Mastery-Aligned Maths Tutoring
“The best thing has been the increase in confidence and tutors being there to deal with any misunderstandings straight away."
FREE daily maths challenges
A new KS2 maths challenge every day. Perfect as lesson starters - no prep required!


25 Time Word Problems for Year 2 to Year 6 With Tips On Supporting Pupils’ Progress
Emma Johnson
Time word problems are an important element of teaching children how to tell the time. Children are introduced to the concept of time in Year 1. At this early stage, they learn the basics of analogue time; reading to the hour and half past and learn how to draw hands on the clocks to show these times.
As they move through primary school, pupils progress onto reading the time in analogue, digital and 24 hour clocks and being able to compare the duration of events. By the time children reach upper Key Stage 2, they should be confident in reading the time in all formats and solving problems involving converting between units of time.
Time in Year 1
Time in year 2, time in year 3, time in year 4, time in year 5 & 6.
- Why are word problems important for children’s understanding of time
How to teach time word problem solving in primary school
Time word problems for year 2, time word problems for year 3, time word problems for year 4, time word problems for year 5, time word problems for year 6, more time and word problems resources.

Let's Practice Telling The Time
Download this free printable worksheet to let your students practice telling the time.
When students are first introduced to time and time word problems , it is important for them to have physical clocks, to hold and manipulate the hands. Pictures on worksheets are helpful, but physical clocks enable them to work out what is happening with the hands and to solve word problems involving addition word problems and subtraction word problems .
Time word problems are important for helping children to understand how time is used in the real-world. We have put together a collection of 25 time word problems, which can be used with pupils from Year 2 to Year 6.
Time word problems in the National Curriculum
In Year 1, students are introduced to the basics of time. They learn to recognise the hour and minute hand and use this to help read the time to the hour and half past the hour. They also draw hands on clock faces to represent these times.
By the end of Year 2, pupils should be able to tell the time to five minutes, including quarter past/to the hour and draw the hands on a clock face to show these times. They should also know the number of minutes in an hour and the number of hours in a day.
In Year 3, children read the time in analogue (including using Roman Numerals). By this stage they are also learning to read digital time in 12 and 24 hour clock, using the AM and PM suffixes. Pupils record and compare time in terms of seconds, minutes and hours; know the number of seconds in a minute, days in a month and year and compare durations of events.
By Year 4, pupils should be confident telling the time in analogue to the nearest minute, digital and 24 hour clock. They also need to be able to read, write and convert time between analogue and digital 12 and 24 hour clocks and solve problems involving converting from hours to minutes; minutes to seconds; years to months and weeks to days.
By Year 5 and 6, there is only limited mention of time in the curriculum. Pupils continue to build on the knowledge they have picked up so far and should be confident telling the time and solving a range of problems, including: converting units of time; elapsed time word problems, working with timetables and tackling multi-step word problems .
Time word problems have been known to appear on Year 6 SATs tests. Third Space Learning’s online one-to-one SATs revision programme incorporates a wide range of word problems to develop students’ problem solving skills and prepare them the SATs tests. Available for all primary year groups as well as Year 7 and GCSE, our online tuition programmes are personalised to suit the needs of each individual student, fill learning gaps and build confidence in maths.
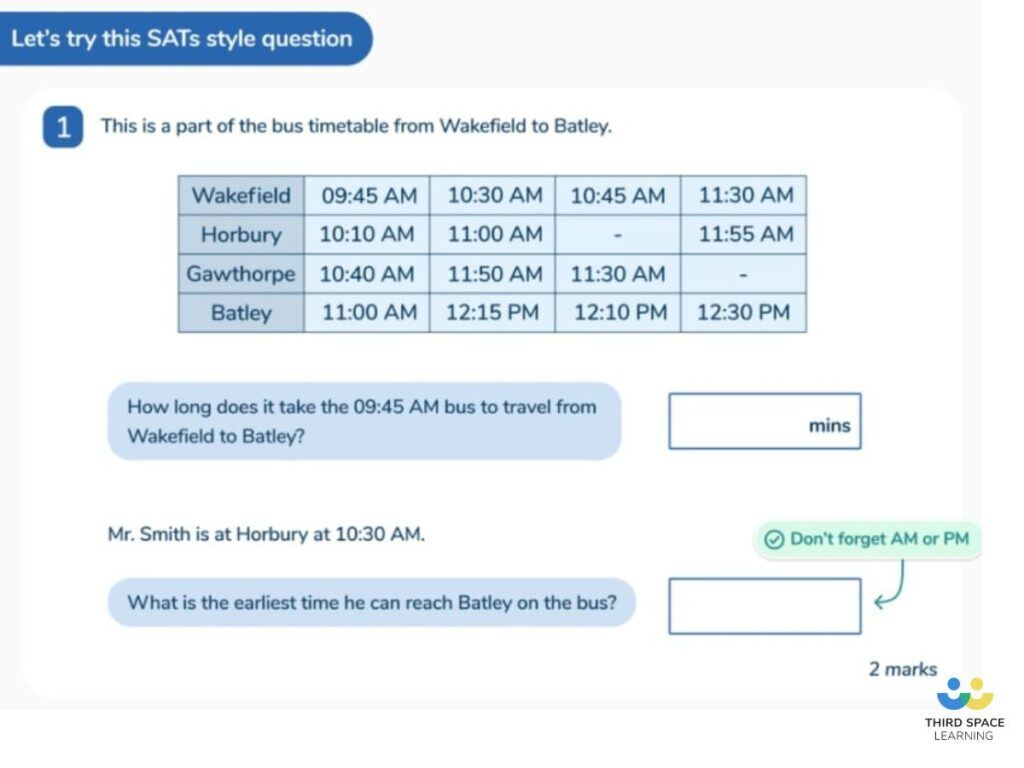
Why are word problems important for children’s understanding of time
Word problems are important for helping children to develop their understanding of time and the different ways time is used on an every-day basis. Confidence in telling the time and solving a range of time problems is a key life skill. Time word problems provide children with the opportunity to build on the skills they have picked up and apply them to real-world situations.
It’s important children learn the skills needed to solve word problems. Key things they need to remember are: to make sure they read the question carefully; to think whether they have fully understood what is being asked and then identify what they will need to do to solve the problem and whether there are any concrete resources or pictorial representations which will help them.
Here is an example:
Mr Arrowsmith drives to Birmingham. He sets off at 3:15pm. He stops for a break of 15 minutes at 4:50 and arrives in Birmingham at 6:15pm.
How long did Mr Arrowsmith spend driving?
How to solve:
What do you already know?
- We know that he set off at 3:15pm and stopped for a break at 4:50. We can calculate how long the first part of his journey was, by counting on from 3:15 to 4:50.
- He had a break at 4:50pm for 15 minutes, so we won’t include that in our driving time calculation.
- He then must have set off again at 5:05pm, before arriving at 6:15pm. We can use this information to work out the length of the second part of his journey.
- We can then add the 2 journey times together, to calculate the total amount of time spent driving.
How can this be represented pictorially?
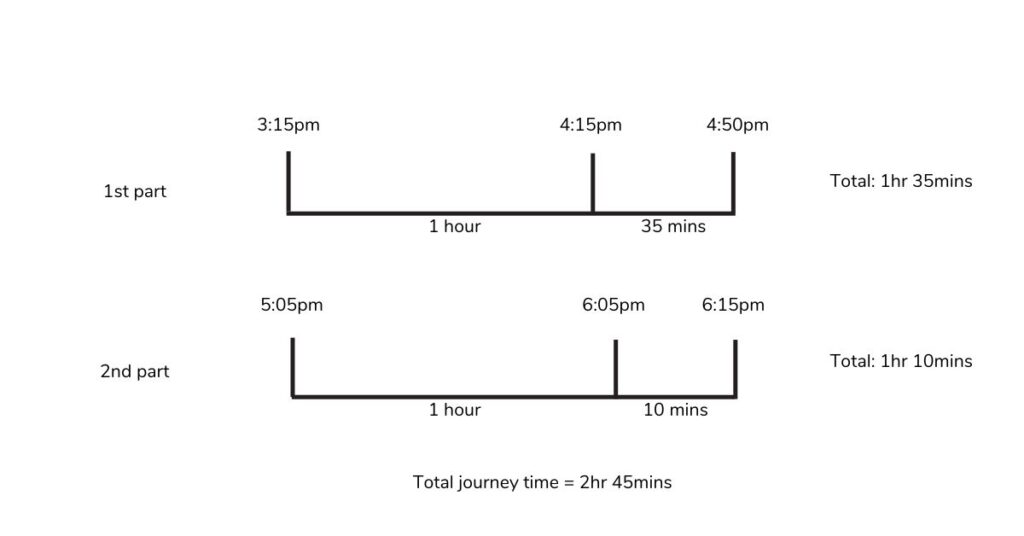
- We can use a number line to calculate the length of time each journey takes.
- If we start by adding on an hour, we can then calculate how many more minutes for each section of the journey.
- Once we have calculated the journey time for each part of the journey, we can add these together to calculate the total journey time.
Time word problems in Year 2 require students to read the time to o’clock and half past the hour and compare and sequence time intervals.
Oliver went for a bike ride with his friend.
He left home at 2 o’clock and came home at 4 o’clock.
How long was he out on his bike for?
Answer: 2 hours
Count on from 2 o’ clock to 4 o’clock or subtract 2 from 4.
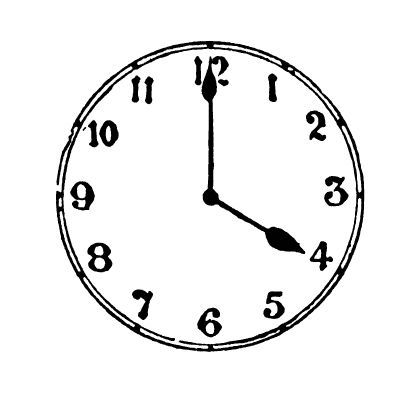
Mum went shopping at 3 o’clock and got home an hour later.
Draw the time she got home on the clock below.
Tom baked a cake.
The cake was in the oven for one hour.
If he took the cake out at half past 11, what time did he put the cake in?
Answer: Half past 10
Use an hour from half past 11.
Arlo starts school at 9 o’clock and has his first break at half past 10.
How long does he have to wait for his first break?
Answer: One and a half hours.
(Use a number line to count on from 9 to half past 10)
The Smith family are going to the beach.
They plan to leave home at 10 o’clock and the journey take two hours.
What time will they arrive at the beach?
Answer: 12 o’clock
(Use a number line to count on 2 hours from 10 o’clock)
With time word problems for year 3 , students build on their understanding of analogue time from Year 2 and also begin to read the time in digital (12 and 24 hour clock). Children also need to be able to compare time and durations of events.
Chloe is walking to football training.
She sets off at 8:40am and takes 17 minutes to get there.
What time does she arrive?
Answer: 8:57
(Count on 17 minutes from 8:40 – use a number line if needed)
(Picture of analogue clock with 2:30 showing here)
Maisie says that in 1 hour and 48 minutes it will be 4:28.
Do you agree? Explain how you worked out your answer.
Answer: Maisie is wrong. It will be 4:18.
This can be worked out by counting on an hour from 2:30 to 3:30 and then another 48 minutes to 4:18.
The Baker family are driving to their campsite.
They set off at 8:30 am, drive for 2 hours and 15 minutes, then had a 30 minute break.
If they drive for another 1 hour and 45 minutes, what time do they arrive at the campsite?
Answer: 12:45pm
Use a number line to show what time they arrive at the break. From 8:30, count on 2 hours and 15 minutes to get to 10:45. Add on the 30 minute break. It is now 11:15. They count on another hour and a half to 12:45
Ahmed looks at his watch and says ‘it is half past 4 in the afternoon’
Jude says that it is 17:30 in a 24 hour clock.
Is Jude correct? Explain your answer.
Answer: Jude is not correct. Half past 4 in the afternoon is 16:30 not 17:30
How many minutes are there in 2 hours and 30 minutes?
Answer: 150 minutes
60 + 60 + 30 = 150
When solving time word problems for year 4 , pupils need to be confident telling time in analogue, and digital, as well as converting between analogue, 12 hour and 24 hour clock. They also begin to solve more challenging problems involving duration of time and converting time.
If there are 60 seconds in 1 minute. How many seconds are there in 8 minutes?
Answer: 480 seconds
60 x 8 = 480 seconds (calculate 6 x 8, then multiply by 10)
Mason played on his VR from 3:35 to 5:25.
How long did he play on his VR?
Answer: 1 hour and 50 minutes.
Count on from 3:35 (using a numberline if needed)
Jamie started his homework at 3:45pm. He finished 43 minutes later.
What time did Jamie finish? Give your answer in 24 hour clock.
Answer: 16:28
Count on 43 minutes from 3:45 (use a number line, if needed) = 4:28. Convert to 24 hour clock.
Chloe and Freya went to the cinema to watch a film. The film started at 2:05pm and lasted for 1 hour and 43 minutes.
What time did the film end?
Answer: 3:48pm
Count on one hour from 2:05 pm to 3:05pm, then add another 43 minutes – 3:48pm
A family is driving on their holiday.
They drive for 2 hours and 28 minutes, stop for 28 minutes and then drive a further 1 hour and 52 minutes.
If they left at 8:30am, what time did they arrive?
Answer: 1:18pm
2 hours and 28 minutes from 8:30am = 10:58am
10:58am with a 28 minute break = 11:26am
1 hour 52 minute drive from 11:26 am = 1:18pm
With word problems for year 5 , pupils should be confident telling the time in analogue and digital and solving a wider range of time problems including: converting units of time; interpreting and answering questions on timetables and elapsed time.
The sun set at 19:31 and rose again at 6:28.
How many hours passed between the sun setting and rising again?
Answer: 10 hours and 57 minutes
Count on from 19:31 to 5:31 (10 hours)
Then count on from 5:31 to 6:28 (57 minutes)
A play started at 14:45 and finished at 16:58.
How long was the play?
Answer: 2 hours and 13 minutes
Count on 2 hours from 14:45 to 16:45, then add another 13 minutes to get to 16:58
How many seconds are there in 23 minutes?
Answer: 1380 seconds
Show as column method: 60 x 23 = 1380
Max ran a race in 2 minutes 13 seconds, Oscar ran it in 125 seconds.
What was the difference in time between Max and Oscar?
Answer: Oscar was 8 seconds faster.
Max – 2 minutes 13 seconds, Oscar – 2 minutes 5 seconds (difference of 8 seconds)
4 children take part in a freestyle swimming relay.
There times were:
Maisie: 42.8 seconds
Amber 36.3 seconds
Megan 48.7 seconds
Zymal 45.6 seconds
What was the final time for the relay in minutes and seconds?
Answer: 2:53.4
(Show as column method) 42.8 + 36.3 + 48.7 + 45.6 = 173.4 seconds
173.4 seconds = 2:53.4
No new time concepts are taught to pupils in word problems for year 6 . By this stage they are continuing to build confidence and develop skills within the concepts already taught.
Chess: 25 minutes
Basketball: 40 minutes.
Trampolining: 30 minutes
Gymnastics: 50 minutes
Tennis 40 minutes
Tri golf – 45 minutes
Hamza is choosing activities to take part in at his holiday club.
The activities can’t add up to more than 2 hours.
Which 3 activities could he do, which add up to exactly 2 hours?
Answer: Trampolining, gymnastics and tennis: Trampolining: 30 minutes, gymnastics: 50 minutes, tennis: 40 minutes.
5 children took part in a sponsored swim. The children swam for the following lengths of time:
Sam: 27 minutes 37 seconds
Jemma: 33 minutes 29 seconds.
Ben: 23 minutes 18 seconds
Lucy: 41 minutes 57 seconds
Oliver: 39 minutes 21 seconds
Answer: 18 minutes 30 seconds
Longest: Lucy: 41 minutes 57 seconds
Shortest: Ben: 23 minutes 18 seconds.
Difference – count up from 23 minutes 18 seconds to 41 minutes 57 seconds = 18 minutes 39 seconds
What is 6 minutes 47 seconds in seconds?
Answer: 407 minutes
60 x 6 = 360
360 + 47 = 407 minutes
Bethany’s goal is to run round her school running track in under 8 minutes.
She runs it in 440 seconds. Does she achieve her goal? How far above or below the target is she?
Answer: Bethany beats her target by 40 seconds
8 minutes = 8 x 60 = 480 minutes
Lucy’s favourite programme is on TV twice a week for 35 minutes.
In 6 weeks, how many hours does Lucy spend watching her favourite programme?
Answer: 7 hours
420 minutes = 7 hours
(Show as column method) 35 x 12 = 420 minutes
420 ÷ 60 = 7
For more time resources, take a look at our collection of printable time worksheets. Third Space Learning also offers a wide collection of word problems covering a range of topics such as place value, decimals and fractions word problems , percentages word problems , division word problems , ratio word problems , addition and subtraction word problems , multiplication word problems , money word problems and other word problem challenge cards.
DO YOU HAVE STUDENTS WHO NEED MORE SUPPORT IN MATHS?
Every week Third Space Learning’s specialist primary maths tutors support thousands of students across hundreds of schools with weekly online 1 to 1 maths lessons designed to plug gaps and boost progress.
Since 2013 these personalised one to one lessons have helped over 150,000 primary and secondary students become more confident, able mathematicians.
Learn how tutors develop pupils’ maths fluency or request a personalised quote for your school to speak to us about your school’s needs and how we can help.
Related articles

