsia. 45
Et-1 in Experimental Pregnancy-Induced Hypertension
Placental ischemia/hypoxia.
In recent years, several excellent animal models of experimental preeclampsia have been developed. 25 One which has proven to mimic many of the hallmarks of preeclampsia is the reduced uterine perfusion pressure (RUPP) model. In this model, blood flow to the uterus is partially occluded during the later stages of gestation, resulting in severe placental hypoxia and ischemia. This model has been recapitulated in rodent, canine, and nonhuman primates, with hypertension, elevated sFlt-1, endothelial dysfunction, renal injury, and resulting proteinuria being common findings. 46 – 48 Initial findings in the rodent RUPP model demonstrated that both the cortical and medullary concentrations of preproendothelin mRNA were significantly elevated when compared to healthy pregnant controls, as assayed by RNAse protection assays.
Moreover, though administration of an ET A specific antagonist had no effect on normal pregnant animals, in the RUPP treated animals, the associated hypertension was completely normalized by ET A antagonism. Further, though not statistically significant, effective renal plasma flow was decreased in the RUPP treated rats, which was normalized in those treated with ET A receptor blockade. 49 Follow-up studies utilizing human umbilical vein endothelial cells and sera from RUPP treated rats demonstrated that ET-1 release was stimulated by RUPP serum when compared to serum obtained from normal pregnant rats. 50 It is clear then that the hypertension seen in the RUPP placental ischemia/hypoxia model is heavily dependent on increased ET-1 production and signaling through the ET A receptor.
Angiogenic imbalance
The role of ET-1 signaling in the rodent sFlt-1 infusion model of pregnancy induced hypertension has also been studied. Several reports have emerged that in pregnant rats, recombinant sFlt-1 or adenoviral delivery of the protein result in significant hypertension, proteinuria, and endothelial dysfunction. 15 , 19 , 20 A recent report demonstrated that the administration of sFlt-1 to pregnant rats also significantly increases the production of preproendothelin message levels in the renal cortex. As a result of sFlt-1 infusion in this model, mean arterial pressure is increased ~20 mm Hg. With coadministration of an ET A antagonist however, this hypertensive response was completely abolished. 20
In a related study, one group has reported that administration of the compound suramin elicits a preeclampsia-like phenotype. Suramin is a potent anti-angiogenic compound thought to inhibit, among other things, VEGF. In this model of hypertension, circulating levels of ET-1 are significantly elevated, demonstrating again the importance of VEGF signaling in the maintenance of a healthy endothelium. 51 As it has been shown that VEGF signaling through the VEGFR2 receptor is important for the production of endothelium-derived NO, it is likely that one mechanism by which sFlt-1 induces hypertension in the pregnant rat is by decreasing the amount of bioavailable NO, thereby increasing systemic peripheral resistance. 52 Whatever the mechanism or mechanisms involved, it seems clear that the hypertension associated with angiogenic imbalance, namely loss of bioavailable VEGF, is heavily reliant on the production of ET-1, showing again its importance in the development of preeclampsia.
Autoimmunity and inflammatory factor administration
There is a growing realization that autoimmunity plays in important role in the symptoms, and perhaps the origins, of preeclampsia. 53 – 55 Included in this broad heading are two separate, but equally important maternal responses; the innate autoimmune response mediated by inflammatory cytokines and the production of agonistic autoantibodies to the angiotensin II type-1 receptor (AT1-AA). The recognition of these pathogenic pathways has given rise to several new animal models of experimental gestational hypertension, and once again implicated ET-1 production as a central agent in the disease progression.