Maths Problem Solving: Engaging Your Students And Strengthening Their Mathematical Skills

Free Year 7 Maths Test With Answers And Mark Scheme: Mixed Topic Questions

What Is A Number Square? Explained For Primary School Teachers, Parents & Pupils
What Is Numicon? Explained For Primary School Teachers, Parents And Pupils
FREE Guide to Maths Mastery
All you need to know to successfully implement a mastery approach to mathematics in your primary school, at whatever stage of your journey.
Ideal for running staff meetings on mastery or sense checking your own approach to mastery.
Privacy Overview
Time Worksheets for Year 2 (age 6-7)
Children should enter Year 2 (age 6-7) familiar with an analogue clock face and being able to read the time using a clock face to the hour and to the half hour. They should also be familiar with the order of the days of the week and the months of the year, although this will need to be revisited in Year 2. We have some great pages on both days of the week and months of the year for Year 2 children.
Work on the analogue clock face continues in Year 2, firstly with telling the time to the quarter hour and then reading the time to 5 minutes. Again, a large clock face with hands is an invaluable resource. A good way to practise this is for children to draw the hands on a clock face to show these times and we have templates showing clock faces without hands which can be easily printed out. Children will need a good deal of practice reading the time to 5 minutes (e.g. 5 to nine, 10 past nine, 25 to ten etc.) and learning about the complexities of to and from the hour. It is interesting to note that we don’t say 40 minutes past eight, but we do say eight forty or 20 to nine.
Further work will be carried out on ordering sequences of time, such as putting these times in order, starting with the smallest:
20 minutes a quarter of an hour 10 minutes
This will need the knowledge that an hour is made up of 60 minutes, a half hour is thirty minutes and a quarter of an hour is 15 minutes.
There may not seem quite as much to do in Year 2 on time as there is in other areas of maths, but it is important that children gain confidence. It is surprising how many struggle with analogue clocks, especially now that fewer children wear a watch, preferring to use digital displays on phones etc. Reading a time on a digital clock comes later.
Revise telling the time to the half hour.
Revision of earlier work on reading analogue clock faces to the half hour.

A further look at reading clock faces to the half hour.

More on telling the time to the half hour.

Matching clock faces showing time to their written counterparts.

More on reading the time to the half hour.

Draw the times of the trains on the clock faces.

Draw the times of the boats on the clock faces.

Draw the times of the planes on the clock faces.

Where should the hands go on the clocks - including the hour hand between hours.
Telling the time to a quarter of an hour
Using analogue clock faces to read the time to a quarter of an hour.

Telling the time to a quarter of an hour.

Begin to read the time to a quarter hour.

Draw the hands on the clock faces to show the correct times.

Try writing times in words using quarter to and quarter past.

Writing times for a digital clock display, using quarter to and quarter past.

Matching times shown in words with times shown on clock faces.

More matching times shown in words with times shown on clock faces.

Using clock faces to read the time to the quarter of an hour.

More practice at reading clock faces. Each is either quarter to or quarter past the hour.

Tricky questions on where both hands of a clock should be.
Telling the time to five minutes
Using analogue clock faces, telling the time to five minutes.

Begin to read the time to 5 minute intervals.

Matching times shown on analogue clock faces to written times.

More matching times shown on analogue clock faces to written times.

Write down in words the times shown on the clock faces.
Time problems
Putting times in order, days of the week, months of the year and other time problems.

Questions all about days of the week.

More on days of the week.

Questions all about the months of the year.

More on months of the year.

Some research might need to be done to answer all these questions.

Order birthdays, starting with the earliest in the year.

Working out how long it is between two times: hour and half hour only.

Comparing lengths of time written in different ways.

Order lengths of time, including quarter and half hour.

How long has it taken our maths rats to complete their tasks?

Who has read the analogue clock correctly?

Solving word problems involving time.

More time problem solving involving time to the half/quarter hour.

Compare lengths of time written in different ways using <>=.

More comparing lengths of time written in different ways using <>=.

Blank clock faces to help with telling the time.
Subscribe to our newsletter
The latest news, articles, and resources, sent to your inbox weekly.
© Copyright 2011 - 2024 Route One Network Ltd. - URBrainy.com 11.1.7
Popular searches in the last week:
Problem-solving maths investigations for year 2.
Hamilton provide an extensive suite of problem-solving maths investigations for Year 2 to facilitate mathematical confidence, investigative inquiry and the development of maths meta skills in 'low floor – high ceiling' activities for all.
Explore all our in-depth problem solving investigations for Year 2 .
Use problem-solving investigations within every unit to encourage children to develop and exercise their ability to reason mathematically and think creatively.
Investigations provide challenges that offer opportunities for the development of the key mathematical skills while deepening conceptual understanding. They are designed to be accessible in different ways to all children. An added bonus is the substantial amount of extra calculation practice they often incorporate! The problems are designed to help children identify patterns, to explore lines of thinking and to reason and communicate about properties of numbers, shapes and measures.
Hamilton provide a mix of our own specially commissioned investigations, that include guidance for teachers together with a child-friendly sheet to guide your pupils through the investigation, as well as links to investigations on other highly regarded websites.
I am very grateful for Hamilton Trust resources, particularly the maths investigations. Julia, teacher in Wiltshire
You can find Hamilton's investigations for Year 2:
- Individually, they are incorporated into every unit in our Year 2 flexible maths blocks .
- Collectively, they appear on our resources page where you can explore all our in-depth problem solving investigations for Year 2 .
Do read our extensive range of advice for more information about the investigations and for tips on how to use them effectively.
Hamilton’s problem-solving investigations are 'low floor, high ceiling' activities that give all children opportunities to develop mastery and mathematical meta-skills. Explore a set for a whole year group.
Hamilton’s Problem-solving Investigations provide school-wide solutions to the challenges of building investigative skills from Early Years to Year 6.
This site uses cookies to give you the most relevant information. Learn more
Log in or sign up to get access to this resource
School subscription, reduce teacher workload.
From £155 (+ VAT) per year. Access to all key stages for multiple users.
Individual Subscription
For inspirational teaching.
Just £45 (£37.50 + VAT) per year to get access to all resources.

Early Career Teacher
Develop your teaching.
Just £33 (£27.50 + VAT) to get access to all resources for 2 years.
Taster Account
100s of resources.
Register to access all free resources.
Already subscribed?
Log in to get access.
- International
- Education Jobs
- Schools directory
- Resources Education Jobs Schools directory News Search

Reasoning and Problem Solving Questions Collection - KS1 and KS2
Subject: Mathematics
Age range: 5-7
Resource type: Worksheet/Activity
Last updated
10 March 2023
- Share through email
- Share through twitter
- Share through linkedin
- Share through facebook
- Share through pinterest

These booklets each contain over 40 reasoning and problem solving questions suitable for KS1, KS2 and KS3 classes. These are the questions that we have been putting out each day in March 2016 on Twitter in the run up to SATS.
The answers are provided with some simple notes at the back of the booklet and for some problems supplementary questions and variation has been provided.
As always we welcome any feedback on the work we are doing and the materials that we are releasing. Thank you for taking an interest in our work. The White Rose Maths Hub Team
Creative Commons "Sharealike"
Your rating is required to reflect your happiness.
It's good to leave some feedback.
Something went wrong, please try again later.
TES Resource Team
We are pleased to let you know that your resource Reasoning and Problem Solving Questions Collection - KS1 and KS2, has been hand-picked by the Tes resources content team to be featured in https://www.tes.com/teaching-resources/blog/fluency-reasoning-and-problem-solving-primary-maths in April 2024 on https://www.tes.com/teaching-resources/blog. Congratulations on your resource being chosen and thank you for your ongoing contributions to the Tes Resources marketplace.
Empty reply does not make any sense for the end user
graceamfo18
A very good and engaging way to teach mastery of maths. Thank you for sharing
thank you for sharing, this is really good
Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch.
Not quite what you were looking for? Search by keyword to find the right resource:
HYPOTHESIS AND THEORY article
Teaching abductive reasoning for use as a problem-solving tool in organic chemistry and beyond.