One of the best characterized inflammatory cytokines known to be elevated in the circulation of preeclamptic women is tumor necrosis factor-α (TNF-α). TNF-α has been shown to be elevated not only in preeclamptic women, but also at comparable levels in the circulation of RUPP-induced rodents. 56 – 58 Intriguingly, administration of the soluble TNF-α receptor Etanercept resulted in the partial abrogation of RUPP-induced hypertension. Coincidentally, tissue expression of preproendothelin was significantly reduced with Etanercept administration, suggesting that ET-1 expression is at least partially driven by increases in TNF-α. 58 In order to study the importance of TNF-α in the progression of preeclampsia, a model of TNF-α infusion has been developed in rodents. 25
In response to TNF-α infusion, pregnant rats demonstrate ~20 mm Hg increase in mean arterial pressure. There was also a concomitant increase in the expression of preproendothelin expression in the aorta, placenta, and renal tissue from TNF-α infused rats. Indeed, coadministration of an ET A receptor antagonist completely abolished the hypertensive response to TNF-α infusion. As an intriguing side, TNF-α infusion had no significant effect on blood pressure or preproendothelin expression in virgin rats, suggesting that pregnancy conveys a heightened sensitization to circulating TNF-α. 59 This data is consistent with earlier in vitro data which demonstrated that ET-1 expression from endothelial cells is regulated in part by TNF-α signaling. 60 Whatever the additional factors involved in TNFα-induced hypertension during pregnancy, it appears clear that endothelin signaling plays an important role in the symptomatic consequences of this pathway.
One of the most intriguing aspects of preeclampsia to emerge in recent years is the identification of the circulating AT1-AA. Originally identified in the circulation of preeclamptic patients, these circulating autoantibodies have been shown to induce reactive oxygen production in vitro , and there appears to be a direct correlation between the level of the AT1-AA and sFlt-1 in preeclamptic women. 61 , 62 The production of the AT1-AA seems to be directly related to placental ischemia through the induction of TNFα, as the autoantibody appears in the circulation of both the RUPP rat and the TNFα infusion model at levels approximately equal to those of preeclamptic women. 63 In follow-up studies, it was demonstrated that infusion of the purified AT1-AA into pregnant rats resulted in an ~20% increase in the mean arterial pressure. Analysis of tissue expression of preproendothelin levels showed a dramatic increase in expression, showing that AT1 receptor activation by AT1-AA could play a critical role in ET-1 production during preeclampsia. Again, pharmacologic blockade of the ET A receptor completely abolished the hypertensive response to AT1-AA infusion, demonstrating yet again that ET-1 is a central agent in the pathology of preeclampsia. 64
ET-1: A possible therapeutic target?
It appears clear that ET-1, specifically acting through the ET A receptor, plays an important role in the etiology of preeclampsia. As seen in Figure 1 , multiple mechanisms, all mediated by placental ischemia, induce ET-1 expression. Experimentally, ET-1 antagonism in each of these models can reduce the pathophysiological hypertension. Is this then a potential target for therapeutic intervention? A great of consternation has surrounded ET A blockade during pregnancy due to the fact that ET A receptor deletion in mice has proven to cause birth defects and embryonic lethality in mice, and the administration of endothelin receptor antagonists is contraindicated during pregnancy. 65 , 66 However, succeeding studies in which pharmacological blockade of the receptor initiated throughout gestation identified specific times in early to mid-gestation which resulted in phenotypical birth defects similar to those seen in the knockout model. ET A blockade during mid-gestation was implicated as the pivotal window for the previously characterized birth defects, as administration of the antagonist before and after mid-gestation resulted in differential effects. Administration only in late pregnancy was not performed, so the exact effect of ET A blockade only in late pregnancy is not clear. 67 The possibility remains that ET A receptor antagonists might be successfully administered in later gestation to control the most severe symptoms of preeclampsia. Research into the safety and efficacy of ET A receptor blockade during pregnancy and preeclampsia is warranted, and may provide an effective target for this complicated and difficult disorder.