- 1 Chemistry Program, Department of Natural Sciences, Central College, Pella, IA, United States
- 2 Department of Chemistry, Augsburg University, Minneapolis, MN, United States
- 3 Department of Chemistry, University of Saint Joseph, West Hartford, CT, United States
- 4 Department of Chemical and Environmental Sciences, United States Coast Guard Academy, New London, CT, United States
The second-year undergraduate Organic Chemistry course sequence is often cited as one of the most, if not the most, challenging for students in the US. Thus, a persistent question remains: What is it about Organic Chemistry that makes the course so difficult for students? Herein, we put forward the hypothesis that a new mode of thinking and problem solving is expected of the students; these skills have not yet been developed in their prior scientific coursework and are often not deliberately taught in Organic Chemistry. This form of reasoning and problem solving, known as abductive reasoning, is highlighted for its connection to medical diagnosis and scientific thinking. We provide examples to showcase how instructors could explicitly foreground the reasoning process in their classroom. Ultimately, we argue that teaching how to reason using abduction may benefit students in both the short term (in the course) and the long term (in their careers as scientists and medical practitioners).
“What changes must be made in the kind of science that we teach and the way that we teach it so that the fundamental ideas of our discipline can be used outside the classroom?” – Herron & Greenbowe
1 Introduction
1.1 background.
Organic Chemistry, as traditionally taught in the US as a primarily second-year undergraduate course sequence, is often considered a course for “weeding out pre-meds” ( Moran, 2013 ) that “strik[es] fear in the hearts of students” ( Garg, 2019 ). This socially constructed barrier adds an additional level of pedagogical challenge for instructors. We, the authors, are instructors of Organic Chemistry and also write and review questions for standardized exams that are required for entrance into specialized medical programs; 1 thus, we are at a position in both the content delivery and assessment where we find ourselves continually asking the question: What do we want students to learn in the Organic Chemistry course sequence?
While some students may think the answer to this question is “to know, understand, and recite back the course material,” this is an unsatisfying response for a number of reasons. First, such a response would imply that only memorization and algorithmic problem-solving skills are necessary for success in Organic Chemistry ( Stowe and Cooper, 2017 ). 2 However, expert organic chemists recognize that the interconnected complexities within chemical systems means that simply following basic rules (i.e., deductive inference) will not necessarily lead to a set outcome (e.g., bulky bases do not always react via E2) ( Achet et al., 1986 ). Second, while the students enter our classrooms as novices, some of them will go on to become practicing, expert organic chemists. We owe it to them, and the future of scientific discovery, to build a sound foundation of both fundamental (e.g., understanding the aldol condensation) and higher order (e.g., performing retrosynthetic analysis) skills within the discipline. Third, most US health professions (e.g., MD, DO, PA, DDS, DMD, OD, PharmD) require this course to be taken as a prerequisite for admission into their graduate programs ( Kovac, 2002 ). These students should be presented, within their undergraduate education, the chance to improve their scientific reasoning and critical thinking skills. We think that these three features, which might not be clear to all students entering the course, illustrate that students are expected to learn and problem solve in new ways—essentially to begin to “think like a chemist” (e.g., Platt, 1964 ).
While certain ideas within this article were presented in a preceding paper ( Wackerly, 2021 ), we intend to flesh out and expand upon some of those initial assertions in this manuscript and craft a more detailed hypothesis that the use of abductive reasoning is critical in the learning of organic chemistry concepts. Herein we provide support for this hypothesis by viewing it from a few different conceptual angles. First, we provide a science education overview on why learning certain organic chemistry concepts is considered challenging for students. Then, we briefly summarize the medical education viewpoint on the teaching of diagnosis and why this is important to many students in Organic Chemistry. Finally, using the lens of the Organic Chemistry curriculum we provide problem-solving examples of how abductive reasoning can assist in the teaching and learning of organic chemistry.
1.2 Why is science difficult to learn?
Johnstone asked this titular question in his seminal 1991 paper ( Johnstone, 1991 ). One conclusion that he drew, which has since been supported by a variety of other work (e.g., Graulich, 2015 ; Tiettmeyer et al., 2017 ; Reid, 2020 ; Dood and Watts, 2022 ), is that the nature and complexity of scientific concepts strain the working memory of students. To assist instructors in conceptualizing the strain of a given concept, he created the “triangle model” which illustrated three levels of thought ( Figure 1 ). He argued that the more levels a concept included the more cognitive load was placed on students.
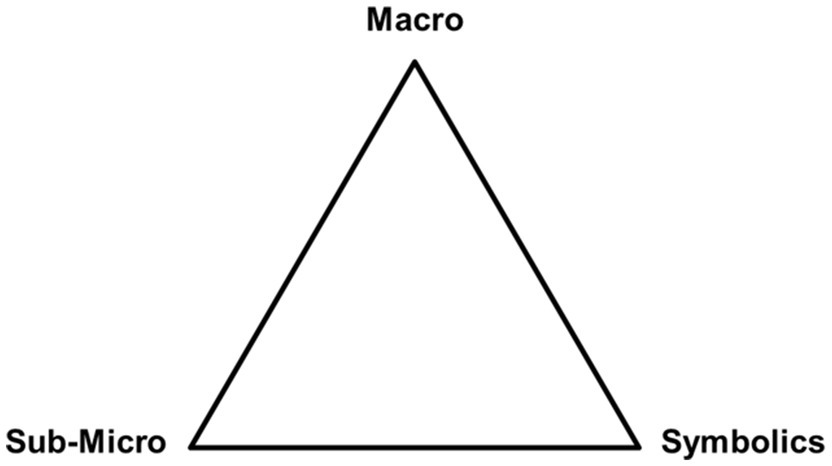
Figure 1 . Reproduction of Johnstone’s model: “Triangle of Levels of Thought”.
One feature that might make learning science difficult is that the instructor, or expert, may not be aware of the extent of cognitive load they are placing on students, or novices. When “multicomponent phenomena that are invisible, dynamic, and interdependent” are presented to students, a large demand is placed on the working memory of novices ( Hmelo-Silver et al., 2007 ). However, experts are able to easily connect two or more cognitive components by “chunking several pieces of information together” ( Overton and Potter, 2008 ) and through years of practice ( Randles and Overton, 2015 ). Specialization within a discipline that requires connecting multiple levels will lower cognitive load for such repetitive tasks over time ( Tiettmeyer et al., 2017 ; Price et al., 2021 ). However, students have typically not been exposed to such tasks, let alone have the opportunity to consistently repeat them, and thus instructors need to disentangle new concepts that might cause cognitive overload for students so they can process and incorporate new material starting from their present knowledge base and scientific models. 3
“[R]easoning [is the] knowledge of some facts [which] leads to a belief in others not directly observed.” – C. S. Peirce
1.3 Why is organic chemistry so difficult to learn?
Here we argue that it should come as no surprise when former and current students of organic chemistry cite that organic chemistry is difficult to learn, because they are asked to problem solve and reason in new ways utilizing new content without prior exposure to, or repetition of, these scientific tasks. 4 Naturally, when a student enters a course they are expected to be ignorant of the course content since they enroll to learn it. However, students might feel that a bait-and-switch has occurred in Organic Chemistry because not only is the content new, but the logical processes required to be successful are also typically new to the students as well.
In prior scientific courses, which for most pre-health ( vide infra ) US students are two courses in general biology and two in general chemistry, students are typically required to perform recall (memorization) or reason algorithmically on summative assessment items ( Raker and Towns, 2010 ). While these skills hold value in organic chemistry, current organic chemistry education research shows that skills such as multivariate ( Kraft et al., 2010 ; Christian and Talanquer, 2012 ) and mechanistic reasoning ( Bhattacharyya, 2013 ) are more important. 5 Thus, inspired by the work in chemistry education research, the philosophy of science, and Johnstone’s seminal triangle, here we propose a tetrahedron model of layered reasoning strategies that are important for consideration by instructors when teaching novice organic chemistry students.
The bottom-most point of the tetrahedron ( Figure 2 ) was chosen to be memorization because it is not a reasoning skill. However, terms and chemical facts still need to be learned by students, which is often not a problem because they have developed this skill during their general biology and chemistry coursework. Algorithmic reasoning is a skill many students leaving General Chemistry assume they will utilize in Organic Chemistry because it was employed so frequently in that course. For example, if a student knows the pressure, temperature, and number of moles of an ideal gas, these students will likely be able to provide the volume of the gas’s container. While these mathematical and deductive reasoning skills remain relevant in the laboratory portion of Organic Chemistry and even for the IUPAC naming of organic molecules (i.e., there is a definitive rule set), they start to break down when chemical systems become more complex and chemical formulas evolve to contain more meaning in the form of chemical structures.
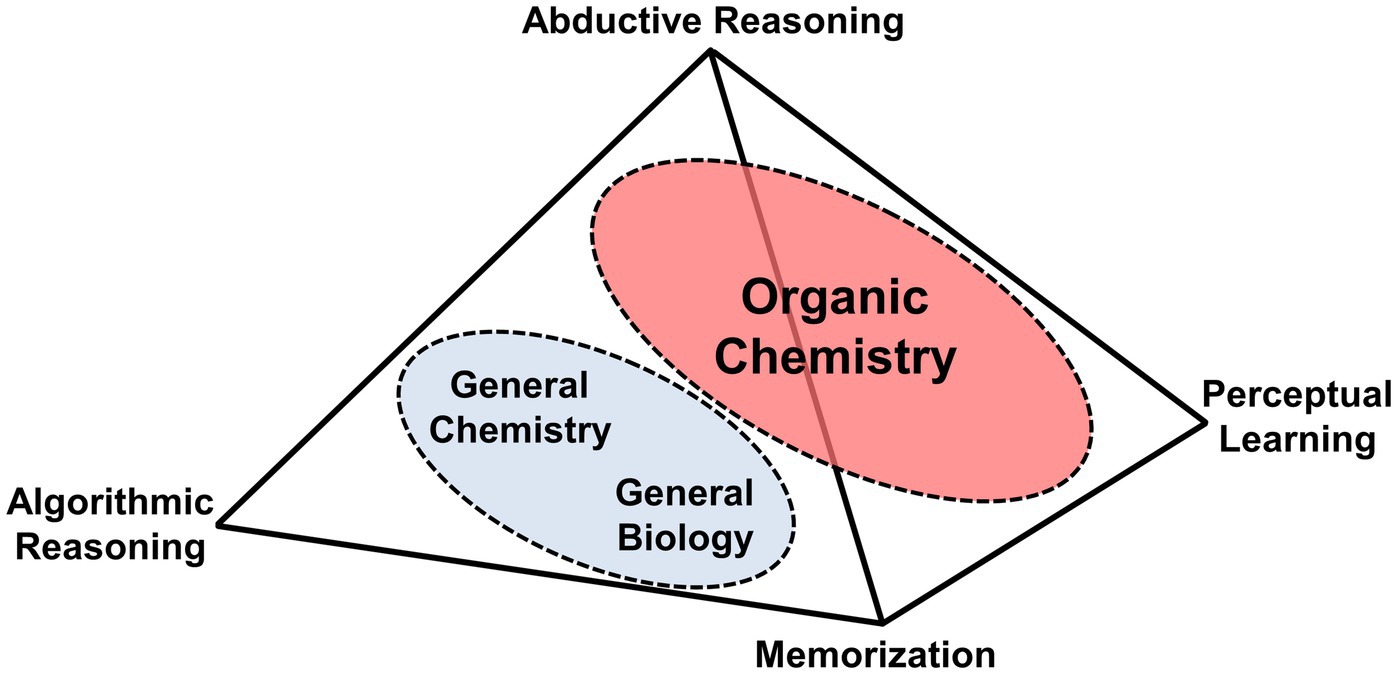
Figure 2 . Tetrahedron model of problem-solving in Organic Chemistry.
The right corner of the tetrahedron is for the set of competencies required to interpret diagrams in organic chemistry, such as visualization ( Gilbert, 2005 ), visuo-spatial reasoning ( Pribyl and Bodner, 1987 ; Habraken, 1996 ), and representational competence ( Kozma and Russell, 1997 ). In lieu of individually listing these skills, we designate this corner as perceptual learning, which integrates conceptual knowledge with a broad set of skills, including those related to visualization and representational competence ( Van Dantzig et al., 2008 ; Kellman and Massey, 2013 ). Perceptual learning “refers, roughly, to the long-lasting changes in perception that result from practice or experience” ( Connolly, 2017 ), and is beginning to be more deeply explored in organic chemistry pedagogy (e.g., Kim et al., 2019 ).
We briefly illustrate how changes associated with perceptual learning might take place with students. Consider, for example, that in General Chemistry students might be asked to calculate the heat of combustion of hexane (denoted at C 6 H 14 ). For most students at that stage, the sole association they would have with the compound’s name is its molecular formula, whereas its “zig-zag” structure might represent nothing more than a crooked line. As these students progress into Organic Chemistry and learn about different representational systems and constitutional isomers, the verbal representation “hexane” changes, this is because the term is now associated with five unique isomers each with unique connectivity, properties, and reactivity (e.g., radical reaction with Br 2 ). Through this process, the students’ perception for the term “hexane” changes from representing a single molecular formula to representing a family of five constitutional isomers each with a unique bond-line structure. This process continues as students advance to more complex structures (e.g., stereochemistry) and learn additional concepts like three-dimensionality, IMFs, physical properties, etc. We propose that the three corners of the tetrahedron discussed thus far are often directly connected to abductive reasoning which focuses on solving problems by generating the most likely most likely outcome of a chemical situation.
Our hypothesis includes the postulation that abductive reasoning is a complex reasoning skill for students in Organic Chemistry and should explicitly be taught in the classroom. While this idea has been presented by us previously ( Wackerly, 2021 ), here we will just provide a brief overview so we can move on to discuss the relevance of this reasoning skill within the Organic Chemistry classroom and to highlight some examples. Firstly, the term “abduction” ( Douven, 2021 ) is often used interchangeably with the terms “inference to the best explanation” ( Lipton, 2017 ) and “scientific hypothesis”—and below we will argue “diagnosis.” All of these terms hold common ground in that they use reasoning that connects various (similar or dissimilar) pieces of evidence/observations together in a way where a plausible conclusion can causally describe the collection of phenomena. 6 For example, say you are inside of grain windmill by the grindstone, and then you begin to see the stone rotating and producing flour. You will abduce that the weather outside has become windy. While this is a simple example only requiring you to understand that outside wind turns the sails and the sails, via a series of machinery, turn the grindstone, it is similar to the reasoning employed by expert organic chemists. Leaving the windmill and heading into your synthetic laboratory, let us say you wish to publish a new compound in the Journal of Organic Chemistry . According to the journal, to conclude that you have made this new compound you must “establish both identity and degree of purity.” Minimally, this means you will need to obtain a 1 H NMR spectrum, 13 C NMR spectrum, and HRMS spectrum then interpret the data present in the spectra to abduce the molecular structure of your new compound. This exact same skill that is required of expert organic chemists, is typically required of students in Organic Chemistry ( Stowe and Cooper, 2019a ). Thus, these students should be taught how to reason like expert scientists in order for them to develop into scientists ( Cartrette and Bodner, 2010 ). Just as the spectroscopic analysis example highlights, instructors of Organic Chemistry often profess a goal is for students to develop critical thinking and scientific problem-solving skills: Our hypothesis presented here is that instructors must explicitly utilize the abductive reasoning process within their teaching and assessment.
Solving problems that require abductive reasoning will also require skills from the three other points of the tetrahedron, which will render them cognitively complex. Teaching abductive reasoning in the classroom should not require additional formal training for instructors/experts since abductive reasoning skills have already been developed over the course of their careers. Further, philosophers have long held ( Harman, 1965 ) that humans utilize abductive reasoning as a matter of course in their day-to-day lives. Paralleling human logic, abductive reasoning has likely been utilized ( Pareschi, 2023 ) and will continue to be ( Dai and Muggleton, 2021 ) an integral part of artificial intelligence. This reasoning skill is particularly important for students required to take Organic Chemistry. It might be obvious that future scientists will need the skills to create new hypotheses and design experiments that could potentially refute current hypotheses, but in our experience, it seems less obvious to pre-health students that using abductive reasoning for problem solving in Organic Chemistry will play a critical role in their desired careers.
2 Framing for pre-health students (diagnosis)
2.1 why is organic chemistry relevant for pre-health students.
In a post-COVID world where test-optional admissions are on the rise and the future of post-graduate education feels increasingly uncertain, convincing students of the importance of Organic Chemistry goes beyond just passing the course. This is especially true for the majority of students taking Organic Chemistry who are pre-health majors. Instructors need to show students the connection between organic chemistry and the health field.
Thus, problem solving in Organic Chemistry can be framed as a diagnostic problem-solving tool–similar to what medical practitioners do when making a diagnosis ( Stowe and Cooper, 2019b ). By overtly showing students the parallels between medical diagnosis and organic chemistry problem solving, instructors demonstrate that students are not just being taught a bunch of facts–they are developing critical thinking skills they can use in the real world. Bridging the gap between theory and practice helps students see the bigger picture and gives them the tools they need to succeed in both their studies and future careers.
The parallels between medical diagnosis and organic chemistry problem solving should be readily apparent ( Table 1 ). Both involve analyzing complex systems (human body/chemical reactions) to identify patterns and relationships, emphasizing the importance of critical thinking and logic-based problem-solving skills, as well as using evidence. Both fields rely on the use of abductive reasoning ( Wackerly, 2021 ; Martini, 2023 ), although typically neither field explicitly states it to students. Table 1 uses simplified language accessible to students that describes the abductive theory of method (ATOM) in clinical diagnosis ( Vertue and Haig, 2008 ), and its parallel to expert thinking in organic chemistry.
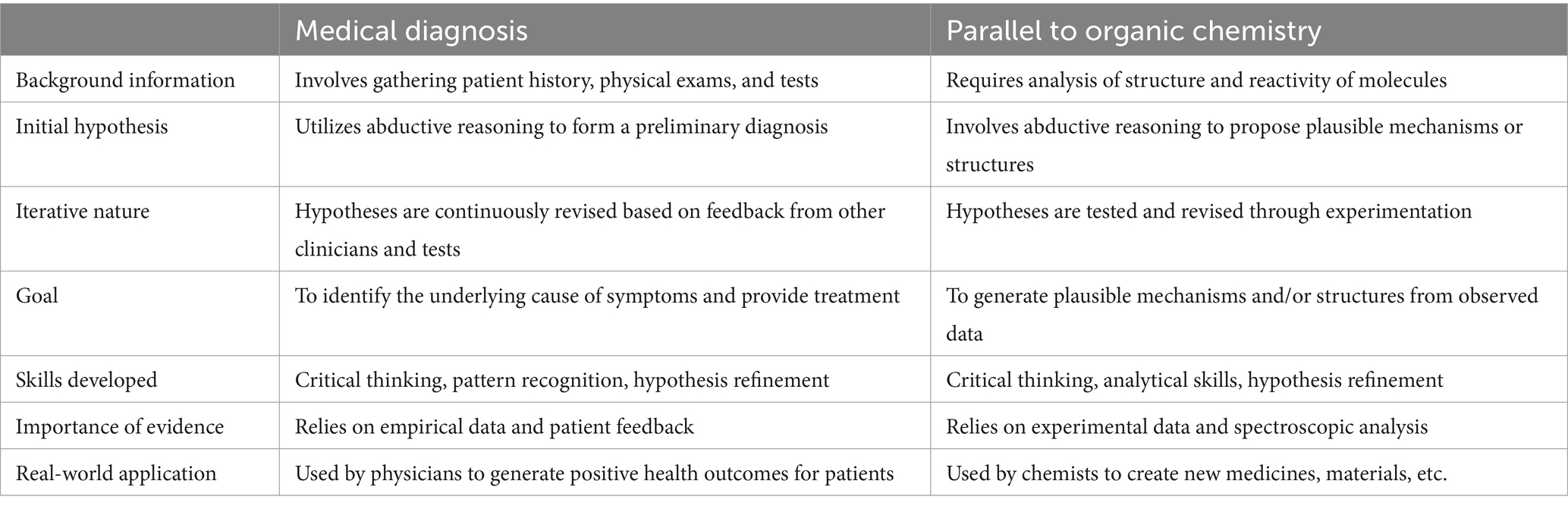
Table 1 . Comparison of medical diagnosis to skills developed in Organic Chemistry.
For example, to “diagnose” the product of an organic chemistry reaction, first the background information, including structure, reactivity, and stability of the starting materials and reagents must be analyzed, which is similar to how medical professionals take patient history. Abductive reasoning is then used to generate the most likely answer. Finally, the hypothesis is tested through gathering evidence such as utilizing spectroscopic analysis which is similar to a physician ordering lab work or imaging. This is an iterative process, wherein multiple pieces of spectroscopic evidence are needed to point to the same answer. Similarly, a physician may order additional studies or perform physical exams to support or refute their medical diagnosis. Although the goals appear different, the same skills are developed such as drawing hypothesis based on empirical evidence. By explicitly demonstrating how these thought processes are parallel, instructors of Organic Chemistry may help students to appreciate the mental training they are receiving in the course.
Organic Chemistry has been deemed essential as a prerequisite for medical school by a panel of medical school professors of biochemistry ( Buick, 1995 ). While many current medical students do not think that the material covered in Organic Chemistry was a valuable part of their undergraduate curriculum, the majority agree that the critical thinking skills learned in the course were valuable ( Dixson et al., 2022 ). While there are those in the field of medicine who think that Organic Chemistry should be de-emphasized in the pre-med curriculum, those that defend Organic Chemistry do so for some of the same reasons we discuss herein, namely that the critical thinking and problem-solving skills in the course directly align with patient diagnosis ( Higgins and Reed, 2007 ).
This process of abductive reasoning coupled with framing for the medical field may serve the students better in both the short term and long term. Students who employ more metacognitive strategies such as the type we are advocating for here are better able to solve problems in Organic Chemistry ( Blackford et al., 2023 ). Connecting course material to students’ future career aspirations also leads to better engagement and course performance ( Hulleman et al., 2010 ). Additional benefits of this diagnostic reasoning process include students’ ability to apply this metacognitive strategy in other courses in their majors, such as biology ( Morris Dye and Dangremond Stanton, 2017 ), and their future medical careers ( Friel and Chandar, 2021 ). Therefore, diagnostic reasoning should be explicitly modeled and assessed in Organic Chemistry courses.
2.2 Using “diagnosis” in examples for students
While there are a variety of ways to teach students how to approach organic chemistry problems like an expert, we would like to present how to do this through the lens of “diagnosis.” Other ways of describing argumentation and the process of problem solving have been discussed in the chemical education literature (e.g., Cruz-Ramírez De Arellano and Towns, 2014 ; Stowe and Cooper, 2019a ; Walker et al., 2019 ) as well as the philosophy of chemistry literature (e.g., Kovac, 2002 ; Goodwin, 2003 ). While they differ in the number of steps and what those steps are called, the processes have a similar logical flow. First, gather evidence and make observations ( What you see ), link this to previous knowledge ( What you know ), and finally make a reasoned conclusion ( Hypothesis ) which is a logical consequence—often via abductive inference.