Placental ischemia induces endothelin-1 (ET-1) expression through multiple pathways during preeclampsia. As a result of placental ischemia, soluble fms-like tyrosine kinase-1 (sFlt-1) is increased in the circulation, directly antagonizing vascular endothelial growth factor (VEGF). Circulating levels of tumor necrosis factor-α (TNF-α) and the agonistic angiotensin II type-1 receptor auto-antibody (AT1-AA) are increased. Each of these pathways in turn increase ET-1 production resulting in maternal hypertension.
Acknowledgments
This work was supported by NIH grants 1T32HL105324 and HL51971.
Disclosure: The authors have no conflicts of interest to disclose.

IMAGES
COMMENTS
The prevalence of hypertension in reproductive-aged women is estimated to be 7.7%. 1 Hypertensive disorders of pregnancy, an umbrella term that includes preexisting and gestational hypertension, preeclampsia, and eclampsia, complicate up to 10% of pregnancies and represent a significant cause of maternal and perinatal morbidity and mortality. 2 ...
The Pregnancy-Induced Hypertension Essay. Pregnancy-induced hypertension (PIH) is among the major causes of maternal mortality and a significant contributor to maternal and perinatal morbidity. Preeclampsia is characterized by hypertension that develops throughout pregnancy and disappears after birth, suggesting that the placenta is a critical ...
Hypertensive disorders of pregnancy (HDP) remain one of the major causes of pregnancy-related maternal and fetal morbidity and mortality worldwide. Affected women are also at increased risk for cardiovascular disease later in life, independently of traditional cardiovascular disease risks. Despite the immediate and long-term cardiovascular disease risks, recommendations for diagnosis and ...
Management. 1. Regardless of the hypertensive disorder of pregnancy, BP requires urgent treatment in a monitored setting when severe (>160/110 mm Hg); acceptable agents for this include oral nifedipine or intravenous labetalol or hydralazine. Oral labetalol may be used if these treatments are unavailable. 2.
Pregnancy-induced hypertension (PIH), which includes both gestational hypertension and preeclampsia, is a common and morbid pregnancy complication for which the pathogenesis remains unclear. Emerging evidence suggests that insulin resistance, which has been linked to essential hypertension, may play a role in PIH. Conditions associated with increased insulin resistance, including gestational ...
Pregnancy-induced hypertension (PIH) is estimated to affect 7% to 10% of all pregnancies in the United States. 1-4 Despite being one of the leading causes of maternal death and a major contributor of maternal and perinatal morbidity, the mechanisms responsible for the pathogenesis of PIH are unclear. Hypertension associated with preeclampsia develops during pregnancy and remits after ...
This term was widely used until 2005, when it was changed to "pregnancy-induced hypertension." In 2018, the classification was standardized with those of other countries, ...
1 INTRODUCTION. Pregnancy-induced hypertension (PIH) is regarded as a multi-system disorder that is defined as persistent hypertension of more than 140/90 mmHg during the gestational period. 1, 2 Based on the presence of edema, proteinuria, seizures, and other related symptoms, PIH can be classified into four distinct categories: preeclampsia (PE)/eclampsia, gestational hypertension (GH ...
Pregnancy-induced hypertension (PIH) complicates 6-10% of pregnancies. It is defined as systolic blood pressure (SBP) >140 mmHg and diastolic blood pressure (DBP) >90 mmHg. It is classified as mild (SBP 140-149 and DBP 90-99 mmHg), moderate (SBP 150-159 and DBP 100-109 mmHg) and severe (SBP ≥ …
Physiological Changes in Blood Pressure During Pregnancy. Due to vasodilation induced by local mediators such as prostacycline and nitric oxide, there is a fall in blood pressure (BP) early in the first trimester. ... Diagnosis of hypertension in pregnancy is based on BP values (SBP ≥ 140 mmHg and/or diastolic DBP ≥ 90 mmHg) measured in the ...
What you need to know. Hypertension affects about 10% of pregnant women, including those with pre-existing hypertension, chronic hypertension that is first diagnosed during pregnancy, and hypertension related to pregnancy (gestational hypertension and pre-eclampsia) Target blood pressure during the antenatal period should be 135/85 mm Hg for ...