The following examples ( Figures 3 – 6 ) are designed to highlight the use of these three steps to explicitly diagnose problems from across the two semester Organic Chemistry sequence. This process can be used in the classroom as a model to guide students through the abduction process and could be used to explicitly scaffold problems. Moreover, instructors can use this model to ascertain the complexity of their assessments including the required prerequisite factual knowledge and the multiple steps required. The complexity of organic chemistry questions is determined by the number of “subtasks” the student must complete ( Raker et al., 2013 ), factual knowledge required, and facets of perceptual learning ( vide supra ). A number of explicit decisions were made in formulating the below questions. The discussion points are certainly not exhaustive, and practitioners should adapt questions to their own students and situations. The amount of information provided or not provided, such as the exclusion of lone-pairs and inorganic by-products, was chosen to be consistent with the information provided by practicing organic chemists and one goal of teaching organic chemistry is to facilitate the development toward expert-level practice. We intentionally included one example of additional information, Figure 4 entry marked with a *, to highlight that there are many more subtasks that could be utilized to assist with arriving at a probable conclusion, but we tried to exclude all other non-essential explanations. We do not suggest that all students should solve each problem from top to bottom as outlined here; in reality expert chemists often take different routes, based on the same evidence and premises, to reach similar conclusions. Although these problems are multiple-choice, we have modeled how to solve them as either multiple-choice or open format. The complexity of these questions can also be adjusted, for example in Figure 4 the mechanistic arrows could be included in the distractors and answer instead of in the stem. This type of alteration can allow for the assessment of mechanistic thinking (e.g., Bodé et al., 2019 ; Finkenstaedt-Quinn et al., 2020 ; Watts et al., 2020 ; Dood and Watts, 2022 ). The following examples demonstrate that when the diagnosis/abduction process is utilized, students can develop and enhance their problem-solving skills.
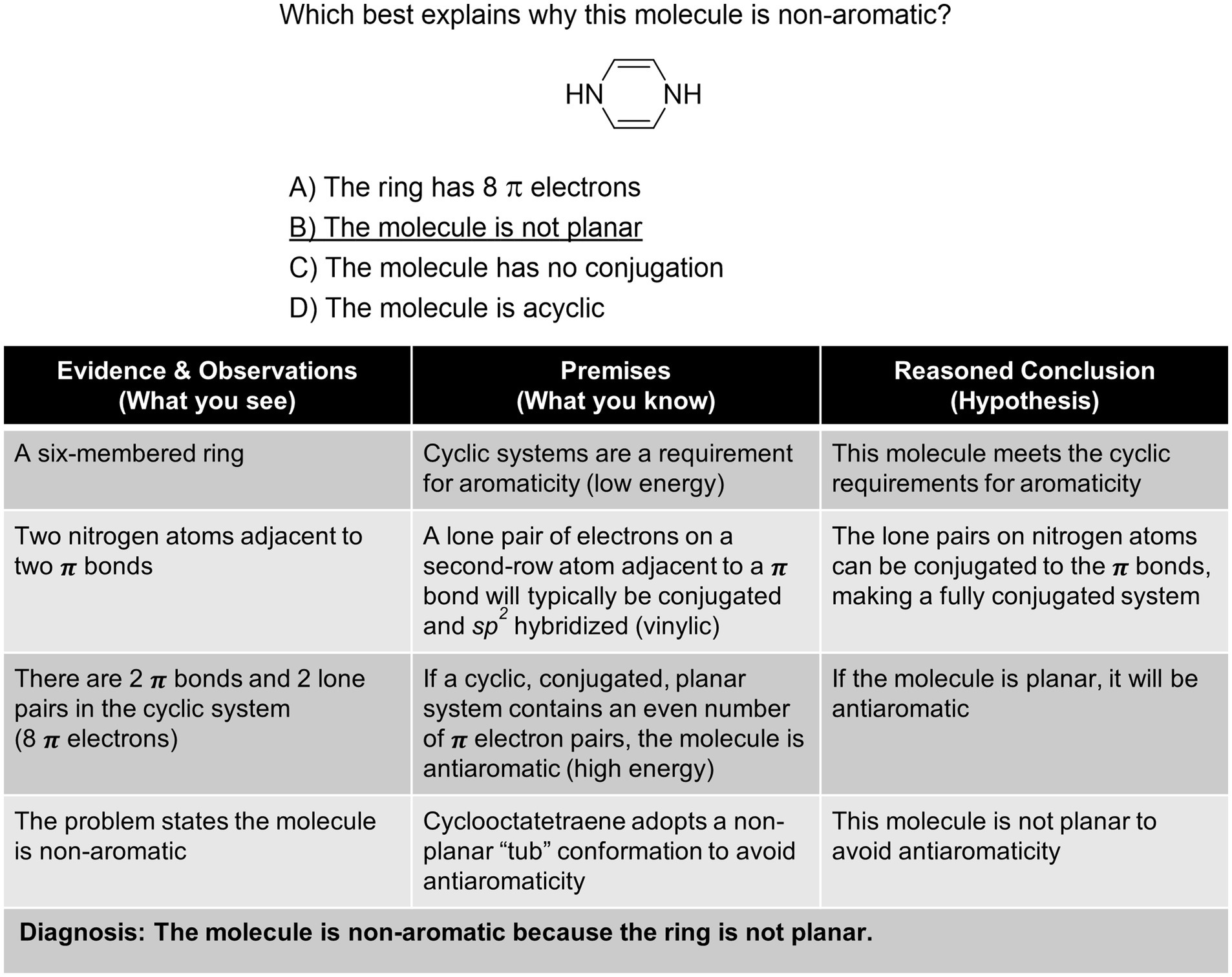
Figure 3 . Diagnosis of an aromaticity problem.
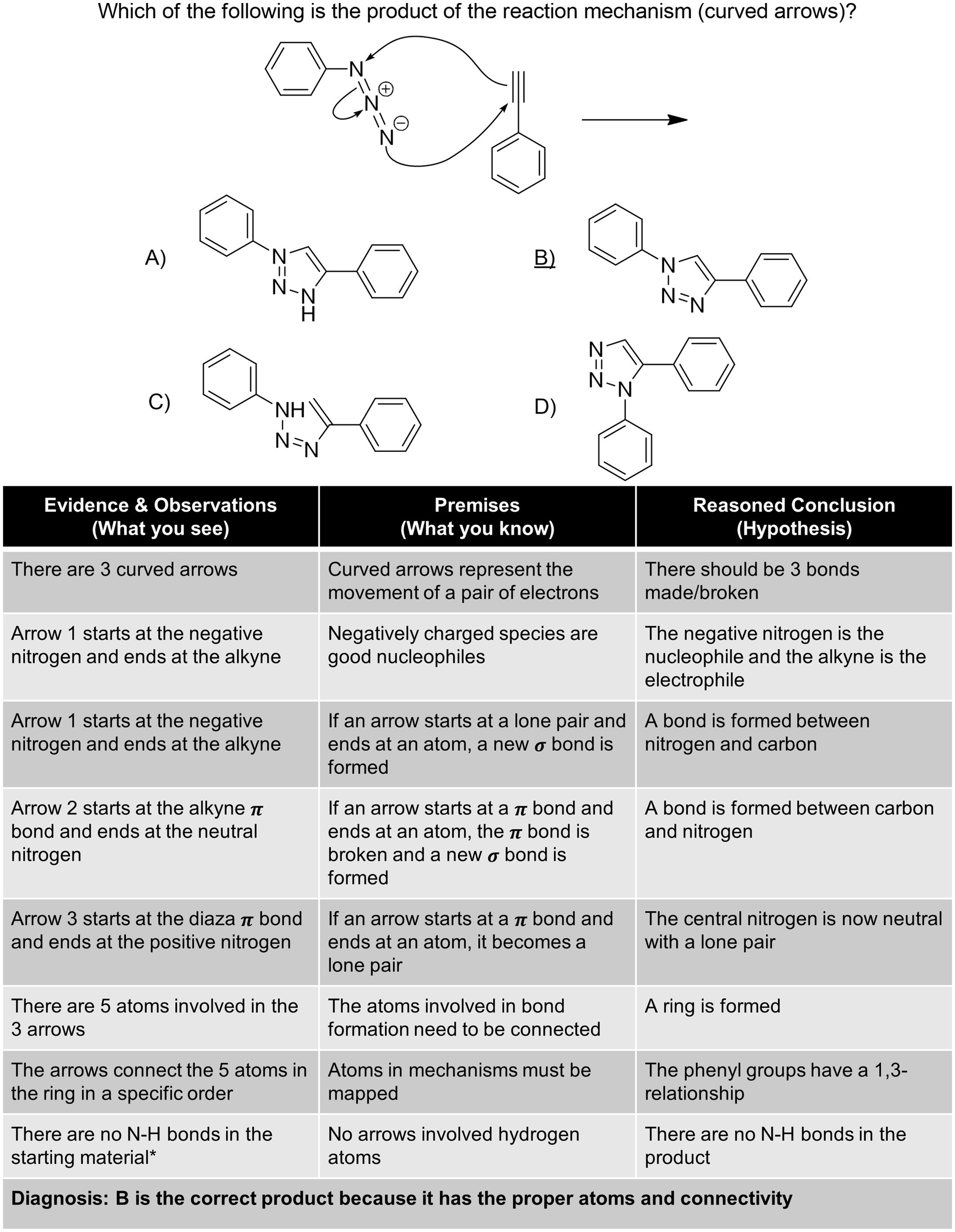
Figure 4 . Diagnosis of a mechanism problem.
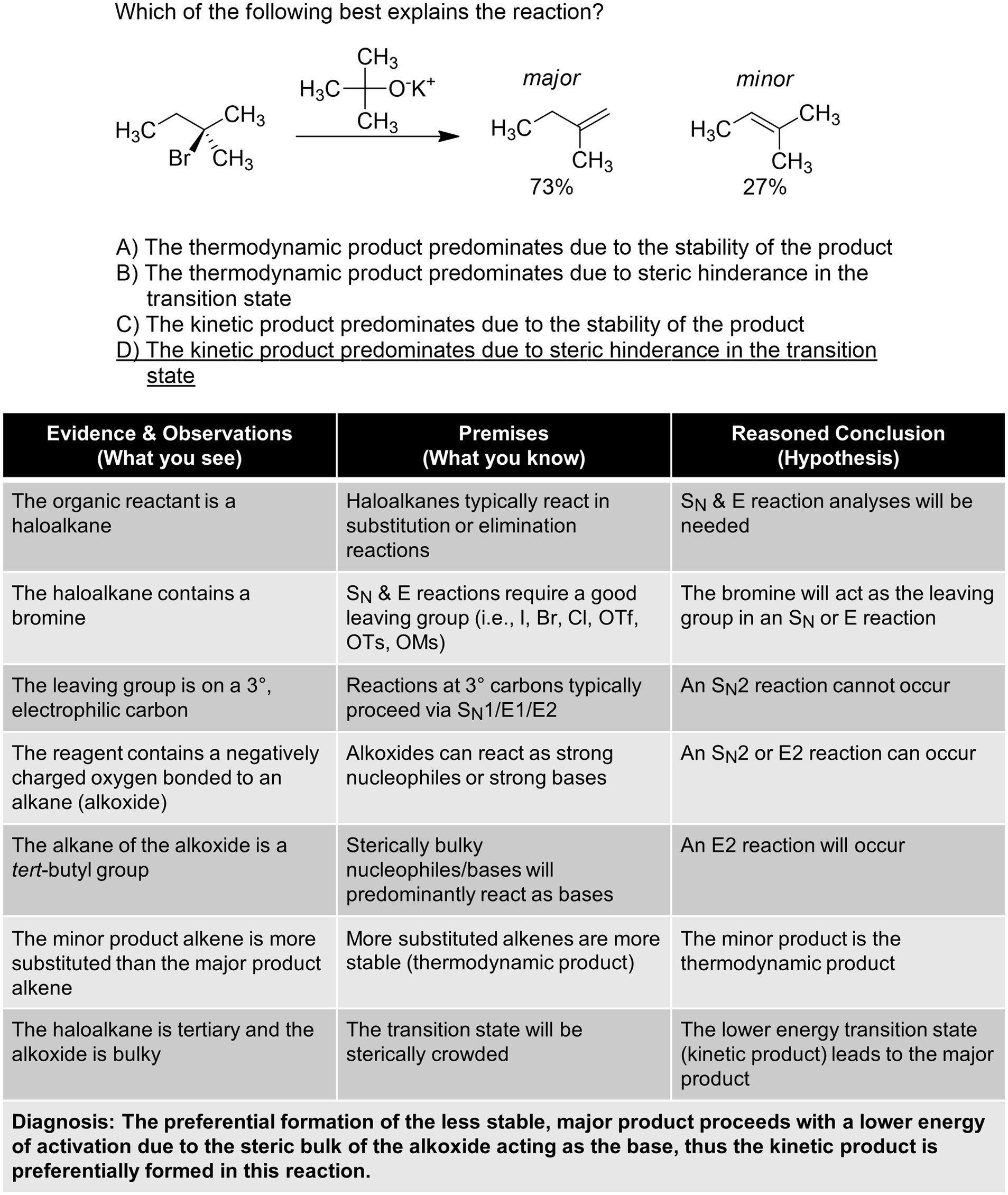
Figure 5 . Diagnosis of a substitution/elimination question.
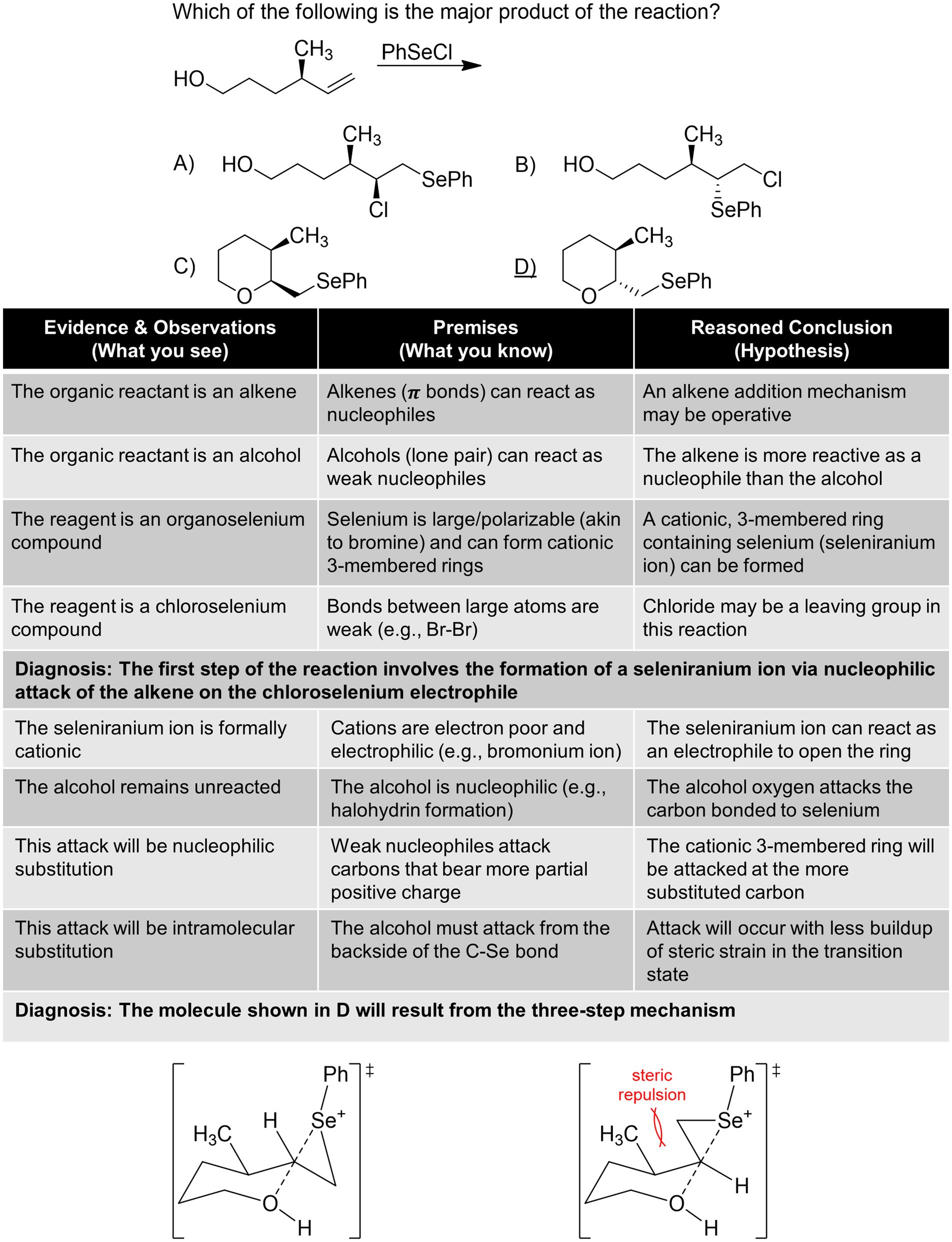
Figure 6 . Diagnosis of a predict the product reaction.
The first example shown in Figure 3 is a case of aromaticity ( Jin et al., 2022 ). Students will typically memorize the requirements and check the structure for being cyclic, planar, containing Huckel’s number (4 n + 2) electrons, and a p orbital at every vertex (i.e., conjugated). However, this problem does not ask for a simple definition of aromaticity, but an application of the ruleset to a structure students would not have typically encountered. The diagnosis requires observations about the structure including recognition of the implicit lone pairs on the nitrogen atoms and the carbon–carbon π bonds, recall of the requirements of aromaticity, and then application of abductive reasoning to the concepts learned (e.g., in class) and perceived by the structural representation. It is easy to see that the 1,4-dihydropyrazine is cyclic, has 8 π electrons, and a p orbital at each vertex. However, this simple analysis would result in the structure being anti-aromatic, so the student must recognize that in order for it to be non-aromatic as the problem states, planarity must be disrupted.
The second example shown in Figure 4 is a curved arrow mechanism problem for a reaction not typically covered in the Organic Chemistry course sequence ( Sarode et al., 2016 ). Students must apply the rules of curved arrows and properly atom map to diagnose the correct product. 7 The pre-existing conditions for a mechanism question with arrows shown include the nature of curved arrows and the examination of the scheme will require atom mapping and keeping track of which bonds are broken and formed.
The third example shown in Figure 5 is a substitution/elimination problem ( Brown et al., 1956 ). Students frequently find these reactions challenging and may employ a variety of heuristic models to approach them. Just as medical diagnosis begins with gathering information (taking patient information), solving this problem begins with direct observation and application of what is known about the structure and reactivity of these molecules. The alkyl halide has a good leaving group and has tertiary electrophilic carbon classification while the t -butoxide reagent is electron-rich, bulky, and reactive. Students must reason abductively how these characteristics interact with each other. This iterative process first eliminates S N 2 due to the nature of the alkyl halide, then identifies E2 as the mechanism with the bulky alkoxide. Next, an understanding of thermodynamics vs. kinetics to differentiate the two possible E2 pathways. Finally, a re-examination of the problem indicates the less stable product is formed preferentially; this is best explained by steric crowding in the transition state of the reaction between the alkyl halide and alkoxide.
The final, and most complex, example shown in Figure 6 is a predict the product, addition reaction problem ( Inoue and Murata, 1997 ) that is analogous to halohydrin formation. The problem requires separate diagnoses as it is layered where advancement to the second part is necessitated by the successful completion of the first addition step. Students would need to differentiate between the nucleophilicity of the alcohol and π bond after recognizing them as potential nucleophiles. After using abduction to recognize the higher reactivity of the π bond, students should then reason that selenium is electrophilic, akin to bromine in Br 2 due to being polarizable and bonded to a leaving group. This diagnosis is supported when taking into account the stereospecificity of the transformation, which precludes carbocation intermediates. The second diagnosis requires that students recall the regioselectivity of reactions with 3-membered cationic rings at the more substituted carbon. The remaining nucleophilic oxygen atom can now react with a higher energy seleniranium ion. However, conformational analysis of the transition state is needed to discern the pseudo axial/equatorial approach of the oxygen atom on the seleniranium ion ( Figure 6 , bottom). Students would then need to apply their knowledge of chair conformations and the lower energy state when having ring substituents equatorial. Thus, the trans -oxacyclohexane is formed.
3 Conclusion and future work
While Organic Chemistry is often regarded as the most challenging undergraduate course in the US, we argue it has gotten a “bad rap” because students are not always prepared for the challenges that lie ahead when they enter the course. Students generally perform better on assessments when they employ metacognitive strategies (i.e., “thinking about thinking”). This has been demonstrated in a variety of courses ( Arslantas et al., 2018 ), including Organic Chemistry (e.g., Graulich et al., 2021 ; Blackford et al., 2023 ). The consensus is that students who employ more metacognitive strategies in Organic Chemistry are more successful in problem-solving tasks and are better able to use those strategies when they are explicitly modeled and scaffolded. We have argued that instructors of Organic Chemistry should teach and demonstrate how to think and problem solve via “diagnosis” (i.e., abductive reasoning) in their classrooms. We hypothesize that students may score higher on metrics that assess scientific learning when these types of diagnostic models are utilized.
As constructors of nationally standardized exams, we fully acknowledge that a lot of growth on organic chemistry knowledge assessment still remains to be achieved. For Organic Chemistry course instructors, we hope the above insight into abductive reasoning can also be used on the assessment side of teaching requirements. Namely, that the cognitive load placed on students when solving each problem be carefully considered when constructing summative assessment items. Though this point has been frequently made previously e.g., (see Raker et al., 2013 ), we believe it is worthwhile for all writers of questions in Organic Chemistry to map out, step-by-step, the logic required to solve each question to determine the cognitive load. This can, in turn, help these instructors teach from a novice-focused perspective—as opposed to the “sage on the stage.” The prior section provided examples with varying levels of complexity and demonstrated that cognitive load can be approximated by the number of reasoning steps (subtasks) required when the assessment piece is broken down. Further, this process could potentially also help the exam writer identify if items require little to no scientific reasoning (e.g., pure memorization questions).
The above manuscript merely outlines a hypothesis that we have generated over the course of our time teaching Organic Chemistry with this “diagnosis” method of abduction. To fully explore its validity, educational research is needed. This will be a precarious endeavor, because measuring the efficacy of teaching abductive reasoning will require assessment of scientific thinking skills in Organic Chemistry, and, as we just pointed out, there are already strong arguments that we are still quite far away from such valid assessments. However, we can be sure that if you are teaching Organic Chemistry from the perspective of your experience and expertise as an organic chemist, then opening a window for your students into how you think and problem solve will benefit your students. Our position is that instructors of Organic Chemistry should not only be explicitly teaching students the abductive reasoning skills to tackle complex problems, but they should also frame it as “diagnosing” the chemical situation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JW: Writing – review & editing, Writing – original draft, Conceptualization. MW: Writing – review & editing, Writing – original draft. SZ: Writing – review & editing, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank Gautam Bhattacharyya for helpful discussions during revisions of this manuscript, specifically regarding perceptual learning theory. JW would like to acknowledge his undergraduate Organic Chemistry professor, Thomas Nalli, on the recent occasion of his 65th birthday for teaching him scientific problem-solving skills and fostering his interest in the discipline.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^ We wish to keep the focus of this manuscript on the relevant student population of the Organic Chemistry course sequence. Students intending to pursue medically relevant careers which require advanced degrees (e.g., medical, dental, optometry, pharmacy, etc.) are a large portion of this population. However, if the reader is curious, we specifically write for the dental and optometric admissions exams.
2. ^ In this manuscript we attempt to provide the reader a broad overview of important chemical education and philosophy of chemistry publications. Since this is not a review article and the scope is quite a bit smaller, all possible relevant literature has not been cited.
3. ^ Cognitive overload could also stem from misconceptions and oversimplified concepts, such as the oft-stated “breaking bonds in ATP releases energy” from introductory biology courses.
4. ^ This can be contrasted with General Chemistry which repeats some of the content of the high school chemistry.
5. ^ Multivariate and mechanistic reasoning are highlighted as examples because they often require combining features from all four points of the tetrahedron.
6. ^ The conclusion need not explain the entire collection of evidence as some may be irrelevant, and they are unrelated to the conclusion. However, the entire collection may not contain a piece of evidence that refutes the conclusion. Thus, abductive reasoning can be useful in differentiating science from non-science and pseudoscience.
7. ^ While one could argue that the diagnosis/answer to the problem presented in Figure 4 does not require abductive reasoning, we have included it because the skills required here can be applied to more complex problems that, for example, include mechanistic reasoning ( vide infra ).
Achet, D., Rocrelle, D., Murengezi, I., Delmas, M., and Gaset, A. (1986). Reactions in slightly hydrated solid/liquid heterogeneous media: the methylation reaction with dimethyl sulfoxide. Synthesis 1986, 642–643. doi: 10.1055/s-1986-31729
Crossref Full Text | Google Scholar
Arslantas, F., Wood, E., and Macneil, S. (2018). “Metacognitive foundations in higher education chemistry” in International perspectives on chemistry education research and practice (American Chemical Society).
Google Scholar
Bhattacharyya, G. (2013). From source to sink: mechanistic reasoning using the Electron-pushing formalism. J. Chem. Educ. 90, 1282–1289. doi: 10.1021/ed300765k
Blackford, K. A., Greenbaum, J. C., Redkar, N. S., Gaillard, N. T., Helix, M. R., and Baranger, A. M. (2023). Metacognitive regulation in organic chemistry students: how and why students use metacognitive strategies when predicting reactivity. Chem. Educ. Res. Pract. 24, 828–851. doi: 10.1039/D2RP00208F
Bodé, N. E., Deng, J. M., and Flynn, A. B. (2019). Getting past the rules and to the why: causal mechanistic arguments when judging the plausibility of organic reaction mechanisms. J. Chem. Educ. 96, 1068–1082. doi: 10.1021/acs.jchemed.8b00719
Brown, H. C., Moritani, I., and Okamoto, Y. (1956). Steric effects in elimination reactions. Vii. The effect of the steric requirements of alkoxide bases on the direction of bimolecular elimination. J. Am. Chem. Soc. 78, 2193–2197. doi: 10.1021/ja01591a047
Buick, P. E. (1995). An evaluation of organic chemistry as a prerequisite for admission to Florida medical schools. Doctorate, Waldon University.
Cartrette, D. P., and Bodner, G. M. (2010). Non-mathematical problem solving in organic chemistry. J. Res. Sci. Teach. 47, 643–660. doi: 10.1002/tea.20306
Christian, K., and Talanquer, V. (2012). Modes of reasoning in self-initiated study groups in chemistry. Chem. Educ. Res. Pract. 13, 286–295. doi: 10.1039/C2RP20010D
Connolly, K. (2017). Perceptual learning [online] . Metaphysics Research Lab, Stanford University. Available at: https://plato.stanford.edu/archives/sum2017/entries/perceptual-learning/ (Accessed 2024).
Cruz-Ramírez De Arellano, D., and Towns, M. H. (2014). Students' understanding of alkyl halide reactions in undergraduate organic chemistry. Chem. Educ. Res. Pract. 15, 501–515. doi: 10.1039/C3RP00089C
Dai, W.-Z., and Muggleton, S. (2021). Abductive knowledge induction from raw data. arxiv:2010.03514v2 . doi: 10.48550/arXiv.2010.03514
Dixson, L., Pomales, B., Hashemzadeh, M., and Hashemzadeh, M. (2022). Is organic chemistry helpful for basic understanding of disease and medical education? J. Chem. Educ. 99, 688–693. doi: 10.1021/acs.jchemed.1c00772
Dood, A. J., and Watts, F. M. (2022). Mechanistic reasoning in organic chemistry: a scoping review of how students describe and explain mechanisms in the chemistry education research literature. J. Chem. Educ. 99, 2864–2876. doi: 10.1021/acs.jchemed.2c00313
Douven, I. (2021). How explanation guides belief change. Trends Cogn. Sci. 25, 829–830. doi: 10.1016/j.tics.2021.07.009
PubMed Abstract | Crossref Full Text | Google Scholar
Finkenstaedt-Quinn, S. A., Watts, F. M., Petterson, M. N., Archer, S. R., Snyder-White, E. P., and Shultz, G. V. (2020). Exploring student thinking about addition reactions. J. Chem. Educ. 97, 1852–1862. doi: 10.1021/acs.jchemed.0c00141
Friel, D. D., and Chandar, K. (2021). Teaching diagnostic reasoning to medical students: a four-step approach. Perspect. Biol. Med. 64, 557–586. doi: 10.1353/pbm.2021.0041
Garg, N. K. (2019). How organic chemistry became one of Ucla's most popular classes. J. Biol. Chem. 294, 17678–17683. doi: 10.1074/jbc.AW119.008141
Gilbert, J. K. (2005). Visualization: A Metacognitive Skill in Science and Science Education. In: GILBERT, J. K. (ed.) Visualization in Science Education. Dordrecht: Springer Netherlands.
Goodwin, W. (2003). Explanation in organic chemistry. Ann. N. Y. Acad. Sci. 988, 141–153. doi: 10.1111/j.1749-6632.2003.tb06093.x
Graulich, N. (2015). The tip of the iceberg in organic chemistry classes: how do students deal with the invisible? Chem. Educ. Res. Pract. 16, 9–21. doi: 10.1039/C4RP00165F
Graulich, N., Langner, A., Vo, K., and Yuriev, E. (2021). “Scaffolding metacognition and resource activation during problem solving: a continuum perspective” in Problems and problem solving in chemistry education: Analysing data, looking for patterns and making deductions . ed. G. Tsaparlis (The Royal Society of Chemistry).
Habraken, C. L. (1996). Perceptions of chemistry: Why is the common perception of chemistry, the most visual of sciences, so distorted?. J sci educ technol, 5, 193–201.
Harman, G. H. (1965). The inference to the best explanation. Philos. Rev. 74, 88–95.
Higgins, T. S., and Reed, S. F. (2007). Changing premedical requirements. JAMA 297, 37–39. doi: 10.1001/jama.297.1.37-b
Hmelo-Silver, C. E., Marathe, S., and Liu, L. (2007). Fish swim, rocks sit, and lungs breathe: expert: novice understanding of complex systems. J. Learn. Sci. 16, 307–331. doi: 10.1080/10508400701413401
Hulleman, C. S., Godes, O., Hendricks, B. L., and Harackiewicz, J. M. (2010). Enhancing interest and performance with a utility value intervention. J. Educ. Psychol. 102, 880–895. doi: 10.1037/a0019506
Inoue, H., and Murata, S. (1997). Novel cyclization of unsaturated alcohols by phenyl selenocyanate in the presence of copper bis(trifluoromethanesulfonate). Heterocycles 45, 847–850.
Jin, X., Li, S., Guo, L., Hua, J., Qu, D.-H., Su, J., et al. (2022). Interplay of steric effects and aromaticity reversals to expand the structural/electronic responses of Dihydrophenazines. J. Am. Chem. Soc. 144, 4883–4896. doi: 10.1021/jacs.1c12610
Johnstone, A. H. (1991). Why is science difficult to learn? Things are seldom what they seem. J. Comput. Assist. Learn. 7, 75–83. doi: 10.1111/j.1365-2729.1991.tb00230.x
Kellman, P. J., and Massey, C. M. (2013). “Chapter four - perceptual learning, cognition, and expertise” in Psychology of learning and motivation . ed. B. H. Ross (Academic Press).
Kim, T., Wright, L. K., and Miller, K. (2019). An examination of students' perceptions of the Kekulé resonance representation using a perceptual learning theory lens. Chem. Educ. Res. Pract. 20, 659–666. doi: 10.1039/C9RP00009G
Kovac, J. (2002). Theoretical and practical reasoning in chemistry. Found. Chem. 4, 163–171. doi: 10.1023/A:1016035726186
Kozma, R. B., and Russell, J. (1997). Multimedia and understanding: Expert and novice responses to different representations of chemical phenomena. Journal of Research in Science Teaching, 34, 949–968.
Kraft, A., Strickland, A. M., and Bhattacharyya, G. (2010). Reasonable reasoning: multi-variate problem-solving in organic chemistry. Chem. Educ. Res. Pract. 11, 281–292. doi: 10.1039/C0RP90003F
Lipton, P. (2017). “Inference to the best explanation” in A companion to the philosophy of science . ed. W. H. Newton-Smith (Blackwell).
Martini, C. (2023). “Abductive reasoning in clinical diagnostics” in Handbook of abductive cognition . ed. L. Magnani (Cham: Springer International Publishing). doi: 10.1007/978-3-031-10135-9_13
Moran, B. (2013). How to get an A- in organic chemistry. New York Times .
Morris Dye, K., and Dangremond Stanton, J. (2017). Metacognition in upper-division biology students: awareness does not always Lead to control. CBE Life Sci. Educ. 16:ar31. doi: 10.1187/cbe.16-09-0286
Overton, T., and Potter, N. (2008). Solving open-ended problems, and the influence of cognitive factors on student success. Chem. Educ. Res. Pract. 9, 65–69. doi: 10.1039/B801307C
Pareschi, R. (2023). Abductive reasoning with the Gpt-4 language model: case studies from criminal investigation, medical practice, scientific research. Sistemi Intelligenti 35, 435–444. doi: 10.48550/arXiv.2307.10250
Platt, J. R. (1964). Strong inference. Science 146, 347–353. doi: 10.1126/science.146.3642.347
Pribyl, J. R., and Bodner, G. M. (1987). Spatial ability and its role in organic chemistry: A study of four organic courses. Journal of Research in Science Teaching, 24, 229–240.
Price, A. M., Kim, C. J., Burkholder, E. W., Fritz, A. V., and Wieman, C. E. (2021). A detailed characterization of the expert problem-solving process in science and engineering: guidance for teaching and assessment. CBE Life Sci. Educ. 20:ar43. doi: 10.1187/cbe.20-12-0276
Raker, J. R., and Towns, M. H. (2010). Benchmarking problems used in second year level organic chemistry instruction. Chem. Educ. Res. Pract. 11, 25–32. doi: 10.1039/C001043J
Raker, J. R., Trate, J. M., Holme, T. A., and Murphy, K. (2013). Adaptation of an instrument for measuring the cognitive complexity of organic chemistry exam items. J. Chem. Educ. 90, 1290–1295. doi: 10.1021/ed400373c
Randles, C. A., and Overton, T. L. (2015). Expert vs. novice: approaches used by chemists when solving open-ended problems. Chem. Educ. Res. Pract. 16, 811–823. doi: 10.1039/C5RP00114E
Reid, N. (2020). The triangle model: the contribution of the late professor Alex H. Johnstone. J. Sci. Educ. 2, 47–61.
Sarode, P. B., Bahekar, S. P., and Chandak, H. S. (2016). Dabco/Acoh jointly accelerated copper(I)-catalysed cycloaddition of Azides and alkynes on water at room temperature. Synlett 27, 2681–2684. doi: 10.1055/s-0036-1588590