The definitions of gestational hypertension (pregnancy-induced hypertension) and preeclampsia are shown in Figure 1. Serious or long-term complications may result when preeclampsia turns into a severe type or is left without being sufficiently treated. Multiorgan involvement may be seen in such cases, and the impairment of uteroplacental ...
See related article, pp 901-909. The population-based study by Birukov et al in this issue of Hypertension 1 described maternal blood pressure patterns during pregnancy in >2400 women, 10% of whom developed a hypertensive disorder of pregnancy (HDP; gestational hypertension or preeclampsia). The authors then examined associations between ...
Hypertension affects 8-10% of pregnancies and is the most common medical disorder in pregnancy. Despite continuing research and guideline development, maternal mortality from hypertensive diseases in pregnancy has increased in the last 6 years. It is vital that physicians are aware of the signs and symptoms, along with differing maternal treatment thresholds, of hypertension in pregnancy to ...
Pregnancy Induced Hypertension. A woman with pregnancy induced hypertension (PIH) experiences high blood pressure and protein deposits are found in her urine. In most cases, this condition occurs after twenty weeks of pregnancy and is common among first time mothers, teenagers and old mothers above forty years and who have had multiple pregnancies.
Pregnancy Induced Hypertension Essay. 463 Words 2 Pages. Pregnancy induced hypertension is a complication characterized by high blood pressure (more than 140/90) swelling due to fluid retention and presence of protein in urine (Proteinuria). Pregnancy induced hypertension affects 5-10% of pregnant woman.
Pregnancy-induced hypertension is different from other types of hypertensions because it affects women only, occurs after 20 weeks of pregnancy, and there are no proteins in the urine. However, when not treated, it progresses into a disease called preeclampsia, which causes urine to appear in urine. ... Need an essay on Complication of ...
Gestational Hypertension also referred to as Pregnancy-Induced Hypertension (PIH) is a condition characterized by high blood pressure during pregnancy. Gestational Hypertension can lead to a serious condition called Preeclampsia, also referred to as Toxemia. Hypertension during pregnancy affects about 6-8% of pregnant women.
e22 February 2022 Hypertension. 2022;79:e21-e41. DOI: 10.1161/HYP.0000000000000208 Garovic et al Hypertension in Pregnancy: Diagnosis, BP Goals, and Pharmacotherapy Association (AHA) Hypertension Clinical Practice Guide-lines, the threshold for the diagnosis of stage 1 hyper-tension was further lowered to 130/80 from 140/90
Pregnancy Induced Hypertension (PIH) is a multi-organ disease process that develops as a result of pregnancy and regresses in the postpartum period. It usually develops after 20 weeks of gestation in a woman who had normal blood pressure. It is defined as an elevation of systolic and diastolic pressures equal to or above 140/90 mm Hg.
Gestational hypertension is defined by BP readings of ≥140/90 mmHg on two occasions at least 4 hours apart after 20 weeks' gestation in a previously normotensive woman, without the presence of proteinuria (<300 mg in 24 hours) or other clinical features (thrombocytopenia, impaired renal or kidney function, pulmonary oedema, or new-onset headache) suggestive of pre-eclampsia.
New-onset hypertension is common during pregnancy, with gestational hypertension affecting an estimated 3% and preeclampsia 2% to 3% of pregnancies in the United States. 1 Although both conditions are characterized by new-onset high blood pressure late in gestation, the development of systemic organ dysfunction during preeclampsia 2 is associated with significant maternal and infant morbidity ...
Preeclampsia is a pregnancy-induced hypertensive disorder characterized by proteinuria and widespread maternal endothelial dysfunction. It remains one of the most common disorders in pregnancy and remains one of the leading causes of maternal and fetal morbidity. Recent research has revealed that placental insufficiency, resulting in hypoxia ...