Stowe, R. L., and Cooper, M. M. (2017). Practicing what we preach: assessing “critical thinking” in organic chemistry. J. Chem. Educ. 94, 1852–1859. doi: 10.1021/acs.jchemed.7b00335
Stowe, R. L., and Cooper, M. M. (2019a). Arguing from spectroscopic evidence. J. Chem. Educ. 96, 2072–2085. doi: 10.1021/acs.jchemed.9b00550
Stowe, R. L., and Cooper, M. M. (2019b). Assessment in chemistry education. Israel J. Chem. 59, 598–607. doi: 10.1002/ijch.201900024
Tiettmeyer, J. M., Coleman, A. F., Balok, R. S., Gampp, T. W., Duffy, P. L., Mazzarone, K. M., et al. (2017). Unraveling the complexities: an investigation of the factors that induce load in chemistry students constructing Lewis structures. J. Chem. Educ. 94, 282–288. doi: 10.1021/acs.jchemed.6b00363
Van Dantzig, S., Pecher, D., Zeelenberg, R., and Barsalou, L. W. (2008). Perceptual processing affects conceptual processing. Cogn. Sci. 32, 579–590. doi: 10.1080/03640210802035365
Vertue, F. M., and Haig, B. D. (2008). An abductive perspective on clinical reasoning and case formulation. J. Clin. Psychol. 64, 1046–1068. doi: 10.1002/jclp.20504
Wackerly, J. W. (2021). Abductive reasoning in organic chemistry. J. Chem. Educ. 98, 2746–2750. doi: 10.1021/acs.jchemed.1c00295
Walker, J. P., Van Duzor, A. G., and Lower, M. A. (2019). Facilitating argumentation in the laboratory: the challenges of claim change and justification by theory. J. Chem. Educ. 96, 435–444. doi: 10.1021/acs.jchemed.8b00745
Watts, F. M., Schmidt-Mccormack, J. A., Wilhelm, C. A., Karlin, A., Sattar, A., Thompson, B. C., et al. (2020). What students write about when students write about mechanisms: analysis of features present in students’ written descriptions of an organic reaction mechanism. Chem. Educ. Res. Pract. 21, 1148–1172. doi: 10.1039/C9RP00185A
Keywords: abduction, abductive reasoning, organic chemistry, diagnosis, metacognition, problem solving, pre-health education
Citation: Wackerly JW, Wentzel MT and Zingales SK (2024) Teaching abductive reasoning for use as a problem-solving tool in organic chemistry and beyond. Front. Educ . 9:1412417. doi: 10.3389/feduc.2024.1412417
Received: 04 April 2024; Accepted: 05 July 2024; Published: 06 August 2024.
Reviewed by:
Copyright © 2024 Wackerly, Wentzel and Zingales. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jay Wm. Wackerly, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
COMMENTS
Reasoning and Problem Solving Telling Time to 5 Minutes Reasoning and Problem Solving Telling Time to 5 Minutes Developing 1a. A -5 past 6, B -10 to 5 2a. Talia is not correct because the minute hand points to the 8 when it is 20 minutes to the hour. If the minute hand was pointing to the 9 then it would have been quarter to 3. 3a. 25 to 2 ...
Develop fluency, reasoning and problem-solving skills using these structured time inspired challenge cards. The time challenge cards covers time sequences, before and after, time word problems, true or false statements relating to days of the week, months of the year and seconds in a minute. Each time activity question prompts children to use ...
Twinkl Key Stage 1 - Year 1, Year 2 Maths Activities and Games Year 2 Measurement. Develop fluency, reasoning and problem-solving skills using this structured time inspired PowerPoint. The PowerPoint covers time sequences, before and after, time word problems, true or false statements relating to days of the week, months of the year and seconds ...
Time word problems for year 4. When solving time word problems for year 4, pupils need to be confident telling time in analogue, and digital, as well as converting between analogue, 12 hour and 24 hour clock. They also begin to solve more challenging problems involving duration of time and converting time.
Reasoning and Problem Solving - Time - Year 2. Splish, Splash, Tick and Tock! You are in charge of managing time at the local swimming pool and sports centre. From parties to pool-time, gymnastics to ping pong and babies to daddies, everyone will stick to your time plan! PARTY TIME! 1. The pool parties all happen on a Sunday afternoon.
The lesson pack includes Diving into Mastery resources to provide reasoning and problem-solving challenges. This lesson supports the year 2 national curriculum aim 'Tell and write the time to five minutes, including quarter past/to the hour and draw the hands on a clock face to show these times' and the White Rose Maths small step ...
Reasoning and Problem Solving Step 6: Compare Durations of Time National Curriculum Objectives: Mathematics Year 2: (2M4a) Tell and write the time to five minutes, including quarter past/to the hour and draw the hands on a clock face to show these times Mathematics Year 2: (2M4b) Compare and sequence intervals of time Differentiation:
Expected Draw hands on clocks to tell the time to 5 minutes. Greater Depth Draw hands on clocks to tell the time to 5 minutes when a clock face only has the numbers 12, 3, 6 and 9. Questions 3, 6 and 9 (Reasoning and Problem Solving) Developing Calculate a duration of time to determine if a statement is correct. Includes
When learning how to tell the time in Year 2, children often struggle with a particular format. This worksheet is perfect for children who find analogue clocks particularly challenging. ... Time Fluency Reasoning and Problem-Solving Maths Mastery Challenge Cards. Months of the Year Handwriting Practice Worksheet. Year 2 Maths Same-Day ...
This worksheet includes a range of varied fluency and reasoning and problem solving questions for pupils to further extend and practise the main skill of using the language of position. Language of Position - Discussion Problem. ... Year 2 Tell the Time Past the Hour Teaching PowerPoint.
Time Worksheets for Year 2 (age 6-7) Telling the time using analogue clock faces and solving problems including finding the time between events.. Children should enter Year 2 (age 6-7) familiar with an analogue clock face and being able to read the time using a clock face to the hour and to the half hour. They should also be familiar with the ...
Mathematics Year 2: (2M4a) Tell and write the time to five minutes, including quarter past/to the hour and draw the hands on a clock face to show these times. Mathematics Year 2: (2M4b) Compare and sequence intervals of time. Differentiation: Questions 1, 4 and 7 (Problem Solving) Developing Work out how many quarter hours are between two times ...
By Nick Barwick - 7 Aug 2018. Hamilton provide an extensive suite of problem-solving maths investigations for Year 2 to facilitate mathematical confidence, investigative inquiry and the development of maths meta skills in 'low floor - high ceiling' activities for all. Explore all our in-depth problem solving investigations for Year 2.
Put the plates in a cross. Use all 15 counters. Put a different number on each plate. Make each line add up to 10. Do it again. This time make each line add up to 8. Solve mathematical problems or puzzles. Know addition and subtraction facts up to 10. Add three small numbers mentally.
Reasoning and. - Mixed Problems - Year 2 Expected. coin only once, but you must use all the coins. Draw lines to show which piggy bank each coin should go in. 6.Lionel is painting a shape picture by using 3D shapes as stamps. List all the 3D shapes he could have used to stamp each of the following 2D shapes: Reasoning and Problem Solving ...
Reasoning and Problem Solving Divide by 2 Reasoning and Problem Solving Divide by 2 Developing 1a. Kyle is correct. 6 ÷ 2 = 3 2a. 10 ÷ 2 = 5 3a. 4 Expected 4a. Emma is incorrect. 18 ÷ 2 = 9 5a. 40 ÷ 2 = 20; 20 ÷ 2 = 10; 10 ÷ 2 = 5; 16 ÷ 2 = 8 6a. He can make 8 pairs of socks. Yes, he can still make pairs. He can now make 11 pairs ...
This investigative year 2 lesson pack teaches children how to tell the time to quarter to the hour. The children investigate the role of the hour and minute hands when reading and showing quarter to times on analogue clocks. They apply this knowledge to solve time challenges. The lesson pack includes Diving into Mastery resources to provide reasoning and problem-solving challenges.This lesson ...
pptx, 2.35 MB. pdf, 3.51 MB. These booklets each contain over 40 reasoning and problem solving questions suitable for KS1, KS2 and KS3 classes. These are the questions that we have been putting out each day in March 2016 on Twitter in the run up to SATS. The answers are provided with some simple notes at the back of the booklet and for some ...
Reasoning and Problem Solving Telling the Time to the Minute Reasoning and Problem Solving Telling the Time to the Minute Developing 1a. 1 = A; 2 = B 2a. B -because it is 'past' the hour and is 1 minute before quarter past which would be at 3. 3a. 16 minutes past 6. Expected 4a. 1 = A; 2 = C; 3 = B 5a. B -because it is 1 minute past the 4,
7b. 10 x 10 and 4 x 10 = 140, 10 x 10 and 6. 10 = 160, 9 x 10 and 10 x 10 = 190; The odd one out is 170: various answers, for example: 9 and 10 and 8 x 10 = 170 8b. Laura is correct because 9 x 10 = 90, 7. 10 = 70 and 90 + 70 = 160. 9b. Milo bought 6 bunches of grapes. If Tim eats 3 bunches of grapes there will be.
The answer should be 22, not 32. 8b. Various possible answers, for example: 81 - 65 = 16, 85 - 16 = 69 and 56 - 18 = 38 9b. Aisha is correct as she has correctly exchanged 1 ten for 10 ones so she has correctly subtracted 7 ones from 13 ones to leave her with 2 tens and 6 ones - 26. Reasoning and Problem Solving - Subtract with 2 ...
Oscar can feed 8 pigs because he has already used 35 of the carrots; 7 x 5 = 35, 75 - 35 = 40. He has 40 carrots left; 40 = 8 x. 5. 9b. Various answers, for example: The number could be 95 because 9 and 10 are both bigger than 8 but smaller than 12; 9 x 5 = 45, 10 x 5 = 50, 45 + 50 = 95. Reasoning and Problem Solving - The 5 Times Table ...
Johnson said CMS is actually coming off its best overall on-time performance year in recent history. He said of those buses that were late, 95% arrived within five minutes of the start of school.
This handy selection of year 2 reasoning questions is the perfect way to prepare your children for the year 2 SATs reasoning paper. Take your time and look over our questions to find the perfect ones for you or your children's needs. Our year 2 reasoning questions have all been created by our team of experienced teachers and follow national ...
Keywords: abduction, abductive reasoning, organic chemistry, diagnosis, metacognition, problem solving, pre-health education. Citation: Wackerly JW, Wentzel MT and Zingales SK (2024) Teaching abductive reasoning for use as a problem-solving tool in organic chemistry and beyond. Front. Educ. 9:1412417. doi: 10.3389/feduc.2024.1412417